Abstract
目的
观察程序性细胞死亡配体1(programmed death-ligand 1,PD-L1)抑制剂阿替利珠单抗在中国高发实体瘤,包括食管癌(esophageal cancer,EC)、胃癌(gastric cancer,GC)、肝细胞癌(hepatocellular carcinoma,HCC)、鼻咽癌(nasopharyngeal cancer,NPC)和非小细胞肺癌(non-small cell lung cancer,NSCLC)患者中的药代动力学(pharmacokinetics,PK)、疗效和安全性数据。
方法
本研究为开放标签的Ⅰ期临床试验,于2016年8月4日至2019年4月15日在中国6个研究中心进行。入组患者年龄≥18岁,患有经组织学证实的无法治愈或转移性的实体瘤,且既往抗肿瘤治疗失败。PK阶段研究了阿替利珠单抗单药治疗的PK和安全性;扩展阶段研究了阿替利珠单抗单药治疗(入组EC、GC、HCC、NPC患者)和联合化疗(入组NSCLC患者)的安全性和有效性。
结果
共入组120例患者(PK阶段20例;扩展期每队列20例)。阿替利珠单抗单药组患者(n=100)中有42例(42.0%)为PD-L1阳性,9例(9.0%)为微卫星高度不稳定性。阿替利珠单抗的清除率为0.219 L/d,重复给药6~9周(2~3个周期)后达到稳态。EC、GC、HCC、NPC和NSCLC的客观缓解率(objective response rate,ORR)分别为10.0%、15.0%、10.0%、5.0%和40.0%。在PD-L1阳性的肿瘤患者中,阿替利珠单抗的ORR为11.9%,阿替利珠单抗联合吉西他滨和顺铂的ORR为46.2%。2例GC患者在假性进展后获得了持久的肿瘤缩小。阿替利珠单抗单药组最常见的治疗相关不良事件是疲劳、贫血、发热和白细胞计数减少,联合组最常见的治疗相关AE是贫血、白细胞计数减少和食欲下降。本试验没有发现新的安全信号。
结论
中国患者应用阿替利珠单抗的PK、疗效和安全性与之前研究中入组的全球患者的数据相似。
Keywords: 阿替利珠单抗, 药代动力学, 中国, 肿瘤
Abstract
Objective
To evaluate pharmacokinetics (PK), efficacy, and safety of atezolizumab (anti-PD-L1) in high interest cancers in China, including esophageal cancer (EC), gastric cancer (GC), hepatocellular carcinoma (HCC), nasopharyngeal cancer (NPC), and non-small cell lung can-cer (NSCLC).
Methods
This phase I, open-label study was conducted at 6 Chinese sites from August 4, 2016 to April 15, 2019. The patients were ≥18 years old with a histologically documented incurable or metastatic solid tumor that was advanced or recurrent and had progressed since the last anti-tumor the-rapy. The PK phase characterized PK and safety of atezolizumab following multiple-dose administration when atezolizumab was administered as a single agent. The extension phase studied safety and efficacy of atezolizumab, as monotherapy (EC, GC, HCC, NPC) and with chemotherapy (NSCLC).
Results
This study enrolled 120 patients (PK phase: n=20; extension phase: n=20/cohort). Fourty-two patients (42.0%) were PD-L1 positive in atezolizumab monotherapy group (100 patients), of the 9 patients (9.0%) with microsatellite instability-high (MSI-H) tumors. Atezolizumab clearance was 0.219 L/d, and steady state was reached after 6 to 9 weeks (2-3 cycles) of repeated dosing. Objective response rates (ORRs) in EC, GC, HCC, NPC, and NSCLC were 10.0%, 15.0%, 10.0%, 5.0%, and 40.0%, respectively. In the patients with PD-L1 positive tumors, ORR was 11.9% with atezolizumab and 46.2% with atezolizumab plus gemcitabine and cisplatin. Two GC patients achieved durable response after pseudo-progression. The most common treatment-related adverse events in the atezolizumab monotherapy group were fatigue, anemia, fever, and decreased white blood cell count. The most common treatment-related adverse events in the combination group were anemia, decreased white blood cell count, and decreased appetite. No new safety signals were identified.
Conclusion
Atezolizumab's PK, efficacy, and safety were similar in Chinese patients vs. global patients in previous studies.
