Abstract
Cytokines and soluble immune activation markers that reflect cytokine activities in vivo are increasingly being measured in plasma, serum, and other body fluids. They provide useful diagnostic and prognostic information as well as insight into disease pathogenesis. Assays of neopterin, β2-microglobulin, soluble interleukin-2 receptor, and soluble tumor necrosis factor receptor type II as well as of the cytokines tumor necrosis factor alpha and gamma interferon (IFN-γ) were evaluated by using serum and plasma samples of human immunodeficiency virus (HIV)-positive and HIV-negative subjects. Many factors were found to influence the outcomes of these assays. Substantial differences in apparent levels of analytes were frequently found when enzyme-linked immunosorbent assay (ELISA) kits from different manufacturers were used. In some cases, differences were found in the standards provided by separate manufacturers. Furthermore, the analytic results from different lots of ELISA kits supplied by single manufacturers differed by as much as 50%. The need for uniformity in the standards for quantitative assays was clearly illustrated. International reference standards are available for cytokines but not for soluble cytokine receptors or soluble activation markers. Marker levels in serum or in plasma were similar except those for IFN-γ. Most of the analytes were stable under several storage conditions. Thus, batch testing of frozen stored samples is feasible. The findings indicate that for longitudinal studies, the levels of cytokines and immune activation markers in plasma or serum should be measured by using preverified reagents from one manufacturer. The quality of laboratory performance can have an impact on clinical relevance. Proficiency testing and external quality assurance programs can help to develop the needed consensus.
Cytokines are involved in the pathology of a wide range of disorders (2). Measurements of levels of cytokines and/or the soluble markers of immune activation that are products of cytokine activities (3, 24, 26, 46, 49, 51) are useful for understanding pathogenesis and as diagnostic and prognostic indicators in many diseases, including those induced by human immunodeficiency virus (HIV) infection (15, 16, 18, 19, 25, 36, 42, 45, 56). The use of appropriately collected and stored test samples of body fluids and of sensitive and accurate methods is critical to these analyses (14, 30, 48, 54). However, complications and difficulties in these assays have been reported (7, 21, 33, 55, 58), and a number of factors have been shown to affect the quality and validity of the measurements (4, 10, 37, 38, 57). Differences in levels were found in studies that compared the major techniques, enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), and bioassays (3, 13). Among the factors that have been implicated as causing the differences are compounds known to bind to cytokines, such as serum albumin and α2-microglobulin (6, 27, 32, 53), autoantibodies (11, 23, 31), and soluble receptors that inhibit cytokine activity, i.e., soluble tumor necrosis factor (TNF) receptor types I and II for TNF-α (sTNF-RI and sTNF-RII, respectively) (1, 3, 52).
At present, immunoassays are the most widely used techniques, although there are known pitfalls of the procedure (28) as well as additional limitations for cytokine measurements (43, 44). In immunoassays, the pairs of antibodies used in different assay kits have been shown to have unique recognition patterns for different forms (monomeric or polymeric) or different epitopes of the antigen molecule (7, 38, 44). Differences in the profiles have been shown to cause differences of up to 100-fold in apparent cytokine levels of identical samples (12, 29, 39, 40). Several World Health Organization-sponsored collaborative studies for cytokine standardization have shown that at least a part of such variations was due to differences in standards used in the assays (5, 20, 47, 50). In addition, the importance of sample collection, processing, and storage in affecting the validity of the measurements and levels of cytokines in biological fluids has been demonstrated (48, 54).
The present study was undertaken to identify factors that influence the results of immunologic assays for seven cytokines, soluble cytokine receptors, and soluble immune activation markers. The findings indicate that reagent sources and the reference standards used have a major impact on the results. The effects of additional factors, such as measurement of levels in serum versus in plasma and the conditions of sample storage, were also investigated. The normal reference ranges in our laboratory for these analytes are reported.
MATERIALS AND METHODS
Samples.
Serum and plasma samples were obtained from healthy volunteers and HIV-negative and HIV-positive subjects participating in the Multicenter AIDS Cohort Study (MACS) of the natural history of AIDS at the University of California, Los Angeles (UCLA), or in AIDS Clinical Trial Unit studies at the UCLA AIDS Clinical Research Center. Blood was collected and processed following our established lab protocol. Blood was collected by venipuncture into 15-ml sterile vacutainers either containing heparin or EDTA as anticoagulant for plasma samples or without anticoagulant for serum samples. The anticoagulant of choice for determination of levels of cytokines in plasma was EDTA. If heparin was used as anticoagulant, each batch of heparin was confirmed by testing to be pyrogen free to avoid unintentional stimulation of blood cells. Also, only pyrogen-free collection tubes were used. Collected blood was kept at 4°C (serum) or at room temperature (plasma). Serum and plasma samples were separated from blood within 1 to 3 h following blood collection: serum was separated by centrifugation at 500 × g for 10 min, and plasma was separated at 350 × g for 15 min at room temperature. Separated samples were aliquoted and stored at −70°C for subsequent batch testing.
Quantitation of levels of cytokines and soluble activation markers in plasma.
