Abstract
Mice fed Bifidobacterium breve YIT4064 and immunized orally with influenza virus were more strongly protected against influenza virus infection of the lower respiratory tract than ones immunized with influenza virus only. The number of mice with enhanced anti-influenza virus immunoglobulin G (IgG) in serum upon oral administration of B. breve YIT4064 and oral immunization with influenza virus was significantly greater than that upon oral immunization with influenza virus only. These findings demonstrated that the oral administration of B. breve YIT4064 increased anti-influenza virus IgG antibodies in serum and protected against influenza virus infection. The oral administration of B. breve YIT4064 may enhance antigen-specific IgG against various pathogenic antigens taken orally and induce protection against various virus infections.
Influenza is an acute viral respiratory infection that results in high morbidity and significant mortality in humans as well as in animals. The site of the virus entry is the mucosa of the upper respiratory tract, and there is evidence that influenza viruses can be spread by coughing and as a small-particle aerosol directly into the lower respiratory tract (28). An increase in specific antibody production at this site is important for preventing infection in the upper and lower respiratory tracts. In several studies, the degree of protection against influenza virus infection was found to be correlated with the levels of mucosal immunoglobulin A (IgA) in the respiratory tract and serum IgG (4, 5, 14, 22, 23). Mucosal anti-influenza virus IgA inhibits viral attachment to epithelial cells in the mucosa, thereby preventing infection in the upper respiratory tract and stopping the spread of the infection. Furthermore, serum anti-influenza virus IgG prevents infection in the lower respiratory tract and protects the lungs against viral infection and thereby prevents death from pneumonia (17). Subcutaneous vaccination with influenza virus, which is being performed at present, enhances the level of anti-influenza virus IgG in serum and prevents infection by the virus with the same surface hemagglutinin (HA) on the influenza virus virion (12); however, this present method has some troublesome problems. If there is a safe oral adjuvant that enhances serum anti-influenza virus IgG and mucosal anti-influenza virus IgA, an oral vaccine will become efficacius. Further, if there are foods that enhance the immune response against influenza virus, these may be functional foods that could prevent influenza virus infection.
In healthy breast-fed (but not formula-fed) infants, numerous bifidobacteria inhabit the intestines (11). These bacteria are thought to play a role in the resistance to infection in humans and animals (8, 16, 29). The intestines of adults have fewer of these organisms (15), and some persons replace them with yogurt and bifidobacteria cultures. We have found one strain of Bifidobacterium (Bifidobacterium breve YIT4064) that can induce large quantities of IgA among the many strains of bifidobacteria isolated from human feces by the murine Peyer’s patch (PP) cell culture method (30). The organism enhances the production of anti-influenza virus HA (31), antirotavirus, and antipoliovirus antibodies (unpublished data) by PP cells in response to the addition of HA, rotavirus, and poliovirus, respectively. When the organism was administered orally to mice with cholera toxin (CT), the amount of anti-CT IgA in feces and the levels of anti-CT IgA production and proliferation in PP cells were significantly greater than those after the administration of CT alone or of CT and B. breve YIT4079, which did not induce IgA in the in vitro PP cell culture (30). Furthermore, the level of anti-rotavirus IgA in milk in mouse dams fed the organism orally with rotavirus was significantly higher than that in dams immunized with rotavirus only, and pups born to and nursed by dams fed the organism and immunized orally with rotavirus were more strongly protected against rotavirus-induced diarrhea than those born to and nursed by dams immunized with rotavirus only (32).
In the present study, we investigated whether the oral administration of B. breve YIT4064 augmented the level of anti-influenza virus IgG in serum and whether the antibody-enhanced mice were protected against influenza virus infection in the lower respiratory tract and were saved from death.
MATERIALS AND METHODS
Mice.
BALB/c female mice, 6 and 9 weeks old, were obtained from Japan SLC, Inc. (Hamamatsu-shi, Japan) and used for the experiments.
Virus.
Influenza A/PR/8/34 (PR8, H1N1) virus was grown in the allantoic sacs of 11-day-old chicken embryos at 34°C for 2 days by the method of Takemoto et al. (20), with modifications. The allantoic fluid was removed and stored at −80°C. The virus titer of allantoic fluid was expressed as the 50% egg-infecting doses (EID50) (27). Serial 10-fold dilutions of the allantoic fluid were injected into five embryonated eggs, and the presence of virus in the allantoic fluid of each egg was determined on the basis of the hemagglutinating capacity 2 days after injection. The virus titer, expressed as a magnification of dilution with EID50, was 109.2 EID50/ml. The various dilutions of the allantoic fluid were used for oral immunization and nasal infection. Oral immunization with various concentrations of live PR8 via a stomach tube was performed in mice that had been pretreated with an intramuscular injection of cimetidine (Tagamet; Smith Kline & French Laboratories, Philadelphia, Pa.) (1.2 mg per mouse) 1 h before (2) and with 3% NaHCO3 via a stomach tube just before the oral immunization. Nasal infection was performed by dropping 10 or 1 μl of fluid containing various concentrations of PR8 into each nostril (injection with 20 or 2 μl per mouse) after the mice were anesthetized by an intraperitoneal injection of sodium amobarbital (0.25 μg per mouse). Mice were infected in the upper and lower respiratory tracts by dropping 10 and 1 μl of PR8 solution into each nostril, respectively (21).
PR8 antigen.
The purified PR8 antigen was prepared from the allantoic fluid that included PR8 grown in 11-day-old embryonated eggs. The PR8 in the fluid was concentrated by centrifugation (174,500 × g, 4°C, 1 h) and then purified by zonal centrifugation through a noncontinuous 30 and 60% sucrose gradient (174,500 × g, 4°C, 50 min) and then a noncontinuous 35, 42, 52, and 60% sucrose gradient (174,500 × g, 4°C, 50 min). The virus preparation was treated with 0.01% formalin, stored at −80°C, and used as the antigen in an enzyme-linked immunosorbent assay (ELISA) for antibody detection.
B. breve YIT4064.
B. breve YIT4064, isolated from the feces of a healthy breast-fed infant, was treated with heat and used in this study. B. breve YIT4064 was incubated in prereduced modified PSYL broth medium at 37°C for 24 h. The composition of the modified PSYL medium (per liter) was as follows: skim milk, 10 g; protease, 0.2 g; yeast extract, 10 g; CH3COONa, 3 g; (NH4)2SO4, 3 g; lactose, 50 g; silicon, 0.1 ml. B. breve YIT4064 was harvested by centrifugation at 7,000 × g for 10 min at 4°C, washed three times with sterilized water, and allowed to autolyse by incubation in sterilized water overnight at 15°C. B. breve YIT4064 was heated at 100°C for 30 min and lyophilized. A 0.05% (wt/wt) concentration of B. breve YIT4064 was added to MM-3 diet (Funabashi Farms, Funabashi-shi, Japan).
Protocol.
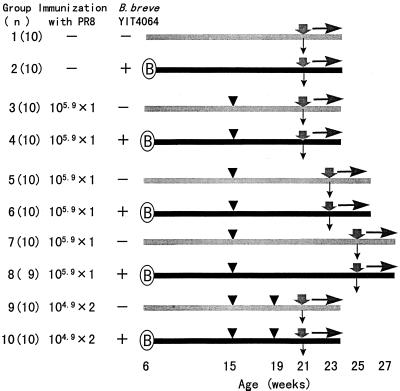
Six-week-old female BALB/c mice were given food orally ad libitum (Fig. 1). Each group consisted of 10 mice, except that group 8 consisted of 9 mice. Groups 2, 4, 6, 8, and 10 received food with 0.05% B. breve YIT4064 added, and groups 1, 3, 5, 7, and 9 received food without the organism. Groups 3 and 4, 5 and 6, and 7 and 8 were immunized via a stomach tube with a single dose of 105.9 EID50s of PR8 at 6, 8, and 10 weeks before nasal infection, respectively. Groups 9 and 10 were immunized via a stomach tube with two doses of 104.9 EID50 of PR8 at 6 and 2 weeks before nasal infection. Groups 1 and 2 were not immunized before nasal infection. These mice were monitored for symptoms and survival every day after nasal infection. The symptoms monitored were erect hair and a decrease in body weight. Nasal infection was performed by dropping 10 μl of fluid containing a virus concentration of 103.5 EID50/20 μl into each nostril. Blood was obtained from retroorbital blood vessels just before the nasal injection of PR8, and the serum was stored at −30°C until tested.
FIG. 1.
Protocol for feeding mice with B. breve YIT4064: B, start; ▾, oral immunization with PR8; , inoculation with PR8; , collection of sera; -➤, observation.
Hyperimmune serum.
Hyperimmune antiserum to PR8 was prepared after four injections of PR8 (108.9 EID50) via a stomach tube and one nasal injection of PR8 antigen (104.5 EID50) into BALB/c mice. By comparing the A492 values against various dilutions of the hyperimmune antiserum to PR8, we determined the dilution values that best represented a straight line. At A492, 1 U of anti-PR8 IgG was equivalent to a 104 dilution of the hyperimmune antiserum to PR8.
Measurement of antibodies.
The antibody titers in serum were measured by ELISA. The purified PR8 antigen (2 μg of protein/ml) was coated onto the wells of a 96-well ELISA plate (Nunc, Roskilde, Denmark) with carbonate buffer (pH 9.6). An experimental sample of serum or a standard sample of hyperimmune antiserum against PR8 was added to each well, and then the plate was incubated at 37°C for 1.5 h. Horseradish peroxidase-labeled goat anti-mouse IgG (1:2,000; Organon Teknika-Cappel) was then added to the wells. The wells were extensively washed between incubations. After an o-phenylenediamine solution (0.4 mg/ml citrate buffer, pH 5.0) containing 0.02% H2O2 had been added to each well, the plates were held at 37°C for 10 min. The reaction was stopped by the addition of 2.5 M H2SO4, and the A492 of the resulting solution was determined with a Titertek Multiscan (Flow Laboratories, McLean, Va.).
Statistical analysis.
The statistical significance of the difference between the groups fed B. breve YIT4064 and those not fed the bacteria in the experiments was examined by means of Fisher’s exact test or the χ2 test.
RESULTS
Virus dose dependence of infection in mice after intranasal challenge with PR8.
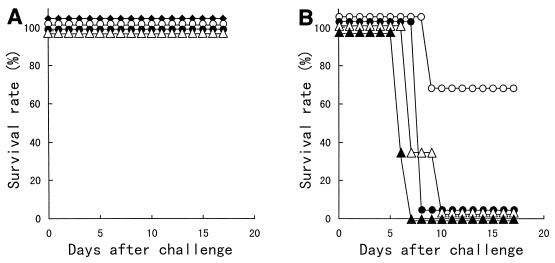
Nine-week-old mice were inoculated with 1 or 10 μl of a PR8 solution at various concentrations into each nostril (inoculation with 2 or 20 μl of PR8 per mouse) and then monitored for survival every day after inoculation. None of the mice given various concentrations of PR8 in the upper respiratory tract, i.e., with a 2-μl solution of PR8 given per mouse, were infected, and therefore these mice did not die (Fig. 2A). Regarding infection of the lower respiratory tract (inoculation with 20 μl of PR8 per mouse), all mice inoculated with 103.5 to 105.5 EID50 of PR8 died between 7 and 10 days after intranasal inoculation. Of the mice inoculated intranasally with 102.5 EID50 of PR8, 66.7% survived (Fig. 2B). Therefore, 103.5 EID50, a lethal dose with regards to infection of the lower respiratory tract (inoculation with 20 μl of PR8 per mouse), was used as the infectious dose to determine the ability of B. breve YIT4064 to protect against influenza virus infection.
FIG. 2.
Kinetics of survival rate after inoculation with 1 (A) or 10 (B) μl of a PR8 solution containing various doses into each nostril. Mice were inoculated with 1 μl of a PR8 solution into each nostril for infection of the upper respiratory tract (A) and with 10 μl of a PR8 solution into each nostril for infection of the lower respiratory tract (B). Virus dose group symbols: ⧫, 101.5 EID50 of PR8 (n = 6); ○, 102.5 EID50 of PR8 (n = 6); ●, 103.5 EID50 of PR8 (n = 6); ▵, 104.5 EID50 of PR8 (n = 6); ▴, 105.5 EID50 of PR8 (n = 6).
Protection against influenza virus infection by oral immunization with influenza virus.
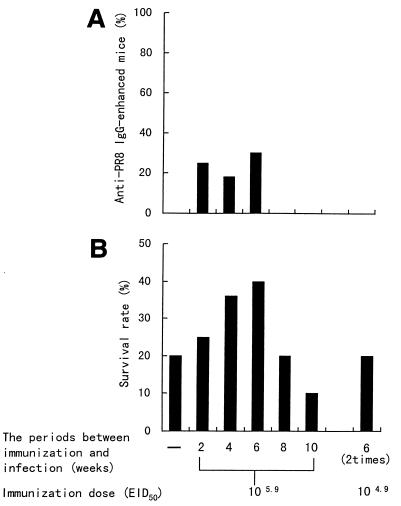
We investigated whether influenza virus infection was prevented in mice immunized with various doses of PR8 orally. Nine-week-old mice were immunized via a stomach tube with a single dose of 105.9 to 108.9 EID50 of PR8 at 2 weeks before nasal inoculation or with two doses of 104.9 EID50 of PR8 at 6 and 2 weeks before nasal inoculation of PR8 (103.5 EID50) and were monitored for survival for 14 days after nasal inoculation. As shown in Table 1, 100% of the mice immunized orally with 108.9 EID50 of PR8 showed enhancement of the level of anti-PR8 IgG in serum just before and survival after nasal inoculation of PR8, and 25.0% of the mice immunized orally with 105.9 EID50 of PR8 showed enhancement of the level of anti-PR8 IgG just before and survival after nasal inoculation of PR8. Therefore, immunization with 108.9 and 105.9 EID50 of PR8 protected mice against infection perfectly and partially, respectively. All the mice immunized orally with two doses of 104.9 EID50 of PR8 died. Therefore, correlation was observed among the oral immunization dose of PR8, augmentation of anti-PR8 IgG production in serum, and survival rate with regards to PR8 infection. Therefore, we studied, by using immunization doses of 105.9 and 104.9 EID50 of PR8, whether B. breve YIT4064 enhanced the level of anti-PR8 IgG in serum and the survival rate after virus infection.
TABLE 1.
Relationship among immunization dose, antibody production, and survival ratea
| Immunization dose (EID50) | No. of immunizations | No. of
mice
|
|
|---|---|---|---|
| With >400 U of Ab/total no. tested | Survived/total no. tested | ||
| 0/5 (0.0) | 0/5 (0.0) | ||
| 108.9 | 1 | 5/5 (100.0) | 5/5 (100.0) |
| 107.9 | 1 | 3/6 (50.0) | 4/6 (66.7) |
| 106.9 | 1 | 4/10 (40.0) | 4/10 (40.0) |
| 105.9 | 1 | 2/8 (25.0) | 2/8 (25.0) |
| 104.9 | 2 | 0/10 (0.0) | 0/10 (0.0) |
Female BALB/c mice, 9 weeks old, were immunized with various single doses of PR8 via a stomach tube and with two doses of 104.9 EID50 of PR8 at 2 weeks and at 6 and 2 weeks before nasal inoculation (103.5 EID50 of PR8), respectively. After nasal injection of PR8, the mice were observed for survival for 14 days. The numbers in parentheses are the ratios of antibody-enhanced mice to surviving mice.
In the next study, we observed differences in the serum IgG titers and survival rates for the period between oral immunization and nasal inoculation. Nine-week-old mice were immunized with a single dose of 105.9 EID50 of PR8 or with two doses of 104.9 EID50 of PR8 at various numbers of weeks or at 6 and 2 weeks before nasal inoculation of PR8, respectively. After nasal inoculation, the mice were monitored for survival for 14 days. As shown in Fig. 3, the number of mice with enhanced anti-PR8 IgG in serum was increased at 2, 4, and 6 weeks after oral immunization with 105.9 EID50 of PR8, but mice with the antibody-enhanced serum were not observed at 8 or 10 weeks after oral immunization with 105.9 EID50 of PR8 or after immunization with two doses of 104.9 EID50 of PR8 (Fig. 3A). Mice infected intranasally at 6 weeks after oral immunization with 105.9 EID50 of PR8 showed the highest survival rate. The survival rate in mice infected at 8 and 10 weeks after immunization with 105.9 EID50 of PR8 was decreased and was the same as that in nonimmunized mice. Mice immunized with two doses of 104.9 EID50 of PR8 did not show an increase in survival rate but showed the same survival rate as that of nonimmunized mice, too (Fig. 3B). The decrease in the survival rate was thought to depend on a decrease or absence of anti-PR8 IgG in serum. Therefore, we investigated whether B. breve YIT4064 augmented antibody production in serum and enhanced the survival rate in mice infected in the lower respiratory tract when the effectiveness of the immunization was decreased for long periods between immunization and infection (8 and 10 weeks) or when the immunization was not effective because a small amount of the immunogen (two doses of 104.9 EID50 of PR8) was used.
FIG. 3.
Differences in percentages of anti-PR8 IgG production in serum (A) and survival rates (B) based on the period between oral immunization and nasal infection or on the quantity of immunogen. Nine-week-old female BALB/c mice were immunized with a single dose of 105.9 EID50 and with two doses of 104.9 EID50 of PR8 at various weeks and at 6 and 2 weeks before nasal inoculation (103.5 EID50 of PR8), respectively. The anti-PR8 IgG titer in serum was measured just before nasal inoculation of PR8. The survival rates were observed for 14 days after nasal inoculation of PR8.
Protection against PR8-induced influenza virus infection by oral administration of B. breve YIT4064.
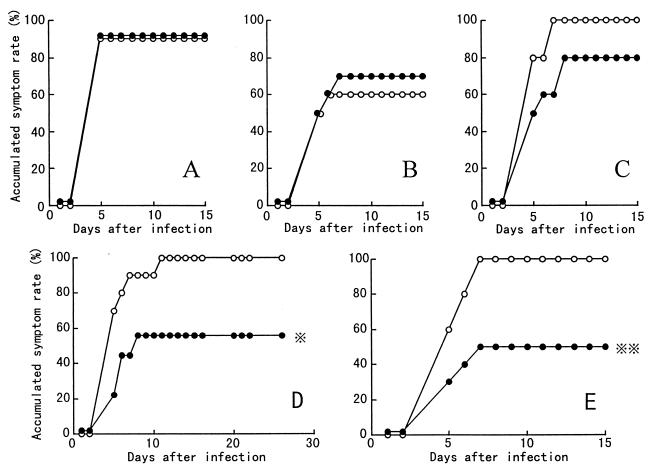
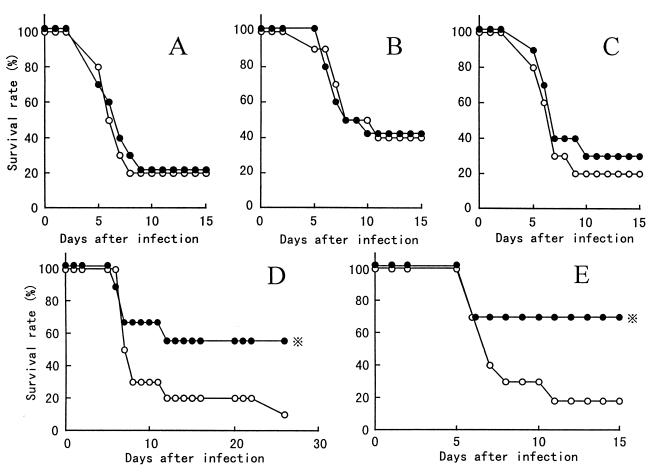
We studied the protection against influenza virus infection by oral administration of B. breve YIT4064 according to the protocol shown in Fig. 1. Figure 4 shows the time courses of the accumulated symptom rate (percentage of mice showing erect hair and/or decrease in body weight) in mice that were nonimmunized (Fig. 4A) and in mice that were immunized with 105.9 EID50 of PR8 at 6, 8, and 10 weeks (Fig. 4B to D) and with two doses of 104.9 EID50 of PR8 (Fig. 4E) before nasal inoculation of PR8 (103.5 EID50). In the mice immunized with 105.9 EID50 of PR8 at 10 weeks (Fig. 4D) and with two doses of 104.9 EID50 of PR8 (Fig. 4E) before nasal inoculation of PR8, oral administration of B. breve YIT4064 significantly decreased the accumulated symptom rate of influenza virus infection. In the nonimmunized mice (Fig. 4A) and in the mice immunized with 105.9 EID50 of PR8 at 6 and 8 weeks before nasal inoculation of PR8 (Fig. 4B and C), the accumulated symptom rate of influenza virus infection did not decrease upon oral administration of B. breve YIT4064. As shown in Fig. 5, in the mice immunized with 105.9 EID50 of PR8 at 10 weeks (Fig. 5D) and with two doses of 104.9 EID50 of PR8 (Fig. 5E) before nasal inoculation of PR8 (103.5 EID50), the survival rate of mice fed B. breve YIT4064 (groups 8 and 10) was significantly greater than that of those not fed the organism (groups 7 and 9). In the nonimmunized mice (Fig. 5A) and in the mice immunized with 105.9 EID50 of PR8 at 6 and 8 weeks before nasal inoculation of PR8 (Fig. 5B and C), the survival rate of mice fed B. breve YIT4064 was nearly the same as that of those not fed B. breve YIT4064. Thus, mice fed B. breve YIT4064 and immunized with PR8 were better protected than mice immunized only with PR8, when the efficiency of immunization was decreased for long periods between immunization and infection (8 and 10 weeks), and the effect of immunization was not recognized when a small amount of the immunogen (two doses of 104.9 EID50 of PR8) was used. Our next experiment sought to determine whether the protection against influenza virus infection by oral administration of B. breve YIT4064 was due to an increase in serum antibodies.
FIG. 4.
Protection against morbidity due to influenza virus infection in mice administered B. breve YIT4064 orally. Mice were not immunized (A) (groups 1 and 2), were immunized with a single dose of 105.9 EID50 of PR8 at 6 (B) (groups 3 and 4), 8 (C) (groups 5 and 6), and 10 (D) (groups 7 and 8) weeks, or were immunized with two doses of 104.9 EID50 of PR8 at 6 and 2 weeks (E) (groups 9 and 10) before nasal inoculation (103.5 EID50 of PR8). Mice were not fed (○) or were fed (●) B. breve YIT4064 throughout these experiments. After nasal inoculation of PR8, the mice were observed for accumulated symptom rate for 14 to 21 days. and , P < 0.02 and P < 0.05, respectively, versus accumulated symptom rate in groups 9 and 7, respectively, by Fisher’s exact test.
FIG. 5.
Protection against mortality due to influenza virus infection in mice administered B. breve YIT4064 orally. Oral immunization and nasal inoculation in each group were performed in the same manner as described in the legend to Fig. 4. After nasal injection of PR8, the mice were observed for survival for 14 to 21 days. , P < 0.05 versus survival rate in group 7 or 9, by Fisher’s exact test.
Antibody titers in sera of mice.
We measured serum antibodies to determine whether oral administration of B. breve YIT4064 augmented anti-PR8 IgG production in serum, which plays a role in the prevention of infection in the lower respiratory tract and mortality. As shown in Table 2, the number of mice with an enhanced level of anti-PR8 IgG in serum increased significantly upon oral administration of B. breve YIT4064 in mice immunized with 105.9 EID50 of PR8 at 10 weeks (groups 7 and 8) or with two doses of 104.9 EID50 of PR8 (groups 9 and 10) before nasal inoculation of PR8. But, it did not increase upon oral administration of B. breve YIT4064 in nonimmunized mice (groups 1 and 2) or in mice immunized with 105.9 EID50 of PR8 at 6 and 8 weeks before nasal inoculation of PR8 (groups 3 to 6).
TABLE 2.
Number of mice with serum antibodies enhanced by oral administration of B. breve YIT4064a
| Mouse group | No. of mice with >400 U of anti-PR8/total no. testedb | % of mice with >400 U of anti-PR8 IgG |
|---|---|---|
| 1 | 0/10 | 0 |
| 2 | 0/10 | 0 |
| 3 | 3/10 | 30 |
| 4 | 2/10 | 20 |
| 5 | 0/10 | 0 |
| 6 | 1/10 | 10 |
| 7 | 0/10 | 0 |
| 8 | 3/9* | 33 |
| 9 | 0/10 | 0 |
| 10 | 5/10** | 50 |
Mice were not immunized (groups 1 and 2), were immunized orally with a single dose of 105.9 EID50 of PR8 at 6 (groups 3 and 4), 8 (groups 5 and 6), and 10 weeks (groups 7 and 8) before nasal inoculation (103.5 EID50 of PR8), or were immunized orally with two doses of 104.9 EID50 of PR8 at 6 and 2 weeks before nasal inoculation (103.5 EID50 of PR8) (groups 9 and 10). Mice were not fed (groups 1, 3, 5, 7, and 9) or were fed (groups 2, 4, 6, 8 and 10) B. breve YIT4064 throughout these experiments. The anti-PR8 IgG titer in serum was measured just before nasal inoculation (103.5 EID50 of PR8), and the number of mice with anti-PR8 IgG levels of >400 U was determined.
**, P < 0.02 versus number of antibody-enhanced mice in group 9, by Fisher’s exact test. *, P < 0.05 versus number of antibody-enhanced mice in group 7, by χ2 test.
Relationship between the level of anti-PR8 IgG in serum just before nasal infection and the survival of mice after nasal infection.
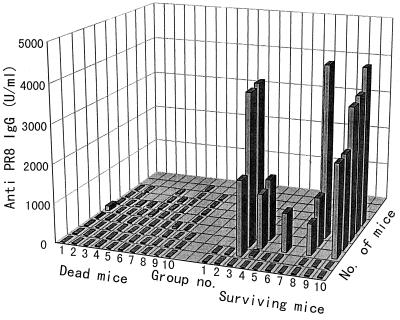
As shown in Fig. 6, all mice in which the level of anti-PR8 IgG in serum was enhanced were protected against influenza virus infection in the lower respiratory tract and death. Anti-PR8 IgG was not detected in the mice that died in any group. One or two mice survived in all groups, and anti-PR8 IgG was not detected in the serum of these mice. We thought that since the activity of nonspecific immune cells and NK cells, etc., differed among the lots of mice, the sensitivity to infection with different lethal doses of PR8 might differ among the lots of mice and noninfected mice might appear in some lots of mice.
FIG. 6.
Relationship between level of anti-PR8 IgG in serum and survival of mice after infection. The anti-PR8 IgG titers in sera of mice from various groups were measured just before nasal inoculation of PR8 (103.5 EID50). The anti-PR8 IgG titers in dead and surviving mice are shown individually.
Therefore, when the efficiency of immunization was decreased for long periods between immunization and infection or was not recognized due to the use of a small quantity of the immunogen, the oral administration of B. breve YIT4064 enhanced antigen-specific IgG production in serum and prevented influenza virus infection in the lower respiratory tract.
DISCUSSION
All mice injected with 103.5 to 105.5 EID50 of PR8 in the lower respiratory tract died. But, for infection of the upper respiratory tract (inoculation with 2 μl of PR8 per mouse), 100% of the mice survived even with inoculation of 105.5 EID50 of PR8 (Fig. 2). It was thought that if PR8 was injected directly into the lower respiratory tract, a small amount of PR8 (103.5 EID50) proliferated sufficiently in the lungs to kill all mice. Serum anti-influenza virus IgG is important in preventing pneumonia and in protecting mice from death after lower respiratory infection, although it is not always effective in preventing infection in the upper respiratory tract (1, 17). We found that oral vaccination with PR8 produced anti-PR8 IgG in serum, prevented infection in the lower respiratory tract, and protected mice from death. The observations in our present study resemble the results of Chen and Quinnan (3); i.e., intragastric administration of inactivated influenza virus vaccine induced a predominance of HA-specific IgG in serum. The level of anti-PR8 IgG in serum and the survival rate of mice after nasal inoculation of PR8 depended on the dose of PR8 given orally (Table 1).
In the next study, we observed the relation between the decrease in the serum IgG titer just before nasal inoculation of PR8 and the increase in the infection rate after nasal inoculation of PR8. The anti-PR8 IgG level in serum at 2, 4, and 6 weeks after oral immunization and the survival rate of mice after infection increased at the same time and peaked at 6 weeks after immunization (Fig. 3). These results are similar to those of Murphy et al. (13), who reported that after inoculation of influenza virus, the IgM and IgA antibody levels peaked at 2 weeks and then began to decrease, whereas IgG antibodies, first detectable during the same period, continued to increase in titer until the maximal level was reached at 4 to 7 weeks.
The oral administration of B. breve YIT4064 with the oral injection of rotavirus augmented anti-rotavirus IgA antibodies in milk secreted from the mammary glands and prevented rotavirus-induced diarrhea in pups (32). Anti-influenza virus IgG in serum prevented influenza virus infection in the lower respiratory tract and protected mice from death (Table 1). We examined whether the oral administration of B. breve YIT4064 augmented anti-PR8 IgG production in serum and prevented influenza virus infection in the lower respiratory tract. When the efficiency of immunization was decreased for long periods after oral immunization (8 and 10 weeks), and when immunization was not recognized due to the small amount of the immunogen (two doses of 104.9 EID50 of PR8), the oral administration of B. breve YIT4064 augmented anti-PR8 IgG production in serum and prevented influenza virus infection (Table 2; Fig. 4 and 5).
The number of surface-IgG-positive cells was very low in PP (6), and anti-HA IgG was not produced upon the addition of HA antigen to a PP cell culture (31). Therefore, it was not thought that after PR8 was taken up by M cells in PP, anti-PR8 IgG was produced in PP. The mechanism underlying the appearance of anti-PR8 IgG in serum after oral vaccination of PR8 was thought to be that the HA of PR8 might bind to sialic acid (13) on epithelial cells in the lamina propria of the intestine and be absorbed by the epithelial cells and then act as an immunogen and produce anti-PR8 IgG in serum. Umesaki and Setoyama have shown that substances with the ability to bind to intestinal epithelial cells are absorbed into the lamina propria, are strong immunogens, and produce antigen-specific IgG in serum (26). The mechanism underlying the enhancement of anti-PR8 IgG upon oral administration of B. breve YIT4064 is not clear. The adjuvant activity of B. breve YIT4064 was not observed when B. breve YIT4064 was administered orally and HA antigen was injected nasally (unpublished data), being found only when B. breve YIT4064 was given orally with an antigen (30, 32). B. breve YIT4064 administered orally was taken up by M cells in PP and was scarcely taken up by epithelial cells on the lamina propria of the intestine (19). Therefore, it was assumed that the PR8 antigen was absorbed by epithelial cells in the intestine and that B. breve YIT4064 was taken up by M cells in PP encountered in mesenteric lymph nodes or another immune tissue and then anti-PR8 IgG in serum was produced in an amount greater than that upon oral injection of PR8 only. Furthermore, it is presumed that B. breve YIT4064 fed orally induces the production of some cytokines in epithelial cells on the lamina propria and accelerates the immune response and antibody production in response to absorbed PR8 at that site.
The attractive attributes of an oral vaccine include the simplicity of administration: no need for sterile syringes, needles, or trained personnel, less stringent requirements for the preparation of orally delivered antigens than for injectable ones, and fewer problems with the storage of dry, lyophilized oral vaccines than with liquid injectable vaccines. However, because antigens administered orally are inactivated by gastric acid and digestive enzymes, it is difficult for them to be taken up and processed by PP. Therefore, the immune response after oral administration of an antigen is weaker than that after systemic immunization. The immunogenicity of vaccines is augmented by the administration of potent adjuvants. A number of promising new adjuvants, such as immunostimulating complexes, CT subunit B, proteoliposomes, 6-o-acylmuramyldipeptide, a lipid A analogue, and others, enhance the immune responses of animals and humans to vaccines (7, 10, 18, 23–25). However, adjuvants that stimulate mucosal immunity have some undesirable side effects after oral administration. Because B. breve YIT4064 has been isolated from the intestines of healthy breast-fed infants and shows weak antigenicity (30), it is safe as an adjuvant for oral vaccination. Furthermore, B. breve YIT4064 may be used as the host in the development of a new oral vaccine which involves rearrangement of a gene. If it is possible to transfer various antigenic genes of a pathogen into B. breve YIT4064 and for the bacteria to inhabit the intestine, one may obtain a new hopeful oral vaccine. We are now studying whether the oral administration of B. breve YIT4064 enhances the secretory anti-PR8 IgA level in nasal and bronchoalveolar washings and prevents the growth of influenza virus in the upper respiratory tract. In preliminary experiment, oral administration of B. breve YIT4064 enhanced antigen-specific IgA against an influenza virus HA vaccine administered orally in a nasal wash (unpublished data). Since B. breve YIT4064 exhibits a protective effect against influenza virus infection, it can be used as a probiotic for protection against infection and is a candidate for production as a kind of “physiologic function food” that was recently defined in Japan (9).
ACKNOWLEDGMENTS
We thank S. Tamura and T. Kurata (National Institute of Health, Tokyo, Japan) and Y. Suzuki (Kitasato Institute, Saitama, Japan) for help with the study and M. Watanuki for helpful discussions.
REFERENCES
- 1.Balkovic E S, Six H R. Pulmonary and serum isotypic antibody response of mice to live and inactivated influenza virus. Am Rev Respir Dis. 1986;134:6–11. doi: 10.1164/arrd.1986.134.1.6. [DOI] [PubMed] [Google Scholar]
- 2.Chen K S, Burlington D B, Quinnan G V. Active synthesis of hemagglutinin-specific immunoglobulin A by lung cells of mice that were immunized intragastrically with inactivated influenza virus vaccine. J Virol. 1987;61:2150–2154. doi: 10.1128/jvi.61.7.2150-2154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen K S, Quinnan G V. Induction, persistence and strain specificity of haemagglutinin-specific secretory antibodies in lungs of mice after intragastric administration of inactivated influenza virus vaccines. J Gen Virol. 1988;69:2779–2784. doi: 10.1099/0022-1317-69-11-2779. [DOI] [PubMed] [Google Scholar]
- 4.Clements M L, Betts R F, Tierney E L, Murphy B R. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24:157–160. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couch R B, Kasal J A. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- 6.Durkin H G, Bazin H, Waksman B H. Origin and fate of IgE-bearing lymphocytes. 1. Peyer’s patches as differentiation site of cells simultaneously bearing IgA and IgE. J Exp Med. 1981;154:640–648. doi: 10.1084/jem.154.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guink N, Kris R M, Goodman-Snitloff G, Small P A, Jr, Mannino R J. Intranasal immunization with proteoliposomes protects against influenza. Vaccine. 1989;7:147–151. doi: 10.1016/0264-410x(89)90055-8. [DOI] [PubMed] [Google Scholar]
- 8.Homma N. Intestinal flora of infants and resistance to infection. Jpn J Pediatr Soc. 1974;27:1266–1275. [Google Scholar]
- 9.Kitazawa H, Nomura M, Itoh T, Yamaguchi T. Functional alteration of macrophages by a slime-forming Lactococcus lactis ssp. cremoris. J Dairy Sci. 1991;74:2082–2088. doi: 10.3168/jds.S0022-0302(91)78380-X. [DOI] [PubMed] [Google Scholar]
- 10.Lovgren K. The serum antibody response distributed in subclasses and isotypes after intranasal and subcutaneous immunization with influenza virus immunostimulating complexes. Scand J Immunol. 1988;27:241–245. doi: 10.1111/j.1365-3083.1988.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 11.Mitsuoka T, Kaneuchi C. Ecology of bifidobacteria. Am J Clin Nutr. 1977;30:1799–1810. doi: 10.1093/ajcn/30.11.1799. [DOI] [PubMed] [Google Scholar]
- 12.Mozdzanowska K, Furchner M, Washko G, Mozdzanowska J, Gerhard W. A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. J Virol. 1997;71:4347–4355. doi: 10.1128/jvi.71.6.4347-4355.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy B R, Nelson D L, Wright P F, Tierney E L, Phelan M A. Secretory and systemic immunological response in children infected with live attenuated influenza A virus vaccines. Infect Immun. 1982;36:1102–1108. doi: 10.1128/iai.36.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy B R, Webster R G. Orthomyxoviruses. In: Fields B N, Knipe D M, Chanock R M, Hirsch M S, Melnick J L, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 1091–1152. [Google Scholar]
- 15.Mutai M, Tanaka R. Ecology of Bifidobacterium in human intestinal flora. Bifidobacteria Microflora. 1987;6:33–41. [Google Scholar]
- 16.Poupard J A, Husain I, Norrits R F. Biology and bifidobacteria. Bacterial Rev. 1973;37:136–165. doi: 10.1128/br.37.2.136-165.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramphal R, Cogliano R C, Shands J W, Jr, Small P A., Jr Serum antibody prevents lethal murine influenza pneumonitis but not tracheitis. Infect Immun. 1979;25:992–997. doi: 10.1128/iai.25.3.992-997.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundquist B, Lovgren K, Morein B. Influenza virus ISCOMs: antibody response in animals. Vaccine. 1988;6:49–53. doi: 10.1016/0264-410x(88)90014-x. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi H, Iwata S, Yamazaki N, Fujiwara H. Phagocytosis of the lactic acid bacteria by M cells in the rabbit Peyer’s patches. J Clin Electron Microsc. 1991;24:532–533. [Google Scholar]
- 20.Takemoto K K, Lynt R K, Rowe W P, Huebner R J, Bell J A, Mellin G W, Davis D J. Primary isolation of influenza A, B, and C viruses in monkey kidney tissue cultures. Proc Soc Exp Biol Med. 1955;89:308–311. doi: 10.3181/00379727-89-21794. [DOI] [PubMed] [Google Scholar]
- 21.Tamura S, Asanuma H, Ito Y, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T, Oya A. Superior cross-protective effect of nasal vaccination to subcutaneous inoculation with influenza hemagglutinin vaccine. Eur J Immunol. 1992;22:477–481. doi: 10.1002/eji.1830220228. [DOI] [PubMed] [Google Scholar]
- 22.Tamura S, Funato H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol. 1991;21:1337–1344. doi: 10.1002/eji.1830210602. [DOI] [PubMed] [Google Scholar]
- 23.Tamura S, Kurata H, Funato H, Nagamine T, Aizawa C, Kurata T. Protection against influenza virus infection by a two-dose regimen of nasal vaccination using vaccine combined with cholera toxin B subunit. Vaccine. 1989;7:314–320. doi: 10.1016/0264-410x(89)90192-8. [DOI] [PubMed] [Google Scholar]
- 24.Tamura S, Samegai Y, Kurata H, Kikuta K, Nagamine T, Aizawa C, Kurata T. Enhancement of protective antibody responses by cholera toxin B subunit inoculated intranasally with influenza vaccine. Vaccine. 1989;7:257–262. doi: 10.1016/0264-410x(89)90240-5. [DOI] [PubMed] [Google Scholar]
- 25.Tsujimoto M, Kotani S, Okunaga T, Kubo T, Takada H, Kubo T, Shiba T, Kusumoto S, Takahashi T, Goto Y. Enhancement of humoral immune responses against virus vaccines by a non-pyrogenic 6-o-acylmuramyldipeptide and synthetic low toxicity analogues of lipid A. Vaccine. 1989;7:39–48. doi: 10.1016/0264-410x(89)90009-1. [DOI] [PubMed] [Google Scholar]
- 26.Umesaki Y, Setoyama H. Immune responses of mice to orally administered asialo GM1-specific rabbit IgG in the presence or absence of cholera toxin. Immunology. 1992;75:386–388. [PMC free article] [PubMed] [Google Scholar]
- 27.Webster R G, Askonas B A. Cross-protection and cross-reactive cytotoxic T cells induced by influenza virus vaccines in mice. Eur J Immunol. 1980;10:396–401. doi: 10.1002/eji.1830100515. [DOI] [PubMed] [Google Scholar]
- 28.Wijburg O L C, Dinatale S, Vadolas J, Rooijen N, Strugnell R A. Alveolar macrophages regulate the induction of primary cytotoxic T-lymphocyte responses during influenza virus infection. J Virol. 1997;71:9450–9457. doi: 10.1128/jvi.71.12.9450-9457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamazaki S, Kamimura H, Momose H, Kawashima T, Ueda K. Protective effect of Bifidobacterium monoassociation against lethal activity in Escherichia coli. Bifidobacteria Microflora. 1982;1:55–59. [Google Scholar]
- 30.Yasui H, Nagaoka N, Mike A, Hayakawa K, Ohwaki M. Detection of Bifidobacterium strains that induce large quantities of IgA. Microb Ecol Health Dis. 1992;5:155–162. [Google Scholar]
- 31.Yasui H, Nagaoka N, Hayakawa H. Augmentation of anti-influenza virus hemagglutinin antibody production by Peyer’s patch cells with Bifidobacterium breveYIT4064. Clin Diagn Lab Immunol. 1994;1:244–246. doi: 10.1128/cdli.1.2.244-246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasui H, Kiyoshima J, Ushijima H. Passive protection against rotavirus-induced diarrhea of mouse pups born to and nursed by dams fed Bifidobacterium breveYIT4064. J Infect Dis. 1995;172:403–409. doi: 10.1093/infdis/172.2.403. [DOI] [PubMed] [Google Scholar]