In their recent article (2), Coetsier and colleagues described the preparation of polyclonal and monoclonal antibodies directed against the 13.6-kDa carboxyl-terminal portion of the 34-kDa protein of Mycobacterium avium subsp. paratuberculosis. They used these immunoglobulins for specific histopathological diagnosis of Johne’s disease (paratuberculosis).
This 34-kDa protein was previously identified as an immunodominant antigen located at the surface of M. avium subsp. paratuberculosis (5, 7). The protein was proved to contain both species-specific and cross-reacting B-cell epitopes, and it was suggested that proteins homologous to the 34-kDa antigen are present in M. bovis and in M. avium (4–6). The 13.6-kDa carboxyl end of the 34-kDa protein (peptide a362) was produced by genetic engineering (5, 10). This part of the protein is immunodominant, hydrophilic, and exposed outside the bacteria, the hydrophobic amino-terminal moiety being presumably anchored in the cell envelope (5, 6, 10). By using on one hand sera of rabbits hyperimmunized against a362 (in incomplete Freund’s adjuvant) and on the other hand sera of cows with tuberculosis or with paratuberculosis, it was observed that peptide a362 contains only B-cell epitopes specific to M. avium subsp. paratuberculosis and no epitopes common to 10 other tested mycobacterial species, including M. bovis BCG (5, 10). This peptide was thus a good candidate for an enzyme-linked immunosorbent assay (ELISA) for paratuberculosis, and such a test was developed (10).
Unexpectedly, the rabbit polyclonal antibodies raised against a362 by Coetsier and colleagues also react with some components of M. bovis BCG (particularly with a 38-kDa protein), but none of their monoclonal antibodies react with M. bovis BCG (2). Whereas the possibility of cross-reactivity between peptide a362 of M. avium subsp. paratuberculosis and some M. bovis components could not be rigorously excluded, this unexpected reaction most probably derives from the inadequate immunization protocol. Indeed, the polyclonal antibodies directed against a362 that Coetsier et al. used for a specific histopathological diagnostic test for paratuberculosis were raised in rabbits following two inoculations of a362 emulsified in complete Freund’s adjuvant (CFA). CFA is an emulsion of mycobacteria in oil (1). Thus, it is not surprising that antibodies to mycobacterial antigens develop in response to CFA, and this has already been demonstrated in animals immunized with CFA alone (9). Preabsorption of the above-mentioned anti-a362 polyclonal serum with the a362 polypeptide and the disappearance of immunostaining in the M. bovis BCG Western blot would have provided support for the stated cross-reactivity between a362 and M. bovis BCG components.
Recently, the complete genome of M. tuberculosis H37 Rv was sequenced (3). As M. tuberculosis and M. bovis BCG are two very closely related species (8), proteins homologous to the M. avium subsp. paratuberculosis 34-kDa protein were searched among those encoded by the M. tuberculosis genome. As expected from our previous experiments (5), a BLAST search in protein data banks identified one protein of M. tuberculosis H37 Rv (a 30,225-Da protein under reference Rv 0954 in the classification of Cole et al. [3]) which is highly similar to the 34-kDa protein of M. avium subsp. paratuberculosis. Apart from this 30,225-Da protein of M. tuberculosis, no significant homology was found with any 38-kDa protein such as the one identified in M. bovis BCG by Coetsier and colleagues. Nevertheless, an M. leprae DNA sequence encoding a protein homologous to the 34-kDa protein of M. avium subsp. paratuberculosis was recently deposited in GenBank under accession no. U8211.
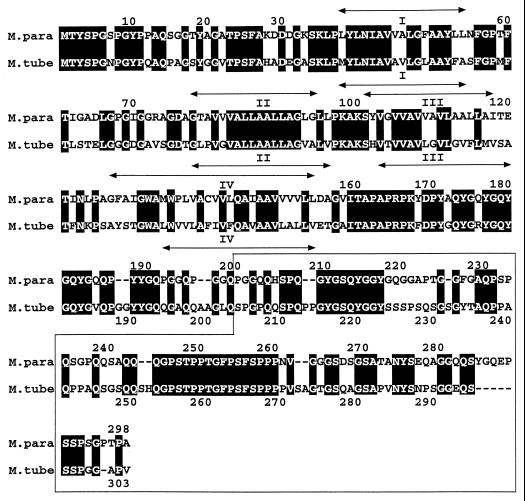
The alignment of the protein identified in M. tuberculosis with the 34-kDa protein of M. avium subsp. paratuberculosis shows that regions of identical amino acids (Fig. 1) are interspersed between regions containing dissimilar amino acids. The carboxyl ends of the two proteins (from 188 to 303) nevertheless seem to be more dissimilar than their amino-terminal parts, as 16 gaps were necessary for their correct alignment (Fig. 1). The B-cell epitope(s) specific to M. avium subsp. paratuberculosis expressed in peptide a362 must be present in the regions of dissimilarity (Fig. 1). Their exact locations could easily be tested with synthetic peptides corresponding to these regions and the monoclonal antibodies produced by Coetsier and colleagues.
