Abstract
Coxiellosis, also known as Q fever, is a zoonotic disease caused by Coxiella burnetii, a gram-negative bacterium that exerts a significant deleterious impact on the productive and reproductive capabilities of livestock, severely effecting the economics of this sector. In this study, 448 sera samples from cattle (n = 224) and buffalo (n = 224) were collected from 112 farms in Pakistan and examined for antibodies against C. burnetii using an indirect ELISA. Ticks were also collected from these animals. Serological analysis revealed a 23.66% and 27.23% seroprevalence of Q fever in cattle and buffalo, respectively. Odds ratio (OR) analysis of the factors associated with C. burnetii seropositivity was performed, and a multivariable logistic model identified five main variables associated with the seropositivity for coxiellosis. These were: (i) the absence of acaricide use (OR 5.61; 95% CI 2.97–10.94); (ii) the presence of ticks (OR 3.23; 95% CI 1.87–5.69); (iii) the abortion history during the preceding year on the farm (OR 14.96; 95% CI 8.09–29.34); (iv) the presence of sheep and goats (OR 2.47; 95% CI 1.20–5.35); and (v) the absence of a separate parturition area (OR 3.17; 95% CI 1.76–5.86). This study provides new insights into the seroprevalence of Q fever in large ruminants across seven studied districts of Punjab, Pakistan, also providing baseline data to inform improved herd management and on-farm practices for the prevention and control of Q fever in large ruminants in the region. Results of this work suggest that further molecular investigation of coxiellosis is warranted to provide a more thorough evaluation of C. burnetii epidemiology in Pakistan.
Subject terms: Microbiology, Zoology, Diseases, Medical research, Risk factors
Introduction
Coxiellosis, also known as Q fever, is a worldwide zoonotic disease caused by Coxiella burnetii, an intracellular gram-negative bacterium categorized as a biological (Type B) warfare agent1. Coxiellosis is known to occur throughout the globe, with the only countries not reporting cases to date being New Zealand, French Polynesia, Ireland, the Netherlands, Poland, and the Scandinavian countries2–4. Several epidemiological studies have increased public health awareness of Q fever5, 6, with cases reported in humans worldwide, including 14 in Switzerland7 and 10 (in military personnel) in France8. A staggering 4,000 acute and 284 chronic human Q fever cases have been reported in the Netherlands, with cases reaching epidemic proportions as a probable result of both seropositive blood donors and affected populations living in close proximity to ovine herds9. Some domestic animals, mainly sheep and goats, can act as reservoirs for Q fever10, with asymptomatic infection suggested in ruminants based on reports of serologically negative dairy cows11. In large ruminants, coxiellosis causes premature birth, sporadic abortions, dead or weak calf, plancentitis, and subclinical mastitis, causing significant economic losses in herds12. The signs and symptoms observed in animals per se include abortion, infertility, stillbirth, mastitis, metritis, weak-offspring, and induced reproductive disorder in domestic ruminants, culminating again in economic impacts13–17. Where Q fever causes ovine and caprine abortions in herds18, 19, C. burnetii are shed via birth products (e.g. birth fluids and the placenta). This creates a source of contamination that increases the risk of related outbreaks in humans19, 20, though the bacterium may also be shed through ruminant urine21, milk products and vaginal mucus and feces22. Ticks also play a role in the transmission of C. burnetii in animals, but their role as vectors of this bacterium in humans is not fully understood23.
Q fever is highly contagious for humans, especially those working in close contact with infected ruminants, such as abattoir staff, veterinarians, and farmers24, 25. In humans, Q fever can lead to an acute self-limiting disease with flu-like symptoms, or to a chronic disease associated with hepatitis, endocarditis (in immuno-compromised patients), encephalitis, abortions and stillbirth in pregnant women26. Coxiella-infected cows may also develop metritis, infertility, and mastitis, and may have the potential to shed C. burnetii through milk for up to 32 months27. Infected animals are thought to transmit Q fever to humans via an aerosol route28, though some, albeit negligible, oral transmission has been reported (often via consumption of contaminated dairy products)29, with sexual and vertical transmission also being possible once an individual is infected30, 31.
