Abstract
When nanoparticles (NPs) enter a physiological environment, a complex coating of biomolecules is absorbed onto their surface, known as the biocorona (BC). This coating alters nanomaterial physical properties, modulating cellular viability, internalization, and immune responses. To safely utilize NPs within medical settings, it is necessary to understand the influence of the BC on cellular responses. Due to the variety of cell types, NPs, and physiological environments, responses are variable; though trends do exist. This review article critically evaluates the currently available literature regarding the influence of the BC on NP interactions with prominent cell types that they are likely to encounter during biomedical applications. Specifically, we will examine responses related to interactions with endothelial cells, macrophages, and epithelial cells of the digestive tract and lung. Further, we will evaluate how the BC may influence interactions with bacteria and fungi, as NPs have been proposed as antimicrobial agents in medical settings. The information reviewed and discussed here may enhance the development of effective of NP-based therapeutics and diagnostic tools.
This article is categorized under: Therapeutic Approaches and Drug Discovery > Emerging Technologies, Toxicology and Regulatory Issues in Nanomedicine > Toxicology of Nanomaterials, Diagnostic Tools > Diagnostic Nanodevices
Keywords: cell interactions, corona, disease environments, nanomedicine, toxicology
1 |. INTRODUCTION
While nanoparticles (NPs) possess a number of intrinsic qualities that make them appealing for biomedical applications, several barriers exist to their clinical utilization. Specifically, many nanotherapeutics that have shown significant therapeutic potential in controlled laboratory conditions have had issues transitioning to utilization in more complex clinical scenarios (Bisht & Maitra, 2009; Schutz, Juillerat-Jeanneret, Mueller, Lynch, & Riediker, 2013). One potential obstacle in the translation of NPs is likely the formation of the NP–biocorona (BC). The BC is a coating of biomolecules that forms on the NP surface following entry into a physiological environment. The composition of the BC can be altered depending on a multitude of factors, including the character of the surrounding physiological environment, time, and NP physiochemical properties such as coating, size, charge, hydrophobicity, surface curvature, etc. (Kobos, Adamson, Evans, Gavin, & Shannahan, 2018; Sahneh, Scoglio, & Riviere, 2013; J. H. Shannahan et al., 2016; Walkey & Chan, 2012). Previously, the BC was modeled as an entity that, while dynamic, consisted primarily of proteins (Lundqvist et al., 2008; Lynch & Dawson, 2008; Orco, Lundqvist, Oslakovic, Cedervall, & Linse, 2010). Recently, newer models of the BC are being utilized that capture its complexity by incorporating interactions between the NP and a variety of biomolecules including proteins, lipids, and others (Hellstrand et al., 2009; Landry et al., 2015; Müller et al., 2018; Raesch et al., 2015). Modern computational kinetic models have begun to understand the NP–biomolecule interactions that dictate BC formation (Monopoli, Åberg, Salvati, & Dawson, 2012; R. Li et al., 2013; Sahneh, Scoglio, Monteiro-Riviere, & Riviere, 2015; Tavanti, Pedone, & Menziani, 2019). A number of methods exist for the study of NP–biomolecular interactions, including ultracentrifugation, gel electrophoresis, and mass spectrometry (Tenzer et al., 2011, 2013; Tiede, Hassellöv, Breitbarth, Chaudhry, & Boxall, 2009). Previously, a comprehensive review detailed the advantages and disadvantages of each of these methods, as well as many others (Docter et al., 2015). Determining the exact mechanisms of how proteins bind the NP surface is challenging, though in recent years our understanding has significantly expanded. A number of excellent reviews have previously been published on the subject of NP–biomolecular interactions which thoroughly detail what is known regarding mechanisms involved in the formation of the BC on the NP surface (Lin et al., 2018; Monopoli et al., 2012; Neagu, Piperigkou, Karamanou, & Engin, 2017; J. Shannahan, 2017; Walkey & Chan, 2012). Modification of human serum albumin has helped to elucidate the influence of protein charge distribution on NP adsorption (Treuel et al., 2014). Additionally, other studies have utilized NP antibody labeling and analytical techniques such as X-ray absorption spectroscopy to determine specific binding sites and examine alterations in protein structure (Kelly et al., 2015; Wang et al., 2013). It is necessary to understand these initial NP–biomolecule interactions to develop and safely utilize NPs within medical scenarios.
Ultimately, the addition of the BC hinders the medical utilization of NPs by modifying targeting, function, clearance, biodistribution, and toxicity (Alex, Chandrasekaran, & Mukherjee, 2017; Amiri et al., 2013; Chakraborty et al., 2018; Salvati et al., 2013; J. Shannahan, 2017; Westmeier, Stauber, & Docter, 2016). These unpredicted variations in biological response result in the loss of nanotechnology benefits and raise concerns of adverse health effects. These variations are consequences of BC-induced alterations in NP–cellular interactions. Overall, there is a degree of controversy regarding the role of the BC in biomedical applications. While some researchers consider the BC solely as a barrier to clinical adoption of NP therapeutics, others hypothesize that the BC may be modified and utilized as an asset to NP-based biomedical applications.
Our current review focuses on the impact of the BC on NP–cellular interactions and the influence of these modified interactions on the medical usage of NPs. Given the increasing prevalence of NP therapeutics in medicine, this knowledge is essential to the development and improved translation of nano-enabled therapeutics and diagnostic tools. Based on proposed biomedical applications and delivery routes of NPs, BC-induced alterations in NP interactions with endothelial cells, macrophages, and epithelial cells are discussed within this review (Figure 1). Specifically, the review will focus on BC-induced alterations in cellular responses, including viability, NP internalization, and immune response. Additionally, this review high-lights what is known regarding the impact of the BC on the use of NPs to target bacterial and fungal cells. Identified deficiencies within our current knowledge that require additional evaluation will be highlighted throughout, as will the medical implications of these modified cellular interactions.
FIGURE 1.
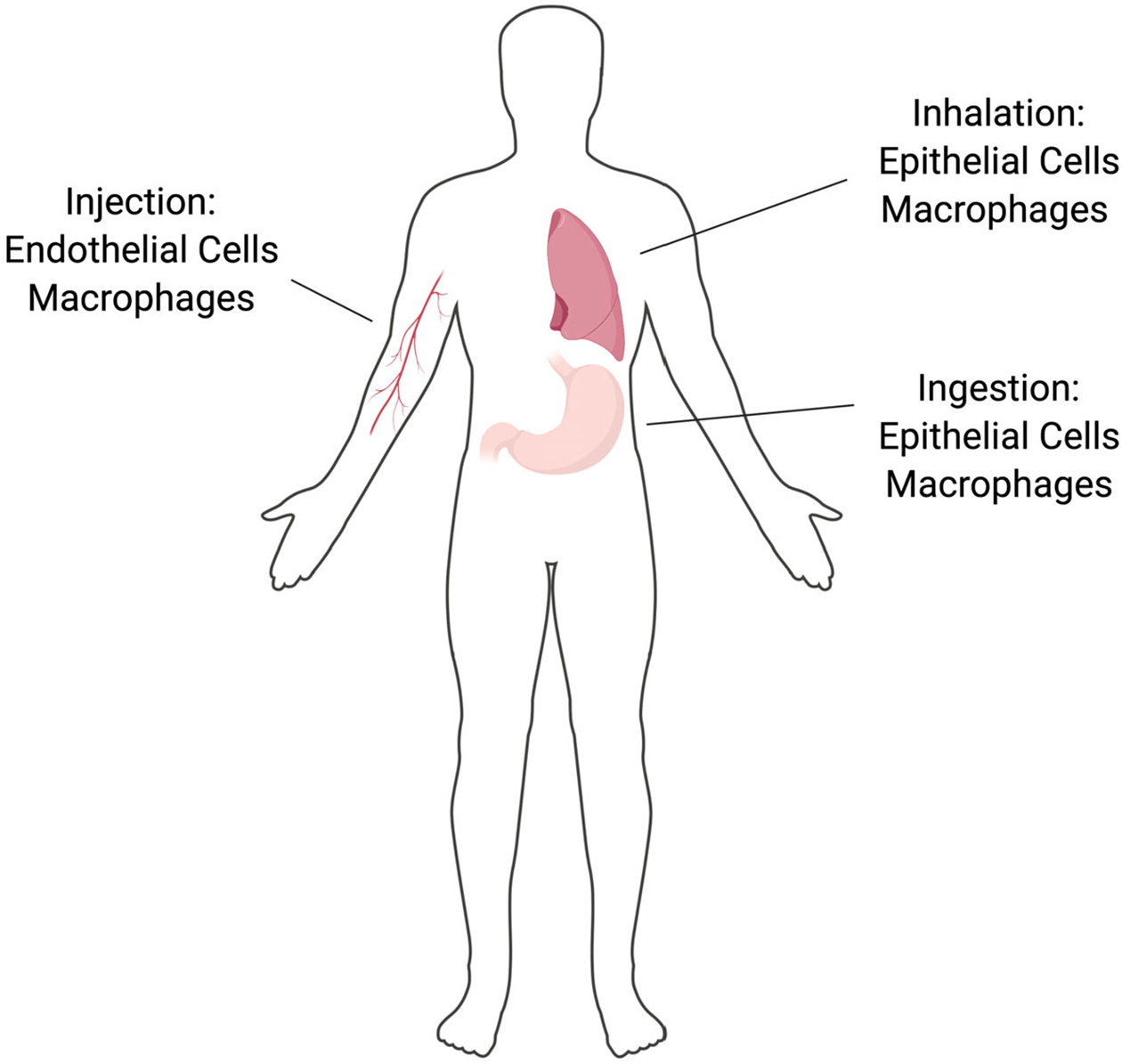
NPs are proposed for a number of biomedical applications which will result in their introduction into the body via intravenous injection, inhalation, or ingestion. This results in interactions with specific cell populations including endothelial cells, macrophages, gastrointestinal epithelial cells, or alveolar epithelial cells. NP, nanoparticle
2 |. ENDOTHELIAL CELLS
Endothelial cells are highly relevant in the discussion of nanomedicine, as many NP therapeutics, including imaging agents and some cancer treatments, are intended to be directly injected into the circulatory system. Once within the circulation, the NPs adsorb biomolecules, which are hypothesized to alter NP–endothelial cell interactions, inducing changes in cellular uptake, cytotoxicity, and other parameters. Further, the addition of the BC can disrupt NP targeting, reducing therapeutic efficacy.
2.1 |. The influence of the BC on endothelial cell internalization of NPs
Experimental results regarding the BC’s effect on NP internalization by endothelial cells lack consistency. While a positive correlation has been observed between the amount of albumin protein adsorbed to the surfaces of several different polystyrene NPs and uptake by endothelial cells, other studies have shown the opposite (Ehrenberg, Friedman, Finkelstein, & Mcgrath, 2009). Specifically, the addition of a BC formed from human plasma reduced the internalization of 40 and 80 nm gold NPs by human umbilical vein endothelial cells (HUVECs; Chandran, Riviere, & Monteiro-Riviere, 2017). The implication of this altered endothelial internalization due to addition of the BC is that the bioavailability of the NP may be altered, resulting in modified delivery to target tissues and differential endothelial responses. These results regarding BC-induced alterations in NP-endothelial cell internalization suggest that albumin-rich BCs facilitate uptake, while other proteins may reduce it. Therefore, development of NPs that associate high amounts of albumin could be utilized to more efficiently deliver endothelial-targeted treatments. Conversely, NPs with naturally formed BCs containing less albumin and more of other proteins are less likely to interact with endothelial cells, thereby increasing circulation time. Modification of the BC may be accomplished by coating NPs with polyethylene glycol (PEG), causing a reduction in the association of highly abundant proteins in the serum such as albumin and thus reducing endothelial internalization (Zhou et al., 2018). With less protein binding and increased internalization, it may seem that PEG-coated NPs are an ideal solution for the targeting of endothelial cells. However, there is some controversy regarding the use of PEG as a NP coating in therapeutic applications, as antibodies will eventually form against the PEG, resulting in internalization by macrophages rather than endothelial cells (B. Lee & Nguyen, 2017). This issue is discussed in further detail in Section 3 of this review.