β2-Microglobulin (β2M) was quantitated by using microparticle enzyme immunoassay (MEIA) (Abbott Laboratories, Abbott Park, Ill.) and enzyme immunoassay (EIA) kits (Pharmacia AB, Uppsala, Sweden, and ORGenTec Diagnostika GmbH, Mainz, Germany) (see Table 1). Neopterin was measured by competitive EIA kit (ICN, Costa Mesa, Calif.) and RIA kit (IMMUtest Neopterin; BRAHMS, Berlin, Germany). Soluble interleukin-2 receptor (sIL-2R) was determined by EIA kits (Endogen Inc., Cambridge, Mass., and Immunotech, Marseilles, France). sTNF-RII was quantitated in plasma at 1:20 dilution by using EIA kits (HyCult, Uden, The Netherlands, and Genzyme, Cambridge, Mass.). TNF-α was measured by EIA kits (Medgenix, Fleurus, Belgium, and Innogenetics, Zwijndecht, Belgium). IL-10 was measured by EIA kits (Immunotech and Endogen). Gamma interferon (IFN-γ) was determined by EIA kits with and without CIRID modifications of the protocols of the manufacturers (Immunotech, T-Cell Diagnostics, Endogen, Biosource International, Genzyme, and R&D Systems). All other assays were performed according to the manufacturers’ instructions. CIRID modifications of each manufacturer’s protocol consisted mainly of increasing the times of incubation to overnight and the number of washes between steps to 10.
TABLE 1.
Differences due to reagent sources in cytokine and soluble activation marker levels in plasma from HIV-positive subjects
| Analyte (units of concn) | Commercial kit (type) from: | n | Mean level ± SD | P value (paired t test) | Pearson’s correlation coefficient |
|---|---|---|---|---|---|
| β2M (mg/liter) | Pharmacia (EIA) | 14 | 3.00 ± 1.25 | 0.0004a | 0.66a |
| Abbott (MEIA) | 1.91 ± 0.70 | 0.0099b | 0.58b | ||
| ORGenTec (EIA) | 2.25 ± 1.00 | 0.0085c | 0.91c | ||
| Neopterin (nmol/liter) | ICN (EIA) | 17 | 28.1 ± 13.2 | 0.00001 | 0.95 |
| BRAHMS (RIA) | 22.2 ± 10.9 | ||||
| sIL-2R (pg/ml) | Endogen (EIA) | 20 | 8,708 ± 6,118 | 0.00002 | 0.58 |
| Immunotech (EIA) | 2,205 ± 1,295 | ||||
| sTNF-RII (ng/ml) | HyCult (EIA) | 42 | 4.59 ± 1.59 | 0.00001 | 0.96 |
| Genzyme (EIA) | 3.82 ± 1.97 | ||||
| TNF-α (pg/ml) | Innogenetics (EIA) | 35 | 335.8 ± 435 | 0.1032 | 0.91 |
| Medgenix (EIA) | 398.0 ± 638 | ||||
| IFN-γ (units/liter) | Immunotech (EIA) | 12 | 532 ± 598 | 0.0166 | 0.98 |
| T-Cell Diagnostics (EIA) | 452 ± 532 | ||||
| IL-10 (pg/ml) | Immunotech (EIA) | 23 | 49.8 ± 30.1 | 0.00004 | 0.03 |
| Endogen (EIA) | 12.6 ± 9.3 |
Pharmacia β2M versus Abbott β2M.
Pharmacia β2M versus ORGentec β2M.
ORGentec β2M versus Abbott β2M.
Statistical analysis.
The statistical analyses were done by calculating the Pearson correlation coefficients and performing paired t tests with Statview and SAS software. A sigma plot was used for the graphs.
RESULTS
Effects of different reagent sources.
Plasma samples from HIV-seropositive patients with a range of CD4 levels were evaluated for their contents of cytokines and soluble immune activation markers by reagents from two or more suppliers (Table 1). The two commercial kits used for TNF-α assays were quite comparable. However, substantial and significant differences in levels in plasma were found when test kits from different manufacturers were used for β2M, neopterin, sIL-2R, sTNF-RII, IFN-γ, and IL-10 assays. These data suggest that the major difference between the compared kits is probably in the standards provided for quantitation. In addition to the differences in standards, the IL-10 levels determined by the two kits were poorly correlated, indicating that there probably are substantial differences in the pairs of antibodies utilized in the two IL-10 assay kits. Generally, similar effects were found when plasma samples from HIV-seronegative donors were tested and analyzed (data not shown).
Comparison of standards from different manufacturers.
Standard preparations provided by one supplier were tested with the reagents and standards from a second supplier. Two comparisons are summarized in Table 2. IFN-γ results agreed well, but those for β2M and sIL-2R were substantially different. The standards from observed concentrations of β2M (NIBSC [National Institute for Biological Standards and Control] 80123200 standard) were 1.00 mg/liter with Abbott IMx assay and 1.36 mg/liter with ORGenTec assay.
TABLE 2.
