Abstract
Antiphospholipid syndrome and the coagulopathy of COVID-19 share many pathophysiologic features, including endotheliopathy, hypercoagulability, and activation of platelets, complement pathways, and neutrophil extracellular traps, all acting in concert via a model of immunothrombosis. Antiphospholipid antibody production in COVID-19 is common, with 50% of COVID-19 patients being positive for lupus anticoagulant in some studies, and with non-Sapporo criteria antiphospholipid antibodies being prevalent as well. The biological significance of antiphospholipid antibodies in COVID-19 is uncertain, as such antibodies are usually transient, and studies examining clinical outcomes in COVID-19 patients with and without antiphospholipid antibodies have yielded conflicting results. In this review, we explore the biology of antiphospholipid antibodies in COVID-19 and other infections and discuss mechanisms of thrombogenesis in antiphospholipid syndrome and parallels with COVID-19 coagulopathy. In addition, we review the existing literature on safety of COVID-19 vaccination in patients with antiphospholipid antibodies and antiphospholipid syndrome.
Keywords: COVID-19 coagulopathy, Antiphospholipid syndrome, Antiphospholipid antibodies, Lupus anticoagulant, Anticardiolipin antibodies, Beta-2 glycoprotein-1 antibodies, COVID-19 vaccination, Immunothrombosis
1. Introduction
One of the major mediators of morbidity and mortality in coronavirus disease 2019 (COVID-19) is a coagulopathy characterized by increased risks of arterial, venous, and microvascular thrombosis [1,2]. A multitude of pathophysiologic processes drive this hypercoagulable state, including endotheliopathy, cellular activation and inflammation, complement activation, autoantibody generation, cytokine dysregulation, and fibrinolytic derangements, all supporting a model of immunothrombosis as observed in other microvascular inflammatory diseases [[3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]].
Antiphospholipid syndrome (APS) is an autoimmune, thromboinflammatory disease characterized by thrombosis and/or pregnancy loss in the presence of one or more antiphospholipid antibodies (aPL) [16]. APS can occur as a primary disorder or concomitantly with another underlying autoimmune disease such as systemic lupus erythematosus [17]. A diagnosis of APS may be established on the basis of the revised Sapporo criteria, which require thrombotic or obstetrical complications and persistent positivity for lupus anticoagulant (LA) or high-titre anti-cardiolipin (aCL) or anti-beta-2 glycoprotein I (aβ2GPI) IgG or IgM antibodies [18]. The primary pathogenic antibodies in APS are aβ2GPI, which activate endothelial cells, leading to a hypercoagulable state. Other “non-Sapporo” criteria aPL such as antiphosphatidylserine and prothrombin antibodies (aPS/PT) have also been described in APS, but their clinical significance is uncertain [19].
LA testing is a complex, multi-step process [20]. The first test, a screening test, measures clotting times in the presence of a reagent exquisitely sensitive to the presence of phospholipids (e.g., Russell's viper venom) [20]. If the clotting time as measured in the screening test is prolonged, a mixing study is done utilizing an equal volume of the patient's plasma with normal pooled plasma; failure of the mixing study to correct the prolonged clotting time is suggestive of the presence of an inhibitor. The final confirmatory test involves the addition of excess phospholipid to shorten or correct the prolonged coagulation test; correction of a prolonged clotting time with added phospholipid and not with control plasma is characteristic of LA. A number of factors may lead to false positive LA testing, most commonly anticoagulant medications that prolong clotting time measurements.
The presence of aPL in COVID-19 was first reported early in the pandemic by investigators in Beijing, who identified three patients with COVID-19, multiple cerebral infarctions, and, in one case, limb ischemia, who tested positive for aCL IgA and aβ2GPI IgA and IgG antibodies [21]. Several subsequent studies followed exploring potential links between APS and COVID-19 based on similar immunothrombotic features of both diseases [22]. In this review, we explore the prevalence and significance of aPL in COVID-19, the shared mechanisms of thrombosis in APS and COVID-19 coagulopathy, and the implications of COVID vaccination in APS patients.
2. Mechanisms of thrombogenesis in APS
aPL are not thrombogenic on their own, and a multiple-hit model is believed to explain the progression from aPL to thrombotic or obstetrical sequelae. Binding of aPL to endothelial cells and monocytes induces expression of cellular adhesion molecules and tissue factor, triggering activation of endothelial cells and of the coagulation cascade [[23], [24], [25]]. aPL also cause production of inflammatory cytokines such as interleukin-6, interleukin-8, and vascular endothelial growth factor [26]. aPL binding to β2GPI activates apolipoprotein E receptor 2, leading to upregulation of protein phosphatase 2A and down regulation of Akt and endothelial nitrous oxide synthase, reducing levels of nitric oxide, an anti-inflammatory and vasodilatory substance [27]. Together, these factors all lead to increased oxidant injury, activating the endothelium for thrombus generation [28,29]. Binding and endosomal uptake of aPL by monocytes and dendritic cells activates NADPH oxidase, producing superoxide and upregulating expression of Toll-like receptors 7 and 8, amplifying oxidative stress and inflammation and further driving thrombosis in APS [30]. aβ2GPI disrupt annexin A5, an anticoagulant protein found in placental and vascular endothelium, augmenting the risks of thrombosis and miscarriage in APS [31,32].
Platelets are also a major mediator thrombosis in APS [33]. aβ2GPI bind to multiple receptors on platelet surfaces, including platelet glycoprotein (GP) Ibα, GP IIb/IIIa (integrin aIIbβ3), and apolipoprotein E receptor 2, with p38MAPK phosphorylation and release of multiple procoagulant molecules including thromboxane A2 and platelet factor 4, all leading to platelet activation [34]. In murine models, infusion of aβ2GPI leads to activation of endothelial cells, platelets, and monocytes [35]; aβ2GPI-β2GPI complexes selectively bind the platelet thrombus rather than the endothelium, amplifying platelet activation, leading to enhanced endothelial activation and fibrin generation [36]. P-selectin on platelets and endothelial cells as well as endothelial cell surface markers such as intercellular cell adhesion molecule-1 and vascular endothelial cell adhesion molecule-1 recruit leukocytes to platelet thrombi and endothelial cells in the presence of aPL [34,37].
In addition, aPL may activate the complement system, particularly the alternative complement pathway [38]. In murine models, complement deficient-mice are resistant to aPL-induced thrombosis or fetal loss [[39], [40], [41]]. APS patients near the time of a thrombotic event demonstrate increased complement activation as measured via a modified Ham assay and cell surface deposition of terminal complement C5b-9, while germline mutations can be identified in patients with catastrophic APS [42]. aβ2GPI complexes trigger the classical complement pathway by binding to C1q, activating C3b and engaging the alternative pathway [43]. A role for complement activation is further underscored by the successful use of eculizumab, a terminal complement inhibitor, in treating some patients with obstetrical or catastrophic APS [38].
The production of extracellular webs of chromatin by neutrophils, a.k.a. neutrophil extracellular traps (NETs), is an emerging hallmark of APS. NETs are complexes of decondensed chromatin with histones and neutrophil granule proteins released by neutrophils in response to various infectious and non-infectious stimuli. This process of NET production and release, known as NETosis, leads to activation of platelets, endothelial cells, and complement proteins [44]. Under normal conditions, NETs physically immobilize microbes and release antimicrobial substances such as antimicrobial peptides, histones and proteases [44]. When dysregulated, NETs may precipitate endothelial damage and thrombosis, contributing to the microvascular complications seen in numerous autoimmune diseases. Mice treated with IgG fractions from APS patients have higher circulating levels of cell-free DNA compared to controls, while thrombi from APS mice are enriched for citrullinated histone H3, a NET marker [45]; moreover, selective agonism of the adenosine A2A receptor in a mouse APS model suppresses aPL-mediated NETosis and reduces thrombosis [46]. These studies highlight a potential role for NETosis in the pathogenesis of thrombosis in APS.
3. aPL and infections
aPL are known to develop transiently in the setting of numerous types of infections. Syphilis was the first infection to be linked with aPL [47,48]; historically, a falsely positive Rapid Plasma Reagin test was a hallmark of the presence of LA and aCL antibodies due to the presence of cardiolipin in the reagent. In one systematic review and meta-analysis, human immunodeficiency virus (HIV) and hepatitis C virus (HCV) were the two most frequent viruses reported to be involved in aPL generation, with HIV, hepatitis B virus (HBV), HCV, and Epstein-Barr virus (EBV) associated with the development of aCL antibodies and HCV and EBV additionally associated with aβ2-GPI antibody formation [47]. The prevalence of aCL in HIV, EBV, and HCV appears to be much higher than LA [[49], [50], [51]].
Among bacterial infections, Hansen's disease, Staphylococci, Streptococci, tuberculosis, Coxiella, Mycoplasma, Salmonella, Lyme disease, leptospirosis, and various forms of bacterial endocarditis have also been implicated in aPL production [47]. Parasitic infections like malaria, kala azar, and toxoplasmosis also lead to aPL production [47].
One mechanism for production of aPL in infections may be molecular mimicry due to antigenic similarity and cross-reacting antibodies between the infectious agent and β2GPI in host tissue [48]. Haemophilus influenzae, Neisseria gonorrhoea, and Clostridium tetani share epitope homology with the β2GPI molecule, which may explain production of aβ2GPI antibodies in these and other bacterial infections [47]. However, the breadth of microbial agents associated with aPL generation is striking, and additional processes may account for aPL production across a range of different infections.
In most instances, aPL that arise in the setting of infection are transient phenomena restricted to active infection, while chronic infections such as HIV and HCV may demonstrate persistence of aPL whose clinical significance is unknown [48]. Some studies of aPL report increased rates of thromboembolic events with HBV or HCV and increased pregnancy-related events with parvovirus [48], while most other studies suggest that aPL in the setting of infection are clinically quiescent [47].
4. aPL in COVID-19
Numerous studies in the literature have described high rates of aPL in hospitalized patients with COVID-19 [[52], [53], [54], [55], [56], [57], [58], [59], [60]]. The rates of aPL in these studies are higher than those reported in other viral infections, but the analyses are all potentially confounded by reporting bias and the use of anticoagulation at the time that LA testing was performed, leading to the potential for falsely positive LA results [61]. In addition, acute phase reactants such as C-reactive protein may increase the risk of a false-positive LA result [60].
In the largest meta-analysis of aPL in COVID-19, which analyzed 1159 hospitalized patients with COVID-19 and at least one aPL across 21 different studies, the pooled prevalence rate of one or more aPL (aCL IgM or IgG, aβ2GPI, LA, or aPS/PT) was 46.8% [49]. The pooled prevalences for each of LA, aCL IgM or IgG, and aβ2GPI IgM or IgG were 50.7%, 13.9%, and 6.7% respectively. Of the total study population, 14.3% of patients were double positive for two Sapporo criteria aPL tests while 6.1% were triple positive for all three. The prevalence of aCL (28.8 versus 7.1%) and aβ2GPI (12.0 versus 5.8%) was significantly higher in critically ill versus non-critically ill patients, mirroring other analyses of aPL in critical illness outside of COVID-19 [62]. Another systematic review observed a wide range of prevalence of aPL in COVID-19 patients across different studies in the literature, with LA positivity reported in 35–90% of COVID-19 patients in the intensive care unit (ICU) and 20–66% for ICU and medical ward patients combined, and with 1–12% of patients being triple positive [22]. In this study, a high prevalence of non-Sapporo criteria antibodies was also observed (aCL IgA, 20% to more than 90% of patients; aβ2GPI IgA, 0–86%; aPS/PT, 0–24%; anti-annexin V antibodies, 3–19%) [21].
