Abstract
Background
Reports of local and systemic side-effects of COVID-19 vaccination may play an important role in public confidence in the acceptance of the COVID-19 vaccine booster dose.
Methods
We conducted a retrospective cross-sectional study among adults living in Eastern Province of Saudi Arabia. A link to the survey was distributed to community members via WhatsApp, SMS, or e-mail. Participants' general and demographic information was also collected, as well as information about any local and systemic side-effects reported following vaccination.
Results
A total of 370/390 (94.87%) of respondents reported one or more side-effects. Pain or redness at the site of injection (88.92%), fatigue (43.78%), body pain fever (37.84%), and headache (15.95%) were the most commonly reported side-effects. Moreover, 2.43% of the participants reported side-effects needed to see a physician; only four were admitted to the hospital. The non-healthcare respondents (n=273 (97.15%), OR (95% CI) = 5.22 (2.02, 13.48, P <0.001) were more likely to report side-effects compared to the healthcare related respondents (n=36 (85.71%), OR (95% CI) = 0.25 (0.10, 0.70), P=0.013).
Conclusion
According to this study, the Pfizer-BioNTech (BNT162b2) COVID-19 vaccine was safe when given to Saudi Arabian adults. All reported side-effects were mild to moderate. The findings will likely persuade vaccine-hesitant individuals and pessimists to accept booster dose of COVID-19 vaccine.
Keywords: COVID-19 vaccine, side-effects, Adults, Eastern province, Saudi Arabia
Background
Globally, at the time of writing over 600 million people have been infected by SARS-CoV-2, and around 6.54 have million died from the infection [1,2]. As of September 30th 2022, 816,498 cases, and 9,352 deaths, have been reported in the kingdom of Saudi Arabia [2]. The clinical presentation of SARS-CoV-2 infection ranges from asymptomatic infection or minor illness to severe life-threatening disease. The threats posed by the pandemic prompted pharmaceutical companies to speed up vaccine development, either by using old technologies or by rapidly developing innovative technology [[3], [4], [5]].
A vaccine could be one of the ways to fight the COVID-19 pandemic. Over 5.5 billion people have received a COVID-19 vaccine worldwide until today. Pfizer-BioNTech (BNT162b2) was the first COVID-19 vaccine. It was developed in a very short time and was given FDA (US Food and Drug Administration) approval after just 7 months following its phase I/II trial that took place in May 2020. It was granted EUA (emergency use authorization) in December 2020, and received full FDA approval on August 23, 2021, after having met all efficiency and safety requirements [6,7]. Originally, a large proportion of people in the Middle East were reluctant to accept the vaccine [7,8].
An mRNA vaccine developed by Pfizer and BioNTech encodes the spike glycoprotein of the SARS-CoV-2 virus with its nucleoside mRNA, and can be delivered via lipid-based nanoparticle drug delivery systems that enable efficient extravasation into host cells [9]. COVID-19 is approved for use as a two-dose vaccine by BioNTech (BNT162b2) to be administered between 21 and 28 days after the first dose to all people 12 years and older. With the rise of new variants of the Coronavirus, from Alpha to Omicron such as Delta, Mu, Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), Omicron (B.1.1.529), there is a worry that the decline in immunity over time will reduce the potency of the vaccine and that booster dose will be required to help stimulate immunity against the new strains [[10], [11], [12], [13]].
After FDA approval, the Centers for Disease Control and Prevention (CDC) recommended a booster dose for every 17 years and over. Later the eligibility was expanded to other groups. The World Health Organization is also promoting the importance of obtaining a third booster dose of the vaccine, to emphasize immunization efforts in order decrease the death rates and reduce the severity of disease, so as to protect the health care system in general [14].
The Saudi Arabian government has recommended a booster shot for everyone over 17 years old who has already received two doses. Saudi Arabia's Ministry of Health created public awareness about the importance of a booster dose of COVID-19 vaccine through a campaign called “Maintain Your Level with booster dose” in order to achieve maximum immunity within the community [15].
Although clinical trials assured the efficacy and safety of the vaccine, the had limitations, such as using small numbers of participants and healthier than real world samples of the population. Thus the side-effects of vaccines may have been underestimated. Public confidence may also have been affected by the use of new technologies for vaccine development. Post-marketing evaluation is necessary to increase the public trust in vaccine and encourage them to accept the vaccine [[16], [17], [18], [19]].
Further epidemiological studies are needed to assess the short-term side-effects of COVID-19 vaccine and to increase public confidence in the safety and effectiveness of the current vaccine and any future boosters. Therefore, the objective of this study was to identify short-term side-effects following the administration of a booster dose of Pfizer-BioNTech (BNT162b2) COVID-19 in adults in the Eastern Province of Saudi Arabia.
Methods
Study settings
We conducted a retrospective, cross-sectional study in the Eastern Province of Saudi Arabia, using a self-administered online survey to look for adverse reactions reported by adults aged 18–72 who received the booster dose of Pfizer-BioNTech (BNT162b2) vaccine. Minimum and maximum age distribution has been done on the basis of vaccine eligibility and expectation for the participation in the survey. Study participants were residents of Eastern Province, Saudi Arabia, and needed to have received their booster dose of COVID-19 vaccine prior to completing the survey questionnaire. All respondents who did not receive the booster dose were removed from the study. The survey team identified community members in Eastern Province. Those who were eligible and willing to participate were identified, and their contact information was collected.
