Abstract
Introduction
Intensive care unit patients are at risk for post-intensive care syndrome (PICS), which includes psychological, physical and/or cognitive sequelae after their hospital stay. Our aim was to investigate PICS in adult patients with out-of-hospital cardiac arrest (OHCA).
Methods
In this prospective observational cohort study, we assessed risks for PICS at 3 and 12-month follow-up within the following domains: a) physical impairment (EuroQol [EQ-5D-3L]), b) cognitive functioning (Cerebral Performance Category [CPC] score >1, modified Rankin Scale [mRS] >2) and c) psychological burden (Hospital Anxiety and Depression Scale [HADS], Impact of Event Scale-Revised [IES-R]).
Results
At 3 months, 69/139 patients (50%) met the definition of PICS including 37% in the physical domain, 25% in the cognitive domain and 13% in the psychological domain. Intubation (OR 2.3, 95%CI 1.1 to 5,0 p = 0.03), sedatives (OR 3.4, 95%CI 1 to 11, p = 0.045), mRS at discharge (OR 4.3, 95%CI 1.70 to 11.01, p = 0.002), CPC at discharge (OR 3.3, 95%CI 1.4 to 7.6, p = 0.005) and post-discharge work loss (OR 13.4, 95%CI 1.7 to 107.5, p = 0.014) were significantly associated with PICS. At 12 months, 52/110 (47%) patients had PICS, which was associated with prolonged duration of rehabilitation, higher APACHE scores, and higher mRS and CPC scores at hospital discharge.
Conclusions
Nearly half of long-term OHCA survivors show PICS after 3 and 12 months. These high numbers call for more emphasis on appropriate screening and treatment in this patient population. Future studies should evaluate whether early identification of these patients enables preventive strategies and treatment options.
Introduction
Out-of-hospital cardiac arrest (OHCA) remains an important cause of death worldwide [1]. Less than a quarter of OHCA patients survive to hospital admission, and only half of initial survivors are discharged from the hospital alive [2]. Although therapeutic advances in intensive care medicine result in a higher number of ICU survivors, the overall ICU mortality decreased only very slightly over time due to the steadily increase of patients’ age and the number of comorbidities upon ICU admission [3]. Also, the risk of severe neurological deficits in ICU patients remains high [4,5] particularly in survivors of an out-of-hospital cardiac arrest (OHCA). In consequence, long-term physical, neurological and mental health status of ICU survivors has become an increasing concern in recent years [6]. These long-term impairments have been summarized under the term post-intensive care syndrome (PICS), which is commonly defined as new or aggravated dysfunction(s) in the physical, cognitive and/or mental (psychiatric) domain after critical illness [7]. Several studies suggest that more than 50% of ICU survivors suffer from at least one component of PICS [6,8]. Accordingly, PICS is becoming a more widely used concept in current clinical practice, even though attempts to define it with clinical accuracy are still ongoing [9].
Importantly, there is insufficient research data regarding the risk for PICS in the population of OHCA patients, although this population of patients is clearly at increased risk to suffer from long-term impairments and have worse physical and social functioning compared to the general population [10]. Studies have suggested that a relevant number of OHCA patients have moderate disabilities, poor autonomy and cognitive impairments particularly in regard to memory, attention and executive functioning [10–14]. In addition, OHCA patients are at increased risk for symptoms of depression, anxiety and posttraumatic stress disorder (PTSD) [15,16]. There are several well-known risk factors for adverse long-term health after OHCA including low-flow time, clinical severity at ICU admission, prolonged coma duration, and mechanical ventilation [11]. Also, young age and female gender was associated with higher risk for poor health [12].
Yet, to the best of our knowledge, only few studies have addressed the concept of PICS in OHCA patients. Better understanding the risk of PICS in OHCA patients is important for adequate future screening and treatment of patients at risk and may help to prevent PICS. Herein, we investigated the prevalence and potential risk factors for PICS in a well-defined cohort of adult OHCA survivors among the domains of physical, cognitive and psychological symptoms.
Materials and methods
Study setting
The COMMUNICATE study is an ongoing prospective observational cohort study (from 10/2012 to 10/2025) at ICU of the University Hospital Basel, a Swiss tertiary care hospital with ongoing sampling. The aim of the trial is to investigate the prognosis and long-term outcomes in consecutive adult patients after cardiac arrest. The methods applied in this study have been published previously [17–19]. The COMMUNICATE trial was approved by the local Ethics Committee (Ethics Committee Northwest and Central Switzerland, EKNZ; approval reference number: 2019–01162) and is conducted in accordance with the declaration of Helsinki. All patients, or in case of unconsciousness, patients’ next of kin provided written informed consent for study participation.
Study population
We included adult patients after OHCA who were admitted to the ICU and who participated in the 3-month and/or 12-month follow-up assessment after hospital/ICU admission. Further, we also included patients with in-hospital cardiac arrest (IHCA) if these were not monitored and had thus a similar risk for adverse outcome compared to OHCA patients. No exclusion criteria regarding patient characteristics, e.g., consciousness, type, severity, or duration of cardiac arrest were used.
Data collection
Data were prospectively collected upon ICU admission. Patients’ medical characteristics were extracted from hospital medical records. Further, we conducted predefined and structured telephone interviews with patients 3 and 12 months after ICU admission to evaluate outcomes. To assess outcomes, the research team performed systematic telephone interviews with patients lasting for around 20 minutes. Thereby, questionnaires were read aloud and patients’ answers were recorded.
Measures
Baseline predictor variables
We calculated all clinical scores at ICU arrival as suggested in original publications [20,21]. From hospital medical records, we collected patients’ sociodemographic information (e.g., age, gender, working status), the setting of cardiac arrest (e.g., location, initial rhythm, no-flow time, low-flow time), adrenaline (epinephrine) dose given), the reason for OHCA (i.e., coronary heart disease, arrhythmogenic, other reason), the ICU treatment received (e.g., intubation, targeted temperature management, use of vasoactive or sedative medication), medical complications during ICU stay (e.g., delirium), comorbidities (e.g., smoking status, hyperlipidemia, coronary disease, diabetes, renal failure, malignant disease), and ICU and hospital length of stay. Further, we assessed the number of weeks in rehabilitation and working status three months after hospitalization.
Outcome measures
All outcome measures were assessed at 3-month and 12-month follow-up. The primary outcome PICS was defined as symptoms or impairment in at least one of the following domains, as previously defined [7]: physical impairment, cognitive impairment and/or psychological distress. The primary endpoint was PICS measured at 3 months and secondary endpoint was PICS at 12 months follow-up.
Physical impairment. Physical impairment was evaluated with the EuroQol questionnaire (EQ-5D-3L), an established, extensively validated, as well as time-efficient self-report measure which assesses general health-related quality of life [22]. The EQ-5D-3L comprises five dimensions, i.e., mobility, self-care, usual activities, pain/discomfort and anxiety/depression, which can be rated on three levels, i.e. no problems, some problems and extreme problems. These dimensions can be summarized in an index ranging from -0.5 “worse than death” to 1 “full health” [23]. We used a cut-off score of ≤0.8 to determine relevant physical impairment. Cronbach’s alpha for this sample was α = 0.65.
Cognitive impairment. To assess cognitive impairment, we used the Cerebral Performance Category (CPC) [24] and the modified Rankin Scale (mRS) [25], two expert-rated and time-efficient scales.
