Abstract
Background:
There are good data to support using a single high-sensitivity cardiac troponin T (hs-cTnT) below the limit of detection (LoD) of 5 ng/L to exclude acute myocardial infarction. Per the United States (US) Food and Drug Administration (FDA), hs-cTnT can only report to the limit of quantitation (LoQ) of 6 ng/L, a threshold for which there is limited data. Our goal was to determine whether a single hs-cTnT below the LoQ of 6 ng/L is a safe strategy to identify patients at low-risk for acute myocardial injury and infarction.
Methods:
The efficacy (proportion identified as low-risk based on baseline hs-cTnT<6 ng/L) of identifying low-risk patients was examined in a multicenter (n=22 sites) US cohort study of emergency department patients undergoing at least one hs-cTnT (CV Data Mart Biomarker cohort). We then determined the performance of a single hs-cTnT<6 ng/L (biomarker alone) to exclude acute myocardial injury (subsequent hs-cTnT >99th percentile in those with an initial hs-cTnT<6 ng/L). The clinically intended rule-out strategy combining a nonischemic electrocardiogram with a baseline hs-cTnT<6 ng/L was subsequently tested in an adjudicated cohort in which the diagnostic performance for ruling-out acute myocardial infarction and safety (myocardial infarction or death at 30-days) were evaluated.
Results:
A total of 85,610 patients were evaluated in the CV Data Mart Biomarker cohort, amongst which 24,646 (29%) had a baseline hs-cTnT<6 ng/L. Women were more likely than men to have hs-cTnT<6 ng/L (38% vs. 20%, p<0.0001). Among 11,962 patients with baseline hs-cTnT<6 ng/L and serial measurements, only 1.2% developed acute myocardial injury, resulting in a negative predictive value of 98.8% (95% CI 98.6, 99.0) and sensitivity of 99.6% (95% CI 99.5, 99.6). In the adjudicated cohort, a nonischemic electrocardiogram with hs-cTnT<6 ng/L identified 33% of patients (610 of 1849) as low-risk and resulted in a negative predictive value and sensitivity of 100% and a 30-day rate of 0.2% for 30-day myocardial infarction or death.
Conclusions:
A single hs-cTnT below the LoQ of 6 ng/L is a safe and rapid method to identify a substantial number of patients at very low risk for acute myocardial injury and infarction.
Keywords: High-sensitivity cardiac troponin, Cardiac troponin, Myocardial infarction, Myocardial injury
There are over 6.5 million emergency department visits for symptoms suspicious for acute myocardial infarction yearly across the United States (US)1. High-sensitivity cardiac troponin (hs-cTn) assays permit earlier evaluation of these patients2–3. Studies indicate that a single hs-cTn measurement with a concentration below the limit of detection (LoD), or for some assays at higher optimized concentrations, is safe to exclude acute myocardial infarction4–13. Since 2015 European Society of Cardiology (ESC) guidelines endorse algorithms that allow single sample rule-out of acute myocardial infarction with class I recommendations when the initial hs-cTn is very low14,15. For hs-cTnT, these guidelines14,15 recommend rule-out in those with a baseline hs-cTnT below the LoD of 5 ng/L. There are good data4,6,11–13 from outside the US supporting the approach. These include meta-analyses4,6 and 2 randomized trials11,12 using hs-cTnT. Most studies in this field have been designed to exclude acute myocardial infarction rather than myocardial injury. In the US, however, the LoD cannot be reported for clinical use per the US Food and Drug Administration (FDA)16,17 despite recent guidelines1 from the American Heart Association (AHA) / American College of Cardiology (ACC) that support this approach to exclude myocardial injury with a class 2a recommendation (level of evidence B – nonrandomized) for patients with suspected acute coronary syndrome with symptom onset at least 3 hours (h) prior to presentation.
In the US, hs-cTn assays were FDA-cleared for clinical use to report down to the limit of quantitation (LoQ) where imprecision is 20% or less 16,17, which for high-sensitivity assays is a concentration threshold above the LoD. For hs-cTnT, this means that results can only be reported to hs-cTnT<6 ng/L17. Whether a single sample rule-out approach using the LoQ is safe for clinical use is unclear.
There are a few small studies evaluating hs-cTnT below the LoQ threshold of 6 ng/L. They often use investigational samples18,19 or are secondary analyses from outside the US20,21. Some of these studies have been inconclusive and lack an adequate gold-standard assay18,19 for acute myocardial infarction diagnosis, including some data suggesting a single hs-cTnT <6 ng/L may not be safe22. In the US, hs-cTn testing is used more broadly23,24 than in Europe, so European data from more selected chest pain populations21 may not be as informative for US practice, and validation of the LoQ approach is needed. Further, concerns exist surrounding the analytical performance of hs-cTnT, especially below the LoQ25,26. Despite these uncertainties and a paucity of definitive data, some US centers have implemented this approach27–29. Thus, there is an urgent need for more data to inform whether the single sample rule-out strategy using a hs-cTnT <6 ng/L is an efficient and safe strategy to identify patients at low-risk for acute myocardial infarction.
To address this unmet need, we examined our multicenter US experience with hs-cTnT. Our goals were two-fold. First, we examined the efficacy (proportion of patients identified as low-risk) and safety of a single hs-cTnT<6 ng/L to identify low-risk patients based on its ability to rule-out acute myocardial injury in a large multicenter biomarker cohort. Second, to evaluate the use of this approach to identify patients at low-risk for acute myocardial infarction, we examined the combined use of a nonischemic electrocardiogram with a hs-cTnT<6 ng/L in an adjudicated cohort to assess the diagnostic performance for index acute myocardial infarction and 30-day safety (myocardial infarction or death).
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
CV Data Mart Biomarker Cohort: efficacy of a single hs-cTnT below the 6 ng/L LoQ for identifying low-risk patients by excluding acute myocardial injury
The CV Data Mart Biomarker cohort is a multicenter, observational, biomarker study involving consecutive, adult patients who presented to one of the Mayo Clinic emergency departments (Rochester, Florida, and Arizona campuses or the 19 Mayo Clinic Health System emergency departments across Minnesota and Wisconsin) in whom at least one hs-cTnT measurement was obtained within 12h of presentation from the date of site-specific hs-cTnT implementation until 12/08/20. It does not include electrocardiographic data. Eligible patients including their baseline characteristics and outcomes were identified using the Mayo Clinic CV Data Mart platform. The CV Data Mart is a collection of cardiology databases that facilitates analyses of existing data from the clinical systems. Patients without a required Minnesota Research Authorization form, those that did not present through the emergency department, aged less than 18 years old, with ST-elevation myocardial infarction, cardiac arrest, and/or pregnancy were excluded (Figure S1). In patients with multiple presentations, the earliest was used. The study was approved by the Institutional Review Board (IRB) (ID 20–009951).
In the CV Data Mart Biomarker cohort, the efficacy a single hs-cTnT<6 ng/L to identify low-risk patients was examined. Efficacy was defined as the proportion of patients with a baseline hs-cTnT<6 ng/L that qualified as low-risk among the entire CV Data Biomarker cohort and such proportions were examined across major sites, in patients with chest pain, and according to sex. The safety of a single hs-cTnT<6 ng/L to reliably identify these low-risk patients was evaluated by examining the frequency of acute myocardial injury30 (defined as any subsequent hs-cTnT increase above the sex-specific 99th percentile during the initial 24h) among those with a baseline hs-cTnT<6 ng/L with serial measurements. Analyses were also performed using an overall 99th percentile threshold of 14 ng/L. Given that acute myocardial infarction diagnoses were not adjudicated in the large CV Data Mart biomarker cohort, acute myocardial injury served as an objective measure because it is a central criterion necessary to make any acute myocardial infarction diagnosis30. Previous hs-cTnT and hs-cTnI studies have evaluated single-sample rule-out strategies using this approach31,32. Subgroup evaluations included analyses according to age, sex, chest pain, comorbidities, and site were performed. Given concerns25,26 surrounding the analytical performance and imprecision of hs-cTnT at the LoQ, among patients with an initial hs-cTnT<6 ng/L with 0/2h serial measurements, we also examined whether the second sample concentration remained at <6 ng/L.