While most studies of aPL in COVID-19 have focused on hospitalized patients, one retrospective multicenter study examining both hospitalized and ambulatory COVID-19 patients found no significant difference in prevalence of aPL between these two populations (50% versus 43.3%, respectively) [[49], [50], [51],[63], [64], [65]].
A few mechanisms have been proposed to explain the development of aPL in COVID-19: molecular mimicry, neoepitope formation, and phosphatidylserine exposure [31,66]. Coronaviruses are structurally comprised of four proteins: spike (S), membrane, envelope, and nucleocapsid [67]. The S glycoprotein, containing two subunits (S1 and S2), determines antigenic diversity of the virus and host tropism [68]. The S1 subunit allows the virus to attach to the host cell receptor while S2 mediates fusion of the viral capsid with the host cell membrane [31]. In molecular mimicry, the S1 and S2 glycoprotein subunits of the SARS-CoV-2 viral S protein form a phospholipid-like epitope that induces formation of aPL, which can trigger an autoimmune response if the antigenic determinants are similar to host human tissue [69]. The neoepitope model postulates that oxidative stress in COVID-19 causes a change in confirmation of β2GPI, a plasma protein involved in hemostasis and immunity, creating a neoepitope for the generation of aβ2GPI [70]. During pathogenesis of APS, oxidative stress leads to a thiol exchange reaction and formation of disulfide bonds in domains I and V of the β2GPI protein, leading to a conformational change that renders β2GPI immunogenic [71,72]. It is possible that oxidative stress from COVID-19 may induce similar conformational changes in β2GPI and consequent generation of aPL. Phosphatidylserine, located on the inner surface of the lipid bilayer, may become exposed during infection with COVID-19 or other viruses through the action of phospholipid scramblase, which may trigger aPL production as well as inflammatory and thrombogenic responses [66].
In the above studies, the ∼50% reported prevalence of LA in COVID-19 is much higher than in other viral infections [[49], [50], [51]]. At first glance, this difference appears intriguing based on the increased association of LA with thrombosis compared to aCL or aβ2-GPI in APS, but reporting bias confounds any direct comparisons [63].
aPL generated during COVID-19 infection tend to demonstrate only transient elevation. One study of 31 ICU patients with COVID-19 found 23 (74.2%) with at least one aPL, but on repeat testing 1 month later, only one of the 10 patients retested had persistent aPL [73]. Another study of 79 hospitalized COVID-19 patients (mostly in the ICU) with an initially positive LA found that none of 42 who were retested between 3 and 6 months later had a positive LA, while 10 patients (23.8%) remained positive for other aPL [74]. Another study of critically ill COVID-19 patients examined aPL at multiple time points in six patients and observed distinct patterns of aPL positivity over time [75]; in some patients, aPL peaked around 30–50 days after disease onset, then declined over subsequent days, while other patients demonstrated more transient aPL positivity [75].
Compared to APS, aPL titres generated in COVID-19 are generally lower, with a preponderance of weakly reactive antibodies against domains 1 and domains 4–5 of β2-GPI, in contrast to strong reactivity against β2GPI domain 1 as seen in APS [76]. In studies of APS, aβ2GPI with strong reactivity against domain 1 are believed to be thrombogenic, while aβ2GPI with reactivity to domains 4–5 are generally not thrombogenic [[76], [77], [78]]. In light of this and the transient nature of aPL in COVID-19, the thrombogenicity of these antibodies is uncertain.
5. aPL and clinical outcomes in COVID-19
Individual studies have drawn a diverse range of conclusions about correlations between aPL in COVID-19 and clinical outcomes such as disease severity, laboratory markers, thromboembolic complications, and mortality (Table 1 ). Some studies have described either a strong and statistically significant association between aPL and disease severity or mortality, or an enrichment of aPL positivity among hospitalized COVID-19 patients as a function of disease severity, while others have not [74,[79], [80], [81], [82], [83], [84], [85], [86]]. Elevated levels of acute phase reactants such as C-reactive protein and fibrinogen have been observed in COVID-19 patients with a positive LA test in a couple prospective studies [82,87]. Other studies, however, have not observed differences in these parameters [42,71,83,88,89].
Table 1.
Summary of studies of antiphospholipid antibodies in patients with COVID-19. Abbreviations: aPL, antiphospholipid antibodies; CVC, central venous catheter; DVT, deep venous thrombosis; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; MI, myocardial infarction; PE, pulmonary embolism.
| Study [reference] | Study location | Setting | Number of patients | aPL tests performed | Number of patients with positive aPL, n | aPL persistent, type (ratio) |
Thrombotic Events, n |
|---|---|---|---|---|---|---|---|
| Zhang et al. [21] | China | ICU | 3 |
|
0 | N/a | Strokes, MI, LI |
|
3 | ||||||
|
3; 3 | ||||||
| Helms et al. [52] | France | ICU | 57 |
|
50 | N/a | N/a |
| Pineton de Chambrum et al. [115] | France | ICU | 25 |
|
23 | n/a | 6 PE |
|
7; 13; 5 | ||||||
|
3; 1;0 | ||||||
|
15; 14 | ||||||
| Fan et al. [93] | China | ICU | 86 |
|
12 | n/a | 6 strokes |
| Amezcua-Guerra et al [116]. | Mexico | ICU | 21 |
|
2; 3 | n/a | 2 PE |
|
1; 0 | ||||||
|
2; 4 | ||||||
|
0; 0 | ||||||
|
1; 4 | ||||||
| Devreese et al. [117] | Belgium | ICU | 31 |
|
21 | At 1 month: 1/10 LA 0/4 aCL 1/2 aβ2-GPI tested again |
4 CVC thrombosis, 2 Clotting of dialysis circuit, 3 Clotting ofECMO circuit, 2 DVT 1 Stroke |
|
3 | ||||||
|
6; 1 | ||||||
|
3 | ||||||
|
3; 1 | ||||||
|
3; 4 | ||||||
| Borghi et al. [118] | France | ICU | 122 |
|
7; 8 | n/a | N/a |
|
19; 11 | ||||||
|
8 | ||||||
| Zhang et al. [119] | China | ICU | 19 |
|
16 | n/a | 4 ATE 1 VTE 7 micro-thrombi |
|
2; 1 | ||||||
|
7 | ||||||
|
6; 0 | ||||||
| Fan et al. [120] | Singapore | ICU | 12 for LA, 4 for others aPL among 12 patients |
|
6 | n/a | n/a |
|
1; 2 | ||||||
|
2 | ||||||
| Alharthy et al. [121] | Saudi Arabia |
ICU | 3 |
|
3 | n/a | 1 DVT |
|
3; 3 | ||||||
| Siguret et al. [122] | France | ICU | 74 |
|
63 | n/a | 26 DVT, 4 PE, 1 stroke, 1 CV thrombosis |
|
9 | ||||||
| Frapard et al. [123] | France | ICU | 37 |
|
7 | n/a | 21 VTE 11 circuit thrombosis |
|
6 | ||||||
| Van der Linden et al. [124] | Sweden | ICU | 23 |
|
19 | n/a | 9 PE 3 DVT |
|
7; 9 | ||||||
|
20 | ||||||
|
7; 8 | ||||||
| Vlachoyiannopoulos et al. [125] | Greece | ICU | 29 |
|
7; 3 | n/a | n/a |
|
5; 7 | ||||||
| Karahan et al. [126] | Turkey | ICU | 26 for LA, 31 for other aPL,among 31 patients |
|
6 | n/a | 1 stroke 1 MI 2 others thrombotic events |
|
0; 2 | ||||||
|
2 | ||||||
|
0; 0 | ||||||
| Mullaguri et al. [127] | USA | ICU | 2 |
|
2,1 | n/a | 2 strokes, 2 PE |
| Trahtemberg et al. [128] | Canada | ICU | 22 |
|
13; 7 | n/a | n/a |
|
0; 0 | ||||||
|
0 | ||||||
|
0; 1 | ||||||
| Najim et al. [129] | Qatar | ICU | 60 |
|
21 | NA | 1 VTE 2 ATE |
|
0; 0 | ||||||
|
1; 1 | ||||||
| Harzallah et al. [130] | France | NA | 56 |
|
25 | n/a | n/a |
|
5 | ||||||
| Bowles et al. [131] | UK | NA | 34 |
|
31 | n/a | 1 VTE |
| Gazzaruso et al. [82] | Italy | MW | 45 |
|
21 | n/a | n/a |
|
1; 1 | ||||||
|
2; 3 | ||||||
| Popovic et al. [132] | France | NA | 11 |
|
3 | n/a | 11 MI |
|
1 | ||||||
| Galeano-Valle et al. [133] |
Spain | MW | 24 |
|
0; 2 | n/a | 24 VTE |
|
0; 2 | ||||||
| Gatto et al. [65] | Italy | NA | 72 for LA 121 for IgA 112 for other isotype,among 122 patients |
|
16 | n/a | 17 VTE 1 stroke |
|
2 | ||||||
|
15; 3 | ||||||
|
4 | ||||||
|
7; 8 | ||||||
| Reyes et al. [91] | USA | NA | 68 |
|
38 | n/a | 17 DVT, 7 PE 6 ATE 2 strokes |
|
0; 1 | ||||||
|
0; 1 | ||||||
| Rothstein et al. [94] | USA | NA | 9 |
|
9 | NA | strokes |
| Hossri et al. [134] | USA | NA | 2 |
|
0 | NA | Stroke, LI, SI |
|
2 | ||||||
|
0 | ||||||
| Previtali et al. [135] | Italy | NA | 35 |
|
0 | Autopsy series | 10 thromboembolic events 4 PE 2 strokes |
|