The CPC measures patients’ neurological status. It distinguishes five levels. In line with previous studies, we defined level 1 (good recovery) as favorable neurological outcome, and 2 (moderate disability), 3 (severe disability), 4 (vegetative state) and 5 (death) as poor neurological outcome [26].
The mRS scale assesses neurological function on a scale from 0 to 6. We defined levels 0 (no symptoms), 1 (no significant disability despite symptoms; able to carry out all usual duties and activities) and 2 (slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance) as favorable outcome; and levels 3 (moderate disability; requiring some help, but able to walk without assistance) 4 (moderately severe disability, unable to walk and attend to bodily needs without assistance), 5 (severe disability; bedridden, incontinent and requiring constant nursing care and attention) and 6 (dead) were defined as unfavourable outcome [25,27].
Psychological distress. Psychological distress was defined as clinically relevant symptoms of anxiety, depression and/or PTSD. Symptoms of depression and anxiety were assessed with the Hospital Anxiety and Depression Scale (HADS) [28], a self-report instrument specifically developed for hospitalized patients with medical conditions. Good reliability and validity with a Cronbach’s alpha of 0.83 and 0.82 for the subscales anxiety and depression, respectively, has been demonstrated, as well as an optimal balance between sensitivity and specificity of approximately 0.80 when applying a cut-off score of ≥8 on both subscales [29]. Therefore, a score of ≥8 on the depression and/or anxiety subscale (range 0 to 21) of the HADS was considered as clinically relevant for the purpose of the study [28,29]. The HADS consists of 14 items and Cronbach’s alpha for this population was α = .84. PTSD symptoms were assessed by the Impact of Event Scale-Revised (IES-R) [30]. This self-report measure with 22 items covers three symptom domains, i.e., intrusion, avoidance, and hyperarousal. It shows high internal consistency with a Cronbach’s alpha of 0.96 and good diagnostic accuracy at a cut-off score of 1.5 [31], which we applied in this study. Cronbach’s alpha for this population was α = .92.
Statistical analysis
Descriptive statistics, i.e., frequencies and percentages for binary and categorical variables, as well as medians and interquartile ranges for continuous variables were used to present sociodemographic and clinical characteristics of the study population. The primary endpoint was PICS defined as a physical, cognitive and/or psychological impairment measured with different scales as defined above (i.e., in one of the five outcome measures). To evaluate associations between potential risk factors and PICS at 3- and 12-month follow-up, logistic regression analyses were performed for the primary endpoint and separately for the three domains of PICS. As a measure of association, we report odds ratios (OR) and 95% confidence intervals (CI). In addition, univariable logistic regression analyses were adjusted for age and gender. We did not perform further multivariable analyses due to the low number of events to avoid overfitting. Further, a chi-square test and cross-tables were used to determine the persistence of patients with PICS between 3- and 12-month follow-up. Pearson correlations were calculated between the PICS domains physical, cognitive and psychological symptoms in a correlation matrix at 3 and 12 months. Stata 15 (StataCorp, College Station, Texas, USA) was used for all statistical analyses. Statistical significance was defined as a p-value of <0.05 (two-tailed).
Results
Study sample and baseline characteristics
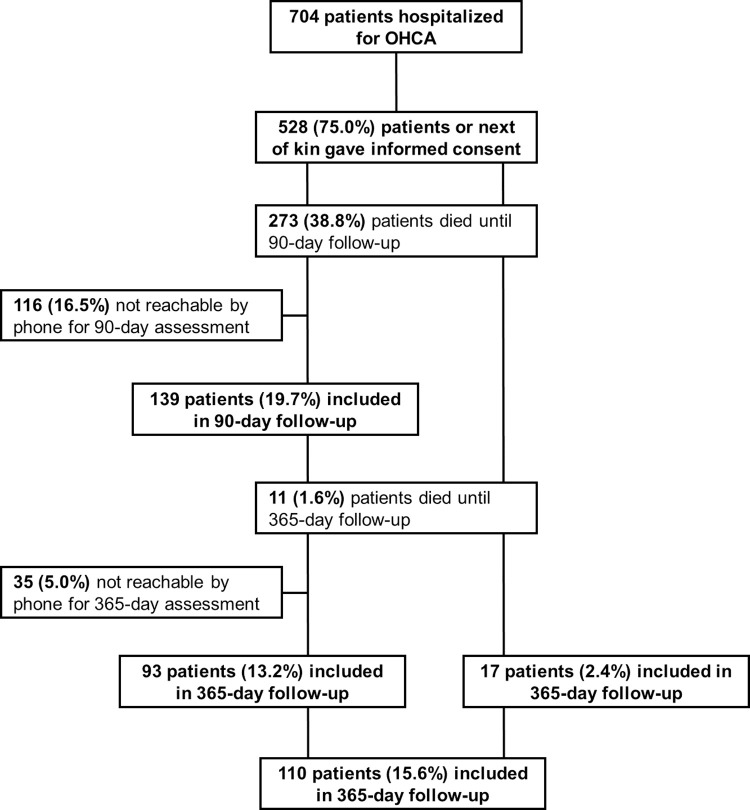
One-hundred fifty-six patients completed at least one of two follow-up interviews; 139 (89.1%) patients completed the 3-month interview, and 110 (70.5%) the 12-month interview. Ninety-three (59.6%) participants completed both interviews. Eleven (7.1%) patients died between the 3- and 12-month follow-up. A flow diagram of the study population is shown in Fig 1.
Fig 1. Flow diagram of the study population.
Sociodemographic and clinical characteristics of the study population and for patients included in the 3-month and 12-month follow-ups are shown in Table 1. Median age of patients was 62.8 years and 17% were female. The median duration of ICU stay was 4 days and median hospital length of stay was 13 days. Patients had a high burden of comorbidities and cardiovascular risk factors.
Table 1. Sociodemographic and clinical characteristics of the study population.