To further corroborate the safety of identifying low-risk patients, secondary analyses evaluated the proportion of index presentation deaths and acute myocardial infarction diagnoses based on International Classification of Disease (ICD-10) among patients with baseline hs-cTnT<6 ng/L as have commonly been used in other rule-out studies22,33. Because ICD-10 have limitations34,35, they were not the primary focus of analyses addressing acute myocardial infarction, which are addressed in the adjudicated cohort. ICD-10 codes are summarized in Table S1.
Adjudicated cohort: diagnostic performance and safety for ruling-out acute myocardial infarction
The MAyo Southwest WisConsin 5th Gen Troponin T ImplementatiON (ACTION) study was used to evaluate the diagnostic performance and safety of ruling-out acute myocardial infarction. The study was approved by the IRB (ID 19–002668). The primary results and methods have been described36. It is a multicenter (n=2), retrospective, observational cohort study across the Southwest Mayo Clinic Health System that evaluated consecutive encounters of adult patients presenting to these emergency departments in whom at least one cTnT measurement was obtained. Data was abstracted and reviewed from the electronic health records by trained study staff following a standardized data collection process and entered in Research Electronic Data Capture (REDCap). For the present study, analyses addressed unique patients based on their 1st presentation during the hs-cTnT study period and excluded patients aged less than 18 years old, those without a 12-lead electrocardiogram, with ischemic ST-elevation on the presenting electrocardiogram, or in whom hs-cTnT was not measured within 12h of emergency department presentation (Figure S1). All cases with at least one hs-cTnT above the sex-specific 99th percentile upper-reference limit (URL) were adjudicated using the Fourth Universal Definition of Myocardial Infarction30 criteria by trained physicians. Details regarding the adjudication are included in the Supplemental Material.
This adjudicated cohort permitted the evaluation of the intended clinical rule-out pathway that combines a nonischemic electrocardiogram with a baseline hs-cTnT<6 ng/L and allowed analyses that examined diagnostic performance and safety for ruling-out acute myocardial infarction, including subgroup analyses according to sex, chest discomfort, early presenters (<3h), electrocardiographic findings, and risk-strata based on HEAR (History, Electrocardiography, Age, Risk Factors) scores37. Given the robust follow-up (median follow-up of 23.3 months available in 1866 patients), it also allowed 30-day safety outcomes analyses based on a composite of myocardial infarction or death, as well as 2-year outcomes. Following guidance from the 2018 American College of Emergency Physicians (ACEP) clinical policy38 and other survey39 analyses on acceptable missed rates, we established that patients identified to be low-risk based on their nonischemic electrocardiogram and baseline hs-cTnT<6 ng/L should have no more than 1–2% adverse event rates during 30-day follow-up to consider the rule-out pathway acceptable.
High-sensitivity cardiac troponin T assay
High sensitivity cardiac troponin T (hs-cTnT) was measured using the Elecsys Troponin T Gen 5 STAT assay (Roche Diagnostics). Per FDA clearance17, concentrations are reported down to the LoQ of <6 ng/L. For results <6 ng/L, the laboratory inputs a concentration of 5 ng/L for delta calculations. Sex-specific 99th percentile URLs of 10 ng/L for women and 15 ng/L for men are used17,40–43. Results are reported as whole units (no decimals) in ng/L. The 0/2h hs-cTnT protocol for the evaluation of MI has been described17,36,43 and is summarized in Figure S2. Except for patients with symptom onset >6h in whom myocardial injury is considered ruled-out if the initial hs-cTnT is below the sex-specific 99th percentile, a single sample rule-out strategy was not recommended by institutional protocols.
Statistical Analysis
Continuous variables are presented as means (standard deviations) or medians (interquartile range, IQR). Between groups comparisons were performed using the Kruskal-Wallis test. Discrete variables are summarized as frequency (percentage) and between groups comparisons analyzed with the chi-square test. Negative predictive values (NPV) and sensitivities with corresponding 95% confidence intervals (CI) were calculated using exact binomial methods44. Diagnostic performance for acute myocardial infarction and 30-safety were determined in the adjudicated cohort. False omission rates defined as false negatives among those with a negative test result (i.e.: baseline hs-cTnT<6 ng/L) are considered “missed” events. Forest plots evaluated NPVs across subgroups. Kaplan-Meier curves compared hs-cTnT groups. The time to event was defined as the arrival date of the index hospitalization to the time of first event. Long term event rates were calculated using Kaplan-Meier methods. Analyses were conducted using SAS version 9.4 (Cary, NC) and R version 4.0.3.
RESULTS
CV Data Mart Biomarker Cohort: efficacy of a single hs-cTnT below the 6 ng/L LoQ for identifying low-risk patients by excluding acute myocardial injury
A total of 85,610 patients were included in the CV Data Mart Biomarker cohort. Baseline characteristics are shown in Table 1 and Tables S2–4. The mean age was 63 (18) years and women represented 50% of the cohort. A total of 24,646 (29%) patients had baseline hs-cTnT<6 ng/L. Similar proportions of patients with baseline hs-cTnT<6 ng/L were observed across major sites (range 28–30%) (Figure 1). Among the 24,646 patients with a baseline hs-cTnT<6 ng/L, 49 (0.2%) acute myocardial infarction diagnoses based on ICD-10 codes and 19 (0.1%) deaths occurred during the index presentation. Among 27,198 patients with chest pain, a total of 11,725 (43%) had baseline hs-cTnT<6 ng/L. Similar proportions were observed across sites (range 41–44%) (Figure 1). In 11,725 patients with chest pain and a baseline hs-cTnT<6 ng/L, 24 (0.2%) acute myocardial infarction diagnoses based on ICD-10 codes and 3 (0.03%) deaths were identified during the index presentation.
Table 1.
Baseline characteristics for the CV Data Mart Biomarker cohort and according to baseline hs-cTnT concentrations. LoQ: limit of quantitation.
| Total (n=85610) |
<LoQ (n=24646) |
LoQ-99th percentile (n=25200) |
>99th percentile (n=35764) |
|
|---|---|---|---|---|
| Age, mean (SD) | 63 (18) | 47 (15) | 63 (15) | 74 (14) |
| Women, n (%) | 43043 (50) | 16143 (66) | 9281 (37) | 17619 (49) |
| Chest pain, n (%) | 27198 (32) | 11725 (48) | 8281 (33) | 7192 (20) |
| Coronary artery disease, n (%) | 23636 (28) | 1891 (7.7) | 6361 (25) | 15384 (43) |
| Prior myocardial infarction, n (%) | 9114 (11) | 665 (2.7) | 2257 (9.0) | 6192 (17) |
| Hypertension, n (%) | 48717 (57) | 7088 (29) | 14230 (57) | 27399 (77) |
| Diabetes mellitus, n (%) | 30213 (35) | 4604 (19) | 8385 (33) | 17224 (48) |
| Chronic kidney disease, n (%) | 17070 (20) | 693 (2.8) | 2939 (12) | 13438 (38) |
| Dialysis, n (%) | 1459 (1.7) | 26 (0.1) | 112 (0.4) | 1321 (3.7) |
| Peripheral vascular disease, n (%) | 21394 (25) | 1483 (6) | 5050 (20) | 14861 (42) |
| Heart failure, n (%) | 14986 (18) | 625 (2.5) | 2589 (10) | 11772 (33) |
| Atrial fibrillation, n (%) | 15298 (18) | 851 (3.5) | 3406 (14) | 11041 (31) |
| Liver cirrhosis, n (%) | 10547 (12) | 2152 (8.7) | 3296 (13) | 5099 (14) |
| Index presentation death, n (%) | 775 (0.9) | 19 (0.1) | 57 (0.2) | 699 (2.0) |
Figure 1.
