Abstract
A marked decrease in the content of ceramide has been reported in the horny layer of the epidermis in atopic dermatitis (AD). This decrease impairs the permeability barrier of the epidermis, resulting in the characteristic dry and easily antigen-permeable skin of AD, since ceramide serves as the major water-holding molecule in the extracellular space of the horny layer. On the other hand, the skin of such patients is frequently colonized by bacteria, most typically by Staphylococcus aureus, possessing genes such as those for sphingomyelinase, which are related to sphingolipid metabolism. We therefore tried to identify a possible correlation between the ceramide content and the bacterial flora obtained from the skin of 25 patients with AD versus that of 24 healthy subjects, using a thin-layer chromatographic assay of the sphingomyelin-associated enzyme activities secreted from the bacteria. The findings of the assay demonstrated that ceramidase, which breaks ceramide down into sphingosine and fatty acid, was secreted significantly more from the bacterial flora obtained from both the lesional and the nonlesional skin of patients with AD than from the skin of healthy subjects; sphingomyelinase, which breaks sphingomyelin down into ceramide and phosphorylcholine, was secreted from the bacterial flora obtained from all types of skin at similar levels for the patients with AD and the healthy controls. The finding that the skin of patients with AD is colonized by ceramidase-secreting bacteria thus suggests that microorganisms are related to the deficiency of ceramide in the horny layer of the epidermis, which increases the hypersensitivity of skin in AD patients by impairing the permeability barrier.
In the mammalian epidermis, ceramide is a major end product of differentiation, namely the keratinization process, and is secreted in the extracellular space to form a mantle surrounding individual horny (keratinized) cells (19, 26). Such extracellular ceramide, which is arranged in a lamellar structure (11), is a major determinant of the permeability barrier and the water reservoir of the skin (18). In addition, it is well known that ceramide functions as a modulator of cell kinetics in the cytoplasm and thus is related to proliferation, differentiation, and apoptosis (6, 11, 25). Ceramidase (CDase), which catalyzes the cleavage of ceramide to fatty acids and sphingosine, is a known inhibitor of the protein kinase C. In addition, dendritic cells reduce their antigen-presenting capacity when the ceramide concentration level is high (24). Based on such findings regarding the roles of ceramide (2), the content of both ceramide and sphingosine may play a role in modifying the features of the epidermis, including the permeability barrier, immune reaction, and other physical features determined by the proliferation and differentiation of epidermal keratinocytes.
A ceramide deficiency in the stratum corneum is considered to be responsible for the characteristic dry skin of patients with atopic dermatitis (AD) (12, 17, 27). Such dry skin helps ease the penetration of allergens into the skin through numerous fissures in the horny layer, giving rise to frequent and potent allergic responses and resulting in the chronic and recurrent eczematous skin lesions of AD (5). However, the cause of such ceramide deficiency has yet to be elucidated.
In a clinical study (14–16), we found the secretory form of immunoglobulin A to be deficient on the surface of the skin of patients with AD (16). This finding provides a basis for the adhesion and proliferation of bacteria on the skin in AD. Actually, the bacterial count on the skin of AD patients is about 100- to 1,000-fold higher than that on the skin of healthy individuals. On the other hand, nearly 90% of patients with AD are either colonized or infected by Staphylococcus aureus, a bacterium which is found in only approximately 5% of healthy subjects (10, 20).
Although S. aureus possesses the sphingomyelinase gene (SMase) (7), which catalyzes the cleavage of sphingomyelin to ceramide and phosphorylcholine (21, 28), the SMase activity of the bacterial flora of the skin of patients with AD has yet to be investigated. We recently identified a bacterium other than mammalian cells that is able to produce CDase—the first of its type reported in a microorganism; this bacterial enzyme hydrolyzes various species of ceramides, including those from human skin (22). In the present study, we thus investigated the CDase and SMase activities in the bacterial flora obtained from the skin of patients with AD and compared our findings with those for the skin of healthy controls. We also performed the same investigation with the bacterial flora obtained from the erythematous skin lesions of patients with psoriasis.
MATERIALS AND METHODS
Subjects.
