Abstract
Background and Aims
Kidney dysfunction is associated with increased mortality among patients with cirrhosis. We investigated whether kidney dysfunction types [e.g., acute kidney injury (AKI), chronic kidney disease (CKD), and AKI on CKD] were differentially associated with inpatient mortality.
Methods
We utilized the nationwide inpatient sample, a nationally representative database, from 2007 to 2014. We included all hospitalizations with previously validated codes for cirrhosis or associated decompensated cirrhosis diagnoses. We defined kidney dysfunction types also from previously validated codes, and we grouped hospitalizations into the following diagnoses: normal, AKI, CKD, and AKI on CKD. Our primary outcome was inpatient mortality.
Results
There were 1,293,779 hospitalizations with cirrhosis sampled in this study. Of these hospitalizations, 849,193 (66%) had normal kidney function, 176,418 (14%) had AKI, 157,600 (12%) had CKD, and 110,568 (9%) had AKI on CKD. We found that the proportion of hospitalizations with AKI, CKD, and AKI on CKD increased significantly throughout the study period (p < 0.001, test for trend for all). Kidney dysfunction type was differentially associated with inpatient mortality, even after adjustment: as compared to those with CKD, normal kidney function: OR 0.75 [95 CI 0.73–0.78], AKI: OR 2.40 [95 CI 2.32–2.48], and AKI on CKD: OR 1.66 [95 CI 1.60–1.72].
Discussion
Using a nationally representative cohort of all hospitalizations with cirrhosis, our study highlights that the burden of kidney dysfunction, especially AKI, among hospitalizations with cirrhosis is rising, and the inclusion of kidney dysfunction type may be an opportunity to improve prognostication.
Keywords: Acute kidney injury, Chronic kidney disease, Cirrhosis, Hospitalizations, Mortality, National inpatient sample
Introduction
Kidney dysfunction among patients with cirrhosis is common—impacting nearly 50% of hospitalized patients [1] and 40% of outpatients [2]. The frequency of this complication is particularly concerning when up to 50% of patients with cirrhosis will die within 30 days of developing kidney failure needing renal replacement therapy (RRT) [3, 4]. However, kidney dysfunction in the context of cirrhosis arises from a broad spectrum of pathologies, including acute kidney injury (AKI) from alterations in perfusion or nephrotoxic insults (e.g., pre-renal azotemia, acute tubular necrosis, hepatorenal syndrome) to chronic kidney disease (CKD) from kidney parenchymal damage unrelated to liver disease or its complications (e.g., diabetic nephropathy, hypertensive nephrosclerosis). Further, although it has been shown among liver transplant candidates that these pathologies differentially impact mortality—patients with CKD have less than half the risk of waitlist mortality compared to those with AKI or AKI on CKD [5]—it is not well established if this association exists among all hospitalized patients with cirrhosis.
Understanding this association among all hospitalized patients with cirrhosis, not just liver transplant candidates, is important because it is clear that the burden of CKD is increasing among patients hospitalized with cirrhosis at liver transplant centers regardless of waitlist status [6, 7]. Only a single study has investigated the differential impact of kidney function patterns on outcomes among hospitalized patients with cirrhosis—a study from Europe that was limited by subjective definitions of kidney dysfunction but suggested that those with CKD, as compared to other, more acute, types of kidney dysfunction, had a lower risk of mortality [8]. Since there are greater than 600,000 patients with cirrhosis in the USA, but only a small minority are hospitalized at liver transplant centers, there is a need to better understand the impact of these findings for all patients with end-stage liver disease, regardless of transplant candidacy and hospitalization location [9, 10].
Herein, we utilize an extensive, nationally representative database to address this need. More specifically, we capitalize on the nationwide inpatient sample (NIS) to determine the prevalence over time and the impact of all kidney dysfunction types on hospitalization outcomes among all patients with cirrhosis.
Methods
Data Source
This study used the nationwide inpatient sample (NIS; called national inpatient sample from 2012 to 2014) from the Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, from 2007 to 2014 [11]. The NIS represents a national database of hospital discharges for all payers. NIS data include demographic information, discharge disposition, discharge diagnoses, procedures, length of stay, hospital charges, and inpatient mortality. The discharges are weighted to provide nationally representative estimates. Diagnosis and procedure codes were utilized in any order.
Study Population
We included all hospitalizations of patients aged 18–99 years with either a diagnosis code of cirrhosis (defined by International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 571.2, 571.5, and 571.6) or the presence of a decompensating event (defined by ICD-9-CM code of bleeding esophageal varices [456.0, 456.21], spontaneous bacterial peritonitis [567.23 along with the code for paracentesis 54.91], ascites [789.5, 789.59], and hepatic encephalopathy [HE; 572.2]). This identification algorithm is similar to previously validated studies that highlight that an algorithm including both an ICD-9-CM code for cirrhosis and a hepatic decompensation event has the highest positive predictive value to identify patients with end-stage liver disease [10, 12].
Kidney Dysfunction Types
According to previously reported algorithms, we defined kidney dysfunction types as follows [13-16]: AKI: acute kidney failure (ICD-9-CM codes 584.5, 584.6, 584.7, 584.8, 584.9); CKD: chronic kidney disease (ICD-9-CM codes 585.C, 585.2, 585.3, 585.4, 585.5, 585.6, 585.9); renal sclerosis, unspecified (587), hypertensive chronic kidney disease (403.00, 403.01, 403.10, 403.90); AKI on CKD: ICD-9-CM code for AKI and CKD; and normal: not meeting any of the above criteria.
Renal Replacement Therapy
We determined RRT status using the following diagnosis and procedure codes—procedure codes: 39.95 and 54.98 and diagnosis codes: V451.1, V56.0, and V56.1. RRT was attributed to a kidney dysfunction type based on the aforementioned ICD-9-CM codes (i.e., AKI, CKD, AKI on CKD). We excluded the 1349 (0.1% of the cohort) hospitalizations without a kidney dysfunction type, but on RRT because we were unable to ascertain the diagnosis of their kidney dysfunction.
