Abstract
The highest incidence of severe pneumococcal infections in children occurs in the first 6 months of life; however, immunization of infants with the existing polysaccharide vaccines is ineffective. We wished to determine the prevalence of immunoglobulin G (IgG) pneumococcal antibodies in unimmunized Brazilian mothers and their transplacental transmission to term and preterm infants. Total IgG, IgG1 and -2 subclass levels, and IgG antibodies against Streptococcus pneumoniae serotypes 1, 3, 6B, 9V, and 14 were determined in 15 pairs of mothers and term newborns (gestational age, ≥37 weeks) and in 18 pairs of mothers and preterm newborns (gestational age, 32 to 36 weeks). Serotype-specific anti-pneumococcal antibodies were detected by a recently standardized enzyme-linked immunosorbent assay calibrated with the 89-SF reference serum. Varying percentages of the mothers had antibody concentrations below arbitrarily defined protective levels: 33% for serotype 1, 67% for serotype 3, 30% for serotype 6B, 52% for serotype 9V, and 22% for serotype 14. In term newborns, IgG1 concentrations were slightly higher than maternal concentrations; in preterm newborns, the concentrations were much lower. Concentrations of IgG2 in term and preterm infants were significantly lower than in the mothers. Transplacental transmission of antibodies to serotypes 3 and 14 was clearly different from that of antibodies to serotypes 1, 6B, and 9V. Concentrations of IgG antibodies against serotypes 3 and 14 were similar to or higher than those of the mothers; against serotypes 1, 6B, and 9V they ranged from 77 to 83% of maternal concentrations in term newborns and also in preterm infants, although transplacental transmission of antibodies was proportionally lower for each specific serotype in preterm than in term infants. These data are relevant for developing strategies to protect infants against pneumococcal infections in the first months of life. Our findings and a review of existing information stress the importance of understanding the relationships among pneumococcal immunization, IgG subclass antibodies to individual serotypes, transplacental transport, half-life, and antibody function and their protective values against infection.
Pneumococcal infections cause high morbidity and mortality in children during the first 2 years of life, with the highest incidence of severe, systemic pneumococcal infections occurring in the first year of life (15, 18). Immunization of infants with the existing polysaccharide vaccines, however, becomes effective only after 2 years of age.
Conjugate pneumococcal vaccines containing five or seven polysaccharides have been found to be immunogenic in the first year of life in several studies (1, 3, 31). However, these vaccines are still experimental and the small number of serotype polysaccharides included in the vaccines may limit the coverage they offer. A 10-year study of 308 cases of meningitis in children under 2 years of age in São Paulo, Brazil (48), reveals that vaccine formula B, which includes serotypes 1, 5, 6B, 14, 18C, 19F, and 23F (47), would cover only 68% of the infections caused by 42 different strains, even taking into account the cross-reactivity of vaccine and nonvaccine serotypes (41). Two recent studies in Brazil estimate that the 23-valent polysaccharide vaccine offers protection against 85.7 and 89.6% of these infections, respectively, if vaccine-related cross-reactive serotypes are taken into account (11, 44).
The diversity of serotypes causing invasive infections in Brazil, the limitations affecting the development of conjugated vaccines to multiple serotypes, and the cost of a single polysaccharide conjugate vaccine (28) led us to explore other avenues for protecting infants against pneumococcal infections. One of these strategies is the immunization of mothers to provide passive immunization to infants (45).
As a preliminary step in evaluating the possibility of increasing infant protection against invasive pneumococcal infections through maternal immunization, we determined the presence of pneumococcal immunoglobulin G (IgG) antibodies in an unimmunized population of Brazilian mothers and studied the transplacental transmission of these antibodies in term and preterm deliveries.
MATERIALS AND METHODS
Population.
The study involved 33 mother-child pairs. All mothers were well nourished. None of the mothers had been immunized with a pneumococcal vaccine. Gestational age was estimated by a neonatologist based on a somatic and neurological examination (12). Fifteen mothers (age range, 18 to 38 years) had term pregnancies (≥37 weeks); 18 mothers (age range, 18 to 37 years) had preterm pregnancies (32 to 36 weeks). After informed consent was obtained, blood samples were collected from the mothers at the time of delivery and from the umbilical cords of their infants. The serum was frozen at −20°C until analysis. All mother-infant pairs were analyzed simultaneously.
IgG subclass determinations.
