Abstract
A cDNA obtained from Grimsby virus (GRV), a Norwalk-like virus, purified from a stool sample of a symptomatic adult associated with a gastroenteritis outbreak in the United Kingdom, was used to obtain the complete nucleotide sequence of the second open reading frame (ORF2). The ORF2 sequence of GRV predicts a capsid of 539 amino acids (aa) which exhibits aa identities of 96% to Lordsdale virus, 67% to Mexico virus (MXV), and 43% to Norwalk virus (NV). The GRV capsid protein was expressed in insects cells by using a recombinant baculovirus, and the resulting virus-like particles (VLPs) possessed a protein with an apparent molecular weight of 58,000. Hyperimmune antisera raised against purified GRV, MXV, and NV VLPs were tested in an indirect enzyme-linked immunosorbent assay (ELISA) against GRV, NV, and MXV VLPs, revealing that GRV is antigenically distinct from both NV and MXV. The antigenic specificity of the GRV-hyperimmune antiserum was confirmed in an antigen capture ELISA using GRV-, NV-, or MXV-containing fecal specimens. The expression of the GRV capsid protein has, for the first time, allowed the antigenic comparison of three distinct recombinant Norwalk-like viruses.
The genetic characterization of Norwalk virus (NV) and other human caliciviruses (HuCVs), previously called small round-structured viruses or classical HuCVs, has revealed a high degree of diversity within the family Caliciviridae (1–3, 8, 9, 12, 14, 19, 21–23, 27, 30). Phylogenetic analyses indicate an ancestral division of the family into four genera. Two of these genera, called Norwalk-like viruses and Sapporo-like viruses, currently contain viruses from humans (8). Viruses in the Norwalk-like virus genus were previously divided into two genogroups based on phylogenetic analyses of both structural (capsid region) and nonstructural (polymerase) genes (1–3, 7, 8, 20, 21, 33), although the biological significance of this subgrouping was unknown. For the polymerase region, viruses within a genogroup show >80% amino acid (aa) similarity. For the capsid region, viruses within a genogroup share >64% aa homology. The cutoff value for the two genogroups was arbitrary (2, 3, 12). Overall comparative analyses of most Norwalk-like strains (reviewed in references 2, 3, and 10) have been hindered by the lack of published sequence data and type-specific reagents. Viruses from the Sapporo-like virus genus include viruses with a Star of David or classical HuCV appearance by electron microscopy (EM), as represented by the prototype classical HuCV Sapporo virus (30).
Prior to the production of recombinant HuCV (rHuCV) capsid proteins, the antigenic diversity of these noncultivable agents was investigated by immune EM (IEM) and cross-protection studies in volunteers (18, 35). NV, Hawaii virus (HV), and Snow Mountain agent (SMA) were found to be antigenically distinct in volunteer challenge studies, and these three strains can also be differentiated by solid-phase IEM (SPIEM) (26, 35). In the United States, there are at least four antigenic groups of Norwalk-like viruses, represented by NV, HV, SMA, and Taunton virus, based on SPIEM (12). The scheme described by Lewis (26) has now been extended to include seven types: UK1 (Taunton virus), UK2, UK3 (HV), UK4 (SMA), NV, Oklahoma virus, and Mexico virus (MXV) (10, 25). Independently, nine antigenic types have been determined by IEM in Japan (31), but the relationship of these antigenic types to Lewis’ scheme is not clear because the antigenic characterization was performed with different immunological reagents.
The observation that expression of NV capsid protein in insect cells results in self-assembly into virus-like particles (VLPs) has allowed the production of reagents to further study antigenic relationships among the Norwalk-like viruses and other HuCVs (15). The use of antisera prepared against expressed VLPs has shown that NV is antigenically distinct from MXV (16). However, despite reports of the self-assembly of a number of other HuCVs, few data on the specificity of hyperimmune sera raised against these VLPs have been published (1, 7, 20, 21). Antigen detection enzyme-linked immunosorbent assays (ELISAs) based on the use of hyperimmune antisera to rNV and rMXV are mainly type specific (10, 29). In contrast, antibodies to VLPs in individuals infected with HuCVs appear to be more broadly reactive, especially between strains belonging to the same genogroup (11, 24, 28). We now report the expression of Grimsby virus (GRV), a virus in the same genogroup as MXV, using recombinant baculovirus technology and the production and use of hyperimmune antiserum raised against self-assembled GRV VLPs to characterize the antigenic relatedness of this virus to other prototype HuCVs.