Keywords: Atezolizumab, Pharmacokinetics, China, Neoplasms
非小细胞肺癌(non-small cell lung cancer,NSCLC)、胃癌(gastric cancer,GC)、肝细胞癌(hepatocellular carcinoma,HCC)、食管癌(esophageal cancer,EC)和鼻咽癌(nasopharyngeal cancer,NPC)是中国人群的高发癌症,受到的关注度很高[1-3]。患有这些癌症的患者可能会从程序性细胞死亡蛋白-1 (programmed cell death protein-1,PD-1)和程序性细胞死亡配体1(programmed death-ligand 1,PD-L1)抑制剂的治疗中获益。PD-1抑制剂在EC、GC、HCC和NPC患者中的临床疗效已得到证实[4-7]。另外,PD-1和PD-L1抑制剂已经被推荐作为一线治疗与化疗联合用于晚期或转移性NSCLC的患者[8]。
阿替利珠单抗(atezolizumab)是一种阻断PD-L1的人源化单克隆抗体,已经被美国食品和药品监督管理局(US Food and Drug Administration,FDA)批准用于治疗多种癌症患者[9]。2016年10月,阿替利珠单抗被批准用于治疗在含铂化疗期间或之后疾病进展的转移性NSCLC患者[10]。2019年,欧盟委员会和FDA批准阿替利珠单抗联合化疗作为一线治疗转移性非鳞状NSCLC[11-12]。2020年,包括美国在内的许多国家批准了阿替利珠单抗与贝伐珠单抗联合作为一线治疗不可切除的HCC[13],这种组合在中国HCC患者中也显示出具有临床意义的结果[14]。在我国其他高发肿瘤中进行的阿替利珠单抗临床试验,包括一项大型的Ⅰ期试验,均证实阿替利珠单抗单药具有广泛的临床活性[9]。
尽管阿替利珠单抗已显示出较好的临床疗效,但这些研究主要是在全球非中国人群中进行的。总体而言,由于生活方式或遗传的差异,中国癌症患者和其他地区的癌症患者在流行病学和临床特点等方面存在种族差异[15-16]。因此,我们开展了这项临床试验,以探索阿替利珠单抗在中国晚期癌症患者中的药代动力学(pharmaeokineties,PK)、疗效和安全性。
1. 资料与方法
本研究为开放标签的Ⅰ期临床试验,于2016年8月4日至2019年4月15日在中国的6个研究中心进行。初始PK阶段探索了阿替利珠单抗单药治疗局部晚期或转移性实体瘤患者的PK和安全性(临床数据截止日期:2017年11月19日);扩展阶段进一步探索了在中国高发实体瘤患者中,单药治疗和联合化疗的安全性和有效性(临床数据截止日期:2019年4月15日)。这些肿瘤包括Ⅳ期的NSCLC,局部晚期或转移性EC、GC、HCC和NPC。
在PK、EC、GC、HCC和NPC队列中,阿替利珠单抗单药治疗的剂量是1 200 mg,静脉输注(intravenous,IV),每3周[q3w;(21±2) d]重复,此剂量是根据阿替利珠单抗单药的Ⅰ期临床研究中,在1~20 mg/kg(q3w)剂量范围内,未达到最大耐受剂量时选择固定剂量1 200 mg(q3w)的治疗方案[相当于基于平均体质量的剂量15 mg/kg(q3w)][9]。在联合化疗的队列,由于吉西他滨联合顺铂双药化疗是鳞状NSCLC一线治疗的推荐方案[8],因此,纳入NSCLC队列的患者接受了以下治疗:阿替利珠单抗1 200 mg(IV,第1天)、吉西他滨1 250 mg/m2(IV,第1天和第8天)、顺铂75 mg/m2(IV,第1天),q3w。阿替利珠单抗的治疗一直持续到无临床获益、疾病进展[在初始评估疾病进展后,需在≥4周的重复评估中通过实体瘤疗效评估标准1.1(Response Evaluation Criteria in Solid Tumors,RECIST 1.1)确认疾病进展]、出现不可接受的毒性、患者或医生决定停止治疗或死亡。根据RECIST 1.1,每6周(±3 d;大约每2个周期)评估所有肿瘤的变化情况。如果满足特定标准,可以在疾病进展后继续治疗。在停药后和随访期,每3个月对所有患者进行生存随访。
1.1. 患者入组和排除标准
入组标准:患者年龄≥18岁,患有经组织学证实的无法治愈或转移性实体瘤,既往抗肿瘤治疗后进展(不可切除)或复发,且不存在公认的标准治愈性治疗方案或拒绝标准治疗(其中,NSCLC队列入组了既往未接受化疗的Ⅳ期NSCLC患者),居住在中华人民共和国的中国患者,根据RECIST 1.1要求有可测量的病灶,东部肿瘤协作组(Eastern Cooperative Oncology Group,ECOG)状态评分为0/1,有福尔马林固定、石蜡包埋的肿瘤标本蜡块或白片。排除标准:患者在入组治疗开始前距末次给药(任何已批准的或处于临床研究阶段的药物)的洗脱期≤5个半衰期,同时存在活动性的或未经治疗的中枢神经系统转移或自身免疫性疾病的病史或风险。
所有患者均在研究开始前签署知情同意书。已完成≥1个周期的阿替利珠单抗和相关PK采样的患者,如由于某些原因中止研究,则不再予以补充。
1.2. 研究的主要终点
PK研究的内容包括最大血清浓度(maximum serum concentration,Cmax)、最小血清浓度(minimum serum concentration,Cmin)、基于首次给药后和稳态浓度的蓄积率(accumulation ratio,AR)、浓度-时间曲线下面积(area under the concentration-time curve,AUC)、清除率(systemic clearance,CL)、稳态条件下的分布容积(volume of distribution under steady-state conditions,Vss)和半衰期(half-life,t1/2)。本研究采用已验证的免疫测定法定量检测阿替利珠单抗血清浓度。PK采样时间点为:第1周期给药前,阿替利珠单抗输注结束后30 min(±10 min),24 h(±6 h),72 h(±12 h),第8天(±1天),第15天(±2天),第2、3、4、8、12、16周期,以及从第8周期之后每8个周期的第1天给药前,阿替利珠单抗输注结束后30 min(±10 min),研究完成或提前终止试验时。
