Molnupiravir and ritonavir-boosted nirmatrelvir are the 2 novel oral antiviral agents that have recently been authorized for the treatment of mild to moderate COVID-19 in adults who are at increased risk for progressing to severe COVID-19. Molnupiravir is a prodrug of the ribonucleoside analogue N-hydroxycytidine with little hepatic metabolism. Ritonavir-boosted nirmatrelvir is metabolized by the cytochrome P450 (CYP) system (largely CYP 3A4). There were no reported episodes of clinically apparent liver injury in preregistration clinical trials, and hence, a likelihood score of E (ie, unlikely cause of clinically apparent liver injury) was assigned to both drugs.1 Despite a favorable hepatic safety profile of both antivirals observed in clinical trial settings, their risk of drug-induced liver injury (DILI) in the real-world setting is unclear. This would be particularly relevant in Asia, where chronic viral hepatitis and nonalcoholic fatty liver disease are highly prevalent.2 , 3
In this territory-wide study, we determined the real-world hepatic safety profile and risk of DILI with molnupiravir and ritonavir-boosted nirmatrelvir in COVID-19 patients amidst the peak of the COVID-19 pandemic. COVID-19 patients who attended public hospitals or clinics in Hong Kong between January 1, 2022, and March 31, 2022 were identified. Patients were followed up until the occurrence of the clinical endpoint of interest, death, date of data retrieval (April 30, 2022), or up to 30 days of follow-up, whichever came first. The primary endpoint was alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) elevation, which was defined as ALT and/or AST of ≥2 times the upper limit of normal (2×ULN) (ie, 80 U/L).4 The secondary endpoints included any abnormal liver enzymes, acute liver injury, and DILI of categories 1–4.
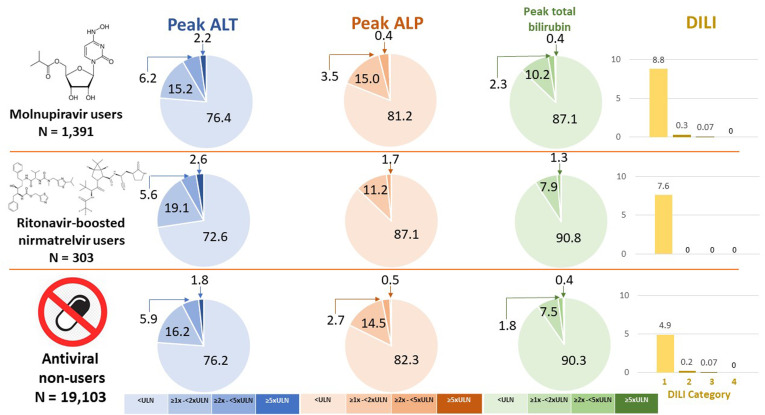
We analyzed the data from 183,041 patients (13,041 molnupiravir users, 4408 ritonavir-boosted nirmatrelvir users, and 165,592 oral antiviral nonusers). The median (interquartile range) interval between COVID-19 diagnosis and antiviral initiation was 0 (0–2) days, and the duration of antiviral treatment was 5 days in >95% of patients. The majority of the patients in the 3 groups had normal liver biochemistries at baseline. Serial liver biochemistries during COVID-19 were checked in 20,797 COVID-19 patients (Figure 1 ). Abnormal ALT level occurred in 23.6%, 27.4%, and 23.8% of molnupiravir users, ritonavir-boosted nirmatrelvir users, and nonusers, respectively; 8.4%, 8.3%, and 7.7% had peak ALT of ≥2×ULN, respectively (P > .20); and 2.2%, 2.6%, and 1.8% had peak ALT of ≥5×ULN, respectively. Similar rates of abnormal alkaline phosphatase (ALP) (12.9%–19.0%) were observed in 3 groups (P > .20). There was a higher rate of elevated peak total bilirubin in molnupiravir users (12.9%) than nonusers (9.7%, P = .003), whereas the rate in ritonavir-boosted nirmatrelvir users was similar to that in nonusers (9.2% vs 9.7%, P = .97) (Figure 1).
Figure 1.
Liver biochemistries in the first 30 days after the use of COVID-19 drugs. ALP, alkaline phosphatase; ALT, alanine aminotransferase; DILI, drug-induced liver injury; ULN, upper limit of normal.
According to the International DILI Expert Working Group classification of the severity of DILI,5 8.8%, 0.3%, 0.07%, and 0% of molnupiravir users had category 1, category 2, category 3 and category 4 DILI, respectively; 7.6%, 0%, 0%, and 0% of ritonavir-boosted nirmatrelvir users had category 1, category 2, category 3, and category 4 DILI, respectively. Category 1, category 2, category 3, and category 4 DILI occurred in 4.9%, 0.2%, 0.04%, and 0% of COVID-19 antiviral nonusers. There were no liver-related deaths or liver transplantations. On univariate analysis, molnupiravir and ritonavir-boosted nirmatrelvir were both associated with a lower risk of ALT and/or AST of ≥2×ULN, whereas chronic viral hepatitis, use of a corticosteroid, advanced age, male sex, diabetes mellitus, and hypertension were associated with a higher risk of ALT and/or AST of ≥2×ULN. Category 1–3 DILI more frequently occurred in patients with 1 or more of these contributors: 6.8%–8.9% in patients with chronic viral hepatitis, corticosteroid users, and/or patients with diabetes vs 3.5%–4.7% without. Use of molnupiravir (adjusted odds ratio, 0.36; 95% confidence interval, 0.28–0.47; P < .001) and ritonavir-boosted nirmatrelvir (aOR, 0.16; 95% CI, 0.08–0.34; P < .001) were 2 independent protective factors after adjustment for significant confounding factors in multivariable analysis.