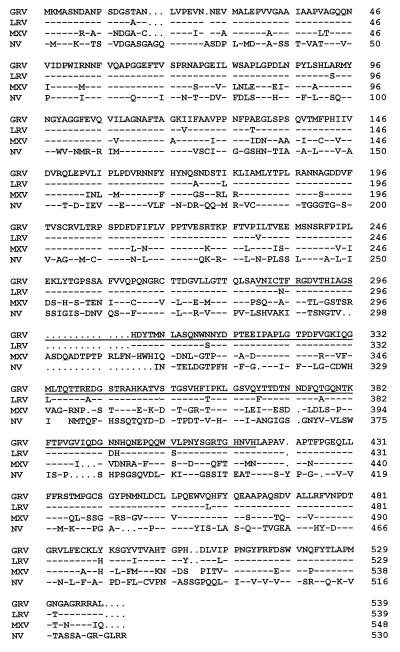
GRV was associated with a hospital outbreak of gastroenteritis which occurred in February 1995 at Grimsby District General Hospital, Grimsby, United Kingdom. Prospective molecular typing of outbreak strains indicated that this was the predominant strain circulating in the United Kingdom during the HuCV season of 1995–1996, and similar strains had also been seen in The Netherlands at the same time (32). Viral nucleic acid was extracted and purified from a fecal specimen of a symptomatic adult by the guanidinium thiocyanate-silica method. A single-stranded cDNA was produced by using SuperScript II reverse transcriptase (Life Technologies, Paisley, United Kingdom) after priming of the polyadenylated positive-strand RNA genome with the primer Linker-T20VN (27). The entire second open reading frame 2 (ORF2) of GRV, flanked by small regions of ORF1 and ORF3, was amplified by a series of seminested PCRs, using the Expand Long Template PCR System (Boehringer Mannheim UK Limited, Lewes, East Sussex, United Kingdom) with forward primers GII and GV4 and reverse primers Linker and GV7 (4, 5). The resulting amplicon of approximately 1.6 kb was cloned into the pTAg vector (R&D Systems, Abingdon, United Kingdom). The ORF2 sequence of GRV predicted a capsid of 539 aa which exhibited 96% aa identity to Lordsdale virus (LRV) within the capsid region. In contrast, GRV exhibited only 67 and 43% aa identity to MXV and NV, respectively, over this same region. An alignment of the capsid regions of GRV, LRV, MXV, and NV is shown in Fig. 1.
FIG. 1.
Amino acid sequence alignments of the GRV, LRV, MXV, and NV putative capsid proteins. Alignments were generated by the Clustal method. The hypervariable region of the GRV sequence is underlined, and residues matching GRV exactly are indicated by dashes. GenBank accession numbers for the sequences used in the alignments are as follows: LRV, X86557; MXV, U22498; and NV, M87661.
The GRV capsid cDNA was recloned into pFastbac1 (Bac-to-Bac; Life Technologies, Gaithersburg, Md.), and a recombinant “bacmid” vector containing ORF2 of GRV downstream from the polyhedron promoter was produced by site-specific transposition between this pFastbac1 recombinant and a baculovirus shuttle vector in the presence of a helper plasmid in Escherichia coli. The resulting composite bacmid was transfected into Spodoptera frugiperda 9 (Sf9) cells, and recombinant baculovirus was isolated by plaque purification. VLPs were obtained by infecting Sf9 cells with recombinant baculovirus at a multiplicity of infection of between 5 and 10, with harvesting of cell cultures at 7 days postinfection. VLPs were concentrated by ultracentrifugation (120,000 × g) of culture supernatants followed by purification in 20 to 60% sucrose gradients in 10 mM Tris-HCl (pH 6.0). VLPs were finally pelleted by ultracentrifugation (120,000 × g) and the protein content was estimated by using a bicinchoninic acid kit (Bio-Rad, Hercules, Calif.) and bovine serum albumin as a standard. The purity and capsid integrity of rGRV preparations were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining, endotoxin quantification (Associates of Cape Cod Inc., Woods Hole, Mass.), and EM.