1; 2 | ||||||
|
0 | ||||||
|
1; 2 | ||||||
| Gazzaruso et al. [58] | Italy | NA | 192 |
|
95 | NA | |
| Kanso et al. [136] | France | MW | 2 |
|
1 | NA | 1 PE |
| Guillet et al. [137] | France | NA | 4 |
|
1 | NA | 4 ATE (MI, LI,aortic thrombosis) |
|
0; 1 | ||||||
| Cristiano et al. [138] | Italy | MW | 92 |
|
3; 1 | NA | NA |
|
0; 2 | ||||||
|
2; 3 | ||||||
|
4; 3 | ||||||
| Balanchivadze et al. [139] |
USA | NA | 2 |
|
2; 2 | At 3 months: 0/2 tested again |
2 PE |
|
2 | ||||||
| Le Joncour et al. [54] |
France | MW | 53 for LA 104 for other aPL,among 104 patients |
|
21 | NA | 9 PE 1 DVT 1 aortic thrombus |
|
31 | ||||||
|
8; 8 | ||||||
|
6 | ||||||
|
5; 3 | ||||||
| Anaya et al. [140] | Colombia | NA | 120 |
|
2; 22 | NA | NA |
|
0; 17 | ||||||
| Beyrouti et al. [141] | UK | Mixed | 6 |
|
5 | NA | 6 strokes |
|
0; 1 | ||||||
|
1; 1 | ||||||
| Pascolini et al. [142] | Italy | Mixed | 33 |
|
3; 5 | NA | NA |
|
2; 2 | ||||||
| Bertin et al. [80] | France | Mixed | 56 |
|
16; 3 | NA | Strokes |
| 1; 4 | |||||||
| Zuo et al. [98] | USA | Mixed | 172 |
|
6 | NA | NA |
|
8; 39 | ||||||
|
7 | ||||||
|
5; 9 | ||||||
|
42; 31 | ||||||
| Lerma et al. [143] | USA | Mixed | 64 |
|
1; 1 | NA | NA |
|
1; 2 | ||||||
|
1; 3 | ||||||
| Ferrari et al. [83] | France | Mixed | 89 |
|
59 | NA | 14 VTE |
|
7 | ||||||
|
6 | ||||||
| Gutiérrez et al. [144] | Spain | Mixed | 27 |
|
6 | NA | 2 LI 6 DVT 10 PE 2 strokes |
|
0 | ||||||
|
1 | ||||||
|
1 | ||||||
| Xiao et al. [57] | China | Mixed | 79 |
|
2 | NA | 19 DVT 5 strokes 1 MI |
|
17; 19 | ||||||
|
4; 2 | ||||||
|
12; 1 | ||||||
|
2 | ||||||
|
0; 7 | ||||||
| Tvito et al. [145] | Israel | Mixed | 43 |
|
16 | NA | 3 thrombotic events |
|
0 | ||||||
| Bauer et al. [146] | Germany | Mixed | 17 |
|
3 | NA | NA |
| Serrano et al. [59] | Spanish | Mixed | 474 |
|
28 | NA | 9 thrombotic events |
|
71 | ||||||
|
22 | ||||||
| Vollmer et al. [74] | France | Mixed | 79 patients withLA positivity 56 for aCL andaβ2-GPI, 53 for other aPL |
|
79 |
At 3 months: 0/42 LA tested again |
30 VTE, 27 PE 5 DTP or superficial VT 10 ATE, 9 strokes, 0 MI, 1 mesenteric infarction 5 CT, 5 ECMO or RRT circuit Clotting |
|
1; 13 | ||||||
|
0; 3 | ||||||
|
1 | ||||||
|
1 | ||||||
|
10 | ||||||
|
1 | ||||||
| Gendron et al. [87] | France | Mixed | 115 for LA, 97 for aCL IgA, 98 for aβ2-GPI IgA, 109 for aPT 148 for other aPL among 154 patients |
|
70 | NA | Only for LA positivity: 19 VTE 15 symptomatic PE 6 symptomatic DVT |
|
3 | ||||||
|
9; 2 | ||||||
|
5; 3 | ||||||
|
2 | ||||||
|
0; 7 | ||||||
|
11; 10 | ||||||
| Benjamin et al. [147] | UK | Mixed | 77 |
|
11; 41 | NA | 12 VTE |
|
6; 10 | ||||||
|
3; 8 | ||||||
|
10 | ||||||
| Hollerbach et al. [148] | Germany | Mixed | 174 for aCL and aβ2-GPI 53/174 had aPS/PT IgG, IgM |
|
11; 0 | NA | NA |
|
1; 0 | ||||||
|
0; 4 | ||||||
| Lee et al. [149] | Korea | Mixed | 105 |
|
2; 29 | 2 in hospital thrombosis | |
|
4; 4 | ||||||
|
0; 3 | ||||||
| Gil-Etayo et al. [96] | Spain | Mixed | 390 |
|
8; 10 | 5; 11 | 24 PE, 8 thrombotic stroke, 4 DVT and 1 arterial thrombosis |
|
4; 10 | 12; 16 | |||||
|
7; 9 | 8; 9 | |||||
| Constans et al. [85] | Spain | Mixed | 211 |
|
128 | NA | 2 PE, 2 MI, 2 ischemic stroke |
| Emmenegger et al. [150] | Germany | Mixed | 95 |
|
0,12% | NA | NA |
|
2.04,42.67% | ||||||
|
0,28% | ||||||
|
2.04,29.33% | ||||||
| Shi et al. [151] | USA | Mixed | 118 |
|
4.29 | NA | NA |
|
2,5 | ||||||
|
28,18 | ||||||
| Atalar et al. [152] | Turkey | Mixed | 73 |
|
0,3 | 3 thrombosis | |
|
0,7 | ||||||
|
12 | ||||||
| Shah et al. [153] | USA | Mixed | 20 (Hospitalized COVID-19 patients with a thromboembolic event) |
|
1,10 | NA | |
|
1 | ||||||
| Bertin et al. [154] | France | Mixed | 157 |
|
41,13 | NA | 8 Thromboembolic events |
|
6,10 | ||||||
|
25,6 | ||||||
|
1,17 | ||||||
| Espinosa et al. [155] | Spain | Mixed | 158 for first sample,58 for second sample |
|
11,5 | 5,10 | 27 PE, 1 CVA |
|
6,2 | 5,4 | |||||
|
24 | 17 | |||||
|
3,9 | 1,7 | |||||
| Rosales-Castillo et al. [156] | Granada | Mixed | 189 for first sample,69 for second sample |
|
3,7 | 2,6 | No thromboembolic event |
|
6,9 | 4,6 | |||||
|
24 | 10 |
In the largest meta-analysis of aPL in COVID-19, no association between aPL positivity and mortality was found [49]. A number of studies have described either an association between aPL and thrombosis in COVID-19, or an increased representation of aPL in COVID-19 patients with thrombotic complications including deep venous thrombosis, pulmonary embolism, stroke, or myocardial infarction [54,[90], [91], [92], [93], [94], [95], [96]]. By contrast, other studies, including the largest meta-analysis of aPL in COVID-19, have found no association with thrombosis [49,97]. Differences in aPL titres, persistence, and structural biology in COVID-19 compared to APS as described earlier may contribute to some of this variation.
One study found elevated markers of neutrophil function and NET formation in sera from 172 hospitalized patients with COVID-19 and high aPL titres [98]. In this study, IgG antibodies purified from COVID-1 patients with high aPL titres triggered NETosis in vitro, suggesting that aPL in some patients with COVID-19 may display biological activity, although their clinical relevance remains uncertain [86].
6. Pathophysiologic similarities between APS and COVID-19
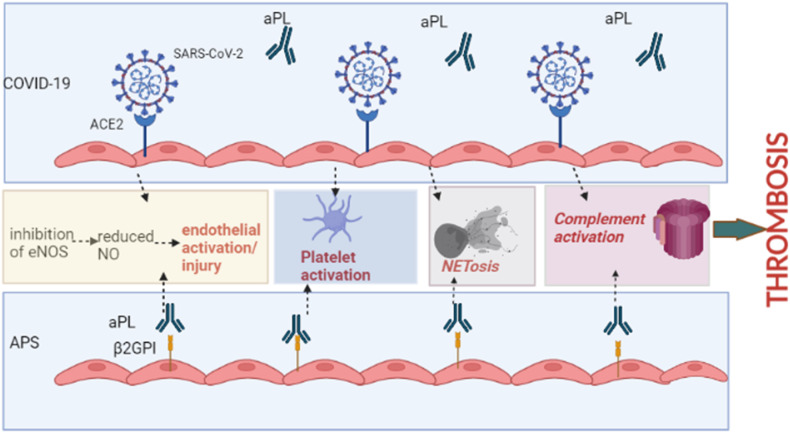
APS and the coagulopathy of COVID-19 share many similar mechanisms that are believed to drive microvascular injury and thrombosis [99] (Fig. 1 ). In both APS and COVID-19, the production of nitric oxide is reduced due to inhibition of endothelial nitric oxide synthase, predisposing the endothelium to injury [99]. Markers of endothelial activation and damage including von Willebrand factor, tissue-type plasminogen activator, and soluble thrombomodulin correlate with disease severity in COVID-19, with upregulated expression of angiogenesis genes in lung tissue, underscoring the importance of the endothelium in COVID-19, similar to APS [88,99,100]. Complement activation has a significant role in both APS and COVID-19. SARS-CoV-2 activates all three complement pathways; viral antigens form immune complexes that activate the classical pathway while the spike protein of SARS-CoV-2 binds mannose-binding lectin, activating the lectin pathway [99]. The alternative complement pathway on cell surfaces is triggered by F-spike proteins (subunit 1 and 2), a mechanism that can be blocked using a Factor D inhibitor [43]. C3 convertase production by binding of the pathogen to a component of the alternative pathway can also activate the alternative pathway [43]. Skin and lung samples as well as elevated membrane attack complex levels in sera of patients, delineate the activation of these pathways in COVID-19, similar to thrombotic APS [43,89,101].
Fig. 1.
Common mechanisms of thrombosis shared by antiphospholipid syndrome and COVID-19. Abbreviations: ACE2, angiotensin converting enzyme-2; aPL, antiphospholipid antibodye; β2GPI, beta-2 glycoprotein-I; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; NETosis, neutrophil extracellular trap formation and release. (Figure created using BioRender.com.)
NETosis may be a central part of the pathogenesis of both APS and COVID-19 coagulopathy. In COVID-19, hyperstimulation of the immune system leads to NET production and microvascular occlusion as evidenced by myeloperoxidase-DNA and citrullinated histone H3 complexes, similar to APS [102]. NETosis may also function in mediating acute lung injury in COVID-19 [103].
Platelet activation is a hallmark of both APS and COVID-19 thrombosis. COVID-19 infection alters platelet transcriptosomes and leads to aggregate complexes of platelets with neutrophils, monocytes, and lymphocytes and platelet-monocyte aggregates in severe COVID-19 express tissue factor [5,104]. Moreover, sera from COVID-19 patients has been shown to lead to increased platelet apoptosis via IgG-mediated mechanisms [105]. Activated platelets in COVID-19 also express S100A8/S100A9 (MRP8/MRP14, calprotectin), correlating with markers of endothelial cell activation [106]. Hence, through various mechanisms platelet activation leads to increased thrombosis in COVID-19, similar to APS.