Sample size
Based on the Eastern Province population size (around 4.9 million), the sample size was determined using Raosoft.com. To determine the sample size, a margin of error of 5%, a 50% response rate, and a 95% confidence interval, for a population of 4.9 million people were used. During the study period, 450 eligible respondents completed the survey. As a result, 385 responses in this study were considered sufficient. Taking into account a drop-out rate of 10%, the final sample size was set at 424.450 eligible respondents participated in the survey during the study period.
Instrument and data collection
A review of literature, including PubMed, Medline, Google Scholar, and other databases, was conducted with the goal of identifying short-term and potential side-effects that might occur after the Pfizer-BioNTech (BNT162b2) vaccination. The questionnaire was designed to have multiple sections. The first section included an explanation of the purpose of the study, contact details for the investigators, and the consent for participants to agree to take part in the survey. The second section was designed to collect general information about the study participants including age, gender, marital status, educational qualification, occupation, chronic diseases such as hypertension, diabetes mellitus, asthma, and history of having had COVID-19. The third section of the study focused on the COVID-19 vaccine, specifically side-effects experienced after Pfizer-BioNTech's (BNT162b2) COVID-19 vaccine, their timing, and duration. Participants were able to leave the box unchecked if no symptoms were reported. There was a subsection with a list of the most frequently side-effects reported in other studies [6,[20], [21], [22], [23]], including pain or redness at the injection site, body pain, fatigue, fever, chills, headaches, allergies, nausea, and vomiting. Moreover, a section was provided for reporting other possible unlisted side-effects the study participants may have experienced. Furthermore, participants were asked to report visits to doctors and any hospitalizations after vaccination, as well as any medications taken after vaccination. The target group for this study was citizens and residents aged 18 years and above in the Eastern Province of Saudi Arabia. The survey team identified the community for the survey. The survey link was sent to the participants through WhatsApp, SMS, or e-mail and in person to set an appointment. WhatsApp numbers were obtained from different WhatsApp groups of the community identified by researchers; e-mail address were obtained from the authors' affiliation institute. Before starting the survey researchers gave clear instruction about survey to the respondents, ensuring that it was only undertaken by residents of the Eastern province. Non-respondents were followed up by the survey team to encourage participation. The survey was carried-out from 15th November 2021 to 15th January 2022, in the community of Eastern Province, Saudi Arabia.
Only data for those who had received a booster dose of the Pfizer-BioNTech (BNT162b2) vaccine were included in study. Out of the 450 responses 31 (6.88%) were excluded because they did not complete the survey, and 29 (6.44%) were excluded because they received the Moderna COVID-19 vaccine, leaving 390 responses eligible for the final analysis.
Ethical approval
For the conduct of this study, approval was obtained from the standing ethical review committee at the Mohammed Al-Mana College for Medical Sciences (reference number: SR/RP/77). The study excluded responses with no informed consent, incomplete responses, those who failed to take a booster dose, and those who received a COVID-19 vaccine other than the Pfizer-BioNTech (BNT162b2) vaccine.
Statistical analysis
We exported the data from Google Forms (Mountain View, California, USA) into Microsoft Excel (Version. 2016) and then exported it into Statistical Package for Social Sciences (SPSS) version 26.0 (IBM, Inc., Armonk, NY, USA) for statistical analysis. To depict the distribution of categorical variables, descriptive analysis, frequency, and proportion were used, whereas to depict quantitative variables, mean ± standard deviation (SD) or median (interquartile range, IQR) were used. Shapiro-Wilk tests were performed before the analysis to determine the normality of all quantitative variables. We used Pearson's chi-square (χ2) or Fisher's exact test as appropriate for examining the association between COVID-19 post-vaccination side-effects and the independent variables (demographics and background characteristics). In the same way, Mann-Whitney U tests and Kruskal-Wallis tests were used to measure the number of COVID-19 side effects that respondents experienced after vaccination as an ordinal dependent variable depending on the independent variables.
Additionally, multivariate logistic regression analysis was conducted to determine the factors associated with a complaint about side-effects following administration of the Pfizer -BioNTech (BNT162b2) COVID-19 vaccine, which was coded as a dummy dependent variable (no = 0 and yes = 1). In order to determine if certain factors are associated with side effects following vaccination, we performed a multivariate ordinal logistic regression analysis. A multivariate logistic regression model was created based on all variables that were associated with P ≤ 0.25 in univariate analyses [24]. In all analyses, odds ratios, and intervals of confidence, were calculated. The significance of a P-value was defined as less than 0.05. Multiple comparisons were corrected for using the sequential Bonferroni correction (Bonferroni-Holm) method [25].
Results
Socio-demographics and general characteristics data of respondents
Table I shows the socio-demographics and general characteristics data of the 390 eligible respondents. The average ± SD age of the respondents was 30.23±4.81 years; 92 (23.6%) were female, and 298 (76.4%) were male. The majority (303, 77.7%) of respondents were unmarried. Sixty-nine (17.7%) were married, and 7, 6 and 5 were respectively widowed, divorced, or separated. Two hundred and forty-five (62.8%) respondents were Diploma or post-high school qualified, 69 (17.7%) were Bachelor degree holders, 49 (12.6%) were high school-qualified only. Only 6.92% (n=27) were Master/Ph.D. holders. Two hundred and eighty-one (72.0%) respondents worked outside healthcare, 59 (15.1%) were unemployed, 42 (10.1%) were healthcare workers, and 8 (2.0%) were housewives. Two hundred and eighty-one (68.2%) of respondents had chronic condition disease such as hypertension (HTN), diabetes mellitus (DM) and asthma. Two-hundred and nineteen (56.5%) of respondents had a history of having had COVID-19.
Table I.