Comparison of different standards
| Preparation (units of concn) | Listed concn of T-Cell Diagnostics calibrator | Observed concn in Immunotech assays of T-Cell Diagnostics calibrator |
|---|---|---|
| sIL-2R (pg/ml) | 2,159 | 808 |
| 4,318 | 1,954 | |
| 8,174 | 4,435 | |
| 16,345 | 8,264 | |
| IFN-γ (units/liter) | 380 | 393 |
| 720 | 853 | |
| 1,540 | 1,783 | |
| 3,090 | 3,338 |
Every time that a new lot is obtained from a manufacturer, it is compared with the prior lots directly by using the in-house quality assurance reference standards. Generally, there is good agreement. However, two lots of kits of IFN-γ from one manufacturer were compared and found to differ significantly. The mean levels (± standard deviations) for 13 samples were 3,099 (± 3,201) pg/ml with lot A and 1,465 (± 1,303) pg/ml with lot B.
Failure to detect cytokines in normal plasma.
In general, cytokines could not be detected in the plasma of many healthy individuals with kits available to our laboratory. IFN-γ, IL-10, and TNF-α could not be detected in 77%, 45%, and 7% of the healthy subjects, respectively. However, our findings with soluble immune activation markers indicated that all samples from healthy subjects had measurable levels of β2M, neopterin, sIL-2R, and sTNF-RII.
Serum versus plasma measurements.
Both serum and plasma samples from 16 to 52 HIV-seropositive individuals were tested for β2M, neopterin, sIL-2R, sTNF-RII, TNF-α, IFN-γ, and IL-10 (Table 3). The mean levels of all analytes in serum and those in plasma were very close (the coefficients of variation [CVs] were less than 10%) except for IFN-γ, for which levels in plasma were higher than those in serum (CV was 39%), and for IL-10, for which levels in serum were higher than those in plasma (CV was 17%). Correlation analyses demonstrated a strong correlation between plasma levels and serum levels of all analytes except IL-10, for which the correlation was weak (Table 3). These data indicate that there are no significant differences between levels of cytokines and immune activation markers in plasma and those in serum except for IFNγ and to some extent for IL-10. Thus, most of the levels could be measured by using either of the two sample types.
TABLE 3.
Measurements of serum and plasma cytokine and soluble activation marker levels
| Analyte (units of concn) | No. of subjects | Mean value in:
|
% CV between serum and plasma levels | Correlation coefficienta | |
|---|---|---|---|---|---|
| Serum | Plasma | ||||
| β2-M (mg/liter) | 18 | 2.16 | 2.10 | 2 | 0.987 |
| Neopterin (nmol/liter) | 52 | 11.5 | 10.3 | 8 | 0.961 |
| sIL-2R (U/ml) | 52 | 918 | 956 | 3 | 0.866 |
| sTNF-RII (ng/ml) | 51 | 2.95 | 3.42 | 10 | 0.914 |
| TNF-α (pg/ml) | 17 | 32.7 | 33.5 | 2 | 0.913 |
| IFN-γ (U/liter) | 16 | 22.1 | 39.1 | 39 | 0.929 |
| IL-10 (pg/ml) | 18 | 13.7 | 10.7 | 17 | 0.715 |
P values for all these comparisons are <0.0001.
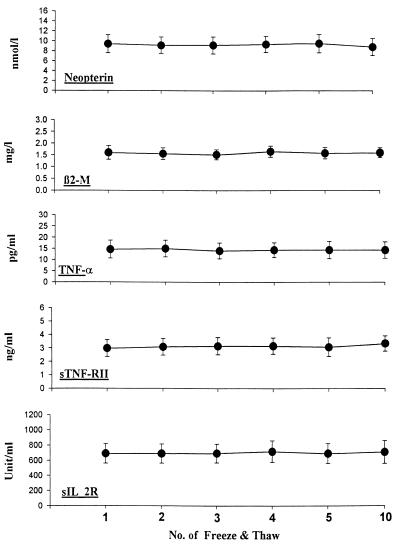
Influence of freeze-and-thaw cycles on levels in plasma of cytokines and soluble activation markers.
The effects of repeated cycles of freezing of plasma and/or serum samples at −70°C and thawing at room temperature were evaluated for five analytes (Fig. 1). One milliliter of each plasma and serum sample from three HIV-seropositive and four HIV-seronegative individuals was tested. Portions of each sample were pipetted into six identical 1-ml cryovials, frozen at −70°C, and kept frozen for 20 h. Samples were brought to room temperature and kept over a 3- to 4-h period to thaw and then refrozen. Such freeze-thaw cycles were conducted 1, 2, 3, 4, 5, and 10 times on sequential days for the sample from each subject. After all freeze-thaw cycles were completed, the aliquots from all seven subjects were tested on the same day and with the same plate and reagent for β2M, neopterin, sIL-2R, sTNF-RII, and TNF-α. The results (Fig. 1) indicated that there were no significant differences in mean levels of any of those analytes in plasma and serum after up to 10 repeated freeze-thaw cycles. The CV between mean values for the repeated cycles for each of the analytes was below 4%.
FIG. 1.
Effects of repeated freezing and thawing on plasma levels of neopterin, β2-microglobulin, TNF-α, sTNF-RII, and sIL-2R. The data are the means ± standard errors for seven subjects (four healthy donors and three HIV-seronegative subjects) for each analyte.
Influence of storage temperature on levels of cytokines and soluble activation markers in plasma.