7. COVID-19 vaccination in APS
Questions regarding the safety and efficacy of COVID vaccines in patients with aPL or APS have been frequently been raised, with a few studies reporting overall favorable tolerance and minimal complications [107]. One multicenter Italian survey study evaluated 161 patients with triple positive APS who received either the Moderna or the Pfizer-BioNTech COVID-19 vaccine [107]. Following the first vaccine dose, 83% experienced either no adverse reaction or minimal local signs/symptoms at the site of injection, while 12% had flu-like symptoms for less than 1 day and 4% for more than 1 day; 1% sought medical care, and no patients required hospitalization. Following the second dose, 68% had a minimal local reaction at the injection site; 22% had flu-like symptoms for less than 1 day and 8% for more than 1 day, while 2% sought medical care. One patient developed deep venous thrombosis 39 days after receiving the second dose. No patients required hospitalization or developed a severe allergic reaction after either dose [107].
A separate single-institution Italian survey study evaluated 102 patients who received either the Moderna or Pfizer-BioNTech COVID-19 vaccine, included 52 patients with APS and 50 with aPL and no clinical APS features [108]. Of the total study patients, 76% experienced injection-site pain, fatigue, or headache; all reported symptoms were transient and resolved within 10 days. Overall, 71% of patients classified their symptoms as mild and 29% as moderate. One patient with thrombotic APS and chronic thrombocytopenia on long-term vitamin K antagonist therapy experienced self-limiting purpuric lesions on her calves 10 days after the second vaccine dose. Together, these two studies suggest that adverse events following COVID-19 vaccination with either the Moderna or Pfizer-BioNTech vaccine in patients with aPL or APS are mostly mild and self-limited [108].
A rare complication of COVID-19 vaccination is vaccine-induced thrombotic thrombocytopenia (VITT), which may arise within several weeks following vaccination with adenovirus vector-based formulations such as ChAdOx1 nCoV-19 (AstraZeneca) or Ad26.COV2.S (Johnson & Johnson/Janssen) [109]. VITT arises as a result of antibodies against platelet factor 4 (PF4), similar to heparin-induced thrombocytopenia (HIT). Parallels among VITT, HIT, and APS have often been described, as all three are antibody-mediated processes associated with thromboembolic manifestations. Patients with VITT or HIT may be positive for aPL, although the clinical significance of aPL in these conditions is uncertain [[110], [111], [112], [113]]. One study of 126 aPL-positive patients (89 with APS, 37 with asymptomatic aPL) who mostly received the Pfizer-BioNTech COVID-19 vaccine found anti-PF4 antibodies in 9 patients, with no significant change in anti-PF4 antibody titres before or after vaccination in either APS or asymptomatic aPL-positive patients [114]. Sera from patients with high-titre anti-PF4 antibodies did not alter in vitro platelet aggregation, and no cases of VITT were observed, even in the few study patients who received an adenovirus vector-based COVID-19 vaccine [114].
8. Conclusions and summary
APS and COVID-19 share many pathophysiologic features common in microvascular and immunothrombotic diseases, including endotheliopathy, platelet activation, complement activation, and NETosis, among others. Despite these shared features, a role for aPL in pathogenesis of COVID-19 remains uncertain. aPL occur at high prevalence in patients with COVID-19, with LA reported in ∼50% of patients with COVID-19 and non-Sapporo criteria aPL being common; however, studies suggest that aPL titres in COVID-19 are usually only transiently elevated, and overall aPL titres in COVID-19 appear to be lower than those reported in APS. Biological differences in aβ2GPI antibody epitopes in APS compared to COVID-19 may underlie some of the differences in pathogenicity of aPL in these two conditions. A correlation between aPL positivity and disease outcomes in COVID-19 such as thrombosis or mortality remains unclear with different studies reporting varying results. Further investigation is required to delineate the significance of aPLs in mediating disease severity, thrombosis, and other outcomes in COVID-19. COVID-19 vaccination has been established to be generally safe in APS patients.
Practice points
-
•
Antiphospholipid syndrome and COVID-19 share many pathophysiologic features common in immunothrombotic diseases
-
•
Antiphospholipid antibodies are common in COVID-19, yet their clinical relevance is uncertain
-
•
COVID-19 vaccination is generally safe in patients with antiphospholipid syndrome or antiphospholipid antibodies.
Research agenda
-
•
Further studies are needed to explore the biological similarities and differences of antiphospholipid antibodies in COVID-19 and antiphospholipid syndrome and to understand the clinical implications of antiphospholipid antibodies in COVID-19
Declaration of competing interest
None of the authors report any conflicts of interest.
References
- 1.Jiménez D., García-Sanchez A., Rali P., Muriel A., Bikdeli B., Ruiz-Artacho P., Le Mao R., Rodríguez C., Hunt B.J., Monreal M. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2021 Mar 1;159(3):1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nopp S., Moik F., Jilma B., Pabinger I., Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost. 2020 Oct 13;4(7):1178–1191. doi: 10.1002/rth2.12439. PMID: 33043231; PMCID: PMC7537137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfister F., Vonbrunn E., Ries T., Jäck H.M., Überla K., Lochnit G., Sheriff A., Herrmann M., Büttner-Herold M., Amann K., Daniel C. Complement activation in kidneys of patients with COVID-19. Front Immunol. 2021 Jan 29;11 doi: 10.3389/fimmu.2020.594849. PMID: 33584662; PMCID: PMC7878379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurence J., Mulvey J.J., Seshadri M., Racanelli A., Harp J., Schenck E.J., Zappetti D., Horn E.M., Magro C.M. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin Immunol. 2020 Oct;219 doi: 10.1016/j.clim.2020.108555. Epub 2020 Aug 6. PMID: 32771488; PMCID: PMC7410014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C., Petrey A.C., Tolley N.D., Guo L., Cody M., Weyrich A.S., Yost C.C., Rondina M.T., Campbell R.A. Platelet gene expression and function in patients with COVID-19. Blood. 2020 Sep 10;136(11):1317–1329. doi: 10.1182/blood.2020007214. PMID: 32573711; PMCID: PMC7483430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., Cody M.J., Manne B.K., Portier I., Harris E.S., Petrey A.C., Beswick E.J., Caulin A.F., Iovino A., Abegglen L.M., Weyrich A.S., Rondina M.T., Egeblad M., Schiffman J.D., Yost C.C. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020 Sep 3;136(10):1169–1179. doi: 10.1182/blood.2020007008. PMID: 32597954; PMCID: PMC7472714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma L., Sahu S.K., Cano M., Kuppuswamy V., Bajwa J., McPhatter J., Pine A., Meizlish M., Goshua G., Chang C.H., Zhang H., Price C., Bahel P., Rinder H., Lei T., Day A., Reynolds D., Wu X., Schriefer R., Rauseo A.M., Goss C.W., O'Halloran J.A., Presti R.M., Kim A.H., Gelman A.E., Cruz C.D., Lee A.I., Mudd P., Chun H.J., Atkinson J.P., Kulkarni H.S. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. bioRxiv [Preprint] 2021 Feb 23 doi: 10.1101/2021.02.22.432177. 2021.02.22.432177, Update in: Sci Immunol. 2021 May 13;6(59): PMID: 33655244; PMCID: PMC7924264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meizlish M.L., Pine A.B., Bishai J.D., Goshua G., Nadelmann E.R., Simonov M., Chang C.H., Zhang H., Shallow M., Bahel P., Owusu K., Yamamoto Y., Arora T., Atri D.S., Patel A., Gbyli R., Kwan J., Won C.H., Dela Cruz C., Price C., Koff J., King B.A., Rinder H.M., Wilson F.P., Hwa J., Halene S., Damsky W., van Dijk D., Lee A.I., Chun H.J. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021 Mar 9;5(5):1164–1177. doi: 10.1182/bloodadvances.2020003568. PMID: 33635335; PMCID: PMC7908851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goshua G., Butt A., Lee A.I. Immunothrombosis: a COVID-19 concerto. Br J Haematol. 2021 Aug;194(3):491–493. doi: 10.1111/bjh.17666.PMID:34114208. [DOI] [PubMed] [Google Scholar]
- 10.Chun H.J., Coutavas E., Pine A.B., Lee A.I., Yu V.L., Shallow M.K., Giovacchini C.X., Mathews A.M., Stephenson B., Que L.G., Lee P.J., Kraft B.D. Immunofibrotic drivers of impaired lung function in postacute sequelae of SARS-CoV-2 infection. JCI Insight. 2021 Jul 22;6(14) doi: 10.1172/jci.insight.148476. PMID: 34111030; PMCID: PMC8410030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw R.J., Bradbury C., Abrams S.T., Wang G., Toh C.H. COVID-19 and immunothrombosis: emerging understanding and clinical management. Br J Haematol. 2021 Aug;194(3):518–529. doi: 10.1111/bjh.17664. Epub 2021 Jul 7. PMID: 34114204. [DOI] [PubMed] [Google Scholar]
- 12.Gu S.X., Tyagi T., Jain K., Gu V.W., Lee S.H., Hwa J.M., Kwan J.M., Krause D.S., Lee A.I., Halene S., Martin K.A., Chun H.J., Hwa J. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021 Mar;18(3):194–209. doi: 10.1038/s41569-020-00469-1. Epub 2020 Nov 19. PMID: 33214651; PMCID: PMC7675396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerber G.F., Chaturvedi S. How to recognize and manage COVID-19-associated coagulopathy. Hematology Am Soc Hematol Educ Program. 2021 Dec 10;2021(1):614–620. doi: 10.1182/hematology.2021000297. PMID: 34889412; PMCID: PMC8791093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaumenhaft R., Enjyoji K., Schmaier A.A. Vasculopathy in COVID-19. Blood. 2022 Jul 21;140(3):222–235. doi: 10.1182/blood.2021012250. PMID: 34986238; PMCID: PMC8736280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonaventura A., Vecchié A., Dagna L., et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limper M., Scirè C.A., Talarico R., Amoura Z., Avcin T., Basile M., Burmester G., Carli L., Cervera R., Costedoat-Chalumeau N., Doria A., Dörner T., Fonseca J.E., Galetti I., Hachulla E., Launay D., Lourenco F., Macieira C., Meroni P., Montecucco C.M., Moraes-Fontes M.F., Mouthon L., Nalli C., Ramoni V., Tektonidou M., van Laar J.M., Bombardieri S., Schneider M., Smith V., Vieira A., Cutolo M., Mosca M., Tincani A. Antiphospholipid syndrome: state of the art on clinical practice guidelines. RMD Open. 2018 Oct 18;4(Suppl 1) doi: 10.1136/rmdopen-2018-000785. PMID: 30402272; PMCID: PMC6203101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negrini S., Pappalardo F., Murdaca G., Indiveri F., Puppo F. The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med. 2017 Aug;17(3):257–267. doi: 10.1007/s10238-016-0430-5. Epub 2016 Jun 22. PMID: 27334977. [DOI] [PubMed] [Google Scholar]