Other distinct protein components of the BC other than albumin have also been studied. One study examined three major human serum and BC proteins (albumin, fibrinogen, and immunoglobulin) to assess how each individually influenced gold and silver NP uptake by HUVECs. It was determined that a fibrinogen BC markedly decreased uptake when compared to either the albumin or immunoglobulin BCs (Sasidharan, Riviere, & Monteiro-riviere, 2015). The ability of superparamagnetic iron-oxide NPs to bind apolipoproteins was determined to assist in their uptake by endothelial cells forming the blood brain barrier and facilitate their entrance into the neuronal system (Mahmoudi et al., 2015). Some researchers have attempted to utilize the BC to their advantage, creating an artificial biomimetic BC. This is typically done by coating the NP with specific cellular membranes which can alter NP–cellular interactions. The functionalization of silica NPs with leukocyte membranes was found to enhance interactions with HUVECs, allowing the NPs to more efficiently cross an inflamed endothelial barrier to deliver their therapeutic payload (Parodi et al., 2013). The transmembrane protein LFA-1 was determined to be a vital component of the NP precoating, as it induced clustering of ICAM-1 on the HUVEC surface, allowing the NP to bind to and interact with the endothelium, facilitating internalization. Overall, this demonstrates that the identity of the BC may alter endothelial internalization. Specifically, modulations in endothelial cell uptake of NPs may be dependent upon the presence/absence or ratio of distinct proteins within the NP–BC. Certain components such as immunoglobulins, apolipoproteins, and albumin appear to promote NP internalization by endothelial cells, while others such as fibrinogen inhibit it. The ability of specific proteins to promote certain outcomes may indicate their importance in not only understanding the BC and its influence over NP–cellular interactions, but in the future design and engineering of NP therapeutics.
Interactions between biomolecules and the NP surface may induce conformational modifications in structure, influencing endothelial cell interactions and internalization. Specifically, association of albumin with the NP surface results in structural and conformational changes, including both misfolding and changes to the secondary protein structure (Ding et al., 2013; Podila, Vedantam, Ke, Brown, & Rao, 2012; J. H. Shannahan et al., 2015). This can result in increased interactions with scavenger receptors gp30 and gp18 as compared to unmodified albumin, contributing to NP uptake by endothelial cells (Figure 2; Bravo & Schnitzer, 1993). NPs can be produced that exhibit diverse properties, which may result in a variety of biomolecule structural modifications that alter NP interactions with specific cell surface receptors, such as scavenger receptors. In designing NPs for clinical applications, modifications to the NP’s surface coating, charge, surface curvature, and other properties may be able to control endothelial cell interactions via differential changes in specific protein adsorption and structural alterations.
FIGURE 2.
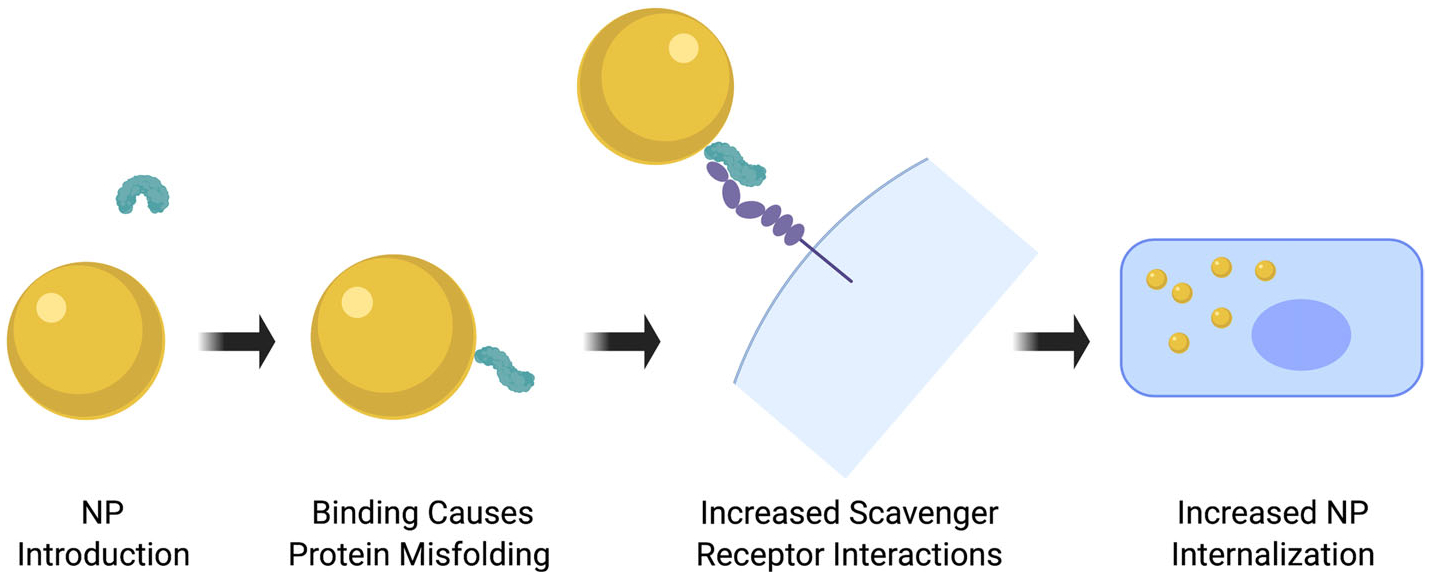
Biomolecules associated with the NP surface can be recognized by cell surface receptors facilitating interactions and internalization. Association with the NP surface can induce conformational alterations in biomolecule structure, allowing for receptor interactions. NP, nanoparticle
2.2 |. The impact of the BC on endothelial cell viability and responses
The BC has the capacity to alter the toxicity of the NP, resulting in differential cellular responses as compared to the bare NP. A number of studies have demonstrated that a plasma BC increases viability of HUVECs and ISO-HAS-1 cells following NP exposure (Chandran et al., 2017; Tenzer et al., 2013). This inhibition of cell death was suggested to be a result of BC-related reductions in cellular internalization. Additionally, gold NPs coated with branched polyethylenimine (BPEI) cause increased expression of genes associated with DNA damage and repair, heat shock response, mitochondrial energy metabolism, oxidative stress and antioxidant response, and ER stress in HUVECs (Chandran et al., 2017). Specific genes which were observed to be elevated included dual oxidase 1 and thioredoxin interacting protein, both of which are known to alter the production of reactive oxygen species through regulation of oxidation–reduction reactions. Addition of a plasma BC to the BPEI-coated gold NPs attenuated these endothelial cell responses, demonstrating the capacity of the BC to alter responses to NP exposure. This suggests that in vitro studies evaluating NP toxicity may overestimate cellular effects if the BC is not included, potentially resulting in premature and erroneous removal of effective NPs from production and development for clinical applications.
The role of specific BC proteins in terms of endothelial toxicity has been examined by utilizing simplified BCs consisting of only bovine serum albumin (BSA). The simplified BC had no impact on rat aortic endothelial cell (RAEC) viability following exposure to carbon nanotubes (CNTs), but did appear to decrease the expression of the proinflammatory gene IL-6 and the oxidative stress marker HMOX-1 when compared to CNTs with no BC (Bai, Wu, Mitra, & Brown, 2016). Multiple studies have performed comparisons of BCs formed of distinct proteins that are prevalent within the circulation to evaluate their contribution to HUVEC responses following CNT exposure. While albumin and immunoglobulin have both been found to reduce CNT cytotoxicity, fibrinogen is the most effective (Ge et al., 2011; Lu, Sui, Tian, & Peng, 2018). However, the toxicity of some NPs is not attenuated by a BC. For example, iron-oxide NPs and multiwalled CNTs with a BC formed in either serum or pulmonary surfactant caused a greater inflammatory response than their respective bare NPs (J. H. Shannahan et al., 2016; Vidanapathirana et al., 2012). Additionally, the bare multiwalled CNTs induced greater endothelial expression of the cellular adhesion molecules VCAM-1, ICAM-1, and SELE compared to CNTs with a BC (Vidanapathirana et al., 2012). Taken together, these studies indicate a number of conclusions regarding the impact of the BC on NP toxicity in endothelial cells. Though some proteins are found in abundance in the BC, they are not the sole contributors to the cellular response. BC components which are found in lower abundances play a critical role in determining the cellular response. Secondly, some proteins appear to be more protective as BC components compared to others. Specifically, fibrinogen within the BC appears to increase cellular viability of endothelial cells, which is generally seen as a favorable outcome for biomedical applications. This may have implications for the future design and use of NP therapeutics, in either the design of the NP itself to attract specific proteins or in the preformation of custom BCs to reduce toxicity. Overall, these studies indicate that the BC can attenuate or exacerbate endothelial toxicity of NPs depending on the properties of the NP and the composition of the BC. Therefore, it is essential to utilize the BC in experiments prior to their introduction into animal models and humans, as in vitro studies of only bare NPs may incorrectly identify adverse responses.
2.3 |. The impact of disease on BC-induced endothelial cell responses
The majority of experimentation regarding biological responses to NPs has been performed in normal, healthy scenarios. However, clinical applications will result in the introduction of NPs into diseased environments with altered circulating biomolecules, which may influence NP–BC formation and subsequent cellular responses (Raghavendra, Fritz, Fu, Brown, & Shannahan, 2017; J. H. Shannahan et al., 2016). A study of iron-oxide NPs compared the differential cellular effects of BCs formed within healthy or hyperlipidemic serum (J. H. Shannahan et al., 2016). While addition of both BCs reduced NP internalization, they also increased the gene expression of proinflammatory mediators such as IL-6, TNF-α, VCAM-1, IL-1β, and others compared to the bare particle, with the hyperlipidemic BC being more proinflammatory (J. H. Shannahan et al., 2016). Distinct alterations in circulating biomolecules are known to occur in disease, which may influence BC formation. For example, lipoprotein levels are modified in metabolic diseases. A study of the differential toxicological effects of silver NPs on RAECs investigated the impact of lipoprotein, a specific component of the BC (Persaud et al., 2019). BCs were formed on 20 or 100 nm silver particles using fetal bovine serum (FBS) to form a complex BC, or single protein BCs consisting of either BSA or high-density lipoproteins (HDL). The BSA and FBS BCs reduced uptake of 100 nm particles compared to NPs without a BC. An HDL BC did not significantly reduce internalization compared to NPs without a BC. This suggests that lipoprotein-rich BCs that form in disease scenarios may increasingly interact with endothelial cells, resulting in intracellular accumulation and exacerbation of cellular responses. When endpoints of ER stress were normalized to NP uptake, it was determined that the addition of the BSA, FBS, and HDL BCs enhanced ER stress via activation of the IREα and PERK pathways through nonoxidative stress mechanisms. These findings suggest that NPs with a BC may be more potent in inducing cellular responses such as ER stress compared to equivalent amounts of NPs without a BC following internalization. The researchers hypothesize, though do not confirm, that these effects may be the result of the NP causing structural alterations to the proteins that adsorb to its surface. This would align with previous research, which has demonstrated the capacity of misfolded proteins to cause ER stress via the unfolded protein response, a process which has been implicated in a number of chronic diseases (Haeri & Knox, 2012; Rao & Bredesen, 2014). In general, these studies demonstrate that the differential biomolecule content of disease environments forms unique BCs that may induce unique endothelial cell responses. Interestingly, these studies demonstrate that the exacerbated cellular response may result from addition of the BC, independent of altered internalization. This suggests that NP internalization cannot always be used as a surrogate for cellular response, as the BC may alter cellular signaling due to the delivery of misfolded proteins into the cell.