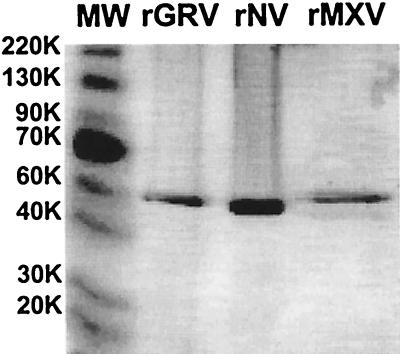
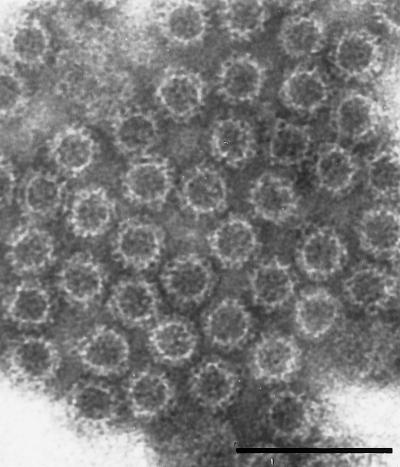
The putative capsid protein of GRV had a predicted molecular weight of 59,000, and Coomassie blue staining of 10% polyacrylamide gels following electrophoresis of purified rGRV revealed migration of the capsid protein to a position slightly above that of the 58,000-molecular-weight capsid protein of rNV (Fig. 2). In addition, similar to rNV, high-molecular-weight forms of rGRV capsid protein were observed when purified VLPs were not boiled prior to electrophoresis (data not shown). These more-slowly migrating bands are thought to represent oligomeric forms (13). EM of purified rGRV particles revealed characteristic VLPs of 38 nm in diameter (Fig. 3). Some preparations of rGRV particles revealed both 38- and 23-nm VLPs by EM, similar to those observed in rNV preparations (data not shown) (34).
FIG. 2.
Coomassie blue-stained 10% polyacrylamide gel following sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified rGRV, rNV, and rMXV capsid proteins. Preparations were boiled prior to electrophoresis.
FIG. 3.
Electron micrograph of rGRV VLPs showing 38-nm-diameter VLPs. Bar, 100 nm.
Two New Zealand White rabbits and four CD-1 mice were immunized with purified rGRV (four doses of 50 μg/dose for rabbits and 20 μg/dose for mice), using Freund’s complete adjuvant for the first dose and Freund’s incomplete adjuvant for the subsequent doses. Indirect ELISAs were performed as described previously for rNV except that VLPs were used at a concentration of 1 μg/ml to coat ELISA plates (6). Rabbit (n = 2) and mouse (n = 4) hyperimmune sera raised against purified rGRV VLPs and rabbit hyperimmune serum raised against purified rNV or rMXV (10, 15, 17) VLPs were tested by indirect ELISAs against rGRV, rNV, and rMXV VLPs (Table 1 and data not shown). Titers of the hyperimmune sera to control preparations from insect cells infected with a wild-type baculovirus were ≤100 (data not shown). Titers of the anti-rGRV rabbit and mouse hyperimmune sera against individual VLPs differed by up to 16-fold between animals, but in all cases, titers to rGRV were at least ≥256-fold higher than those to heterologous rNV or rMXV VLPs (Table 1 and data not shown). The titers of the anti-rMXV hyperimmune serum when tested against rGRV and rNV were <8- and <128-fold lower, respectively, than that obtained against the homologous rMXV (Table 1). The titers of the anti-rNV hyperimmune serum when tested against rMXV and rGRV were >512-fold lower than that against rNV (Table 1). The low cross-reactivity of the anti-rGRV hyperimmune sera with rMXV was rather unexpected because GRV and MXV exhibited 68% aa identity over the capsid region, although the level of aa identity was 47% within the hypervariable region. The titers of anti-rGRV hyperimmune sera obtained when tested against rNV and rMXV were similar, although NV exhibited only 43 and 20% aa identities to rGRV over the complete and hypervariable capsid regions, respectively.