The stability of cytokines and soluble activation markers in plasma and/or serum samples from six HIV-seropositive and five HIV-seronegative individuals was evaluated after storage for 20 days at room temperature (24°C), refrigerated (4°C), or frozen (−70°C). Samples from each subject were aliquoted into three 1-ml cryovials and stored at the three temperatures. Twenty days later, all samples were batch tested for β2M, neopterin, sTNF-RII, sIL-2R, IFN-γ, and TNF-α. No significant differences among the mean levels at the three storage temperatures were seen for the first five analytes (Fig. 2). The CVs between levels at the three storage temperatures were below 14%. However, the mean level of TNF-α in samples kept at room temperature was 55% lower than the mean levels in samples stored at 4 or −70°C (CV was 38%).
FIG. 2.
Influence of storage temperature on plasma levels of β2M, sIL-2R, neopterin, IFN-γ, sTNF-RII, and TNF-α. Box plots are shown for the analyte levels in serum and plasma samples from 11 subjects (6 HIV-seropositive patients and 5 HIV-seronegative donors) that were stored for 20 days at room temperature (24°C), refrigerated (4°C), and frozen (−70°C). For TNF-α and IFN-γ, the data are for the five donors and for five of the HIV-seropositive subjects. The line inside each box represents the median; the lower and upper edge of each box indicate the 25th and 75th percentiles, respectively; the vertical lines begin and end at the 10th and 90th percentile points; and the lower and upper circles indicate the 5th and 95th percentile points, respectively.
Reference ranges for levels in plasma of cytokines and soluble activation markers.
Donor characteristics may influence the reference (normal) ranges for plasma cytokine and immune activation marker levels. Data for two HIV-seronegative populations are presented in Table 4. The mean levels of several markers were found to be higher in the largely Caucasian seronegative homosexual men (age range, 30 to 54 years) than in a medical center employee population (age range, 24 to 65 years; 32% male and 68% female) that included Asian, Hispanic, African-American, and Caucasian donors. However, of the five parameters, only β2M and TNF-α levels were significantly different (P < 0.05) between the two populations.
TABLE 4.
Reference ranges for levels in plasma of cytokine and soluble activation markers
| Assay (units of concn) | Level in medical center employees
|
Level in Caucasian seronegative homosexual men
|
||||||
|---|---|---|---|---|---|---|---|---|
| Na | Mean | SD | Analyte range | Na | Mean | SD | Analyte range | |
| Neopterin (nmol/liter) | 78 | 5.74 | 3.16 | 3.56–8.45 | 30 | 5.94 | 2.00 | 3.86–8.58 |
| β2M (mg/liter) | 138 | 1.27 | 0.34 | 0.94–1.61 | 132 | 1.59 | 0.72 | 1.09–2.15 |
| sIL-2R (units/ml) | 29 | 548 | 255 | 320–720 | 16 | 663 | 223 | 460–1080 |
| sTNF-RII (ng/ml) | 39 | 1.87 | 0.80 | 1.13–2.74 | 37 | 2.49 | 2.02 | 1.30–3.20 |
| TNF-α (pg/ml) | 55 | 11.5 | 9.7 | 4.8–20.5 | 94 | 16.0 | 11.4 | 6.04–28.8 |
Number of different subjects.
DISCUSSION
The purpose of this study was to determine the influence of various factors on the measurement of plasma cytokines and soluble activation markers. The comparability between kits for measurements of cytokine levels in samples obtained from a diverse population, including HIV-positive subjects with different stages of HIV disease as reflected by CD4 counts, was generally good. This may relate to the fact that international standards are available for many cytokines (5, 9, 20, 41, 47, 50).
Measurements of the soluble products of immune activation provide different information than measurements of cytokine levels in plasma. Cytokine levels reflect the production, removal, and retention of these molecules. The soluble markers of immune activation, however, indicate the biological effects of cytokines. Unfortunately, significant differences in measurements of soluble activation markers were observed when commercial kits from different manufacturers were used. This was especially noteworthy for sIL-2R and β2M. The studies reported here emphasize the differences between reference standards provided by manufacturers. In some instances they are similar but not in every case. However, there are not international standards for the soluble receptors, such as sTNF-RII and sIL-2R, or for other products of cytokine activity, such as neopterin or β2M.
Another problem has been changes made in reagents by manufacturers, which may give different quantitative results or may change the level of sensitivity of assays. Unfortunately, many workers in this field have discovered these changes when their results change, without having received forewarning by the manufacturers or suppliers.
Other reports have noted that some monoclonal antibodies recognize only monomers rather than naturally occurring polymers, a fact which can account for differences between one kit and another (38, 44). Soluble cytokine receptors in plasma and/or serum samples may affect the recognition of cytokines by immunoassay (3, 52, 58). Cytokines bound to soluble receptors may or may not be detected by any particular immunoassay, and methodologies such as EIA, RIA, and bioassay may provide different results (3, 13, 37).
Collection and storage procedures may influence the detectable levels of cytokines and soluble markers. To avoid breakdown of some cytokines, collection of blood into endotoxin-free tubes and prompt processing before storage as cell-free plasma or serum at −70°C has been recommended, especially for TNF-α (14, 30, 33, 55). By and large, the cytokines and soluble markers studied here were quite stable if refrigerated or frozen. Samples kept at room temperature for 20 days, however, had TNF-α levels appreciably lower than the mean values in samples kept at 4 and −70°C. Plasma and serum cytokine and soluble marker levels were almost the same. In our laboratory the correlation coefficient for a comparison of serum and plasma neopterin levels was 0.961 (Table 3). Only the mean level in plasma for IFN-γ was higher (77%) than the level in serum.