| Factor | All patients | 3 months | 12 months | |
|---|---|---|---|---|
| N | 156 | 139 | 110 | |
| Sociodemographics | ||||
| Age, median (IQR) | 62.8 (54, 73.2) | 63.2 (54.3, 73.5) | 62.6 (53.9, 73.2) | |
| Female, n (%) | 27 (17.3%) | 22 (15.8%) | 17 (15.5%) | |
| In partnership, n (%) | 123 (80.9%) | 109 (80.7%) | 90 (82.6%) | |
| Children, n (%) | 128 (82.1%) | 114 (82.0%) | 92 (83.6%) | |
| Highest education | School, n (%) | 14 (11.0%) | 12 (10.8%) | 12 (11.9%) |
| Diploma/ apprenticeship, n (%) | 90 (70.9%) | 79 (71.2%) | 72 (71.3%) | |
| University, n (%) | 23 (18.1%) | 20 (18.0%) | 17 (16.8%) | |
| Employed at baseline, n (%) | 72 (48.3%) | 61 (46.2%) | 54 (50.0%) | |
| Setting of cardiac arrest | ||||
| Setting of cardiac arrest | At home | 43 (28.7%) | 38 (28.6%) | 31 (29.0%) |
| In public | 95 (63.3%) | 84 (63.2%) | 69 (64.5%) | |
| IHCA | 12 (8.0%) | 11 (8.3%) | 7 (6.5%) | |
| Observed cardiac arrest | 143 (91.7%) | 126 (90.6%) | 106 (96.4%) | |
| Bystander CPR | 124 (79.5%) | 112 (80.6%) | 83 (75.5%) | |
| Professional bystander | 39 (48%) | 37 (47%) | 26 (57%) | |
| Initial rhythm | Ventricular tachycardia | 8 (5.2%) | 5 (3.6%) | 7 (6.4%) |
| Ventricular fibrillation | 114 (73.5%) | 102 (73.9%) | 81 (74.3%) | |
| Asystole | 7 (4.5%) | 6 (4.3%) | 6 (5.5%) | |
| Pulseless electrical activity | 9 (5.8%) | 9 (6.5%) | 7 (6.4%) | |
| Unknown | 17 (11.0%) | 16 (11.6%) | 8 (7.3%) | |
| No-flow (min), median (IQR) | 0 (0, 2) | 0 (0, 2) | 0 (0, 2) | |
| Low-flow (min), median (IQR) | 11 (8, 20) | 12 (9, 20) | 11.5 (8, 20) | |
| Time until ROSC (min), median (IQR) | 15 (10, 23) | 15 (10, 25) | 15 (10, 25) | |
| Adrenaline | No adrenaline | 77 (55.0%) | 70 (56.0%) | 56 (57%) |
| >0 and <3 mg | 33 (23.6%) | 26 (20.8%) | 23 (23%) | |
| ≥3 mg | 30 (21.4%) | 29 (23.2%) | 19 (19%) | |
| Clinical scores at ICU arrival | ||||
| APACHE II, median (IQR) | 25 (19, 30) | 25 (20, 30) | 25 (19, 30) | |
| SAPS II, median (IQR) | 58 (45, 66) | 58 (43, 66) | 58 (45, 68) | |
| GCS, median (IQR) | 4 (3, 14) | 4 (3, 14) | 5 (3, 14) | |
| Days in ICU, median (IQR) | 4 (2, 7) | 4 (2, 7) | 4 (2, 7) | |
| Total days of hospital stay, median (IQR) | 13 (8, 18) | 13 (8, 17) | 13 (8, 19) |
Note: Data are presented as n (%) or median (interquartile range). Abbreviations: APACHE II, Acute Physiology And Chronic Health Evaluation Score II; SAPS II, Simplified Acute Physiology Score II; GCS, Glasgow Coma Scale; OHCA, out-o-hospital cardiac arrest; IHCA, in-hospital cardiac arrest; IQR, interquartile range; CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation; IABP, intra-aortal balloon pump; mRS, modified Rankin Scale; CPC, Cerebral Performance Category.
Primary endpoint: PICS 3 months after hospitalization
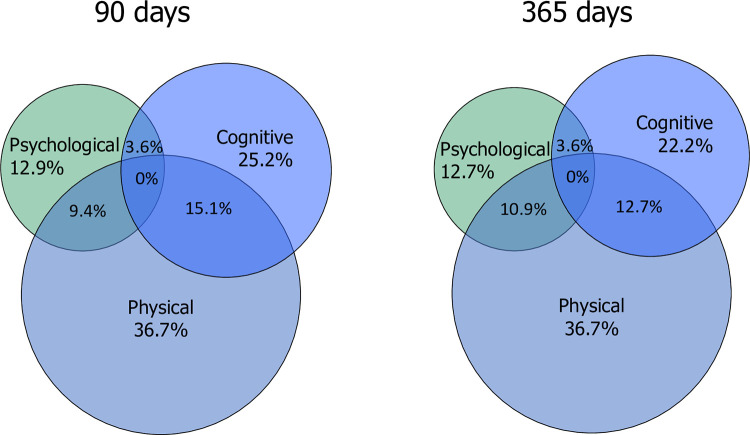
Of 139 patients, 69 patients (49.6%) showed evidence of PICS 3 months after OHCA. Of those, 36.7% showed physical impairment, 25.2% cognitive impairment, and 12.9% psychological distress. Fig 1 shows the distribution of impairments among the different domains.
We assessed the association of several potential predictors with the risk for PICS at 3 months adjusted for age and gender (Table 2). Several factors were associated with PICS including baseline severity of illness scores (APACHE II: OR 1.07, 95%CI 1.02 to 1.12, p = 0.007 and SAPS II: OR 1.03, 95%CI 1.01 to 1.06, p = 0.006), intubation (OR 2.21, 95%CI 1.02 to 4.78, p = 0.043) and duration of intubation (in days) (OR 1.21, 95%CI 1 to 1.46, p = 0.046), length of ICU stay (in days) (OR 1.11, 95%CI 1.01 to 1.21, p = 0.022), functionality at discharge (poor mRS score: OR 4.35, 95%CI 1.7 to 11.1, p = 0.002 and CPC score: OR 3.39, 95%CI 1.46 to 7.88, p = 0.005), as well as work loss within the observed 3 months (OR 14.53, 95%CI 1.8 to 117.56, p = 0.012).
Table 2. Associations of predictor variables and post-intensive care syndrome at 3 months.
| Factor | No PICS | PICS | OR (95%CI) | p | OR adjusted for age and gender (95%CI) | p | |
|---|---|---|---|---|---|---|---|
| N | 70 | 69 | |||||
| Sociodemographics | |||||||
| Age, median (IQR) | 65.4 (58.6, 73.5) | 61.1 (53.3, 73.3) | 0.99 (0.97, 1.01) | 0.46 | NA | NA | |
| Female, n (%) | 9 (13%) | 13 (19%) | 1.57 (0.62, 3.96) | 0.34 | NA | NA | |
| In partnership, n (%) | 54 (79%) | 55 (82%) | 1.19 (0.5, 2.8) | 0.69 | 1.25 (0.53, 2.99) | 0.61 | |
| Children, n (%) | 55 (79%) | 59 (86%) | 1.61 (0.67, 3.88) | 0.29 | 1.73 (0.7, 4.28) | 0.24 | |
| Highest education | School, n (%) | 7 (12%) | 5 (9%) | 0.73 (0.22, 2.45) | 0.61 | 0.95 (0.45, 2.02) | 0.89 |
| Diploma/Apprenticeship, n (%) | 38 (67%) | 41 (76%) | 1.58 (0.69, 3.62) | 0.28 | 1.61 (0.7, 3.72) | 0.27 | |
| University, n (%) | 12 (21%) | 8 (15%) | 0.65 (0.24, 1.75) | 0.40 | 0.68 (0.25, 1.85) | 0.45 | |
| Employed at baseline, n (%) | 27 (41%) | 34 (52%) | 1.53 (0.77, 3.05) | 0.22 | 1.69 (0.65, 4.36) | 0.28 | |
| Setting of cardiac arrest | |||||||
