Abstract
Pulmonary hypertension (PH) constitutes one of the main contraindications to heart transplantation (OHT), and elevated pulmonary vascular resistance (PVR) is associated with high risk of posttransplant right heart failure (RVF). In the present case report, a patient with PH is introduced who qualified for heart lung transplantation (HLT) and underwent successful OHT with temporary right ventricle assist device (tRVAD) due to the lack of a suitable heart-lung donor. Temporary RVAD support coupled with optimal medical management may help reverse pulmonary vascular resistance, which was previously deemed as permanent in patients requiring heart transplantation.
Keywords: heart transplantation, pulmonary hypertension, right ventricle assist device, RVAD
1. Introduction
Pulmonary hypertension (PH) constitutes one of the main contraindications to heart transplantation (OHT). Elevated pulmonary vascular resistance (PVR) is associated with a high risk of posttransplant right heart failure (RVF) [1]. Several reports have indicated that in patients with PH-associated with left heart failure, implantation of a left ventricle assist device (LVAD) decreases PVR, facilitating successful bridging to OHT [2,3].
2. Materials and Methods
Herein, a patient with PH is described who qualified for heart lung transplantation (HLT) and underwent successful OHT with a temporary right ventricle assist device (tRVAD) due to the lack a of suitable heart-lung donor.
3. Case Report
A 54-year-old woman previously diagnosed with heart failure, possibly due to Lyme disease, was admitted to a hospital with signs of circulatory decompensation. The patient, having already been on optimal medial therapy, was presented to a cardiac transplant team one year ago and did not qualify for OHT because of significant PVR (7.0 WU) with insufficient response to sodium nitroprusside (3.8 WU). Her left ventricular cavity was already severely diminished (34 mm), and its apex was calcified massively, prohibiting LVAD placement. Given the above, the patient was placed on the HLT waiting list and remained hospitalized due to catecholamine dependence. Unfortunately, due to the catecholamine dependence, the patient had to remain hospitalized for the next 6 months. When waiting for the HLT, she experienced intermittent episodes of circulatory deterioration, with the last one occurring in the moment when a matching donor was presented. Unfortunately, the donor’s lungs were not suitable for donation due to infection. After a thoughtful discussion with the patient, the decision was made to proceed with OHT coupled with temporary RVAD support as depicted in Figure 1.
Figure 1.
The treatment plan with the tRVAD timeframe.
OHT was performed using the bicaval technique. A 10mm reinforced graft (Vascutek, Therumo) was sewn proximal to pulmonary trunk’s bifurcation and distal to vascular anastomosis. It was tunneled to an exit point located 3cm below the right costal margin in the mid axillary line, where it was connected to a CentriMag (Abbott Inc., Abbott Park, IL, USA) outflow tubing line. Venous return was facilitated through a multistage femoral cannula (24F) introduced percutaneously at the beginning of the procedure. tRVAD was initiated immediately after extracorporeal circulation ceased and was set to provide blood flow at the rate of 3 liters per minute. Apical calcification proved to be massive, underestimated by imaging tests (Figure 2).
Figure 2.
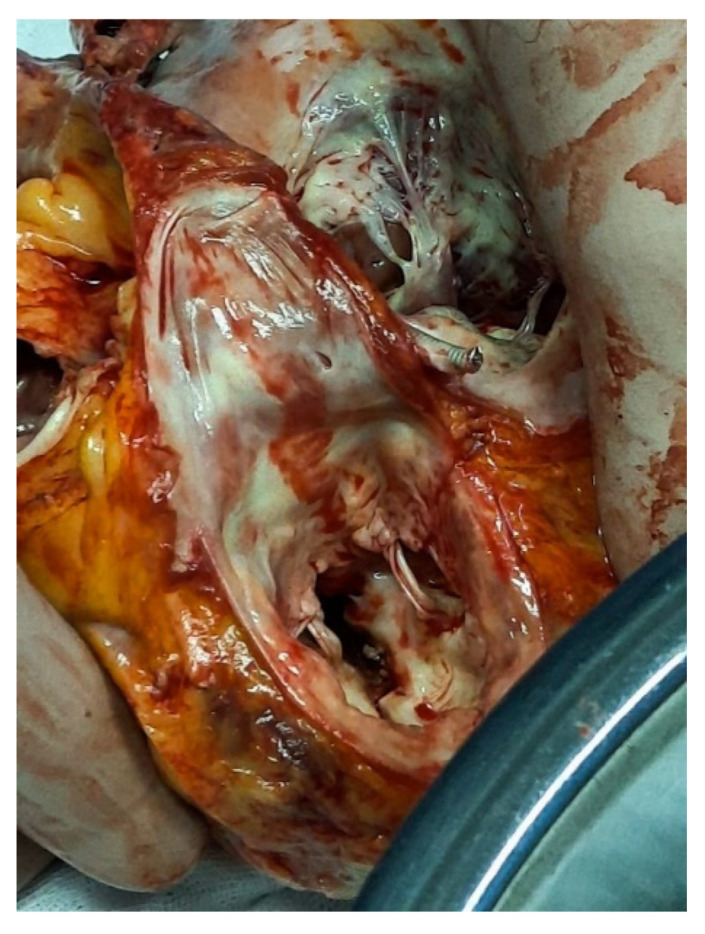
Explanted heart with massive calcification.