Several studies have demonstrated substantial differences between laboratories that were using the same kits for soluble marker measurements (reference 4 and unpublished observations). Thus, factors relating to assay performance can contribute to differences in observed values for cytokines and for immune activation markers. Furthermore, the quality of laboratory data can be important in clinical evaluations of prognosis or prediction of disease progression. We have studied a set of 594 plasma samples that originated at UCLA and were tested for neopterin levels at UCLA and another site. The correlation between the data was not strong (r was 0.614). The log-likelihood for the regression model (17) of AIDS occurrence in these patients within the 3 years following the sample draw was −248 based on the UCLA neopterin data and −264 based on the data at the second laboratory. The larger log-likelihood number in the second set of data indicates poorer precision of prediction. Thus, it is evident that the quality of laboratory data can have an impact on clinical relevance. Furthermore, the difference in the proportion with AIDS at 3 years between subject groups in the lowest and the highest quartiles of Kaplan-Meyer plots for neopterin was 0.58 for UCLA and 0.38 for the other laboratory. Similar experiences in the early days of CD4 T-cell measurement (8, 22) indicated that superior laboratory performance related to clinical outcome, whereas poor relationships were found with data from laboratories that demonstrated more erratic testing. Also, differences in laboratory performance could have been responsible for the differences in prognostic value of CD4 levels vis-à-vis HIV viral load, where the CD4 level was found to have no value (34) and substantial value (35).
Research laboratories and service laboratories may differ significantly in their approaches to and capacities to conduct clinical investigations. Research laboratories may not have the resources or not be attuned to the needs for determination of intraassay, interassay, and intrasubject variation and interlaboratory differences, for CV calculations, for routine quality control procedures, for factors relevant to testing of serially obtained samples, or for measurements in appropriate reference (normal) populations.
Some service laboratories may need to modify preexisting procedures or reagent selection in order to meet the needs for uniform testing at multiple sites, as is required in clinical trials. Frozen storage of samples for long periods and determinations of intrasubject variability are not part of the routine in most service laboratories. Also, established charges for clinical tests may be outside the budget allowances of clinical studies.
Attention to the issues noted in this report is needed to avoid conclusions that provide misinformation, in terms of both inappropriate claims and failures to detect significant relationships between immunological changes and clinical events. Measuring cytokines and soluble activation markers is not quite as simple as ordering kits out of the catalogue and following the manufacturer’s instructions. Potential problems can be avoided by conducting assays with careful attention to quality control procedures (4) and issues reported here. Suggested evaluation steps for ELISA kits and comparison of different suppliers of reagents are summarized in Table 5.
TABLE 5.
Suggested evaluation steps for EIA kits and comparison of two different suppliers
| Step | Procedure |
|---|---|
| 1. Assess analytical sensitivity or minimum detectable level | Determined from 10 replicates of the standard without any amount of the analyte (zero calibrator) (A). The mean for the 10 replicates of the zero standard is calculated in net p, rates, absorbance, or counts per minute. The mean for the zero standard is plotted on graph paper. The mean duplicate for the standard with the lowest value (B) is also plotted on the graph. A line is drawn between points A and B. Two times the standard deviation for 10 replicates of the zero standard is calculated, and the value is added to the mean for 10 replicates. This number is read off of the graph to give the concentration which, by definition, is the minimum detection limit. |
| 2. Intraassay variability | Determined from mean, SD, and % CV for 10 replicates of at least two plasma samples, one in the normal range and the other in the abnormal (high) range, in the same assay. |
| 3. Interassay variability | Determined from mean, SD, and % CV for runs (at least three different runs) of two plasma samples, one each in the normal range and abnormal high range. |
| 4. Comparison of the assays from different suppliers | Assay 10 or more normal and 10 or more abnormal samples with two or more different EIA kits; calculate the correlation coefficient and P values and perform paired t-test. |
| 5. Establish reference range, median, mean, SD, and 5th and 95th percentiles | For this purpose, assay 30 or more serum and/or plasma samples from a reference population that has age, sex, and ethnic distribution representative of the population affected by disease. |
Institution of external proficiency testing and/or quality assurance programs could assist laboratories that are conducting cytokine and soluble activation marker measurements. The field will benefit by the establishment of international reference standards for the soluble cytokine receptors and other immune activation products. The use of resources now available, e.g., the international reference standards for cytokines, can also be recommended.
ACKNOWLEDGMENTS
We express our appreciation of the participants and the staff of the Adult AIDS Clinical Trial Unit and the Advanced Technology Laboratory at UCLA and Jonathan Kagan and Daniella Livnat of the Drug Development and Clinical Sciences Branch, TRP, DAIDS, NIAID, National Institutes of Health, who made these studies possible. Some samples discussed in this manuscript were collected by the Multicenter AIDS Cohort Study with centers (principal investigators) at the following institutions: The Johns Hopkins School of Public Health (Joseph B. Margolick and Alvaro Muñoz); Howard Brown Health Center and Northwestern University Medical School (John Phair); UCLA (Roger Detels and Janis V. Giorgi); and University of Pittsburgh (Charles Rinaldo). We are grateful for the organizational support of Susan Stehn, technical assistance of Hripi Nishanian, statistical efforts of Joanie Chung, and manuscript preparation by Deborah Mathieson.