IgG subclass concentrations (in milligrams per deciliter) were measured by the single radial immunodiffusion technique with the following specific monoclonal antibodies: clone JL512 for IgG1 and clone GOM1 for IgG2 (Unipath, Hampshire, United Kingdom) (20). The results were calibrated with the standard serum WHO 67/97, and secondary controls were kindly provided by Lars A. Hanson, Göteberg University, Sweden.
Pneumococcal polysaccharide antibody assay.
Using a modified enzyme-linked immunosorbent assay (ELISA) protocol (25, 40), we measured IgG antibodies against pneumococcal serotypes 1, 3, 6B, 9V, and 14, which include some of the most prevalent serotypes isolated from children with invasive infections in the São Paulo area of Brazil (8, 10). This procedure involves the binding of optimal concentrations of individual pneumococcal serotype polysaccharides (American Type Culture Collection, Rockville, Md.) on the surface of microtiter plates (Nunc Maxisorp catalog no. 439454). Standard, control, and serum samples, diluted in 0.01 M phosphate-buffered saline containing 0.05% polyoxyethylenesorbitan monolaurate (Tween 20) and 1.0% bovine serum albumin, were preabsorbed for 30 min at 37°C with Streptococcus pneumoniae C polysaccharide (500 μg/ml in undiluted serum) (Statens Seruminstitut, Copenhagen, Denmark). Four twofold dilutions of both patient and control samples were then added to their respective wells, and the plates were incubated for 2 h at room temperature. The wells were then washed three times with phosphate-buffered saline–0.05% Tween 20. A titrated amount of horseradish peroxidase-labeled mouse anti-human IgG Fc monoclonal antibody (HP6043HRP; Hybridoma Reagent Laboratories, Baltimore, Md.) was added, and the plates were incubated in the dark for 2 h at room temperature. Following another washing step, the bound enzyme was detected by the addition of tetramethyl-benzidine-dihydrochloride (Sigma Chemical Co., St. Louis, Mo.) in citrate phosphate buffer. The serotype-specific IgG antibody concentration (in micrograms per milliliter) was calculated by measuring the absorbance (optical density at 450 nm) against a standard curve obtained by using twofold serial dilutions of a serum pool (standard) prepared from six healthy adults who had been immunized with the 23-valent polysaccharide vaccine (Lederle-Praxis Biologicals, West Henrietta, N.Y.). Serotype-specific IgG levels in this standard had been previously determined with the FDA 89-SF reference sample Center for Biological Evaluation and Research, U.S. Food and Drug Administration, Rockville, Md.). The concentration of antibody against serotype 3, which is not included in the conjugate vaccines, was determined by cross-standardization of our standard against the assigned IgG values for the other serotypes in the FDA 89-SF sample. Prior to clinical use, evaluation of linearity in this assay showed an excellent correlation (r = 0.99) between observed and expected results, with minimum detectable concentrations ranging from 0.04 to 0.1 μg/ml for all nine serotypes tested. The interassay precision (percent coefficient of variation), determined by assaying a sample in 20 separate tests, ranged from 10.2 to 14.4%, for the same serotypes.
Statistical methods.
All results of IgG subclass and specific antibody concentrations were analyzed by following logarithmic transformation in order to decrease the influence of a few extreme measurements. The results were expressed as geometric means and confidence intervals (CI). Maternal and newborn IgG subclass and serotype-specific antibody concentrations were compared by the nonparametric Wilcoxon signed-rank test. Transplacental transmission of IgG subclasses and specific antipneumococcal antibodies was calculated by dividing the cord blood concentrations by the concentration found in the corresponding maternal blood. Since the ratios were normally distributed, means and standard deviations were used for the description of results. Least-squares linear regression was used for mother-newborn pair correlations.
RESULTS
All mothers studied had IgG subclass concentrations within the normal limits established for the Brazilian population (Table 1). The concentrations of IgG1 in term newborns were similar to the maternal concentrations (P = 0.4631), while the concentrations in preterm newborns were significantly lower than those in the mothers (P = 0.0019). The concentration of IgG2 in term infants was significantly lower than in the mothers (P = 0.0302), with an even larger difference between maternal and newborn concentrations in preterm pregnancies (P = 0.0006).
TABLE 1.