We studied 25 Japanese outpatients with AD (13 males and 12 females; mean age, 17.6 years; range, 1 to 33 years) and 24 nonallergic university students (16 males and 7 females; mean age, 22.0 years). An additional control group consisted of eight subjects with psoriasis who were inpatients at our university hospital (males; mean age, 35.4 years; range, 18 to 45). The severity of AD skin lesions was graded from 1 to 4 (Table 1) based on a modification of the severity index established by Rajka and Langeland (23). The laboratory findings for patients with AD showed the following mean values: total immunoglobulin E, 5,917.6 U/ml; leukocytes, 8,164.2/ml; eosinophil, 14.9%.
TABLE 1.
Grading of the severity of eczema in patients with AD
| Grade | Type of eczema | No. of patients |
|---|---|---|
| 1 | Dry skin with minimal inflammation | 0 |
| 2 | Localized areas of more extensive inflammation (four or fewer sites) | 9 |
| 3 | Widespread areas of inflammation with excoriations and erosions | 11 |
| 4 | Erythroderma (affecting most areas of the body) | 5 |
Sampling of the skin flora.
Bacteria were collected from the skin surface by using cotton-tipped swabs moistened with saline. We rubbed such swabs about 10 to 15 times over the eczematous and the normal-appearing skin of patients with AD, the erythematous skin lesions of patients with psoriasis, and the normal skin of healthy controls. The bacteria thus obtained were then multiplied by incubating each swab in the media described below with suitable substrates.
Selective culture of bacteria in SM-Cer-PY culture media.
The tip of each swab was incubated at 25°C for 5 days in 500 μl of synthetic medium A (0.5% peptone yeast, 0.1% extract, 0.5% NaCl, and 0.05% taurodeoxycholic acid [pH 7.2]) (22), which contained 0.045% sphingomyelin and 0.005% ceramide. We used this medium in an attempt to selectively culture CDase-producing and SMase-producing bacteria by providing essential substrates (ceramide and sphingomyelin) for the bacteria to degrade.
Ceramide is generated through three metabolic pathways which are regulated by rate-limiting enzymes: (i) sphingolipid base synthesis serine-palmitoyl transferase (12); (ii) SMase, which catalyzes the cleavage of sphingomyelin to ceramide and phosphorylcholine (21, 28); and (iii) β-glucocerebrosidase, which breaks glucosylceramide down into ceramide and glucose (13).
Preparation of [14C]ceramide.
[14C]ceramide was synthesized by the reverse hydrolysis reaction of sphingolipid ceramide N-deacylase. In brief, 100 nmol of [14C]fatty acid was incubated with 200 nmol of sphingosine in the presence of 500 mU of the enzyme in 1 ml of 25 mM phosphate buffer, pH 7.0, containing 0.3% (wt/vol) Triton X-100. After being incubated at 37°C for 20 h, the reaction mixture was dried with a Speed Vac concentrator. The unreacted fatty acid or sphingosine was separated from [14C]ceramide by using the combination of Sep-Pak Plus Silica, Sep-Pak C18, and Sep-Pak CM cartridges (22).
Assay of CDase and SMase activity.
The activity of CDase was measured by using [14C]ceramide (C16:0, d18:1) as a substrate as follows. First, 100 μl of culture fluid that had been incubated for 5 days was centrifuged at 15,000 × g for 5 min. The rest of each culture medium was frozen at −80°C and later was used to count the bacteria. Thirty-five microliters of the supernatant was recentrifuged. Next, 10 μl of the recentrifuged fluid was mixed with 10 μl of 50 mM NaAacOH, 0.5% Triton X-100 (pH 6.0), and 1 μl of 25 pmol of [14C]ceramide as the substrate. This mixture was incubated at 37°C for 16 h. Thereafter, the samples were evaporated, dissolved in 10 μl of chloroform-methanol (2:1 [vol/vol]), centrifuged at 15,000 × g for 5 min, and applied to a thin-layer chromatography (TLC) plate that was developed with solvent A (chloroform-methanol-ammonia, 90:25:0.5 [vol/vol]). The [14C]palmitic acid released by the action of the enzyme and the remaining [14C]ceramide were separated by TLC and then analyzed and quantified with an imaging analyzer (BAS Model 1000; Fuji Film, Tokyo, Japan). The activity of SMase was measured under the same conditions as those described above, except that choline-radiolabeled [14C]sphingomyelin was used as the substrate, and TLC was developed with solvent B (chloroform-methanol-H2O, 2.5:1:1 [vol/vol]) (22).