2.4 |. The influence of the BC on NP–endothelial cell interactions: Conclusions
The BC impacts NP–endothelial interactions, which may influence NP-based therapeutics delivered intravenously into the circulation. While the BC may be seen as desirable in that it often improves endothelial cell viability following NP exposure, issues have also been identified. The BC may disrupt targeting and induce inflammatory responses through the absorption of specific proteins within the BC and alterations in protein structure. The BC also alters NP interactions with cell surface receptors and intracellular effects such as ER stress. Together, these altered interactions with endothelial cells may result in the development of certain inflammatory diseases, such as atherosclerosis, in which scavenger receptor are known to be increasingly expressed (Moore & Freeman, 2006). Intravenously delivered NPs, such as single-walled CNTs, have been shown to alter vascular tone and may contribute to adverse cardiovascular effects (Vlasova et al., 2014). Currently, there is a lack of evaluation examining the influence of the BC regarding alterations in vascular tone via changes in endothelial inducible nitric oxide synthase, endothelial nitric oxide synthase, and endothelin-1. Further, limited research has been performed in prevalent diseased models such as obesity, diabetes, cancer, and others. These conditions likely alter BC formation via disease-induced modifications in the biomolecule content of the circulation and subsequent endothelial interactions. The information from current and future studies investigating the influence of the BC on NP–endothelial interactions will be useful for the development and use of NP therapeutics intended for intravenous use, such as imaging agents and cancer treatments.
3 |. MACROPHAGES
The interactions between NPs and immune cells are perhaps the most extensively studied of all cell types. Macrophages are prevalently utilized for in vitro nanotoxicity studies due to their role in phagocytosis of foreign materials and their ability to initiate the immune response (Mills, 2012; Okabe & Medzhitov, 2016). The BC can enhance NP–macrophage interactions through opsonization, resulting in increased recognition and phagocytosis (Wolfram et al., 2015). It is hypothesized that BC-induced alterations in NP–macrophage interactions could present numerous potential challenges for medical use of NPs. Specifically, these interactions may influence therapeutic targeting and delivery by modifying NP clearance and biodistribution while also inducing an immune response.
3.1 |. Impact of the BC on macrophage internalization of NPs
Immune cells, such as macrophages, internalize NPs by a variety of mechanisms, which have been found to vary depending on the presence of the BC on the NP’s surface. Macrophages primarily internalize NPs with BCs via phagocytosis, while NPs without a BC are mostly taken up by alternative mechanisms such as clatharin- or dynamin-mediated endocytosis (Yan et al., 2013). Researchers hypothesize that this may be due to the BC changing the size of the particle, or proteins present on the particle surface activating specific surface receptors (Lunov et al., 2011). While the biological outcome of both uptake mechanisms is the same, not all cell types are capable of both. Endocytosis is a more general process which is carried out by many eukaryotic cell types, while phagocytosis is more specific and primarily performed by specialized cells of the immune system (Alberts, Johnson, & Lewis, 2002). This may indicate that the presence of a BC may result in increased internalization by macrophages, and that a reduction in protein binding may be a method by which NP therapeutics could avoid detection and uptake by the immune system. The degree to which NPs are internalized also appears to be BC-dependent, though studies have demonstrated variability based on BC components. For example, addition of a serum BC on silver NPs increased uptake of NPs by RAW 264.7 mouse macrophages (J. H. Shannahan, Podila, & Brown, 2015). This increased uptake was observed to be more pronounced for larger particles (110 nm) than smaller ones (20 nm). However, a separate study using the same macrophage cell line found that association of a serum BC inhibited uptake of 5, 20, and 50 nm gold NPs (Cheng et al., 2015). These results serve to underline the complexity of the influence of the BC on macrophage–NP interactions, as two metallic NPs of similar size in the same environment do not produce the same effect. This indicates that the effect of the BC cannot be inferred for each NP based upon previous evaluations of similar NPs.
Biocorona effects on NP–macrophage interactions also appear to be modified across time points in cell culture experiments. A study of iron-oxide NPs examined the effects of either a normal serum BC or an LDL-supplemented serum BC on the uptake in RAW 264.7 macrophages (X.-F. S. Adamson, Lin, Chen, Kobos, & Shannahan, 2014). The study determined that the addition of both BCs reduced NP association with macrophages at early time points compared to NPs without a BC. However, at later time points, cellular association of NPs with the LDL-BC was increased in comparison to the other NPs. It is possible that NPs without a BC interact with macrophages at early time points in culture due to faster settling velocities and/or charge-driven interactions. Specifically, addition of the BC to the NP surface has been found to increase NP agglomeration, which may decrease settling velocity while association of biomolecules decreases the NP’s surface charge, impacting charge-driven interactions. BC-induced alterations in these parameters impact how in vitro screening studies should be performed, and may influence the translatability of research findings. This demonstrates the importance of time course assessments in the evaluation of BC-induced alterations in macrophage interactions. The increased uptake of NPs with LDL-rich BCs at later time points suggests preferential internalization that is LDL-dependent. Other studies also support the role of LDL as a promoter of cellular uptake. Lipoprotein-depleted serum (LPDS) and delipidated LPDS were used to examine the effects of LDL on the uptake of quantum dots by RAW 264.7 macrophages (Prapainop, Witter, & Wentworth, 2012). The study demonstrated reduced NP uptake in conditions where LDL was absent, indicating the importance of LDL in NP–macrophage interactions. The researchers also elucidated the mechanism by which LDL facilitates macrophage uptake of NPs. The NP coating (5,6-secosterol atheronal-B) binds apolipoprotein B-100 within LDL, causing misfolding and exposing specific epitopes. These exposed epitopes can interact with the CD-36 scavenger receptor on the macrophage cell surface and facilitate uptake (Prapainop et al., 2012). This suggests that BCs with a higher LDL content may result in increased internalization of NPs by macrophages, impacting clearance, biodistribution, and immune activation. However, LDL is not the only component of the BC which may influence internalization. A study examining how the absence of glycans (polysaccharides) affects the interaction of THP-1 derived M1 and M2 macrophages with silica NPs determined that the in situ removal of glycans from the BC decreased colloidal stability of the NP, resulting in modified internalization (Wan et al., 2015). Specifically, NP–BCs that underwent an enzyme treatment to remove the glycans in the BC adhered more strongly to the macrophage membrane and were internalized more than NP–BCs that did not experience such a treatment.
Together, these studies demonstrate that NP internalization by macrophages is influenced by many factors, including the specific biomolecules present within the BC, duration of exposure, and NP properties. LDL present within the BC appears to be involved in immune cell recognition and internalization of NPs. This is likely due to the expression of lipoprotein receptors, such as scavenger receptors, on macrophages. These lipoprotein-mediated responses may have implications for the use of NP therapeutics in patients with high circulating levels of LDL. Specifically, NPs within circulation the circulation of patients with elevated LDL may be more readily recognized and internalized by immune cells. This presents an issue if the NP is intended to avoid immune detection, as it may result in enhanced clearance. However, this increased uptake could potentially be a benefit if the immune system is the therapeutic target.
3.2 |. Specific biomolecular components of the BC involved in NP–macrophage inflammation and cytotoxicity
While there are numerous biomolecules associated with the BC on NPs, albumin often represents a major component of the BC due to its abundance in serum. Therefore, certain studies have attempted to utilize an albumin-only BC as a surrogate for a complex BC. Comparison of a complex BC to an albumin-only BC on silver NPs demonstrated that, although a major component of the complex BC, albumin was not the sole contributor macrophage responses (J. H. Shannahan, Podila, & Brown, 2015). A NP with a complex BC formed from serum stimulated different levels of cellular uptake, activation, and cytotoxicity than a NP with a BC of only albumin. This implies that although albumin is the primary component of many BCs, other biomolecules contribute to macrophage responses. In a separate study, incorporation of LDL into the BC was determined to enhance iron-oxide NP inflammatory response compared to BCs free of LDL (S. X. F. Adamson et al., 2014). LDL is not the only biomolecule which has been shown to alter the BC’s influence on NP–immune cell interactions. Some studies have attempted to correlate the abundance of specific BC components, or a lack thereof, to certain cellular outcomes. It has been determined that a NP–BC that has been treated with enzymes to remove glycans is slightly more cytotoxic than the untreated, stimulating greater release of proinflammatory cytokines IL-1β in M1-type macrophages and TNF-α in M2-type (Wan et al., 2015). Together, these evaluations demonstrate the influence of specific proteins within the BC on NP–macrophage interactions. In vitro experiments that utilize complex BCs are likely more relevant to clinical scenarios than those using BCs made of singular proteins; however, studies support the conclusion that specific proteins within the BC do appear to influence interactions and responses. Specifically, the presence of LDL and other lipoproteins appear to induce an inflammatory response by macrophages, and NPs with BCs rich in glycans may more efficiently interact with immune cells. This information can be applied to generate solutions to barriers impeding the clinical application of nanotechnology by assisting in the design of NPs that can either engage or evade the immune system. Continued evaluation of BC-mediated NP–immune cell interactions is needed to synthesize therapeutics to attract or repel specific biomolecules to ensure formation of a BC that will assist in treatments.
3.3 |. Alteration of the macrophage response through BC modification
Attempts have been made in recent years to engineer solutions to the issues of NP internalization and cytotoxicity through modifications to the BC such that it may be utilized as an aspect of treatment, rather than a hindrance. One of the main obstacles facing the use of NP therapeutics is clearance via the immune system, preventing NPs from reaching their target and decreasing their efficacy. For this reason, the development of NPs and/or coatings to evade the immune system is valuable for biomedical function and applications. It has been shown that NPs designed for drug delivery associate circulating antibodies, resulting in increased phagocytosis by the immune system, limiting their therapeutic potential (Corbo & Toledano, 2016). Making the NP less visible to the immune system has therefore been a goal of biomedical engineers, because a NP that evades immune clearance is often more therapeutically active. Addition of PEG to the surface of NPs has been increasingly utilized in attempts to limit protein binding to the NP, thus allowing for NPs to evade the immune system and enhancing circulation time. These attempts have had moderate success, as PEG coating reduces nonspecific protein binding and increases blood circulation time (Besnard, Noel, Appel, Angelo, & Couvreur, 1999; Papi et al., 2017; Runa et al., 2014). However, the benefit of PEG is limited, as repeated administration of PEG-coated NPs results in accelerated clearance due to an increase in circulating antibodies against PEG (B. Lee & Nguyen, 2017). It is likely that other coatings could be developed to overcome the limitations associated with PEG. Further, PEG could be utilized for the acute administration of NP-based therapeutics.
Some researchers have attempted to evade the immune system by preforming BCs on NPs prior to introducing them into the biological system. Multiple approaches have been utilized toward this end. Some studies have been measured in their attempts, using only a single protein or a small selection in order to try to evade immune detection. One such study utilized CD-47 as a model to design a minimalistic peptide that would result in the cell recognizing the NP as endogenous (Rodriguez et al., 2014). CD-47, also known as integrin associated protein, is highly conserved between cell types and species and acts as a self-identifier, allowing the immune system to distinguish between foreign material and the self. Attachment of the synthesized simplified CD-47 to the surface of the polystyrene nanobeads inhibited macrophage uptake and prolonged circulation time (Rodriguez et al., 2014). Other researchers have eschewed simplified biomimetic coatings in favor of more complex composites consisting of NPs, drugs, and additional compounds. For example, BCs containing endogenous compounds, or substances meant to resemble endogenous compounds, have generated interest in recent years. One of the main advantages conferred by such biomimetic coatings is prolonged circulation. With the NPs disguised by the biomimetic coating, the immune system is temporarily unable to label them as foreign, and the NPs can remain active in the body for longer periods of time. Biomimetic coatings may also enhance targeting capabilities and reduce nontarget effects. A variety of coatings have been investigated for this purpose, including platelet, cancer cell, leukocyte, red blood cell, stem cell, and bacterial membranes (Li, He, Zhang, Qin, & Wang, 2018). Much of the research has shown promise; one study found that functionalization of silica NPs with leukocyte membranes allowed the NPs to evade phagocytic clearance (Parodi et al., 2013). Additionally, silica NPs surrounded by a lipid bilayer, deemed “protocells,” have been used to evade the immune system, allowing for enhanced delivery of mock payloads to human carcinoma cells. Experiments demonstrated that protocells have a 10,000-fold greater affinity for human hepatocellular carcinoma than for immune cells, resulting in enhanced chemotherapeutic drug delivery (Ashley et al., 2011). These studies demonstrate that decreasing and increasing the complexity of the BC may be valid approaches to enhance NP functionality.
