Abstract
Objective
To assess the clinical effectiveness of the BNT162b2 vaccine during pregnancy in preventing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) hospitalizations of infants.
Study design
A retrospective, multicenter, 1:3 case-control (test-negative) study. Symptomatic hospitalized infants less than 6 months of age, with a positive SARS-CoV-2 polymerase chain reaction test between January 3, 2021, and March 11, 2021, were matched by age and time to negative controls, hospitalized with symptoms compatible with SARS-CoV-2 infection. Mothers were defined as fully vaccinated who received 2 doses of BNT162b2 with the second given 2 weeks to 6 months before delivery; or partially vaccinated, if they received only 1 dose or 2 doses with the second given more than 6 months or less than 2 weeks before delivery. Severe SARS-CoV-2 was defined as a need for assisted ventilation.
Results
We matched 116 SARS-CoV-2 positive infants with 348 negative controls with symptoms compatible with SARS-CoV-2 infection. The effectiveness of fully vaccinated mothers was 61.6% (95% CI, 31.9-78.4) and the effectiveness of partially vaccinated mothers was not significant. Effectiveness was higher in infants 0-2 vs 3-6 months of age. The effectiveness (57.1%; 95% CI, 22.8-76.4) was similar when excluding mothers who were infected with SARS-CoV-2 during pregnancy. The OR of severe infection in infants born to unvaccinated vs fully vaccinated mothers was 5.8.
Conclusions
At least 2 doses of BNT162b2 vaccine administered during the second or third trimester of pregnancy had an effectiveness of 61.6% in decreasing hospitalization for SARS-CoV-2 infection in infants less than 6 months of age.
Keywords: Maternal vaccination, SARS-CoV-2, infants' hospitalizations, pregnancy
Pregnant women are at high risk for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection mainly during the third trimester.1 Infants 3 or fewer months of age usually have a benign clinical course and, although age less than 1 year has been associated with increased rates of hospitalization, it may not reflect the severity of the illness.2
Vaccination of very young infants by direct immunization might be limited by poor immunogenicity and interference from maternal antibodies.3 One approach for protecting young infants against vaccine-preventable infections is to vaccinate their mothers during pregnancy, as is currently recommended for the pertussis, tetanus, and influenza virus vaccines.
A national SARS-CoV-2 immunization program started in Israel on December 20, 2020, and by February, 2021 50% of the population more than 30 years old had received 2 doses of the vaccine.4 Data from vaccinated pregnant women have not shown safety concerns, and vaccine-induced immune responses in pregnant females were equivalent to nonpregnant females.5, 6, 7 SARS-CoV-2-specific antibodies (Immunoglobulin G) cross the placenta from the mother to the fetus, particularly during the final weeks of pregnancy.8 Additionally, SARS-CoV-2 antibodies (Immunoglobulin G and IgA) were found in breast milk of mothers as early as 2 weeks after vaccination.5 , 9 Nevertheless, the potential role of maternal immunization in protecting young infants against SARS-CoV-2 hospitalizations is less clear.10, 11, 12
According to the Israeli ministry of health, pregnant women are eligible for BNT162b2 vaccination during all trimesters of pregnancy based on growing evidence of safety of mRNA SARS-CoV-2 vaccine in pregnancy and a decreased risk of SARS-CoV-2 infection in vaccinated pregnant women. Of note, no SARS-CoV-2 vaccine other than BNT162b2 was commercially available in Israel during the study period.
The aim of this study was to assess the clinical effectiveness of the BNT162b2 SARS-CoV-2 vaccination during pregnancy in preventing SARS-CoV-2 hospitalizations of infants less than 6 months of age.
Methods
SARS-CoV-2 in Israel
Since the first SARS-CoV-2 case occurred in Israel on February 21, 2020, Israel has had 5 waves of SARS-CoV-2 (during February and March 2020 and May through November 2020) and more than 8000 deaths occurring during November 2020 to April 2021 and June to November 2021 were predominantly due to the Delta variant. The fifth wave, which is predominantly caused by the Omicron variant, started in January 2022 and is ongoing.4 , 6 Israel launched its BNT162b2 SARS-CoV-2 vaccination program in December 2020.