Covariates
Similar to prior studies, we defined covariates as follows [17-21]: Shock was identified by the presence of an ICD-9-CM code for shock (785.52, 785.59) or vasopressor use (572.2); respiratory failure requiring mechanical ventilation as defined as ICD-9-CM codes for invasive mechanical ventilation (96.05, 96.70, and 96.72) or tracheostomy placement (31.1, 31.2, 31.21, and 31.29); we identified hospitalizations with diabetes (250.XX) and hypertension (401.X, 405.X). We identified hospitalizations with infection using ICD-9-CM codes from previous studies [10, 22]: pneumonia (480–486), urinary tract infection (590x, 595.0, 595.3, 599.0), Clostridium difficile infection (008.45), spontaneous bacterial peritonitis (567.23), sepsis (038x, 785.52, 995.91, 995.92), cellulitis (680–686), or cholangitis (576.1). We identified potential causes of cirrhosis as follows: hepatitis C virus (HCV) (70.44, 70.41, 70.44, 70.51, 70.54, 70.7), hepatitis B virus (HBV) (70.2X, 70.3X, 70.42, 70.52), and alcohol-related (571.2, 571.3, 303.XX, and DXCCS 660). The following variables were extracted directly from the NIS: age, sex, race, hospital location, and year of admission. We generated North American Consortium for the Study of End-Stage Liver Disease (NACSELD) acute on chronic liver failure (ACLF) scores by defining organ failure by the presence of shock, presence of encephalopathy, requiring RRT, or respiratory failure requiring mechanical ventilation. Because of limitations in the NIS, we were not able to grade hepatic encephalopathy, and to better understand the impact of kidney dysfunction types, we treated the requirement of dialysis as kidney failure [10, 23].
Outcomes
The primary outcome was inpatient mortality, as reported to the NIS. The primary objective was to determine the association between kidney dysfunction type and inpatient mortality. In a priori analyses, we planned to understand the interaction between kidney dysfunction types and RRT on inpatient mortality. We also planned a subgroup analysis among those with NACSELD ACLF score 2 or higher to investigate the impact of kidney dysfunction types.
Statistical Analysis
Survey-Specific Analysis
Survey-specific analyses (STATA: svy package) were used for all analyses to account for sampling. This includes a weighted description of descriptive and trend analyses and necessary weighting, clustering, and stratification needed in logistic modeling.
Descriptive Data Analysis
Continuous variables were expressed as weighted means with 95% confidence intervals and compared between groups by adjusted Wald test. Categorical variables were expressed as weighted proportions and analyzed by the Chi-square test.
Trend Analysis
To test for statistical trends over time, we utilized weighted linear regression models to evaluate the outcome—the percentage of each of the kidney dysfunction types—and the exposure—calendar year as a continuous variable. In 2012, the sampling methodology of the NIS changed from a sample of 100% of discharges from 20% of hospitals in the USA to a national 20% sample of patients [24]—to address this change, we adjusted by including a dichotomous variable based on the period in each of the regression analyses. Finally, to address the possibility of “code creep” (i.e., the increased awareness the impact of kidney dysfunction diagnoses leading to rising diagnosis code utilization over time), we determined both the weighted mean of diagnosis codes utilized per year and the weighted annual proportion of diagnosis codes that were kidney related (e.g., any disease of the genitourinary syndrome) [25, 26].
Logistic Regression Analysis
Logistic regression assessed the association between the available variables, including kidney dysfunction types, and the primary outcome inpatient mortality, accounting for clustering, stratification, and weighting. To highlight the differential impact of kidney dysfunction types on outcomes, CKD was treated as the reference group for logistic regression analyses. Unadjusted models were used to assess the association of covariates with the outcomes of interest. All covariates with a p < 0.2 in univariate analysis were considered for inclusion in multivariate models. Sequential backward selection eliminated those not reaching significance of p < 0.05.
Significance
Two-sided p-values < 0.05 were considered statistically significant. Analyses were performed using Stata 15.0 statistical software (College Station, TX). This study was approved by the institutional review board at Weill Cornell Medicine.
Results
Population Characteristics
A total of 1,293,779 hospitalizations with cirrhosis were sampled in this study to represent 6,417,747 total hospitalizations (Table 1). Of 6,417,747 projected hospitalizations, the mean age was 59.5 years (95% confidence interval [95 CI] 59.4–59.5), 43% were female, 60% were white, 32% were hospitalized in a metropolitan city center, 22% had HCV, 36% had alcohol use documented, 67% had decompensated cirrhosis, 30% had diabetes mellitus, 33% had a documented infection, and 8% received RRT.
Table 1.