This is one of the first studies describing the real-world hepatic safety profile of the 2 novel oral antiviral agents for COVID-19. Compared to no antiviral treatment, both molnupiravir and ritonavir-boosted nirmatrelvir did not increase the risk of abnormal liver enzymes or DILI. Significant DILI occurred only in a tiny proportion of the patients, with incidence rates similar to those in antiviral nonusers. Although the hepatic safety profile of both of the oral antivirals was favorable in clinical trials, our territory-wide, real-world cohort has provided additional safety data in patients infected mostly by the omicron instead of delta variant of SARS-CoV-2, with the latter mainly represented in the MOVe-OUT and EPIC-HR trials, respectively.6 , 7 The rapid surge of COVID-19 in the first quarter of 2022 in Hong Kong was primarily related to the highly transmissible nature of the omicron variant. This wave in Hong Kong would be a suitable setting to determine the real-world safety of these 2 oral antivirals in COVID-19 infections caused predominantly by the omicron variant.
Abnormal liver function tests (hepatocellular, cholestatic, or mixed) are commonly observed in COVID-19 patients in the absence of 2 oral antivirals.8 Liver cirrhosis is associated with increased mortality in COVID-19 patients; fortunately, neither current nor past hepatitis B virus infection is associated with COVID-19–related mortality.9 Patients with decompensated liver disease and abnormal liver biochemistries are, at the same time, at risk of DILI when using specific drugs such as protease inhibitors, like nirmatrelvir-ritonavir, and severe COVID-19. Hence, the very low risk (<1%) of significant DILI in our real-world cohort reassures both health care practitioners and patients of the safety of COVID-19 antivirals even in the presence of risk factors of DILI. Our study has a few limitations, namely, the lack of a formal DILI adjudication panel, prescription bias, ascertainment bias, its retrospective nature, and missing data. In conclusion, this territory-wide, real-world study confirms the favorable hepatic safety profile of the 2 novel oral antiviral agents for COVID-19, molnupiravir and ritonavir-boosted nirmatrelvir. In view of the ongoing outbreak worldwide, we are able to reassure health care practitioners and patients that the risk of DILI with these drugs is minimal even in the presence of risk factors. Hence, at-risk COVID-19 patients should use these antivirals to reduce adverse clinical outcomes.
Acknowledgments
CRediT Authorship Contributions
Grace Lai-Hung Wong, MD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Funding acquisition: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Resources: Lead; Supervision: Lead; Validation: Lead; Visualization: Lead; Writing – original draft: Lead; Writing – review & editing: Lead).
Vicki Wing-Ki Hui, MPhil(Med) (Data curation: Lead; Formal analysis: Lead; Software: Lead; Writing – original draft: Lead; Writing – review & editing: Lead).
Terry Cheuk-Fung Yip, PhD (Data curation: Equal; Formal analysis: Equal; Software: Equal; Supervision: Equal; Writing – original draft: Equal; Writing – review & editing: Equal).
Grace Chung-Yan Lui, MBChB(Hons) (Conceptualization: Equal; Supervision: Equal; Writing – original draft: Equal; Writing – review & editing: Equal).
David Shu-Cheong Hui, MD (Conceptualization: Equal; Supervision: Equal; Writing –original draft: Equal; Writing – review & editing: Equal).
Vincent Wai-Sun Wong, MD (Data curation: Equal; Funding acquisition: Lead; Supervision: Lead; Writing – original draft: Lead; Writing – review & editing: Lead).
Footnotes
Conflicts of interest These authors disclose the following: Grace Lai-Hung Wong has served as an advisory committee member for Gilead Sciences and Janssen; has served as a speaker for Abbott, AbbVie, Ascletis, Bristol-Myers Squibb, Echosens, Gilead Sciences, Janssen, and Roche, and has received a research grant from Gilead Sciences. Terry Cheuk-Fung Yip has served as an advisory committee member and a speaker for Gilead Sciences. Grace Chung-Yan Lui has served as an advisory committee member for Gilead, Merck, and GSK; has served as a speaker for Merck and Gilead; and has received research grants from Gilead, Merck, and GSK. Vincent Wai-Sun Wong has served as a consultant or advisory committee member for AbbVie, Boehringer Ingelheim, Echosens, Gilead Sciences, Intercept, Inventiva, Merck, Novo Nordisk, Pfizer, ProSciento, Sagimet Biosciences, and TARGET PharmaSolutions; has served as a speaker for Abbott, AbbVie, Echosens, Gilead Sciences, and Novo Nordisk; has received a research grant from Gilead Sciences; and is a cofounder of Illuminatio Medical Technology Limited. The remaining authors disclose no conflicts.
Funding This study was funded in part by the Health and Medical Research Fund–Food and Health Bureau Commissioned Research on COVID-19 (project reference number: COVID1903002) to Grace Lai-Hung Wong.
References
- 1.National Institute of Diabetes and Digestive and Kidney Diseases, 2012. https://www.ncbi.nlm.nih.gov/books/NBK547852/. Accessed August 31, 2022.
- 2.Yip T.C., et al. Hepatol Int. 2022;16:257–268. doi: 10.1007/s12072-022-10306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yip T.C., et al. J Hepatol. 2022;76:726–734. doi: 10.1016/j.jhep.2021.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Sarin S.K., et al. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontana R.J., et al. Hepatology. 2010;52:730–742. doi: 10.1002/hep.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayk Bernal A., et al. N Engl J Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond J., et al. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong G.L., et al. Lancet Gastroenterol Hepatol. 2020;5:776–787. doi: 10.1016/S2468-1253(20)30190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yip T.C., et al. Hepatology. 2021;74:1750–1765. doi: 10.1002/hep.31890. [DOI] [PMC free article] [PubMed] [Google Scholar]