Socio-demographics and general characteristics data of respondents (n= 390)
| Characteristics | n (%) | |
|---|---|---|
| Age (years) (Mean± SD: 30.23±4.81) | 18–36 | 338 (86.67) |
| 37–54 | 34 (8.72) | |
| 55–72 | 18 (4.62) | |
| Gender | Male | 298 (76.41) |
| Female | 92 (23.59) | |
| Marital status | Unmarried | 303 (77.69) |
| Married | 69 (17.69) | |
| Widow | 7 (1.79) | |
| Divorce | 6 (1.54) | |
| Separated | 5 (1.28) | |
| Educational Qualification | High school | 49 (12.56) |
| Diploma or post high school | 245 (62.82) | |
| Bachelor | 69 (17.69) | |
| Master/Ph.D. | 27 (6.92) | |
| Occupation | Non-Healthcare related | 281 (72.05) |
| Healthcare related | 42 (10.77) | |
| Unemployed | 59 (15.13) | |
| House wife | 8 (2.05) | |
| Suffering from any chronic disease such as HTN, DM and Asthma or others | Yes | 266 (68.21) |
| No | 124 (31.79) | |
| History of COVID-19 infection before taking booster dose-vaccine | Yes | 171 (43.85) |
| No | 219 (56.48) | |
Reported Side-Effects after COVID-19 booster dose-vaccine of Pfizer-BioNTech (BNT162b2)
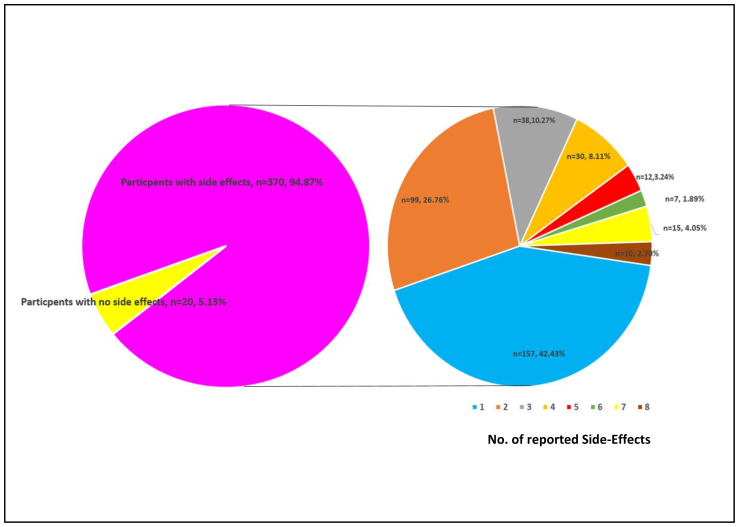
Three hundred and seventy (94.9%) of those receiving the COVID-19 booster dose vaccine from BioNTech (BNT162b2) reported experiencing at least one side-effect. One hundred and fifty-seven (42.4%) recipients reported one side-effect (%; n=157), and at the other extreme 10 (2.8%) reported experiencing eight side-effects (Figure 1).
Figure 1.
Total number of COVID-19 vaccine side-effects reported among the participants associated with COVID-19 Booster dose-vaccine of Pfizer-BioNTech (BNT162b2) among respondents.
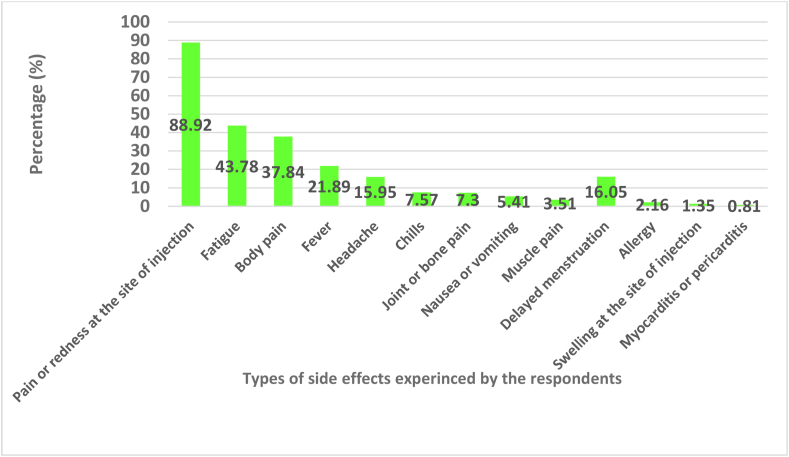
Numbers of patients with different side effects are shown in Figure 2; pain and redness at the site of injection (88.92%) was much the commonest side effect. Side effects according to gender are shown in Table II. The distribution of different COVID-19 booster dose-vaccine side-effects according to respondents' gender in shown in Table II. There were some significant differences between the sexes. For example, males were more likely to experience pain or redness at the injection site (93.1% vs 74.1%,; χ2 = 132.76; P=0.001), whereas females were more likely to complain of fatigue (65.4% vs 37.7%, χ2 =19.74; P=0.001).
Figure 2.
Frequency and types of side-effects associated with COVID-19 Vaccines among respondents.
Table II.