Preformed BCs also have the capacity for targeting cells of the immune system, though the results of research to date have been mixed. For instance, a silica NP precoated with gamma globulins was created in an attempt to modify opsonization and uptake by RAW macrophages (Mirshafiee, Kim, Park, Mahmoudi, & Kraft, 2016). It was found that additional proteins bound the surface of the modified NP, impeding the opsonins and preventing them from interacting with the target receptors to initiate phagocytosis (Mirshafiee et al., 2016). These attempts to design NP-based therapeutics that take advantage of the BC have, overall, shown limited success. PEG may be a useful solution for biomedically active NPs that are delivered acutely, though benefits are lost when used repeatedly. The use of preformed engineered BCs is a newer approach to evade the immune system, and while experiments so far have yielded varying degrees of success, the fundamental concept shows promise. The future of NP therapeutic design should likely embrace the BC and engineer it to assist in clinical applications.
3.4 |. The impact of the BC on NP–macrophage interactions: Conclusions
Taken together, these studies demonstrate the complexity of the interaction of the NP–BC with immune cells. Macrophage internalization appears to be particularly variable in regards to the BC, though cytotoxicity appears to be more predictable, with the endogenous BC decreasing NP toxicity in most situations. Research has demonstrated the capacity of specific components of the BC to influence NP–immune cell interactions. It appears as though a BC rich in fibrinogen is typically protective, increasing cellular viability following particle exposure. Immunoglobulins appear to have the opposite effect, exacerbating the cellular reaction to particle exposure and enhancing their toxic effects. Other biomolecule components of the BC are also vital to NP–cellular interactions, such as LDL. Studies have demonstrated the importance of LDL in NP’s interactions with immunological cells, showing that the presence of LDL facilitates uptake by macrophages. The mechanism of such interactions has been partially elucidated, as well. Misfolding of proteins following association with the NP surface can allow for interactions to occur between specific epitopes and immune cell receptors, resulting in internalization. Based on these previous studies, attempts are now being undertaken to develop solutions to engineer the BC for evasion or engagement of the immune system. These biomedical applications utilizing the BC for therapeutic benefit appear promising and should continue to be expanded. Based upon our current knowledge, NPs which adsorb high amounts of fibrinogen and/or low amounts of immunoglobulins would be a logical starting point for designing NPs with BCs that evade immune clearance and may assist in therapeutic effectiveness.
4 |. PULMONARY AND GASTROINTESTINAL EPITHELIAL CELLS
Epithelial cell-NP interactions have been the subject of multiple nanotoxicology studies. This is appropriate, as NPs have been proposed for a variety of applications in medical settings, many of which include their delivery via inhalation or ingestion(W. Lee, Loo, Traini, & Young, 2015; Miragoli et al., 2018). Both inhalation and ingestion of NPs would lead to the formation of unique BCs, which researchers hypothesize could result in altered NP–cellular interactions. Despite these proposed uses, little is known regarding the BC that forms via these routes compared to intravenous delivery, or the impact of this BC on subsequent cellular reactions following inhalation and ingestion exposures.
4.1 |. BC-induced alterations in epithelial cell internalization of NPs
Epithelial cells provide the initial physical barrier to inhaled and ingested exogenous materials and are often the first to interact with NPs and other exposures. Interactions and internalization by epithelial cells are vital in determining the systemic distribution of nanotherapeutics delivered via the pulmonary system or GI tract. Experiments have been performed to examine how the presence or absence of a BC affects the response of A549 adenocarcinomic human alveolar basal epithelial cells to NP exposure. Carboxylated polystyrene and silica NPs lacking a BC were internalized more efficiently and had greater adhesion to the cellular surface than NPs with a serum BC (Lesniak et al., 2012, 2013). This suggests that NP-based therapeutics delivered to the pulmonary system may not be internalized to the same degree as observed in cell culture studies due to differential BC formation.
Inhaled NPs have the ability to penetrate the lung epithelium and translocate to cause systemic toxicity (Yang, Peters, & Williams, 2008). This translocation may possibly be facilitated by the presence of a BC, contributing to systemic responses such as vascular inflammation and the development of cardiovascular disease (Miller et al., 2017). One study examining titanium dioxide (TiO2) NPs demonstrated that the formation of a BC from the biomolecules of the endothelial cell membrane caused disruption and allowed NPs to cross through the barriers of LA4 cells, a mouse adenoma epithelial lung cell line. Specifically, experiments were performed that demonstrated the capacity of TiO2 particles to disrupt lipid membranes and form lipid wraps, as well as their destructive interactions with the membrane of live cultured epithelial lung cells (Urbančič et al., 2018). Tissue factor, a transmembrane protein abundant in lung epithelium, binds to the NP surface as the NP disrupts the membrane of the epithelial cell. The NP then translocates the protein through the damaged epithelial cell to the endothelial cell surrounding the capillary, where it can subsequently enter the bloodstream and activate the coagulation cascade. The researchers hypothesize that this is a contributing mechanism to the enhanced blood coagulation that contributes to development of cardiovascular disease following inhalation of nano-sized particulate matter. However, precoating of NPs delivered via inhalation with endothelial membrane components could be therapeutically utilized to deliver NPs systemically via inhalation.
The ability of the BC to reduce cellular internalization will likely decrease bioavailability, potentially creating an additional barrier for the use of NPs in biomedical applications and reducing the potential of NP therapeutic utilization though oral administration. Regarding the effects of NPs on the digestive system, specifically on colorectal cells, experimental results have been mixed. In some studies, the BC appears to increase uptake of magnetite NPs (Di Silvio, Rigby, Bajka, MacKie, & Baldelli Bombelli, 2016) while others demonstrate decreased internalization of silica and chitosan NPs (Varnamkhasti et al., 2015; Ye et al., 2017). These seemingly contradictory results could be due to a number of factors. The former study utilized simulated digestive fluids to form a BC, while the latter used serum. The variable results may also be attributed to differences in core NP material. Specifically, addition of the BC on magnetite NPs enhanced internalization, while the BC reduced uptake of aptamer-functionalized chitosan and silica NPs (Di Silvio et al., 2016; Varnamkhasti et al., 2015; Ye et al., 2017). However, while all of the discussed studies are robust in their design, the evaluation of magnetite NP uptake by colorectal cells is perhaps most pertinent to the cellular response to ingested NPs, as the BC was formed using simulated in vitro digestive fluids rather than serum, resulting in a BC more accurate to what would be observed in an in vivo exposure.
Although numerous studies have investigated BC-induced alterations in NP internalization by cells, few have examined the BC’s impact on NP translocation across epithelial barriers. An understanding of mechanisms by which the BC modifies epithelial cell uptake and translocation is necessary to control off-target effects and delivery of nano-enable therapeutics via the routes of pulmonary and gastrointestinal administration.
4.2 |. Impact of the BC on epithelial cell viability and responses
The BC has been demonstrated to alter how epithelial cells interact with NPs; for instance, differential toxicity-related effects have been examined in A549 alveolar epithelial cells following exposure to NPs with a serum BC (Hu et al., 2011; Lesniak et al., 2012; Panas et al., 2013). While this BC is not accurate to that which would form naturally following inhalation, and thus the study conclusions may not be viewed as definitive, the presence of a BC still confers an enhanced degree of biological relevance as compared to a NP with no BC. The A549 cell cytotoxicity of graphene oxide nanosheets, silica NPs, and poly-3-hydroxybutyrate-co-3-hydrozyhexanoate NPs is mitigated by the presence of a BC, demonstrating the protective effect of the BC (Hu et al., 2011; Lesniak et al., 2012; Panas et al., 2013; Peng et al., 2013). However, multiple studies utilizing relatively inert gold NPs have determined no alterations in cell viability due to addition of the BC (Cheng et al., 2015; Connor, Mwamuka, Gole, Murphy, & Wyatt, 2005). These studies support the assertion that the core material of the NP appears to have the greatest influence on toxicity, with the BC appearing to attenuate the effects of NPs which inherently cause toxicity. Additionally, these studies effectively demonstrate the protective effect of the BC, as epithelial cells exposed to bare NPs frequently experience adverse toxic outcomes.
While A549 cells are a relatively popular in vitro epithelial model to study lung effects, less attention is given to colorectal cells, such as HT29 and Caco-2, for evaluation of intestinal responses. HT29 colon cells were used to demonstrate that the presence of a FBS BC increased viability following exposure to chitosan NPs functionalized with aptamer (Varnamkhasti et al., 2015). Others have attempted to generate a more physiologically relevant BC though the use of simulated digestive fluids. One such study incubated iron-oxide NPs in a mixture of salts, enzymes, and other substances to form a BC prior to examining their effect on Caco-2 colorectal cells. The uncoated NP and NP with a simulated digestive BC-induced differential cellular morphological changes, with the pristine NP causing slight alterations to the actin network and the NP with a BC causing an increase in the number of vesicles in the apical membrane (di Silvio et al., 2016). This altered morphology did not appear to translate to differences in cytotoxicity, as cell viability was constant following exposure to bare NPs or NPs with a simulated digestive BC (Böhmert et al., 2014). These evaluations establish that the presence of a digestive BC alters NP-induced epithelial responses. Additional studies are needed to understand intestinal cell effects beyond morphology. Although the digestive system often contains consumed items, few studies have incorporated food into the study design evaluating the BC. The presence of food components was determined to alter the internalization and cytotoxic response of Caco-2 cells (Lichtenstein et al., 2015). Complex processes involved in the digestion (pH changes, presence of food, active absorption by the GI tract, etc.) likely modify the NP–BC and subsequent interactions with epithelial cells of the digestive system and thus require significant future study. Oral administration of NP therapeutics is a goal worth pursuing, though the digestive BC may interfere and require certain NPs to be delivered via alternate routes, such as injection and inhalation.
4.3 |. Impact of BC composition on NP–epithelial cell interactions
While overall the BC appears to reduce the toxic effects of the NP, the BC is not homogenous across conditions, and its effects can change depending on its composition. Specific components of the BC have the capacity to alter cellular interactions, as seen in a study utilizing silver NPs which were incubated in human albumin or HDL to form a BC prior to exposure to rat lung epithelial cells (J. H. Shannahan, Podila, Aldossari, et al., 2015). The researchers found that while all three BCs tested improved cellular viability as compared to a bare particle, a BC formed from HDL elicited a much greater release of IL-6 than a BC of albumin (J. H. Shannahan, Podila, Aldossari, et al., 2015). HDL is not the only BC component which has been examined as a potential influence on cellular behavior; a comprehensive evaluation of protein components of the BC and cellular interactions examined the contents of the BC formed on 105 types of silver and gold NPs following incubation in serum and their impact on A549 alveolar epithelial cell internalization (Walkey et al., 2014). The proteins present in the BC were isolated, identified, and quantified, and the amount of associated metal (internalized metal as well as membrane-associated) was measured. Statistical analysis was then used to identify specific proteins correlated directly or inversely with NP association. It was determined that certain proteins, including inter-alpha trypsin inhibitor heavy chains H1, H2, and H3, R-1 micro-globulin, and hyaluronan-binding protein 2, seemed to act as promoters of NP association, while others, such as complement component C3, seemed to inhibit (Walkey et al., 2014). Another analysis of this data set identified BC proteins APOB, A1AT, ANT3, and PLMN as significant to the degree of epithelial cell association of gold NPs (Liu, Jiang, Walkey, Chan, & Cohen, 2015). Together, these studies demonstrate BC profiles and specific individual components that may facilitate differential epithelial cell responses.