The numbers of bacteria in each medium were quite similar among the four groups, and the findings (means ± standard deviations) were as follows: for involved skin with AD, (2.51 ± 0.64) × 106/ml; for uninvolved skin with AD, (2.32 ± 0.88) × 106/ml; for erythematous skin lesions of psoriasis, (2.22 ± 0.63) × 106/ml; for the normal skin of healthy controls, (2.80 ± 0.70) × 106/ml. The activities of CDase and SMase thus obtained were compared by calculating the absolute values of degraded substrates according to the number of bacteria in each sample. The absolute values of the degraded substrates were calculated in advance by subtracting the values of the naturally degradating substrates when there were no bacteria in the medium from the values of total radiolabeled degrading products.
Statistical analysis.
The data for the degrading activity were statistically analyzed by both Dunnett’s method and Spearman’s rank correlation.
Materials.
[14C]sphingomyelin and [14C]fatty acids (stearic acid, palmitic acid, and lauric acid) were purchased from American Radiolabeled Chemicals Inc. Ceramide, sphingomyelin, and Triton X-100 were purchased from Sigma. Precoated silica gel 60 TLC plates were obtained from Merck. The agar media for bacterial counts were obtained from Wako. All other reagents were of the highest purity available.
RESULTS
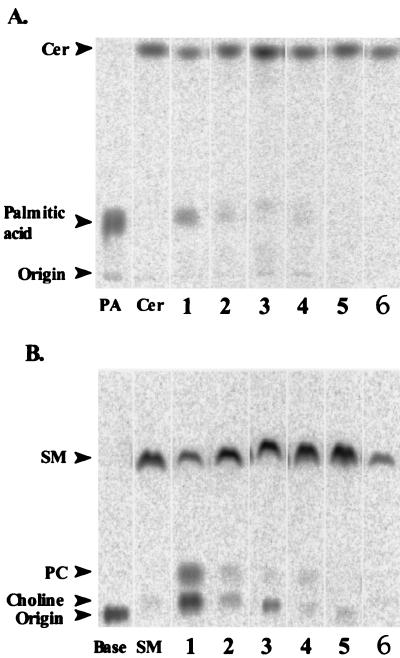
The activity of bacterial CDase or SMase present in each culture supernatant made from cotton swabs was determined by quantifying the reaction product on TLC following incubation with the medium containing [14C]ceramide or [14C]sphingomyelin. CDase catalyzed the cleavage of [14C]ceramide to nonradiolabeled sphingosine and 14C-radiolabeled palmitic acid. We detected clear, relatively thin bands of the reaction product, which corresponded to palmitic acid, in the lanes of bacterial enzymes isolated from the lesional and the normal-appearing skin of patients with AD. However, only a slight amount of reaction product was seen in the lane representing the normal skin of healthy subjects and in the lane representing the erythematous skin lesions of psoriasis (Fig. 1A).
FIG. 1.
Activities of SMase and CDase secreted from bacterial flora. (A) Degradation of ceramide by CDase is shown as bands of 14C-radiolabeled palmitic acid on TLC after incubation with a mixture of [14C]palmitic acid-radiolabeled ceramide and a solution of skin bacteria. (B) Degradation of sphingomyelin by SMase is shown as bands of [14C]choline-radiolabeled phosphorylcholine on TLC after incubation with a mixture of [14C]choline-radiolabeled sphingomyelin and a solution of bacteria. Shown are actuarial enzymes isolated from skin lesion specimens in patients with AD (lanes 1 and 2), normal-appearing skin of patients with AD (lanes 3 and 4), erythematous skin of patients with psoriasis (lane 5), and normal skin of healthy control subjects (lane 6). Cer, ceramide; PA, palmitic acid; SM, sphingomyelin; PC, phosphorylcholine.
SMase catalyzed the cleavage of [14C]choline-radiolabeled sphingomyelin to [14C]choline-radiolabeled phosphorylcholine and ceramide whose composition was not radiolabeled. The bacterial enzymes isolated from both the lesional and nonlesional skin of patients with AD produced a relatively large amount of phosphorylcholine. Nevertheless, the bands of phosphorylcholine were less dense in the lanes of the controls consisting of normal skin and psoriasis (Fig. 1B).