- 18.Miyakis S., Lockshin M.D., Atsumi T., Branch D.W., Brey R.L., Cervera R., Derksen R.H., De Groot P.G., Koike T., Meroni P.L., Reber G., Shoenfeld Y., Tincani A., Vlachoyiannopoulos P.G., Krilis S.A. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemostasis. 2006 Feb;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. PMID: 16420554. [DOI] [PubMed] [Google Scholar]
- 19.Sciascia S., Sanna G., Murru V., Roccatello D., Khamashta M.A., Bertolaccini M.L. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. A systematic review. Thromb Haemostasis. 2014 Feb;111(2):354–364. doi: 10.1160/TH13-06-0509. Epub 2013 Oct 31. PMID: 24172938. [DOI] [PubMed] [Google Scholar]
- 20.Rasool Z.S., Tiwari V. StatPearls. StatPearls Publishing; Treasure Island (FL): 2022 Jan. Biochemistry, lupus anticoagulant.https://www.ncbi.nlm.nih.gov/books/NBK544357/ [Updated 2022 Jul 18] [Internet] Available from: [PubMed] [Google Scholar]
- 21.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foret T., Dufrost V., Salomon Du Mont L., et al. Systematic review of antiphospholipid antibodies in COVID-19 patients: culprits or bystanders? Curr Rheumatol Rep. 2021;23(8):65. doi: 10.1007/s11926-021-01029-3. Published 2021 Jul 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amengual O., Atsumi T., Khamashta M., Hughes G. The role of the tissue factor pathway in the hypercoagulable state in patients with the antiphospholipid syndrome. Thromb Haemostasis. 1998;79:276–281. [PubMed] [Google Scholar]
- 24.Edgington T.S., Mackman N., Brand K., Ruf W. The structural biology of expression and function of tissue factor. Thromb Haemostasis. 1991;66:67–79. [PubMed] [Google Scholar]
- 25.Müller-Calleja N., Hollerbach A., Ritter S., Pedrosa D.G., Strand D., Graf C., Reinhardt C., Strand S., Poncelet P., Griffin J.H., Lackner K.J., Ruf W. Tissue factor pathway inhibitor primes monocytes for antiphospholipid antibody-induced thrombosis. Blood. 2019 Oct 3;134(14):1119–1131. doi: 10.1182/blood.2019001530. Epub 2019 Aug 21. PMID: 31434703; PMCID: PMC6776793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harper B.E., Wills R., Pierangeli S.S. Pathophysiological mechanisms in antiphospholipid syndrome. Int J Clin Rheumatol. 2011 Apr 1;6(2):157–171. doi: 10.2217/ijr.11.9. PMID: 23487578; PMCID: PMC3593246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sacharidou A., Chambliss K.L., Ulrich V., Salmon J.E., Shen Y.M., Herz J., Hui D.Y., Terada L.S., Shaul P.W., Mineo C. Antiphospholipid antibodies induce thrombosis by PP2A activation via apoER2-Dab2-SHC1 complex formation in endothelium. Blood. 2018 May 10;131(19):2097–2110. doi: 10.1182/blood-2017-11-814681. Epub 2018 Mar 2. PMID: 29500169; PMCID: PMC5946764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierangeli S., Chen P., Raschi E., et al. Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. Semin Thromb Hemost. 2008;34:236–250. doi: 10.1055/s-0028-1082267. [DOI] [PubMed] [Google Scholar]
- 29.Mineo C. Inhibition of nitric oxide and antiphospholipid antibody-mediated thrombosis. Curr Rheumatol Rep. 2013 May;15(5):324. doi: 10.1007/s11926-013-0324-4. PMID: 23519891; PMCID: PMC3625922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prinz N., Clemens N., Strand D., Pütz I., Lorenz M., Daiber A., Stein P., Degreif A., Radsak M., Schild H., Bauer S., von Landenberg P., Lackner K.J. Antiphospholipid antibodies induce translocation of TLR7 and TLR8 to the endosome in human monocytes and plasmacytoid dendritic cells. Blood. 2011 Aug 25;118(8):2322–2332. doi: 10.1182/blood-2011-01-330639. Epub 2011 Jul 6. PMID: 21734241. [DOI] [PubMed] [Google Scholar]
- 31.Tung M.L., Tan B., Cherian R., Chandra B. Anti-phospholipid syndrome and COVID-19 thrombosis: connecting the dots. Rheumatol Adv Pract. 2021 Feb 4;5(1) doi: 10.1093/rap/rkaa081. PMID: 33615129; PMCID: PMC7882149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber R., Berendes R., Burger A., Luecke H., Karshikov A. Annexin V-crystal structure and its implications on function. Behring Inst Mitt. 1992;91:107–125. [PubMed] [Google Scholar]
- 33.Vega-Ostertag M.E., Pierangeli S.S. Mechanisms of aPL-mediated thrombosis: effects of aPL on endothelium and platelets. Curr Rheumatol Rep. 2007;9:190–197. doi: 10.1007/s11926-007-0031-0. [DOI] [PubMed] [Google Scholar]
- 34.Baroni G., Banzato A., Bison E., Denas G., Zoppellaro G., Pengo V. The role of platelets in antiphospholipid syndrome. Platelets. 2017 Dec;28(8):762–766. doi: 10.1080/09537104.2017.1280150. Epub 2017 Mar 7. PMID: 28267395. [DOI] [PubMed] [Google Scholar]
- 35.Romay-Penabad Z., Aguilar-Valenzuela R., Urbanus R.T., Derksen R.H., Pennings M.T., Papalardo E., Shilagard T., Vargas G., Hwang Y., de Groot P.G., et al. Apolipoprotein E receptor 2 is involved in the DOI: 10.1080/09537104.2017.1280150 Platelets in antiphospholipid syndrome 765 thrombotic complications in a murine model of the antiphospholipid syndrome. Blood. 2011;117(4):1408–1414. doi: 10.1182/blood-2010-07-299099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Proulle V., Furie R.A., Merrill-Skoloff G., Furie B.C., Furie B. Platelets are required for enhanced activation of the endothelium and fibrinogen in a mouse thrombosis model of APS. Blood. 2014;124(4):611–622. doi: 10.1182/blood-2014-02-554980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bontadi A., Ruffatti A., Giannini S., Falcinelli E., Tonello M., Hoxha A., Gresele P., Punzi L. In vitro effect of anti-beta(2) glycoprotein I antibodies on P-selectin expression, a marker of platelet activation. Reumatismo. 2012;64(1):35–39. doi: 10.4081/reumatismo.2012.35. [DOI] [PubMed] [Google Scholar]
- 38.Chaturvedi S., Braunstein E.M., Brodsky R.A. Antiphospholipid syndrome: complement activation, complement gene mutations, and therapeutic implications. J Thromb Haemostasis. 2021 Mar;19(3):607–616. doi: 10.1111/jth.15082. Epub 2021 Feb 10. PMID: 32881236; PMCID: PMC8080439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Girardi G., Berman J., Redecha P., Spruce L., Thurman J.M., Kraus D., Hollmann T.J., Casali P., Caroll M.C., Wetsel R.A., Lambris J.D., Holers V.M., Salmon J.E. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003 Dec;112(11):1644–1654. doi: 10.1172/JCI18817. Erratum in: J Clin Invest. 2004 Feb;113(4):646. PMID: 14660741; PMCID: PMC281643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holers V.M., Girardi G., Mo L., Guthridge J.M., Molina H., Pierangeli S.S., Espinola R., Xiaowei L.E., Mao D., Vialpando C.G., Salmon J.E. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002 Jan 21;195(2):211–220. doi: 10.1084/jem.200116116. PMID: 11805148; PMCID: PMC2193604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischetti F., Durigutto P., Pellis V., Debeus A., Macor P., Bulla R., Bossi F., Ziller F., Sblattero D., Meroni P., Tedesco F. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005 Oct 1;106(7):2340–2346. doi: 10.1182/blood-2005-03-1319. Epub 2005 Jun 14. PMID: 15956288. [DOI] [PubMed] [Google Scholar]
- 42.Chaturvedi S., Braunstein E.M., Yuan X., Yu J., Alexander A., Chen H., Gavriilaki E., Alluri R., Streiff M.B., Petri M., Crowther M.A., McCrae K.R., Brodsky R.A. Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood. 2020 Jan 23;135(4):239–251. doi: 10.1182/blood.2019003863. PMID: 31812994; PMCID: PMC6978159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu J., Yuan X., Chen H., et al. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boeltz S., Amini P., Anders H.J., Andrade F., Bilyy R., Chatfield S., et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019;26(3):395–408. doi: 10.1038/s41418-018-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng H., Yalavarthi S., Kanthi Y., Mazza L.F., Elfline M.A., Luke C.E., Pinsky D.J., Henke P.K., Knight J.S. In vivo role of neutrophil extracellular traps in antiphospholipid antibody-mediated venous thrombosis. Arthritis Rheumatol. 2017 Mar;69(3):655–667. doi: 10.1002/art.39938. PMID: 27696751; PMCID: PMC5329054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali R.A., Gandhi A.A., Meng H., Yalavarthi S., Vreede A.P., Estes S.K., Palmer O.R., Bockenstedt P.L., Pinsky D.J., Greve J.M., Diaz J.A., Kanthi Y., Knight J.S. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun. 2019 Apr 23;10(1):1916. doi: 10.1038/s41467-019-09801-x. PMID: 31015489; PMCID: PMC6478874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asherson R.A. Cervera R Antiphospholipid antibodies and infections. Ann Rheum Dis. 2003;62:388–393. doi: 10.1136/ard.62.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdel-Wahab N., Talathi S., Lopez-Olivo M.A., Suarez-Almazor M.E. Risk of developing antiphospholipid antibodies following viral infection: a systematic review and meta-analysis. Lupus. 2018;27(4):572–583. doi: 10.1177/0961203317731532. [DOI] [PubMed] [Google Scholar]
- 49.Taha M., Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7(2) doi: 10.1136/rmdopen-2021-001580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uthman I.W., Gharavi A.E. Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31:256–263. doi: 10.1053/sarh.2002.28303. [DOI] [PubMed] [Google Scholar]
- 51.Abdel-Wahab N., Talathi S., Lopez-Olivo M.A., et al. Risk of developing antiphospholipid antibodies following viral infection: a systematic review and meta-analysis. Lupus. 2018;27:572–583. doi: 10.1177/0961203317731532. [DOI] [PubMed] [Google Scholar]