TABLE 1.
ELISA cross-reactivity of hyperimmune sera raised against rHuCVs NV, MXV, and GRV
| rHuCV | Titer with antiserum to rHuCVa:
|
|||
|---|---|---|---|---|
| NV (rabbit) | MXV (rabbit) | GRV (rabbit) | GRV (mouse) | |
| NV | 6,553,600 | 3,200 | 1,600 | 400 |
| MXV | 1,600 | 409,600 | 1,600 | 800 |
| GRV | 6,400 | 51,200 | 1,638,400 | 6,553,600 |
Values in the table represent the reciprocals of ELISA titers of sera tested with the indicated rHuCVs. Homologous titers are given in boldface type.
To confirm the type specificity of rGRV antisera, an antigen capture ELISA similar to those described for NV was developed (13). Preimmune and postimmune rabbit antisera at a dilution of 1:5,000 were used to coat the ELISA plates, and a 1:2,000 dilution of pooled mouse hyperimmune antisera was used as the detection antibody. One percent normal rabbit serum was added to the goat anti-mouse conjugate to reduce background reactivity. NV and MXV antigen capture ELISAs were used as previously described except that normal rabbit or guinea pig serum was added to the conjugate as deemed appropriate (13, 15). Specimens were considered positive if P > 0.1 and P/N > 2 (where P is the optical density [OD] with hyperimmune antiserum and N is the OD with preimmune serum). The sensitivity of the assay was approximately 2 ng/ml of purified rGRV. Nevertheless, the assay was unable to detect either rNV or rMXV at concentrations up to 2 μg/ml.
Five fecal specimens containing NV, obtained from volunteers challenged with NV, were tested in parallel in the GRV and NV antigen ELISAs. Four fecal specimens from symptomatic patients involved in three outbreaks of gastroenteritis associated with MXV-like viruses were tested in both GRV and MXV assays, and four fecal specimens containing viruses closely related to GRV, as determined by sequence analysis (data not shown), were tested in all three antigen ELISAs (Table 2). These ELISAs recognized only homotypic strains. One MXV specimen, 67RBH/93/MXV, was positive in the GRV assay. However, this sample was also highly positive in the MXV assay. Further studies are needed to determine if this specimen contains two viruses. Finally, it will be of interest to test recombinant LRV particles in the GRV ELISA, since we anticipate that LRV particles will react given the high level of predicted aa identity between LRV and GRV.
TABLE 2.