| Setting of cardiac arrest | At home | 20 (29%) | 18 (28%) | 1.20 (0.66, 2.18) | 0.55 | 1.27 (0.69, 2.35) | 0.43 |
| In public | 45 (65%) | 39 (61%) | |||||
| IHCA | 4 (6%) | 7 (11%) | |||||
| Observed cardiac arrest | 63 (90%) | 63 (91%) | 1.16 (0.37, 3.67) | 0.79 | 1.15 (0.37, 3.66) | 0.80 | |
| Bystander CPR | 57 (81%) | 55 (80%) | 0.90 (0.39, 2.08) | 0.80 | 0.89 (0.38, 2.09) | 0.80 | |
| Professional bystander | 23 (55%) | 14 (38%) | 0.50 (0.20, 1.24) | 0.13 | 0.48 (0.18, 1.31) | 0.15 | |
| Initial rhythm | Ventricular tachycardia | 4 (6%) | 1 (1%) | 1.05 (0.77, 1.44) | 0.75 | 1.04 (0.77, 1.43) | 0.77 |
| Ventricular fibrillation | 52 (74%) | 50 (74%) | |||||
| Asystole | 0 (0%) | 6 (9%) | |||||
| Pulseless electrical activity | 6 (9%) | 3 (4%) | |||||
| Unknown | 8 (11%) | 8 (12%) | |||||
| No-flow (min), median (IQR) | 0 (0, 4) | 0 (0, 2) | 1.01 (0.91, 1.12) | 0.89 | 1.01 (0.91, 1.12) | 0.91 | |
| Low-flow (min), median (IQR) | 12 (10, 20) | 12 (7, 23) | 1.01 (0.99, 1.04) | 0.36 | 1.01 (0.99, 1.04) | 0.39 | |
| Time until ROSC (min), median (IQR) | 15 (10, 21) | 14 (8, 30) | 1.01 (0.99, 1.04) | 0.28 | 1.01 (0.99, 1.04) | 0.32 | |
| Adrenaline | No adrenaline | 38 (58%) | 32 (53%) | 1.19 (0.78, 1.82) | 0.42 | 1.16 (0.75, 1.80) | 0.50 |
| >0 and <3 mg | 14 (22%) | 12 (20%) | |||||
| ≥3 mg | 13 (20%) | 16 (27%) | |||||
| Clinical scores at ICU arrival | |||||||
| APACHE II, median (IQR) | 24 (17, 29) | 26 (21, 31) | 1.06 (1.02, 1.11) | 0.01 | 1.07 (1.02, 1.12) | 0.007 | |
| SAPS II, median (IQR) | 55 (36, 65) | 61 (51, 68) | 1.03 (1.01, 1.05) | 0.01 | 1.03 (1.01, 1.06) | 0.006 | |
| GCS, median (IQR) | 4 (3, 15) | 4 (3, 8) | 0.95 (0.89, 1.02) | 0.17 | 0.96 (0.89, 1.02) | 0.19 | |
| Reason for OHCA | |||||||
| Coronary heart disease, n(%) | 45 (66%) | 47 (69%) | 1.14 (0.56, 2.35) | 0.71 | 1.24 (0.59, 2.6) | 0.57 | |
| Rhythmogenic, n(%) | 13 (19%) | 9 (13%) | 0.65 (0.26, 1.63) | 0.35 | 0.62 (0.24, 1.58) | 0.32 | |
| Other or unclear reason, n (%) | 10 (15%) | 12 (18%) | 1.24 (0.5, 3.11) | 0.64 | 1.15 (0.45, 2.92) | 0.77 | |
| Intensive care treatment | |||||||
| Intubation, n (%) | 44 (63%) | 55 (80%) | 2.32 (1.08, 4.97) | 0.03 | 2.21 (1.02, 4.78) | 0.04 | |
| Total days of intubation, median (IQR) | 2 (1, 2) | 2 (2, 6) | 1.21 (1.01, 1.45) | 0.04 | 1.21 (1, 1.46) | 0.046 | |
| Targeted temperature management (TTM), n (%) | 34 (49%) | 43 (62%) | 1.75 (0.89, 3.44) | 0.10 | 1.74 (0.86, 3.5) | 0.12 | |
| Vasoactives, n (%) | 56 (80%) | 51 (74%) | 0.71 (0.32, 1.57) | 0.40 | 0.68 (0.31, 1.52) | 0.35 | |
| Impella / IABP, n (%) | 4 (6%) | 5 (7%) | 1.29 (0.33, 5.02) | 0.71 | 1.21 (0.3, 4.84) | 0.79 | |
| Sedatives, n (%) | 58 (83%) | 65 (94%) | 3.36 (1.03, 11) | 0.05 | 3.18 (0.97, 10.48) | 0.06 | |
| Coronary angiography, n (%) | 61 (87%) | 63 (91%) | 1.55 (0.52, 4.61) | 0.43 | 1.59 (0.53, 4.79) | 0.41 | |
| Medical complications during ICU stay | |||||||
| Aspiration, n (%) | 29 (41%) | 28 (41%) | 0.97 (0.49, 1.9) | 0.92 | 0.99 (0.5, 1.96) | 0.98 | |
| Pneumonia, n (%) | 31 (44%) | 33 (48%) | 1.15 (0.59, 2.25) | 0.68 | 1.19 (0.61, 2.35) | 0.61 | |
| Hemorrhage, n (%) | 5 (7%) | 7 (10%) | 1.47 (0.44, 4.87) | 0.53 | 1.52 (0.46, 5.1) | 0.50 | |
| Delirium, n (%) | 25 (36%) | 22 (32%) | 0.84 (0.42, 1.7) | 0.63 | 0.87 (0.43, 1.78) | 0.71 | |
| Renal failure, n (%) | 7 (10%) | 11 (16%) | 1.71 (0.62, 4.7) | 0.30 | 1.7 (0.61, 4.74) | 0.31 | |
| Seizure, n (%) | 2 (3%) | 7 (10%) | 3.84 (0.77, 19.18) | 0.10 | 4.13 (0.82, 20.84) | 0.09 | |
| Days in ICU, median (IQR) | 4 (2, 5) | 4 (2, 7) | 1.1 (1.02, 1.2) | 0.02 | 1.11 (1.01, 1.21) | 0.02 | |
| Total days of hospital stay, median (IQR) | 12 (7, 16) | 14 (9, 18) | 1.03 (0.99, 1.07) | 0.14 | 1.03 (0.99, 1.08) | 0.13 | |
| Poor mRS score at ICU discharge, n (%) | 7 (10%) | 22 (33%) | 4.33 (1.7, 11.01) | 0.002 | 4.35 (1.7, 11.1) | 0.002 | |
| Poor CPC score at ICU discharge, n (%) | 10 (14%) | 24 (36%) | 3.29 (1.43, 7.6) | 0.01 | 3.39 (1.46, 7.88) | 0.005 | |
| Follow-up on patients after 3 months | |||||||
| Rehabilitation | None, n (%) | 24 (34%) | 19 (28%) | 0.73 (0.35, 1.5) | 0.39 | 0.72 (0.35, 1.5) | 0.38 |
| Up to 3 weeks, n (%) | 25 (36%) | 25 (36%) | 1.02 (0.51, 2.04) | 0.95 | 1.04 (0.52, 2.09) | 0.91 | |
| More than 3 weeks, n (%) | 21 (30%) | 25 (36%) | 1.33 (0.65, 2.69) | 0.44 | 1.31 (0.64, 2.67) | 0.46 | |
| Working status | Still working, n (%) | 26 (42%) | 22 (36%) | 0.78 (0.38, 1.61) | 0.51 | 0.49 (0.18, 1.35) | 0.17 |
| Work lost, n (%) | 1 (2%) | 11 (18%) | 13.42 (1.67, 107.53) | 0.01 | 14.53 (1.8, 117.56) | 0.01 | |
| No work prior to OHCA, n (%) | 35 (56%) | 28 (46%) | 0.65 (0.32, 1.33) | 0.24 | 0.51 (0.18, 1.46) | 0.21 |
Note: Data are presented as n (%) or median (interquartile range). Abbreviations: IQR, interquartile range; ROSC, return to spontaneous circulation; IABP, intra-aortal balloon pump; mRS, modified Rankin Scale; CPC, Cerebral Performance Category; APACHE II, Acute Physiology And Chronic Health Evaluation Score II; SAPS II, Simplified Acute Physiology Score II.