FIG. 1.
Alignment of the amino acid sequences of the 34-kDa protein of M. avium subsp. paratuberculosis (M.para) and of the Rv0954 protein of M. tuberculosis H37Rv (M.tube). The two homologous proteins were aligned by using the Align program (http://vega.igh.cnrs.fr/bin/align-guess.cgi). Black background indicates regions containing identical amino acids. Arrows indicate locations of potential transmembrane helices as predicted by the TMpred program (http://www.isrec.isb-sib.ch/software/TMPRED_form.html). The sequence corresponding to the a362 peptide is boxed.
In conclusion, although the work of Coetsier and colleagues proves again that the 34-kDa protein of M. avium subsp. paratuberculosis contains species-specific B-cell epitopes, and albeit it is highly probable that a similar protein does exist in M. bovis BCG, the fact that B-cell epitope(s) cross-reacting with M. bovis BCG are present in the carboxyl end of the 34-kDa protein of M. avium subsp. paratuberculosis (peptide a362) needs further examination.
REFERENCES
- 1.Claassen E, de Leeuw W, de Greeve P, Hendriksen C, Boersma W. Freund’s complete adjuvant: an effective but disagreeable formula. Res Microbiol. 1992;143:478–483. doi: 10.1016/0923-2494(92)80057-r. [DOI] [PubMed] [Google Scholar]
- 2.Coetsier C, Havaux X, Mattelard F, Sadatte S, Cormont F, Buergelt K, Limbourg B, Latinne D, Bazin H, Denef J-F, Cocito C. Detection of Mycobacterium avium subsp. paratuberculosisin infected tissues by new species-specific immunohistological procedures. Clin Diagn Lab Immunol. 1998;5:446–451. doi: 10.1128/cdli.5.4.446-451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole S, Brosch R, Parkhil J, Garnier T, Churcher C, Harris D, Gordon S, Eiglmeier K, Gas S, Bary III C, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares S, Sulston J, Taylor K, Whitehead S, Barrell B. Deciphering the biology of Mycobacterium tuberculosisfrom the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 4.De Kesel M, Gilot P, Coene M, Cocito C. Composition and immunological properties of the protein fraction of A36, a major antigen complex of Mycobacterium paratuberculosis. Scand J Immunol. 1992;36:201–212. doi: 10.1111/j.1365-3083.1992.tb03092.x. [DOI] [PubMed] [Google Scholar]
- 5.De Kesel M, Gilot P, Misonne M-C, Coene M, Cocito C. Cloning and expression of portions of the 34-kilodalton protein gene of Mycobacterium paratuberculosis: its application to serological analysis of Johne’s disease. J Clin Microbiol. 1993;31:947–954. doi: 10.1128/jcm.31.4.947-954.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilot P, De Kesel M, Machtelinckx L, Coene M, Cocito C. Isolation and sequencing of the gene coding for an antigenic 34-kilodalton protein of Mycobacterium paratuberculosis. J Bacteriol. 1993;175:4930–4935. doi: 10.1128/jb.175.15.4930-4935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilot P, Coene M. Thermostable macromolecular antigens from mycobacteria. Can J Microbiol. 1994;40:605–611. doi: 10.1139/m94-097. [DOI] [PubMed] [Google Scholar]
- 8.Imaeda T. Deoxyribonucleic acid relatedness among strains of Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium bovis BCG, Mycobacterium microti, and Mycobacterium africanum. Int J Syst Bacteriol. 1985;35:147–150. [Google Scholar]
- 9.Ramos-Ruiz R, Lopez-Bote J P, Pelayo F, Larraga V, van der Zee R, Bernabeu C. Cellular and humoral reactivity pattern to the mycobacterial heat shock protein hsp 65 in adjuvant arthritis susceptible and resistant Wistar rats. Autoimmunity. 1991;9:1–5. doi: 10.3109/08916939108997117. [DOI] [PubMed] [Google Scholar]
- 10.Vannuffel P, Gilot P, Limbourg B, Naerhuyzen B, Dieterich C, Coene M, Machtelinckx L, Cocito C. Development of species-specific enzyme-linked immunosorbent assay for diagnosis of Johne’s disease in cattle. J Clin Microbiol. 1994;32:1211–1216. doi: 10.1128/jcm.32.5.1211-1216.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]