Baseline demographics of hospitalizations by kidney diagnosis type
| Total (no.* = 1,293,779) |
Normal (no.* = 849,193) |
AKI (no.* = 176,418) |
CKD (no.* = 157,600) |
AKI on CKD (no.* = 110,568) |
p | |
|---|---|---|---|---|---|---|
| Female sex, no.* (%) | 553,345 (43) | 372,418 (44) | 72,722 (41) | 65,236 (41) | 42,969 (39) | < 0.001 |
| Age, m (95 CI) | 59.5 (59.4–59.5) | 58.0 (57.9–58.0) | 60.2 (60.2–60.3) | 62.7 (62.6–62.8) | 65.4 (65.4–65.5) | < 0.001 |
| Race, no.* (%) | ||||||
| White | 769,477 (60) | 521,015 (61) | 107,282 (61) | 77,102 (49) | 64,078 (58) | |
| Black | 145,137 (13) | 75,915 (9) | 19,993 (11) | 32,540 (21) | 16,689 (15) | < 0.001 |
| Hispanic | 171,419 (15) | 114,143 (13) | 20,439 (12) | 22,926 (15) | 13,911 (14) | |
| Asian | 26,558 (2) | 16,052 (2) | 3693 (2) | 4172 (3) | 2641 (2) | |
| Other/unknown | 181,188 (14) | 122,068) | 25,011 (14) | 20,860 (13) | 13,249 (12) | |
| Hospital location, no.* (%) | ||||||
| Metropolitan city center | 417,920 (32) | 268,393 (32) | 57,584 (33) | 54,524 (35) | 37,419 (34) | |
| Metropolitan county | 285,243 (22) | 186,234 (23) | 39,991 (23) | 33,356 (22) | 25,662 (24) | |
| Large county | 241,301 (19) | 158,275 (19) | 33,546 (19) | 28,981 (19) | 20,499 (19) | < 0.001 |
| Medium county | 108,833 (8) | 72,545 (9) | 14,218 (8) | 13,229 (9) | 8841 (8) | |
| Micropolitan county | 128,013 (10) | 86,385 (10) | 16,775 (10) | 14,948 (10) | 9905 (9) | |
| Other | 112,469 (9) | 56,010 (7) | 10,548 (6) | 9728 (6) | 6467 (6) | |
| HCV, no.* (%) | 285,116 (22) | 201,154 (24) | 34,781 (20) | 29,247 (19) | 19,934 (18) | < 0.001 |
| EtOH-related, no.* (%) | 467,225 (36) | 345,182 (41) | 63,700 (36) | 30,239 (19) | 28,104 (25) | < 0.001 |
| Decompensated, no.* (%) | 865,500 (67) | 530,481 (63) | 138,167 (78) | 112,581 (71) | 84,271 (76) | |
| Ascites | 594,926 (46) | 355,309 (42) | 98,518 (56) | 75,902 (48) | 65,197 (59) | |
| HE | 210,388 (16) | 120,818 (14) | 44,127 (25) | 22,409 (14) | 23,034 (21) | < 0.0 |
| Esophageal variceal | 58,497 (5) | 44,658 (5) | 8252 (5) | 2768 (2) | 2819 (3) | |
| Diabetes mellitus, no.* (%) | 387,492 (30) | 235,909 (28) | 40,397 (23) | 72,344 (46) | 39,207 (36) | < 0.001 |
| Sepsis, no.* (%) | 173,226 (13) | 67,485 (8) | 61,675 (35) | 19,940 (13) | 24,126 (22) | < 0.001 |
| Infection, no.* (%) | 431,152 (33) | 228,951 (27) | 98,509 (56) | 51,171 (33) | 52,521 (48) | < 0.001 |
| RRT, no.* (%) | 99,455 (8) | 0 (0) | 14,177 (8) | 67,782 (43) | 17,496 (16) | < 0.001 |
| Years of admission, no.* (%) | ||||||
| 2007–2008 | 285,712 (22) | 204,940 (24) | 32,719 (19) | 32,372 (21) | 15,681 (14) | |
| 2009–2010 | 310,870 (24) | 207,980 (25) | 41,276 (23) | 36,884 (23) | 24,730 (22) | < 0.001 |
| 2011–2012 | 345,430 (27) | 221,531 (26) | 47,783 (27) | 43,455 (28) | 32,661 (30) | |
| 2013–2014 | 351,767 (27) | 214,742 (25) | 54,640 (31) | 44,889 (29) | 37,496 (34) | |
| Days of stay, m (95 CI) | 7.5 (7.4–7.5) | 6.3 (6.2–6.3) | 11.8 (11.8–11.9) | 6.9 (6.8–6.9) | 10.7 (10.6–10.8) | < 0.001 |
| Inpatient mortality, no (%) | 103,456 (8) | 34,225 (4) | 43,675 (25) | 9805 (6) | 15,751 (14) | < 0.001 |
AKI acute kidney injury, CKD chronic kidney disease, AKI on CKD acute kidney injury on chronic kidney disease, m weighted mean; 95 CI 95% confidence Interval, no. number, % weighted proportion, EtOH alcohol-related disease, HE hepatic encephalopathy, RRT renal replacement therapy
The sampled number
Of these projected hospitalizations, 66% had normal kidney function, 14% had AKI, 12% had CKD, and 9% had AKI on CKD. Those without kidney dysfunction had the following significant differences from the AKI, CKD, and AKI on CKD groups, respectively: They were more likely to be female (normal: 44% v. AKI: 41% v. CKD: 41% v. AKI on CKD: 39%, p < 0.001); they were younger (58.0 v. 60.2 v. 62.7 v. 65.4 years, p < 0.001); they were less likely to be hospitalized at a metropolitan city center (32 v. 33 v. 35 v. 34%, p < 0.001); they were more likely to have HCV (24 v. 20 v. 19 v. 18%, p < 0.001); they were more likely to have alcohol-related liver disease (41 v. 36 v. 19 v. 25%, p < 0.001); they were less likely to be decompensated (63 v. 78 v. 71 v. 76%, p < 0.001); and they had significantly lower inpatient mortality (4 v. 25 v. 6 v. 14%, p < 0.001) (Table 1).