Reactivity of fecal specimens containing GRV, MXV, or NV in antigen detection ELISAs using hyperimmune antisera to rVLPs
| Specimen | Reactivity in ELISA fora:
|
||
|---|---|---|---|
| rGRV | rMXV | rNV | |
| 85ELH/96/GRV | 4.41 | 0.01 | 0.00 |
| 60RHCH/96/GRV | 4.18 | 0.01 | 0.00 |
| 64RHCH/96/GRV | 4.59 | 0.01 | 0.00 |
| 69Bai/96/GRV | 3.77 | 0.01 | 0.00 |
| 52909/NV/8FIIa | 0.01 | NTb | 2.90 |
| 54009/NV/8FIIa | 0.00 | NT | 2.45 |
| 54504/NV/8FIIa | 0.00 | NT | 3.76 |
| 55503/NV/8FIIa | 0.00 | NT | 2.83 |
| 55005/NV/8FIIa | 0.00 | NT | 3.64 |
| 47RBH/93/MXV | 0.08 | 1.86 | NT |
| 67RBH/93/MXV | 0.11 | 3.19 | NT |
| 65Car/94/MXV | 0.09 | 2.82 | NT |
| 54AGH/94/MXV | 0.08 | 3.45 | NT |
The demonstration of the type specificity of antigen ELISAs using polyclonal antisera raised against VLPs confirms earlier observations based on NV and MXV assays. However, the expression of GRV has, for the first time, allowed comparison of three antigenically distinct rHuCV ELISAs, two of which are based on viruses within the same genogroup. The high level of sequence similarity in the capsid region of GRV to the corresponding regions of Bristol virus (BV) and LRV suggests that these three viruses are antigenically related. However, neither BV nor LRV has been typed under Lewis’ scheme (26); therefore, the antigenic relationship of GRV to LRV and BV awaits confirmation. Antigenic typing of HuCVs has been hampered by the lack of an in vitro culture system. Although SPIEM and IEM studies using human convalescent-phase sera have provided valuable information on antigenic variation within HuCVs, such reagents are poorly defined and cross-reactivity with more than one strain has been observed (7). ELISAs using hyperimmune antisera raised against VLPs offer an alternative approach for antigenic typing of HuCVs and allow the rapid testing of large numbers of strains with standardized reagents. It is clear, however, that we are still at an early stage in this process. The expression and production of monotypic antisera to VLPs based on genetically distinct HuCVs are essential prerequisites to further our understanding of the antigenic heterogeneity of this group of viruses.
Nucleotide sequence accession number.
The EMBL accession number for ORF2 of GRV is AJ004864.
Acknowledgments
We thank Sharon Krater for excellent technical assistance.
This work was supported by funding from the U.S. Public Health Service (grant AI38036), and A.D.H. was funded, in part, by a fellowship from The Pathological Society of Great Britain and Ireland.
REFERENCES
- 1.Dingle K E, Lambden P R, Caul E O, Clarke I N. Human enteric Caliciviridae: the complete genome sequence and expression of virus-like particles from a genetic group II small round structured virus. J Gen Virol. 1995;76:2349–2355. doi: 10.1099/0022-1317-76-9-2349. [DOI] [PubMed] [Google Scholar]
- 2.Estes M K, Hardy M E. Norwalk virus and other enteric caliciviruses. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. 1st ed. New York, N.Y: Raven Press, Ltd.; 1995. pp. 1009–1034. [Google Scholar]
- 3.Estes M K, Atmar R L, Hardy M E. Norwalk and related diarrhea viruses. In: Richman D, Whitley R, Hayden F, editors. Clinical virology. New York, N.Y: Churchill Livingstone; 1997. pp. 1073–1095. [Google Scholar]
- 4.Gray J J, Green J, Gallimore C, Lee J V, Neal K, Brown D W G. Mixed genogroup SRSV infections among a party of canoeists exposed to contaminated recreational water. J Med Virol. 1997;52:425–429. [PubMed] [Google Scholar]
- 5.Green, J., J. Vinjé, C. Gallimore, M. P. Koopmans, A. Hale, and D. W. G. Brown. Capsid diversity among small round structured viruses. Submitted for publication.
- 6.Green K Y, Lew J F, Jiang X, Kapikian A Z, Estes M K. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J Clin Microbiol. 1993;31:2185–2191. doi: 10.1128/jcm.31.8.2185-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green K Y, Kapikian A Z, Valdesuso J, Sosnovtsev S, Treanor J J, Lew J F. Expression and self-assembly of recombinant capsid protein from the antigenically distinct Hawaii human calicivirus. J Clin Microbiol. 1997;35:1909–1914. doi: 10.1128/jcm.35.7.1909-1914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green, K. Y., T. Ando, M. S. Balayan, I. N. Clarke, M. K. Estes, D. O. Matson, S. Nakata, J. D. Neill, M. J. Studdert, and H.-J. Thiel.In M. van Regenmortel, C. Fauquet, D. Bishop, E. Carsten, M. K. Estes, S. Lemon, J. Maniloff, M. Mayo, D. McGeoch, C. R. Pringle, and R. Wickner (ed.), Virus taxonomy: 7th report of the International Committee on Taxonomy of Viruses. Academic Press, Orlando, Fla.