Secondary endpoint: PICS 12 months after hospitalization
Of 110 patients, 52 patients (47.3%) showed evidence of PICS after 12 months with 36.7% showing physical impairment, 22.2% cognitive impairment, and 12.7% psychological distress (Fig 2). We assessed potential predictors for PICS (Table 3) and found initial severity of illness scores (APACHE II: OR 1.08, 95%CI 1.02 to 1.14, p = 0.008) and functionality at discharge (poor mRS score: OR 3.97, 95%CI 1.42 to 11.12, p = 0.009; and CPC score: OR 3.22, 95%CI 1.29 to 8.04, p = 0.012) to be associated with PICS. In addition, risk for PICS was lower in patients not needing rehabilitation (OR 0.31, 95%CI 0.12 to 0.82, p = 0.019) and in turn increased with longer duration of the rehabilitation (in days) (OR 1.24, 95%CI 1.03 to 1.5, p = 0.027).
Fig 2. Co-occurrence of post-intensive care syndrome domains at 3 and 12 months.
Note: Post-intensive care syndrome domains, i.e. physical, cognitive and psychological symptoms, and overlaps between the different domains.
Table 3. Associations of predictor variables and post-intensive care syndrome at 12 months.
| Factor | No PICS | PICS | OR (95% CI) | p | OR adjusted for age and gender (95% CI) | p | |
|---|---|---|---|---|---|---|---|
| N | 58 | 52 | |||||
| Sociodemographics | |||||||
| Age, median (IQR) | 63.4 (54, 72.4) | 61.1 (53.4, 74.5) | 1.01 (0.98, 1.04) | 0.49 | NA | NA | |
| Female, n (%) | 8 (14%) | 9 (17%) | 1.31 (0.46, 3.69) | 0.61 | NA | NA | |
| In partnership, n (%) | 48 (84%) | 42 (81%) | 0.79 (0.29, 2.12) | 0.64 | 0.78 (0.29, 2.11) | 0.62 | |
| Children, n (%) | 46 (79%) | 46 (88%) | 2 (0.69, 5.78) | 0.20 | 1.85 (0.62, 5.5) | 0.27 | |
| Highest education | School, n (%) | 6 (12%) | 6 (12%) | 1.07 (0.32, 3.57) | 0.91 | 1.17 (0.31, 4.34) | 0.82 |
| Diploma/apprenticeship, n (%) | 34 (65%) | 38 (78%) | 1.83 (0.76, 4.42) | 0.18 | 1.79 (0.73, 4.36) | 0.2 | |
| University, n (%) | 12 (23%) | 5 (10%) | 0.38 (0.12, 1.17) | 0.09 | 0.37 (0.12, 1.15) | 0.09 | |
| Employed at baseline, n (%) | 29 (51%) | 25 (49%) | 0.93 (0.44, 1.98) | 0.85 | 1.19 (0.45, 3.1) | 0.73 | |
| Setting of cardiac arrest | |||||||
| Setting of cardiac arrest | At home | 14 (25%) | 17 (33%) | 0.83 (0.41, 1.64) | 0.58 | 0.82 (0.41, 1.66) | 0.59 |
| In public | 39 (70%) | 30 (59%) | |||||
| IHCA | 3 (5%) | 4 (8%) | |||||
| Observed cardiac arrest | 54 (93%) | 52 (100%) | 1 | - | 1 | - | |
| Bystander CPR | 46 (79%) | 37 (71%) | 0.64 (0.27, 1.54) | 0.32 | 0.59 (0.24, 1.45) | 0.25 | |
| Professional bystander | 15 (54%) | 11 (61%) | 1.36 (0.41, 4.54) | 0.62 | 1.71 (0.42, 6.90) | 0.45 | |
| Initial rhythm | Ventricular tachycardia | 5 (9%) | 2 (4%) | 0.77 (0.51, 1.16) | 0.21 | 0.77 (0.51, 1.17) | 0.22 |
| Ventricular fibrillation | 41 (71%) | 40 (78%) | |||||
| Asystole | 1 (2%) | 5 (10%) | |||||
| Pulseless electrical activity | 3 (5%) | 4 (8%) | |||||
| Unknown | 8 (14%) | 0 (0%) | |||||
| No-flow (min), median (IQR) | 0 (0, 2) | 0 (0, 2) | 1.07 (0.96, 1.20) | 0.24 | 1.08 (0.96, 1.21) | 0.20 | |
| Low-flow (min), median (IQR) | 11 (9, 17) | 12 (6, 30) | 1.02 (0.99, 1.05) | 0.21 | 1.02 (0.99, 1.06) | 0.13 | |
| Time until ROSC (min), median (IQR) | 15 (10, 20) | 20 (8, 30) | 1.02 (0.99, 1.05) | 0.13 | 1.03 (1, 1.06) | 0.07 | |
| Adrenaline | No adrenaline | 36 (69%) | 20 (43%) | 2.03 (1.19, 3.47) | 0.01 | 2.30 (1.30, 4.09) | 0.004 |
| >0 and <3 mg | 10 (19%) | 13 (28%) | |||||
| ≥3 mg | 6 (12%) | 13 (28%) | |||||
| Clinical scores at ICU arrival | |||||||
| APACHE II, median (IQR) | 24 (17, 28) | 28 (22, 32) | 1.08 (1.02, 1.14) | 0.01 | 1.08 (1.02, 1.14) | 0.01 | |
| SAPS II, median (IQR) | 58 (39, 66) | 60 (50, 70) | 1.02 (1, 1.05) | 0.11 | 1.02 (0.99, 1.05) | 0.13 | |
| GCS, median (IQR) | 5 (3, 15) | 4 (3, 9) | 0.97 (0.90, 1.04) | 0.38 | 0.96 (0.89, 1.04) | 0.30 | |
| Reason for OHCA at ICU admission | |||||||
| Coronary heart disease, n (%) | 38 (70%) | 33 (63%) | 0.73 (0.32, 1.65) | 0.45 | 0.75 (0.32, 1.73) | 0.49 | |
| Rhythmogenic, n (%) | 10 (19%) | 11 (21%) | 1.18 (0.45, 3.07) | 0.73 | 1.17 (0.44, 3.12) | 0.75 | |
| Other or unclear reason, n (%) | 6 (11%) | 8 (15%) | 1.45 (0.47, 4.52) | 0.52 | 1.39 (0.44, 4.39) | 0.58 | |
| Intensive care treatment | |||||||
| Intubation, n (%) | 37 (64%) | 41 (79%) | 2.12 (0.9, 4.97) | 0.09 | 2.34 (0.97, 5.64) | 0.06 | |
| Total days of intubation, median (IQR) | 2 (1, 2) | 2 (1, 6) | 1.21 (0.98, 1.49) | 0.08 | 1.25 (0.99, 1.58) | 0.07 | |
| Targeted temperature management (TTM), n (%) | 30 (52%) | 31 (60%) | 1.38 (0.65, 2.94) | 0.41 | 1.61 (0.71, 3.63) | 0.25 | |
| Vasoactives, n (%) | 47 (81%) | 38 (73%) | 0.64 (0.26, 1.56) | 0.32 | 0.65 (0.26, 1.59) | 0.34 | |
| Impella / IABP, n (%) | 5 (9%) | 7 (13%) | 1.65 (0.49, 5.55) | 0.42 | 1.89 (0.54, 6.59) | 0.32 | |
| Sedatives, n (%) | 49 (84%) | 48 (92%) | 2.2 (0.64, 7.64) | 0.21 | 2.28 (0.65, 8.02) | 0.20 | |
| Coronary angiography, n (%) | 50 (86%) | 45 (87%) | 1.03 (0.35, 3.06) | 0.96 | 1.14 (0.37, 3.51) | 0.82 | |
| Medical complications during ICU stay | |||||||
| Aspiration, n (%) | 25 (43%) | 20 (38%) | 0.83 (0.38, 1.77) | 0.62 | 0.87 (0.4, 1.9) | 0.73 | |
| Pneumonia, n (%) | 28 (48%) | 25 (48%) | 0.99 (0.47, 2.1) | 0.98 | 1.05 (0.49, 2.25) | 0.90 | |