4.4 |. The influence of the BC on NP–epithelial cell interactions: Conclusions
Taken together, our assessment of the current scientific literature demonstrates that the BC is frequently protective for epithelial NP exposures, diminishing and delaying deleterious cellular responses. In the absence of a BC, NPs can cause damage to the epithelial cells via membrane disruption, ROS formation, and loss of viability. Formation of specific BCs on inhaled NPs may assist in the translocation of NP across the alveolar epithelia, contributing to the systemic responses. While research has been done regarding the effects of NP exposure on lung epithelial cells, there remain numerous avenues of future investigation required for pulmonary delivery of NP-enabled therapeutics. More research is needed regarding the impact of the BC on the NP’s ability to penetrate the epithelial cell membranes and cross through to the circulatory system. Also, many studies examining the effects of the BC on NP-epithelial cell exposure have utilized a serum BC, which may not be appropriate for all epithelial cell types. Specifically, future evaluations of the alveolar epithelium should utilize collected bronchoalveolar lavage fluid or lung surfactants to more appropriately mimic biological conditions within the respiratory system. Although it is a primary route of drug delivery, very few studies have examined the effect of the BC on the epithelial cell reactions induced following ingestion of NPs. In these studies, digestion processes and the presence of food components within the digestive system that could influence BC formation should be taken into account within the experimental design. Through a thorough understanding of how the BC influences NP interactions with epithelial cells, safe and effective therapies can be developed that allow for their delivery via inhalation and ingestion.
5 |. BACTERIAL/FUNGAL CELLS
Due to the rise in antibiotic resistance, NP antimicrobials have generated a great deal of interest among the medical and scientific communities. A number of nanomaterials have been investigated for their antibacterial/antifungal properties, including chitosan, copper, zinc, and silver (Kanhed et al., 2014; Qi, Xu, Jiang, Hu, & Zou, 2004; Sirelkhatim, Mahmud, & Seeni, 2015). Of these, silver has been the subject of the most scientific testing and commercial use (Kuek-Jun et al., 2014; W. Li, Xie, & Shi, 2010; Martinez-Castanon, Nino-Martinez, Martinez-Gutierrez, Martinez-Mendoza, & Ruiz, 2008; Monteiro et al., 2011; Panacek et al., 2006). Silver’s popularity is due to its high degree of antimicrobial effectiveness coupled with its reputation for having comparatively low toxicity to humans, though the extent of its toxic effects in higher organisms is still of concern (Clement & Jarrett, 1994; Lansdown, 2006). Silver NPs induce cytotoxicity primarily through dissolution and ion-mediated damage to the cell components including membranes, DNA, enzymes, and so forth (Kittler, Greulich, Diendorf, Kollwe, & Epple, 2010; W. Li et al., 2010; Monteiro et al., 2011; Morones et al., 2005). However, dissolution, and thus the antimicrobial properties of silver and other NPs, may be altered by the presence of a BC, influencing their use. Specifically, the composition of the BC changes how dissolution occurs; addition of human or bovine albumin reduces silver NP dissolution, while the addition of HDL enhances it (J. H. Shannahan, Podila, Aldossari, et al., 2015). It has been shown that the presence of a BC reduces both the antibacterial and antifungal activity of NPs. For bacteria, the presence of a BC prevents the NP from binding the bacterial membrane and subsequent internalization, thus reducing the antibiotic effect (Siemer et al., 2019). NPs function as antifungals by binding fungal spores and inhibiting fungal growth. The BC reduces antimycotic activity by inhibiting the association of fungal spores with the NP surface (Siemer et al., 2018). Though distinct, both mechanisms are similar in that they both act primarily through preventing the NP from binding and interacting with pathogens (Figure 3).
FIGURE 3.
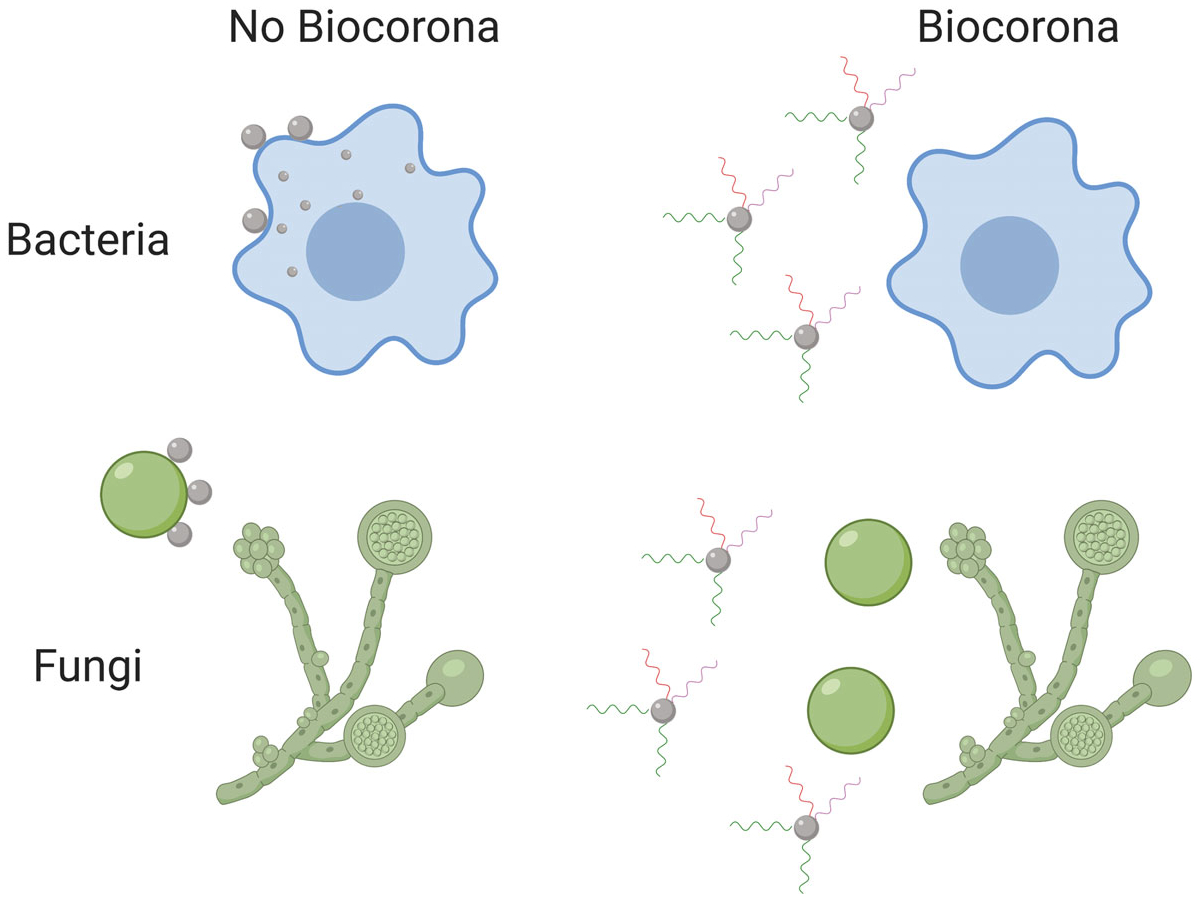
The BC can inhibit NP antimicrobial properties. The BC can reduce NP interactions with bacteria, reducing internalization and antibacterial activity. Antifungal activity is related to the NP’s ability to associate fungal spores. The BC can inhibit these interactions, decreasing NP antifungal properties. BC, biocorona; NP, nanoparticle
Using this information, researchers have attempted to design NP therapeutics which retain their antimicrobial effects within biomolecule-rich, physiologically relevant conditions via the use of capping agents. In a study of the antibacterial activity of silver NPs, it was determined that while a BC significantly inhibits the in vitro antibacterial activity of uncapped silver NPs, this reduction in activity can be attenuated by a PVP or citrate coating on the NP surface (Gnanadhas, Thomas, Thomas, Raichur, & Chakravortty, 2013). The researchers attribute this to the capping agents increasing the stability of the NPs and decreasing the interactions with the surrounding proteins, leading to decreased NP aggregation. By reducing aggregation, more NPs were available to be internalized by bacteria, resulting in greater antimicrobial effects. This is further supported by additional experiments demonstrating that BSA-NP interaction had an inverse relationship with antibiotic activity; the silver NP with the least amount of BSA interaction also had the greatest antibacterial effects (Gnanadhas et al., 2013). Attempts have also been made to create a synthetic BC to enhance antimicrobial effects while minimizing off-target toxicity. Researchers have used an artificial corona of polyoxometalate (POM) in conjunction with silver, gold, and TiO2 NPs to increase bactericidal activity (Daima, Selvakannan, Shukla, Bhargava, & Bansal, 2013; Daima et al., 2016). The addition of POM coatings to the NP surface was found to enhance the NP’s antibacterial effects against both gram-negative and gram-positive bacteria (Daima et al., 2014). Additional study is needed to translate these primarily in vitro studies into in vivo environments.
While NP therapeutics have the potential to act as potent antibacterial and antifungal agents, the BC represents a barrier to in vivo efficacy and clinical utilization. The presence of a BC on the NP surface prevents the NPs from acting on the bacteria or fungi in question, reducing the effectiveness of the NP. While several initial approaches have been identified for the design and utilization of NP antimicrobials, further research is needed to refine and perfect these strategies such that any products which are used clinically or commercially are as safe and effective as possible.
6 |. OVERALL CONCLUSIONS
The impact of the BC on NP–cellular interactions is clearly significant in terms of the biological response, impacting nanosafety research and the translation of findings to clinical applications. Failure to account for the BC in early assessments of biomedically utilized NPs may result in inconsistent experimental results between laboratories while also contributing to unexpected biological responses when NPs are evaluated clinically, thus reducing their therapeutic potential. Specifically, the addition of a BC may cause altered NP functionality, biodistribution, and toxicity, resulting in inconsistent outcomes outside of tightly controlled in vitro experimental conditions. Current research of the BC and its effects on cellular interactions is far from comprehensive, and large gaps still exist in our knowledge. Studies to date have almost exclusively focused on the proteins present within the BC; however, other biomolecules such as lipids are present which may contribute to cellular interactions and responses. Specifically, copolymer and magnetite NPs have been shown to associate multiple classes of lipids, the quantity of which varied based on HDL content of the surrounding environment and NP hydrophobicity, respectively (Hellstrand et al., 2009; Raesch et al., 2015). These studies indicate that lipids are also present within the BC and that their contribution to NP–cellular interactions requires significant future evaluation.
Examination of the NP–BC has primarily concentrated on variations in NP physicochemical properties that influence association of the BC in healthy physiological conditions. However, due to their proposed utilization in therapeutic diseases, NPs will be introduced into disease environments. The progression of many diseases, including obesity, diabetes, cancer, and others, results in alterations of biomolecule content, which may influence BC formation and subsequent cellular interactions and responses. The limited previous research on this topic has demonstrated that the content of the BC is modified in disease states compared to healthy, and that these variations result in differential cellular responses (J. H. Shannahan et al., 2016). Due to the increasing prevalence of chronic diseases, expanding research regarding disease-induced variations in NP–BC formation and cellular responses will enhance our ability to translate in vitro-based laboratory examinations to clinical applications. In addition to disease, other factors may modify the biological milieu and consequently the formation of the BC, including gender, diet, age, activity level, and others (Gordon, Castelli, Hjortland, Kannel, & Dawber, 1977; Jenkins et al., 1993; Murphy, 2014; Tran, Weltman, Glass, & Mood, 1983). Recently, exercise was determined to alter formation of the iron-oxide NP–BC and influence macrophage interactions and responses (Kobos et al., 2018). This suggests that lifestyle modifications by an individual may introduce variability in the association of biomolecules with the NP surface that impact cellular interactions and responses. From a clinical perspective, this implies that the functionality and biological response to biomedically utilized NPs may change over time due to common alterations in an individual’s behavior. The existence of intraindividual variations in the formation of the BC that may occur due to lifestyle changes is an area for future evaluation that may assist in the development of personalized medical treatments.