Trends in Kidney Dysfunction Types by Admission Year
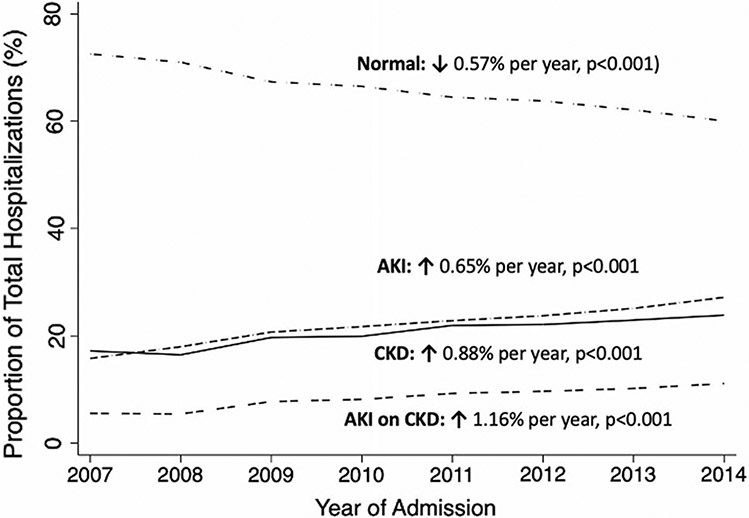
In this analysis from 2007 to 2014, we found that the proportion of projected hospitalizations with normal kidney function decreased 0.48% per year (p < 0.001), while AKI increased 0.55% per year (p < 0.001), CKD increased 0.69% per year (p < 0.001), and AKI on CKD increased 0.94% per year (p < 0.001) (Fig. 1). Similarly, the percentage of projected hospitalizations with the utilization of RRT increased 2.4% per year (p < 0.001). To address the concerns of “code creep,” we did see an increase in the number of diagnoses by year: weighted mean increase of 0.59 diagnoses per year (p < 0.001); however, we found that the weighted proportion of diagnoses that were kidney related only increased 0.11% per year (p < 0.001), significantly less than each of the kidney dysfunction types (p < 0.001 for all).
Fig. 1.
Kidney function diagnosis code trends among patients with cirrhosis. Legend: acute kidney injury (AKI); chronic kidney disease (CKD); acute kidney injury on chronic kidney disease (AKI on CKD)
Association Between Kidney Dysfunction Types and Inpatient Mortality
In univariable analysis, the kidney dysfunction type was significantly associated with inpatient mortality: As compared to those with CKD, those with normal kidney function had lower odds of inpatient mortality (OR 0.63, 95% confidence interval [95 CI] 0.62–0.65) and those with AKI (OR 4.95, 95 CI 4.86–5.07) and AKI on CKD (OR 2.50, 95 CI 2.44–2.57) had higher odds of inpatient mortality compared with those with CKD. The other factors that were significantly associated with inpatient morality in univariable analysis are shown in Table 2. In the final multivariable model even after adjusting for confounders including age, female sex, race, HCV, alcohol-related, diabetes mellitus, hospital location, infectious status, shock, mechanical ventilation, year of admission, decompensated cirrhosis, RRT status, and the interaction between RRT and kidney dysfunction type, the kidney dysfunction type was significantly associated with inpatient mortality (as compared to CKD, normal kidney function: OR 0.75 [95 CI 0.73–0.78], AKI: OR 2.40 [95 CI 2.32–2.48], and AKI on CKD: OR 1.66 [95 CI 1.60–1.72]) (Table 2).
Table 2.
Logistic regression for kidney function pattern and associated inpatient mortality
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Kidney diagnosis | ||||||
| Normal | 0.63 | 0.62–0.65 | < 0.001 | 0.75 | 0.73–0.78 | < 0.001 |
| AKI | 4.95 | 4.84–5.07 | < 0.001 | 2.40 | 2.32–2.48 | < 0.001 |
| CKD | – | – | – | – | – | – |
| AKI on CKD | 2.50 | 2.44–2.57 | < 0.001 | 1.66 | 1.60–1.72 | < 0.001 |
| Age per year | 1.02 | 1.02–1.02 | < 0.001 | 1.02 | 1.02–1.02 | < 0.001 |
| Female Sex | 0.96 | 0.94–0.97 | < 0.001 | 0.93 | 0.92–0.95 | < 0.001 |
| Race | ||||||
| Caucasian | – | – | – | – | – | – |
| African-American | 1.04 | 1.01–1.06 | 0.001 | 1.06 | 1.03–1.09 | < 0.001 |
| Hispanic | 0.85 | 0.83–0.86 | < 0.001 | 1.00 | 0.98–1.03 | 0.90 |
| Asian | 1.28 | 1.23–1.34 | < 0.001 | 1.23 | 1.16–1.29 | < 0.001 |
| Other | 1.01 | 0.99–1.02 | 0.55 | 0.99 | 0.97–1.01 | 0.38 |
| Hepatitis C | 0.66 | 0.65–0.67 | < 0.001 | 0.95 | 0.93–0.97 | < 0.001 |
| EtOH-related | 0.79 | 0.77–0.80 | < 0.001 | 1.08 | 1.06–1.10 | < 0.001 |
| Diabetes mellitus | 0.46 | 0.45–0.47 | < 0.001 | 0.65 | 0.63–0.66 | < 0.001 |
| Hospital location | ||||||
| Metropolitan city center | – | – | – | – | – | – |
| Metropolitan county | 0.98 | 0.96–1.00 | 0.04 | 1.00 | 0.98–1.03 | 0.75 |
| Large county | 1.00 | 0.98–1.02 | 0.89 | 1.11 | 1.09–1.14 | < 0.001 |
| Medium county | 1.02 | 0.99–1.04 | 0.14 | 1.20 | 1.16–1.23 | < 0.001 |
| Micropolitan county | 1.06 | 1.04–1.09 | < 0.001 | 1.27 | 1.23–1.31 | < 0.001 |
| Other | 1.07 | 1.04–1.10 | < 0.001 | 1.30 | 1.25–1.34 | < 0.001 |
| Infectious status | ||||||
| None | – | – | – | – | – | – |
| Infection | 1.39 | 1.37–1.42 | < 0.001 | 1.07 | 1.05–1.09 | < 0.001 |