Distribution of COVID-19 vaccine side-effects reported among the respondents according to gender (n =370)
| Outcome | Male (n = 289) | Female (n = 81) | Total (n =370) | χ2 (P) |
|---|---|---|---|---|
| Body pain | 83 (28.72) | 57 (70.37) | 140 (37.84) | 4.82 (0.02) |
| Pain or redness at the site of injection | 269 (93.08) | 60 (74.07) | 329 (88.92) | 132.76 (0.001) |
| Fatigue | 109 (37.72) | 53 (65.43) | 162 (43.78) | 19.74 (0.001) |
| Fever | 42 (14.53) | 39 (48.15) | 81 (21.89) | 0.111 (0.74) |
| Chills | 9 (3.11) | 19 (23.46) | 28 (7.57) | 3.57 (0.06) |
| Headache | 22 (7.61) | 37 (45.68) | 59 (15.95) | 3.81 (0.05) |
| Joint or bone pain | 9 (3.11) | 18 (22.22) | 27 (7.30) | 3 (0.08) |
| Nausea or vomiting | 6 (2.08) | 14 (17.28) | 20 (5.41) | 3.2 (0.07) |
| Muscle pain | 9 (3.11) | 4 (4.94) | 13 (3.51) | 0.492 (0.497)† |
| Delayed menstruation | - | 13 (16.05) | 13 (16.05) | - |
| Myocarditis or pericarditis | 1 (0.35) | 2 (2.47) | 3 (0.81) | 0.51 (0.122)† |
| Allergy | 2 (0.69) | 6 (7.41) | 8 (2.16) | 0.003 (0.002)† |
| Swelling at the site of injection | 4 (1.38) | 1 (1.23) | 5 (1.35) | 1 (0.062)† |
χ2, chi-square test statistic. Significant difference between the two groups (unadjusted P < 0.05). †Fisher's exact test.
Associations of the reported COVID-19 booster dose-vaccine of Pfizer-BioNTech (BNT162b2) Side-Effects
Table III compares the reporting of COVID-19 vaccine side-effects with respondents' background characteristics. Of particular note, respondents with non-healthcare related employment were more likely to report side-effects compared with healthcare workers.
Table III.
Associations of the reported COVID-19 vaccine side-effects with the respondents' background characteristics (n = 390)
| Characteristic | Respondents with side Effects, n=370 |
Respondents with no Side-effects, n=20 | OR (95% CI) | P-Value |
|---|---|---|---|---|
| Age, years 18-36 (n=338) 37-54 (n=34) 55-72 (n=18) |
328 (97) 29 (85.29) 13 (72.22) |
10 (3) 5 (14.70) 5 (27.78) |
7.80 (3.07, 19.86) 0.26 (0.09, 0.75) 0.11 (0.03, 0.34) |
0.001∗ 0.022∗ 0.001∗ |
| Gender Male (n=298) Female (n= 92) |
289 (96.97) 81 (88.05) |
9 (1.67) 11 (11.95) |
4.36 (1.75, 10.9) 0.22 (0.09, 0.5) |
0.001∗ 0.001∗ |
| Marital status Unmarried (n=303) Married (n= 69) Widow (n= 7) Divorce (n= 6) Separated (n=5) |
296 (97.68) 59 (85.50) 6 (85.71) 5 (83.33) 4 (80) |
7 (2.31) 10 (14.92) 1 (14.28) 1 (16.66) 1 (20) |
7.42 (2.86, 19.27) 0.18 (0.073, 0.45) 0.32 (0.04, 2.81) 0.27 (0.29, 2.40) 4.94 (0.52, 46.43) |
<0.001∗ 0.005∗ 0.317 0.280 0.227 |
| Occupation Non-Healthcare related (n=281) Healthcare related (n=42) Unemployed (n= 59) House wife (n=8) |
273 (97.15) 36 (85.71) 54 (91.52) 7 (87.50) |
7 (2.49) 6 (14.28) 6 (10.16) 1 (12.5) |
5.22 (2.02, 13.48) 0.25 (0.10, 0.70) 0.39 (0.14, 1) 0.36 (0.04, 3.1) |
<0.001∗ 0.013∗ 0.103 0.342 |
| Highest educational qualification High school (n= 49) Diploma or post high school (n= 245) Bachelor (n= 69) Master/Ph.D. (n=27) |
46 (93.87) 239 (96.32) 63 (91.30) 22 (81.48) |
3 (6.122) 6 (2.44) 6 (8.69) 5 (18.51) |
0.80 (0.22, 2.8) 4.25 (1.60, 11.34) 0.47 (0.18, 1.30) 0.19 (0.06, 0.56) |
0.001∗ 0.002∗ 0.221 0.008∗ |
| History of comorbidities such as DM/HTN/Asthma or others Yes (n= 266) No (n=124) |
260 (97.74) 110 (88.70) |
6 (2.25) 14 (11.29) |
5.51 (2.06, 14.72) 0.18 (0.06, 0.48) |
0.001∗ 0.001∗ |
| History of COVID-19 infection before booster dose-vaccine Yes (n=171) No (n=219) |
166 (97.07) 204 (93.15) |
5 (2.92) 15 (6.84) |
2.44 (0.86, 6.85) 0.40 (0.15, 1.119) |
0.102 0.063 |
OR: Odds ratio; CI: Confidence interval. Significant association (unadjusted P < 0.05). ∗ Significant association (using the Fisher exact test correction for multiple comparisons).
Differences in the number of COVID-19 Booster dose-vaccine side-effects reported by respondent characteristics are shown in Table IV. Numbers of side effects experienced were higher in unmarried respondents (U = 16872; P = 0.001), those with comorbidities (U = 28600; P = 0.001), and those with a history of previous COVID-19. Multivariate logistic regression analysis showed that only the presence of comorbidities was associated with an increased risk of side-effects (adjusted odds ratio (aOR) = 3.434; 95% CI = 1.116, 10.569, P-value=0.031) (Table V).
Table IV.