| Hemorrhage, n (%) | 3 (5%) | 8 (15%) | 3.33 (0.83, 13.31) | 0.09 | 3.36 (0.83, 13.54) | 0.09 | |
| Delirium, n (%) | 18 (31%) | 18 (35%) | 1.18 (0.53, 2.61) | 0.69 | 1.18 (0.53, 2.64) | 0.68 | |
| Renal failure, n (%) | 5 (9%) | 10 (19%) | 2.52 (0.8, 7.95) | 0.11 | 2.46 (0.77, 7.81) | 0.13 | |
| Seizure, n (%) | 2 (3%) | 4 (8%) | 2.33 (0.41, 13.3) | 0.34 | 2.63 (0.45, 15.27) | 0.28 | |
| Days in ICU, median (IQR) | 4 (2, 8) | 4.5 (2, 7) | 1.03 (0.96, 1.11) | 0.39 | 1.05 (0.97, 1.13) | 0.27 | |
| Total days of hospital stay, median (IQR) | 13 (8, 16) | 14 (7, 21) | 1.03 (0.98, 1.07) | 0.22 | 1.03 (0.98, 1.07) | 0.20 | |
| Poor mRS score at ICU discharge, n (%) | 6 (11%) | 17 (33%) | 4.05 (1.45, 11.29) | 0.01 | 3.97 (1.42, 11.12) | 0.01 | |
| Poor CPC score at ICU discharge, n (%) | 9 (16%) | 20 (38%) | 3.26 (1.32, 8.08) | 0.01 | 3.22 (1.29, 8.04) | 0.01 | |
| Follow-up on patients after 3 months | |||||||
| Rehabilitation | None | 19 (33%) | 7 (13%) | 0.32 (0.12, 0.84) | 0.02 | 0.31 (0.12, 0.82) | 0.02 |
| Up to 3 weeks | 21 (36%) | 16 (31%) | 0.78 (0.35, 1.74) | 0.55 | 0.79 (0.36, 1.77) | 0.57 | |
| More than 3 weeks | 18 (31%) | 29 (56%) | 2.8 (1.28, 6.11) | 0.01 | 2.88 (1.3, 6.38) | 0.01 | |
| Working status | Still working | 26 (48%) | 17 (36%) | 0.61 (0.27, 1.36) | 0.23 | 0.69 (0.23, 2.01) | 0.49 |
| Work lost | 3 (6%) | 7 (15%) | 2.98 (0.72, 12.24) | 0.13 | 3.07 (0.74, 12.82) | 0.12 | |
| No work prior to OHCA | 25 (46%) | 23 (49%) | 1.11 (0.51, 2.43) | 0.79 | 0.72 (0.24, 2.11) | 0.55 |
Note: Data are presented as n (%) or median (interquartile range). Abbreviations: IQR, interquartile range; ROSC, return to spontaneous circulation; IABP, intra-aortal balloon pump; mRS, modified Rankin Scale; CPC, Cerebral Performance Category; APACHE II, Acute Physiology And Chronic Health Evaluation Score II; SAPS II, Simplified Acute Physiology Score II.
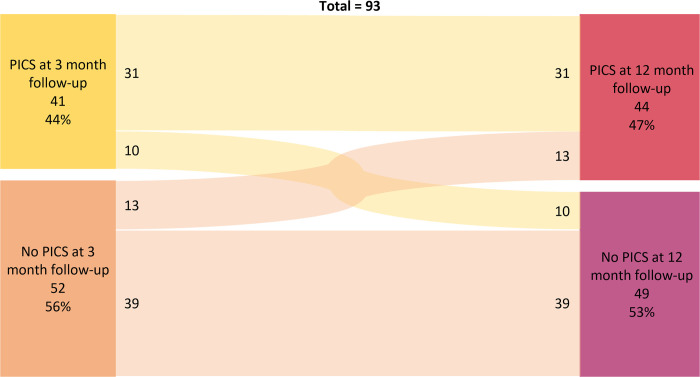
We also investigated, in the 93 patients that were assessed at both time points, whether PICS at 3-month would persist after 12-month. Results stratified according to PICS at both time points are shown in Fig 3. Chi-square test between PICS at 3 and 12 months was significant, X2(1, N = 93) = 23.6, p < .001. Further, we investigated how the different domains of PICS were inter-correlated by calculation of a correlation matrix at 3- and 12-month as shown in Table 4. Correlations between PICS domains at 3-month follow-up showed significant correlations between the physical and psychological domain and between the physical and cognitive domain. Similar results were found at 12-month follow-up.
Fig 3. Sankey diagram of occurrence of PICS or no PICS at 3 and 12 months.
Table 4. Correlation matrix of physical, cognitive and psychological domain at 3- and 12-month follow-up.
| PICS domains at 3 months | PICS domains at 12 months | |||||
|---|---|---|---|---|---|---|
| Physical domain | Cognitive domain | Psychological domain | Physical domain | Cognitive domain | Psychological domain | |
| Physical domain | - | - | . | - | - | - |
| Cognitive domain | 0.28, p<0.001 | - | - | 0.25, p<0.01 | - | - |
| Psychological domain | 0.28, p<0.001 | 0.02, p = 0.79 | - | 0.39, p<0.001 | 0.06, p = 0.55 | - |
Note: Data reported in Pearson correlation coefficient r.
Discussion
In this prospective observational cohort study, we found that nearly half of our OHCA survivors suffered from long-term health impairments after their ICU stay. One in three patients showed physical impairments, one in four had cognitive impairments, and one in eight patients psychological distress. These findings were comparable at 3 and 12 months following cardiac arrest with similar percentages overall and within domains. We found weak, yet significant correlations between domains except for the psychological and cognitive domain. Furthermore, several baseline predictors were identified as potential risk factors.
This study has several important implications. First, the prevalence of PICS found in our cohort of OHCA surviors is comparable to other cohorts of general ICU patients at 3 and 12 months [8]. Yet, there are differences in the distribution among PICS domains. We found similar rates of physical impairments of almost 40% in our cohort compared to studies from the general ICU patient population [32]. In contrast to other reports showing an improvement in self-assessed health at long-term [11,12], our cohort was fairly stable within the 12 months of investigation. Furthermore, we found cognitive impairments in 25% and 22% of patients at 3 and 12 months. Importantly, these numbers may be influenced by the instrument used for assessment: objective assessments of cognitive impairment have found higher prevalences compared to subjective assessments [13]. We used a subjective instrument for assessing cognitive impairment [33], which may explain the lower risks, which is again in line with other reports [14]. Also, one third to nearly half of ICU patients have been found to suffer from mental health issues [34,35]. For OHCA patients, previous reports ranged between 14% to 45% for depression and from 13% to 61% for anxiety, again dependent on the instrument and cut-offs used [15]. Our findings of 13% at both time points are thus in the lower range of these studies [15].