主要疗效终点是根据RECIST 1.1由研究者评估确定的客观缓解率(objective response rate,ORR),其需要在≥4周进行重复评估以确认疗效。次要疗效终点包括按照RECIST 1.1评估的缓解持续时间(duration of response,DOR)和无进展生存期(progression-free survival,PFS)、总生存期(overall survival,OS)以及6个月和/或1年的总生存率。安全性终点包括根据美国国家癌症研究所不良事件通用术语标准(National Cancer Institute Common Terminology Criteria for Adverse Events,NCI CTCAE)第4版分级的不良事件(adverse events,AE)的发生率和严重程度、主要生命体征以及临床实验室结果相对于基线的变化情况。另外,本试验还收集了特别关注的AE以分析阿替利珠单抗已获知的及潜在的风险。本试验还设立了中心实验室以协调收集归档肿瘤组织标本、新鲜肿瘤组织标本以及剩余的肿瘤组织和血液样本。
1.3. 统计学分析
此项Ⅰ期研究没有进行统计学设计和Ⅰ类错误的考量。基于之前类似的研究,并为了与监管指南保持一致,每个队列的样本量为20例,用于与既往数据进行间接比较。对PK可评估人群,包括所有进入PK阶段并接受了任何剂量阿替利珠单抗的患者进行了非房室模型分析来描述PK特征。将阿替利珠单抗PK参数的估计值制成表格并进行汇总。使用第1天和稳态值比较患者间差异性和药物积累。
本试验应用Clopper-Pearson方法来测算ORR的精确95%置信区间(confidence intervals,CI)。Kaplan-Meier方法用于估计DOR、PFS、OS和6个月和/或1年总生存率,Brookmeyer-Crowley方法用于构建中位DOR、PFS和OS的95%CI。
1.4. 研究满足的监管政策
本研究完全符合国际协调人用药品技术要求委员会E6临床试验规范、赫尔辛基宣言原则、中华人民共和国法律法规和每个研究中心的伦理委员会要求。该研究还符合国际协调人用药品技术要求E2A指南(临床安全数据管理:加速报告的定义和标准)和FDA法规的要求。
2. 结果
2.1. 患者的一般情况
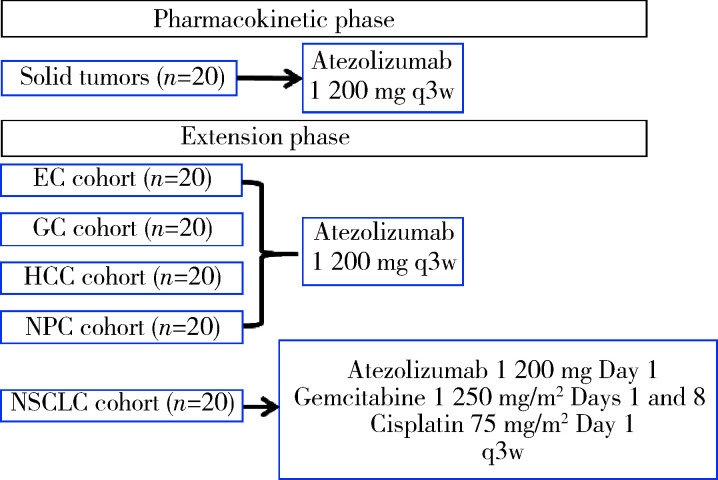
20例患者参加了PK阶段的研究,100例患者参加了扩展期研究(图 1),EC、GC、HCC、NPC和NSCLC队列各入组了20例患者。
图 1.
研究流程图
Study schema
EC, esophageal cancer; GC, gastric cancer; HCC, hepatocellular carcinoma; NPC, nasopharyngeal cancer; NSCLC, non-small cell lung can-cer; q3w, every 3 weeks.
阿替利珠单抗单药组(n=100)的患者主要为男性(80.0%),中位年龄为55.0岁(表 1),多数患者(81.0%)年龄小于65岁。42例(42.0%)患者为PD-L1阳性,其中34例(34.0%)为免疫细胞PD-L1阳性,16例(16.0%)为肿瘤细胞PD-L1阳性。在9例(9.0%)微卫星高度不稳定性(microsatellite instability-high,MSI-H)的肿瘤患者中,5例(5.0%)为GC。
表 1.
入组患者的一般基线特征
Demographic and baseline characteristics
| Items | Atezolizumab (n=100) | Atezolizumab+gemcitabine+cisplatin (n=20) |
| CRC, colorectal cancer; UC, urothelial cancer; PK, pharmacokinetics; PD-L1, programmed death-ligand 1; EBV, Epstein-Barr virus; MSI-H, microsatellite instability-high; BTC, biliary tract carcinoma; NET, neuroendocrine tumor; ECOG, Eastern Cooperative Oncology Group; IC, immune cells; TC, tumor cells; NA, not applicable. Other abbreviations as in Figure 1. a, PD-L1+ status is defined as IC or TC≥1%; b, PD-L1 IC score at baseline: IC < 1% (IC0), 1% < IC < 5% (IC1), 5% < IC < 10% (IC2), IC≥10% (IC3); c, PD-L1 TC score at baseline: TC < 1% (TC0), 1% < TC < 5% (TC1), 5% < TC < 50% (TC2), TC≥50% (TC3). | ||
| Age/years, median (range) | 55.0 (19.0-77.0) | 60.0 (38.0-77.0) |
| Age < 65 years, n(%) | 81 (81.0) | 14 (70.0) |
| Male, n(%) | 80 (80.0) | 18 (90.0) |
| Weight/kg, Median (Range) | 60.0 (33.0-96.0) | 64.5 (47.0-94.0) |
| Study cohort | ||
| EC (PD-L1+/PD-L1-), n | 20 (10/10) | NA |
| GC (PD-L1+/PD-L1-/EBV+/MSI-H), n | 20 (6/14/10/5) | NA |
| HCC (PD-L1+/PD-L1-), n | 20 (8/12) | NA |
| NPC (PD-L1+/PD-L1-), n | 20 (15/5) | NA |
| NSCLC (PD-L1+/PD-L1-), n | NA | 20 (13/7) |
| PK (NSCLC/CRC/GC/UC/EC/HCC/BTC/NET),n | 20 (7/4/4/1/1/1/1/1) | NA |
| ECOG performance score, n(%) | ||
| 0 | 21 (21.0) | 0 |
| 1 | 79 (79.0) | 20 (100.0) |
| PD-L1+a, n(%) | 42 (42.0) | 13 (65.0) |
| PD-L1 IC scoreb, n(%) | ||
| IC0 | 63 (63.0) | 10 (50.0) |