Study Design
We performed a multicenter, observational 1:3 case-control, test-negative study involving 3 tertiary pediatric hospitals in central and southern Israel (Soroka Medical Center, Schneider Medical Center, and Shamir Medical Center). The patients enrolled were selected from all hospitalized infants 0-6 months of age who underwent SARS-CoV-2 PCR testing between March 1, 2021, and November 31, 2021. Infants whose test was part of a screening protocol (before a procedure or surgery, before transport, or after delivery) were excluded from this analysis.
Variables and Definitions
Cases were defined as all SARS-CoV-2 reverse transcriptase PCR-positive infants. Three PCR negative controls were selected for each case using frequency matching with cases based on age (by week), date of test (by week), and ethnicity (Jews vs non-Jews). Prior data indicated that the probability of exposure among controls and cases were 68% and 84%, respectively.11 For power calculations, we estimated a sample size of 103 cases and 309 matched controls to reject the null hypothesis that the OR equals 1 with a power of 80%, assuming that the type I error probability associated with the test of this null hypothesis is 5%.13 , 14 A positive SARS-CoV-2 PCR test was defined according to the Israeli Ministry of Health. Two testing methods were used in the hospitals' virology laboratories during the study period. BGI's Real-Time Fluorescent PCR kit (MGI [a subsidiary of BGI Group]) was used for detection of SARS-CoV-2 spike protein (S gene), results were reported as positive with a cycle time (Ct) value of less than 36, as negative with a Ct value of greater than 40, or borderline with a Ct value of 36-40. Seegene SARS-COV-2 PCR kit (AllplexTM2019-nCoV Assay) was used for the detection of the E, RdRP, and N genes. Results were reported as positive when positive for all 3 genes, any 2 genes, or 1 gene for E or RdRP genes, and as negative when negative for all genes, or as borderline when positive only for the N gene. Borderline test results were excluded; only infants with a positive SARS-COV-2 PCR result were enrolled as cases.
Severe acute SARS-CoV-2 infection was defined as need for assisted ventilation.
The study was approved by the ethics committee at each institution.
SARS-CoV-2 PCR test results and date of testing were retrieved from the virology laboratory at each institution. Demographic and clinical data of the infants were collected from the patients’ computerized medical records and included age, sex, ethnicity, premature birth (<37 weeks of gestation), oxygen saturation during hospital admission, use of supplemental oxygen, noninvasive or invasive ventilation, mortality within 30 days of admission, and lactation before or during the time of hospital admission. The maternal BNT162b2 vaccination status by number of doses and dates of vaccination were collected from the computerized medical records and by phone interviews (Figure 1; available at www.jpeds.com).
Figure 1.
Telephone questionnaire for mothers.
A fully vaccinated mother was defined as a mother who had received at least 2 doses of the BNT162b2 vaccine, with the second dose given 2 weeks to 6 months before delivery. A partially vaccinated mother was defined as one who had received only 1 dose of the vaccine or 2 doses with the second dose given more than 6 months or less than 2 weeks before delivery. Mothers with a positive SARS-CoV-2 PCR test were considered recovered.
Statistical Analyses
All data were described using summary statistics. Comparisons of demographic and clinical characteristics between infants with a SARS-CoV-2-positive PCR test and infants with a SARS-CoV-2-negative PCR test, and of demographic and clinical characteristics between unvaccinated, fully vaccinated, and partially vaccinated mothers were performing using the Student t test for quantitative variables and the χ2 or Fisher exact tests for categorical variables. Vaccine effectiveness with 95% CI against infant hospitalization for coronavirus disease 2019 was calculated using the equation: Vaccine effectiveness = 100% × (1 – OR). Multivariate logistic regression model was performed to evaluate the mother's vaccine status on infant hospitalization for coronavirus disease 2019, adjusted for variables found to be statistically significant relating the mothers' vaccine status (infant age at PCR date and ethnic group). All statistical analyses were perform using SPSS 28.0 software (IBM). A P value of less than .05 was considered statistically significant.