Transplacental transmission of IgG1 and IgG2 in preterm and term pregnancies
| Concn (mg/dl)
| ||||
|---|---|---|---|---|
| Parameter | Term (n = 15)
|
Preterm (n = 18)
|
||
| IgG1 | IgG2 | IgG1 | IgG2 | |
| GMa (CI) | ||||
| Mothers | 758 (640–1,017) | 164 (134–236) | 751 (650–960) | 168 (144–218) |
| Newborns | 852 (764–993) | 112 (86–180) | 423 (360–594) | 83 (74–96) |
| Ratio | 112 (95–145) | 68 (56–99) | 58 (50–77) | 47 (41–62) |
| r | 0.4976 | 0.6077 | 0.4224 | 0.0849 |
| Correlation P value | 0.0591 | 0.0163 | 0.0912 | 0.7460 |
GM, geometric mean.
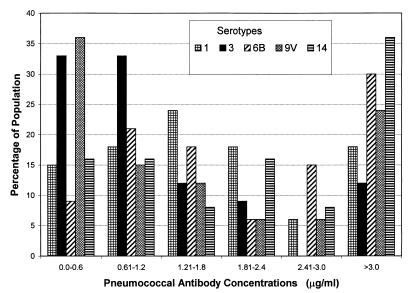
The geometric mean maternal pneumococcal antibody concentrations (in micrograms per milliliter) varied for the five serotypes studied: serotype 1, 1.54 (95% CI, 1.50 to 2.89); serotype 3, 0.96 (95% CI, 0.81 to 2.67); serotype 6B, 1.96 (95% CI, 1.95 to 2.67); serotype 9V, 1.13 (95% CI, 1.19 to 2.52); and serotype 14, 2.93 (95% CI, 2.49 to 6.32). A detailed analysis of the distribution of maternal antibody concentrations reveals varying percentages of mothers in arbitrarily-defined groups (Fig. 1). Concentrations of antibodies against the different serotypes of ≤1.2 μg/ml were found in 22 to 67% of the unimmunized mothers studied: 33% for serotype 1, 67% for serotype 3, 30% for serotype 6B, 52% for serotype 9V, and 22% for serotype 14.
FIG. 1.
Distribution of concentrations of pneumococcal antibodies against five serotypes in 33 unimmunized Brazilian women.
In term newborns, concentrations of serotype-specific IgG antibodies against serotypes 3 and 14 were similar to or higher than those of the mothers (Table 2); however, the mean percentage of transmission of antibodies against serotypes 1, 6B, and 9V ranged from 77 to 83%. A difference in the transplacental transmission between serotypes 3 and 14 and serotypes 1, 6B, and 9V was also noted in preterm infants (Table 3). The ratios of transplacental transmission were lower for preterm newborns than for term newborns for each of the serotypes studied (Tables 2 and 3). There was a significant correlation between maternal and infant antibody concentrations for both term and preterm infants (Tables 2 and 3). There was no difference between the transplacental transmissions of high and low maternal antibody concentrations (data not shown). There was no correlation between serotype-specific antibody concentrations and IgG1 or IgG2 subclass concentrations in either the mothers or the newborns or between the ratios of transplacental transmission of specific antibodies and IgG subclasses (data not shown).
TABLE 2.
Transplacental transmission of pneumococcal serotype antibodies in term newborns
| Parameter | Value in serotype:
|
||||
|---|---|---|---|---|---|
| 1 (n = 15) | 3 (n = 15) | 6B (n = 15) | 9V (n = 15) | 14 (n = 14) | |
| Mothers | |||||
| GMa (μg/ml) | 1.73 | 0.94 | 2.09 | 1.30 | 3.21 |
| 95% CI | 1.28–4.14 | 0.44–2.48 | 1.63–5.00 | 0.92–3.44 | 1.95–6.98 |
| Infants | |||||
| GM (μg/ml) | 1.38 | 1.03 | 1.60 | 0.98 | 3.09 |
| 95% CI | 0.87–3.53 | 0.17–3.25 | 0.99–4.00 | 0.63–2.59 | 2.24–5.76 |
| P valueb | 0.0151 | 0.5995 | 0.0031 | 0.0012 | 0.9515 |
| Ratioc (mean % ± SD) | 83 ± 23 | 116 ± 44 | 79 ± 20 | 77 ± 18 | 99 ± 24 |
GM, geometric mean.
P value represents the comparison of maternal and infant antibody concentrations (Wilcoxon signed-rank test).
There was a high correlation (r ≥ 0.884; P < 0.0001) between maternal and term-newborn antibody concentrations for the serotypes tested (least-squares linear regression).