- 9.Green S M, Lambden P R, Caul E O, Ashley C R, Clarke I N. Capsid diversity in small round-structured viruses: molecular characterization of an antigenically distinct human enteric calicivirus. Virus Res. 1995;37:271–283. doi: 10.1016/0168-1702(95)00041-n. [DOI] [PubMed] [Google Scholar]
- 10.Hale A D, Lewis D, Green J, Jiang X, Brown D W G. Evaluation of an antigen capture ELISA based on recombinant Mexico virus capsid protein. Clin Diagn Virol. 1996;5:27–35. doi: 10.1016/0928-0197(95)00200-6. [DOI] [PubMed] [Google Scholar]
- 11.Hale A D, Lewis D C, Jiang X, Brown D W G. Homotypic and heterotypic IgG and IgM antibody responses in adults infected with small round structured viruses. J Med Virol. 1998;54:305–312. [PubMed] [Google Scholar]
- 12.Hardy M E, Kramer S F, Treanor J J, Estes M K. Human calicivirus genogroup II capsid sequence diversity revealed by analyses of the prototype Snow Mountain agent. Arch Virol. 1997;142:1469–1479. doi: 10.1007/s007050050173. [DOI] [PubMed] [Google Scholar]
- 13.Hardy M E, Tanaka T N, Kitamoto N, White L J, Ball J M, Jiang X, Estes M K. Antigenic mapping of recombinant Norwalk virus capsid protein using monoclonal antibodies. Virology. 1996;217:252–261. doi: 10.1006/viro.1996.0112. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, Wang M, Estes M K. Sequence and genomic organization of Norwalk virus. Virology. 1993;195:51–61. doi: 10.1006/viro.1993.1345. [DOI] [PubMed] [Google Scholar]
- 15.Jiang X, Wang M, Graham D Y, Estes M K. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992;66:6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang X, Matson D O, Ruiz-Palacios G M, Hu J, Treanor J, Pickering L K. Expression, self-assembly, and antigenicity of a Snow Mountain agent-like calicivirus capsid protein. J Clin Microbiol. 1995;33:1452–1455. doi: 10.1128/jcm.33.6.1452-1455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang X, Cubitt D, Hu J, Treanor J, Matson D O, Pickering L K. Development of an ELISA to detect MX virus, a human calicivirus in the Snow Mountain agent genogroup. J Gen Virol. 1995;76:2739–2747. doi: 10.1099/0022-1317-76-11-2739. [DOI] [PubMed] [Google Scholar]
- 18.Kapikian A Z, Wyatt R G, Dolin R, Thornhill T S, Kalica A R, Chanock R M. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972;10:1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambden P R, Caul E O, Ashley C R, Clarke I N. Sequence and genome organization of a human small round structured (Norwalk-like) virus. Science. 1993;259:516–519. doi: 10.1126/science.8380940. [DOI] [PubMed] [Google Scholar]
- 20.Leite J P, Ando T, Noel J S, Jiang B, Humphrey C D, Lew J F, Green K Y, Glass R I, Monroe S S. Characterization of Toronto virus capsid protein expressed in baculovirus. Arch Virol. 1996;141:865–875. doi: 10.1007/BF01718161. [DOI] [PubMed] [Google Scholar]
- 21.Lew J F, Kapikian A Z, Jiang X, Estes M K, Green K Y. Molecular characterization and expression of the capsid protein of a Norwalk-like virus recovered from a Desert Shield troop with gastroenteritis. Virology. 1994;200:319–325. doi: 10.1006/viro.1994.1194. [DOI] [PubMed] [Google Scholar]
- 22.Lew J F, Kapikian A Z, Valdesuso J, Green K Y. Molecular characterization of Hawaii virus and other Norwalk-like viruses: evidence of genetic polymorphism among human caliciviruses. J Infect Dis. 1994;170:535–542. doi: 10.1093/infdis/170.3.535. [DOI] [PubMed] [Google Scholar]