| IC1 | 26 (26.0) | 6 (30.0) |
| IC2 | 3 (3.0) | 2 (10.0) |
| IC3 | 5 (5.0) | 1 (5.0) |
| Unknown | 3 (3.0) | 1 (5.0) |
| PD-L1 TC scorec, n(%) | ||
| TC0 | 81 (81.0) | 13 (65.0) |
| TC1 | 5 (5.0) | 1 (5.0) |
| TC2 | 10 (10.0) | 1 (5.0) |
| TC3 | 1 (1.0) | 4 (20.0) |
| Unknown | 3 (3.0) | 1 (5.0) |
| Tobacco use, n(%) | ||
| Current | 6 (6.0) | 0 |
| Previous | 40 (40.0) | 14 (70.0) |
| Never | 54 (54.0) | 6 (30.0) |
| Alcohol use, n(%) | ||
| Current | 5 (5.0) | 0 |
| Previous | 32 (32.0) | 11 (55.0) |
| Never | 63 (63.0) | 9 (45.0) |
| Prior radiotherapy, n(%) | 43 (43.0) | 4 (20.0) |
| MSI-H (all cancer types), n(%) | 9 (9.0) | 0 |
| Prior therapy for recurrent/metastatic disease, n(%) | 91 (91.0) | 1 (5.0) |
在阿替利珠单抗联合吉西他滨和顺铂组(即NSCLC队列,n=20)中,患者也主要是男性(90.0%),中位年龄为60.0岁(表 1),多数患者(70.0%)年龄小于65岁。13例(65.0%)PD-L1阳性患者中,9例(45.0%)为免疫细胞PD-L1阳性,6例(30.0%)为肿瘤细胞PD-L1阳性。
2.2. 患者的PK特征
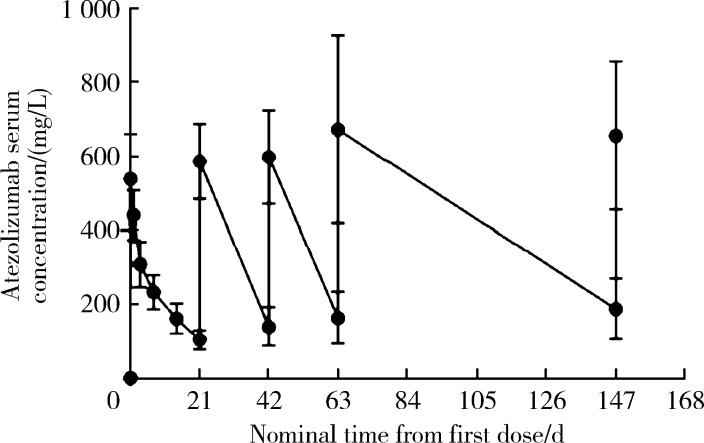
多次阿替利珠单抗q3w给药后Cmin增加,并在2~3个给药周期间达到平台期(图 2)。治疗后阿替利珠单抗的药代动力学参数总结在表 2中。在每个治疗周期中,基于Cmin和Cmax的阿替利珠单抗的AR几何平均值>1。第1周期阿替利珠单抗的几何平均值AUC0-21(n=20)和AUC0-∞(n=13)分别为4 450 d·(mg/L)[变异系数(coefficient of variation,CV)=17.2%]和5 600 d·(mg/L)(CV=20.7%)。平均药物清除率为0.219 L/d(CV=21.2%)。由于在第1周期中只有21天的密集PK数据,t1/2可能被低估了,因此,AUC0-∞、CL和Vss也是大致估计值。
图 2.
阿替利珠单抗在PK组的血药浓度
Atezolizumab concentrations in PK cohort
Atezolizumab 1 200 mg administered every 3 weeks.
表 2.
阿替利珠单抗PK组患者在第1周期获得的血浆PK参数(n=20)
Summary statistics for serum atezolizumab PK parameters in cycle 1 in PK cohort (n=20)
| Items | Cmax/(mg/L) | Cmin/(mg/L) | AUC0-21/[d·(mg/L)] | AUC0-∞a/[d·(mg/L)] | CLb/(mL/d) |
| AUC0-21, area under the serum concentration-time curve from Day 1 to 21; AUC0-∞, area under the serum concentration-time curve from Day 1 to infinity; Cmax, maximum serum concentration; Cmin, minimum serum concentration; CL, clearance; CV, coefficient of variation; GM, geometric mean; PK, pharmacokinetics; SD, standard deviation. a, patients with AUC extrapolation (last PK sample to infinity in cycle 1) >30% were excluded from summary statistics; b, total clearance of drug was calculated by dose/AUC0-∞. | |||||
| n | 20 | 20 | 20 | 13 | 13 |
| Mean (CV) | 542 (22.3%) | 102 (26.0%) | 4 510 (16.6%) | 5 710 (19.6%) | 219 (21.2%) |
| SD | 121 | 26.5 | 749 | 1 120 | 46.4 |
| Minimum | 377 | 56.1 | 2 970 | 3 780 | 165 |
| Median | 520 | 105 | 4 530 | 5 970 | 201 |
| Maximum | 887 | 143 | 6 100 | 7 280 | 317 |
| GM (CV) | 530 (20.9%) | 98.7 (28.7%) | 4 450 (17.2%) | 5 600 (20.7%) | 214 (20.7%) |
2.3. 患者的疗效结果
本研究中,EC、GC、HCC、NPC和NSCLC队列的ORR分别为10.0%、15.0%、10.0%、5.0%和40.0%(表 3),DOR、PFS和OS数据及每个队列的中位随访时间也列于表 3。在PD-L1阳性患者中,阿替利珠单抗单药组的ORR为11.9%,患者的中位PFS和OS分别为2.8个月和14.9个月;阿替利珠单抗联合吉西他滨和顺铂组的ORR为46.2%,患者的中位PFS为6.9个月,因累积率未达到50%,故未计算中位OS(表 4)。对于PD-L1阴性患者,阿替利珠单抗单药组的ORR为8.9%,患者中位PFS和OS分别为2.7个月和9.7个月;阿替利珠单抗联合吉西他滨和顺铂组的ORR为33.3%,患者的中位PFS为5.8个月,因累积率未达到50%,故未计算中位OS(表 4)。
表 3.