Results
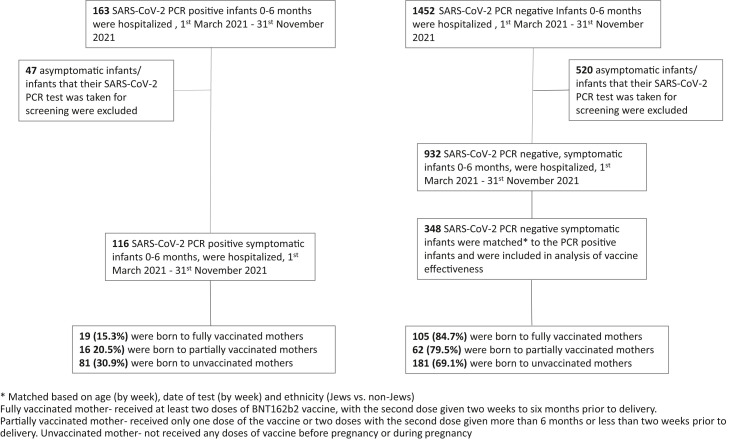
There were 163 SARS-CoV-2 PCR-positive infants aged 0-6 months who were hospitalized during the study period, of which 116 symptomatic SARS-CoV-2 PCR-positive infants were included in the study group; 42 asymptomatic infants whose SARS-CoV-2 PCR test was taken for screening were excluded. There were 1452 hospitalized infants aged 0-6 months who had a negative SARS-CoV-2 PCR test during the same period; however, only 932 infants had symptoms compatible with SARS-CoV-2 infection (fever, respiratory or gastrointestinal symptoms). Each of the 116 SARS-CoV-2-positive infants was successfully matched with 3 SARS-CoV-2-negative infants (348 controls) by the matching criteria of week of birth, week of PCR testing, and ethnicity (Figure 2 ).
Figure 2.
Study enrollment.
The SARS-CoV-2-positive infants had a median age of 6.2 weeks (IQR, 3.7-9.1 weeks); 59 (50.9%) were males, 74 (63.8%) Jewish, 21 (18.1%) were born prematurely (<37 weeks of gestation), and 17 (14.7%) required supplementary oxygen or assisted ventilation. None of the infants died within 30 days of admission. There were no significant differences in demographic and clinical characteristics between case and control infants (Table I ).
Table I.
Demographic and clinical characteristics, comparison between positive and negative SARS-CoV2 PCR hospitalized infants
| SARS-COV-2 PCR positive infants (n = 116) | SARS-COV-2 PCR negative infants (n = 348) | P value | |
|---|---|---|---|
| Age at PCR test, weeks | 6.2 (3.7-9.1) | 6.6 (3.9-10.1) | .814 |
| Males | 59 (50.9) | 206 (59.2) | .116 |
| Ethnicity, Jews | 74 (63.8) | 222 (63.8) | 1.00 |
| Prematurity, <37 weeks | 21 (18.1) | 54 (15.5) | .512 |
| Lactation | 43/81 (53.1) | 165/347 (47.6) | .369 |
| Saturation <94 | 15 (12.9) | 61 (17.5) | .247 |
| Supplementary oxygen/assistant ventilation | 17 (14.7) | 61 (17.5) | .474 |
| Mortality within 30 days | 0 (0.0) | 1 (0.3) | .563 |
| Mother positive for SARS-COV-2 PCR before pregnancy | 0 (0.0) | 6 (1.7) | .343 |
| Mother positive for SARS-COV-2 PCR during pregnancy | 3 (2.6) | 22 (6.3) | .192 |
Values are median (IQR) or number (%).
Nineteen mothers of SARS-CoV-2-positive infants were fully vaccinated during pregnancy—2 (10.5%) and 17 (89.5%) in the second and third trimesters, respectively—compared with 105 mothers of SARS-CoV-2-negative infants—29 (27.6%) and 76 (72.4%) in the second and third trimesters, respectively. The effectiveness of fully vaccinated mothers was 59.6% (95% CI, 29.6-76.8; P = .001) and the effectiveness of partially vaccinated mothers was 42.3% (95% CI, 0.0-68.6; P = .045) (Table II ). Six mothers had received 3 doses of the BNT162b2 vaccine before delivery (all during the second or third trimesters of pregnancy). When excluding those 6 mothers from the fully vaccinated group, vaccine effectiveness was 57.1% (95% CI, 22.8%-76.4%; P = .004).
Table II.