| Sepsis | 8.88 | 8.75–9.01 | < 0.001 | 2.14 | 2.09–2.18 | < 0.001 |
| Shock | 14.44 | 14.21–14.67 | < 0.001 | 2.53 | 2.46–2.59 | < 0.001 |
| Mechanical ventilation | 15.92 | 15.68–16.15 | < 0.001 | 6.07 | 5.95–6.20 | < 0.001 |
| Year of admission | ||||||
| 2007–2008 | – | – | – | – | – | – |
| 2009–2010 | 0.94 | 0.92–0.95 | < 0.001 | 0.80 | 0.78–0.82 | < 0.001 |
| 2011–2012 | 0.86 | 0.84–0.88 | < 0.001 | 0.68 | 0.67–0.70 | < 0.001 |
| 2013–2014 | 0.85 | 0.83–0.86 | < 0.001 | 0.61 | 0.60–0.62 | < 0.001 |
| Decompensated cirrhosis | 2.17 | 2.14–2.21 | < 0.001 | 1.41 | 1.39–1.44 | < 0.001 |
| RRT | 2.52 | 2.48–2.57 | < 0.001 | 1.14 | 1.09–1.19 | < 0.001 |
| Kidney pattern and dialysis# | ||||||
| Normal | – | – | – | – | – | – |
| AKI | 2.62 | 2.47–2.75 | < 0.001 | 1.39 | 1.30–1.48 | < 0.001 |
| CKD | Ref | Ref | Ref | Ref | Ref | Ref |
| AKI on CKD | 1.73 | 1.63–1.83 | < 0.001 | 1.10 | 1.03–1.17 | 0.005 |
AKI acute kidney injury, CKD chronic kidney disease, AKI on CKD acute kidney injury on chronic kidney disease, EtOH alcohol-related disease, RRT renal replacement therapy
This is the interaction term between variables
Interaction of Renal Replacement Therapy and Kidney Dysfunction Type
We wanted to investigate the interaction between RRT and kidney dysfunction type—specifically with the hypothesis that RRT was associated with a lower odds of inpatient mortality among those started for CKD, as compared to those started for AKI. To start, we found the weighted proportion of inpatient mortality by kidney dysfunction type and RRT status to be the following: no kidney dysfunction (4.0%); AKI not requiring RRT (22.5%); AKI requiring RRT (50.3%); CKD not requiring RRT (5.5%); CKD requiring RRT (7.2%); AKI on CKD not requiring RRT (12.3%); and AKI on CKD requiring RRT (24.5%). Additionally, we found in the final multivariable model, even after adjusting for confounders and the interaction between RRT and kidney dysfunction type, that RRT was associated with an increased odds of inpatient mortality (OR 1.14, [1.09–1.19]). We next tested for the interaction between kidney function pattern and RRT. We found even after adjustment in the final multivariable model, and as compared to those with CKD, RRT conferred greater odds of inpatient mortality among each of the other kidney dysfunction types (AKI: OR 1.39 [95 CI 1.30–1.48]; AKI on CKD: OR 1.10 [95 CI 1.03–1.17]).
Association of Kidney Dysfunction Type and Inpatient Mortality Among Those with ACLF
Next, to determine whether kidney dysfunction type was associated with inpatient mortality among those with ACLF, we performed a logistic regression analysis among the hospitalizations with a NACSELD ACLF score of 2 or greater (6.6% of the cohort). We found in univariable analysis that compared to those with CKD, normal kidney function (OR 1.39 [95 CI 1.32–1.45]), AKI (OR 3.33 [95 CI 3.20–3.47], and AKI on CKD (OR 2.11 [95 CI 2.02–2.22]) were each associated with higher odds of inpatient mortality. Similarly in the adjusted analysis, accounting for age, ethnicity, HCV infection, alcohol-related disease, diabetes, infectious status, presence of shock, presence of mechanical ventilation, year of hospitalization, decompensated liver disease, utilization of RRT, and the interaction of RRT and kidney dysfunction type, we found that as compared to those with CKD, only those with normal kidney function (OR 0.58, 95 CI 0.51–0.64) and those with AKI (OR 1.21, 95 CI 1.08–1.33) had significantly different odds of inpatient mortality (Table 3). Additionally, among those with an NACSELD ACLF score ≥ 2, we found a protective effect of RRT, which persisted in the final multivariable model (OR 0.72, 95 CI 0.64–0.80). Finally, as seen in our complete cohort, we found that RRT conferred greater odds of inpatient mortality among those who were initiated with acute diagnoses of kidney dysfunction, as compared to CKD: AKI (OR 2.10 [95 CI 1.87–2.37]) and AKI on CKD (OR 1.47 [95 CI 1.29–1.67]).
Table 3.