Support was provided by grants AI-36086, AI-38858, AI-35040, and AI-27660 from the National Institutes of Health.
REFERENCES
- 1.Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992;175:323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal B B, Puri R K. Common and uncommon features of cytokines and cytokine receptors: an overview. In: Aggarwal B B, Puri R K, editors. Human cytokines: their role in disease and therapy. Cambridge, Mass: Blackwell Scientific; 1995. pp. 3–24. [Google Scholar]
- 3.Aukrust P, Liabakk N B, Müller F, Lien E, Espevik T, Froland S S. Serum levels of tumor necrosis factor-alpha (TNF-α) and soluble TNF receptors in human immunodeficiency virus type 1 infection: correlations to clinical immunologic and virologic parameters. J Infect Dis. 1994;169:420–424. doi: 10.1093/infdis/169.2.420. [DOI] [PubMed] [Google Scholar]
- 4.Aziz N, Nishanian P, Fahey J L. Levels of cytokines and immune activation markers in plasma in human immunodeficiency virus infection: quality control procedures. Clin Diagn Lab Immunol. 1998;5:755–761. doi: 10.1128/cdli.5.6.755-761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bienvenu J, Coulon L, Doche C, Gutowski M C, Grau G. Analytical performances of commercial ELISA kits for IL-2, IL-6 and TNFα. A WHO study. Eur Cytokine Netw. 1993;4:447–451. [PubMed] [Google Scholar]
- 6.Borth W, Luger T A. Identification of α2-macroglobulin as a cytokine binding plasma protein. J Biol Chem. 1989;264:5818–5825. [PubMed] [Google Scholar]
- 7.Cannon J G, Nerad J L, Poutsiaka D D, Dinarello C A. Measuring circulating cytokines. J Appl Physiol. 1993;75:1897–1902. doi: 10.1152/jappl.1993.75.4.1897. [DOI] [PubMed] [Google Scholar]
- 8.Choi S, Lagakos S W, Schooley R T, Volberding P A. CD4 lymphocytes are an incomplete surrogate marker for clinical progression in persons with asymptomatic HIV infection taking zidovudine. Ann Intern Med. 1993;118:674–680. doi: 10.7326/0003-4819-118-9-199305010-00003. [DOI] [PubMed] [Google Scholar]
- 9.De Kossodo S, Houba V, Grau G E the WHO Collaborative Study Group. Assaying tumor necrosis factor concentrations in human serum. A WHO international collaborative study. J Immunol Methods. 1995;182:107–114. doi: 10.1016/0022-1759(95)00028-9. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello C A. ELISA kits based on monoclonal antibodies do not measure total IL-1β synthesis. J Immunol Methods. 1992;148:255–259. doi: 10.1016/0022-1759(92)90179-w. [DOI] [PubMed] [Google Scholar]
- 11.Duff G W. Cytokines and anti-cytokines. Br J Rheumatol. 1993;32:15–20. [PubMed] [Google Scholar]
- 12.Engelberts I, Moller A, Schoen G J M, Linden C J V D, Buurman W A. Evaluation of measurement of human TNF in plasma by ELISA. Lymphokine Cytokine Res. 1991;10:69–76. [PubMed] [Google Scholar]
- 13.Engelberts I, Stephens S, Francot G J M, Van Der Linden C J, Buurman W A. Evidence for different effects of soluble TNF receptors on various TNF measurements in human biological fluids. Lancet. 1991;338:515–516. doi: 10.1016/0140-6736(91)90591-c. [DOI] [PubMed] [Google Scholar]
- 14.Exley A R, Cohen J. Optimal collection of blood samples for the measurement of tumor necrosis factor-alpha. Cytokine. 1990;2:353–356. doi: 10.1016/1043-4666(90)90065-2. [DOI] [PubMed] [Google Scholar]
- 15.Fahey J L. Cytokines, plasma immune activation markers, and clinically relevant surrogate markers in human immunodeficiency virus infection. Clin Diagn Lab Immunol. 1998;5:597–603. doi: 10.1128/cdli.5.5.597-603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahey J L, Taylor J M G, Detels R, Hoffmann B, Melmed R, Nishanian P, Giorgi J V. The prognostic value of cellular and serological markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 17.Fahey J L, Taylor J M G, Manna B, Nishanian P, Aziz N, Giorgi J V, Detels R. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS. 1998;12:1581–1590. doi: 10.1097/00002030-199813000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Fauci A S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs D, Jager H, Popescu M, Reibnegger G, Werner E R, Dierich M P, Kaboth W, Tilz G P, Wachter H. Immune activation markers to predict AIDS and survival in HIV-1 seropositives. Immunol Lett. 1990;26:75–80. doi: 10.1016/0165-2478(90)90178-s. [DOI] [PubMed] [Google Scholar]
- 20.Gaines-Das R E, Poole S. The international standard for interleukin-6—evaluation in an international collaborative study. J Immunol Methods. 1993;160:147–153. doi: 10.1016/0022-1759(93)90172-4. [DOI] [PubMed] [Google Scholar]