Number of side-effects reported by the respondents following the booster dose-vaccine of Pfizer-BioNTech (BNT162b2) COVID-19 vaccine according to the respondents' background characteristics (n = 370)
| Characteristic | No. of Reported side- Effects |
Statistics | P-Value |
|---|---|---|---|
| Median (IQR) | |||
| Age, years 18-36 (n=328) 37-54 (n=29) 55-72 (n=13) |
3 (1,3) 2 (1,3) 2 (2,3) |
H=2.0 | 0.367 |
| Gender Male (n=289) Female (n= 81) |
3 (3,4) 2 (1,2) |
H=1.0 | 0.30 |
| Marital status Unmarried (n=296) Married (n= 59) Widow (n= 6) Divorce (n= 5) Separated (n=4) |
4 (3,4) 3 (3,4) 2 (1,2) 1 (1,2) 1 (1,2) |
U=16872 | 0.001 |
| Occupation Non-Healthcare related (n=273) Healthcare related (n=36) Unemployed (n= 54) House wife (n=7) |
3 (2,4) 2 (1,2) 3 (2,4) 2 (1,2) |
H=4.0 | 0.406 |
| Highest educational qualification High school (n= 46) Diploma or post high school (n= 239) Bachelor (n= 63) Master/Ph.D. (n=22) |
2 (1,2) 3 (2,4) 2 (2,4) 1 (1,2) |
H=3.0 | 0.391 |
| History of comorbidities such as DM/HTN/Asthma or others Yes (n= 260) No (n=110) |
3 (1,4) 2 (1,4) |
U= 28600 | 0.001 |
| History of COVID-19 infection before taking booster dose-vaccine of Pfizer-BioNTech (BNT162b2) Yes (n=166) No (n=204) |
3 (1,4) 2 (1,4) |
U= 18659 | 0.001 |
IQR: Interquartile range; U: Mann–Whitney U statistic; H: Kruskal–Wallis test statistic. Significant difference (unadjusted P < 0.05). ∗ Significant difference (using the Bonferroni correction for multiple comparisons).
Table V.
Multivariate analysis of factors associated with reporting of side-effects following booster dose of Pfizer-BioNTech (BNT162b2) vaccine among the participants (n = 370)
| Variable | aOR | 95% CI | P-value |
|---|---|---|---|
| Gender (female) | 2.286 | 0.803, 6.508 | 0.121 |
| History of comorbidities | 3.434 | 1.116, 10.569 | 0.031∗ |
| History of COVID-19 infection | 1.860 | 0.642, 5.384 | 0.253 |
Table VI estimates that multivariate ordinal logistic regression and logistic regression analysis are factors that affect the number of side effects reported by non-healthcare workers following vaccination with COVID-19 vaccine. The model had Goodness-of-Fit and was statistically significant (χ2 = 27.592, P<0.001) for all variables that found differences with P≤0.25 in the univariate analysis presented in Table IV. The number of side effects reported among study participants was significantly related to their occupations and genders. Even when other variables were kept constant, healthcare workers reported fewer side-effects than other respondents (aOR = 0.174; 95% CI = 0.133; P< 0.001). Similarly, female participants reported a lower number of side effects than male participants (aOR = 0.205; 95% CI = 0.353, 0.97; P<0.001).
Table VI.
Results of ordinal logistic regression for the factors related with the number of reported side-effects following booster dose of Pfizer-BioNTech (BNT162b2) COVID-19 vaccine (n = 370)
| Variable | aOR | 95% CI | P-value |
|---|---|---|---|
| Age (year) | 0.068 | 0.308, 0.403 | <0.001∗ |
| Gender (Female) | 0.205 | 0.353, 1.863 | <0.001∗ |
| Occupation (Healthcare related) | 0.174 | 0.133,0.403 | <0.001∗ |
aOR, adjusted odds ratio; CI, confidence interval. ∗ Significant association (P < 0.05).
The onset, duration, and management of COVID-19 vaccine side-effects reported among the participants in the study are given in Table VII. Ninety-six-point-two percent of respondents reported side-effects occurring on the day of vaccine administration. Side effects generally lasted for no more than two days. Nine (2.4%) of respondents with side effects attended hospital, and 4 (1.1%) were hospitalized as a result of side-effects. Most respondents reported taking medication for alleviating post-vaccination side-effects.
Table VII.
The onset, duration, and management of COVID-19 vaccine side-effects reported among the participated in the study (n = 370)
| Onset and duration of Side-effects | Male (n = 289) | Female (n = 81) | Total (n = 370) | χ2 (P) |
|---|---|---|---|---|
| Duration of the side effect symptom One day From 1-2 days From 3-5 days More than 5 days |
7 (2.42) 247 (85.47) 29 (10.03) 6 (2.08) |
2 (2.47) 38 (46.91) 27 (33.33) 14 (17.28) |
9 (2.43) 287 (77.57) 56 (15.14) 20 (5.41) |
61.97 (0.001) |
| Time of the side-effects started to appear Day 0 Day 1 Day 2 |
281 (97.23) 7 (2.42) 1 (0.35) |
75 (92.59) 4 (4.94) 2 (2.47) |
356 (96.22) 11 (2.97) 3 (0.81) |
5.06 (0.080) |
| Taking medication to alleviate side-effects | 277 (95.84) | 65 (80.24) | 342 (92.43) | 28.38 (0.001) |
| Visiting a hospital due to side-effects | 3 (1.04) | 6 (7.41) | 9 (2.43) | 1 (0.312)† |
| Hospitalization because of side-effects | 2 (0.69) | 2 (2.47) | 4 (1.08) | 0 (0.209)† |
χ2: Chi-square test statistic; †Fisher's exact test statistic.