Serological diagnosis of Q fever is achieved using immunofluorescence assays (IFA), enzyme linked immunosorbent assays (ELISA) or complement fixation tests (CFT)32. Direct detection of C. burnetii can be achieved by polymerase chain reaction (PCR) or in vitro culture of the bacterium. Isolation of the Q fever pathogen is a reliable diagnostic method, but is difficult, time consuming and hazardous, also requiring access to BSL 3 facilities26. According to the World Animal Health Organization (OIE), CFT and ELISA have better sensitivity and specificity than IFA for Q fever detection33. The CFT is, however, time consuming, tedious and requires specific laboratory conditions, while ready-to-use ELISA kits are widely available and the preferred diagnostic test according to recommendations from the OIE (made in 2015). In veterinary medicine, indirect diagnostic methods such as ELISA are commonly used to detect antibodies against C. burnetii, and for that purpose different kits are available with different sensitivity and specificity ranges34–44. Such kits are not without potential flaws, however, and it must be borne in mind that cross-reactions with other pathogens, such as Bartonella quintana, Bartonella henselae, and Legionella micdadei, can influence test specificity44–48.
In Pakistan, the agricultural sector (including crops and livestock) employs 45% of the national workforce and produces 21% of the national Gross Domestic Production (GDP)49. Livestock farming constitutes 56% of the agriculture sector and 12% of the GDP, playing an important role in poverty alleviation and economic growth49. In rural areas, the majority of the population relies on livestock for their livelihood50, with Pakistan being the 4th largest milk-producing country in the world, and milk being known nationally as ‘White Gold’50. Almost 48 million cattle and 40 million buffalo are owned by rural families or smallholder farmers in Pakistan51, though the first case of Q fever in the country, in 1955, was reported in camels12. Across Pakistan, other diseases such as brucellosis also cause reproductive disorders in livestock52–54, making the diagnosis and differentiation of Coxiella challenging.
Coxiellosis is considered a neglected disease in both humans and animals in Pakistan. The majority of coxiellosis cases in the country are overlooked, due to either the lack of a proper diagnosis or misdiagnosis for other diseases with similar symptoms, for example brucellosis that can present with fever and abortion1. From 1955 to 2019, only two studies of coxiellosis (Q fever) were undertaken in large ruminants of Pakistan, and in both cases antigen was detected using CFT and ELISA55, 56. According to the limited available literature, the seroprevalence of Q fever in Pakistan ranges from 4.6 to 40% in all livestock species, and from 10.2 to 26.8% in humans55, 56. The rates of premature births and weak calves are particularly high in certain districts, including those selected for study in the current work57 which was designed to fully assess Q fever seroprevalence and associated factors in large ruminants on livestock farms in Punjab, Pakistan. In estimating the seroprevalence of coxiellosis in seven districts not previously targeted in preceding research, this study provides essential information for policy makers and concerned authorities to implement prevention and control strategies against coxiellosis in Pakistan.
Materials and methods
Ethical approval
This study obtained ethical approval from the Institutional Research Ethics Committee of the City University of Hong Kong with internal reference number A-0672. All procedures were performed in accordance with the relevant guidelines and regulations. This study was conducted in accordance with (ARRIVE) Animal Research: Reporting In Vivo Experiments.
Study population
Pakistan has five provinces with Punjab being the largest, containing 36 districts and the highest animal and human populations in the country. This study was conducted across various smallholder livestock farms from October 2020 to January 2021, surveying sites where large ruminants were present (n = 112) across the following seven districts of Punjab: Khushab, Vehari, Sheikhupura, Muzaffargarh, Kasur, Gujranwala and Bahawalnagar. Livestock farms and animals residing on them were selected randomly using the software Survey Toolbox (Ausvet, The Australian Biosecurity Cooperative Research Centre for Emerging Infectious Disease, Australia)58. Farms were chosen on the basis of operational convenience in all districts, where selected farms-maintained herds of between 5 and 60 animals. From 112 farms (16 from each district), 448 animals were sampled; 224 cattle and 224 buffaloes. Geographically, this province covers an area of 205,344 km2 and is located 31.1704° N and 72.7097° E in a semiarid lowland region. The average temperature ranges from a minimum of − 2 °C to a maximum of 46 °C, but can reach − 10 °C in winter and 50–52 °C in summer1. Livestock is the primary source of income in rural areas of Pakistan and, as previously noted, is a major contributor to the country’s economy. Punjab itself is a key production region for many species, being home to 24% of Pakistan’s sheep, 37% of its goats, 49% of its buffalo and 65% of the country’s cattle.