A statistical analysis of the degrading activity among the individual groups revealed that both the lesional ([3.51 ± 0.12] × 10−21 M/bacterial amount) and nonlesional ([3.59 ± 0.15] × 10−21 M/bacterial amount) skin of patients with AD showed a significantly higher activity of bacterial CDase than that of the healthy controls ([3.03 ± 0.09] × 10−21 M/bacterial amount, P < 0.05, Dunnett’s method). As for the bacterial SMase activity, however, no significant difference was found among the groups studied (involved skin of AD, 3.65 ± 0.28; uninvolved skin of AD, 3.17 ± 0.29; control, 3.74 ± 0.16; unit, ×10−21 M/bacterial amount) (Fig. 2). In addition, no significant correlation was found between the activity of bacterial SMase and that of CDase (P = 0.277, Spearman’s rank correlation method).
FIG. 2.
The degrading activities of CDase and SMase of the culture supernatant obtained from the skin lesions and in the normal-appearing skin of patients with AD, lesions of psoriasis, and the skin of healthy subjects. The degrading activity of ceramide is expressed as the ratio of 14C-radiolabeled palmitic acid/total radiolabeled substrates, and that of sphingomyelin is expressed as the [14C]choline-radiolabeled phosphorylcholine/total radiolabeled substrates. The horizontal dotted line represents the mean response for all samples. The line across each diamond represents the mean for each group. The height of each diamond represents the 95% confidence interval for each group, and the width of the diamond represents the size of the sample.
We further analyzed the possible correlation between the enzyme activity and the clinical features of AD. However, no significant relationship was observed between the severity of AD and the activity of bacterial SMase or CDase (P values = 0.8313 and 0.7226, Spearman’s rank correlation method).
DISCUSSION
The present study showed three key finding. (i) CDase-secreting bacteria were present in the skin of patients with AD, regardless of the condition of their skin condition. The enzyme activity was present at similar levels on the skin lesions and the normal-appearing, nonlesional skin of the patients. (ii) SMase was secreted relatively strongly from the bacterial flora of both the AD and healthy skin. (iii) No correlation was found between the activity of bacterial CDase and that of SMase, suggesting that the enzymes were secreted from different bacteria. In addition, such enzyme activities appeared to be unrelated to the severity of AD in the patients in the present study.
The presence of bacterial CDase may thus be one possible mechanism for the deficiency of ceramides in the stratum corneum of AD skin. Ceramide deficiency is responsible for the dry skin of patients with AD (12, 17, 27). We also suspected that the source of the CDase might be Pseudomonas aeruginosa and/or a related strain, because the gram-negative bacterium AN17, which was formerly isolated as CDase-secreting bacteria from atopic skin, was P. aeruginosa (22), and some type strains such as IFO 12689 retained the ability to produce CDase (data not shown). On the other hand, neither the S. aureus type strain, which represents the majority of the skin flora in the patients with AD, nor Staphylococcus epidermis has CDase activity (data not shown). P. aeruginosa is well known to be an opportunistic pathogen (4) but represents a minority of the microbial flora in AD (1, 10). We thus counted P. aeruginosa colonies in all of the samples by using an NAC agar medium, which is a selective culture medium for P. aeruginosa. While we were unable to find P. aeruginosa colonies in either the healthy controls or the psoriasis samples, we did find them in the AD samples, but the amount was very low; five samples of AD had P. aeruginosa strains, and the CDase activity of the five samples was (3.90 ± 0.50) × 10−21 M/bacterial amount, while the other samples of AD had unknown related strains; the maximum content of P. aeruginosa was 2.90 × 1010/ml. These findings suggest that other kinds of bacteria may also secrete CDase but that the dry skin of AD patients is induced only by P. aeruginosa because of the bacterial secretion of CDase.
The ceramide deficiency thus produced may also lead to the proliferation of S. aureus in the skin of patients with AD (10, 20). S. aureus may be predominant because it is resistant to dry environments such as the dry skin of AD patients and to the high-potassium environment produced by sweat concentration on the surface of skin where the water-holding capacity is considerably reduced. In addition, S. aureus adheres more easily to the keratinocytes of AD skin than to those of normal skin or to those of lesions of psoriasis (3, 8).
Sphingolipids are now thought to be secondary messengers that are involved in the differentiation, proliferation, and apoptosis of cells (6). In cultured human keratinocytes, the exogenously added, short-chained, cell-permeated analogues of ceramides significantly promote cellular differentiation, while sphingosine, at an appropriate concentration, modestly stimulates cellular proliferation (25). Mature dendritic cells, which are antigen-presenting cells that are involved in the immune response, reduce their capacity to take up soluble antigens and their antigen-presenting ability in response to the addition of ceramides (24). If this is the case with Langerhans cells, which are dendritic antigen-presenting cells in the epidermis, the reduced amount of ceramide may accelerate the antigen-presenting ability against the penetrating antigens to induce an excessive reaction in the immune response, which is another major characteristic of AD.