- 21.Gearing A J H, Cartwright J E, Wadhwa M. Biological and immunological assays for cytokines. In: Thomson A, editor. The cytokine handbook. London, United Kingdom: Academic Press Ltd.; 1991. pp. 339–355. [Google Scholar]
- 22.Giorgi J V, Cheng H-L, Margolick J B, Bauer K D, Ferbas J, Waxdel M, Schmid I, Hultin L E, Jackson A L, Park L, Taylor L M the Multicenter AIDS Cohort Study Group. Quality control in the flow cytometric measurement of T-lymphocyte subsets: the multicenter AIDS cohort study experience. Clin Immunol Immunopathol. 1990;55:173–186. doi: 10.1016/0090-1229(90)90096-9. [DOI] [PubMed] [Google Scholar]
- 23.Hansen M B, Svenson M, Diamant M, Bendizen K. High affinity IgG autoantibodies to IL-6 in sera of normal individuals are competitive inhibitors of IL-6 in vitro. Cytokine. 1993;5:72–80. doi: 10.1016/1043-4666(93)90026-2. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann B, Bass H, Nishanian P, Faisal M, Figlin R A, Sarna G P, Fahey J L. Different lymphoid cell populations produce varied levels of neopterin, β2-microglobulin and soluble IL-2 receptor when stimulated with IL-2, interferon-gamma or tumor necrosis factor-alpha. Clin Exp Immunol. 1992;88:548–554. doi: 10.1111/j.1365-2249.1992.tb06485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofmann B, Wang Y X, Cumberland W C, Detels R, Bozorgmehri M, Fahey J L. Serum β2-microglobulin level increases in HIV-1 infection: relation to seroconversion, CD4 T cell fall and prognosis. AIDS. 1990;4:207–214. [PubMed] [Google Scholar]
- 26.Huber C, Batchelor J R, Fuchs D, Hausen A, Lang A, Niederwieser D, Reibnegger G, Swetly P, Troppmair J, Wachter H. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med. 1984;160:310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James K, Milne I, Cunningham A, Elliott S-F. The effect of α2-macroglobulin in commercial cytokine assays. J Immunol Methods. 1993;168:33–37. doi: 10.1016/0022-1759(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 28.Kapadia S, Torre-Amione G, Mann D L. Pitfalls in measuring cytokines. Ann Intern Med. 1994;121:149–150. doi: 10.7326/0003-4819-121-2-199407150-00016. [DOI] [PubMed] [Google Scholar]
- 29.Ledur A, Fitting C, David B, Hamberger C, Cavaillon J-M. Variable estimates of cytokine levels produced by commercial ELISA kits improved results using international cytokine standards. J Immunol Methods. 1995;186:171–179. doi: 10.1016/0022-1759(95)00184-c. [DOI] [PubMed] [Google Scholar]
- 30.Leroux-Roels G, Offner F, Philippe J, Vermeulen A. Influence of blood collecting systems on concentrations of tumor necrosis factor in serum and plasma. Clin Chem. 1988;34:2373–2374. [PubMed] [Google Scholar]
- 31.Mae N, Liberato D J, Chizzonite R, Satoh H. Identification of high-affinity anti-IL-1α autoantibodies in normal human serum as an interfering substance in a sensitive enzyme-linked immunosorbent assay for IL-1α. Lymphokine Cytokine Res. 1991;10:61–68. [PubMed] [Google Scholar]
- 32.Matsuda T, Hirano T, Nagasawa S, Kishimoto T. Identification of α2-macroglobulin as a carrier protein for IL-6. J Immunol. 1989;142:148–152. [PubMed] [Google Scholar]
- 33.Meager A, Leung H, Wooley J. Assays for tumor necrosis factor and related cytokines. J Immunol Methods. 1989;116:1–17. doi: 10.1016/0022-1759(89)90306-2. [DOI] [PubMed] [Google Scholar]
- 34.Mellors J W, Rinaldo C R, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 35.Mellors J W, Munoz A, Giorgi J V, Margolick J B, Tassoni C J, Gupta P, Kingsley L A, Todd J A, Saah A J, Detels R, Phair J P, Rinaldo C R., Jr Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 36.Melmed R N, Taylor J M G, Detels R, Bozorgmehri M, Fahey J L. Serum neopterin changes in HIV-1 infected subjects: indicator of significant pathology, CD4 T cell changes, and the development of AIDS. J Acquired Immune Defic Syndr. 1989;2:70–76. [PubMed] [Google Scholar]
- 37.Mire-Sluis A R. Cytokines—protein structure and biological activity: a complex relationship with implication for biological assays and standardization. Biologicals. 1993;21:131–144. doi: 10.1006/biol.1993.1062. [DOI] [PubMed] [Google Scholar]
- 38.Mire-Sluis A R, Gaines-Das R, Thorpe R. Immunoassays for detecting cytokines: what are they really measuring? J Immunol Methods. 1995;186:157–160. doi: 10.1016/0022-1759(95)00128-w. [DOI] [PubMed] [Google Scholar]
- 39.Mire-Sluis A R, Gaines-Das R, Thorpe R Participants of the Collaborative Study. The international standard for granulocyte-macrophage colony stimulating factor (GM-CSF)—evaluation in an international collaborative study. J Immunol Methods. 1995;179:127–135. doi: 10.1016/0022-1759(94)00273-y. [DOI] [PubMed] [Google Scholar]