Effectiveness∗ of maternal BNT162b2 SARS-CoV-2 vaccination against SARS-CoV2-associated hospitalization in infants <6 months, for fully and partially vaccinated mothers by infant age at admission
| SARS-COV-2 PCR negative infants (n = 348) | SARS-COV-2 PCR positive infants (n = 116) | Vaccine effectiveness∗ (CI) | P value | |
|---|---|---|---|---|
| All infants unadjusted | ||||
| Unvaccinated mothers | 181 (69.1%) | 81 (30.9%) | ||
| Partially vaccinated mothers | 62 (79.5%) | 16 (20.5%) | 42.3% (0.0-68.6) | 0.045 |
| Fully vaccinated mothers | 105 (84.7%) | 19 (15.3%) | 59.6% (29.6-76.8) | 0.001 |
| All infants adjusted | ||||
| Unvaccinated mothers | 181 (69.1%) | 81 (30.9%) | ||
| Partially vaccinated mothers† | 62 (79.5%) | 16 (20.5%) | 41.7% (0.0-68.6) | .087 |
| Fully vaccinated mothers‡ | 105 (84.7%) | 19 (15.3%) | 61.6% (31.9-78.4) | .001 |
| All infants excluding mothers who received a third dose of the vaccine | ||||
| Unvaccinated mothers | 181 (69.1%) | 81 (30.9%) | ||
| Partially vaccinated mothers | 62 (79.5%) | 16 (20.5%) | 42.3% (0.0-68.6) | .045 |
| Fully vaccinated mothers | 105 (84.7%) | 19 (15.3%) | 57.1% (22.8-76.4) | .004 |
| Infants <2 months | ||||
| Unvaccinated mothers | 119 (67.2%) | 58 (32.8%) | ||
| Partially vaccinated mothers | 43 (78.2%) | 12 (21.8%) | 42.7% (0.0-71.9) | .122 |
| Fully vaccinated mothers | 85 (85.0%) | 15 (15.0%) | 63.8% (31.9-80.8) | .001 |
| Infants 2-6 months of age | ||||
| Unvaccinated mothers | 62 (72.9%) | 23 (27.1%) | ||
| Partially vaccinated mothers | 19 (82.6%) | 4 (17.4%) | 43.3% (0.0-82.6) | .342 |
| Fully vaccinated mothers | 20 (83.3%) | 4 (16.7%) | 46.1% (0.0-83.4) | .298 |
| Infants with severe infection | ||||
| Unvaccinated mothers | 36 (73.4%) | 13 (26.6%) | ||
| Partially vaccinated mothers | 9 (75%) | 3 (25%) | 7.7 (0.0-78.0) | 1.0 |
| Fully vaccinated mothers | 16 (94.1%) | 1 (5.9%) | 82.7 (0.0-97.9) | .093 |
| All infants excluding infants of SARS-COV-2 infected mothers during pregnancy | ||||
| Unvaccinated mothers | 166 (67.5%) | 80 (32.5%) | ||
| Partially vaccinated mothers | 58 (80.6%) | 14 (19.4%) | 49.9% (4.9-73.6) | .032 |
| Fully vaccinated mothers | 102 (84.3%) | 19 (15.7%) | 61.4% (32.5-77.9) | <.001 |
Vaccine effectiveness estimates were based on odds of antecedent maternal vaccination during pregnancy in case infants vs control-infants.
Adjusted for ethnicity and infant age.
Adjusted for ethnicity and prematurity.
Unvaccinated mothers were more likely to be non-Jewish and their infants were older at time of PCR testing compared with fully vaccinated mothers. When using a multivariable analysis controlling for ethnicity and infant age vaccine effectiveness for fully vaccinated mothers was 61.6% (95% CI, 31.9-78.4). The infants of unvaccinated mothers had higher rates of prematurity compared with those of partially vaccinated mothers, and their mothers were more likely to be non-Jewish. However, when using a multivariable analysis controlling for ethnicity and prematurity, vaccine effectiveness for partially vaccinated mothers was not significant (Figure 3 , Table III; available at www.jpeds.com). Unvaccinated and vaccinated mothers had similar breastfeeding rates.