Discussion
In the context of the ongoing global COVID-19 pandemic, and due to emergence of variants of SARS-CoV-2 [26], the Ministry of Health, Saudi Arabia announced the nationwide administration of the booster dose of COVID-19 vaccine as a preventive measure to confront ‘fast-spreading’ variants [27,28]. According to the announcement, booster shots will be mandatory for priority groups (age group of 17 years and older) [27]. Even though Saudi Arabians have access to the vaccine, they still hesitate to get vaccinated. Some have suggested that this is because the vaccine was approved so quickly, and/or suspicion of the new mRNA vaccine technology [[28], [29], [30]]. We evaluated the short-term side effects of a booster dose of Pfizer-BioNTech (BNT162b2) in Eastern Province, Saudi Arabia. To date few Saudi Arabian studies have been conducted to gauge the safety and efficacy of booster doses of Pfizer (BNT162b2) COVID-19 vaccine. Although we gave respondents the opportunity to report any possible side effects, participants reported only previously reported common side effects. According to the statistics, the Eastern Province individuals reacted differently to the vaccine with variances in reaction rates. Results revealed similar side-effects reported during the first day among groups, and comparable with previously reported findings among individuals [31].
In this study, it was observed that there was a high incidence of side-effects after taking the booster dose around 95%, similar to after the first and second doses of Pfizer-BioNTech's (BNT162b2) COVID-19 Vaccine. Comparing our results to a previous study, in which we examined short-term side-effects of BioNTech (BNT162b2) in individuals aged 18–70 years old, we found fewer overall adverse reactions (40%, n = 208) [32]. More side-effects were reported in a younger population [9,33]. Pain redness at the injection site (88.9%), fatigue (43.8%), body pain (37.8%), fever (21.9%), and headache (15.9%) were the most frequently reported side-effects, consistent with other real world studies [6,16,17,34,15], A phase 3 clinical trial with the Pfizer-BioNTech (BNT162b2) vaccine revealed that injection site pain was the most common complication (71–83%), fatigue was the second (34–47%), and headache was the third (25–42%).
Also consistent with previous studies was that 96.2% of side-effects occurred on the day of vaccination, and only 0.81% persisted for more than two days [29,30]. 92.4% of our participants took medication (mainly analgesics) to alleviate the side effects, which has previously been reported to be common practice amongst Saudi Arabians [37]. Despite the high frequency of side effects only small numbers of respondents concluded a physician, or required hospital admission, because of side effects, which strongly supports the safety of these vaccines. The existing literature had generally reported that side-effects are more common among females and among people younger than 55 years [6,16,17,[34], [35], [36], [37]], whereas we found that side effects were more common amongst males. However, according to a study conducted in southwestern Saudi Arabia, Pfizer-BioNTech's (BNT162b2) COVID-19 caused more side-effects in male patients [38].
The number of side-effects was also greater among individuals with past exposure to SARS-CoV-2, in accordance with several prior reports of more side-effects following second vaccine doses as well as in people with a history of COVID-19 [6,34,35,39]. There is evidence that the humoral immunity in individuals with prior SARS-CoV-2 infection following a single dose of the vaccine is equal to or stronger than in SARS-CoV-2-naïve individuals following a second dose [40]. This can induce cytokine production that mediates mediating more systemic adverse effects after repeated vaccine doses, especially in individuals who had previously been infected with SARS-CoV 2 [41].
Previous studies have suggested a gender-based difference in reporting adverse events following various viral and bacterial vaccines [33,42]. In general, females respond to vaccines more strongly with inflammatory, antibody, and cell-mediated immune responses, which may explain the differences in reactions and immunogenicity [43]. Moreover, behavioural, genetic, and hormonal factors might also contribute to gender-based variation in adverse vaccination reactions [[42], [43], [44]]. Some studies have found a significant relationship between the development of post-vaccination side-effects and advancing age. Studies among healthcare workers in Jordan [36], Germany [45], and Slovakia [46], found that young adult participants had a significantly higher incidence of COVID-19 vaccine side-effects. In contrast to other studies conducted in Saudi Arabia and elsewhere in multivariate analysis we found no association between age and side effects [7,47].
Our study can contribute to increasing public confidence in the safety profile of COVID-19 Pfizer-BioNTech vaccine, which is important in improving vaccine coverage in a population with a high degree of scepticism. However, our study is not without limitations. First, the self-administered online questionnaire could have resulted in reporting bias. We chose to conduct the study as a web-based study to ensure the safety of all participants at a time when social distancing still applied in Saudi Arabia. The online nature of the study, and the use of the investigators' networks meant that younger and male participants were over-represented. Our study only assessed short-term side-effects and was not larger enough to assess rare events such as thromboembolic profiles and myocarditis [48]. (immediately after injection). Finally, we restricted our study to recipients of the Pfizer-BioNTech (BNT162b2) vaccine, and our. At the time of the study supply issues with the alternative Moderna vaccine meant that there were insufficient recipients to allow meaningful analysis.
Conclusions
Our study showed that participants in the Eastern province of Saudi Arabia receiving a booster dose of the Pfizer-BioNTech (BNT162b2) COVID-19 vaccine commonly experienced short-lived side effects, especially pain or redness at the infection site, but also fatigue, body pain, fever and headache. 2.4% of recipients had sought medical attention for their side effects, although only four participants (1.1%) were admitted to the hospital after experiencing side effects. Findings from this study support the vaccine's safety as well as provide important baseline data for healthcare workers and the general public to be aware of possible side effects following the COVID-19 vaccine. In light of the current study, its results support the vaccine's safety and provide important baseline data that will raise healthcare workers' and the general public's awareness of COVID-19 vaccine expected side-effects. In this way, vaccine-hesitant individuals and pessimists might be convinced to accept the COVID-19 booster dose.