不同组间患者的治疗疗效汇总
Efficacy summary by cohorts
| Items | Atezolizumab (n=100) | Atezolizumab+ gemcitabine+ cisplatin (n=20) | |||||
| EC (n=20) | GC (n=20) | HCC (n=20) | NPC (n=20) | PK (n=20) | NSCLC (n=20) | ||
| ORR, objective response rate; DOR, duration of response; PFS, progression-free survival; OS, overall survival; CR, complete response; PR, partial response; SD, stable disease; DCR, disease control rate; PD, progressive disease; CI, confidence interval; NE, not evaluable; NA, not applicable; Other abbreviations as in Figure 1 and Table 1. a, DCR is calculated by taking the sum of CR, PR, and SD. | |||||||
| ORR (95%CI)/% | 10.0 (1.23-31.70) | 15.0 (3.21-37.89) | 10.0 (1.23-31.70) | 5.0 (0.13-24.87) | 10.0 (1.23-31.70) | 40.0 (19.12-63.95) | |
| CR, n(%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| PR, n(%) | 2 (10.0) | 3 (15.0) | 2 (10.0) | 1 (5.0) | 2 (10.0) | 8 (40.0) | |
| SD, n(%) | 6 (30.0) | 7 (35.0) | 10 (50.0) | 12 (60.0) | 7 (35.0) | 10 (50.0) | |
| DCRa, n(%) | 8 (40.0) | 10 (50.0) | 12 (60.0) | 13 (65.0) | 9 (45.0) | 18 (90.0) | |
| PD, n(%) | 7 (35.0) | 7 (35.0) | 7 (35.0) | 7 (35.0) | 11 (55.0) | 1 (5.0) | |
| NE, n(%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Missing, n(%) | 5 (25.0) | 3 (15.0) | 1 (5.0) | 0 | 0 | 1 (5.0) | |
| Median DOR (95%CI)/months | 14.4 (8.0-20.8) | 18.4 (NE) | NE (6.9-NE) | 8.3 (NE) | NA | NE (5.6-NE) | |
| Median PFS (95%CI)/months | 1.4 (1.4-2.8) | 2.7 (1.4-4.4) | 2.8 (1.4-7.8) | 3.5 (1.4-7.0) | NA | 5.8 (5.5-9.6) | |
| Median OS (95%CI)/months | 4.8 (2.1-10.9) | 9.7 (3.7-27.6) | 7.8 (4.7-NE) | 13.9 (8.2-21.3) | NA | NE (9.6-NE) | |
| 6-month OS rate (95%CI)/% | 40 (19-61) | 52 (29-75) | 61 (39-84) | 90 (77-100) | NA | 89 (74-100) | |
| 1-year OS rate (95%CI)/% | 25 (6-44) | 37 (13-61) | 43 (19-66) | 57 (34-80) | NA | 66 (27-100) | |
| Duration of follow-up/months, M (Range) | 4.76 (0.3-29.9) | 7.41 (1.2-29.2) | 12.16 (0.7-29.2) | 14.93 (1.7-26.3) | 15.21 (2.4-27.5) | 6.95 (0.8-14.6) | |
表 4.
不同生物标志物表达组间患者疗效汇总
Efficacy summary by biomarker status
| Items | PD-L1+ (n=55) | PD-L1- (n=62) | MSI-H (n=9) | ||||
| Atezolizumab (n=42) | Atezolizumab + gemcitabine + cisplatin (n=13) | Atezolizumab (n=56) | Atezolizumab + gemcitabine + cisplatin (n=6) | Atezolizumab (n=9) | |||
| Abbreviations and explain as in Figure 1, Table 1 and 3. a, DCR is calculated by taking the sum of CR, PR, and SD. | |||||||
| ORR (95%CI)/% | 11.9 (3.98-25.63) | 46.2 (19.22-74.87) | 8.9 (2.96-19.62) | 33.3 (4.33-77.72) | 11.1 (0.28-48.25) | ||
| CR, n(%) | 0 | 0 | 0 | 0 | 0 | ||
| PR, n(%) | 5 (11.9) | 6 (46.2) | 5 (8.9) | 2 (33.3) | 1 (11.1) | ||
| SD, n(%) | 16 (38.1) | 5 (38.5) | 25 (44.6) | 4 (66.7) | 4 (44.4) | ||
| DCRa, n(%) | 21 (50.0) | 11 (84.6) | 30 (53.6) | 6 (100.0) | 5 (55.6) | ||
| PD, n(%) | 17 (40.5) | 1 (7.7) | 21 (37.5) | 0 | 4 (44.4) | ||
| NE, n(%) | 0 | 0 | 0 | 0 | 0 | ||
| Missing, n(%) | 4 (9.5) | 1 (7.7) | 5 (8.9) | 0 | 0 | ||
| Median DOR (95%CI)/months | 20.8 (6.9-20.8) | NE (5.6-NE) | 17.1 (8.0-NE) | NE (NE) | NE | ||
| Median PFS (95%CI)/months | 2.8 (1.4-4.4) | 6.9 (5.5-NE) | 2.7 (1.4-3.8) | 5.8 (3.3-NE) | 4.2 (1.4-15.0) | ||
| Median OS (95%CI)/months | 14.9 (6.1-29.9) | NE (9.6-NE) | 9.7 (6.6-13.2) | NE (3.8-NE) | 15.0 (9.7-NE) | ||
| 6-month OS rate (95%CI)/% | 67 (52-82) | 100 (100-100) | 64 (52-77) | 67 (29-100) | 78 (51-100) | ||
| 1-year OS rate (95%CI)/% | 50 (34-67) | 75 (33-100) | 39 (26-52) | NE (NE) | 65 (32-97) | ||
| Duration of follow-up/months, M (Range) | NA | NA | NA | NA | NA | ||
在MSI-H肿瘤患者(n=9)中,1例达到部分缓解,4.2个月后出现病情进展,患者OS达到15.0个月(表 4)。2例GC患者在假性进展后肿瘤达到了部分缓解,并在临床数据收集截止时还在继续治疗。
2.4. 患者的安全性结果
在阿替利珠单抗单药组中,98%的患者发生了至少1次AE(表 5),51例患者(51.0%)报告了3/4级AE,11例(11.0%)发生了5级AE,1例(1.0%)发生了5级治疗相关AE,严重AE和特别关注AE分别为41.0%和64.0%。84例患者(84.0%)出现了各种级别的治疗相关AE,17例(17.0%)发生了导致退出治疗的AE。表 6列出了发生率≥10%的AE,最常见的治疗相关AE是疲劳、贫血、发热和白细胞计数减少。
表 5.