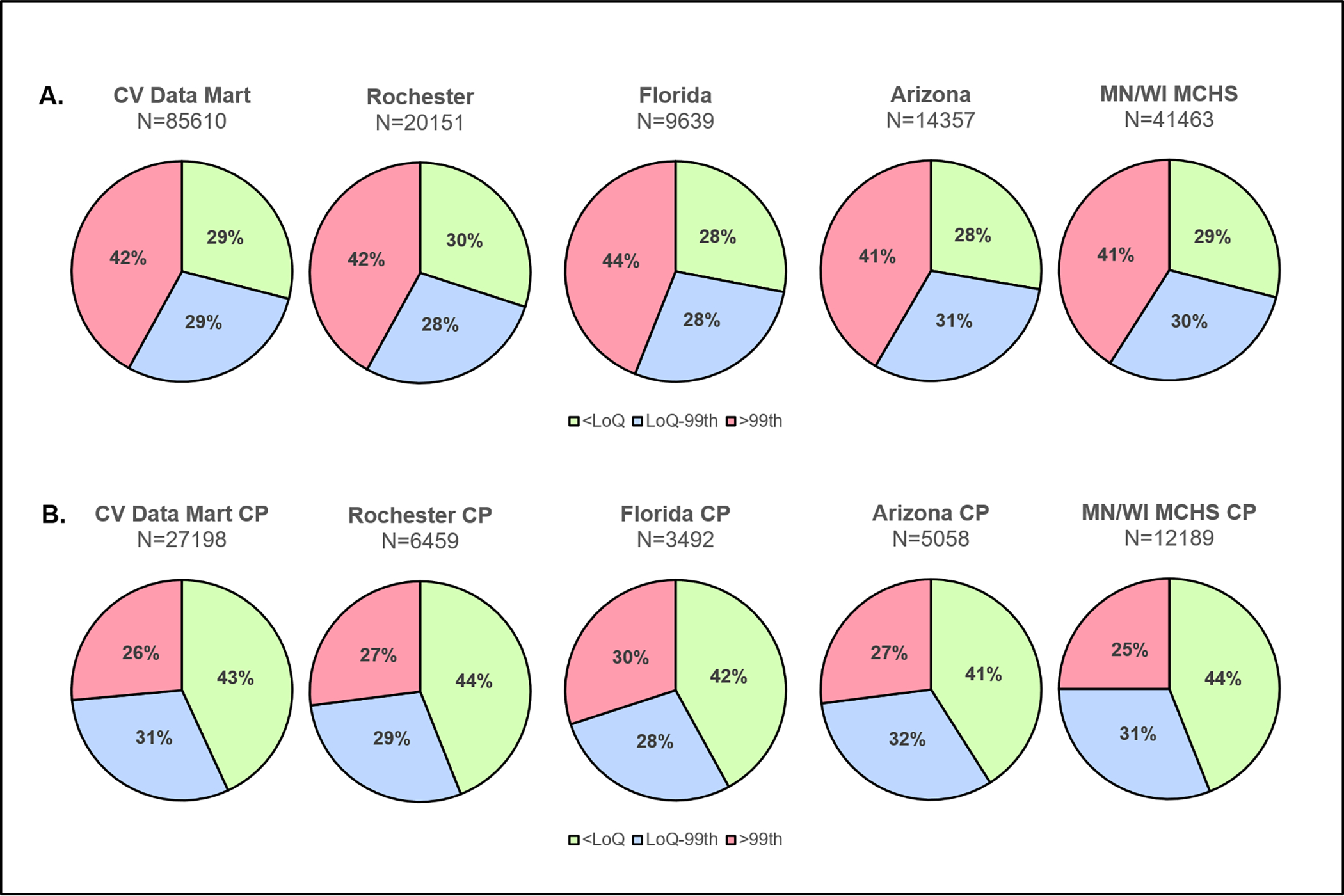
Proportion of patients with baseline hs-cTnT<6 ng/L across the CV Data Mart Biomarker overall cohort (Panel A) and in those with chest pain (Panel B) for the entire population and per-site. CP: chest pain, MCHS: Mayo Clinic Health System.
Baseline characteristics according to baseline hs-cTnT (Table 1) demonstrated that patients with an initial hs-cTnT<6 ng/L were younger, more often women, and less likely to have comorbidities compared to those with quantifiable hs-cTnT, and those with increased concentrations above the sex-specific 99th percentile. Sex-specific analyses demonstrated that women were more likely than men to have baseline hs-cTnT<6 ng/L (38% vs. 20%, p<0.0001). As compared to men with hs-cTnT<6 ng/L, except for prior myocardial infarction and coronary artery disease that were more frequent in men, women with hs-cTnT<6 ng/L were older and more likely to have comorbidities (Table S4).
Baseline hs-cTnT<6 ng/L with serial measurements for acute myocardial injury exclusion
The ability of identifying low-risk patients by excluding acute myocardial injury was evaluated among 11,962 patients with a baseline hs-cTnT<6 ng/L with serial measurements (Table 2). Patients with baseline hs-cTnT<6 ng/L and serial measurements were older and had more comorbidities than those without serial measurements (Table S3). Among these patients, the 2h hs-cTnT remained at <6 ng/L in 85% of cases, whereas 2h hs-cTnT concentrations where within the reference range in 14% of cases. Patients with quantifiable 2h hs-cTnT concentrations within the reference range were older and had more comorbidities than those with 2h hs-cTnT<6 ng/L (Table S5).
Table 2.
Diagnostic performance of a single baseline hs-cTnT below the limit of quantitation of 6 ng/L for acute myocardial injury (defined as any hs-cTnT increase above the sex-specific 99th percentiles within 24-hours of presentation) in patients with a baseline hs-cTnT<6 ng/L with serial measurements in the CV Data Mart Biomarker Cohort. LoQ: limit of quantitation.
| CV Data Mart Biomarker Cohort | Baseline hs-cTnT<6 ng/L (LoQ) with serial hs-cTnT measurements | |||||
|---|---|---|---|---|---|---|
| All-comers | Chest Pain Subgroup | |||||
| All | Men | Women | All | Men | Women | |
| Patients with baseline hs-cTnT<6 ng/L (LoQ) and serial measurements, n (%) | 11962 | 4264 | 7698 | 5840 | 2219 | 3621 |
| Patients with baseline hs-cTnT<6 ng/L with 2h hs-cTnT<6 ng/L (LoQ), n (%) | 10184 (85) | 3469 (81) | 6715 (87) | 5085 (87) | 1865 (84) | 3220 (89) |
| Negative predictive value of hs-cTnT<6 ng/L for acute myocardial injury (hs-cTnT>99th percentile), % (95% CI) | 98.8 (98.6, 99.0) |
99.3 (99.0, 99.6) |
98.5 (98.2, 98.7) |
98.9 (98.6, 99.1) |
99.4 (99.0, 99.7) |
98.6 (98.1, 98.9) |
| Sensitivity of hs-cTnT<6 ng/L for acute myocardial injury (hs-cTnT>99th percentile), % (95% CI) | 99.6 (99.5, 99.6) |
99.8 (99.8, 99.9) |
99.3 (99.1, 99.4) |
99.0 (98.8, 99.2) |
99.6 (99.4, 99.8) |
98.4 (97.9, 98.8) |
| Acute myocardial injury (hs-cTnT >99th percentile) among patients with a baseline hs-cTnT<6 ng/L (LoQ), % | 1.2% (146/11962) | 0.7% (29/4264) | 1.5% (117/7698) | 1.1% (65/5840) | 0.6% (13/2219) | 1.4% (52/3621) |
| Maximum hs-cTnT concentrations in those with acute myocardial injury (ng/L) (median and Q1, Q3) | 20 (14, 43) | 40 (21, 71) | 16 (13, 33) | 25 (14, 48) | 40 (24, 70) | 21 (14, 47) |
Acute myocardial injury (any subsequent hs-cTnT increase above the sex-specific 99th percentile during the initial 24h) occurred in 146 (1.2%) of the 11,962 patients with a baseline hs-cTnT<6 ng/L, which resulted in a NPV of 98.8% (95% CI 98.6, 99.0) and sensitivity of 99.6% (95% CI 99.5, 99.6) (Table 2 and Table S6). Similar findings were observed among patients with chest pain. Among patients that developed acute myocardial injury, maximum hs-cTnT concentrations were 20 (14, 43) ng/L. Adjudication of the 146 false negative cases (117 were women and 29 men) that developed acute myocardial injury demonstrated that most diagnoses (111, 76%) were due to isolated nonischemic acute myocardial injury, with the remaining 35 cases (25 women and 10 men) classified as acute myocardial infarction; most (20/35) were type 2 myocardial infarctions, and 15 cases were adjudicated as type 1 myocardial infarction.