Author's contribution
Writing of the first draft: M.P., N.B. and A.Y. (Munfis Patel, Nuzhat Banu and Ayaz Ahmad). Data analysis: M.A. (Mohammad Daud Ali), J.A. (Jenan Ahmed Al-Matouq). Concept, and design of the study: M.A. (Mohammad Daud Ali). Participated review the manuscript, concept, and editing, writing and review, and revised the manuscript: J.A., M.A. Construction of Questionnaire: M.A., M.P. Review the manuscript: J.A. Data collection: S.A. (Samiah Mohammad Alsomali) and M.A. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Informed consent statement
Informed consent was obtained from all the respondents who participated in the study.
Data availability statement
The data presented in this study are available on request from the corresponding author.
Conflicts of interest
All the author(s) declare no conflict of interest.
Funding
None.
References
- 1.Coronavirus Cases worldwide. Last updated: September 30, 2022, 17:16 GMT. Available from: https://www.worldometers.info/coronavirus/. (Assess on 30th September 2022).
- 2.Coronavirus cases: Saudi Arabia. Last updated: September 30, 2022, 17:16 GMT. Available from: https://www.worldometers.info/coronavirus/country/saudi-arabia/. (Assess on 30th September 2022).
- 3.Hapshy V., Aziz D., Kahar P., Khanna D., Johnson K.E., Parmar M.S. COVID-19 and pregnancy: risk, symptoms, diagnosis, and treatment. SN Compr Clin Med. 2021:1–12. doi: 10.1007/s42399-021-00915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suri J.S., Agarwal S., Gupta S.K., Puvvula A., Biswas M., Saba L., et al. A narrative review on characterization of acute respiratory distress syndrome in COVID-19-infected lungs using artificial intelligence. Comput Biol Med. 2021;104210:130. doi: 10.1016/j.compbiomed.2021.104210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. Symptoms of coronavirus. Centers for Disease Control. Published 2021. Updated 2022. Available from: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. (Assess on 14th March 2022).
- 6.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Publisher correction: phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2021;590(7844):E26. doi: 10.1038/s41586-020-03098-3. E26. [DOI] [PubMed] [Google Scholar]
- 7.FDA. Published 2021 Updated 2021 Aug 23. [Updated 2022 11 March]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine.. (Assess on 14th March 2022).
- 8.Alfageeh E.I., Alshareef N., Angawi K., Alhazmi F., Chirwa G.C. Acceptability of a COVID-19 Vaccine among the Saudi Population. Vaccines. 2021;226:9. doi: 10.3390/vaccines9030226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.From Alpha to Omicron: Everything you need to know about SARS-CoV-2 variants of concern. Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html. (Assess on 30th September 2022).
- 11.Uriu K., Kimura I., Shirakawa K., Takaori-Kondo A., Nakada T.A., Kaneda A., et al. Genotype to Phenotype Japan (G2P-Japan) Consortium. Neutralization of the SARS-CoV-2 Mu Variant by Convalescent and Vaccine Serum. N Engl J Med. 2021;385(25):2397–2399. doi: 10.1056/NEJMc2114706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aouissi H.A. Algeria's preparedness for Omicron variant and for the fourth wave of COVID-19. Glob Health Med. 2021;3(6):413–414. doi: 10.35772/ghm.2021.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen H.L., Sit T.H.C., Brackman C.J., Chuk S.S.Y., Gu H., Tam K.W.S., et al. HKU-SPH study team. Transmission of SARS-CoV-2 delta variant (AY.127) from pet hamsters to humans, leading to onward human-to-human transmission: a case study. Lancet. 2022;399(10329):1070–1078. doi: 10.1016/S0140-6736(22)00326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Interim statement on booster doses for COVID-19 vaccination. Available from: https://www.who.int/news/item/22-12-2021-interim-statement-on-booster-doses-for-covid-19-vaccination–-update-22-december-2021. (Assess on 14th March 2022).
- 15.Saudi Food & Drug Authority Allows the Import and Use of Pfizer-BioNTech (BNT162b2) Covid 19 Vaccine. Available from: https://sfda.gov.sa/en/cnews. (Assess on 14th March 2022).
- 16.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N. Engl. J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ossato A., Tessari R., Trabucchi C., Zuppini T., Realdon N., Marchesini F. Comparison of medium-term adverse reactions induced by the first and second dose of mRNA BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine: A post-marketing Italian study conducted between 1 January and 28 February 2021. Eur. J. Hosp. Pharm. 2021 doi: 10.1136/ejhpharm-2021-002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arce J.S.S., Warren S.S., Meriggi N.F., Scacco A., McMurry N., Voors M., et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021;27:1385–1394. doi: 10.1038/s41591-021-01454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerji A., Wickner P.G., Saff R., Stone C.A., Robinson L.B., Long A.A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: Current evidence and suggested approach. J. Allergy Clin. Immunol In Pract. 2021;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meo S.A., Bukhari I.A., Akram J., Meo A.S., Klonoff D.C. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663–1669. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- 21.Lounis M., Rais M.A., Bencherit D., Aouissi H.A., Oudjedi A., Klugarová J., et al. Side Effects of COVID-19 Inactivated Virus vs. Adenoviral Vector Vaccines: Experience of Algerian Healthcare Workers. Front Public Health. 2022;10:896343. doi: 10.3389/fpubh.2022.896343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuschieri S., Borg M., Agius S., Souness J., Brincat A., Grech V. Adverse reactions to Pfizer-BioNTech vaccination of healthcare workers at Malta’s state hospital. Int J Clin Pract. 2021;75:e14605. doi: 10.1111/ijcp.14605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nittner-Marszalska M., Rosiek-Biegus M., Kope´c A., Pawłowicz R. Kosi´ nska, M.; Łata, A.; Szenborn, L. Pfizer-BioNTech COVID-19 vaccine tolerance in allergic versus non-allergic individuals. Vaccines. 2021;9:553. doi: 10.3390/vaccines9060553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendel R.B., Afifi A.A. Comparison of stopping rules in forward “stepwise” regression. J Am Stat Assoc. 1977;72:46–53. [Google Scholar]
- 25.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 26.Tracking SARS-CoV-2 variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. (Access on 10 Nov. 2021).