TABLE 3.
Transplacental transmission of pneumococcal serotype antibodies in preterm newborns
| Parameter | Value in serotype:
|
||||
|---|---|---|---|---|---|
| 1 (n = 18) | 3 (n = 18) | 6B (n = 18) | 9V (n = 18) | 14 (n = 9) | |
| Mothers | |||||
| GMa (μg/ml) | 1.40 | 0.98 | 1.87 | 1.01 | 2.34 |
| 95% CI | 1.18–2.34 | 0.41–3.55 | 1.41–3.61 | 0.84–2.33 | 0.61–8.02 |
| Infants | |||||
| GM (μg/ml) | 0.70 | 0.69 | 0.82 | 0.52 | 1.82 |
| 95% CI | 0.54–1.22 | 0.35–2.02 | 0.64–1.53 | 0.40–1.18 | 0.78–4.67 |
| P valueb | 0.0007 | 0.4376 | 0.0007 | 0.0007 | 0.0977 |
| Ratio (Mean % ± SD) | 58 ± 32 | 94 ± 69 | 53 ± 30 | 61 ± 34 | 86 ± 44 |
| r | 0.837 | 0.551 | 0.693 | 0.849 | 0.963 |
| P valuec | <0.0001 | 0.0179 | 0.0014 | <0.0001 | <0.0001 |
GM, geometric mean.
P value represents the comparison of maternal and infant antibody concentrations (Wilcoxon signed-rank test).
P value for the correlation between maternal and infant antibody concentrations.
DISCUSSION
All serotypes included in this study can cause invasive disease in infants (11, 23, 44, 48). Four of these serotypes are included in the new experimental conjugate vaccines proposed for different areas of the world (47). This study was performed with unimmunized Brazilian women. Immunization with a 23-valent polysaccharide vaccine is likely to increase serotype-specific antibody concentrations. Maternal immunization during pregnancy was shown to significantly increase the geometric mean titers against serotypes 6B and 19F in one study in Bangladesh (45) and to five of six serotypes tested in The Gambia (38). Since the serotypes tested in these studies are no more immunogenic than other serotypes included in the 23-valent vaccine, antibody concentrations to other serotypes are also likely to increase after immunization.
There was a significant correlation between maternal and newborn antibodies for all serotypes in both term and preterm pregnancies. The ratios of transplacental transmission, however, followed two distinct patterns, one similar to the transmission of total IgG1 for serotypes 3 and 14 and another similar to the transmission of IgG2 for serotypes 1, 6B, and 9V. In some studies, transplacental transmission of IgG2 has been found to be less efficient than that of other IgG subclasses (9, 14, 22), probably due to the low binding ability of placental immunoglobulin receptors for IgG2 (35). Other studies of transplacental transmission have established low ratios for antibodies to serotype 7F (0.63) (13), for antibodies to native type III group B streptococcus polysaccharide (5), and for anti-group A streptococcal polysaccharide antibodies (16). Our results showing different ratios for serotypes 6B and 14 are in agreement with those of Anderson et al., who found much lower transmission ratios for antibodies to serotype 6A (0.54) than for serotype 14 (0.89) (4). Earlier studies measuring both IgM and IgG antibodies had shown transmission ratios lower than 50% for several serotypes (21); however, only IgG antibodies are transmitted transplacentally and measured in cord blood.
The predominant isotype of pneumococcal IgG antibodies is IgG2 (7, 33, 34, 46). However, anti-pneumococcal serotype antibodies have also been found in all other IgG subclasses (6). The preponderance of IgG2 subclass antibodies may be more pronounced in older individuals than in children (19, 33) and may also be more pronounced in the responses to certain serotypes (19). To what extent immunization may alter the isotype and therefore the transplacental transmission pattern for each serotype-specific antibody has yet to be determined. Conjugate Haemophilus influenzae type b vaccines administered during the last trimester of pregnancy resulted in significantly higher PRP antibodies in infants at birth and at 2 months of age than did polysaccharide vaccine (17), suggesting that the immunization of women with conjugate pneumococcal vaccines should be considered in the future.
After transplacental transmission, the half-life of anti-pneumococcal antibodies has been estimated at 35 days (45); the half-life is approximately 30 days after passive administration of intravenous IgG to patients with antibody deficiency syndromes (43). This half-life may be affected by the IgG subclass to which the antibodies belong (36).