- 23.Lew J F, Petric M, Kapikian A Z, Jiang X, Estes M K, Green K Y. Identification of minireovirus as a Norwalk-like virus in pediatric patients with gastroenteritis. J Virol. 1994;68:3391–3396. doi: 10.1128/jvi.68.5.3391-3396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis D C, Hale A, Jiang X, Eglin R, Brown D W. Epidemiology of Mexico virus, a small small-round structured virus in Yorkshire, United Kingdom, between January 1992 and March 1995. J Infect Dis. 1997;175:951–954. doi: 10.1086/513998. [DOI] [PubMed] [Google Scholar]
- 25.Lewis D, Ando T, Humphrey C D, Monroe S S, Glass R I. Use of solid-phase immune electron microscopy for classification of Norwalk-like viruses into six antigenic groups from 10 outbreaks of gastroenteritis in the United States. J Clin Microbiol. 1995;33:501–504. doi: 10.1128/jcm.33.2.501-504.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis D C. Three serotypes of Norwalk-like virus demonstrated by solid phase immune electron microscopy. J Med Virol. 1990;30:77–81. doi: 10.1002/jmv.1890300117. [DOI] [PubMed] [Google Scholar]
- 27.Liu B L, Clarke I N, Caul E O, Lambden P R. Human enteric caliciviruses have a unique genome structure and are distinct from the Norwalk-like viruses. Arch Virol. 1995;140:1345–1356. doi: 10.1007/BF01322662. [DOI] [PubMed] [Google Scholar]
- 28.Noel J S, Ando T, Leite J P, Green K Y, Dingle K E, Estes M K, Seto Y, Monroe S S, Glass R I. Correlation of patient immune responses with genetically characterized small round-structured viruses involved in outbreaks of nonbacterial acute gastroenteritis in the United States, 1990 to 1995. J Med Virol. 1997;53:372–383. doi: 10.1002/(sici)1096-9071(199712)53:4<372::aid-jmv10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 29.Numata K, Nakata S, Jiang X, Estes M K, Chiba S. Epidemiological study of Norwalk virus infections in Japan and Southeast Asia by enzyme-linked immunosorbent assays with Norwalk virus capsid protein produced by the baculovirus expression system. J Clin Microbiol. 1994;32:121–126. doi: 10.1128/jcm.32.1.121-126.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Numata K, Hardy M E, Nakata S, Chiba S, Estes M K. Molecular characterization of morphologically typical human calicivirus Sapporo. Arch Virol. 1997;142:1537–1552. doi: 10.1007/s007050050178. [DOI] [PubMed] [Google Scholar]
- 31.Okada S, Sekine S, Ando T, Hayashi Y, Murao M, Yabuuchi K, Miki T, Ohashi M. Antigenic characterization of small, round-structured viruses by immune electron microscopy. J Clin Microbiol. 1990;28:1244–1248. doi: 10.1128/jcm.28.6.1244-1248.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinjé J, Koopmans M P. Molecular detection and epidemiology of small round-structured viruses in outbreaks of gastroenteritis in The Netherlands. J Infect Dis. 1996;174:610–615. doi: 10.1093/infdis/174.3.610. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Jiang X, Madore H P, Gray J, Desselberger U, Ando T, Seto Y, Oishi I, Lew J F, Green K Y, Estes M K. Sequence diversity of small, round-structured viruses in the Norwalk virus group. J Virol. 1994;68:5982–5990. doi: 10.1128/jvi.68.9.5982-5990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White L J, Hardy M E, Estes M K. Biochemical characterization of a smaller form of recombinant Norwalk virus capsids assembled in insect cells. J Virol. 1997;71:8066–8072. doi: 10.1128/jvi.71.10.8066-8072.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyatt R G, Dolin R, Blacklow N R, Dupont H L, Buscho R F, Thornhill T S, Kapikian A Z, Chanock R M. Comparison of three agents of acute infectious nonbacterial gastroenteritis by cross-challenge in volunteers. J Infect Dis. 1974;129:709–714. doi: 10.1093/infdis/129.6.709. [DOI] [PubMed] [Google Scholar]