It was also interesting that CDase-secreting bacteria were present everywhere on the skin of patients with AD, regardless of its severity. The present findings support the clinical feature of AD that most patients with AD had dry skin even if they are free of eczematous changes.
In human promyelocytic HL-60 cells and U937 monoblastic leukemia cells, treatment with SMase induced apoptosis (9), suggesting that extracellular SMase may alter the intracellular transduction of various functional signals. It can be said concerning exogenous SMase that human skin appears to suffer from relatively strong SMase irrespective of skin condition or disease, suggesting that bacterial SMase is not likely involved in the etiology of AD.
Although the bacterial flora of the psoriasis patients exhibited scaly erythema similar to that seen in the patients with AD, little CDase or SMase activity was observed. These findings suggest that bacterial enzymes concerning sphingolipids are less related to the development of the skin lesions in psoriasis than in AD.
In the present study, we provide evidence that CDase is present in the skin of patients with AD, secreted from P. aeruginosa and/or related strains, suggesting that such exogenous enzymes may be involved in the pathogenesis of AD. The enzyme activities may result in ceramide deficiency, thus disturbing the permeability barrier function of the stratum corneum while accelerating the immune reaction and eventually resulting in the predominance of S. aureus in the skin of patients with AD.
ACKNOWLEDGMENTS
We thank Sandra Pelus and Brian Quinn for their advice on the manuscript and S. Hamanaka of Yamaguchi Rosai Hospital for ceramide samples and helpful discussions.
This work was supported in part by a grant-in aid for scientific research on a priority area (09240101) and a grant-in aid for scientific research (A) (09460051) from the Ministry of Education, Science and Culture of Japan, and the Yamada Science Foundation.
REFERENCES
- 1.Aly R, Maibach H I, Shinefield H R. Microbial flora of atopic dermatitis. Arch Dermatol. 1977;113:780–782. [PubMed] [Google Scholar]
- 2.Bazzi M D, Nelsestuen G L. Mechanism of protein kinase C inhibition by sphingosine. Biochem Biophys Res Commun. 1987;146:203–207. doi: 10.1016/0006-291x(87)90711-x. [DOI] [PubMed] [Google Scholar]
- 3.Bibel D J, Aly R, Shinefield H R, Maibach H I, Strauss W G. Importance of the keratinized epithelial cell in bacterial adherence. J Investig Dermatol. 1982;79:250–253. doi: 10.1111/1523-1747.ep12500072. [DOI] [PubMed] [Google Scholar]
- 4.Bodey G P, Bolivar R, Fainstein V, Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983;5:279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- 5.Bos J D, Kapsenberg M L, Smitt J H. Pathogenesis of atopic eczema. Lancet. 1994;343:1338–1341. doi: 10.1016/s0140-6736(94)92473-2. [DOI] [PubMed] [Google Scholar]
- 6.Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 7.Carroll J D, Cafferkey M T, Coleman D C. Serotype F double- and triple-converting phage insertionally inactivate the Staphylococcus aureus beta-toxin determinant by a common molecular mechanism. FEMS Microbiol Lett. 1993;106:147–155. doi: 10.1111/j.1574-6968.1993.tb05951.x. [DOI] [PubMed] [Google Scholar]
- 8.Cole G W, Silverberg N L. The adherence of Staphylococcus aureus to human corneocytes. Arch Dermatol. 1986;122:166–169. [PubMed] [Google Scholar]
- 9.Cuvillier O, Pirianov G, Kleuser B, Vanek P G, Coso O A, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 10.Goodyear H M, Watson P J, Egan S A, Price E H, Kenny P A, Harper J I. Skin microflora of atopic eczema in first time hospital attenders. Clin Exp Dermatol. 1993;18:300–304. doi: 10.1111/j.1365-2230.1993.tb02202.x. [DOI] [PubMed] [Google Scholar]
- 11.Hamanaka S, Asagami C, Suzuki M, Inagaki F, Suzuki A. Structure determination of glucosyl beta 1-N-(omega-O-linoleoyl)-acylsphingosines of human epidermis. J Biochem. 1989;105:684–690. doi: 10.1093/oxfordjournals.jbchem.a122727. [DOI] [PubMed] [Google Scholar]