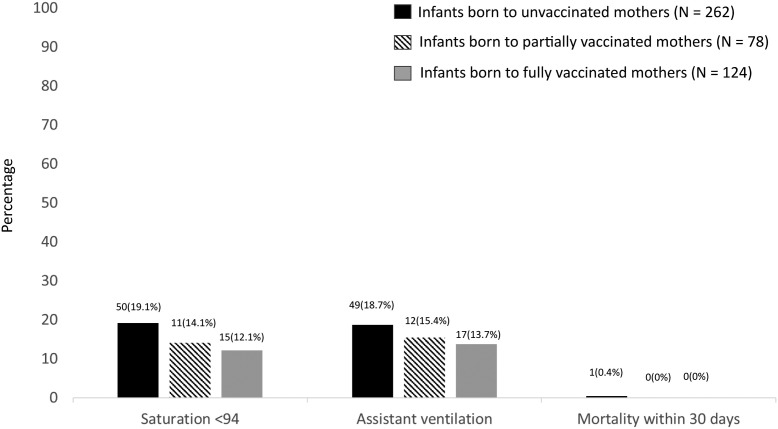
Figure 3.
Outcomes of infants according to maternal vaccination status.
The vaccine effectiveness in fully vaccinated mothers was higher in infants 0-2 months of age (63.8%; 95% CI, 31.9-80.8) compared with infants 3-6 months of age 46.1% (95% CI, 0.0-83.4) (Table II).
Seventeen infants (14.7%) in the case group and 61 infants (17.5%) in the control group had a severe infection, defined as the need for assisted ventilation. In the SARS-CoV-2-positive group, 81 unvaccinated mothers had 13 infants (16.0%) with severe infection compared with 1 infant (5.3%) with severe infection in the 19 fully vaccinated mothers. The OR of severe infection between infants born to unvaccinated vs fully vaccinated mothers was 5.8 (P = .09).
Overall, 130 mothers had a SARS-CoV-2 infection documented by PCR; 6 mothers before conception (4 [66.6%] unvaccinated, 2 [33.4%] partially vaccinated, 0 [0.0%] fully vaccinated), 25 mothers during pregnancy (but ≥2 weeks before delivery) (16 [64%] unvaccinated, 6 [24%] partially vaccinated, 3 [12%] fully vaccinated) and 99 mothers after delivery (61 [61.6%] unvaccinated, 21 [21.2%] partially vaccinated, and 17 [17.2%] fully vaccinated). Mothers who had a SARS-CoV-2 infection during pregnancy had similar rates of infants’ prematurity as mothers who did not have infection during pregnancy (5/25 [20%] vs 70/439 [16%]; P = .592).
When excluding the mothers who were infected with SARS-CoV-2 during pregnancy, the effectiveness of infant protection of fully vaccinated mothers was 61.4% (95% CI, 32.5-77.9; P < .001) and for partially vaccinated mothers it was 49.9% (95% CI, 4.9-73.6; P = .032).
Six mothers had a SARS-CoV-2 infection documented by PCR while breast-feeding (but ≥2 weeks before their infants' PCR test), of which 4 were unvaccinated and had 1 positive SARS-CoV2 infant.
Discussion
Among hospitalized infants, at least 2 doses of maternal BNT162b2 vaccine administered during the second or third trimester had an effectiveness of 61.6% in decreasing hospitalizations for SARS-CoV-2 infection in infants during the first 6 months of life, with greater effectiveness during the first 2 months of life.
Multiple studies on immunogenicity of coronavirus disease 2019 (COVID-19) mRNA vaccination during pregnancy have shown neonatal humoral responses during delivery with a positive association between maternal and neonatal titers, which are highest after late second and early third trimester vaccination.5 , 8 Earlier vaccination, during the first trimester of pregnancy, has been shown to elicit significantly lower maternal anti-SARS-CoV-2 antibody levels and neutralizing titers at the time of delivery; thus, we divided vaccinated mothers into fully vaccinated mothers who had received 2 doses of vaccine during the second or third trimester of pregnancy (months 4-9 of pregnancy) but longer than 2 weeks before delivery, allowing a sufficient time for a complete immune response and placental transmission, and partially vaccinated mothers, who had received their second dose of vaccine during the first trimester of pregnancy or earlier (preconception and first 3 months of pregnancy).5 , 15 We showed significantly greater effectiveness in the fully vaccinated group compared with the partially vaccinated group. For mothers who were vaccinated early, a third booster dose could potentially augment antibody levels; however, because the BNT162b2 booster campaign started in Israel only in September 2021, only 6 mothers in this cohort had received the booster before delivery.