In men, acute myocardial injury occurred in 0.7% (29 out of 4264) of those with a baseline hs-cTnT<6 ng/L with serial measurements, with a corresponding NPV of 99.3% (95% CI 99.0, 99.6) and sensitivity of 99.8% (95% CI 99.8, 99.9). Among men that developed acute myocardial injury, maximum hs-cTnT concentrations were 40 (21, 71) ng/L. Similar findings were observed among men with chest pain. Subgroup analyses in men demonstrated no heterogeneity across NPVs (Figure S3).
In women, acute myocardial injury occurred in 1.5% (117 out of 7698) of those with a baseline hs-cTnT<6 ng/L with serial measurements, with a corresponding NPV of 98.5% (95% CI 98.2, 98.7) and sensitivity of 99.3% (95% CI 99.1, 99.4). Among women that developed acute myocardial injury, maximum hs-cTnT concentrations were 16 (13, 33) ng/L. Similar findings were observed among women with chest pain. Subgroup analyses in women demonstrated lower NPVs, primarily among the elderly and those with coronary artery disease (Figure S4).
Analyses using an overall 99th percentile of 14 ng/L URL to define myocardial injury showed that among those with a baseline hs-cTnT<6 ng/L, acute myocardial injury occurred in 0.9%, with a NPV of 99.1% (95% CI 99.0, 99.3) and sensitivity of 99.6% (95% CI 99.6, 99.7). In men, acute myocardial injury occurred in 0.8%, with a NPV of 99.2% (95% CI 98.9, 99.5) and sensitivity of 99.8% (95% CI 99.7, 99.9) and no heterogeneity was observed among subgroups (Figure S5). In women, acute myocardial injury occurred in 0.9%, with a NPV of 99.1% (95% CI 98.9, 99.3) and sensitivity of 99.4% (95% CI 99.3, 99.6). Lower NPVs were again observed among the elderly and those with coronary artery disease (Figure S6).
Adjudicated cohort: diagnostic performance and safety for ruling-out acute myocardial infarction
A total of 1979 emergency department patients who met inclusion criteria were evaluated. Baseline characteristics are presented in Tables S7–9 The incidence of acute myocardial infarction (including both type 1 and 2 myocardial infarctions) was 7.1% (n=141). Overall, 624 (32%) patients had a baseline hs-cTnT<6 ng/L. Using a single baseline hs-cTnT<6 ng/L in isolation (biomarker alone), the NPV and sensitivity for acute myocardial infarction were 99.8% (95% CI 99.1, 100) and 99.3% (95% CI 96.1, 100) respectively (Table 3 and Table S10). At 30-days post-discharge, including index events, 0.3% (2/624) had an event. False negatives are summarized in Table S11. Sex-specific analyses showed similar diagnostic and safety performance. Subgroup analyses demonstrated no differences in rule-out performance (Figure S7).
Table 3.
Diagnostic performance, efficacy, and safety of a single baseline hs-cTnT below the limit of quantitation of 6 ng/L for index acute MI rule-out in patients in the adjudicated cohort. LoQ: limit of quantitation.
| Total Cohort | Total cohort with nonischemic ECG | |||||
|---|---|---|---|---|---|---|
| Total | Men | Women | Total | Men | Women | |
| Population and incidence of MI | ||||||
| Total patients, n | 1979 | 949 | 1030 | 1849 | 895 | 954 |
| Incidence of MI, n (%) | 141 (7.1) | 70 (7.4) | 71 (6.9) | 95 (5.0) | 51 (5.7) | 44 (4.6) |
| Efficacy: number (percentage) of patients identified as low risk | ||||||
| Patients with baseline hs-cTnT <LoQ (efficacy), n (%) | 624 (32) |
214 (23) |
410 (40) |
610 (33) |
210 (23) |
400 (42) |
| Diagnostic performance of baseline hs-cTnT<LoQ for ruling-out index presentation acute MI | ||||||
| Negative predictive value, % (95% CI) |
99.8 (99.1, 100) |
100 (98.3, 100) |
99.8 (98.7, 100) |
100 (99.4, 100) |
100 (98.3, 100) |
100 (99.1, 100) |
| Sensitivity, % (95% CI) |
99.3 (96.1, 100) |
100 (94.9, 100) |
98.6 (92.4, 100) |
100 (96.2, 100) |
100 (93.0, 100) |
100 (92.0, 100) |
| Negative likelihood ratio, (95% CI) |
0.02 (0.00–0.15) |
0 | 0.03 (0.00–0.23) |
0 | 0 | 0 |
| Missed MI rate among those with a negative test | 0.2% 1/624 |
0% 0/214 |
0.2% 1/410 |
0% 0/610 |
0% 0/210 |
0% 0/400 |
| Safety of baseline hs-cTnT<LoQ based on acute MI or death within 30-days | ||||||
| Negative predictive value, % (95% CI) |
99.7 (98.9, 100) |
100 (98.3, 100) |
99.5 (98.3, 99.9) |
99.8 (99.1, 100) |
100 (98.3, 100) |
99.8 (98.6, 100) |
| Sensitivity, % (95% CI) |
99.0 (96.4, 99.9) |
100 (96.3, 100) |
98.0 (92.8, 99.8) |
99.3 (96.2, 100) |
100 (95.2, 100) |
98.6 (92.3, 100) |
| Negative likelihood ratio, (95% CI) |
0.03 (0.01–0.12) |
0 | 0.05 (0.01–0.18) |
0.02 (0.00–0.14) |
0 | 0.03 (0.00–0.22) |
| Missed acute MI or death within 30-days among those with a negative test | 0.3% 2/624 |
0% 0/214 |
0.5% 2/410 |
0.2% 1/610 |
0% 0/210 |
0.3% 1/400 |
Among those with a nonischemic electrocardiogram (n=1849), 610 (33%) patients had a baseline hs-cTnT<6 ng/L. In this group, the NPV and sensitivity for acute myocardial infarction were 100% and there were no missed diagnoses of acute MI during the index hospitalization (Table 3). At 30-days post-discharge, including index events, 0.2% (1/610) had an event. In early presenters (symptom onset <3h) with a baseline hs-cTnT<6 ng/L and a nonischemic electrocardiogram (145/420, 35%), NPV and sensitivity were 100% with no missed diagnoses at index presentation or during 30-day follow-up (Table S12). Sensitivity analyses in those with chest pain showed similar results (Table S13). At 1- and 2-years, myocardial infarction or death occurred in 2.1% and 2.6% of patients with baseline hs-cTnT<6 ng/L (Figure S8), and 2.0% and 2.3% in those with a baseline hs-cTnT<6 ng/L and a non-ischemic electrocardiogram (Figure 2).
Figure 2.