Second, several clinical and psychosocial factors were related to developing PICS at 3 months including severity of illness, adrenaline, intubation, functionality at discharge and work loss within 3 months post-discharge. These risk factors, however, are challenging to modify. Prolonged mechanical ventilation or deep sedation have previously been found to aggravate symptoms of PICS [36,37]. Thus, daily stop of anesthetics to avoid oversedation, early weaning strategies and use of lower sedative drug doses have become an important goal in any ICU patient care [37,38]. Additionally, we found that the need for rehabilitation and prolonged rehabilitation were associated with PICS 12 months after OHCA. Our data indicate that during rehabilitation, screening for PICS could help identify high-risk patients needing medical and psychological support, which in turn may reduce their risk in the long term. This may be important not only for the individual patient but also on a larger economic and social level [39].
Similarly, cognitive impairment at discharge assessed by the mRS and CPC score was associated with PICS 3 and 12 months following OHCA. This association may at least partially be explained by the cognitive impairments we had already found at baseline persisting in the long-term. This is in line with research, showing that most recovery of cognitive function in ICU patients occurred within the first 3 months with only little improvements after 12 months [40]. Thus, measures of cognitive functioning may be useful in screening patients to predict long-term PICS early on.
Interestingly, no patients had impairments in all three PICS domains at neither time point of assessment. This is in line with other results in general ICU patients: Marra et al. found a 56% prevalence of PICS-related complaints when considering one or more domains, but a much lower prevalence when complaints in all three domains were considered (i.e., 4% after 12 months) [8]. Concerning the co-occurrence of the different PICS domains, we found weak, yet significant correlations between domains except between the psychological and cognitive domains. This coincides with findings in OHCA patients that show health-related quality of life to be associated with cognitive impairments [14,41], as well as with psychological distress [14,16], yet finding mixed results in associations between psychological distress and cognitive impairment [16,42]. Possibly, PICS in OHCA patients falls into two subgroups: physically and cognitively impaired patients, or physically impaired and psychologically distressed patients. However, this hypothesis must be validated in future research.
Our findings suggest that PICS at 3 months is highly predictive for PICS after 12 months. At the same time, our data show that 11 patients newly developed physical impairment, 6 developed cognitive impairment and 8 patients developed psychological distress at twelve-month follow-up. Research shows levels of psychological distress and self-assessed health to improve among OHCA survivors in the long term [11,12], yet only minor improvements have been found in cognitive performance from 3 to 12 months [14]. However, these results are average findings and are comparable to our percentual stability of PICS impairments over time. Yet to the best of our knowledge, no analysis has assessed the course of symptoms as fine-grained as our study, therefore, intraindividual trajectories in other studies remain unclear. Possibly, due to patients’ self-report as only information, subjective health impairment may become more visible in everyday life over time.
This trial is strengthened by the prospective and consecutive inclusion of study patients. Yet, it also has several limitations. As an observational study, the results are in need for interventional research to prove causality. Also, due to the sample size the power of the study is limited. Further, 83% of the study cohort are men, however, we adjusted for gender in the multivariable model to control for possible confounding. Also, patient outcomes were assessed subjectively, therefore outcomes might differ to objective outcome measures. Further, as several patients were not reachable at either 3- or 12-month follow-up, only a subgroup could be assessed for PICS trajectories over time. Also, as a single-center study, there is a lack of generalizability to other institutions and countries. Therefore, multicenter and multinational studies are necessary to validate our findings. Further, since no single definition of PICS exists, comparability with other study findings is limited. We do not expect biased results by the telephone assessment, as no difference has been found between face-to-face and telephone self-report measures [43]. Within this hypothesis generating study, we aimed to understand the possible associations of baseline factors and long-term risk for PICS. Because there is insufficient literature on this topic, we did not preselect variables but present the full list of predictors and due to the limited number of events, we adjusted the analysis only for age and gender. The high number of tests makes type II error possible and prospective validation is needed in an independent cohort. Finally, in our analysis acute physiology parameters wane in importance as time from OHCA passes, but mRS and CPC continue to dominate the associations. This may be indeed specific to the population of OHCA patients with brain injury and may differ in other ICU populations. However, more data is needed to better understand the influence on brain injury on long-term risk for PICS.
Conclusions
With a growing number of patients surviving their ICU stay after an OHCA and nearly half of all OHCA survivors displaying evidence of PICS up to one year after ICU admission, appropriate screening and management is necessary to minimize the risk for PICS and to meet the increased need for its treatment. Future studies should evaluate whether early identification of these patients enables preventive strategies.
Supporting information
(XLSX)
Acknowledgments
We would like to express our gratitude to all patients and their relatives for participating in the COMMUNICATE trial as well as to the medical ICU and laboratory staff of the University Hospital Basel for making this study possible.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Sabina Hunziker and her research team received funding by the Swiss National Science Foundation (SNSF) (Ref 10001C_192850/1 and 10531C_182422) and the Gottfried and Julia Bangerter-Rhyner Foundation (8472/HEG-DSV). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 2.Yan S, Gan Y, Jiang N, Wang R, Chen Y, Luo Z, et al. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: a systematic review and meta-analysis. Critical Care. 2020;24(1):61. doi: 10.1186/s13054-020-2773-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim DY, Lee MH, Lee SY, Yang BR, Kim HA. Survival rates following medical intensive care unit admission from 2003 to 2013: An observational study based on a representative population-based sample cohort of Korean patients. Medicine. 2019;98(37):e17090. doi: 10.1097/MD.0000000000017090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young GB. Clinical practice. Neurologic prognosis after cardiac arrest. N Engl J Med. 2009;361(6):605–11. doi: 10.1056/NEJMcp0903466 [DOI] [PubMed] [Google Scholar]
- 5.Hendriks JM, Brits T, Van der Zijden T, Monsieurs K, de Bock D, De Paep R. U-Shape Kissing Chimney Thoracic Endovascular Aneurysm Repair for a Traumatic Arch Rupture in a Polytraumatized Patient. Aorta (Stamford). 2015;3(1):41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan A. Long-term outcomes from critical care. Surgery (Oxford). 2021;39(1):53–7. doi: 10.1016/j.mpsur.2020.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preiser J-C, Herridge MS, Azoulay E. Post-Intensive Care Syndrome: Springer; 2020. [Google Scholar]