Logistic regression for kidney function pattern and associated inpatient mortality among those with ACLF
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p value | |
| Kidney diagnosis | ||||||
| Normal | 1.39 | 1.32–1.45 | < 0.001 | 0.58 | 0.51–0.64 | < 0.001 |
| AKI | 3.33 | 3.20–3.47 | < 0.001 | 1.21 | 1.08–1.33 | < 0.001 |
| CKD | – | – | – | – | – | – |
| AKI on CKD | 2.11 | 2.02–2.22 | < 0.001 | 1.03 | 0.92–1.16 | 0.52 |
| Age per year | 1.01 | 1.01–1.01 | < 0.001 | 1.01 | 1.01–1.01 | < 0.001 |
| Female sex | 1.00 | 0.98–1.03 | 0.77 | |||
| Race | ||||||
| Caucasian | – | – | – | – | – | – |
| African-American | 1.12 | 1.07–1.67 | < 0.001 | 1.22 | 1.16–1.28 | < 0.001 |
| Hispanic | 1.01 | 0.97–1.06 | 0.50 | 1.20 | 1.15–1.25 | < 0.001 |
| Asian | 1.18 | 1.08–1.28 | < 0.001 | 1.15 | 1.05–1.26 | < 0.001 |
| Other | 1.00 | 0.96–1.04 | 0.91 | 1.05 | 1.01–1.10 | 0.03 |
| Hepatitis C | 1.54 | 1.48–1.60 | < 0.001 | 1.29 | 1.23–1.34 | < 0.001 |
| EtOH-related | 0.99 | 0.96–1.02 | 0.53 | |||
| Diabetes mellitus | 0.54 | 0.52–0.56 | < 0.001 | 0.79 | 0.76–0.82 | < 0.001 |
| Hospital location | ||||||
| Metropolitan city center | – | – | – | |||
| Metropolitan county | 0.95 | 0.91–0.98 | 0.003 | |||
| Large county | 0.88 | 0.84–0.91 | < 0.001 | |||
| Medium county | 0.87 | 0.83–0.92 | < 0.001 | |||
| Micropolitan county | 0.88 | 0.84–0.93 | < 0.001 | |||
| Other | 0.87 | 0.81–0.92 | < 0.001 | |||
| Infectious status | ||||||
| None | – | – | – | – | – | – |
| Infection | 0.87 | 0.82–0.91 | < 0.001 | 0.80 | 0.75–0.84 | < 0.001 |
| Sepsis | 2.42 | 2.34–2.50 | < 0.001 | 1.32 | 1.26–1.37 | < 0.001 |
| Mechanical ventilation | 3.10 | 2.99–3.21 | < 0.001 | 2.70 | 2.59–2.81 | < 0.001 |
| Shock | 2.59 | 2.52–2.67 | < 0.001 | 1.88 | 1.81–1.95 | < 0.001 |
| Year of admission | ||||||
| 2007–2008 | – | – | – | – | – | – |
| 2009–2010 | 0.91 | 0.88–0.95 | < 0.001 | 0.84 | 0.80–0.88 | < 0.001 |
| 2011–2012 | 0.81 | 0.78–0.84 | < 0.001 | 0.73 | 0.70–0.76 | < 0.001 |
| 2013–2014 | 0.76 | 0.73–0.79 | < 0.001 | 0.66 | 0.63–0.69 | < 0.001 |
| Decompensated cirrhosis | 0.56 | 0.53–0.58 | < 0.001 | 0.79 | 0.75–0.83 | < 0.001 |
| RRT | 0.76 | 0.74–0.78 | < 0.001 | 0.72 | 0.64–0.80 | < 0.001 |
| Kidney diagnosis and dialysis# | ||||||
| Normal | – | – | – | – | – | – |
| AKI | 3.08 | 2.74–3.45 | < 0.001 | 2.10 | 1.87–2.37 | < 0.001 |
| CKD | Ref | Ref | Ref | Ref | Ref | Ref |
| AKI on CKD | 1.85 | 1.63–2.09 | < 0.001 | 1.47 | 1.27–1.65 | < 0.001 |
AKI acute kidney injury, CKD chronic kidney disease, AKI on CKD acute kidney injury on chronic kidney disease, EtOH alcohol-related disease, RRT renal replacement therapy
This is the interaction term between variables
Discussion
Using a nationally representative sample, we aimed to describe the trends and determine the impact of kidney dysfunction types among patients hospitalized with cirrhosis. We first demonstrated that the overall burden of kidney dysfunction among patients hospitalized with end-stage liver disease is rising—the prevalence of AKI increased 72% (15.8 to 27.2%), CKD increased 38% (17.2 to 23.9%), and AKI on CKD increased 102% (5.5 to 11.1%) during the 8 years of this study. Additionally, we demonstrate that these diagnoses have a differential impact on inpatient mortality. In adjusted models, those with AKI have 2.40 times the odds and those with AKI on CKD have 1.66 times the odds of inpatient mortality, as compared to those with CKD—an important finding that has implications in how we assign risk of mortality and inform the management of patients with end-stage liver disease.
We hypothesize that the kidney dysfunction types are differentially associated with inpatient mortality because of the underlying pathophysiology of each diagnosis. We suspect those with increased acuity, likely have had a triggering event—infection, bleeding, or hyperinflammatory state—which inherently comes with a higher mortality as compared to those with a more chronic, steady decline in kidney function. Although we could not clearly determine the timing of triggering events, we believe this hypothesis is strengthened by our analysis of the interaction of hemodialysis and kidney dysfunction types—as compared to those started with a diagnosis of CKD, the initiation of hemodialysis was associated with a higher mortality if started with an acute diagnosis—AKI or AKI on CKD. These findings highlight that different kidney dysfunction types have a varying impact on inpatient mortality.
This varying impact is all the more important when you consider the rising prevalence of the burden of kidney dysfunction among hospitalized patients with cirrhosis described here. This is because as the burden rises our tools to prognosticate become even more essential. Currently, kidney function in both the Model for End-Stage Liver Disease-Sodium (MELD-Na) score and all acute on chronic liver failure (ACLF) (e.g., NACSELD, chronic liver failure-sequential organ failure assessment) scores is incorporated as a single laboratory value that does not consider the dynamic changes in renal function or the underlying kidney dysfunction type [23, 27, 28]. Additionally, despite improved survival in cirrhosis patients over time, there was no significant interaction between kidney dysfunction type and year of hospitalization—suggesting that improvements in the management of kidney dysfunction did not drive the improved mortality seen in later years. Likewise, there was no significant interaction between kidney dysfunction type and hospital location—suggesting that locality did not impact the survival of each of the kidney dysfunction types. Therefore, our findings in the entire cohort and in the subgroup analysis among patients hospitalized with NACSELD ACLF highlight that inclusion of kidney dysfunction types, in particular AKI, may serve as tools to further calibrate these diagnostic indices [5].