- 27.The world is Witnessing a Decrease in COVID-19 Critical Cases. https://www.moh.gov.sa/en/Ministry/MediaCenter/News/Pages/News-2022-01-16-003.aspx. (Access on 5th Dec. 2021).
- 28.Bono S.A., Faria de Moura Villela E., Siau C.S., Chen W.S., Pengpid S., Hasan M.T., et al. Factors affecting COVID-19 vaccine acceptance: An international survey among Low- and Middle-Income Countries. Vaccines. 2021;515(5):9. doi: 10.3390/vaccines9050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Available online: Covid vaccine: Period changes could be a short-term side effect. https://www.bbc.com/news/health-56901353. (Access on 5th Dec. 2021).
- 30.Yan Z.P., Yang M., Lai C.L. COVID-19 Vaccines: a review of the safety and efficacy of current clinical trials. Pharmaceuticals. 2021;406(5):14. doi: 10.3390/ph14050406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L., et al. Preliminary findings of mRNA Covid-19 vaccine safety in Pregnant persons. N Engl J Med. 2021;384(24):2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alhazmi A., Alamer E., Daws D., Hakami M., Darraj M., Abdelwahab S., et al. Evaluation of Side-effects Associated with COVID-19 Vaccines in Saudi Arabia. Vaccines (Basel) 2021;9(6):674. doi: 10.3390/vaccines9060674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flanagan K.L., Fink A.L., Plebanski M., Klein S.L. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33:577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- 34.Skowronski D.M., De Serres G. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J Med. 2021;384:1576–1577. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 35.Frenck R.W., Jr., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N. Engl. J Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abu-Hammad O., Alduraidi H., Abu-Hammad S., Alnazzawi A., Babkair H., Abu-Hammad A., et al. Side-effects reported by Jordanian healthcare workers who received COVID-19 vaccines. Vaccines. 2021;577:9. doi: 10.3390/vaccines9060577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alghamdi A.A., Alkazemi A., Alissa A., Alghamdi I., Alwarafi G., Waggas H.A. Adverse events following AstraZeneca COVID-19 vaccine in Saudi Arabia: A cross-sectional study among healthcare and non-healthcare workers. Intervirology. 2021 doi: 10.1159/000519456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adam M., Gameraddin M., Alelyani M., Alshahrani M.Y., Gareeballah A., Ahmad I., et al. Evaluation of post-vaccination symptoms of two common COVID-19 vaccines used in Abha, Aseer Region, Kingdom of Saudi Arabia. Patient Prefer. Adherence. 2021;15:1963–1970. doi: 10.2147/PPA.S330689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flanagan K.L., Fink A.L., Plebanski M., Klein S.L. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33:577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- 40.Frieman M., Harris A.D., Herati R.S., Krammer F., Mantovani A., Rescigno M., et al. SARS-CoV-2 vaccines for all but a single dose for COVID-19 survivors. EBioMedicine. 2021;68:103401. doi: 10.1016/j.ebiom.2021.103401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCartney P.R. Sex-based vaccine response in the context of COVID-19. J Obstet Gynecol Neonatal Nurs. 2020;49:405–408. doi: 10.1016/j.jogn.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vassallo A., Shajahan S., Harris K., Hallam L., Hockham C., Womersley K., et al. Sex and gender in COVID-19 vaccine research: Substantial evidence gaps remain. Front. Glob. Women Health. 2021;761511:2. doi: 10.3389/fgwh.2021.761511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris T., Nair J., Fediurek J., Deeks S.L. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012–2015. Vaccine. 2017;35:2600–2604. doi: 10.1016/j.vaccine.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 45.Klugar M., Riad A., Mekhemar M., Conrad J., Buchbender M., Howaldt H.P., et al. Side-effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. Biology. 2021;752:10. doi: 10.3390/biology10080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riad A., Hocková B., Kantorová L., Slávik R., Spurná L., Stebel A., et al. Side-effects of mRNA-based COVID-19 vaccine: Nationwide phase IV study among healthcare workers in Slovakia. Pharmaceuticals. 2021;873:14. doi: 10.3390/ph14090873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeon M., Kim J., Oh C.E., Lee J.Y. Adverse events following immunization associated with the first and second doses of the ChAdOx1 nCoV-19 vaccine among healthcare workers in Korea. Vaccines. 2021;1096:9. doi: 10.3390/vaccines9101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dionne A., Sperotto F., Chamberlain S., Baker A.L., Powell A.J., Prakash A., et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 Vaccine in a case series of children. JAMA Cardiol. 2021;6(12):1446–1450. doi: 10.1001/jamacardio.2021.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.