- 8.Marra A, Pandharipande PP, Girard TD, Patel MB, Hughes CG, Jackson JC, et al. Co-Occurrence of Post-Intensive Care Syndrome Problems Among 406 Survivors of Critical Illness. Critical care medicine. 2018;46(9):1393–401. doi: 10.1097/CCM.0000000000003218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnbull AE, Rabiee A, Davis WE, Nasser MF, Venna VR, Lolitha R, et al. Outcome Measurement in ICU Survivorship Research From 1970 to 2013: A Scoping Review of 425 Publications. Critical care medicine. 2016;44(7):1267–77. doi: 10.1097/CCM.0000000000001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin YH, Yaow CYL, Teoh SE, Foo MZQ, Luo N, Graves N, et al. Long-term outcomes after out-of-hospital cardiac arrest: A systematic review and meta-analysis. Resuscitation. 2022;171:15–29. doi: 10.1016/j.resuscitation.2021.12.026 [DOI] [PubMed] [Google Scholar]
- 11.Peskine A, Cariou A, Hajage D, Deye N, Guérot E, Dres M, et al. Long-Term Disabilities of Survivors of Out-of-Hospital Cardiac Arrest: The Hanox Study. Chest. 2021;159(2):699–711. doi: 10.1016/j.chest.2020.07.022 [DOI] [PubMed] [Google Scholar]
- 12.Viktorisson A, Sunnerhagen KS, Johansson D, Herlitz J, Axelsson Å. One-year longitudinal study of psychological distress and self-assessed health in survivors of out-of-hospital cardiac arrest. BMJ Open. 2019;9(7):e029756. doi: 10.1136/bmjopen-2019-029756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moulaert VRMP Verbunt JA, van Heugten CM Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: A systematic review. Resuscitation. 2009;80(3):297–305. doi: 10.1016/j.resuscitation.2008.10.034 [DOI] [PubMed] [Google Scholar]
- 14.Ørbo M, Aslaksen PM, Larsby K, Schäfer C, Tande PM, Anke A. Alterations in cognitive outcome between 3 and 12 months in survivors of out-of-hospital cardiac arrest. Resuscitation. 2016;105:92–9. [DOI] [PubMed] [Google Scholar]
- 15.Wilder Schaaf KP, Artman LK, Peberdy MA, Walker WC, Ornato JP, Gossip MR, et al. Anxiety, depression, and PTSD following cardiac arrest: A systematic review of the literature. Resuscitation. 2013;84(7):873–7. doi: 10.1016/j.resuscitation.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 16.Davies SE, Rhys M, Voss S, Greenwood R, Thomas M, Benger JR. Psychological wellbeing in survivors of cardiac arrest, and its relationship to neurocognitive function. Resuscitation. 2017;111:22–5. doi: 10.1016/j.resuscitation.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 17.Isenschmid C, Kalt J, Gamp M, Tondorf T, Becker C, Tisljar K, et al. Routine blood markers from different biological pathways improve early risk stratification in cardiac arrest patients: Results from the prospective, observational COMMUNICATE study. Resuscitation. 2018;130:138–45. doi: 10.1016/j.resuscitation.2018.07.021 [DOI] [PubMed] [Google Scholar]
- 18.Isenschmid C, Luescher T, Rasiah R, Kalt J, Tondorf T, Gamp M, et al. Performance of clinical risk scores to predict mortality and neurological outcome in cardiac arrest patients. Resuscitation. 2019;136:21–9. doi: 10.1016/j.resuscitation.2018.10.022 [DOI] [PubMed] [Google Scholar]
- 19.Metzger K, Gamp M, Tondorf T, Hochstrasser S, Becker C, Luescher T, et al. Depression and anxiety in relatives of out-of-hospital cardiac arrest patients: Results of a prospective observational study. Journal of critical care. 2019;51:57–63. doi: 10.1016/j.jcrc.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 21.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63. doi: 10.1001/jama.270.24.2957 [DOI] [PubMed] [Google Scholar]
- 22.Nowels D, McGloin J, Westfall JM, Holcomb S. Validation of the EQ-5D quality of life instrument in patients after myocardial infarction. Qual Life Res. 2005;14(1):95–105. doi: 10.1007/s11136-004-0614-4 [DOI] [PubMed] [Google Scholar]
- 23.EQ-5D-3L User Guide [Internet]. EuroQol Research Foundation. 2018. Available from: https://euroqol.org/publications/user-guides. [Google Scholar]
- 24.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–4. doi: 10.1016/s0140-6736(75)92830-5 [DOI] [PubMed] [Google Scholar]
- 25.Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin Scale: a systematic review. Stroke. 2009;40(10):3393–5. doi: 10.1161/STROKEAHA.109.557256 [DOI] [PubMed] [Google Scholar]
- 26.Grossestreuer AV, Abella BS, Sheak KR, Cinousis MJ, Perman SM, Leary M, et al. Inter-rater reliability of post-arrest cerebral performance category (CPC) scores. Resuscitation. 2016;109:21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rittenberger JC, Raina K, Holm MB, Kim YJ, Callaway CW. Association between Cerebral Performance Category, Modified Rankin Scale, and discharge disposition after cardiac arrest. Resuscitation. 2011;82(8):1036–40. doi: 10.1016/j.resuscitation.2011.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 29.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 30.Maercker A, Schützwohl M. Erfassung von psychischen Belastungsfolgen: Die Impact of Event Skala-revidierte Version (IES-R). Diagnostica. 1998(44(3)):130–41. [Google Scholar]
- 31.Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale—Revised. Behaviour Research and Therapy. 2003;41(12):1489–96. doi: 10.1016/j.brat.2003.07.010 [DOI] [PubMed] [Google Scholar]
- 32.Appleton RT, Kinsella J, Quasim T. The incidence of intensive care unit-acquired weakness syndromes: A systematic review. J Intensive Care Soc. 2015;16(2):126–36. doi: 10.1177/1751143714563016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brück E, Larsson JW, Lasselin J, Bottai M, Hirvikoski T, Sundman E, et al. Lack of clinically relevant correlation between subjective and objective cognitive function in ICU survivors: a prospective 12-month follow-up study. Critical Care. 2019;23(1):253. doi: 10.1186/s13054-019-2527-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive care medicine. 2009;35(5):796–809. doi: 10.1007/s00134-009-1396-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatch R, Young D, Barber V, Griffiths J, Harrison DA, Watkinson P. Anxiety, Depression and Post Traumatic Stress Disorder after critical illness: a UK-wide prospective cohort study. Critical care (London, England). 2018;22(1):310. doi: 10.1186/s13054-018-2223-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Critical care medicine. 2011;39(2):371–9. doi: 10.1097/CCM.0b013e3181fd66e5 [DOI] [PubMed] [Google Scholar]
- 37.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Critical care medicine. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72 [DOI] [PubMed] [Google Scholar]
- 38.Olsen HT, Nedergaard HK, Strøm T, Oxlund J, Wian KA, Ytrebø LM, et al. Nonsedation or Light Sedation in Critically Ill, Mechanically Ventilated Patients. N Engl J Med. 2020;382(12):1103–11. doi: 10.1056/NEJMoa1906759 [DOI] [PubMed] [Google Scholar]
- 39.Rousseau AF, Prescott HC, Brett SJ, Weiss B, Azoulay E, Creteur J, et al. Long-term outcomes after critical illness: recent insights. Critical care (London, England). 2021;25(1):108. doi: 10.1186/s13054-021-03535-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moulaert VRM, van Heugten CM, Gorgels TPM, Wade DT, Verbunt JA. Long-term Outcome After Survival of a Cardiac Arrest: A Prospective Longitudinal Cohort Study. Neurorehabil Neural Repair. 2017;31(6):530–9. doi: 10.1177/1545968317697032 [DOI] [PubMed] [Google Scholar]
- 41.Geri G, Dumas F, Bonnetain F, Bougouin W, Champigneulle B, Arnaout M, et al. Predictors of long-term functional outcome and health-related quality of life after out-of-hospital cardiac arrest. Resuscitation. 2017;113:77–82. doi: 10.1016/j.resuscitation.2017.01.028 [DOI] [PubMed] [Google Scholar]
- 42.Lilja G, Nilsson G, Nielsen N, Friberg H, Hassager C, Koopmans M, et al. Anxiety and depression among out-of-hospital cardiac arrest survivors. Resuscitation. 2015;97:68–75. doi: 10.1016/j.resuscitation.2015.09.389 [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Kuchinke L, Woud ML, Velten J, Margraf J. Survey method matters: Online/offline questionnaires and face-to-face or telephone interviews differ. Computers in Human Behavior. 2017;71:172–80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.