- 52.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Anglés-Cano E., Sattler L., Mertes P.M., Meziani F. CRICS TRIGGERSEP group (clinical research in intensive care and sepsis trial group for global evaluation and research in sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 Jun;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. Epub 2020 May 4. PMID: 32367170; PMCID: PMC7197634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reyes Gil M., Barouqa M., Szymanski J., Gonzalez-Lugo J.D., Rahman S., Billett H.H. Assessment of lupus anticoagulant positivity in patients with coronavirus disease 2019 (COVID-19) JAMA Netw Open. 2020 Aug 3;3(8) doi: 10.1001/jamanetworkopen.2020.17539. PMID: 32785632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Joncour A., Frere C., Martin-Toutain I., Gougis P., Ghillani-Dalbin P., Maalouf G., Vieira M., Marcelin A.G., Salem J.E., Allenbach Y., Saadoun D., Benveniste O., Cacoub P. Antiphospholipid antibodies and thrombotic events in COVID-19 patients hospitalized in medicine ward. Autoimmun Rev. 2021 Feb;20(2) doi: 10.1016/j.autrev.2020.102729. Epub 2020 Dec 13. PMID: 33321245; PMCID: PMC7834187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan S., Xiao M., Han F., Xia P., Bai X., Chen H., Zhang H., Ding X., Zhao H., Zhao J., Sun X., Jiang W., Wang C., Cao W., Guo F., Tian R., Gao P., Wu W., Ma J., Wu D., Liu Z., Zhou X., Wang J., Guan T., Qin Y., Li T., Xu Y., Zhang D., Chen Y., Xie J., Li Y., Yan X., Zhu Y., Peng B., Cui L., Zhang S., Guan H. Neurological manifestations in critically ill patients with COVID-19: a retrospective study. Front Neurol. 2020 Jul 10;11:806. doi: 10.3389/fneur.2020.00806. PMID: 32754114; PMCID: PMC7365850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rothstein A., Oldridge O., Schwennesen H., Do D., Cucchiara B.L. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. 2020 Sep;51(9):e219–e222. doi: 10.1161/STROKEAHA.120.030995. Epub 2020 Jul 20. PMID: 32684145; PMCID: PMC7386677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao M., Zhang Y., Zhang S., Qin X., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H., Wang C., Zhao J., Sun X., Tian R., Wu W., Wu D., Ma J., Chen Y., Zhang D., Xie J., Yan X., Zhou X., Liu Z., Wang J., Du B., Qin Y., Gao P., Lu M., Hou X., Wu X., Zhu H., Xu Y., Zhang W., Li T., Zhang F., Zhao Y., Li Y., Zhang S. Antiphospholipid antibodies in critically ill patients with COVID-19. Arthritis Rheumatol. 2020 Dec;72(12):1998–2004. doi: 10.1002/art.41425. Epub 2020 Oct 7. PMID: 32602200; PMCID: PMC7361932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gazzaruso C., Mariani G., Ravetto C., Malinverni L., Tondelli E., Cerrone M., Sala V., Bevilacqua L., Altavilla T., Coppola A., Gallotti P. Lupus anticoagulant and mortality in patients hospitalized for COVID-19. J Thromb Thrombolysis. 2021 Jul;52(1):85–91. doi: 10.1007/s11239-020-02335-w. Epub 2020 Nov 7. PMID: 33159639; PMCID: PMC7648549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serrano M., Espinosa G., Lalueza A., Bravo-Gallego L.Y., Diaz-Simón R., Garcinuño S., Gil-Etayo J., Moises J., Naranjo L., Prieto-González S., Ruiz-Ortiz E., Sánchez B., Moreno-Castaño A.B., Díaz-Pedroche C., Viñas-Gomis O., Cervera R., Serrano A. APS-COVID 19 study group/European forum on antiphospholipid antibodies. Beta-2-Glycoprotein-I deficiency could precipitate an antiphospholipid syndrome-like prothrombotic situation in patients with coronavirus disease 2019. ACR Open Rheumatol. 2021 Apr;3(4):267–276. doi: 10.1002/acr2.11245. Epub 2021 Mar 19. PMID: 33738987; PMCID: PMC8063141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gendron N., Dragon-Durey M.A., Chocron R., Darnige L., Jourdi G., Philippe A., Chenevier-Gobeaux C., Hadjadj J., Duchemin J., Khider L., Yatim N., Goudot G., Krzisch D., Debuc B., Mauge L., Levavasseur F., Pene F., Boussier J., Sourdeau E., Brichet J., Ochat N., Goulvestre C., Peronino C., Szwebel T.A., Pages F., Gaussem P., Samama C.M., Cheurfa C., Planquette B., Sanchez O., Diehl J.L., Mirault T., Fontenay M., Terrier B., Smadja D.M. Lupus anticoagulant single positivity during the acute phase of COVID-19 is not associated with venous thromboembolism or in-hospital mortality. Arthritis Rheumatol. 2021 Nov;73(11):1976–1985. doi: 10.1002/art.41777. Epub 2021 Sep 22. PMID: 33881229; PMCID: PMC8250965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Favaloro E.J., Henry B.M., Lippi G. Is lupus anticoagulant a significant feature of COVID-19? A critical appraisal of the literature. Semin Thromb Hemost. 2022 Feb;48(1):55–71. doi: 10.1055/s-0041-1729856. Epub 2021 Jun 15. PMID: 34130341. [DOI] [PubMed] [Google Scholar]
- 62.Vassalo J., Spector N., Meis Ed, Soares M., Salluh J.I. Antiphospholipid antibodies in critically ill patients. Rev Bras Ter Intensiva. 2014 Apr-Jun;26(2):176–182. doi: 10.5935/0103-507x.20140026. PMID: 25028953; PMCID: PMC4103945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galli M., Luciani D., Bertolini G., et al. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood. 2003;101:1827–1832. doi: 10.1182/blood-2002-02-0441. [DOI] [PubMed] [Google Scholar]
- 64.Keeling D., Mackie I., Moore G.W., et al. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157:47–58. doi: 10.1111/j.1365-2141.2012.09037.x. [DOI] [PubMed] [Google Scholar]
- 65.Gatto M., Perricone C., Tonello M., Bistoni O., Cattelan A.M., Bursi R., et al. Frequency and clinical correlates of antiphospholipid antibodies arising in patients with SARS-CoV-2 infection: findings from a multicentre study on 122 cases. Clin Exp Rheumatol. 2020;38:754–759. [PubMed] [Google Scholar]
- 66.Argañaraz G.A., Palmeira J.D.F., Argañaraz E.R. Phosphatidylserine inside out: a possible underlying mechanism in the inflammation and coagulation abnormalities of COVID-19. Cell Commun Signal. 2020 Dec 27;18(1):190. doi: 10.1186/s12964-020-00687-7. PMID: 33357215; PMCID: PMC7765775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bosch B.J., van der Zee R., de Haan C.A.M., Rottier P.J.M. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Beltagi M., Saeed N.K., Bediwy A.S. COVID-19 disease and autoimmune disorders: a mutual pathway. World J Methodol. 2022 Jul 20;12(4):200–223. doi: 10.5662/wjm.v12.i4.200. PMID: 36159097; PMCID: PMC9350728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delgado-Roche L., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.López-Pedrera C., Barbarroja N., Jimenez-Gomez Y., et al. Oxidative stress in the pathogenesis of atherothrombosis associated with anti-phospholipid syndrome and systemic lupus erythematosus: new therapeutic approaches. Rheumatology. 2016;55:2096–2108. doi: 10.1093/rheumatology/kew054. [DOI] [PubMed] [Google Scholar]
- 72.Giannakopoulos B., Krilis S.A. The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368 doi: 10.1056/NEJMra1112830. 1033–44. [DOI] [PubMed] [Google Scholar]
- 73.Devreese K.M.J., Linskens E.A., Benoit D., et al. Antiphospholipid antibodies in patients with COVID-19: a relevant observation? J Thromb Haemostasis. 2020;18:2191–2201. doi: 10.1111/jth.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vollmer O., Tacquard C., Dieudonné Y., Nespola B., Sattler L., Grunebaum L., et al. Follow-up of COVID-19 patients: LA is transient but other aPLs are persistent. Autoimmun Rev. 2021;20 doi: 10.1016/j.autrev.2021.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiao M., Zhang Y., Zhang S. Brief report: anti‐phospholipid antibodies in critically ill patients with coronavirus disease 2019 (COVID‐19) Arthritis Rheumatol. 2020;72:1998–2004. doi: 10.1002/art.41425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Durigutto P., Grossi C., Borghi M.O., Macor P., Pregnolato F., Raschi E., Myers M.P., de Groot P.G., Meroni P.L., Tedesco F. New insight into antiphospholipid syndrome: antibodies to β2glycoprotein I-domain 5 fail to induce thrombi in rats. Haematologica. 2019 Apr;104(4):819–826. doi: 10.3324/haematol.2018.198119. Epub 2018 Nov 15. PMID: 30442725; PMCID: PMC6442945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andreoli L., Chighizola C.B., Nalli C., et al. Clinical characterization of antiphospholipid syndrome by detection of IgG antibodies against β 2 -glycoprotein I domain 1 and domain 4/5: ratio of anti-domain 1 to anti-domain 4/5 as a useful new biomarker for antiphospholipid syndrome. Arthritis Rheumatol. 2015;67:2196–2204. doi: 10.1002/art.39187. [DOI] [PubMed] [Google Scholar]
- 78.Liu T., Gu J., Wan L. Anti- β 2GPI domain 1 antibodies stratify high risk of thrombosis and late pregnancy morbidity in a large cohort of Chinese patients with antiphospholipid syndrome. Thromb Res. 2020;Jan;185:142–149. doi: 10.1016/j.thromres.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 79.Xiao M., Zhang Y., Zhang S., Qin X., Xia P., Cao W., et al. Antiphospholipid antibodies in critically ill patients with COVID-19. Arthritis Rheum. 2020;72:1998–2004. doi: 10.1002/art.41425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bertin D., Brodovitch A., Beziane A., Hug S., Bouamri A., Mege J.L., et al. Anticardiolipin IgG autoantibody level is an independent risk factor for COVID-19 severity. Arthritis Rheum. 2020;72 doi: 10.1002/art.41409. 1953 – 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karahan S., Erol K., Yuksel R.C., Artan C., Celik I. Antiphospholipid antibodies in COVID-19-associated pneumonia patients in intensive care unit. Mod Rheumatol. 2021:1–10. doi: 10.1080/14397595.2021.1892257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gazzaruso C., Mariani G., Ravetto C., Malinverni L., Tondelli E., Cerrone M., et al. Lupus anticoagulant and mortality in patients hospitalized for COVID-19. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02335-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferrari E., Sartre B., Squara F., et al. High prevalence of acquired thrombophilia without prognosis value in patients with coronavirus disease 2019. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serrano M., Espinosa G., Lalueza A., Bravo-Gallego L.Y., DiazSimón R., Garcinuño S., et al. Beta-2-Glycoprotein-I deficiency could precipitate an antiphospholipid syndrome-like prothrombotic situation in patients with coronavirus disease 2019. ACR Open Rheumatol. 2021;3:267–276. doi: 10.1002/acr2.11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Constans M., Santiago R., Jimenez L., et al. Lupus anticoagulant is an independent risk factor for non-thrombotic in-hospital mortality in COVID-19 patients. Thromb Res. 2021;208:99–105. doi: 10.1016/j.thromres.2021.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gkrouzman E., Barbhaiya M., Erkan D., Lockshin M.D. Reality check on antiphospholipid antibodies in COVID-19-associated coagulopathy. Arthritis Rheumatol. 2021 Jan;73(1):173–174. doi: 10.1002/art.41472. Epub 2020 Dec 5. PMID: 32901454. [DOI] [PubMed] [Google Scholar]