Several studies have addressed the dynamics of protective maternal antibodies in the infants. Although a majority of infants had persistence of antibodies at 6 months of age, waning of antibody titers was observed as early as 2 months of age and indeed, in our real-life study, effectiveness was higher in infants 0-2 months of age.16
Completion of a 2-dose vaccination series either with Pfizer-BioNTech or Moderna mRNA COVID-19 vaccine decreased the risk of SARS-CoV-2 hospitalizations of infants 0-6 months in 20 US pediatric hospitals during a period of Delta and Omicron variant circulation by 61%. We showed similar effectiveness of late vaccination of at least 2 doses of Pfizer-BioNTech (Pfizer) of 61.6%; however, the effectiveness of late vaccination was lower in our cohort than in the studies by Halasa et al (61.6% vs 80.0% during the Delta-predominant period).11 , 12 One possible explanation could be differences in the rate of mothers who were infected with SARS-CoV2 during pregnancy in our cohort compared with the Halasa studies; however, the latter did not include data on maternal past infection status.
Our study has several strengths; first, all vaccine doses and specific dates of administration were verified by interviews and review of maternal computerized medical records. In addition, all mothers were vaccinated with the BNT162b2 vaccine because no other COVID-19 vaccine was available in Israel during the study period. Second, we included data that potentially could affect infants' protection against SARS-CoV-2, like maternal infection status before, during, and after pregnancy and lactation. Third, our cohort included only symptomatic infants who were suspected of SARS-CoV-2 infection by a physician, whereas infants who were tested during screening or were asymptomatic were excluded from analysis because SARS-CoV-2 PCR positivity rates might be biased by behavioral differences between parents and the parents’ threshold for testing.
The study also has several limitations; first the sample size was limited. Second, our study was unable to examine the impact of the administration of a booster dose during pregnancy on preventing infant SARS-CoV-2 hospitalizations. Third, other factors that potentially affect maternal immunogenicity and hence neonatal antibody titers, such as maternal age (which is negatively correlated with BNT162b2 vaccine immune response) and presence of immunodeficiency were not addressed in our study.17 Fourth, the study period—March 1, 2021, through November 31, 2021—was dominated by the Delta variant, with the first cases of the Omicron variant reported in Israel only in January 2022. Thus, our findings might not be indicative of vaccine effectiveness against other SARS-CoV-2 variants.
With increasing data on the safety of SARS-CoV-2 mRNA vaccines during pregnancy and the effectiveness against infections of pregnant woman, our findings may further strengthen the recommendation for routine mRNA SARS-CoV-2 vaccination during pregnancy.18, 19, 20, 21, 22, 23
Footnotes
All authors declare no support from any organization for the submitted work. D.D. has received a grant from Pfizer. D.G. has received grants from MSD, serves as scientific consultant to Pfizer, MSD, and GSK. These funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The other authors declare no conflicts of interest.
Supplementary Data
Appendix
Table III.
Demographic and clinical characteristics of hospitalized infants by mothers' vaccination status, comparison between unvaccinated, fully vaccinated, and partially vaccinated mothers
| Unvaccinated mothers (n = 262) | Fully vaccinated mothers (n = 124) | Partially vaccinated mothers (n = 78) | P value∗,† | |
|---|---|---|---|---|
| Age at PCR test <2 months | 177 (67.6) | 100 (80.6) | 55 (70.5) | .008∗, 0.623† |
| Males | 158 (60.3) | 66 (53.2) | 41 (52.6) | .188, .223 |
| Ethnicity, Jews | 142 (54.2) | 99 (79.8) | 55 (70.5) | <.001, .010 |
| Prematurity, <37 weeks | 51 (19.5) | 17 (13.7) | 7 (9.0) | .166, .031 |
| Lactation | 116/241 (48.1) | 61/119 (51.3) | 31/68 (45.6) | .577, .711 |
Values are number (%).
Bold indicates statistical significance.
P value unvaccinated mothers vs fully vaccinated mothers.
P value unvaccinated mothers vs partially vaccinated mothers.