阿替利珠单抗单药或联合化疗治疗患者的安全性数据汇总
Saftey summary on atezolizumab monotherapy and atezolizumab combined with chemotherapy
| Items | Atezolizumaba (n=100) | Atezolizumab+gemcitabine+cisplatina (n=20) |
| AE, adverse event; AESI, adverse event of special interest. a, percentages are based on n in the column heading. Multiple occurrences of the same AE in one individual are counted only once except for in the “AEs” row, in which multiple occurrences of the same AE are counted separately; b, dose modification not allowed with atezolizumab; c, due to disease progression, AE, or other causes; d, death. | ||
| Patients with ≥1 AE, n(%) | 98 (98.0) | 20 (100.0) |
| Serious AEs | 41 (41.0) | 8 (40.0) |
| Leading to treatment discontinuation | 16 (16.0) | 0 |
| Leading to any dose modification/interruptionb | 12 (12.0) | 2 (10.0) |
| Treatment-related | 18 (18.0) | 7 (35.0) |
| AEs leading to treatment discontinuation | 17 (17.0) | 0 |
| AEs leading to any dose modification/interruptionb | 28 (28.0) | 4 (20.0) |
| Treatment-related AEs | 84 (84.0) | 20 (100.0) |
| Leading to treatment discontinuation | 7 (7.0) | 0 |
| Leading to any dose modification/interruptionb | 17 (17.0) | 2 (10.0) |
| Grade 3/4 AEs | 51 (51.0) | 17 (85.0) |
| Treatment-related | 31 (31.0) | 17 (85.0) |
| Grade 5 AEs | 11 (11.0) | 1 (5.0) |
| Treatment-related | 1 (1.0)d | 0 |
| AESIs | 64 (64.0) | 12 (60.0) |
| AEs, n | 913 | 399 |
| Deathsc, n(%) | 71 (71.0) | 3 (15.0) |
| Withdrawal from study due to an AE, n(%) | 0 | 0 |
表 6.
阿替利珠单抗单药组的不良事件
Adverse events in the atezolizumab monotherapy arm
| Items | Atezolizumaba (n=100) | Items | Atezolizumaba (n=100) | |
| AE, adverse event. a, percentages are based on n in the column heading. For frequency counts by preferred term, multiple occurrences of the same AE in an individual are counted only once. | ||||
| AEs (≥10%), n(%) | 98 (98.0) | AEs leading to treatment discontinuation, n(%) | 17 (17.0) | |
| Aspartate aminotransferase increased | 30 (30.0) | Hepatic function abnormal | 3 (3.0) | |
| Anemia | 29 (29.0) | Death | 2 (2.0) | |
| Pyrexia | 28 (28.0) | Dyspnea | 2 (2.0) | |
| Alanine aminotransferase increased | 26 (26.0) | Aspartate aminotransferase increased | 1 (1.0) | |
| Decreased appetite | 24 (24.0) | Blood creatinine phosphokinase increased | 1 (1.0) | |
| Fatigue | 24 (24.0) | Esophagobronchial fistula | 1 (1.0) | |
| Blood bilirubin increased | 24 (24.0) | Fistula | 1 (1.0) | |
| White blood cell count decreased | 22 (22.0) | Hyponatremia | 1 (1.0) | |
| Neutrophil count decreased | 16 (16.0) | Neoplasm progression | 1 (1.0) | |
| Bilirubin conjugated increased | 15 (15.0) | Pneumonia fungal | 1 (1.0) | |
| Hyponatremia | 13 (13.0) | Polymyositis | 1 (1.0) | |
| Weight decreased | 13 (13.0) | Sudden death | 1 (1.0) | |
| Proteinuria | 13 (13.0) | Transaminases increased | 1 (1.0) | |
| Upper respiratory tract infection | 13 (13.0) | |||
| Nausea | 11 (11.0) | |||
| Hypoalbuminemia | 10 (10.0) | |||
阿替利珠单抗联合吉西他滨和顺铂组的所有患者均发生了至少1次AE(表 5),17例患者(85.0%)发生了3/4级AE,1例(5.0%)经历了5级AE。严重AE和特别关注AE分别为40.0%和60.0%。表 7列出了发生率≥10%的AE,全部20例患者(100.0%)发生了各个级别的治疗相关AE,最常见的是贫血、白细胞计数减少和食欲下降,但没有患者因AE退出治疗。
表 7.