- 12.Holleran W M, Man M Q, Gao W N, Menon G K, Elias P M, Feingold K R. Sphingolipids are required for mammalian epidermal barrier function. Inhibition of sphingolipid synthesis delays barrier recovery after acute perturbation. J Clin Investig. 1991;88:1338–1345. doi: 10.1172/JCI115439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holleran W M, Takagi Y, Menon G K, Legler G, Feingold K R, Elias P M. Processing of epidermal glucosylceramides is required for optimal mammalian cutaneous permeability barrier function. J Clin Investig. 1993;91:1656–1664. doi: 10.1172/JCI116374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imayama S, Hashizume T, Miyahara H, Tanahashi T, Takeishi M, Kubota Y, Koga T, Hori Y, Fukuda H. Combination of patch test and IgE for dust mite antigens differentiates 130 patients with atopic dermatitis into four groups. J Am Acad Dermatol. 1992;27:531–538. doi: 10.1016/0190-9622(92)70218-5. [DOI] [PubMed] [Google Scholar]
- 15.Imayama S, Shibata Y, Hori Y. Epidermal mast cells in atopic dermatitis. Lancet. 1995;346:1559. doi: 10.1016/s0140-6736(95)92089-7. [DOI] [PubMed] [Google Scholar]
- 16.Imayama S, Shimozono Y, Hoashi M, Yasumoto S, Ohta S, Yoneyama K, Hori Y. Reduced secretion of IgA to skin surface of patients with atopic dermatitis. J Allergy Clin Immunol. 1994;94:195–200. doi: 10.1016/0091-6749(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 17.Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Investig Dermatol. 1991;96:523–526. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- 18.Imokawa G, Akasaki S, Hattori M, Yoshizuka N. Selective recovery of deranged water-holding properties by stratum corneum lipids. J Investig Dermatol. 1986;87:758–761. doi: 10.1111/1523-1747.ep12456950. [DOI] [PubMed] [Google Scholar]
- 19.Lampe M A, Burlingame A L, Whitney J, Williams M L, Brown B E, Roitman E, Elias P M. Human stratum corneum lipids: characterization and regional variations. J Lipid Res. 1983;24:120–130. [PubMed] [Google Scholar]
- 20.Leyden J J, Marples R R, Kligman A M. Staphylococcus aureus in the lesions of atopic dermatitis. Brit J Dermatol. 1974;90:525–530. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 21.Menon G K, Grayson S, Elias P M. Cytochemical and biochemical localization of lipase and sphingomyelinase activity in mammalian epidermis. J Investig Dermatol. 1986;86:591–597. doi: 10.1111/1523-1747.ep12355263. [DOI] [PubMed] [Google Scholar]
- 22.Okino N, Tani M, Imayama S, Ito M. Purification and characterization of a novel ceramidase from Pseudomonas aeruginosa. J Biol Chem. 1998;273:14368–14373. doi: 10.1074/jbc.273.23.14368. [DOI] [PubMed] [Google Scholar]
- 23.Rajka G, Langeland T. Grading of the severity of atopic dermatitis. Acta Dermato-Venereol Suppl. 1989;144:13–14. doi: 10.2340/000155551441314. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Nicolo C, De Maria R, Corinti S, Testi R. Ceramide inhibits antigen uptake and presentation by dendritic cells. J Exp Med. 1996;184:2411–2416. doi: 10.1084/jem.184.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakita H, Tokura Y, Yagi H, Nishimura K, Furukawa F, Takigawa M. Keratinocyte differentiation is induced by cell-permeant ceramides and its proliferation is promoted by sphingosine. Arch Dermatol Res. 1994;286:350–354. doi: 10.1007/BF00402228. [DOI] [PubMed] [Google Scholar]
- 26.Wertz P W, Swartzendruber D C, Abraham W, Madison K C, Downing D T. Essential fatty acids and epidermal integrity. Arch Dermatol. 1987;123:1381–1384. [PubMed] [Google Scholar]
- 27.Yamamoto A, Serizawa S, Ito M, Sato Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch Dermatol Res. 1991;283:219–223. doi: 10.1007/BF01106105. [DOI] [PubMed] [Google Scholar]
- 28.Yamamura T, Tezuka T. Change in sphingomyelinase activity in human epidermis during aging. J Dermatol Sci. 1990;1:79–83. doi: 10.1016/0923-1811(90)90219-4. [DOI] [PubMed] [Google Scholar]