Transplacental transmission of high maternal antibody concentrations may permit the postponement of infant immunization, which would allow a maturation of the response to protein-polysaccharide conjugates, possibly through enhanced priming for the protein carrier function by diphtheria, pertussis, and tetanus immunization (39). This may be particularly important for preterm infants, whose response to the first doses of a conjugate H. influenzae vaccine was found to be significantly lower than that of older infants (26). The risk of high concentrations of anti-polysaccharide antibodies interfering with the response to immunization with conjugate vaccines is negligible or nonexistent (27, 32).
Antibody concentrations needed for the protection of infants against invasive pneumococcal disease have not yet been defined with the ELISA employed in our study. Antibody concentrations of ≥200 ng/ml were correlated with protection against sepsis in adults and with decreased nasopharyngeal colonization with specific pneumococcal serotypes in children (29, 30). This antibody concentration is approximately equivalent to a concentration of 1.3 μg/ml measured by the ELISA (21a). According to our results, few infants born to unimmunized mothers in Brazil would have antibody concentrations sufficient to offer protection. However, these results will have to be reinterpreted once a better definition of protective concentrations of antibodies against the various S. pneumoniae serotypes has been formulated.
The evaluation of opsonic activity may also allow the assessment of the protection conferred by transplacentally transmitted antibodies. A correlation between the measurement of opsonic activity in vitro and protection against infection has been established for mice (2). In one study of humans, no correlation was found between opsonization and antibody levels in unimmunized adults. After immunization, most sera had increased antibody titers and opsonic activity, but a lack of correlation persisted for individual sera (37). In another study, IgG2 antibodies correlated better with opsonic activity than IgG1 antibodies (24). Recently, we have shown that immunization with a conjugate heptavalent pneumococcal vaccine increases opsonophagocytic activity to some vaccine serotypes (42). We are now extending these studies of opsonophagocytosis to mothers and infants followed during the first year of life.
We conclude that a relatively high percentage of this study population of unimmunized Brazilian mothers have low antibody concentrations against several pneumococcal serotypes. These data are relevant for future strategies for protection against pneumococcal infections in the first months of life. Immunization of pregnant women in high-risk groups may protect both the mother and the infant. Our findings and a review of existing information stress the importance of future studies to clarify the relationships among pneumococcal immunization, IgG subclass antibodies to individual serotypes, transplacental transport, half-life, and antibody function and their protective values against infection.
ACKNOWLEDGMENTS
This research was supported in part by an unrestricted grant from Lederle-Praxis Biologicals and by FAPESP (Fundaca̧ó de Amparo a Pesquisa do Estado de São Paulo).
REFERENCES
- 1.Ahman H, Kayhty H, Tamminen P, Vuorela A, Malinoski F, Eskola J. Pentavalent pneumococcal oligosaccharide conjugate vaccine PncCRM is well-tolerated and able to induce an antibody response in infants. Pediatr Infect Dis J. 1996;15:134–139. doi: 10.1097/00006454-199602000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Alonso De Velasco E, Dekker A T, Verhul A F, Feldman R G, Verhoef J, Snippe H. Anti-polysaccharide immunoglobulin isotype levels and opsonic activity of antisera: relationship with protection against Streptococcus pneumoniae infections in mice. J Infect Dis. 1995;172:562–565. doi: 10.1093/infdis/172.2.562. [DOI] [PubMed] [Google Scholar]
- 3.Anderson E L, Kennedy D J, Geldmacher K M, Donnelly J, Mendelman P M. Immunogenicity of heptavalent pneumococcal conjugate vaccine in infants. J Pediatr. 1996;128:649–653. doi: 10.1016/s0022-3476(96)80130-2. [DOI] [PubMed] [Google Scholar]
- 4.Anderson P, Porcelli S, Pichichero M. Natural maternal and cord serum antibodies to pneumococcal serotypes 6A, 14, 19F and 23F polysaccharides. Pediatr Infect Dis J. 1992;11:677–679. [PubMed] [Google Scholar]
- 5.Baker C J, Edwards M S, Kasper D L. Role of antibody to native type III polysaccharide of group B Streptococcus in infant infection. Pediatrics. 1981;68:544–549. [PubMed] [Google Scholar]
- 6.Bardardottir E, Jonsson S, Jonsdottir I, Sigfusson A, Valdimarsson H. IgG subclass response and opsonization of Streptococcus pneumoniae after vaccination of healthy adults. J Infect Dis. 1990;162:482–488. doi: 10.1093/infdis/162.2.482. [DOI] [PubMed] [Google Scholar]
- 7.Barret D J, Ayoub E M. IgG2 subclass restriction of antibody to pneumococcal polysaccharides. Clin Exp Immunol. 1986;63:127–134. [PMC free article] [PubMed] [Google Scholar]
- 8.Berezin E N, Carvalho E S, Casagrande S, Brandileone M C, Mimica I M, Farhat C K. Streptococcus pneumoniae penicillin-nonsusceptible strains in invasive infections in São Paulo, Brazil. Pediatr Infect Dis J. 1996;15:1051–1053. doi: 10.1097/00006454-199611000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Black C M, Plikaytis B D, Wells T W, Ramirez R M, Carlone G M, Chilmonczyk B A, Reimer C A. Two-site immunoenzymometric assays for serum IgG subclass infant/maternal ratios at full term. J Immunol Methods. 1988;106:71–81. doi: 10.1016/0022-1759(88)90273-6. [DOI] [PubMed] [Google Scholar]
- 10.Brandileone M C, Vieira V S, Casagrande S T, Zanella R C, Guerra M L, Bokerman S, De Moraes J C, Baldacci E R, Chamone C B, Oliveira M A, De Matos D G, Arruda T M, Coelho M F, D’Avila S M, Dos Santos A R, Di Fabio J L. Prevalence of serotypes and antimicrobial resistance of Streptococcus pneumoniae strains isolated from Brazilian children with invasive infections. Microb Drug Resist. 1997;3:141–146. doi: 10.1089/mdr.1997.3.141. [DOI] [PubMed] [Google Scholar]
- 11.Brandileoue M C, Vieira V S, Zanella R C, Landgraf I M, Helles C E A, De Escragnole Taunay A, Moraes J C, Austrian R. Distribution of serotypes of Streptococcus pneumoniae isolated from invasive infections over a 16-year period in the greater São Paulo area, Brazil. J Clin Microbiol. 1995;33:2789–2791. doi: 10.1128/jcm.33.10.2789-2791.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capurro H, Konichezky S, Fonseca D, Caldeyro-Barcia R. Simplified method for diagnosis of gestational age in the newborn infant. J Pediatr. 1978;93:120–122. doi: 10.1016/s0022-3476(78)80621-0. [DOI] [PubMed] [Google Scholar]
- 13.Chudwin D S, Wara D, Schiffman G, Artrip S G, Ammann A J. Maternal-fetal transfer of pneumococcal capsular polysaccharide antibodies. Am J Dis Child. 1985;139:378–380. doi: 10.1001/archpedi.1985.02140060060029. [DOI] [PubMed] [Google Scholar]
- 14.Costa-Carvalho B T, Vieira H M, Dimantas R B R, Arslanian C, Naspitz C K, Solé D, Carneiro-Sampaio M M S. Transfer of IgG subclasses across the placenta in term and preterm pregnancies. Braz J Med Biol Res. 1996;29:201–204. [PubMed] [Google Scholar]
- 15.Dagan R, Englehard D, Piccard E. Epidemiology of invasive childhood pneumococcal infections in Israel. JAMA. 1992;268:3328–3332. [PubMed] [Google Scholar]
- 16.Einhorn M S, Granoff D M, Nahm M H, Quinn A, Schackelford P G. Concentrations of antibodies in paired maternal and infant sera: relationship to IgG subclass. J Pediatr. 1987;111:783–788. doi: 10.1016/s0022-3476(87)80268-8. [DOI] [PubMed] [Google Scholar]
- 17.Englund J A, Glezen P, Thompson C, Anwaruddin R, Turner C S, Siber G R. Haemophilus influenzae type-b-specific antibody in infants after maternal immunization. Pediatr Infect Dis J. 1997;16:1122–1130. doi: 10.1097/00006454-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Eskola J, Takala A K, Kela E, Pekkanen E, Kalliokosi R, Leinonen M. Epidemiology of invasive pneumococcal infections in children in Finland. JAMA. 1992;268:3323–3327. [PubMed] [Google Scholar]
- 19.Freijd A, Hammarstrøm L, Persson M, Smith C. Plasma pneumococcal antibody of the IgG class and subclasses in otitis prone children. Clin Exp Immunol. 1984;56:233–238. [PMC free article] [PubMed] [Google Scholar]
- 20.French M, Harrison G. Serum IgG subclass concentration in healthy adults: a study using monoclonal antisera. Clin Exp Immunol. 1984;56:473–475. [PMC free article] [PubMed] [Google Scholar]
- 21.Grunebaum A M, Minkoff H, Schwartz R, Schiffman G. Pneumococcal polysaccharide antibody levels in patients with term and preterm pregnancies. Obstet Gynecol. 1986;68:483–487. [PubMed] [Google Scholar]
- 21a.Halsey, J. F. (IBT Reference Laboratory). Personal communication.
- 22.Hay F C, Hull M G R, Torrigiani G. The transfer of human IgG subclasses from mother to foetus. Clin Exp Immunol. 1971;9:355–358. [PMC free article] [PubMed] [Google Scholar]
- 23.Inostroza J, Trucco O, Prado V, Vinet A M, Retamal G, Ossa G, Facklam R R, Sorensen R U. Capsular serotype and antibiotic resistance of Streptococcus pneumoniae isolated in two Chilean cities. Clin Diagn Lab Immunol. 1998;5:176–180. doi: 10.1128/cdli.5.2.176-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaniuk A S C, Loran J E, Monteil M A. Specific IgG subclass antibody levels and phagocytosis of serotype 14 pneumococcus following immunization. Scand J Immunol. 1992;36:S96–S98. doi: 10.1111/j.1365-3083.1992.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 25.Koskela M. Serum antibodies to pneumococcal C polysaccharide in children: response to acute pneumococcal otitis media or to vaccination. Pediatr Infect Dis J. 1987;6:519–526. doi: 10.1097/00006454-198706000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Kristensen K, Ghyrs A, Lausen B, Barington T, Heilmann C. Antibody response to Haemophilus influenzae type b capsular polysaccharide conjugated to tetanus toxoid in preterm infants. Pediatr Infect Dis J. 1996;15:525–529. doi: 10.1097/00006454-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Kurikka S, Olander R M, Eskola J, Kayhty H. Passively acquired anti-tetanus and anti-Haemophilus antibodies and the response to Haemophilus influenzae type b-tetanus toxoid conjugate vaccine in infancy. Pediatr Infect Dis J. 1996;15:530–535. doi: 10.1097/00006454-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Lagos R, Horwitz I, Toro J, San Martin O, Abrego P, Bustamante C, Wasserman S S, Levine O S, Levine M M. Large scale, postlicensure, selective vaccination of Chilean infants with PRP-T conjugate vaccine: practicality and effectiveness in preventing invasive Haemophilus influenzae type b infections. Pediatr Infect Dis J. 1996;15:216–222. doi: 10.1097/00006454-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Landesman S H, Schiffman G. Assessment of the antibody response to pneumococcal vaccine in high-risk populations. Rev Infect Dis. 1981;3:S184–S197. doi: 10.1093/clinids/3.supplement_1.s184. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence E M, Edwards K M, Schiffmann G, Thompson J M, Vaughn W K, Wright P F. Pneumococcal vaccine in normal children. Am J Dis Child. 1983;137:846–850. doi: 10.1001/archpedi.1983.02140350024007. [DOI] [PubMed] [Google Scholar]
- 31.Leach A, Ceesay S J, Banya W A S, Greenwood B M. Pilot trial of a pentavalent pneumococcal polysaccharide/protein conjugate vaccine in Gambian infants. Pediatr Infect Dis J. 1996;15:333–339. doi: 10.1097/00006454-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Letson C W, Santosham M, Reid R, Priehs C, Burns B, Jahnke A, Gahagan S, Nienstadt L, Johnson C, Smith D, Siber G. Comparison of active and combined passive/active immunization of Navajo children against Haemophilus influenzae type b. Pediatr Infect Dis J. 1988;7:747–752. doi: 10.1097/00006454-198811000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Lim P L, Lau Y L. Occurrence of IgG subclass antibodies to ovalbumin, avidin, and pneumococcal polysaccharide in children. Int Arch Allergy Appl Immunol. 1994;104:137–143. doi: 10.1159/000236721. [DOI] [PubMed] [Google Scholar]
- 34.Lortan J E, Kaniuk A, Monteil M A. Relationship of in vitro phagocytosis of serotype 14 Streptococcus pneumoniae to specific class and IgG subclass antibody levels in healthy adults. Clin Exp Immunol. 1993;91:54–57. doi: 10.1111/j.1365-2249.1993.tb03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNabb T, Hoh T Y, Dornington K J, Painter R H. Binding of IgG and fragments to placental membrane preparations. J Immunol. 1976;117:882–888. [PubMed] [Google Scholar]
- 36.Morell A, Terry W D, Waldmann T A. Metabolic properties of IgG subclasses in man. J Clin Investig. 1970;49:673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musher D M, Chapman A J, Goree A, Jonsson S, Briles D, Baughn R E. Natural and vaccine-related immunity to Streptococcus pneumoniae. J Infect Dis. 1986;154:245–256. doi: 10.1093/infdis/154.2.245. [DOI] [PubMed] [Google Scholar]
- 38.O’Dempsey T J, McArdle T, Ceesay S J, Banya W A, Demba E, Secka O, Leinonen M, Kayhty H. Immunization with a pneumococcal capsular polysaccharide vaccine during pregnancy. Vaccine. 1996;14:963–970. doi: 10.1016/0264-410x(96)00009-6. [DOI] [PubMed] [Google Scholar]
- 39.Pichichero M E, Shelly M A, Treanor J J. Evaluation of a pentavalent conjugated pneumococcal vaccine in toddlers. Pediatr Infect Dis J. 1997;16:72–74. doi: 10.1097/00006454-199701000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Quataert S A C S, Kirch L J, Quackenbush D C, Phipps S, Strohmeyer C O, Cimino J, Skuse J, Madore D V. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin Diagn Lab Immunol. 1995;2:590–592. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robbins J B, Austrian R, Lee C J, Rastogi S C, Schiffman G, Henrichsen J, Makela P H, Broome C V, Facklam R R, Tiesjema R H, Parke J C. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983;148:1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- 42.Romero-Steiner S, Sorensen R U, Schinsky M F, Leiva L, Giangrosso P, Fenner A L, Carlone G M. Programs and Abstracts of the International Conference on Emerging Infectious Diseases. 1998. Immune responses to pneumococcal CRM197-hepta-valent polysaccharide conjugate vaccine in children who responded poorly to the 23-valent polysaccharide vaccine, abstr. P1-4; p. 78. [Google Scholar]
- 43.Schiff R I. Half-life and clearance of pH 6.8 and pH 4.25 immunoglobulin G intravenous preparations in patients with primary disorders of humoral immunity. Rev Infect Dis. 1986;8:S449–S456. doi: 10.1093/clinids/8.supplement_4.s449. [DOI] [PubMed] [Google Scholar]
- 44.Sessegolo J F, Levin A S S, Levy C E, Asensi M, Facklam R R, Martins Teixeira L. Distribution of serotypes and antimicrobial resistance of Streptococcus pneumoniae strains isolated in Brazil from 1988 to 1992. J Clin Microbiol. 1994;32:906–911. doi: 10.1128/jcm.32.4.906-911.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shahid N S, Steinhoff M C, Hoque S S, Begum T, Thompson C, Siber G R. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet. 1995;346:1252–1257. doi: 10.1016/s0140-6736(95)91861-2. [DOI] [PubMed] [Google Scholar]
- 46.Siber G R, Schur P H, Aisenberg A C, Weitzman S A, Schiffman G. Correlation between serum IgG-2 concentration and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980;303:178–182. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- 47.Sniadak D H, Schwartz B, Lipman H, Bogaerts J, Butler J C, Dagan R, Echaniz-Aviles G, Lloyd-Evans N, Fenoli A, Girgis N I, Henrichsen J, Klugman K, Lehmann D, Takala A K, Vandepitte J, Gove S, Breiman R F. Potential interventions for the prevention of childhood pneumonia: geographic and temporal differences in serotype and serogroup distribution of sterile site pneumococcal isolates from children—implications for vaccine strategies. Pediatr Infect Dis J. 1995;14:503–510. [PubMed] [Google Scholar]
- 48.Taunay A E, Austrian R, Landgraf I M, Vieira M E P, Melles C E A. Sorotipos de Streptococcus pneumoniae isolados de liquido cefaloraquidiano no periodo de 1977–1988 na cidade de São Paulo, Brasil. Rev Inst Med Trop São Paulo. 1990;32:11–15. doi: 10.1590/s0036-46651990000100003. [DOI] [PubMed] [Google Scholar]