We acknowledge the following limitations to this study. First, the NIS lacks the granularity, particularly regarding laboratory data, to calculate MELD-Sodium scores, to validate kidney dysfunction types, or to determine the severity of kidney dysfunction. However, previous studies have utilized validated ICD-9 codes to identify cirrhosis, AKI, and CKD [10, 12-15], and our findings, specifically the differential impact of kidney dysfunction types on inpatient mortality, appear to be consistent with previously published findings seen among patients awaiting liver transplant [5]. Second, given the nature of the NIS database, there may remain residual confounding on how the kidney dysfunction types were made—specifically, the laboratory test used to determine kidney function (i.e., serum creatinine) is known to overestimate kidney function among patients with cirrhosis [2, 29]. Therefore, there is potential that the prevalence of kidney function diagnoses reported here may be an underrepresentation of the actual burden of kidney dysfunction among patients with cirrhosis. On the other hand, the opposite may be true—"code creep” bias is an important consideration in any study that analyzes temporal trends utilizing diagnostic codes [25]. We address this concern by demonstrating that although the proportion of diagnostic codes that were kidney related rose significantly through the study period, they did not rise as much as the kidney dysfunction types. Finally, despite the underlying uncertainty in temporal trends, either of these biases should have impacted the kidney dysfunction types uniformly and should not have affected the differential effect of kidney dysfunction types on inpatient mortality. Additionally, the NIS is a record of hospitalizations, and not individuals—there remains the possibility that several hospitalizations are from the same individual. Although we fully acknowledge these and other weaknesses inherent to large databases, utilization of the NIS provides substantial external validity—it includes a large population of geographically, economically, and racially diverse patients with end-stage liver disease and can provide insights regarding a broader population of patients with cirrhosis that are not included in analyses of the tertiary care centers included in prior analyses of the liver transplant waitlist or among hospitalizations at liver transplant centers.
Therefore, our study offers significant insights into the burden and impact of each of the kidney dysfunction types among all patients with cirrhosis. Specifically, we highlight that not just AKI [30], but each of the types of kidney dysfunction (e.g., AKI, CKD, AKI on CKD) is increasing among all patients hospitalized with cirrhosis. We build on these findings by demonstrating that, as compared to those with CKD, those with AKI and AKI on CKD have more than and nearly double the risk of inpatient mortality, respectively. Finally, we highlight that the acuity of kidney dysfunction determines the risk conferred when a patient with cirrhosis initiates hemodialysis—those started with acute kidney dysfunction types have a significantly greater risk of inpatient mortality, as compared to those started with chronic kidney dysfunction types. The pathophysiologic mechanism and validation of these findings warrant further study in cohorts with more granular data. Nevertheless, our data focusing on the impact of kidney dysfunction types among all hospitalized patients with cirrhosis demonstrate an opportunity to enhance prognostication of mortality and provide added evidence to support a diagnostic framework that considers the etiology and timing of kidney dysfunction when evaluating patients with cirrhosis.
Funding
Louis and Rachel Rudin Foundation was used to purchase the dataset of the nationwide inpatient sample.
Abbreviations
- AKI
Acute kidney injury
- CKD
Chronic kidney disease
- MELD
Model for end-stage liver disease
- HCC
Hepatocellular carcinoma
Footnotes
Conflict of interest The authors of this manuscript have conflicts of interest to disclose: Giuseppe Cullaro—nothing to disclose. Jessica Rubin—nothing to disclose. Brett E. Fortune—no relevant disclosures. Carl V. Crawford—no relevant disclosures. Elizabeth C. Verna—Advisory Committees or Review Panels: Gilead; Grant/Research Support: Salix. Chi-yuan Hsu—no relevant disclosures. Kathleen D. Liu—no relevant disclosures. Robert S. Brown—no relevant disclosures. Jennifer C. Lai—Consultant: Axcella Health, Inc. Russell Rosenblatt—nothing to disclose.
References
- 1.Wong L, O’Leary JG, Reddy KR, Garcia-Tsao G, Fallon MB, Biggins SW, et al. Acute Kidney Injury in Cirrhosis: Baseline Serum Creatinine Predicts Patient Outcomes. Am J Gastroenterol [Internet]. 2017;(October 2016):1–8. Available from: http://www.nature.com/doifinder/ 10.1038/ajg.2017.122. [DOI] [PubMed] [Google Scholar]
- 2.Cullaro G, Park M, Lai JC. “Normal” Creatinine Levels Predict Persistent Kidney Injury and Waitlist Mortality in Outpatients with Cirrhosis. Hepatology [Internet]. 2018. Apr 26 [cited 2018 Jun 22]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/29698588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet [Internet]. 2014. May 17 [cited 2018 Jun 8];383:1749–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24480518. [DOI] [PubMed] [Google Scholar]
- 4.Ginès P, Schrier RW. Renal Failure in Cirrhosis. N Engl J Med [Internet]. 2009. Sep 24 [cited 2017 Nov 4];361:1279–90. Available from: http://www.nejm.org/doi/abs/ 10.1056/NEJMra0809139. [DOI] [PubMed] [Google Scholar]