Overall, the current research demonstrates that NP–cellular interactions are variable between cell types and materials. Specific components of the BC may be linked to specific effects, with fibrinogen in particular serving as protective and immunoglobulins exacerbating NP toxicity. This may indicate that the inclusion of a preformed BC consisting of specific biomolecules represents a solution to many of the problems facing the utilization of NP therapeutics in biomedical applications. Broadly, additional research is needed examining BCs formed following inhalation and ingestion, as fewer studies exist evaluating these routes of exposure. The impact of the BC has implications, both for laboratory studies and real-world applications, that are necessary to understand and utilize nanotechnologies in biomedical settings. The composition of the BC is a factor that investigators must consider when extrapolating their experimental results to human health effects. The complexity of the BC dictates that results from nanosafety studies of therapeutics cannot be generalized to all cell types, NPs, or individuals. Ultimately, the biomedical utilization of NPs is dependent upon both understanding and control of the NP–BC in order to regulate cellular interactions and biological responses.
ACKNOWLEDGMENT
All figures were created with Biorender.com.
Funding information
National Institute of Environmental Health Sciences, Grant/Award Number: R00/ES024392
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
RELATED WIREs ARTICLES
FURTHER READING
- Bonder MJ, & Wang X (2009). Metallic iron nanoparticles for MRI contrast enhancement and local hyperthermia. Small, 4(11), 1925–1929. 10.1002/smll.200800261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Wang G, Griffin JI, Brenneman B, Banda NK, Holers VM, … Simberg D (2017). Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nature Nanotechnology, 12(4), 387–393. 10.1038/nnano.2016.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, & Zhang J (2006). Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. Journal of Nanoscience and Nanotechnology, 6(4), 1159–1166. 10.1166/jnn.2006.327 [DOI] [PubMed] [Google Scholar]
- Choi K, Riviere JE, & Monteiro-Riviere NA (2017). Protein corona modulation of hepatocyte uptake and molecular mechanisms of gold nanoparticle toxicity. Nanotoxicology, 11(1), 64–75 10.1080/17435390.2016.1264638. [DOI] [PubMed] [Google Scholar]
- Coyne DW, & Auerbach M (2010). Anemia management in chronic kidney disease: Intravenous iron steps forward. American Journal of Hematology, 85(5), 311–312. 10.1002/ajh.21682 [DOI] [PubMed] [Google Scholar]
- Deng ZJ, Liang M, Monteiro M, Toth I, & Minchin RF (2011). Nanoparticle-induced unfolding of fibrinogen promotes mac-1 receptor activation and inflammation. Nature Nanotechnology, 6(1), 39–44. 10.1038/nnano.2010.250 [DOI] [PubMed] [Google Scholar]
- Fleischer CC, & Payne CK (2013). Nanoparticle surface charge mediates the cellular receptors used by protein-nanoparticle complexes. Nano,116(30), 8901–8907. 10.1021/jp304630q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, & Gupta M (2017). Synthesis and modification of iron oxide nanoparticles (magnetite) for biomedical applications. Research Journal of Biotechnology, 12(9), 87–95. 10.1016/j.biomaterials.2004.10.012 [DOI] [Google Scholar]
- Hu CMJ, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, … Zhang L (2015). Nanoparticle biointerfacing by platelet membrane cloaking. Nature, 526(7571), 118–121. 10.1038/nature15373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram DT, Runa S, Kemp ML, & Payne CK (2017). Nanoparticle-induced oxidation of corona proteins initiates an oxidative stress response in cells. Nanoscale, 9(22), 7595–7601. 10.1039/c6nr09500c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunov O, Syrovets T, Loos C, Beil J, Delacher M, Tron K, … Simmet T (2011). Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano, 5(3), 1657–1669. 10.1021/nn2000756 [DOI] [PubMed] [Google Scholar]
- Maiorano G, Sabella S, Sorce B, Brunetti V, Malvindi MA, Cingolani R, & Pompa PP (2010). Effects of cell culture media on the protein—Nanoparticle complexes and influence on the cellular response. ACS Nano, 4(12), 7481–7491. [DOI] [PubMed] [Google Scholar]
- Patel PC, Giljohann DA, Daniel WL, Zheng D, Prigodich AE, & Mirkin CA (2010). Scavenger receptors mediate cellular uptake of polyvalent oligonucleotide-functionalized gold nanoparticles. Bioconjugate Chemistry, 21(12), 2250–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salata OV (2004). Applications of nanoparticles in biology and medicine. Journal of Nanobiotechnology, 2(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayes CM, Reed KL, & Warheit DB (2007). Assessing toxicity of fine and nanoparticles: Comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicological Sciences, 97(1), 163–180. 10.1093/toxsci/kfm018 [DOI] [PubMed] [Google Scholar]
- Sobczynski DJ, Charoenphol P, Heslinga MJ, Onyskiw PJ, Namdee K, Thompson AJ, & Eniola-adefeso O (2014). Plasma protein corona modulates the vascular wall interaction of drug carriers in a material and donor specific manner. PLoS One, 9(9), 1–12. 10.1371/journal.pone.0107408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczynski DJ, & Enioloa-Adefeso O (2017). IgA and IgM protein primarily drive plasma corona-induced adhesion reduction of PLGA nanoparticles in human blood flow. Bioengineering & Translational Medicine, 2(2), 180–190. 10.1002/btm2.10064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkey CD, Olsen JB, Guo H, Emili A, & Chan WCW (2012). Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. Journal of the American Chemical Society, 134(4), 2139–2147. 10.1021/ja2084338 [DOI] [PubMed] [Google Scholar]
- Yin H, Chen R, Casey PS, Ke C, & Davis P (2015). Reducing the cytotoxicity of ZnO nanoparticles by a pre-formed protein corona in a supplemented cell culture medium. RSC Advances, 5(90), 73963–73973. 10.1039/c5ra14870g [DOI] [Google Scholar]
REFERENCES
- Alberts B, Johnson A, & Lewis J (2002). Molecular biology of the cell (4th ed.). New York, NY: Garland Science. [Google Scholar]
- Alex SA, Chandrasekaran N, & Mukherjee A (2017). Impact of gold nanorod functionalization on biocorona formation and their biological implication. Journal of Molecular Liquids, 248, 703–712. 10.1016/j.molliq.2017.10.119 [DOI] [Google Scholar]
- Adamson SXF, Lin Z, Chen R, Kobos L, & Shannahan J (2018). Experimental challenges regarding the in vitro investigation of the nanoparticle-biocorona in disease states. Toxicology in Vitro, 51, 40–49 10.1016/j.tiv.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri H, Bordonali L, Lascialfari A, Wan S, Monopoli MP, Lynch I, … Mahmoudi M (2013). Protein corona affects the relaxivity and MRI contrast efficiency of magnetic nanoparticles. Nanoscale, 5(18), 8656–8665 10.1039/c3nr00345k. [DOI] [PubMed] [Google Scholar]
- Ashley CE, Carnes EC, Phillips GK, Padilla D, Durfee PN, Brown PA, … Brinker CJ (2011). The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nature Materials, 10(5), 389 10.1038/NMAT2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai W, Wu Z, Mitra S, & Brown JM (2016). Effects of multiwalled carbon nanotube surface modification and purification on bovine serum albumin binding and biological responses. Journal of Nanomaterials, 4. 10.1186/s40945-017-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard M, Noel JP, Appel M, Angelo J, & Couvreur P (1999). Stealth® PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. Journal of Controlled Release, 60(1), 121–128. [DOI] [PubMed] [Google Scholar]
- Bisht S, & Maitra A (2009). Dextran-doxorubicin/chitosan nanoparticles for solid tumor therapy. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 1(4), 415–425. 10.1002/wnan.043 [DOI] [PubMed] [Google Scholar]
- Böhmert L, Girod M, Hansen U, Maul R, Knappe P, Niemann B, … Lampen A (2014). Analytically monitored digestion of silver nanoparticles and their toxicity on human intestinal cells. Nanotoxicology, 8(6), 631–642. 10.3109/17435390.2013.815284 [DOI] [PubMed] [Google Scholar]
- Bravo J, & Schnitzer JE (1993). High affinity binding, endocytosis, and degradation of conformationally modified albumins. Potential role of gp30 and gp18 as novel scavenger receptors. Journal of Biological Chemistry, 268(10), 7562–7570. [PubMed] [Google Scholar]
- Chandran P, Riviere JE, & Monteiro-Riviere NA (2017). Surface chemistry of gold nanoparticles determines the biocorona composition impacting cellular uptake, toxicity and gene expression profiles in human endothelial cells. Nanotoxicology, 11(4), 507–519. 10.1080/17435390.2017.1314036 [DOI] [PubMed] [Google Scholar]
- Cheng X, Tian X, Wu A, Li J, Tian J, Chong Y, … Ge C (2015). Protein corona influences cellular uptake of gold nanoparticles by phagocytic and nonphagocytic cells in a size-dependent manner. ACS Applied Materials & Interfaces, 7(37), 20568–20575. 10.1021/acsami.5b04290 [DOI] [PubMed] [Google Scholar]
- Chakraborty D, Chauhan P, Ann S, Chaudhary S, Ethiraj KR, Chandrasekaran N, & Mukherjee A (2018). Comprehensive study on biocorona formation on functionalized selenium nanoparticle and its biological implications. Journal of Molecular Liquids, 268, 335–342. 10.1016/j.molliq.2018.07.070 [DOI] [Google Scholar]
- Connor EE, Mwamuka J, Gole A, Murphy CJ, & Wyatt MD (2005). Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small, 1(3), 325–327 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- Corbo C, & Toledano NE (2016). The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine, 11(1), 81–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement JL, & Jarrett PS (1994). Antibacterial silver. Metal Based Drugs, 1(13), 467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daima HK, Selvakannan PR, Kandjani AE, Shukla R, Bhargava SK, & Bansal V (2014). Synergistic influence of polyoxometalate surface corona towards enhancing the antibacterial performance of tyrosine-capped Ag nanoparticles. Nanoscale, 6(2), 758–765. 10.1039/c3nr03806h [DOI] [PubMed] [Google Scholar]
- Daima HK, Selvakannan PR, Shukla R, Bhargava SK, & Bansal V (2013). Fine-tuning the antimicrobial profile of biocompatible gold nanoparticles by sequential surface functionalization using polyoxometalates and lysine. PLoS ONE, 8(10), 1–14. 10.1371/journal.pone.0079676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daima HK, Vinay Kumar P, Rashmi S, Pandey SR, Pn N, Mlowe S, & Revaprasadu N (2016). Formulation, characterization and applications of titanium dioxide, polyoxometalates and silver nanocomposites. Advanced Manufacturing, Electronics and Microsystems: TechConnect Briefs, 1, 37–40. [Google Scholar]
- di Silvio D, Rigby N, Bajka B, MacKie A, & Baldelli Bombelli F (2016). Effect of protein corona magnetite nanoparticles derived from bread in vitro digestion on Caco-2 cells morphology and uptake. International Journal of Biochemistry and Cell Biology, 75, 212–222. 10.1016/j.biocel.2015.10.019 [DOI] [PubMed] [Google Scholar]
- Ding F, Radic S, Chen R, Chen P, Geitner NK, Brown JM, & Ke PC (2013). Direct observation of a single nanoparticle-ubiquitin corona formation. Nanoscale, 5(19), 9162–9169. 10.1039/c3nr02147e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docter D, Westmeier D, Markiewicz M, Stolte S, Knauer SK, & Stauber RH (2015). The nanoparticle biomolecule corona: lessons learned—Challenge accepted? Chemical Society Reviews, 44, 6094–6121. 10.1039/c5cs00217f [DOI] [PubMed] [Google Scholar]