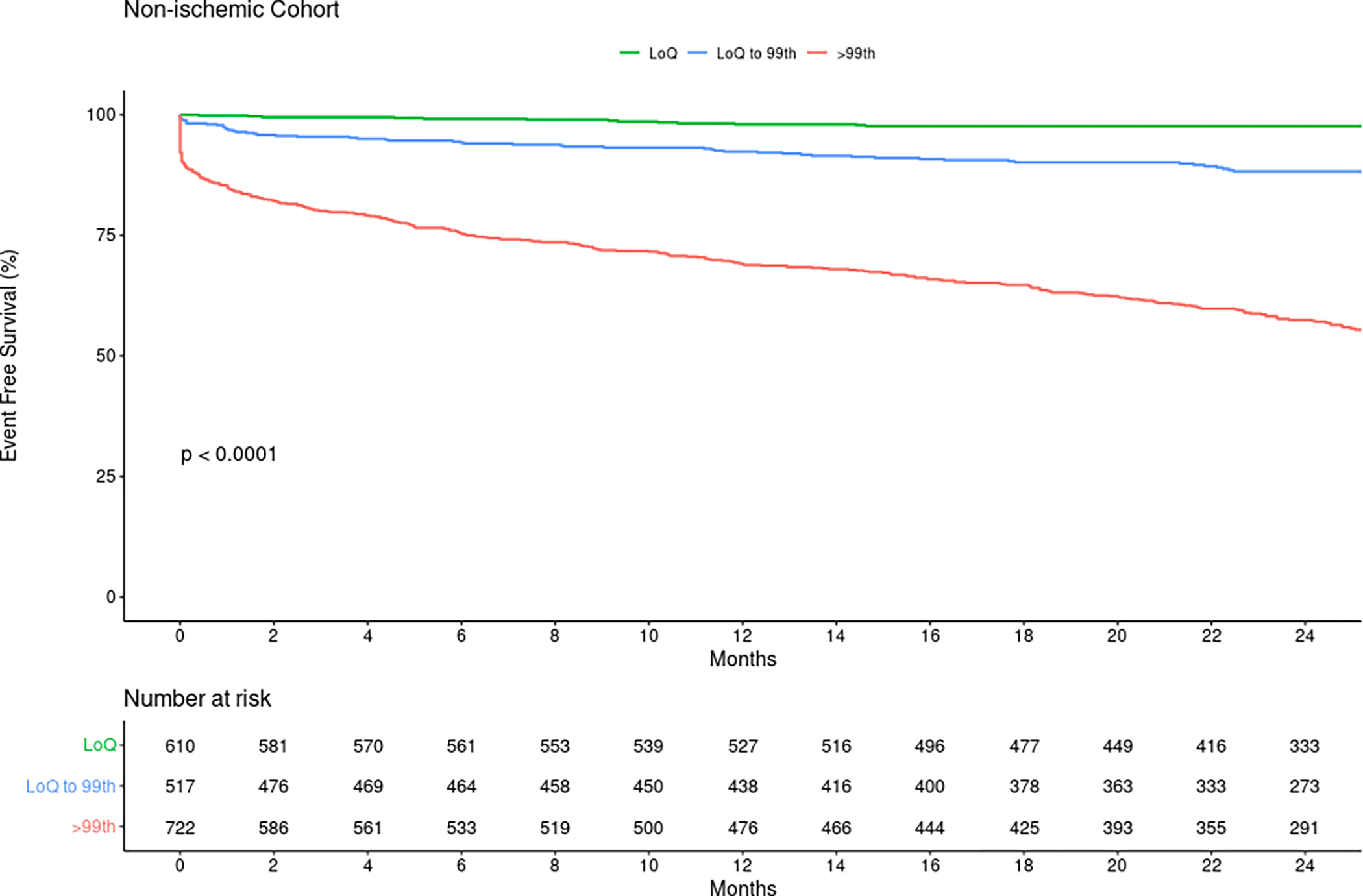
Kaplan-Meier curves for survival free of myocardial infarction or death according to baseline hs-cTnT groups in patients with a nonischemic electrocardiogram from the adjudicated cohort. LoQ: limit of quantitation.
Among the 624 patients with a baseline hs-cTnT<6 ng/L, 206 (33%) had serial hs-cTnT measurements. In those with a 2h serial measurement, concentrations remained <6 ng/L in 84% (173/206). Within 24h following the initial baseline hs-cTnT<6 ng/L, 2 patients (0.97%) developed acute myocardial injury. Among patients with baseline hs-cTnT<6 ng/L, there were no differences in myocardial infarction or death between those with 2h hs-cTnT that remained <6 ng/L as compared to those with 2h hs-cTnT≥6 ng/L but below the sex-specific 99th percentile (Figure S9).
DISCUSSION
This multicenter study is the largest to evaluate the role of a single hs-cTnT below the LoQ of 6 ng/L to identify patients at very low risk for acute myocardial injury and infarction. While extensive data support single-sample rule-out when hs-cTnT concentrations are below the LoD of 5 ng/L, this concentration threshold is not available for clinical use in the US, and limited data exists for the LoQ, which is the lowest reportable concentration for hs-cTnT per the FDA17. Our data therefore provides important and timely information to US clinicians as it demonstrates that a single hs-cTnT below the LoQ of 6 ng/L is a safe method to rapidly triage and identify patients at very low risk for acute myocardial injury and infarction.
This approach identifies a substantial number of low-risk patients that may be eligible for early discharge, which likely would be associated with important reductions in length of stay, emergency department overcrowding, and costs. Our study demonstrates that the proportions of patients identified as low-risk based on hs-cTnT below the LoQ of 6 ng/L were similar across sites, which underscores the generalizable benefits of this approach. In all-comers, our study showed that 28% to 30% of patients can be rapidly identified as low risk using a single hs-cTnT measurement. Critically, the efficacy of this approach is augmented when applied to patients with chest discomfort, with 41% to 44% of such patients identified as low risk across sites.
The safety of a single hs-cTnT<6 ng/L to identify low risk patients was comprehensively examined in multiple ways. In the large CV Data Mart Biomarker cohort, we evaluated the ability of a baseline hs-cTnT<6 ng/L to reliably identify low-risk patients. First, based on analyses performed in 11,962 patients with a baseline hs-cTnT<6 ng/L and serial measurements, we demonstrated that acute myocardial injury is unlikely to occur when the initial hs-cTnT<6 ng/L, with only 1.2% of patients identified to have increases in hs-cTnT above the sex-specific 99th percentiles. This is a robust and objective metric as it is central criterion for the diagnosis of acute myocardial infarction30–32. Second, in this same subset of patients, we demonstrated the subsequent 2h hs-cTnT measurement remained <6 ng/L in 85% of cases. These findings confirm that these results are reliable for clinical decision-making and attenuate concerns about analytical performance25,26. Third, secondary analyses addressing the entire CV Data Biomarker cohort involving 24,646 patients with a baseline hs-cTnT<6 ng/L further corroborated the safety of this approach, with only 0.2% acute myocardial infarction diagnoses based on ICD-10 codes and only 0.1% deaths occurring in such patients during the index presentation. Although ICD-10 codes have recognized limitations34,35, they provided another complementary layer of safety as they allowed us to evaluate whether any acute myocardial infarction diagnoses were coded among patients with a baseline hs-cTnT<6 ng/L. Last, and most importantly, we validated the safety of this approach in an adjudicated cohort where the combination of nonischemic electrocardiogram with a baseline hs-cTnT<6 ng/L resulted in a NPV of 99.8% and sensitivity of 99.3% for acute myocardial infarction or death at 30-days (including index events). These data support the observations from the CV Data Mart using ICD-10 codes. Patients with a nonischemic electrocardiogram and a baseline hs-cTnT<6 ng/L had favorable short- and long-term outcomes, with only about 2% having myocardial infarction or death at 1- and 2-years, which further corroborates the safety of this approach. These findings underscore how a nonischemic electrocardiogram with a baseline hs-cTnT<6 ng/L allows the reliable identification of very low risk patients in whom in most circumstances hospital admission or observation is often unwarranted for ischemic evaluation purposes, and in whom additional testing during the index presentation can be avoided or if clinically needed, deferred to the outpatient setting.