阿替利珠单抗联合吉西他滨和顺铂组的不良事件(≥10%)
Adverse events in the atezolizumab plus gemcitabine and cisplatin arm(≥10%)
| Items | Atezolizumab+ gemcitabine+ cisplatina (n=20) |
| a, percentages are based on n in the column heading. For frequency counts by preferred term, multiple occurrences of the same AE in an individual are counted only once. | |
| Anemia | 15 (75.0) |
| Decreased appetite | 13 (65.0) |
| White blood cell count decreased | 13 (65.0) |
| Nausea | 11 (55.0) |
| Platelet count decreased | 11 (55.0) |
| Neutrophil count decreased | 10 (50.0) |
| Neutropenia | 8 (40.0) |
| Vomiting | 8 (40.0) |
| Alanine aminotransferase increased | 7 (35.0) |
| Hypoalbuminemia | 6 (30.0) |
| Leukopenia | 6 (30.0) |
| Thrombocytopenia | 6 (30.0) |
| Fatigue | 5 (25.0) |
| Pyrexia | 5 (25.0) |
| Rash | 5 (25.0) |
| Aspartate aminotransferase increased | 4 (20.0) |
| Asthenia | 4 (20.0) |
| Hyponatremia | 4 (20.0) |
| Lymphocyte count decreased | 4 (20.0) |
| Productive cough | 4 (20.0) |
| Blood lactate dehydrogenase increased | 3 (15.0) |
| Bone marrow failure | 3 (15.0) |
| Chest pain | 3 (15.0) |
| Constipation | 3 (15.0) |
| Gamma-glutamyltransferase increased | 3 (15.0) |
| Hemoptysis | 3 (15.0) |
| Hypoproteinemia | 3 (15.0) |
| Weight decreased | 3 (15.0) |
| Abdominal discomfort | 2 (10.0) |
| Amylase increased | 2 (10.0) |
| Bilirubin conjugated increased | 2 (10.0) |
| Blood bilirubin increased | 2 (10.0) |
| Blood creatinine increased | 2 (10.0) |
| Hypochloremia | 2 (10.0) |
| Hypokalemia | 2 (10.0) |
| Hypothyroidism | 2 (10.0) |
3. 讨论
本研究很好地探索了阿替利珠单抗在中国人群中的PK数据,没有证据表明中国患者与全球人群之间存在差异。中国人群阿替利珠单抗的CL与美国处方信息中报告的结果类似(0.219 L/d vs. 0.20 L/d),且在重复给药6~9周(2~3个周期)后达到稳态也与群体PK数据分析结果类似[17]。
本研究入组的中国患者与之前研究中入组的非中国患者的疗效结果也具有可比性[9, 18]。GC组的ORR为15%,与非中国患者接受阿替利珠单抗单药治疗的有效率16.7%(1/6)相似[9];HCC组ORR为10%,与GO30140研究中单药阿替利珠单抗组的相比略低,考虑是由于本研究入组了较多的二线治疗及以上患者[19]。PD-L1阳性组和MSI-H组患者的ORR在数值上低于使用帕博利珠单抗的患者,这可能是由于基线和疾病特征的差异导致的[20-21]。一项全球研究中入组的实体瘤患者的中位PFS(18周)与本试验中入组的HCC和NPC队列中的中国患者相似[9, 19, 22]。在NSCLC患者中,阿替利珠单抗联合化疗组的中国患者与非中国患者的中位PFS也是类似的[18]。对于中位OS,中国NPC队列的OS在数值上比全球头颈部肿瘤亚组长[22]。中国NSCLC队列的1年总生存率与全球NSCLC队列数据相似[18]。在非NSCLC队列中,PD-L1阳性和阴性的中国患者的疗效没有显着差异。此外,2例MSI-H的GC患者出现假性进展后达到部分缓解,这超出了RECIST 1.1标准定义的疾病进展的范畴,提示阿替利珠单抗可能实现持久的疗效。
在安全性方面,在中国患者中使用阿替利珠单抗与之前研究中非中国患者的情况基本一致,没有发现新的安全性信号。同样,阿替利珠单抗联合吉西他滨和顺铂的安全性也与其他非中国患者的安全性一致[23]。
本研究扩展阶段入组的各个肿瘤队列的病例数较少,与之前进行的较大规模的试验相比,增加了结果的不确定性。但本研究入组的癌症类型是根据中国的癌症发病率情况设计的,均为中国高发病率的癌症[1, 24]。本研究结果表明,阿替利珠单抗可作为潜在治疗药物应用于中国癌症患者,且治疗效果与全球其他患者不存在种族差异。本研究还为阿替利珠单抗在中国的其他研究提供了重要的安全性和疗效数据基础。总体而言,我们的结果显示,阿替利珠单抗的PK特征、临床疗效和安全特征与之前在非中国患者中进行的临床研究是类似的。
志谢
感谢MediTech Media,Inc. 的Bena LIM博士为本文提供医学写作支持,感谢罗氏有限公司提供研究药物及研究资金。
Biographies
胡夕春,主任医师,教授,博士研究生导师,复旦大学附属肿瘤医院肿瘤内科主任,复旦大学附属肿瘤医院临床试验机构常务副主任。擅长乳腺癌、多原发和不明原发恶性肿瘤的治疗及药物临床试验。 目前担任欧洲肿瘤内科学会乳腺癌专业委员会委员、中国抗癌协会多原发和不明原发肿瘤专委会主任委员、上海抗癌协会乳腺癌专业委员会副主委、上海市疾病预防控制中心乳腺癌防治专业委员会副主委、中国抗癌协会乳腺癌专业委员会常委兼秘书长、国家食品药品监督管理局审评中心审评专家、中国抗癌协会癌症康复和姑息治疗委员会常委、中国抗癌协会临床肿瘤学协作专业委员会学术委员会委员、上海市肿瘤化疗质控中心专家委员会委员、中国研究型医院学会乳腺专业委员会副主委、上海抗癌协会癌症康复和姑息治疗委员会主任委员。任Int J Biol Markers、J Bone Oncol、中华乳腺病杂志、中国癌症杂志、临床肿瘤学杂志编委。在Lancet Oncol、Ann Oncol和J Clin Orthod等期刊发表论文200多篇,主编《肿瘤内科方案的药物不良反应及对策》和《肿瘤科常见诊疗问题问答——胡夕春医生查房实录》等专著。主持过“十三五”重大新药创制科技重大专项、国家自然科学基金面上项目和上海市科委项目等,获得过中国抗癌协会科技奖一等奖、全国妇幼健康科学技术奖一等奖和上海市医学科技进步奖一等奖等奖项
沈琳,主任医师、教授、博士生导师,现任北京大学肿瘤医院消化肿瘤内科主任、Ⅰ期临床试验病房主任。历任北京大学肿瘤医院副院长、北京市肿瘤防治研究所副所长。为国家重点研发计划慢病专项首席专家、全国优秀科技工作者、北京学者,享受国务院政府特殊津贴。担任中国抗癌协会肿瘤药物临床研究专业委员会首届主任委员、中国抗癌协会肿瘤精准治疗专业委员会主任委员、中国临床肿瘤学会临床研究专家委员会主任委员、中国临床肿瘤学会胃癌专家委员会候任主任委员等多个重要学术职务。 致力于消化系统肿瘤精准治疗与多学科综合治疗、抗肿瘤新药临床试验与转化研究。获国家科技进步二等奖、教育部科技进步一等奖、华夏医学科技奖一等奖及二等奖、中国抗癌协会科技奖一等奖、中华医学科技奖一等奖等多项奖项。牵头承担国家或省部级课题近10项;在Nature、Nat Med、Science、BMJ、Lancet Oncol、J Clin Oncol等国际权威期刊发表SCI论文200余篇,出版专著6部,获国家发明专利6项;培养学生70余人
Contributor Information
胡 夕春 (Xi-chun HU), Email: xchu2009@hotmail.com.