- Ehrenberg MS, Friedman AE, Finkelstein JN, & Mcgrath JL (2009). The influence of protein adsorption on nanoparticle association with cultured endothelial cells. Biomaterials, 30(4), 603–610. 10.1016/j.biomaterials.2008.09.050 [DOI] [PubMed] [Google Scholar]
- Ge C, Du J, Zhao L, Wang L, Liu Y, Li D, … Zhou R (2011). Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America, 108(41), 16968–16973. 10.1073/pnas.1105270108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanadhas DP, Thomas MB, Thomas R, Raichur AM, & Chakravortty D (2013). Interaction of silver nanoparticles with serum proteins affects their antimicrobial activity in vivo. Antimicrobial Agents and Chemotherapy, 57(10), 4945–4955. 10.1128/AAC.00152-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, & Dawber TR (1977). Diabetes, blood lipids, and the role of obesity in coronary heart disease risk for women. The Framingham study. Annals of Internal Medicine, 87(4), 393–397. 10.7326/0003-4819-87-4-393 [DOI] [PubMed] [Google Scholar]
- Haeri M, & Knox BE (2012). Endoplasmic reticulum stress and unfolded protein response pathways: Potential for treating age-related retinal degeneration. Journal of Ophthalmic & Vision Research, 7(1), 45–59. [PMC free article] [PubMed] [Google Scholar]
- Hellstrand E, Lynch I, Andersson A, Drakenberg T, Dahlbäck B, Dawson KA, … Cedervall T (2009). Complete high-density lipoproteins in nanoparticle corona. FEBS Journal, 276(12), 3372–3381. 10.1111/j.1742-4658.2009.07062 [DOI] [PubMed] [Google Scholar]
- Hu W, Peng C, Lv M, Li X, Zhang Y, Chen N, … Huang Q (2011). Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano, 5(5), 3693–3700. 10.1021/nn200021j [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Venketeshwer Rao A, Hegele RA, Mitchell SJ, Ransom TP, … Wursch P (1993). Effect on blood lipid of very high intakes of fiber in diets low in saturated fat and cholesterol. The New England Journal of Medicine, 329(1), 21–26. [DOI] [PubMed] [Google Scholar]
- Kanhed P, Birla S, Gaikwad S, Gade A, Seabra AB, Rubilar O, … Rai M (2014). In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Materials Letters, 115, 13–17. 10.1016/j.matlet.2013.10.011 [DOI] [Google Scholar]
- Kelly PM, Åberg C, Polo E, Connell AO, Cookman J, Fallon J, … Dawson KA (2015). Mapping protein binding sites on the biomolecular corona of nanoparticles. Nature Nanotechnology, 10(5), 472–479. 10.1038/nnano.2015.47 [DOI] [PubMed] [Google Scholar]
- Kobos LM, Adamson SXF, Evans S, Gavin TP, & Shannahan JH (2018). Altered formation of the iron oxide nanoparticle-biocorona due to individual variability and exercise. Environmental Toxicology and Pharmacology, 62, 215–226. 10.1016/j.etap.2018.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler S, Greulich C, Diendorf J, Kollwe M, & Epple M (2010). Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chemistry of Materials, 22(16), 4548–4554. 10.1021/cm100023p [DOI] [Google Scholar]
- Kuek-Jun K, Sung WS, Moon S-K, Choi J-S, Kim JG, & Lee DG (2014). Antifungal effect of silver nanoparticles on dermatophytes. Journal of Microbiology and Biotechnology, 18(8), 1482–1484. [PubMed] [Google Scholar]
- Landry MP, Vukovic L, Kruss S, Bisker G, Landry AM, Islam S, … Strano MS (2015). Comparative dynamics and sequence dependence of DNA and RNA binding to single walled carbon nanotubes. The Journal of Physical Chemistry C, 119, 10048–10058. 10.1021/jp511448e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdown A (2006). Silver in health care: Antimicrobial effects and safety in use. Biofunctional Textiles and the Skin, 33, 17–34. [DOI] [PubMed] [Google Scholar]
- Lee B, & Nguyen VH (2017). Protein corona: A new approach for nanomedicine design. International Journal of Nanomedicine, 12,3137–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Loo C, Traini D, & Young PM (2015). Inhalation of nanoparticle-based drug for lung cancer treatment: Advantages and challenges. Asian Journal of Pharmaceutical Sciences, 10(6), 481–489. 10.1016/j.ajps.2015.08.009 [DOI] [Google Scholar]
- Lesniak A, Fenaroli F, Monopoli MP, Åberg C, Dawson KA, & Salvati A (2012). Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano, 6(7), 5845–5857. 10.1021/nn300223w [DOI] [PubMed] [Google Scholar]
- Lesniak A, Salvati A, Santos-Martinez MJ, Radomski MW, Dawson KA, & Aberg C (2013). Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. Journal of the American Chemical Society, 135(4), 1438–1444. 10.1021/ja309812z [DOI] [PubMed] [Google Scholar]
- Li R, Chen R, Chen P, Wen Y, Ke PC, & Cho SS (2013). Computational and experimental characterizations of silver nanoparticle—Apolipoprotein biocorona. The Journal of Physical Chemistry B, 117, 13451–13456. 10.1021/jp4061158 [DOI] [PubMed] [Google Scholar]
- Li R, He Y, Zhang S, Qin J, & Wang J (2018). Cell membrane-based nanoparticles: A new biomimetic platform for tumor diagnosis and treatment. Acta Pharmaceutica Sinica B, 8(1), 14–22. 10.1016/j.apsb.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Xie X, & Shi Q (2010). Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Applied Microbial and CellPhysiology, 85, 1115–1122. 10.1007/s00253-009-2159-5 [DOI] [PubMed] [Google Scholar]
- Lichtenstein D, Ebmeyer J, Knappe P, Juling S, Bohmert L, Selve S, … Lampen A (2015). Impact of food components during in vitro digestion of silver nanoparticles on cellular uptake and cytotoxicity in intestinal cells. Biological Chemistry, 396(11), 1255–1264. [DOI] [PubMed] [Google Scholar]
- Lin S, Mortimer M, Chen R, Kakinen A, Riviere JE, Thomas P, … States U (2018). NanoEHS beyond toxicity—Focusing on biocorona. Environmental Science: Nano, 7(4), 1433–1454. 10.1039/C6EN00579A.NanoEHS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Jiang W, Walkey CD, Chan WCW, & Cohen Y (2015). Prediction of nanoparticles-cell association based on corona proteins and physicochemical properties. Nanoscale, 7(21), 9664–9675. 10.1039/c5nr01537e [DOI] [PubMed] [Google Scholar]
- Lu N, Sui Y, Tian R, & Peng YY (2018). Adsorption of plasma proteins on single-walled carbon nanotubes reduced cytotoxicity and modulated neutrophil activation. Chemical Research in Toxicology, 31(10), 1061–1068. 10.1021/acs.chemrestox.8b00141 [DOI] [PubMed] [Google Scholar]
- Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, & Dawson KA (2008). Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proceedings of the National Academy of Sciences of the United States of America, 105(38), 14265–14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch I, & Dawson KA (2008). Protein-nanoparticle interactions. Nanotoday, 3(1), 40–47. [Google Scholar]
- Mahmoudi M, Sheibani S, Milani AS, Gauberti M, Dinarvand R, & Vali H (2015). Crucial role of the protein corona for the specific targeting of nanoparticles. Nanomedicine, 10(2), 215–226. [DOI] [PubMed] [Google Scholar]
- Martinez-Castanon GA, Nino-Martinez N, Martinez-Gutierrez F, Martinez-Mendoza JR, & Ruiz F (2008). Synthesis and antibacterial activity of silver nanoparticles with different sizes. Journal of Nanoparticle Research, 10, 1343–1348. 10.1007/s11051-008-9428-6 [DOI] [Google Scholar]
- Miller MR, Raftis JB, Langrish JP, McLean SG, Samutrtai P, Connell SP, … Mills NL (2017). Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano, 11(5), 4542–4552. 10.1021/acsnano.6b08551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C (2012). M1 and M2 macrophages: Oracles of health and disease. Critical Reviews in Immunology, 32(6), 463–488. 10.1615/CritRevImmunol.v32.i6.10 [DOI] [PubMed] [Google Scholar]
- Miragoli M, Ceriotti P, Iafisco M, Vacchiano M, Salvarani N, Alogna A, … Catalucci D (2018). Inhalation of peptide-loaded nanoparticles improves heart failure. Science Translational Medicine, 10(424), 1–12. [DOI] [PubMed] [Google Scholar]
- Mirshafiee V, Kim R, Park S, Mahmoudi M, & Kraft ML (2016). Impact of protein pre-coating on the protein corona composition and nanoparticle cellular uptake. Biomaterials, 75, 295–304. 10.1016/j.biomaterials.2015.10.019 [DOI] [PubMed] [Google Scholar]
- Monopoli MP, Åberg C, Salvati A, & Dawson KA (2012). Biomolecular coronas provide the biological identity of nanosized materials. Nature Nanotechnology, 7(12), 779–786. 10.1038/nnano.2012.207 [DOI] [PubMed] [Google Scholar]
- Monteiro DR, Gorup LF, Silva S, Negri M, De Camargo ER, Oliveira R, … De Camargo ER. (2011). Silver colloidal nanoparticles: Antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrata. Biofouling, 27(7), 711–719. 10.1080/08927014.2011.599101 [DOI] [PubMed] [Google Scholar]
- Moore KJ, & Freeman MW (2006). Scavenger receptors in atherosclerosis: Beyond lipid uptake. Arteriosclerosis, Thrombosis, and Vascular Biology, 26(8), 1702–1711. 10.1161/01.ATV.0000229218.97976.43 [DOI] [PubMed] [Google Scholar]
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ram JT, & Yacaman MJ (2005). The bactericidal effect of silver nanoparticles. Nanotechnology, 16, 2346–2353. 10.1088/0957-4484/16/10/059 [DOI] [PubMed] [Google Scholar]
- Müller J, Prozeller D, Ghazaryan A, Kokkinopoulou M, Mailänder V, Morsbach S, & Landfester K (2018). Beyond the protein corona—Lipids matter for biological response of nanocarriers. Acta Biomaterialia, 71, 420–431. 10.1016/j.actbio.2018.02.036 [DOI] [PubMed] [Google Scholar]
- Murphy WG (2014). The sex difference in haemoglobin levels in adults—Mechanisms, causes, and consequences. Blood Reviews, 28(2), 41–47. 10.1016/j.blre.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Neagu M, Piperigkou Z, Karamanou K, & Engin AB (2017). Protein bio-corona: Critical issue in immune nanotoxicology. Archives of Toxicology, 91(3), 1031–1048. 10.1007/s00204-016-1797-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y, & Medzhitov R (2016). Tissue biology perspective on macrophages. Nature Immunology, 17(1), 9–17. 10.1038/ni.3320 [DOI] [PubMed] [Google Scholar]
- Orco DD, Lundqvist M, Oslakovic C, Cedervall T, & Linse S (2010). Modeling the time evolution of the nanoparticle-protein corona in a body fluid. PLoS ONE, 5(6), 1–8. 10.1371/journal.pone.0010949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panacek A, Kvitek L, Prucek R, Kolar M, Vecerova R, Pizurova N, … Zboril R (2006). Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. The Journal of Physical Chemistry B, 110, 16248–16253. 10.1021/jp063826h [DOI] [PubMed] [Google Scholar]
- Panas A, Marquardt C, Nalcaci O, Bockhorn H, Baumann W, Paur HR, … Weiss C (2013). Screening of different metal oxide nanoparticles reveals selective toxicity and inflammatory potential of silica nanoparticles in lung epithelial cells and macrophages. Nanotoxicology, 7(3), 259–273. 10.3109/17435390.2011.652206 [DOI] [PubMed] [Google Scholar]
- Papi M, Caputo D, Palmieri V, Coppola R, Palchetti S, Bugli F, … Caracciolo G (2017). Clinically approved PEGylated nanoparticles are covered by a protein corona that boosts the uptake by cancer cells. Nanoscale, 9(29), 10327–10334. 10.1039/c7nr03042h [DOI] [PubMed] [Google Scholar]
- Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, … Tasciotti E (2013). Biomimetic functionalization with leukocyte membranes imparts cell like functions to synthetic particles. Nature Nanotechnology, 8(1), 61–68. 10.1038/nnano.2012.212.Biomimetic [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q, Zhang S, Yang Q, Zhang T, Wei XQ, Jiang L, … Lin YF (2013). Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials, 34(33), 8521–8530. 10.1016/j.biomaterials.2013.07.102 [DOI] [PubMed] [Google Scholar]
- Persaud I, Shannahan JH, Raghavendra AJ, Alsaleh NB, Podila R, & Brown JM (2019). Biocorona formation contributes to silver nanoparticle induced endoplasmic reticulum stress. Ecotoxicology and Environmental Safety, 170, 77–86. 10.1016/J.ECOENV.2018.11.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podila R, Vedantam P, Ke PC, Brown JM, & Rao AM (2012). Evidence for charge-transfer-induced conformational changes in carbon nanostructure-protein corona. Journal of Physical Chemistry C, 116(41), 22098–22103. 10.1021/jp3085028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prapainop K, Witter DP, & Wentworth P (2012). A chemical approach for cell-specific targeting of nanomaterials: Small-molecule-initiated misfolding of nanoparticle corona proteins. Journal of the American Chemical Society, 134(9), 4100–4103. 10.1021/ja300537u [DOI] [PubMed] [Google Scholar]
- Qi L, Xu Z, Jiang X, Hu C, & Zou X (2004). Preparation and antibacterial activity of chitosan nanoparticles. Carbohydrate Research, 339,2693–2700. 10.1016/j.carres.2004.09.007 [DOI] [PubMed] [Google Scholar]
- Raesch SS, Tenzer S, Storck W, Rurainski A, Selzer D, Ruge CA, … Lehr CM (2015). Proteomic and lipidomic analysis of nanoparticle corona upon contact with lung surfactant reveals differences in protein, but not lipid composition. ACS Nano, 9(12), 11872–11885. 10.1021/acsnano.5b04215 [DOI] [PubMed] [Google Scholar]
- Raghavendra AJ, Fritz K, Fu S, Brown JM, & Shannahan JH (2017). Variations in biocorona formation related to defects in the structure of single walled carbon nanotubes and the hyperlipidemic disease state. Scientific Reports, 7(1), 1–11. 10.1038/s41598-017-08896-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RV, & Bredesen DE (2014). Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Current Opinion in Cell Biology,16(6), 653–662. 10.1016/j.ceb.2004.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PL, Harada T, Christian DA, Pantano DA, Richard K, & Discher DE (2014). Minimal “self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science, 339, 971–975. 10.1126/science.1229568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runa S, Hill A, Cochran VL, Payne CK, Runa S, Hill A, … Payne CK (2014). PEGylated nanoparticles: Protein corona and secondary structure. Physical Chemistry of Interfaces and Nanomaterials, XIII, 9165. 10.1117/12.2062767 [DOI] [Google Scholar]
- Sahneh FD, Scoglio C, & Riviere J (2013). Dynamics of nanoparticle-protein corona complex formation: Analytical results from population balance equations. PLoS ONE, 8(5), 1–10. 10.1371/journal.pone.0064690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahneh FD, Scoglio CM, Monteiro-Riviere NA, & Riviere JE (2015). Predicting the impact of biocorona formation kinetics on interspecies extrapolations of nanoparticle bio distribution modeling. Nanomedicine, 10(1), 25–33. [DOI] [PubMed] [Google Scholar]
- Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, … Dawson KA (2013). Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nature Nanotechnology, 8(2), 137–143. 10.1038/nnano.2012.237 [DOI] [PubMed] [Google Scholar]
- Sasidharan A, Riviere JE, & Monteiro-Riviere NA (2015). Gold and silver nanoparticle interactions with human proteins: Impact and implications in biocorona formation. Journal of Materials Chemistry B, 3(10), 2075–2082. 10.1039/c4tb01926a [DOI] [PubMed] [Google Scholar]
- Schutz CA, Juillerat-Jeanneret L, Mueller H, Lynch I, & Riediker M (2013). Therapeutic nanoparticles in clinics and under clinical evaluation. Nanomedicine, 8(3), 449–467. [DOI] [PubMed] [Google Scholar]
- Shannahan J (2017). The biocorona: A challenge for the biomedical application of nanoparticles. Nanotechnology Reviews, 6(4), 345–353. 10.1515/ntrev-2016-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan JH, Fritz KS, Raghavendra AJ, Podila R, Persaud I, & Brown JM (2016). Disease-induced disparities in formation of the nanoparticle-biocorona and the toxicological consequences. Toxicological Sciences, 152(2), 406–416. 10.1093/toxsci/kfw097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan JH, Podila R, Aldossari AA, Emerson H, Powell BA, Ke PC, … Brown JM (2015). Formation of a protein corona on silver nanoparticles mediates cellular toxicity via scavenger receptors. Toxicological Sciences, 143(1), 136–146. 10.1093/toxsci/kfu217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan JH, Podila R, & Brown JM (2015). A hyperspectral and toxicological analysis of protein corona impact on silver nanoparticle properties, intracellular modifications, and macrophage activation. International Journal of Nanomedicine, 10, 6509–6520. 10.2147/IJN.S92570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemer S, Westmeier D, Barz M, Eckrich J, Wünsch D, Seckert C, … Strieth S (2019). Biomolecule-corona formation confers resistance of bacteria to nanoparticle-induced killing: Implications for the design of improved nanoantibiotics. Biomaterials, 192, 551–559. 10.1016/j.biomaterials.2018.11.028 [DOI] [PubMed] [Google Scholar]
- Siemer S, Westmeier D, Vallet C, Becker S, Voskuhl J, Ding G, … Knauer SK (2018). Resistance to nano-based antifungals is mediated by biomolecule coronas. ACS Applied Materials & Interfaces, 11, 104–114. 10.1021/acsami.8b12175 [DOI] [PubMed] [Google Scholar]
- Sirelkhatim A, Mahmud S, & Seeni A (2015). Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-MicroLetters, 7(3), 219–242. 10.1007/s40820-015-0040-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavanti F, Pedone A, & Menziani MC (2019). Multiscale molecular dynamics simulation of multiple protein adsorption on gold nanoparticles. International Journal of Molecular Sciences, 20(14), 3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenzer S, Docter D, Rosfa S, Wlodarski A, Rekik A, Knauer SK, … Stauber RH (2011). Nanoparticle size is a critical physico-chemical determinant of the human blood plasma corona: A comprehensive quantitative proteomic analysis. ACS Nano, 5(9), 7155–7167. 10.1021/nn201950e [DOI] [PubMed] [Google Scholar]
- Tenzer S, Musyanovych A, Fetz V, Docter D, Hecht R, Schlenk F, … Schild H (2013). Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nature Nanotechnology, 8(10), 772. 10.1038/nnano.2013.181 [DOI] [PubMed] [Google Scholar]
- Tiede K, Hassellöv M, Breitbarth E, Chaudhry Q, & Boxall ABA (2009). Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles. Journal of Chromatography A, 1216, 503–509. 10.1016/j.chroma.2008.09.008 [DOI] [PubMed] [Google Scholar]
- Tran ZV, Weltman A, Glass GV, & Mood DP (1983). The effects of exercise on blood lipids and lipoproteins: A meta-analysis of studies. Medicine & Science in Sports & Exercise, 15(5), 393–402. http://europepmc.org/abstract/MED/6645868 [PubMed] [Google Scholar]
- Treuel L, Brandholt S, Maffre P, Wiegele S, Shang L, & Nienhaus U (2014). Impact of protein modification on the protein corona on nanoparticles and nanoparticle-cell interactions. ACS Nano, 8(1), 503–513. 10.1021/nn405019v [DOI] [PubMed] [Google Scholar]
- Urbančič I, Garvas M, Kokot B, Majaron H, Umek P, Cassidy H, … Štrancar J (2018). Nanoparticles can wrap epithelial cell membranes and relocate them across the epithelial cell layer. Nano Letters, 18(8), 5294–5305. 10.1021/acs.nanolett.8b02291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnamkhasti BS, Hosseinzadeh H, Azhdarzadeh M, Vafaei SY, Atyabi F, & Dinarvand R (2015). Protein corona hampers targeting potential of MUC1 aptamer functionalized SN-38 core—Shell nanoparticles. International Journal of Pharmaceutics, 494(1), 430–444. 10.1016/j.ijpharm.2015.08.060 [DOI] [PubMed] [Google Scholar]
- Vidanapathirana AK, Lai X, Hilderbrand SC, Pitzer JE, Podila R, Sumner SJ, … Brown JM (2012). Multi-walled carbon nanotube directed gene and protein expression in cultured human aortic endothelial cells is influenced by suspension medium. Toxicology, 302(2–3), 114–122. 10.1016/j.tox.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova MA, Tarasova OS, Riikonen J, Raula J, Lobach AS, Borzykh AA, … Järvinen K (2014). Injected nanoparticles: The combination of experimental systems to assess cardiovascular adverse effects. European Journal of Pharmaceutics and Biopharmaceutics, 87(1), 64–72. 10.1016/j.ejpb.2014.02.001 [DOI] [PubMed] [Google Scholar]
- Walkey CD, & Chan WCW (2012). Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chemical Society Reviews, 41(7), 2780–2799. 10.1039/c1cs15233e [DOI] [PubMed] [Google Scholar]
- Walkey CD, Olsen JB, Song F, Liu R, Guo H, Olsen DWH, … Chan WCW (2014). Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano, 8(3), 2439–2455. 10.1021/nn406018q [DOI] [PubMed] [Google Scholar]
- Wan S, Kelly PM, Mahon E, Sto H, Rudd PM, Caruso F, … Monopoli MP (2015). The “sweet” side of the protein corona: Effects of glycosylation on nanoparticle cell interactions. ACS Nano, 9(2), 2157–2166. 10.1021/nn506060q [DOI] [PubMed] [Google Scholar]
- Wang F, Yu L, Monopoli MP, Sandin P, Mahon E, Salvati A, & Dawson KA (2013). The biomolecular corona is retained during nanoparticle uptake and protects the cells from the damage induced by cationic nanoparticles until degraded in the lysosomes. Nanomedicine: Nanotechnology, Biology, and Medicine, 9(8), 1159–1168. 10.1016/j.nano.2013.04.010 [DOI] [PubMed] [Google Scholar]
- Westmeier D, Stauber RH, & Docter D (2016). The concept of bio-corona in modulating the toxicity of engineered nanomaterials (ENM). Toxicology and Applied Pharmacology, 299, 53–57. 10.1016/j.taap.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Wolfram J, Yang Y, Shen J, Moten A, Chen C, Shen H, … Zhao Y (2015). The nano-plasma interface: Implications of the protein corona. Colloids and Surfaces B: Biointerfaces, 124, 17–24. 10.1016/j.colsurfb.2014.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Gause KT, Kamphuis MMJ, Ang C, Brien-simpson NMO, Lenzo JC, … Caruso F (2013). Differential roles of the protein corona in the cellular uptake of nanoporous polymer particles by monocyte and macrophage cell lines. ACS Nano, 7(12), 10960–10970. 10.1021/nn404481f [DOI] [PubMed] [Google Scholar]
- Yang W, Peters JI, & Williams RO (2008). Inhaled nanoparticles—A current review. International Journal of Pharmaceutics, 356(1–2),239–247. 10.1016/j.ijpharm.2008.02.011 [DOI] [PubMed] [Google Scholar]
- Ye D, Bramini M, Hristov DR, Wan S, Salvati A, Åberg C, & Dawson KA (2017). Low uptake of silica nanoparticles in Caco-2 intestinal epithelial barriers. Beilstein Journal of Nanotechnology, 8(1), 1396–1406. 10.3762/bjnano.8.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Fan Z, Li PY, Deng J, Arhontoulis DC, Li CY, … Cheng H (2018). Dense and dynamic polyethylene glycol shells cloak nanoparticles from uptake by liver endothelial cells for long blood circulation. ACS Nano, 12, 10130–10141. 10.1021/acsnano.8b04947 [DOI] [PMC free article] [PubMed] [Google Scholar]