Our data complements the extensive data on the safety of a single hs-cTnT below the 5 ng/L LoD. There are randomized data11,12 using the LoD of hs-cTnT of <5 ng/L that demonstrate that this approach is safe. The RAPID-TNT trial12 (Rapid Assessment of Possible ACS in the Emergency Department with High-Sensitivity Troponin T) was a prospective, randomized, noninferiority evaluation of the ESC 0/1h protocol that involved rule-out when baseline hs-cTnT was <5 ng/L at >3h after onset of symptoms. The trial demonstrated noninferiority compared to standard care, with a NPV for the 0/1h hs-cTnT protocol for 30-day death or myocardial infarction of 99.6% 12. The LoDED (Limit of Detection and ECG Discharge) trial11 enrolled chest pain patients across 8 UK hospitals with a nonischemic electrocardiogram; no patient with an undetectable hs-cTnT had a major adverse event within 30-days11. There are also large meta-analyses4,6 documenting this approach is safe. In a collaborative meta-analysis4 of 9241 patients, Pickering et al. demonstrated that low-risk patients defined as those with a nonischemic electrocardiogram and a hs-cTnT<5 ng/L had a pooled sensitivity of 98.7% for acute myocardial infarction and 98.0% for 30-day major adverse cardiac events and no low-risk patients died. In another recent international collaborative meta-analyses6, both the ESC 0/1h and 0/2h hs-cTnT protocols incorporating single sample rule-out using hs-cTnT<5 ng/L were evaluated. The ESC 0/1h protocol was evaluated in 13,899 patients across 12 cohorts and demonstrated a sensitivity of 99.2% and NPV of 99.8%6. The ESC 0/2h protocol was evaluated in 2488 patients across 5 cohorts and demonstrated a sensitivity of 99.0% and NPV of 99.5%6. Implementation data13 from Scotland also confirms safety with cardiovascular death occurring in only 0.1% at both 30-days and 1-year in those with hs-cTnT<5 ng/L in the intervention group. Our data complements the vast experience on the LoD of hs-cTnT<5 ng/L and demonstrates that the LoQ of hs-cTnT<6 ng/L is also safe.
Limited data exists for hs-cTnT below the LoQ of 6 ng/L as used across the US. Small studies often using investigational samples18,19 or performed outside the US20,21 have probed the performance of hs-cTnT<6 ng/L but no robust data exists. Allen et al. evaluated initial hs-cTnT measurements in 1462 participants in a prospective multicenter observational cohort in which treating clinicians were blinded to the investigational hs-cTnT results and patient care and adjudications were based on local contemporary cTn results19, which is a less sensitive gold standard biomarker for the detection of myocardial injury and diagnosis of myocardial infarction than the hs-cTnT assay used in our study. This has been a common limitation in US studies probing this approach with hs-cTnT18,19. Many either employ a less sensitive assay for adjudication or higher 99th percentile upper-reference limits than we used. Like our findings, Allen et al demonstrated that 32% of patients had a baseline hs-cTnT<6 ng/L and a nonischemic electrocardiogram, with a NPV of 99.1% and 98.9% respectively for index MI and 30-day cardiac death and MI19. They demonstrated that the approach associated with the highest NPV required the combination of hs-cTnT<6 ng/L with a low HEART score19. Our adjudicated cohort analyses showed an excellent performance for acute myocardial infarction rule-out using a baseline hs-cTnT<6 ng/L in combination with a nonischemic electrocardiogram. Further, subgroup analyses demonstrated no heterogeneity among risk-strata using the HEAR score, for which reason our data does not support addition of risk scores for ruling-out acute myocardial infarction.
Several sex-specific findings warrant discussion. First, there is a significant difference between the proportion of men and women with baseline hs-cTnT concentrations below the LoQ, with women almost twice as likely to have hs-cTnT<6 ng/L than men. These differences may be explained in part by the analytical sensitivity of hs-cTnT as several normality studies have shown that women are less likely to have detectable hs-cTnT concentrations than men42,45. Second, sex-specific analyses addressing the exclusion of acute myocardial injury showed no heterogeneity in the rule-out performance among subgroups in men. In women, however, subgroup analyses demonstrated lower NPVs, particularly in the elderly and those with coronary artery disease; similar findings were observed using an overall 99th percentile. Therefore, for the purpose of excluding acute myocardial injury (not acute myocardial infarction), our analyses suggest caution in these patient subsets. When we adjudicated false negative cases with an initial baseline hs-cTnT<6 ng/L that developed myocardial injury, most were due to isolated nonischemic myocardial injury. One possible explanation for the observed differences is that the reduced analytical sensitivity of hs-cTnT in women42,45 may diminish the ability of hs-cTnT to screen for underlying cardiovascular disease as compared to men. Our data suggest this is a plausible explanation as women with hs-cTnT<6 ng/L were older and had more comorbidities than men with hs-cTnT<6 ng/L. Last, while differences were observed between men and women with respect to acute myocardial injury, these differences were not observed for acute myocardial infarction, with NPVs and sensitivities for acute myocardial infarction of 100% in both men and women with a nonischemic electrocardiogram and a hs-cTnT<6 ng/L in the adjudicated cohort.
Our study has multiple strengths. First, it is the largest US study to evaluate the role a single hs-cTnT below the LoQ to identify patients at low-risk for acute myocardial injury and infarction. These findings are of unique importance to clinicians in the US using hs-cTnT because the LoQ is the lowest reportable hs-cTnT concentration for clinical use per the FDA17 and paucity of data exists. Second, our large multicenter analysis addressing the real-life clinical use of hs-cTnT across 22 US sites allowed us to evaluate the efficacy of this approach in a multi-state, diverse population, including 50% women, and informed that a similar proportion of patients are eligible for this approach across sites. Third, the population evaluated exceeds the combined hs-cTnT data using the LoD from previous meta-analyses4,6 and randomized trials11,12 evaluating the “single-sample” rule-out. Fourth, we validated the safety of a baseline hs-cTnT in combination with a nonischemic electrocardiogram to exclude acute myocardial infarction in an adjudicated cohort, where we were also able to demonstrate safety among key subgroups, including in early presenters and in those with chest pain.
Limitations exist. First, while electrocardiographic findings and symptom onset were evaluated in the adjudicated cohort, these findings could be not be interrogated in the CV Data Mart Biomarker cohort. We did exclude patients with ST-elevation MI diagnoses. Second, our efficacy analyses from the CV Data Mart Biomarker cohort address patients with serial measurements. We cannot exclude a component of selection and workup bias in that these patients were potentially perceived to be higher risk than those that only underwent a single hs-cTnT despite an institutional 0/2h protocol. Our data confirms that patients undergoing serial measurements are likely at higher risk given they are older and have more comorbidities than those undergoing single measurements. This demonstrates that the approach is safe in those potentially at higher risk, for which reason performance would likely be enhanced in all-comers. Third, while only 1.2% of patients with a baseline hs-cTnT<6 ng/L developed acute myocardial injury, 14% had 2h hs-cTnT concentrations within the reference range below the 99th percentile. These patients are older and have more comorbidities (Table S5). Concentration changes at these levels can occur due to analytical and/or biological variation. Additional studies are needed to clarify the prognostic implications, but analyses from the adjudicated cohort suggest these patients have favorable outcomes (Figure S9). However, they are limited by small sample size. Irrespective of the potential for small concentration changes in a modest subset of patients with baseline hs-cTnT<6 ng/L, 5-year outcomes data46 from the ADAPT study demonstrate that patients with very low baseline hs-cTnT concentrations have good long-term outcomes. Fourth, larger early presenter studies are needed. Pending such investigations, as recommended by clinical practice guidelines1, single sample rule-out is favored in those with symptoms that began at least 3h before. Fifth, our analyses are relevant to hs-cTnT, with studies required using other assays. Sixth, there is no global consensus on acceptable miss rates. Following ACEP38 guidance, we established that an acceptable rule-out strategy should not have more than 1–2% adverse events within 30-days, but we recognize that practice and geographic differences exist. Our data demonstrates excellent sensitivities and NPVs consistent with what the field considers safe and acceptable. Seventh, the potential for verification (workup) bias exists in all such real-life studies, however, the excellent 30-day clinical outcomes should attenuate such concerns. Eight, our adjudicated cohort has a modest sample size and larger, multicenter, adjudicated cohorts with higher number of events are needed. Last, we recognize ICD-10 codes have limitations34,35, for which reason analyses using ICD-10 codes were restricted to secondary analyses.
CONCLUSIONS
A single hs-cTnT measurement <6 ng/L is a safe method to rapidly triage and identify patients at very low risk for acute MI, particularly among those with a non-ischemic electrocardiogram. This approach can facilitate the identification of a substantial number of low-risk patients that are potentially eligible for early discharge and likely will be associated with important reductions in length of stay and cost.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
Data for excluding acute myocardial infarction with a single hs-cTn relies largely on the limit of detection (LoD). This threshold cannot be reported in the United States per the FDA, where the lowest reportable concentration is the limit of quantitation (LoQ).
This is the largest study evaluating a single hs-cTnT below the LoQ of 6 ng/L to identify patients at low-risk for acute myocardial injury and infarction.
Among 11,962 patients with hs-cTnT<6 ng/L and serial measurements, only 1.2% developed acute myocardial injury. In an adjudicated cohort, among those with a nonischemic electrocardiogram, only 0.2% had myocardial infarction or death at 30-days.
What are the clinical implications?
The latest AHA/ACC clinical practice guidelines recommend the use of a single hs-cTn below the LoD to exclude myocardial injury. This concentration threshold, however, is not available for clinical use in the United States.
For hs-cTnT, this is a particular issue because there are limited data about exclusion of myocardial injury and infarction based on the LoQ of 6 ng/L.
The present study demonstrates that a single hs-cTnT below the LoQ of 6 ng/L is a safe and rapid method to identify a substantial number of patients at very low risk for acute myocardial injury and infarction.
ACKNOWLEDGEMENT
The authors would like to acknowledge the multidisciplinary hs-cTnT implementation team across the Mayo Clinic enterprise that facilitated the introduction of 5th Gen cTnT into clinical practice.
FUNDING SOURCES
This publication was made possible in part by the Mayo Clinic CTSA through grant number UL1TR002377 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- Hs-cTn
high-sensitivity cardiac troponin
- LoD
limit of detection
- US
United States
- FDA
Food and Drug Administration
- LoQ
limit of quantitation
- AHA
American Heart Association
- ACC
American College of Cardiology
- ESC
European Society of Cardiology
- IRB
Institutional Review Board
- ICD
Internal Classification of Disease
- ACTION
MAyo Southwest WisConsin 5th Gen Troponin T ImplementatiON
- REDCap
Research Electronic Data Capture
- URL
upper-reference limit
- HEAR
History, Electrocardiography, Age, Risk Factors
- ACEP
American College of Emergency Physicians
- NPV
negative predictive values
- CI
confidence intervals
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Sandoval has previously served on Advisory Boards for Roche Diagnostics and Abbott Diagnostics without personal financial compensation. He has also been a speaker without personal financial compensation for Abbott Diagnostics.
Dr. Jaffe has consulted or presently consults for most of the major diagnostics companies, including Beckman-Coulter, Abbott, Siemens, Ortho Diagnostics, ET Healthcare, Roche, Radiometer, Sphingotec, RCE Technologies, Astellas, Amgen and Novartis.
All other authors have nothing to disclose.
REFERENCES
- 1.Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021; 144: e368–e454. [DOI] [PubMed] [Google Scholar]
- 2.Apple FS, Sandoval Y, Jaffe AS, Ordonez-Llanos J, IFCC Task Force on Clinical Applications of Cardiac Bio-Markers. Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem 2017; 63: 73–81. [DOI] [PubMed] [Google Scholar]
- 3.Sandoval Y, Smith SW, Apple FS. Present and future of cardiac troponin in clinical practice: a paradigm shift to high-sensitivity assays. Am J Med 2016; 129: 354–365 [DOI] [PubMed] [Google Scholar]
- 4.Pickering JW, Than MP, Cullen L, Aldous S, Ter Avest E, Body R, Carlton EW, Collinson P, Dupuy AM, Ekelund U et al. Rapid rule-ot of acute myocardial infarction with a single high-sensitivity cardiac troponin T measurement below the limit of detection: a collaborative meta-analysis. Ann Intern Med 2017; 166: 715–724. [DOI] [PubMed] [Google Scholar]
- 5.Chapman AR, Lee KK, McAllister DA, Cullen L, Greenslade JH, Parsonage W, Worster A, Kavsak PA, Blankenberg S, Neumann J et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA 2017; 318: 1913–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang CH, Chiang CH, Pickering JW, Stoyanov KM, Chew DP, Neumann JT, Ojeda F, Sörensen NA, Su KY, Kavsak P et al. Performance of the European Society of Cardiology 0/1-hour, 0/2-hour, and 0/3-hour algorithms for rapid triage of acute myocardial infarction: an international collaborative meta-analysis. Ann Intern Med 2022; 175: 101–113. [DOI] [PubMed] [Google Scholar]
- 7.Shah AS, Anand A, Sandoval Y, Lee KK, Smith SW, Adamson PD, Chapman AR, Langdon T, Sandeman D, Vaswani A et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 2015; 386: 2481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandoval Y, Nowak R, deFilippi CR, Christenson RH, Peacock WF, McCord J, Limkakeng AT, Sexter A, Apple FS. Myocardial infarction risk stratification with a single measurement of high-sensitivity troponin I. J Am Coll Cardiol 2019; 74: 271–282. [DOI] [PubMed] [Google Scholar]
- 9.Sandoval Y, Smith SW, Love SA, Sexter A, Schulz K, Apple FS. Single high-sensitivity cardiac troponin I to rule out acute myocardial infarction. Am J Med 2017; 130: 1076–1083. [DOI] [PubMed] [Google Scholar]
- 10.Anand A, Lee KK, Chapman AR, Ferry AV, Adamson PD, Strachan FE, Berry C, Findlay I, Cruikshank A, Reid A et al. High-sensitivity cardiac troponin on presentation to rule-out myocardial infarction: a stepped-wedge cluster randomized controlled trial. Circulation 2021; 143: 2214–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlton EW, Ingram J, Taylor H, Glynn J, Kandiyali R, Campbell S, Beasant L, Aziz S, Beresford P, Kendall J et al. Limit of detection of troponin discharge strategy versus usual care: randomised controlled trial. Heart 2020; 106; 1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew DP, Lambrakis K, Blyth A, Seshadri A, Edmonds MJR, Briffa T, Cullen LA, Quinn S, Karnon J, Chuang A et al. A randomized trial of a 1-hour troponin T protocol in suspected acute coronary syndromes: The Rapid Assessment of Possible Acute Coronary Syndrome in the Emergency Department with High-Sensitivity Troponin T Study (RAPID-TnT). Circulation 2019; 140: 1543–1556. [DOI] [PubMed] [Google Scholar]
- 13.Sandeman D, Syed MBJ, Kimenai DM, Lee KK, Anand A, Joshi SS, Dinnel L, Wenham PR, Campbell K, Jarvie M et al. Implementation of an early rule-out pathway for myocardial infarction using a high-sensitivity cardiac troponin T assay. Open Heart 2021; 8: e001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 267–315. [DOI] [PubMed] [Google Scholar]
- 15.Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021; 42: 1289–1367. [DOI] [PubMed] [Google Scholar]
- 16.Apple FS, Fantz CR, Collinson PO, IFCC Committee on Clinical Application of Cardiac Bio-Markers. Implementation of high-sensitivity and point-of-care cardiac troponin assays into practice: some different thoughts. Clin Chem 2021; 67: 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandoval Y, Jaffe AS. Using high-sensitivity cardiac troponin T for acute cardiac care. Am J Med 2017; 130: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 18.Peacock WF, Baumann BM, Bruton D, Davis TE, Handy B, Jones CW, Hollander JE, Limkakeng AT, Mehrotra A, Than M et al. Efficacy of high-sensitivity troponin T in identifying very-low-risk patients with possible acute coronary syndrome. JAMA Cardiol 2018; 3: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen BR, Christenson RH, Cohen SA, Nowak R, Wilkerson RG, Mumma B, Madsen T, McCord J, Huis In’t Veld M, Massoomi M et al. Diagnostic performance of high-sensitivity cardiac troponin T strategies and clinical variables in multisite US cohort. Circulation 2021; 143: 1659–1672. [DOI] [PubMed] [Google Scholar]