沈 琳 (Lin SHEN), Email: linshenpku@163.com.
References
- 1.International Agency for Research on Cancer. China (Globocan 2020)[EB/OL]. (2021-03)[2021-05-15] https://gco.iarc.fr/today/data/factsheets/populations/160-china-fact-sheets.pdf.
- 2.Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 3.Salehiniya H, Mohammadian M, Mohammadian-Hafshejani A, et al. Nasopharyngeal cancer in the world: Epidemiology, incidence, mortality and risk factors. World Cancer Res J. 2018;5(1):e1046. [Google Scholar]
- 4.Merck. Highlights of prescribing information. Keytruda® (Pembrolizumab)[EB/OL]. (2014)[2022-05-12]. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf.
- 5.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembro-lizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bristol Myers Squibb Company. Highlights of prescribing information. Opdivo (Nivolumab)[EB/OL]. (2014)[2022-05-03]. https://packageinserts.bms.com/pi/pi_opdivo.pdf.
- 7.Ma BBY, Lim WT, Goh BC, et al. Antitumor activity of ni-volumab in recurrent and metastatic nasopharyngeal carcinoma: An international, multicenter study of the Mayo clinic phase 2 consortium (NCI-9742) J Clin Oncol. 2018;36(14):1412–1418. doi: 10.1200/JCO.2017.77.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NCCN Clinical Practice Guidelines in Oncology. Non-small cell lung cancer. Version 3.2022[EB/OL]. (2022-03-16)[2022-05]. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 9.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstock C, Khozin S, Suzman D, et al. U.S. Food and Drug Administration approval summary: Atezolizumab for metastatic non-small cell lung cance. Clin Cancer Res. 2017;23(16):4534–4539. doi: 10.1158/1078-0432.CCR-17-0540. [DOI] [PubMed] [Google Scholar]
- 11.Genentech. FDA approves Genentech's Tecentriq plus chemothe-rapy (abraxane and carboplatin) for the initial treatment of metastatic non-squamous non-small cell lung cancer[N/OL]. (2019-12-03)[2021-05-03] https://www.gene.com/media/press-releases/14827/2019-12-03/fda-approves-genentechs-tecentriq-plus-c.
- 12.F. Hoffmann-La Roche Ltd. European Commission approves Roche's new Tecentriq-based combination therapy as an initial treatment for most common form of advanced lung cancer[N/OL]. (2019-09-06)[2021-05-11] https://www.roche.com/media/releases/med-cor-2019-09-06.htm.
- 13.F. Hoffmann-La Roche Ltd. FDA approves Roche's Tecentriq in combination with Avastin for people with the most common form of liver cancer[N/OL]. (2020-06-02)[2021-05-15] https://www.roche.com/media/releases/med-cor-2020-06-02.htm.
- 14.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 15.Xu ZY, Brown L, Pan GW, et al. Lifestyle, environmental pollution and lung cancer in cities of Liaoning in northeastern China. Lung Cancer. 1996;14(Suppl 1):S149–S160. doi: 10.1016/s0169-5002(96)90220-2. [DOI] [PubMed] [Google Scholar]
- 16.Grenade C, Phelps MA, Villalona-Calero MA. Race and ethnicity in cancer therapy: What have we learned? Clin Pharmacol Ther. 2014;95(4):403–412. doi: 10.1038/clpt.2014.5. [DOI] [PubMed] [Google Scholar]
- 17.Genentech. Highlights of prescribing information. Tecentriq®(atezolizumab)[EB/OL]. (2016)[2022-05-15]. https://www.gene.com/download/pdf/tecentriq_prescribing.pdf.
- 18.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 19.Lee MS, Ryoo BY, Hsu CH, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21(6):808–820. doi: 10.1016/S1470-2045(20)30156-X. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Xie J, Arai S, et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory can-cers: A meta-analysis. Oncotarget. 2016;7(45):73068–73079. doi: 10.18632/oncotarget.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le DT, Kavan P, Kim TW, et al. KEYNOTE-164: Pembrolizu-mab for patients with advanced microsatellite instability high (MSI-H) colorectal cancer. J Clin Oncol. 2018;36(Suppl 15):3514. doi: 10.1200/jco.2018.36.15_suppl.3514. [DOI] [Google Scholar]
- 22.Colevas AD, Bahleda R, Braiteh F, et al. Safety and clinical activity of atezolizumab in head and neck cancer: Results from a phase Ⅰ trial. Ann Oncol. 2018;29(11):2247–2253. doi: 10.1093/annonc/mdy411. [DOI] [PubMed] [Google Scholar]
- 23.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 24.International Agency for Research on Cancer. All cancers (Globocan 2020)[EB/OL]. (2020-12)[2022-05-12] https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf.