Abstract
The management of vertebral compression fractures (VCFs) is based on conservative treatment and minimally invasive vertebral augmentation procedures. However, the role of vertebral augmentation is now being questioned by clinical trials and extensive studies. The aim of this review is to report the most relevant evidences on effectiveness, safety, and indications of the currently available vertebral augmentation techniques. Conservative treatment with bracing is effective in reducing acute but it has no effect on segmental kyphosis progression and pseudoarthrosis can occur. Percutaneous vertebroplasty (PV) was the first vertebral augmentation technique to be proposed for the treatment of VCFs. Two blinded and randomized clinical trials compared PV to a sham procedure and no significant differences in terms of efficacy were reported. More recent studies have suggested that PV can still benefit patients with acute VCFs and severe pain at onset. Balloon kyphoplasty (BK) was developed to improve the segmental alignment restoring the height of collapsed vertebrae. BK allows similar pain relief and disability improvement, as well as greater kyphosis correction compared to PV, moreover BKP seems to reduce cement leakage. Vertebral body stenting (VBS) and the KIVA system are third generation techniques of vertebral augmentation. VBS aims to increase the effectiveness in restoring the segmental alignment, while the KIVA system can prevent cement leakage. These techniques are effective and safe, even if their superiority to BK has yet to be proven by studies with a high level of evidence.
Keywords: Vertebral compression fractures, Osteoporosis, Vertebral augmentation, Vertebroplasty, Kyphoplasty, Vertebral body stenting, KIVA, Review
BACKGROUND
The incidence of vertebral compression fractures (VCFs) is constantly increasing due to a higher prevalence of osteoporosis resulting from the aging of the population. Less frequent causes of vertebral fragility fractures include hemangiomas, multiple myeloma, and metastasis. The annual incidence of VCFs in the United States is of 750000 and data from large populations demonstrate that mortality rates in patients with VCF is 2.5 times higher compared to patients without VCFs.1,2 In Europe it ranges from 5.7/1000 inhabitants for males to 10.7/1000 inhabitants for females with a M:F ratio of about 1:2.3 According to the World Health Organization, the peak prevalence is typically associated with post-menopausal women who present a lifetime risk of osteoporotic fractures that exceeds 40%, which is very close to that of coronary heart disease. The same report identified the most frequent clinical risk factors for VCFs, which include low body mass index (BMI), previous fragility fractures, oral glucocorticoid treatments, current smoking, and alcoholism.4 Secondary causes of osteoporosis include rheumatoid arthritis, hypogonadism, inflammatory bowel diseases, prolonged immobility, organ transplantation, diabetes, thyroid disorders, and chronic obstructive pulmonary disease. Pregnancy-associated osteoporosis is a very specific secondary form whose pathogenesis is still unclear.5 Some authors have tried to identify clinical factors predictive for fragility fractures. In a previous study we analyzed a population of post-menopausal patients with osteoporotic vertebral fractures using the clinical history and diagnostic exams results and we came up with a simple score predictive for fractures. Thus clinical risk factors for VCF include advanced age, low lumbar L1-L4 and femoral neck T-scores, a diminished L4 body volume and smoking abitude.6
Acute back pain is the most common clinical presentation of VCFs. Nevertheless, only one third of cases come to medical attention, suggesting that most are either asymptomatic or have tolerable symptoms.7 It is indeed recognized that only 30% of vertebral fractures are clinically apparent.8 Osteoporotic fractures are burdened by a high rate of complications which prompt a great decrease in the patient’s quality of life. Untreated fractures can lead to severe deformities which cause chronic pain and limit the patient in his everyday life activities. In a large analysis on the Medicare population in the United States, the mortality rate for patients with VCFs was twice that of matched controls.9 Moreover, VCFs have alarming economic implications, representing a heavy burden for public healthcare expenditure. Orsini et al. reported an overall expenditure for osteoporotic fractures of barely less than $16000 per patient in the US, which means an annual national burden exceeding $6 billions.10 Joestl et al. confirmed these results in Europe reporting treatment costs ranging from €3200 to €11500 per patient and an average of 32 working days lost per patient.11 Furthermore, the indirect costs of vertebral fractures, due to productivity loss and decreased everyday activities, have never been thoroughly investigated. The International Osteoporosis Foundation estimates that 20% of all costs are direct ones, amounting to around 4.5 to 6.4 millions of dollars in the US alone, and that lumbar fractures account for the highest rate of morbidity. For these reasons, many treatment strategies have been developed over the last decades. Before the introduction of minimally invasive techniques, surgery had a limited role because of the old age and comorbidities of the patients. Therefore, conservative treatment was often the only option. Open reduction and internal fixation were indicated when neurological impairment arose.12 To avoid the complications related to open surgery, several minimally invasive vertebral augmentation procedures have been developed: percutaneous vertebroplasty (PV), balloon kyphoplasty (BK), and third generation techniques including vertebral body stenting (VBS); the KIVA system; Spine jack and Osseofix. In all of these procedures, vertebral augmentation is achieved by injecting polymethylmethacrylate (PMMA) cement into the collapsed vertebral body using a transpedicular access, with the purpose of reducing pain and of restoring vertebral height. The first evidences on effectiveness and safety led to a relentless spread of vertebral augmentation procedures. The initial enthusiasm was followed by more extensive studies that questioned the actual efficacy and cost-effectiveness of these procedures, leading to a great debate in literature.
The aims of this review were to describe the available minimally invasive vertebral augmentation techniques, to compare their evidence-based efficacy and safety, and to define their proper indications using data from our previous studies as well. When available, cost-analysis of the procedures have been reported.
WHAT IS THE EVIDENCE FOR CONSERVATIVE TREATMENT?
Most osteoporotic vertebral fractures are treated conservatively with bed rest, pain control with systemic analgesics (i.e. paracetamol, NSAIDs, and/or opioids), bracing, and early rehabilitation. Back brace use duration strictly depends on the time it takes for the fracture to heal, which in turn depends on the patient’s age and bone quality. Between 3 to 6 months of bracing are commonly needed.13 Patients treated conservatively need a strict radiographic follow-up to monitor the segmental stability and the healing process.
Conservative treatment is effective in reducing acute pain although more slowly than surgical treatments do.14,15 This treatment usually leads to good results and most of vertebral fractures heal with excellent functional recovery. However, this treatment has no effect on segmental kyphosis progression and, rarely, pseudoarthrosis can occur.6
Complications related to conservative treatment are uncommon and predictable. Bracing and bed rest are risk factors for pneumonia, urinary infections, bedsores, and deep vein thrombosis.16 Prolonged immobilization can lead to further bone demineralization.17 However, if anti-osteoporotic therapy is correctly administered, conservative treatment has an equal or lower rate of new VCFs as compared to surgical treatments.16,17 After hospitalization, 50% of patients require ongoing care and chronic pain occurs in 40%.18,19
Patients’ compliance to bracing and bed rest may determine the success or failure of the treatment. Although it is difficult to define the exact number of patients who arbitrarily suspend the treatment, the cohorts of patients treated conservatively show higher rates of follow-up discontinuation compared to the surgical ones.18
Muratore et al. conducted a systematic review on predictive factors for conservative treatment failure. They reported that the shape of the fracture, the involvement of the thoracolumbar junction, damage to the middle column or posterior wall, a non-homogeneous high MRI signal, and the presence of intravertebral vacuum clefts are associated with higher risks of pseudoarthrosis, progressive kyphotic deformity and chronic back pain.20 Non-conservative treatments should be taken in consideration in the presence of one or more of these factors.
WHAT IS THE EVIDENCE FOR PERCUTANEOUS VERTEBROPLASTY?
PV was firstly described by Galibert in 1987 for the treatment of vertebral angioma and it was the first minimally invasive vertebral augmentation procedure proposed for VCFs.21 This technique consists of a percutaneous injection of PMMA cement into the collapsed vertebral body using a mono- or bilateral transpedicular access. Although originally conducted under general anesthesia, PV is now carried out using conscious sedation by infiltrating the periosteum of the pedicles, the subcutaneous tissues, and the skin with local anesthetics (e.g. 1% lidocaine and 0.25% bupivacaine) before carrying out the percutaneous access. Under constant fluoroscopic guidance, 11-gauge or 13-gauge needles are placed into the collapsed vertebral body through its pedicles. Using a hydraulic injection system, barium-opacified PMMA is forced into the cancellous bone, filling its porous structure (Figure 1). At a given hydraulic pressure, the injected volume strictly depends on cement viscosity and bone compactness at the fracture site. For this reason, PV is primarily aimed at solidifying the fractured vertebral body to prevent further collapse rather than to restore vertebral height.22
Figure 1. Intraoperative fluoroscopic radiographs (LL view) during a single-level and monolateral percutaneous vertebroplasty (PV).
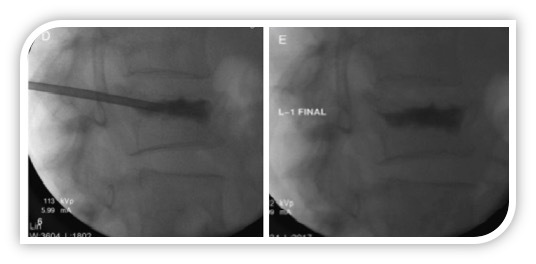
The procedure consists of percutaneous injection of PMMA cement into the collapsed vertebral body under conscious sedation and local anesthesia. Under fluoroscopy-guidance, the cannula is placed inside the vertebral body through a transpedicular access, then the cement is slowly injected with a hydraulic system (left). The correct positioning of cement below the collapsed endplate is verified before completing the procedure (right).
According to a recent study by the Nationwide Inpatients Sample Database, the number of vertebroplasty procedures performed in the USA has decreased by 53% from 2008 to 2014 as a result of the current debate regarding its effectiveness.23 In 2009, two multicenter, blinded, and randomized clinical trials compared the effectiveness of vertebroplasty versus a sham procedure acting as placebo control group. Kallmes et al. evaluated the modified Roland-Morris Disability Questionnaire (RDQ) and Visual Analogic Scale (VAS) scores at 1 month, whilst Buchbinder et al. reported only the VAS scores but at 1 week and 1, 3, and 6 months after surgery. Both studies showed no significant differences between vertebroplasty and the sham procedure in terms of pain relief and disability reduction.24,25 These results have been greeted with disbelief among physicians and concerns have been raised regarding the design of both studies: small sample size, inclusion of patients with subacute and chronic fractures instead of acute ones, crossover between the groups, and especially the possibility that the sham procedure could itself have promoted pain relief by decompressing the fracture hematoma.
More recent randomized clinical trials support the effectiveness of vertebroplasty. The VERTOS II study compared vertebroplasty to conservative treatment, whilst the VAPOUR study involved a sham procedure control group. These trials involved only patients with acute fractures (<6 weeks) and severe pain at onset, and both reported the superiority of vertebroplasty in this more specific group of patients.14,26 Many reviews and meta-analyses with controversial results have followed. Studies with higher level of evidence seem to question the clinical utility of vertebroplasty.27 However, Widmer Soyka et al. have suggested that the effectiveness of PV is determined by patient’s bone condition, and by cement volume, injection technique and location.28 Under this point of view, PV can still be indicated in acute conditions for patients with severe pain and clinical or radiological risk factors for conservative treatment failure.
As for the safety of PV, the rate of major complications is <1% with a mortality rate close to zero.29 These results have been confirmed on a large population of elderly patients.30 Symptomatic cement leakage outside the vertebra is very rare, although the rate of asymptomatic leakage can be higher than 70% when assessed with CT.14 Higher rates of leakage are related to cortical disruption or fracture clefts, the use of low-viscosity cement, and larger volumes of injection. Moreover, PV shows a higher rate of cement leakage as compared to BK.31 PV does not increase the baseline risk of new VCFs. Overall, 1 in 5 patients develop a new fracture within 12 months and the risk is higher in patients with multiple fractures and a lower bone mineral density.32 Two meta-analyses of published prospective trials found no difference in subsequent VCF risk between medical management and vertebroplasty.33,34 In 1998, a report conducted by the EU Commission estimated an average length of hospital stay exceeding 20 days and an increasing trend in the number of beds required.4 A large analysis of French Hospital National Database demonstrated that the introduction of PV reduced both the hospital stay and re-admission rate.35
WHAT IS THE EVIDENCE FOR KYPHOPLASTY?
BK was developed from PV with the aim of improving the vertebral body height restoration by adding the step of cavity creation with the inflation of a balloon. Is case control study by our group, we have demonstrated how kyphoplasty as compared to conservative therapy is able to improve segmental kyphosis, the quality of life and to reduce pain at one month from surgery, although there is no statistical difference at 12 months between the two forms of treatment. Our data confirms the superiority of kyphoplasty over conservative therapy in reducing pain.6 In kyphoplasty a hydraulic balloon tamp is inflated inside the collapsed vertebral body to create a cavity before injecting PMMA (Figure 2). Moreover, the inflation of the balloon into the cancellous bone creates compact bone at the periphery of the cavity, which allows cement injection at lower pressures and higher viscosity, which reduces the rate of leaks.31 Furthermore, as compared to PV whose goal is to reduce pain and to stabilize the fracture, kyphoplasty, as a result of the hydraulic pressure of the balloon tamp, is even able to partially reestablish vertebral height, as reported by biomechanical studies.22
Figure 2. Intraoperative fluoroscopic radiographs (LL view) during a multi-level and bilateral balloon kyphoplasty (BK).
As with the PV, the cannula is placed inside the vertebral body through a transpedicular percutaneous access. Barium-opacified balloon tamps are inflated with a hydraulic system creating a cavity in the cancellous bone (left). The balloons are then deflated and removed. PMMA is injected inside the cavity below the collapsed vertebral endplates (right).
While many studies have been published comparing BK with conservative therapy, there are few studies with high levels of evidence comparing BK with PV. The largest clinical trial was the Kyphoplasty and Vertebroplasty in the Augmentation and Restoration of Vertebral Body Compression Fractures (KAVIAR) study. Although this trial was terminated early due to limited enrollment (33% of the enrollment target), it showed that the mean procedural duration was longer with kyphoplasty, with no difference in clinical outcome or symptomatic complications.36 Similar results have been reported in 2 smaller prospective trials.37,38 A recent large systematic review and meta-analysis compared PV and BK including 2838 patients across 29 studies. There were no differences in back pain or disability pain scores at any time point between the two procedures. There was no difference in the rate of symptomatic cement leakage, but BK was associated with a lower rate of overall cement leakage and greater kyphosis correction (p< 0.01).39 For these reasons, BK is indicated mostly in patients with recent VCFs and with a high risk of segmental kyphosis. Under this point of view, the cost-effectiveness of BK has been confirmed by a nationwide cost analysis conducted in UK.40
Radiofrequency-Targeted Vertebral Augmentation of Radiofrequency Kyphoplasty (RFK) is a novel technique developed from BK. The bone cavity is created using an osteotome instead of the hydraulic balloon. Highly viscous cement is activated using radiofrequencies and then injected in the channels made with the osteotome. The use of highly viscous cement allows the surgeon to fill the cancellous bone in a more targeted way and reduces the risk of cement leakage.41 However, there is still not enough evidence of its clinical advantages as compared to BK.
WHAT IS THE EVIDENCE FOR THIRD GENERATION VERTEBRAL AUGMENTATION TECHNIQUES?
During the last decade, a third generation of vertebral augmentation techniques was developed to deal with the issues that emerged with PV and BK. The focus shifted to restoring the individuals’ segmental and general spinal alignment to prevent chronic pain and deformities. As more and more extensive clinical studies were published, the effectiveness of BK in restoring the height of collapsed vertebrae was poorer than expected. The kyphoplasty implantation technique creates differences between the kyphosis correction achieved during maximum balloon inflation and the final angle achieved after cement application. This technique-related loss of reduction is known as the deflation effect. Vertebral body stenting (VBS) was introduced to maintain height restoration after the deflation of the balloon. In this technique a catheter-mounted metallic stent is expanded around the same inflatable balloon as the one used for kyphoplasty. The expanded stent remains within the cavity when the balloon is deflated preventing the vertebral body from collapsing again. A first biomechanical study showed better results achieved with VBS regarding both deflation effect and final reduction.42 However, these results were only partially confirmed by a second biomechanical study which demonstrated that VBS and BK achieved a comparable initial reduction of segmental kyphosis (5.9° vs. 6.0°) but the deflation effect was slightly lower with VBS (-1.6° vs. -4.4°). Moreover, the same study showed that high flexion moments during implantation also play a significant role and may affect these results.43 The randomized clinical trial conducted by Werner et al. showed no significant differences between BK and VBS in terms of kyphotic correction, cement leakage, radiation exposure time, or neurologic sequelae. However, they reported a significantly higher rate of material-related complications (i.e. failure of the working cannulas, incomplete or no opening of the stent, and balloon rupture) with VBS, although without clinical consequences.44 A systematic review confirmed the comparable effectiveness and safety between VBS and BK.45 In terms of pain relief and quality of life improvement, the evidence sustaining VBS is still scarce.
As for the cost-effectiveness analysis of VBS, more numerous and larger studies are needed. Although, Werner et al. reported comparable material costs for single-level procedures between VBS and BK but higher expenditures for multiple-level procedures with VBS.44
The Osseofix is an expandible titanium mesh that can compact the surrounding trabecular bone which is indicated in the treatment of VCFs at the level of the T6-L5 tract. It acts as a scaffold to induce the stabilization of the vertebral fracture and to favor the interdigitation of the cement inside the cancellous bone. Upasani et al have reported a biomechanical comparison of kyphoplasty using this technique demonstrating how it can maintain vertebral height for a longer period of time using a smaller volume of cement.46
The Jack dilators create a parasagittal vertebral cleft that extends along the entire vertebral soma height. They are inserted cranio-caudally under radioscopic guidance to guarantee the reduction of the fracture before fixating it with cement. Sietsma et al have compared the in vitro biomechanical effectiveness of this technique with kyphoplasty and the results were similar to the ones seen regarding vertebral height retrieval, maintenance and resistance to charges.47 However, using spinejack a smaller amount of cement was enough to achieve the same fixation.
The KIVA system (Benvenue Medical Inc., Santa Clara, CA, USA) is a novel vertebral augmentation procedure proposed a few years ago. Through the usual percutaneous transpedicular approach, a coil made of nitinol is placed inside the vertebral body, where, thanks to its shape-memory, the nitinol coil reconfigures itself into a stack of uniform diameter loops. Using the nitinol coil as a guide, the KIVA implant, made of barium-opacified PEEK-OPTIMA, is placed inside the vertebral body and forms a stack of up to 4 loops. The coil is retracted and PMMA is injected inside the implant which delivers the cement at the center of the loops preventing its leakage. The KAST trial demonstrated that the KIVA system is noninferior to BK in terms of pain relief and safety.48 The cost-effectiveness of this technique is still unclear. However, an economic analysis, using the same KAST trial data, showed that the KIVA system may allow a cost saving attributable to a reduced risk of adjacent-level fractures and thus a reduced number of revision procedures.49
DISCUSSION
In the US, vertebral compression fractures (VCFs) are nearly half of the approximatively 1.5 million osteoporotic fractures that arise yearly.50 In 2017 VCFs were the most common type of osteoporotic fracture, comprising approximately 1.4 million cases worldwide.51 These types of fractures are frequent in the elderly because of their reduced mineral bone density and they can lead to severe pain, progressive spinal deformity, decreased mobility, an increased risk of secondary fractures and age-adjusted mortality, and to a generally decreased quality of life. Medical therapy may temporarily reduce pain, but it is often not enough. In the last 30 years, vertebral augmentation techniques have thus been developed to treat symptomatic patients. These procedures stabilize the fractured segment and restore vertebral height, thereby alleviating pain and disability. On the downside, they comprise many risks including cement leakage, pulmonary embolisms and fractures of the surrounding vertebras. Between 2005 and 2010, as many as 300’000 augmentation surgeries were performed in the US, 73% of which were kyphoplasties and 27% vertebroplasties, however there is still no data concerning third generation implants.52
Some studies sustain that patients with VCF have an 8 year life expectancy which is 40% lower than the one of their healthy counterparts.53,54 This increased mortality risk is associated with a general condition of frailty because of the decreased physical function and the loss of weight or of muscle mass. More recent studies confirm that augmentation surgery leads to better results in terms of pain control, improved quality of life and reduction of acute or subacute (less than 3 months) fractures.9 Vertebral compression fractures can lead to a progressive kyphotic deformity. Aside from pain and reduced mobility, the kyphosis causes a decrease lung vital capacity because of a reduction of thoracic and abdominal volumes. The clinical consequences of these mechanical effects include: a decrease pulmonary function, a decreased appetite with a relative nutritional impact, fragility, an increased risk of new adjacent vertebral fractures and of chronic pain.54–61 Many studies sustain the potential advantages of kyphoplasty over vertebroplasty in terms of vertebral body height recovery, if the intervention is done within four weeks, the sagittal profile recovery is optimal. If the surgery is performed later, there is a loss in correction of around 1° every three days and after one month it is not possible to achieve any grade of correction of the kyphosis .62,63 The reestablishment of the kyphotic angle can ameliorate the pulmonary function and could be one of the reasons for a higher survival rate in these patients.
Furthermore, kyphoplasty is better at ameliorating the sagittal profile if performed with 2-3 weeks when compared to vertebroplasty, and leads to a greater decrease in mortality rates, although at triple the costs.64 From a technical point of view, kyphoplasty has a low risk of leakage of about 10%, whereas vertebroplast has a risk of 10 to 73%; and a fracture risk of 7 to 26% with a risk of 12-52% for vertebroplasty. In the last few years, various new spine implants with the objective of combining the analgesic effect and the stabilization given from the cement injection into the soma with the retrieval of the vertebral height and the correction of the kyphotic angle have been introduced. These implants include stents, jack dilators, PEEK cage and other fracture reduction systems.
Figure 3. Intraoperative fluoroscopic radiographs (LL view) during a single-level and monolateral percutaneous Vertebral body Stenting with the Spine Jack dilators (Vexim).
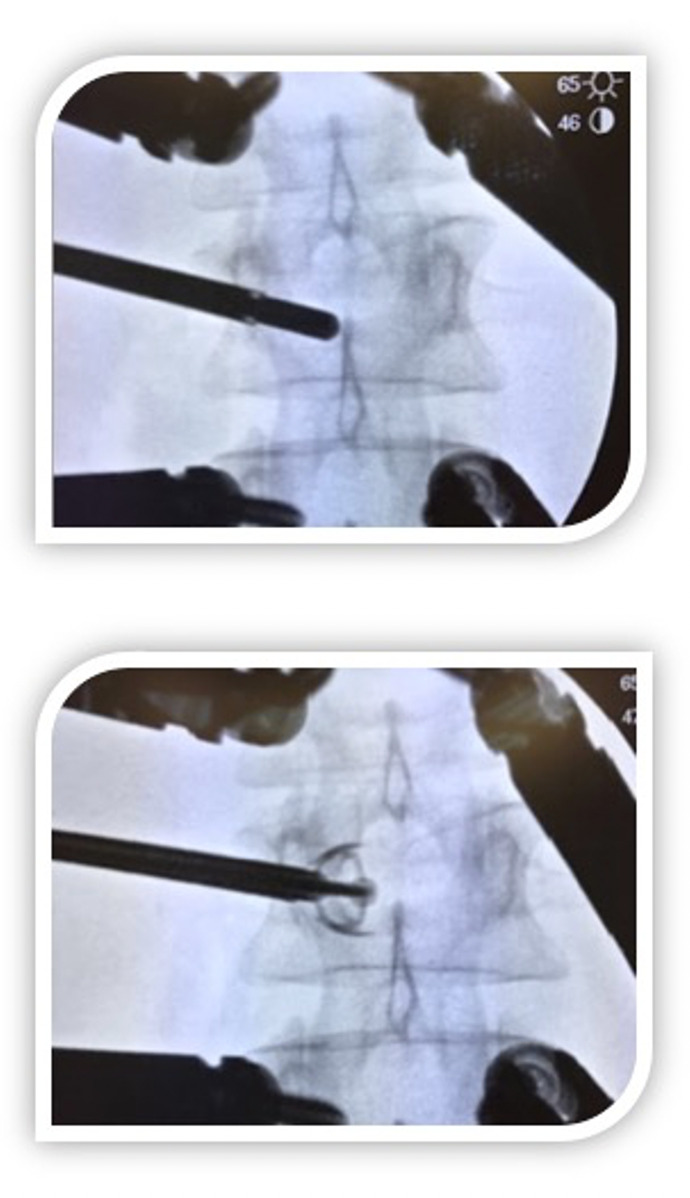
As with the PV and BK, the cannula is placed inside the vertebral body through a transpedicular percutaneous access (3a). A guide wire is inserted and a reamer cannula is introduced and drilled into the vertebral body. The implant’s site is then cleaned with a template and a cannula plug is inserted aiming to stop any potential bleeding; an implant expander is then inserted and cement is injected via cement pusher placed inside the expander (3b).
CONCLUSIONS
Most VCFs are still treated conservatively. Patients’ compliance to bracing is key for a successful conservative treatment. For this reason, patients treated conservatively should be mobilized as early as possible, monitored more strictly, and they must be informed earlier about bracing duration and available alternatives. An adequate treatment plan is needed for CT as well, particularly regarding the radiographical factors associated with a higher risk of failure. Adequate anti-osteoporotic pharmacological therapy is the cornerstone of prevention and should be always administered alongside orthopedic treatment.
Vertebral augmentation procedures still play a significant role in the treatment of VCFs, although evidence of this has evolved with time. The annual number of PV and BK procedures performed has decreased by 53% and 20% over the last 10 years, probably because of the concerns regarding their actual effectiveness raised by some clinical trials.23 In fact, early studies were probably susceptible to bias and placebo effect. More recent studies suggest that vertebral augmentation should no longer be considered an universal strategy for VCFs but an adequate treatment for acute fractures with moderate-severe pain at onset. For these patients, BK shows biomechanical and clinical advantages over PV especially when there is a high risk of kyphosis. Clinical trials with sham procedure should be encouraged to confirm the clinical role of BK. Third generation techniques were developed in response to the concerns regarding PV and BK. VBS aims to increase the effectiveness in restoring the segmental alignment, whilst the KIVA system can prevent cement leakage, although their superiority has not yet been demonstrated by studies with a high level of evidence.
All techniques are generally safe and have very low rates of major complications. The main concern regards their effect on the risk of subsequent fractures, which is still debated. However, in a setting of optimal pharmacological prevention, this risk seems lower with the latest techniques. Cost-effectiveness of vertebral augmentation is confirmed, and it is attributable to the shorter length of stay.
Spine implants are able to guarantee vertebral height restoration and to improve the kyphotic angle. In case of VCFs with a severe vertebral height reduction, the use of these implants is able to correct the kyphotic angle and to maintain it in the long term. Clinical and biomechanical comparative studies have investigated first and second generation augmentation techniques and compared them with third generation ones which ultimately have a similar efficacy with some differences like the quantity of cement to be injected. However, in the literature there are no evidences clearly demonstrating the superiority of one implant above the others.
AUTHORS’ CONTRIBUTION
The authors contributed equally
CONFLICT OF INTEREST STATEMENT
All authors disclose any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations and grants or other funding.
HUMAN AND ANIMAL RIGHT
For this type of study is not required any statement relating to studies on humans and animals.
Acknowledgments
ACKNOWLEDGEMENTS
None
Funding Statement
None
References
- 1. Toy JO, Basques BA, Grauer JN. Morbidity, mortality, and readmission after vertebral augmentation: analysis of 850 patients from the American College of Surgeons National Surgical Quality Improvement Program database. Spine. 2014;39(23):1943-1949. doi:10.1097/brs.0000000000000563 [DOI] [PubMed]
- 2. Melton LJ III, Thamer M, Ray NF, et al. Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12(1):16-23. doi:10.1359/jbmr.1997.12.1.16 [DOI] [PubMed]
- 3. Felsenberg D, Silman A, Lunt M, Armbrecht G, Ismail AA, Finn JD. Incidence of vertebral fracture in europe: results from the European Prospective Osteoporosis Study (EPOS). J Bone Miner Res. 2002;17(4):716-724. doi:10.1359/jbmr.2002.17.4.716 [DOI] [PubMed]
- 4. Kanis JA, McCloskey EV, Johansson H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2012;24(1):23-57. doi:10.1007/s00198-012-2074-y [DOI] [PMC free article] [PubMed]
- 5. Pola E, Colangelo D, Nasto LA, Pambianco V, Autore G, Formica VM, et al. Pregnancy-associated osteoporosis (PAO) with multiple vertebral fragility fractures: diagnosis and treatment in a young primigravid woman. J Biol Regul Homeost Agents. 2016;30:153-158. [PubMed]
- 6. Colangelo D, Nasto LA, Genitiempo M, Formica VM, Autore G, Pambianco V, et al. Kyphoplasty vs conservative treatment: a case-control study in 110 post-menopausal women population. Is kyphoplasty better than conservative treatment? Eur Rev Med Pharmacol Sci. 2015;19:3998-4003. [PubMed]
- 7. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726-1733. doi:10.1007/s00198-006-0172-4 [DOI] [PubMed]
- 8. National Osteoporosis Foundation. America’s Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation. National Osteoporosis Foundation; 2002.
- 9. Lau E, Ong K, Kurtz S, Schmier J, Edidin A. Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. J Bone Joint Surg Am. 2008;90(7):1479-1486. doi:10.2106/jbjs.g.00675 [DOI] [PubMed]
- 10. Orsini LS, Rousculp MD, Long SR, Wang S. Health care utilization and expenditures in the United States: a study of osteoporosis-related fractures. Osteoporos Int. 2005;16(4):359-371. doi:10.1007/s00198-004-1694-2 [DOI] [PubMed]
- 11. Joestl J, Lang N, Bukaty A, Tiefenboeck TM, Platzer P. Osteoporosis associated vertebral fractures-Health economic implications. PLoS ONE. 2017;12(5):e0178209. doi:10.1371/journal.pone.0178209 [DOI] [PMC free article] [PubMed]
- 12. Mikles MR, Stchur RP, Graziano GP. Posterior instrumentation for thoracolumbar fractures. J Am Acad Orthop Surg. 2004;12(6):424-435. doi:10.5435/00124635-200411000-00007 [DOI] [PubMed]
- 13. Longo UG, Loppini M, Denaro L, Maffulli N, Denaro V. Osteoporotic vertebral fractures: current concepts of conservative care. Br Med Bull. 2011;102(1):171-189. doi:10.1093/bmb/ldr048 [DOI] [PubMed]
- 14. Klazen CA, Lohle PN, de Vries J, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet. 2010;376(9746):1085-1092. doi:10.1016/s0140-6736(10)60954-3 [DOI] [PubMed]
- 15. Voormolen MH, Mali WP, Lohle PN, Fransen H, Lampmann LE, van der Graaf Y, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study, AJNR Am J Neuroradiol. 2007;28:555-560. [PMC free article] [PubMed]
- 16. Suzuki N, Ogikubo O, Hansson T. The course of the acute vertebral body fragility fracture: its effect on pain, disability and quality of life during 12 months. Eur Spine J. 2008;17(10):1380-1390. doi:10.1007/s00586-008-0753-3 [DOI] [PMC free article] [PubMed]
- 17. Riek AE, Towler DA. The pharmacological management of osteoporosis. Mo Med. 2011;108:118-123. [PMC free article] [PubMed]
- 18. Chen LX, Li YL, Ning GZ, et al. Comparative efficacy and tolerability of three treatments in old people with osteoporotic vertebral compression fracture: a network meta-analysis and systematic review. PLoS ONE. 2015;10(4):e0123153. doi:10.1371/journal.pone.0123153 [DOI] [PMC free article] [PubMed]
- 19. Zhang H, Xu C, Zhang T, Gao Z, Zhang T. Does Percutaneous Vertebroplasty or Balloon Kyphoplasty for Osteoporotic Vertebral Compression Fractures Increase the Incidence of New Vertebral Fractures? A Meta-Analysis. Pain Phys. 2017;1(21;1):E13-E28. doi:10.36076/ppj.2017.1.e13 [PubMed]
- 20. Muratore M, Ferrera A, Masse A, Bistolfi A. Osteoporotic vertebral fractures: predictive factors for conservative treatment failure. A systematic review. Eur Spine J. 2008;27(10):2565-2576. doi:10.1007/s00586-017-5340-z [DOI] [PubMed]
- 21. Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33:166-168. [PubMed]
- 22. Hiwatashi A, Sidhu R, Lee RK, deGuzman RR, Piekut DT, Westesson PLA. Kyphoplasty versus vertebroplasty to increase vertebral body height: a cadaveric study. Radiology. 2005;237(3):1115-1119. doi:10.1148/radiol.2373041654 [DOI] [PubMed]
- 23. Laratta JL, Shillingford JN, Lombardi JM, et al. Utilization of vertebroplasty and kyphoplasty procedures throughout the United States over a recent decade: an analysis of the Nationwide Inpatient Sample. J Spine Surg. 2017;3(3):364-370. doi:10.21037/jss.2017.08.02 [DOI] [PMC free article] [PubMed]
- 24. Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569-579. doi:10.1056/nejmoa0900563 [DOI] [PMC free article] [PubMed]
- 25. Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557-568. doi:10.1056/nejmoa0900429 [DOI] [PubMed]
- 26. Clark W, Bird P, Gonski P, et al. Safety and Efficacy of Vertebroplasty for Acute Painful Osteoporotic Fractures (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10052):1408-1416. doi:10.1016/s0140-6736(16)31341-1 [DOI] [PubMed]
- 27. Buchbinder R, Johnston RV, Rischin KJ, et al. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database Syst Rev. 2018;4:CD006349. doi:10.1002/14651858.cd006349.pub3 [DOI] [PMC free article] [PubMed]
- 28. Buchbinder R, Johnston RV, Rischin KJ, Homik J, Jones CA, Golmohammadi K, et al. The Effectiveness of Percutaneous Vertebroplasty Is Determined by the Patient-Specific Bone Condition and the Treatment Strategy. PLoS ONE. 2016;11(4):e0151680. doi:10.1371/journal.pone.0151680 [DOI] [PMC free article] [PubMed]
- 29. Chandra RV, Maingard J, Asadi H, et al. Vertebroplasty and Kyphoplasty for Osteoporotic Vertebral Fractures: What Are the Latest Data? AJNR Am J Neuroradiol. 2018;39(5):798-806. doi:10.3174/ajnr.a5458 [DOI] [PMC free article] [PubMed]
- 30. Lin JH, Chien LN, Tsai WL, Chen LY, Chiang YH, Hsieh YC. Early vertebroplasty associated with a lower risk of mortality and respiratory failure in aged patients with painful vertebral compression fractures: a population-based cohort study in Taiwan. Spine J. 2017;17(9):1310-1318. doi:10.1016/j.spinee.2017.05.001 [DOI] [PubMed]
- 31. Zhan Y, Jiang J, Liao H, Tan H, Yang K. Risk Factors for Cement Leakage After Vertebroplasty or Kyphoplasty: A Meta-Analysis of Published Evidence. World Neurosurg. 2017;101:633-642. doi:10.1016/j.wneu.2017.01.124 [DOI] [PubMed]
- 32. Cao J, Kong L, Meng F, Zhang Y, Shen Y. Risk factors for new vertebral compression fractures after vertebroplasty: a meta-analysis. ANZ J Sur. 2016;86(7-8):549-554. doi:10.1111/ans.13428 [DOI] [PubMed]
- 33. Anderson PA, Froyshteter AB, Tontz WLJ. Meta-analysis of vertebral augmentation compared with conservative treatment for osteoporotic spinal fractures. J Bone Miner Res. 2013;28(2):372-382. doi:10.1002/jbmr.1762 [DOI] [PubMed]
- 34. Shi MM, Cai XZ, Lin T, Wang W, Yan SG. Is there really no benefit of vertebroplasty for osteoporotic vertebral fractures? A meta-analysis. Clin Orthop Relat Res. 2012;470(10):2785-2799. doi:10.1007/s11999-012-2404-6 [DOI] [PMC free article] [PubMed]
- 35. Maravic M, Taupin P, Roux C. Hospital burden of vertebral fractures in France: influence of vertebroplasty. Osteoporos Int. 2013;24(7):2001-2006. doi:10.1007/s00198-012-2264-7 [DOI] [PubMed]
- 36. Dohm M, Black CM, Dacre A, Tillman JB, Fueredi G. KAVIAR investigators, A randomized trial comparing balloon kyphoplasty and vertebroplasty for vertebral compression fractures due to osteoporosis. AJNR Am J Neuroradiol. 2014;35(12):2227-2236. doi:10.3174/ajnr.a4127 [DOI] [PMC free article] [PubMed]
- 37. Liu JT, Liao WJ, Tan WC, et al. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporos Int. 2009;21(2):359-364. doi:10.1007/s00198-009-0952-8 [DOI] [PubMed]
- 38. Evans AJ, Kip KE, Brinjikji W, et al. Randomized controlled trial of vertebroplasty versus kyphoplasty in the treatment of vertebral compression fractures. J Neurointerv Surg. 2016;8(7):756-763. doi:10.1136/neurintsurg-2015-011811 [DOI] [PubMed]
- 39. Gu CN, Brinjikji W, Evans AJ, Murad MH, Kallmes DF. Outcomes of vertebroplasty compared with kyphoplasty: a systematic review and meta-analysis. J Neurointerv Surg. 2015;8(6):636-642. doi:10.1136/neurintsurg-2015-011714 [DOI] [PubMed]
- 40. Ström O, Leonard C, Marsh D, Cooper C. Cost-effectiveness of balloon kyphoplasty in patients with symptomatic vertebral compression fractures in a UK setting. Osteoporos Int. 2010;21(9):1599-1608. doi:10.1007/s00198-009-1096-6 [DOI] [PubMed]
- 41. Feng L, Shen JM, Feng C, Chen J, Wu Y. Comparison of radiofrequency kyphoplasty (RFK) and balloon kyphoplasty (BKP) in the treatment of vertebral compression fractures: A meta-analysis. Medicine. 2017;96(25):e7150. doi:10.1097/md.0000000000007150 [DOI] [PMC free article] [PubMed]
- 42. Rotter R, Martin H, Fuerderer S, et al. Vertebral body stenting: a new method for vertebral augmentation versus kyphoplasty. Eur Spine J. 2010;19(6):916-923. doi:10.1007/s00586-010-1341-x [DOI] [PMC free article] [PubMed]
- 43. Disch AC, Schmoelz W. Cement augmentation in a thoracolumbar fracture model: reduction and stability after balloon kyphoplasty versus vertebral body stenting. Spine. 2014;39(19):E1147-E1153. doi:10.1097/brs.0000000000000470 [DOI] [PubMed]
- 44. Werner CML, Osterhoff G, Schlickeiser J, et al. Vertebral body stenting versus kyphoplasty for the treatment of osteoporotic vertebral compression fractures: a randomized trial. J Bone Joint Surg Am. 2013;95(7):577-584. doi:10.2106/jbjs.l.00024 [DOI] [PubMed]
- 45. Martín-López JE, Pavón-Gómez MJ, Romero-Tabares A, Molina-López T. Stentoplasty effectiveness and safety for the treatment of osteoporotic vertebral fractures: a systematic review. Orthop Traumatol Surg Res. 2015;101(5):627-632. doi:10.1016/j.otsr.2015.06.002 [DOI] [PubMed]
- 46. Upasani VV, Robertson C, Lee D, Tomlinson T, Mahar AT. Biomechanical comparison of kyphoplasty versus a titanium mesh implant with cement for stabilization of vertebral compression fractures. Spine. 2010;35(19):1783-1788. doi:10.1097/brs.0b013e3181b7cc5d [DOI] [PubMed]
- 47. Sietsma MS, Hosman AJF, Verdonschot NJJ, Aalsma AMM, Veldhuizen AG. Biomechanical evaluation of the vertebral jack tool and the inflatable bone tamp for reduction of osteoporotic spine fractures. Spine. 2009;34(18):E640-E644. doi:10.1097/brs.0b013e3181b1fed8 [DOI] [PubMed]
- 48. Tutton SM, Pflugmacher R, Davidian M, Beall DP, Facchini FR, Garfin SR. KAST Study: The Kiva System As a Vertebral Augmentation Treatment-A Safety and Effectiveness Trial: A Randomized, Noninferiority Trial Comparing the Kiva System With Balloon Kyphoplasty in Treatment of Osteoporotic Vertebral Compression Fractures. Spine. 2015;40(12):865-875. doi:10.1097/brs.0000000000000906 [DOI] [PubMed]
- 49. Beall DP, Olan WJ, Kakad P, Li Q, Hornberger J. Economic Analysis of Kiva VCF Treatment System Compared to Balloon Kyphoplasty Using Randomized Kiva Safety and Effectiveness Trial (KAST) Data. Pain Phys. 2015;3;18(3;5):E299-E306. doi:10.36076/ppj/2015.18.e299 [PubMed]
- 50. Riggs BL, Melton LJ III. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995;17(5):S505-S511. doi:10.1016/8756-3282(95)00258-4 [DOI] [PubMed]
- 51. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporosis International . 2006;17:1726-1733. [DOI] [PubMed]
- 52. Vadim Goz BA, Errico TJ, Weinreb JH, et al. Vertebroplasty and kyphoplasty: national outcomes and trends in utilization from 2005 through 2010. Spine Journal. 2015;15(5):959-965. doi:10.1016/j.spinee.2013.06.032 [DOI] [PubMed]
- 53. Heini P, Teuscher R. Vertebral body stenting / stentoplasty. Swiss Med Wkly. 2012;142:13658. doi:10.4414/smw.2012.13658 [DOI] [PubMed]
- 54. Kado DM, Duong T, Nevitt MC, et al. Incident vertebral fractures and mortality in older women: a prospective study. Osteoporosis International. 2003;14(7):589-594. doi:10.1007/s00198-003-1412-5 [DOI] [PubMed]
- 55. Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR. Balance disorder and increased risk of falls in osteoporosis and kyphosis: significance of kyphotic posture and muscle strength. Osteoporosis International. 2005;16:8. [DOI] [PubMed]
- 56. Gold DT. The clinical impact of vertebral fractures: Quality of life in women with osteoporosis. Bone. 1996;18(3):S185-S189. doi:10.1016/8756-3282(95)00500-5 [DOI] [PubMed]
- 57. Leidig-Bruckner G, Minnie HW, Schlaich C, Wagner G, Scheidt-Nave C, Bruckner T, et al. Clinical grading of spinal osteoporosis: quality of life components and spinal deformity in women with chronic low back pain and women with vertebral osteoporosis. J Bone Miner Res. 1997;12:663-675. [DOI] [PubMed]
- 58. Leech JA, Dulberg C, Kellie S, Pattee L, Gay J. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis. 1990;141(1):68-71. doi:10.1164/ajrccm/141.1.68 [DOI] [PubMed]
- 59. Culham EG, Jimenez HAI, King CE. Thoracic kyphosis, rib mobility, and lung volumes in normal women and women with osteoporosis. Spine. 1994;19(11):1250-1255. doi:10.1097/00007632-199405310-00010 [DOI] [PubMed]
- 60. Schlaich C, Minne HW, Bruckner T, et al. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos Int. 1998;8(3):261-267. doi:10.1007/s001980050063 [DOI] [PubMed]
- 61. Hillier TA, Lui LY, Kado DM, et al. Height loss in older women: risk of hip fracture and mortality independent of vertebral fractures. J Bone Miner Res. 2011;27(1):153-159. doi:10.1002/jbmr.558 [DOI] [PMC free article] [PubMed]
- 62. Heini PF, Orler R. Kyphoplasty for treatment of osteoporotic vertebral fractures. Spine J. 2004;13:184-192. [DOI] [PMC free article] [PubMed]
- 63. Chen AT, Cohen DB, Skolasky RL. Impact of nonoperative treatment, vertebroplasty, and kyphoplasty on survival and morbidity after vertebral compression fracture in the medicare population. J Bone Joint Surg Am. 2013;95(19):1729-1736. doi:10.2106/jbjs.k.01649 [DOI] [PubMed]
- 64. Blattert TR, Schnake KJ, Gonschorek O, et al. Nonsurgical and Surgical Management of Osteoporotic Vertebral Body Fractures: Recommendations of the Spine Section of the German Society for Orthopaedics and Trauma (DGOU). Global Spine J . 2018;8(2_suppl):50S-55S. doi:10.1177/2192568217745823 [DOI] [PMC free article] [PubMed]