- 20.Chapman AR, Sandeman D, Ferry AV, Stewart S, Strachan FE, Wereski R, Bularga A, Anand A, Shah ASV, Mills NL. Risk-stratification using high-sensitivity cardiac troponin T in patients with suspected acute coronary syndrome. J Am Coll Cardiol 2020; 75: 985–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Twerenbold R, Badertscher P, Boeddinghaus J, Nestelberger T, Wildi K, Rubini Gimenez M, Miró Ò, Martín-Sánchez FJ, Reichlin T, Mueller C. Effect of the FDA regulatory approach on the 0/1-h algorithm for rapid diagnosis of MI. J Am Coll Cardiol 2017; 70: 1532–1534. [DOI] [PubMed] [Google Scholar]
- 22.McRae AD, Innes G, Graham M, Lang E, Andruchow JE, Ji Y, Vatanpour S, Abedin T, Yang H, Southern DA et al. Undetectable concentrations of a Food and Drug Administration-approved High-sensitivity Cardiac Troponin T Assay to Rule Out Acute Myocardial Infarction at Emergency Department Arrival. Acad Emerg Med 2017; 24: 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah ASV, Sandoval Y, Noaman A, Sexter A, Vaswani A, Smith SW, Gibbins M, Griffiths M, Chapman AR, Strachan FE et al. Patient selection for high sensitivity cardiac troponin testing and diagnosis of myocardial infarction: prospective cohort study. BMJ 2017; 359: j4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makam AN, Nguyen OK. Use of cardiac biomarker testing in the emergency department. JAMA Intern Med 2015; 175: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donato LJ, Wockenfus AM, Katzman BM, Baumann NA, Jaffe AS, Karon BS. Analytical and clinical considerations in implementing the Roche Elecsys Troponin T Gen 5 STAT assay. Am J Clin Pathol 2021; 156: 1121–1129 [DOI] [PubMed] [Google Scholar]
- 26.Saenger AK, Beyrau R, Braun S, Cooray R, Dolci A, Freidank H, Giannitsis E, Gustafson S, Handy B, Katus H et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin Chim Acta 2011; 412: 748–54. [DOI] [PubMed] [Google Scholar]
- 27.Vigen R, Kutscher P, Fernandez F, Yu A, Bertulfo B, Hashim IA, Molberg K, Diercks DB, Metzger JC, Soto J et al. Evaluation of a novel rule-out myocardial infarction protocol incorporating high-sensitivity troponin T in a US hospital. Circulation 2018; 138: 2061–2063. [DOI] [PubMed] [Google Scholar]
- 28.Ford JS, Chaco E, Tancredi DJ, Mumma BE. Impact of high-sensitivity cardiac troponin implementation on emergency department length of stay, testing, admissions, and diagnoses. Am J Emerg Med 2021; 45: 54–60. [DOI] [PubMed] [Google Scholar]
- 29.Baugh CW, Scirica BM, Januzzi JL, Morrow DA, Lewandrowski KB, Jarolim P, White BA, Weinfeld MS, Hoffmann U, Nagurney JT. Implementation of an emergency department high-sensitivity troponin chest pain pathway in the United States. Crit Pathw Cardiol 2019; 18: 1–4. [DOI] [PubMed] [Google Scholar]
- 30.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Executive group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Circulation 2018; 138: e618–e651. [DOI] [PubMed] [Google Scholar]
- 31.Body R, Carley S, McDowell G, Jaffe AS, France M, Cruickshank K, Wibberley C, Nuttall M, Mackway-Jones K. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011; 58: 1332–9. [DOI] [PubMed] [Google Scholar]
- 32.Sandoval Y, Smith SW, Shah AS, Anand A, Chapman AR, Love SA, Schulz K, Cao J, Mills NL, Apple FS. Rapid rule-out of acute myocardial injury using a single high-sensitivity cardiac troponin I measurement. Clin Chem 2017; 63: 369: 376. [DOI] [PubMed] [Google Scholar]
- 33.Wassie M, Lee MS, Sun BC, Wu YL, Baecker AS, Redberg RF, Ferencik M, Shen E, Musigdilok V, Sharp AL. Single vs serial measurements of cardiac troponin level in the evaluation of patients in the emergency department with suspected acute myocardial infarction. JAMA Netw Open 2021; 4: e2037930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarthy C, Murphy S, Cohen JA, Rehman S, Jones-O’Connor M, Olshan DS, Singh A, Vaduganathan M, Januzzi JL Jr, Wasfy JH. Misclassification of myocardial injury as myocardial infarction: implications for assessing outcomes in value-based programs. JAMA Cardiol 2019; 4: 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Díaz-Garzón J, Sandoval Y, Smith SW, Love S, Schulz K, Thordsen SE, Johnson BK, Driver B, Jacoby K, Carlson MD et al. Discordance between ICD-coded myocardial infarction and diagnosis according to the Universal Definition of Myocardial Infarction. Clin Chem 2017; 63: 415–419. [DOI] [PubMed] [Google Scholar]
- 36.Ola O, Akula A, De Michieli L, Dworak M, Crockford E, Lobo R, Rastas N, Knott JD, Mehta RA, Hodge DO. Clinical impact of high-sensitivity cardiac troponin T implementation in the community. J Am Coll Cardio 2021; 77: 3160–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahler SA, Lenoir KM, Wells BJ, Burke GL, Duncan PW, Case LD, Herrington DM, Diaz-Garelli JF, Futrell WM, Hiestand BC et al. Safely identifying emergency department patients with acute chest pain for early discharge. Circulation 2018; 138: 2456–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on suspected non-ST-elevation acute coronary syndromes; Tomaszewski CA, Nestler D, Shah KH, Sudhir A, Brown MD. Clinical Policy: Critical issues in the Evaluation and Management of Emergency Department Patients With Suspected Non-ST-Elevation Acute Coronary Syndromes. Ann Emerg Med 2018; 72: e65–e106. [DOI] [PubMed] [Google Scholar]
- 39.Than M, Herbert M, Flaws D, Cullen L, Hess E, Hollander JE, Diercks D, Ardagh MW, Kline JA, Munro Z et al. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the Emergency Department?: a clinical survey. Int J Cardiol 2013; 166; 752–4. [DOI] [PubMed] [Google Scholar]
- 40.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 2010; 56: 254–61. [DOI] [PubMed] [Google Scholar]
- 41.Saenger AK, Beyrau R, Braun S, Cooray R, Dolci A, Freidank H, Giannitsis E, Gustafson S, Handy B, Katus H et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin Chim Acta 2011; 412: 748–54. [DOI] [PubMed] [Google Scholar]
- 42.Apple FS, Wu AHB, Sandoval Y, Sexter A, Love SA, Myers G, Schulz K, Duh SH, Christenson RH. Sex-specific 99th percentile upper reference limits for high sensitivity cardiac troponin assays derived using a Universal Sample Bank. Clin Chem 2020; 66: 434–444. [DOI] [PubMed] [Google Scholar]
- 43.Sandoval Y, Askew JW 3rd, Newman JS, Clements CM, Grube ED, Ola O, Akula A, Dworak M, Wohlrab S, Karon BS et al. Implementing high-sensitivity cardiac troponin T in US regional healthcare system. Circulation 2020; 141: 1937–1939. [DOI] [PubMed] [Google Scholar]
- 44.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1943; 26: 404–413. [Google Scholar]
- 45.Kimenai DM, Henry RM, van der Kallen CJ, Dagnelie PC, Schram MT, Stehouwer CD, van Suijlen JD, Niens M, Bekers O, Sep SJ et al. Direct comparison of clinical decision limits for cardiac troponin T and I. Heart 2016; 102: 610–6. [DOI] [PubMed] [Google Scholar]
- 46.Than MP, Aldous SJ, Troughton RW, Pemberton CJ, Richards AM, Frampton CMA, Florkowski CM, George PM, Bailey S, Young JM et al. Detectable high-sensitivity cardiac troponin within the population reference interval conveys high 5-year cardiovascular risk: an observational study. Clin Chem 2018; 64: 1044–1053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