- 87.Gendron N., Dragon-Durey M.-A., Chocron R., Darnige L., Jourdi G., Philippe A., et al. Lupus anticoagulant single positivity at acute phase is not associated with venous thromboembolism or inhospital mortality in COVID-19. Arthritis Rheum. 2021 doi: 10.1002/art.41777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020 Jul 9;383(2):120–128. doi: 10.1056/NEJMoa2015432. Epub 2020 May 21. PMID: 32437596; PMCID: PMC7412750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of fi ve cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reyes Gil M., Barouqa M., Szymanski J., Gonzalez-Lugo J.D., Rahman S., Billett H.H. Assessment of lupus anticoagulant positivity in patients with coronavirus disease 2019 (COVID-19) JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.17539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le Joncour A., Frere C., Martin-Toutain I., et al. Antiphospholipid antibodies and thrombotic events in COVID-19 patients hospitalized in medicine ward. Autoimmun Rev. 2021;20 doi: 10.1016/j.autrev.2020.102729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fan S., Xiao M., Han F., Xia P., Bai X., Chen H., et al. Neurological manifestations in critically Ill patients with COVID-19: a retrospective study. Front Neurol. 2020;11:806. doi: 10.3389/fneur.2020.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rothstein A., Oldridge O., Schwennesen H., Do D., Cucchiara B.L. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. 2020;51:e219–e222. doi: 10.1161/STROKEAHA.120.030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Popovic B., Varlot J., Metzdorf P.A., Jeulin H., Goehringer F., Camenzind E. Changes in characteristics and management among patients with ST-elevation myocardial infarction due to COVID-19 infection. Cathet Cardiovasc Interv. 2020;97:E319–E326. doi: 10.1002/ccd.29114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gil-Etayo F.J., Garcinuño S., Lalueza A., et al. Anti-phospholipid antibodies and COVID-19 thrombosis: a Co-star, not a supporting actor. Biomedicines. 2021;9(8):899. doi: 10.3390/biomedicines9080899. Published 2021 Jul 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Espinola R.G., Pierangeli S.S., Ghara A.E., Harris E.N. Hydroxychloroquine reverses platelet activation induced by human IgG antiphospholipid antibodies. Thromb Haemostasis. 2002;87:518–522. [PubMed] [Google Scholar]
- 98.Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H., Sule G., Gockman K., Madison J.A., Zuo M., Yadav V., Wang J., Woodard W., Lezak S.P., Lugogo N.L., Smith S.A., Morrissey J.H., Kanthi Y., Knight J.S. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020 Nov 18;12(570) doi: 10.1126/scitranslmed.abd3876. Epub 2020 Nov 2. PMID: 33139519; PMCID: PMC7724273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang X., Gkrouzman E., Andrade D.C.O., Andreoli L., Barbhaiya M., Belmont H.M., Branch D.W., de Jesús G.R., Efthymiou M., Ríos-Garcés R., Gerosa M., El Hasbani G., Knight J., Meroni P.L., Pazzola G., Petri M., Rand J., Salmon J., Tektonidou M., Tincani A., Uthman I.W., Zuily S., Zuo Y., Lockshin M., Cohen H., Erkan D. Aps action. COVID-19 and antiphospholipid antibodies: a position statement and management guidance from AntiPhospholipid syndrome alliance for clinical trials and InternatiOnal networking (APS action) Lupus. 2021 Dec;30(14):2276–2285. doi: 10.1177/09612033211062523. Epub 2021 Dec 16. PMID: 34915764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J., Dela Cruz C.S., Dumont A., Halene S., Hwa J., Koff J., Menninger H., Neparidze N., Price C., Siner J.M., Tormey C., Rinder H.M., Chun H.J., Lee A.I. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020 Aug;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. Epub 2020 Jun 30. PMID: 32619411; PMCID: PMC7326446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cugno M., Meroni P.L., Gualtierotti R., et al. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J Autoimmun. 2021;116 doi: 10.1016/j.jaut.2020.102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M., Woods R.J., Kanthi Y., Knight J.S. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020 Jun 4;5(11) doi: 10.1172/jci.insight.138999. PMID: 32329756; PMCID: PMC7308057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu Y., Chen X., Liu X. NETosis and neutrophil extracellular traps in COVID-19: immunothrombosis and beyond. Front Immunol. 2022 Mar 2;13 doi: 10.3389/fimmu.2022.838011. PMID: 35309344; PMCID: PMC8924116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pão C.R.R., Righy C., Franco S., Souza T.M.L., Kurtz P., Bozza F.A., Bozza P.T. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020 Sep 10;136(11):1330–1341. doi: 10.1182/blood.2020007252. PMID: 32678428; PMCID: PMC7483437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Althaus K., Marini I., Zlamal J., Pelzl L., Singh A., Häberle H., Mehrländer M., Hammer S., Schulze H., Bitzer M., Malek N., Rath D., Bösmüller H., Nieswandt B., Gawaz M., Bakchoul T., Rosenberger P. Antibody-induced procoagulant platelets in severe COVID-19 infection. Blood. 2021 Feb 25;137(8):1061–1071. doi: 10.1182/blood.2020008762. PMID: 33512415; PMCID: PMC7791311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barrett T.J., Cornwell M., Myndzar K., Rolling C.C., Xia Y., Drenkova K., Biebuyck A., Fields A.T., Tawil M., Luttrell-Williams E., Yuriditsky E., Smith G., Cotzia P., Neal M.D., Kornblith L.Z., Pittaluga S., Rapkiewicz A.V., Burgess H.M., Mohr I., Stapleford K.A., Voora D., Ruggles K., Hochman J., Berger J.S. Platelets amplify endotheliopathy in COVID-19. Sci Adv. 2021 Sep 10;7(37) doi: 10.1126/sciadv.abh2434. Epub 2021 Sep 8. PMID: 34516880; PMCID: PMC8442885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pengo V., Del Ross T., Tonello M., et al. Impact of COVID-19 and COVID-19 vaccination on high-risk patients with Antiphospholipid Syndrome: a nationwide survey. Rheumatology. 2022 doi: 10.1093/rheumatology/keac224. [published online ahead of print, 2022 Apr 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sciascia S., Costanzo P., Radin M., et al. Safety and tolerability of mRNA COVID-19 vaccines in people with antiphospholipid antibodies. Lancet Rheumatol. 2021;3(12) doi: 10.1016/S2665-9913(21)00320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klok F.A., Pai M., Huisman M.V., Makris M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022 Jan;9(1):e73–e80. doi: 10.1016/S2352-3026(21)00306-9. Epub 2021 Nov 11. PMID: 34774202; PMCID: PMC8585488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021 Jun 22:1–16. doi: 10.1007/s42399-021-00992-3. Epub ahead of print. PMID: 34179695; PMCID: PMC8218573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chittal A., Rao S., Lakra P., Nacu N., Haas C. A case of COVID-19 vaccine-induced thrombotic thrombocytopenia. J Community Hosp Intern Med Perspect. 2021 Nov 15;11(6):776–778. doi: 10.1080/20009666.2021.1980966. PMID: 34804389; PMCID: PMC8604444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.See I., Su J.R., Lale A., Woo E.J., Guh A.Y., Shimabukuro T.T., Streiff M.B., Rao A.K., Wheeler A.P., Beavers S.F., Durbin A.P., Edwards K., Miller E., Harrington T.A., Mba-Jonas A., Nair N., Nguyen D.T., Talaat K.R., Urrutia V.C., Walker S.C., Creech C.B., Clark T.A., DeStefano F., Broder K.R. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, march 2 to april 21, 2021. JAMA. 2021 Jun 22;325(24):2448–2456. doi: 10.1001/jama.2021.7517. PMID: 33929487; PMCID: PMC8087975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Y., Shao Z., Wang H. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. Thromb Res. 2022 Jan;209:75–79. doi: 10.1016/j.thromres.2021.12.002. Epub 2021 Dec 6. PMID: 34894531; PMCID: PMC8647389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lonati P.A., Bodio C., Scavone M., Martini G., Pesce E., Bandera A., Lombardi A., Gerosa M., Franceschini F., Tincani A., Podda G., Abrignani S., Grifantini R., Cattaneo M., Borghi M.O., Meroni P.L. Production of anti-PF4 antibodies in antiphospholipid antibody-positive patients is not affected by COVID-19 vaccination. RMD Open. 2022 Feb;8(1) doi: 10.1136/rmdopen-2021-001902. PMID: 35131751; PMCID: PMC8822540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pineton de Chambrun M., Frere C., Miyara M., Amoura Z., Martin-Toutain I., Mathian A., Hekimian G., Combes A. High frequency of antiphospholipid antibodies in critically ill COVID-19 patients: a link with hypercoagulability? J Intern Med. 2021 Mar;289(3):422–424. doi: 10.1111/joim.13126. Epub 2020 Jul 13. PMID: 32529774; PMCID: PMC7307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Amezcua-Guerra L.M., Rojas-Velasco G., Brianza-Padilla M., Vázquez-Rangel A., Márquez-Velasco R., Baranda-Tovar F., et al. Presence of antiphospholipid antibodies in COVID-19: case series study. Ann Rheum Dis. 2020;80:e73. doi: 10.1136/annrheumdis-2020-218100. [DOI] [PubMed] [Google Scholar]
- 117.Kmj D., Linskens E.A., Benoit D., Peperstraete H. Antiphospholipid antibodies in patients with COVID-19: a relevant observation? J Thromb Haemostasis. 2020;18:2191–2201. doi: 10.1111/jth.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Borghi M.O., Beltagy A., Garrafa E., Curreli D., Cecchini G., Bodio C., et al. Anti-phospholipid Antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.584241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Y., Cao W., Jiang W., Xiao M., Li Y., Tang N., et al. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J Thromb Thrombolysis. 2020;50:580–586. doi: 10.1007/s11239-020-02182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fan B.E., Ng J., Chan S.S.W., Christopher D., Tso A.C.Y., Ling L.M., et al. COVID-19 associated coagulopathy in critically ill patients: a hypercoagulable state demonstrated by parameters of haemostasis and clot waveform analysis. J Thromb Thrombolysis. 2020;51:663–674. doi: 10.1007/s11239-020-02318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alharthy A., Faqihi F., Balhamar A., Memish Z.A., Karakitsos D. SAGE Open Med Case Rep; 2020. Life-threatening COVID-19 presenting as stroke with antiphospholipid antibodies and low ADAMTS-13 activity, and the role of therapeutic plasma exchange: a case series. 8 2050313X20964089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Siguret V., Voicu S., Neuwirth M., Delrue M., Gayat E., Stépanian A., et al. Are antiphospholipid antibodies associated with thrombotic complications in critically ill COVID-19 patients? Thromb Res. 2020;195:74–76. doi: 10.1016/j.thromres.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Frapard T., Hue S., Rial C., de Prost N., Mekontso Dessap A. Antiphospholipid antibodies and thrombosis in patients with COVID-19. Arthritis Rheum. 2020;73:897–899. doi: 10.1002/art.41634. [DOI] [PubMed] [Google Scholar]