The patient was quickly extubated and remained hemodynamically stable. Echocardiography presented good LV function, but RV remained overloaded despite adjusted pump flow and sildenafil (3 × 25 mg) treatment. Levosimendan was introduced on the 11th postoperative day (POD). In the first 14 PODs, the patient developed a septic shock due to pneumonia, cardiac tamponade complicated by a cardiac arrest and massive venous congestion in canulated lower extremity. Proper treatment was introduced and allowed for tRVAD removal on the 16th POD, in accordance with the protocol (Figure 3).
Figure 3.
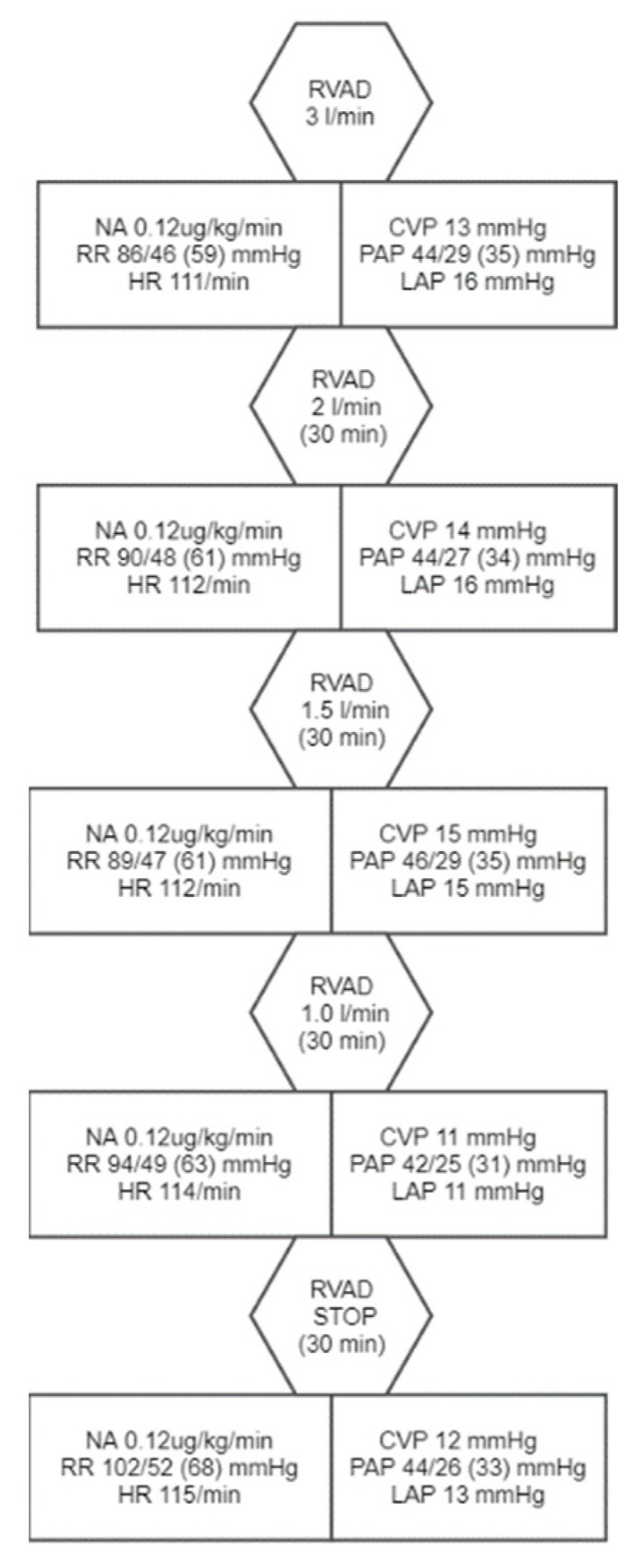
tRVAD weaning protocol with observed vital signs and hemodynamic values. NA—noradrenaline, HR—heart rate, CVP—central venous pressure, PAP—pulmonary arterial pressure, LAP—left atrium pressure.
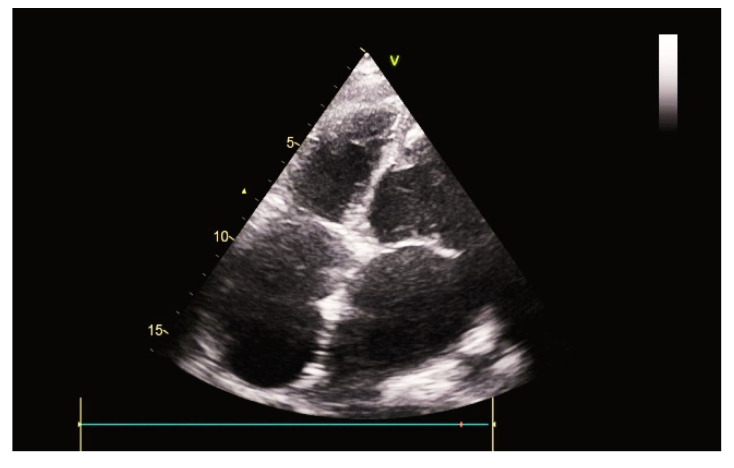
Shortly after RVAD explantation, both RV pressures (92/0–20 mmHg) and PVR (4.5 WU) were elevated and clinically evident, with peripheral oedema and recurring pulmonary infections (Figure 4).
Figure 4.
Ultrasound image after tRVAD explantation.
Renal replacement therapy was used to optimize the circulatory status. Macytentan (1 × 10 mg) and iloprost (6 × 20 mg) were introduced. Meanwhile, severe edema of the lower right extremity evolved into deep venous ulceration and required specialized care. Intensive parenteral nutrition and physiotherapy were carried out continuously.
Three months post OHT, the patient’s condition improved, allowing for a successful discharge on the 123rd POD. Right heart catheterization revealed a complete reversal of PH (RV 72/0–2 mmHg, PVR 1.78 WU), allowing for withdrawal of pharmacotherapy.
4. Discussion
Left heart failure is the most frequent cause of PH and an equally important prognostic factor of poor outcome after OHT. The distinction between reversible and irreversible changes in PVR is crucial for OHT candidates [1,3,4,5]. The ISHLT guidelines suggest that sPAP > 60 mmHg, PVR > 5 Wood or TPG > 16–20 mmHg represent a high risk for postoperative RV failure and may result in 30-day mortality [6,7,8,9].
LVAD implantation is a viable option serving as a bridge to OHT, as the pump helps decrease PVR by unloading LV, reducing pulmonary venous pressure, and augmenting cardiac output [2,3]. The most challenging patients are, however, those with biventricular failure or contraindications to LVAD. In such patients, total artificial heart (TAH) or HLT remains the only treatment option [1].
The presented case is, to our knowledge, the first published example of this approach and was forced by necessity. Our patient had contraindications to LVAD (massive LV apex calcification), TAH (small chest) and a suitable HLT donor was unavailable despite the nearly 5-month waiting time. During such a long hospitalization, the patient’s condition deteriorated, and she could not be optimized at the time of OHT. We might speculate that performing OHT with tRVAD earlier could have prevented some of the clinical complications (malnutrition, impeded wound healing, infections, muscle thinning). On the other hand, it was notably difficult to come up with a successful solution other than HLT in this particular patient.
The use of tRVAD resembled a technique used in patients in whom LVAD was complicated by acute RV failure [10,11,12]. Lower extremity oedema was clearly associated with peripheral venous cannulation. Although 22F multistage cannula was utilized, it caused unexpectedly significant stasis despite systemic anticoagulation. Central cannulation could be used instead, at a higher risk of bleeding and discomfort, due to it tunneling though the thoracic wall.
The problems with RV in the postoperative period indicated that the process of PH reversal may require a longer duration of tRVAD support, coupled with vasoactive treatment. The simultaneous usage of three different drugs for PH (sildenafil, iloprost, macycentan) could have accelerated the process of PVR normalization. We introduced medical treatment after tRVAD explantation in accordance with the protocol. The question remains, whether simultaneous use of PH treatment and tRVAD, could have prevented RV failure after OHT. Yet, this success led us to believe that PVR irreversibility in severe LVH may not necessarily be irrecusable and could be erroneous. However, further studies are required.
5. Conclusions
Temporary RVAD support coupled with optimal medical management may help reverse pulmonary vascular resistance previously deemed as permanent in patients requiring heart transplantation.
Author Contributions
Conceptualization, A.D. and W.M.; resources, K.J. and H.S.; data curation, R.P.; writing—original draft preparation, A.D., W.M. and M.O.Z.; writing—review and editing, M.O.Z.; visualization, J.W.; supervision, M.O.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kanwar M., Raina A., Aponte M.P., Benza R. Pulmonary hypertension in potential heart transplant recipients: Current treatment strategies. Curr. Opin. Organ. Transpl. 2015;20:570–576. doi: 10.1097/MOT.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 2.Biełka A., Kalinowski M., Hawranek M., Małyszek-Tumidajewicz J., Pacholewicz J., Kowalczuk-Wieteska A., Ratman K., Kubiak G., Król B., Przybyłowski P., et al. Mechanical circulatory support restores eligibility for heart transplant in patients with significant pulmonary hypertension. Kardiol. Pol. 2020;78:1008–1014. doi: 10.33963/KP.15518. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez C.A., Rame J.E. Reversible but risky: Pulmonary hypertension in advanced heart failure is the Achilles’ heel of cardiac transplantation. J. Thorac. Cardiovasc. Surg. 2015;150:1362–1363. doi: 10.1016/j.jtcvs.2015.07.098. [DOI] [PubMed] [Google Scholar]
- 4.Matkovic M., Milicevic V., Bilbija I., Aleksic N., Cubrilo M., Nestorovic E., Antic A., Zlatkovic M., Velinovic M., Putnik S. Pulmonary Artery Hypertension as A Risk Factor for Long-Term Survival after Heart Transplantation. Heart Surg. Forum. 2021;24:E544–E549. doi: 10.1532/hsf.3789. [DOI] [PubMed] [Google Scholar]
- 5.Tavares-Silva M., Saraiva F., Pinto R., Amorim S., Silva J.C., Leite-Moreira A.F., Maciel M.J., Lourenço A.P. Comparison of levosimendan, NO, and inhaled iloprost for pulmonary hypertension reversibility assessment in heart transplant candidates. ESC Heart Fail. 2021;8:908–917. doi: 10.1002/ehf2.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobashigawa J., editor. Clinical Guide to Heart Transplantation. Springer International Publishing; 2017. [(accessed on 29 August 2021)]. Available online: https://www.springer.com/gp/book/9783319437712. [Google Scholar]
- 7.Klotz S., Wenzelburger F., Stypmann J., Welp H., Drees G., Schmid C., Scheld H.H. Reversible pulmonary hypertension in heart transplant candidates: To transplant or not to transplant. Ann. Thorac. Surg. 2006;82:1770–1773. doi: 10.1016/j.athoracsur.2006.05.114. [DOI] [PubMed] [Google Scholar]
- 8.Cantero-Pérez E.M., Sayago I., Sobrino-Márquez J.M., Rangel-Sousa D., Grande-Trillo A., Rezaei K., Adsuar-Gómez A. Impact of Preoperative Pulmonary Hypertension on Survival in Patients Undergoing Elective Heart Transplant. Transpl. Proc. 2020;52:580–583. doi: 10.1016/j.transproceed.2019.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Crawford T.C., Leary P.J., Fraser C.D., Suarez-Pierre A., Magruder J.T., Baumgartner W.A., Zehr K.J., Whitman G.J., Masri S.C., Sheikh F., et al. Impact of the New Pulmonary Hypertension Definition on Heart Transplant Outcomes: Expanding the Hemodynamic Risk Profile. Chest. 2020;157:151–161. doi: 10.1016/j.chest.2019.07.028. [DOI] [PubMed] [Google Scholar]
- 10.Lang S.A., O’Neill B., Waterworth P., Bilal H. Can the temporary use of right ventricular assist devices bridge patients with acute right ventricular failure after cardiac surgery to recovery? Interact. Cardiovasc. Thorac. Surg. 2014;18:499–510. doi: 10.1093/icvts/ivt472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berman M., Tsui S., Vuylsteke A., Klein A., Jenkins D.P. Life-threatening right ventricular failure in pulmonary hypertension: RVAD or ECMO? J. Heart Lung Transpl. 2008;27:1188–1189. doi: 10.1016/j.healun.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Carrozzini M., Merlanti B., Olivieri G.M., Lanfranconi M., Bruschi G., Mondino M., Russo C.F. Percutaneous RVAD with the Protek Duo for severe right ventricular primary graft dysfunction after heart transplant. J. Heart Lung Transpl. 2021;40:580–583. doi: 10.1016/j.healun.2021.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.