- 40.Mire-Sluis A R, Gaines-Das R, Thorpe R Participants of the Collaborative Study. The international standard for macrophage colony stimulating factor (M-CSF)—evaluation in an international collaborative study. J Immunol Methods. 1995;179:141–151. doi: 10.1016/0022-1759(94)00306-h. [DOI] [PubMed] [Google Scholar]
- 41.Mire-Sluis A R, Gaines-Das R, Thorpe R Participants of the Collaborative Study. Implications for the assay and biological activity of interleukin-8. J Immunol Methods. 1997;200:1–16. doi: 10.1016/s0022-1759(96)00157-3. [DOI] [PubMed] [Google Scholar]
- 42.Osmond D H, Shiboski S, Bacchetti P, Winger E E, Moss A R. Immune activation markers and AIDS prognosis. AIDS. 1991;5:505–511. doi: 10.1097/00002030-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Pesce A J, Michael J G. Artifacts and limitations of enzyme immunoassays. J Immunol Methods. 1992;150:111–119. doi: 10.1016/0022-1759(92)90070-a. [DOI] [PubMed] [Google Scholar]
- 44.Petyovka N, Lyach L, Voitenok N N. Homologous ELISA for detection of oligomeric human TNF: properties of assay. J Immunol Methods. 1995;186:161–170. doi: 10.1016/0022-1759(95)00183-b. [DOI] [PubMed] [Google Scholar]
- 45.Plaeger, S., H. Z. Bass, P. Nishanian, J. Thomas, N. Aziz, R. Detels, J. King, W. Cumberland, M. Kemeny, and J. L. Fahey. The prognostic significance in HIV infection of immune activation represented by cell surface antigen and plasma activation marker changes. Clin. Immunol. Immunopathol., in press. [DOI] [PubMed]
- 46.Poli G, Fauci A S. Role of cytokines in the pathogenesis of human immunodeficiency virus infection. In: Aggarwal B B, Puri R K, editors. Human cytokines: their role in disease and therapy. Cambridge, Mass: Blackwell Scientific; 1995. pp. 421–450. [Google Scholar]
- 47.Poole S, Gaines-Das R E. The international standards for IL-1α and IL-1β—evaluation in an international collaborative study. J Immunol Methods. 1991;142:1–13. doi: 10.1016/0022-1759(91)90286-o. [DOI] [PubMed] [Google Scholar]
- 48.Riches P, Gooding R, Millar B C, Rowbottom A W. Influence of collection and separation of blood samples on plasma IL-1, IL-6 and TNF-α concentrations. J Immunol Methods. 1992;153:125–131. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- 49.Romagnani S, Del Prete G, Manetti R, Ravina A, Annunziato F, De Carli M, Mazzetti M, Piccinni M P, D’Elios M M, Parronchi P, Sampognaro S, Maggi E. Role of TH1/TH2 cytokine in HIV infection. Immunol Rev. 1994;140:73–92. doi: 10.1111/j.1600-065x.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 50.Roux-Lombard P, Steiner G the Cytokine Consensus Study Group of the European Workshop for Rheumatology Research. Preliminary report on cytokine determination in human synovial fluids: a consensus study of the European Workshop for Rheumatology Research. Clin Exp Rheumatol. 1992;10:515–520. [PubMed] [Google Scholar]
- 51.Rubin L A, Kurman C C, Fitz M E, Boutin B. Soluble interleukin-2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985;135:3172–3177. [PubMed] [Google Scholar]
- 52.Seckinger P, Zhang J H, Hauptmann B, Dayer J M. Characterization of a tumor necrosis factor-α inhibitor: evidence of immunological cross-reactivity with the TNF receptor. Proc Natl Acad Sci USA. 1990;87:5188–5192. doi: 10.1073/pnas.87.13.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teodorescu M, Skosey J L, Schlesinger C, Wallman J. Covalent disulfide binding of IL-1 to α2-macroglobulin. Prog Leukoc Biol. 1988;8:209–212. [Google Scholar]
- 54.Thavasu P W, Longhurst S, Joel S P, Slevin M L, Balkwill F R. Measuring cytokine levels in blood: importance of anticoagulants, processing and storage conditions. J Immunol Methods. 1992;153:115–124. doi: 10.1016/0022-1759(92)90313-i. [DOI] [PubMed] [Google Scholar]
- 55.Thorpe R, Wadhwa M, Bird C R, Mire-Sluis A R. Detection and measurement of cytokines. Blood Rev. 1992;6:133–148. doi: 10.1016/0268-960x(92)90025-l. [DOI] [PubMed] [Google Scholar]
- 56.Tsoukas C M, Bernard N F. Markers predicting progression of human immunodeficiency virus-related disease. Clin Microbiol Rev. 1994;7:14–28. doi: 10.1128/cmr.7.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Kessel K P M, Van Strijp J A G, Verhoeff J. Inactivation of recombinant human tumor necrosis factor-α by proteolytic enzymes released from stimulated human neutrophils. J Immunol. 1991;147:3862–3868. [PubMed] [Google Scholar]
- 58.Whicher J, Ingham E. Cytokine measurements in body fluids. Eur Cytokine Netw. 1990;1:239–243. [PubMed] [Google Scholar]