References
- 1.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yarden Bilavski H., Balanson S., Damouni Shalabi R., Dabaja-Younis H., Grisaru-Soen G., Youngster I., et al. Benign course and clinical features of COVID-19 in hospitalised febrile infants up to 60 days old. Acta Paediatr. 2021;110:2790–2795. doi: 10.1111/apa.15993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Healy C.M., Baker C.J. Prospects for prevention of childhood infections by maternal immunization. Curr Opin Infect Dis. 2006;19:271–276. doi: 10.1097/01.qco.0000224822.65599.5b. [DOI] [PubMed] [Google Scholar]
- 4.Israeli Ministry of Health Up-to-date guidelines for validating and releasing test results for the new Corona virus by the Israeli Ministry of Health. https://govextra.gov.il/media/18274/ld-176449420.pdf
- 5.Collier A.Y., McMahan K., Yu J., Tostanoski L.H., Aguayo R., Ansel J., et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Israeli Ministry of Health COVID-19 data repository of the Israeli Ministry of Health. https://data.gov.il/dataset/covid-19
- 7.Pham A., Aronoff D.M., Thompson J.L. Maternal COVID-19, vaccination safety in pregnancy, and evidence of protective immunity. J Allergy Clin Immunol. 2021;148:728–731. doi: 10.1016/j.jaci.2021.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beharier O., Plitman Mayo R., Raz T., Nahum Sacks K., Schreiber L., Suissa-Cohen Y., et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131 doi: 10.1172/JCI154834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perl S.H., Uzan-Yulzari A., Klainer H., Asiskovich L., Youngster M., Rinott E., et al. SARS-CoV-2-specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA. 2021;325:2013–2014. doi: 10.1001/jama.2021.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlsen E.O., Magnus M.C., Oakley L., Fell D.B., Greve-Isdahl M., Kinge J.M., et al. Association of COVID-19 vaccination during pregnancy with incidence of SARS-CoV-2 infection in infants. JAMA Intern Med. 2022;182:825–831. doi: 10.1001/jamainternmed.2022.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halasa N.B., Olson S.M., Staat M.A., Newhams M.M., Price A.M., Boom J.A., et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months - 17 states, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264–270. doi: 10.15585/mmwr.mm7107e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halasa N.B., Olson S.M., Staat M.A., Newhams M.M., Price A.M., Pannaraj P.S., et al. Maternal vaccination and risk of hospitalization for Covid-19 among infants. N Engl J Med. 2022;387:109–119. doi: 10.1056/NEJMoa2204399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupont W.D. Power calculations for matched case-control studies. Biometrics. 1988;44:1157–1168. [PubMed] [Google Scholar]
- 14.Stevenson M., Nunes T., Heuer C., Jonathon M., Sanchez J., Thornton R., et al. An R package for the analysis of epidemiological data. epiR. 2015 Version 0.9-69. [Google Scholar]
- 15.Mithal L.B., Otero S., Shanes E.D., Goldstein J.A., Miller E.S. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am J Obstet Gynecol. 2021;225:192–194. doi: 10.1016/j.ajog.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shook L.L., Atyeo C.G., Yonker L.M., Fasano A., Gray K.J., Alter G., et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA. 2022;327:1087–1089. doi: 10.1001/jama.2022.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rottenstreich A., Zarbiv G., Oiknine-Djian E., Vorontsov O., Zigron R., Kleinstern G., et al. The effect of gestational age at BNT162b2 mRNA vaccination on maternal and neonatal SARS-CoV-2 antibody levels. Clin Infect Dis. 2022;75:e603–e610. doi: 10.1093/cid/ciac135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldshtein I., Steinberg D.M., Kuint J., Chodick G., Segal Y., Shapiro Ben David S., et al. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. 2022;176:470–477. doi: 10.1001/jamapediatrics.2022.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blakeway H., Prasad S., Kalafat E., Heath P.T., Ladhani S.N., Le Doare K., et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226:236.e1–236.e14. doi: 10.1016/j.ajog.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chavan M., Qureshi H., Karnati S., Kollikonda S. COVID-19 vaccination in pregnancy: the benefits outweigh the risks. J Obstet Gynaecol Can. 2021;43:814–816. doi: 10.1016/j.jogc.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldshtein I., Nevo D., Steinberg D.M., Rotem R.S., Gorfine M., Chodick G., et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728–735. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L., et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wainstock T., Yoles I., Sergienko R., Sheiner E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine. 2021;39:6037–6040. doi: 10.1016/j.vaccine.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.