- 5.Cullaro G, Verna EC, Lai JC. Association Between Renal Function Pattern and Mortality in Patients with Cirrhosis. Clin Gastroenterol Hepatol [Internet]. 2019. Feb [cited 2019 Mar 23]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S1542356519300904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong F, Reddy KR, O’Leary JG, Tandon P, Biggins SW, Garcia-Tsao G, et al. Impact of Chronic Kidney Disease on Outcomes in Cirrhosis. Liver Transpl [Internet]. 2019. Jun 25 [cited 2019 Jun 13];25:870–80. Available from: https://onlinelibrary.wiley.com/doi/abs/ 10.1002/lt.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cullaro G, Verna EC, Lee BP, Lai JC. Chronic Kidney Disease in Liver Transplant Candidates: A Rising Burden Impacting Post–Liver Transplant Outcomes. Liver Transplant [Internet]. 2020. Apr 29 [cited 2020 Mar 29];26:498–506. Available from: https://onlinelibrary.wiley.com/doi/abs/ 10.1002/lt.25694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martín–Llahí M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, et al. Prognostic Importance of the Cause of Renal Failure in Patients With Cirrhosis. Gastroenterology [Internet]. 2011. Feb [cited 2018 Jun 8];140:488–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20682324. [DOI] [PubMed] [Google Scholar]
- 9.Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, et al. The epidemiology of cirrhosis in the United States a population-based study. J Clin Gastroenterol [Internet]. 2015. Sep [cited 2020 Apr 16];49:690–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25291348. [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt R, Shen N, Tafesh Z, Cohen-Mekelburg S, Crawford CV, Kumar S, et al. The North American Consortium for the Study of End-Stage Liver Disease–Acute-on-Chronic Liver Failure Score Accurately Predicts Survival: An External Validation Using a National Cohort. Liver Transplant [Internet]. 2020. Feb 21 [cited 2020 Mar 29];26:187–95. Available from: https://onlinelibrary.wiley.com/doi/abs/ 10.1002/lt.25696. [DOI] [PubMed] [Google Scholar]
- 11.HCUP-US NIS Overview [Internet]. [cited 2020 Mar 29]. Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp
- 12.Goldberg D, Lewis J, Halpern S, Weiner M, Lo Re V. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf [Internet]. 2012. Jul 1 [cited 2020 Mar 29];21:765–9. Available from: http://doi.wiley.com/ 10.1002/pds.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O et al. Validity of international classification of diseases, ninth revision? Clinical modification codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–1694. [DOI] [PubMed] [Google Scholar]
- 14.Navaneethan SD, Jolly SE, Schold JD, Arrigain S, Saupe W, Sharp J et al. Development and validation of an electronic health record-based chronic kidney disease registry. Clin J Am Soc Nephrol. 2011;6:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winkelmayer WC, Schneeweiss S, Mogun H, Patrick AR, Avorn J, Solomon DH. Identification of individuals with CKD from Medicare claims data: a validation study. Am J Kidney Dis. 2005;46:225–232. [DOI] [PubMed] [Google Scholar]
- 16.Vlasschaert MEO, Bejaimal SAD, Hackam DG, Quinn R, Cuerden MS, Oliver MJ et al. Validity of administrative database coding for kidney disease: a systematic review. Am J Kidney Dis. 2011;57:29–43. [DOI] [PubMed] [Google Scholar]
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 18.Tapper EB, Halbert B, Mellinger J. Rates of and reasons for hospital readmissions in patients with cirrhosis: a multistate population-based cohort study. Clin Gastroenterol Hepatol. 2016;14:1181–1188.e2. [DOI] [PubMed] [Google Scholar]
- 19.Goto T, Yoshida K, Tsugawa Y, Filbin MR, Camargo CA, Hasegawa K. Mortality trends in U.S. adults with septic shock, 2005–2011: a serial cross-sectional analysis of nationally-representative data. BMC Infect Dis. 2016. Jun 14;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellinger JL, Richardson CR, Mathur AK, Volk ML. Variation among United States hospitals in inpatient mortality for cirrhosis. Clin Gastroenterol Hepatol. 2015;13:577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G, Khan N, Walker R, Quan H. Validating ICD coding algorithms for diabetes mellitus from administrative data. Diabetes Res Clin Pract. 2010;89:189–195. [DOI] [PubMed] [Google Scholar]
- 22.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: Observational study. BMJ. 2018;362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Leary JG, Reddy KR, Garcia-Tsao G, Biggins SW, Wong F, Fallon MB, et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology [Internet]. 2018. Jun 1 [cited 2019 May 24];67:2367–74. Available from: http://doi.wiley.com/ 10.1002/hep.29773. [DOI] [PubMed] [Google Scholar]
- 24.Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S et al. Adherence to methodological standards in research using the National Inpatient Sample. JAMA - J Am Med Assoc. 2017;318:2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seiber EE. Physician code creep: evidence in Medicaid and State Employee Health Insurance billing. Health Care Financ Rev [Internet]. 2007. [cited 2020 May 4];28:83–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17722753. [PMC free article] [PubMed] [Google Scholar]
- 26.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–248. [DOI] [PubMed] [Google Scholar]
- 27.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and Mortality among Patients on the Liver-Transplant Waiting List. N Engl J Med [Internet]. 2008. Sep 4 [cited 2017 Sep 27];359:1018–26. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18768945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-Chronic Liver Failure Is a Distinct Syndrome That Develops in Patients With Acute Decompensation of Cirrhosis. Gastroenterology [Internet]. 2013. Jun [cited 2019 Apr 2];144:1426–37. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23474284. [DOI] [PubMed] [Google Scholar]
- 29.De Souza V, Hadj-Aissa A, Dolomanova O, Rabilloud M, Rognant N, Lemoine S, et al. Creatinine- versus cystatine C-based equations in assessing the renal function of candidates for liver transplantation with cirrhosis. Hepatology [Internet]. 2014. Apr [cited 2017 Dec 13];59:1522–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24123197. [DOI] [PubMed] [Google Scholar]
- 30.Desai AP, Knapp SM, Orman ES, Ghabril MS, Nephew LD, Anderson M, et al. Changing epidemiology and outcomes of acute kidney injury in hospitalized patients with cirrhosis - a US population-based study. J Hepatol [Internet]. 2020. May 6 [cited 2020 May 26]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32387698 [DOI] [PMC free article] [PubMed] [Google Scholar]