- 124.van der Linden J., Almskog L., Liliequist A., Grip J., Fux T., Rysz S., et al. Thromboembolism, hypercoagulopathy, and antiphospholipid antibodies in critically ill coronavirus disease 2019 patients: a before and after study of enhanced anticoagulation. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vlachoyiannopoulos P.G., Magira E., Alexopoulos H., Jahaj E., Theophilopoulou K., Kotanidou A., et al. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann Rheum Dis. 2020;79:1661–1663. doi: 10.1136/annrheumdis-2020-218009. [DOI] [PubMed] [Google Scholar]
- 126.Karahan Samet, Erol Kemal, Yuksel Recep Civan, Artan Cem, Celik Ilhami. Antiphospholipid antibodies in COVID-19-associated pneumonia patients in intensive care unit. Mod Rheumatol. January 2022;32(1):163–168. doi: 10.1080/14397595.2021.1892257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mullaguri N., Hepburn M., Gebel J.M., Itrat A., George P., Newey C.R. COVID-19 Disease and hypercoagulability leading to acute ischemic stroke. Neurohospitalist. 2021;11:131–136. doi: 10.1177/1941874420960324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trahtemberg U., Rottapel R., Dos Santos C.C., Slutsky A.S., Baker A., Fritzler M.J. Anticardiolipin and other antiphospholipid antibodies in critically ill COVID-19 positive and negative patients. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220206. annrheumdis-2021-220206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Najim M., Rahhal A., Khir F., Aljundi A.H., Abu Yousef S., Ibrahim F., et al. Prevalence and clinical significance of antiphospholipid antibodies in patients with coronavirus disease 2019 admitted to intensive care units: a prospective observational study. Rheumatol Int. 2021;41:1243–1252. doi: 10.1007/s00296-021-04875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Harzallah I., Debliquis A., Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemostasis. 2020;18:2064–2065. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bowles L., Platton S., Yartey N., Dave M., Lee K., Hart D.P., et al. Lupus anticoagulant and abnormal coagulation tests in patients with covid-19. N Engl J Med. 2020;383:288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Popovic B., Varlot J., Metzdorf P.A., Jeulin H., Goehringer F., Camenzind E. Changes in characteristics and management among patients with ST-elevation myocardial infarction due to COVID-19 infection. Cathet Cardiovasc Interv. 2020;97:E319–E326. doi: 10.1002/ccd.29114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Galeano-Valle F., Oblitas C.M., Ferreiro-Mazón M.M., Alonso-Muñoz J., Del Toro-Cervera J., di Natale M., et al. Antiphospholipid antibodies are not elevated in patients with severe COVID-19 pneumonia and venous thromboembolism. Thromb Res. 2020;192:113–115. doi: 10.1016/j.thromres.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hossri S., Shadi M., Hamarsha Z., Schneider R., El-Sayegh D. Clinically significant anticardiolipin antibodies associated with COVID-19. J Crit Care. 2020;59:32–34. doi: 10.1016/j.jcrc.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Previtali G., Seghezzi M., Moioli V., Sonzogni A., Cerutti L., Marozzi R., et al. The pathogenesis of thromboembolic disease in covid-19 patients: could be a catastrophic antiphospholipid syndrome? Thromb Res. 2020;194:192–194. doi: 10.1016/j.thromres.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kanso M., Cardi T., Marzak H., Schatz A., Faucher L., Grunebaum L., et al. Delayed pulmonary embolism after COVID-19 pneumonia: a case report. Eur Heart J Case Rep. 2020;4:1–4. doi: 10.1093/ehjcr/ytaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guillet H., Gallet R., Pham V., D'Humières T., Huguet R., Lim P., et al. Clinical spectrum of ischaemic arterial diseases associated with COVID-19: a series of four illustrative cases. Eur Heart J Case Rep. 2021;5 doi: 10.1093/ehjcr/ytaa488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cristiano A., Fortunati V., Cherubini F., Bernardini S., Nuccetelli M. Anti-phospholipids antibodies and immune complexes in COVID19 patients: a putative role in disease course for anti-annexin-V antibodies. Clin Rheumatol. 2021;1–7 doi: 10.1007/s10067-021-05580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Balanchivadze N., Xie P., Kuriakose P., Barthel B., Dabak V. Transient anti-phospholipid antibodies in two patients with COVID-19. Cureus. 2021;13 doi: 10.7759/cureus.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Anaya J.-M., Monsalve D.M., Rojas M., Rodríguez Y., Montoya-García N., Mancera-Navarro L.M., et al. Latent rheumatic, thyroid and phospholipid autoimmunity in hospitalized patients with COVID-19. J Transl Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y., et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pascolini S., Vannini A., Deleonardi G., Ciordinik M., Sensoli A., Carletti I., et al. COVID-19 and immunological dysregulation: can autoantibodies be useful? Clin Transl Sci. 2020;14:502–508. doi: 10.1111/cts.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lerma L.A., Chaudhary A., Bryan A., Morishima C., Wener M.H., Fink S.L. Prevalence of autoantibody responses in acute coronavirus disease 2019 (COVID-19) J Transl Autoimmun. 2020;3 doi: 10.1016/j.jtauto.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gutiérrez López de Ocáriz X., Castro Quismondo N., Vera Guerrero E., Rodríguez Rodríguez M., Ayala Díaz R., Martínez López J. Thrombosis and antiphospholipid antibodies in patients with SARS-COV-2 infection (COVID-19) Int J Lab Hematol. 2020;42:e280–e282. doi: 10.1111/ijlh.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tvito A., Ben-Chetrit E., Zimmerman F.S., Asher E., Helviz Y. Lupus anticoagulant in patients with COVID-19. Int J Lab Hematol. 2021;43:e17–e18. doi: 10.1111/ijlh.13334. [DOI] [PubMed] [Google Scholar]
- 146.Bauer W., Galtung N., Neuwinger N., Kaufner L., Langer E., Somasundaram R., et al. A matter of caution: coagulation parameters in COVID-19 do not differ from patients with ruled-out SARS-CoV-2 infection in the emergency department. TH Open. 2021;5:e43–e55. doi: 10.1055/s-0040-1722612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Benjamin L.A., Paterson R.W., Moll R., Pericleous C., Brown R., Mehta P.R., Athauda D., Ziff O.J., Heaney J., Checkley A.M., Houlihan C.F., Chou M., Heslegrave A.J., Chandratheva A., Michael B.D., Blennow K., Vivekanandam V., Foulkes A., Mummery C.J., Lunn M.P., Keddie S., Spyer M.J., Mckinnon T., Hart M., Carletti F., Jäger H.R., Manji H., Zandi M.S., Werring D.J., Nastouli E., Simister R., Solomon T., Zetterberg H., Schott J.M., Cohen H., Efthymiou M., UCLH Queen Square COVID-19 Biomarker Study group Antiphospholipid antibodies and neurological manifestations in acute COVID-19: a single-centre cross-sectional study. EClinicalMedicine. 2021 Sep;39 doi: 10.1016/j.eclinm.2021.101070. Epub 2021 Aug 12. PMID: 34401683; PMCID: PMC8358233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hollerbach A., Müller-Calleja N., Pedrosa D., Canisius A., Sprinzl M.F., Falter T., Rossmann H., Bodenstein M., Werner C., Sagoschen I., Münzel T., Schreiner O., Sivanathan V., Reuter M., Niermann J., Galle P.R., Teyton L., Ruf W., Lackner K.J. Pathogenic lipid-binding antiphospholipid antibodies are associated with severity of COVID-19. J Thromb Haemostasis. 2021 Sep;19(9):2335–2347. doi: 10.1111/jth.15455. Epub 2021 Jul 22. PMID: 34242469; PMCID: PMC8420426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee A., Nahm C.H., Lee J.S., Lee M.K., Lee K.R. Assessment of antiphospholipid antibodies and calprotectin as biomarkers for discriminating mild from severe COVID-19. J Clin Lab Anal. 2021 Nov;35(11) doi: 10.1002/jcla.24004. Epub 2021 Oct 5. PMID: 34608677; PMCID: PMC8605160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Emmenegger M., Kumar S.S., Emmenegger V., Malinauskas T., Buettner T., Rose L., Schierack P., Sprinzl M.F., Sommer C.J., Lackner K.J., Aguzzi A., Roggenbuck D., Frauenknecht K.B.M. Anti-prothrombin autoantibodies enriched after infection with SARS-CoV-2 and influenced by strength of antibody response against SARS-CoV-2 proteins. PLoS Pathog. 2021 Dec 3;17(12) doi: 10.1371/journal.ppat.1010118. Erratum in: PLoS Pathog. 2022 Feb 2;18(2):e1010289. PMID: 34860860; PMCID: PMC8673606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shi H., Zuo Y., Navaz S., et al. Endothelial cell-activating antibodies in COVID-19. Arthritis Rheumatol. 2022 Jul;74(7):1132–1138. doi: 10.1002/art.42094. PMID: 35174669; PMCID: PMC9082472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Atalar E., Erden A., Guven S.C., Armagan B., Ates İ., Küçüksahin O., Omma A. The clinical significance of antiphospholipid antibodies in COVID-19 infection. J Infect Dev Ctries. 2022 Feb 28;16(2):276–282. doi: 10.3855/jidc.15423. PMID: 35298422. [DOI] [PubMed] [Google Scholar]
- 153.Shah R., Mohammed Y.N., Koehler T.J., Kaur J., Toufeili M., Pulipati P., Alqaysi A., Khan A., Khalid M., Lee Y., Dhillon P., Dan A.T., Kumar N., Bowen M., Sule A.A., Krishnamoorthy G. Antiphospholipid antibodies and vitamin D deficiency in COVID-19 infection with and without venous or arterial thrombosis: a pilot case-control study. PLoS One. 2022 Jul 14;17(7) doi: 10.1371/journal.pone.0269466. PMID: 35834511; PMCID: PMC9282449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bertin D., Brodovitch A., Lopez A., Arcani R., Thomas G.M., Beziane A., Weber S., Babacci B., Heim X., Rey L., Leone M., Mege J.L., Bardin N. Anti-cardiolipin IgG autoantibodies associate with circulating extracellular DNA in severe COVID-19. Sci Rep. 2022 Jul 22;12(1) doi: 10.1038/s41598-022-15969-y. PMID: 35869087; PMCID: PMC9305055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Espinosa G., Zamora-Martínez C., Pérez-Isidro A., Neto D., Bravo-Gallego L.Y., Prieto-González S., Viñas O., Moreno-Castaño A.B., Ruiz-Ortiz E., Cervera R. Persistent antiphospholipid antibodies are not associated with worse clinical outcomes in a prospective cohort of hospitalised patients with SARS-CoV-2 infection. Front Immunol. 2022 Jun 22;13 doi: 10.3389/fimmu.2022.911979. PMID: 35812410; PMCID: PMC9257245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rosales-Castillo A., Sabio J.M. Assessment of antiphospholipid antibodies during the follow-up of patients after SARS-CoV-2 infection. Med Clin. 2022 May 13;158(9):437–438. doi: 10.1016/j.medcli.2021.09.021. English, Spanish, Epub 2021 Nov 13. PMID: 34895890; PMCID: PMC8590510. [DOI] [PMC free article] [PubMed] [Google Scholar]