Sample size calculation
Coxiellosis seroprevalence in large ruminants in the project study areas was unknown prior to commencing work. As such, sample size was calculated using 95% confidence intervals (CI), with an expected seroprevalence of 50% and an absolute precision of 5%, as recommended by Thursfield59.
N indicates sample size, where 1.96 is the Z value for the selected CI (95%), P is the expected disease seroprevalence and L is the desired absolute precision. Using this approach, from all livestock farms, 384 serum samples were calculated as being required. A total of 112 farms from seven districts were visited, where for each district, sixteen farms were selected randomly, and on each four animals (two cattle and two buffalo) were randomly sampled. In this way, 448 serum samples were collected from study area (224 from cattle and 224 from buffalo). During collection of samples, data was also obtained from the farm owner using a pre-designed questionnaire.
Sample collection
For blood collection, four blood samples were collected per farm from four animals (two from cows, two from buffaloes), with preference given to sampling those animals which were infected with ticks. Approximately 8 to 10 ml of blood was collected from the jugular vein of each animal using disposable needles. Blood was collected in thrombin-containing vacutainers (Catalog No. VP20021S, BD Diagnostic, Oxford, UK) to easily separate serum from red blood cells. Samples were carefully labeled according to each district, farm, and animal. Blood samples were then stored at 4 °C and transported to the University of Veterinary and Animal Sciences, Lahore, Pakistan. The samples were centrifuged at 3000 rpm for 5–10 min for serum separation, with serum then extracted and added into 1.5 ml Eppendorf safe-lock tubes (Catalog No. 0030123328, Sigma-Aldrich, Missouri, United States). Sera samples, conserved at − 20 °C, were then shipped to the Department of Infectious Diseases and Public Health, City University of Hong Kong.
Tick collection
Alongside blood sample collection, 358 ticks (182 from 92 cows and 176 from 87 buffaloes) were collected from the same animals in the seven districts of Punjab, Pakistan. Tick collection was done during the winter season (from October to January) when tick infestation is rare and, consequently, low numbers of ticks were typically present on infested animals, dictating that two ticks per animal were collected to maintain uniformity. The ticks from each animal were placed into labeled Eppendorf tubes (3 ml) containing 70% ethyl alcohol as a preservative, prepared at the University of Veterinary and Animal Sciences, Lahore, Pakistan. Ticks were then shipped to City University of Hong Kong, following international regulations for transportation and after acquisition of a Hong Kong Department of Health import permit, where specimens were identified to species level under a stereomicroscope. Two complementary identification keys where used, these being a hard copy of Walker et al.60 and an online taxonomic key included in Multikey 2.161, 62, with reference also made to original descriptions and re-descriptions of relevant tick species.
Questionnaire data collection
Survey data was collected from each farm using a predesigned questionnaire, with this data subsequently used for odds ratio analysis. Each question was translated into the local language to avoid confusion and maximize accuracy and pretested on selected farmers before circulation. The questionnaire gathered metadata on the district and farm name, the total number of animals kept at the farm, common practices such as feeding methods employed (stallfed, grazed, mixed), acaricidal use and frequency of using these acaracides, abortion history, how many abortions occurred in the preceding year, presence of sheep and goats on the farm, and separate parturition area presence/absence. Details on the age, parity and reproductive status of the sampled animals were also recorded.
Serological test
ID Screen Q Fever Indirect Multi-species (ID vet, France) was used for the detection of antibodies of Q fever in all serum samples, as per manufacturer recommendations. The plates were read at 450 nm with an ELISA reader (SpectraMax iD3, San Jose, California, USA), also as per manufacturer recommendations, with these readngs automatically downloaded in an Excel file connected to the machine. The test was considered to be valid if: (i) the mean positive control optical density (ODPC) was greater than 0.350, and (ii) the ratio of the mean value of the positive control (PC) OD to the negative control OD (ODPC to ODNC) was greater than 3. Results were interpreted as detailed in Table 1.
Table 1.
Result interpretation of ELISA for Q fever.
| S/P% values* | Interpretation of result |
|---|---|
| S/P% ≤ 40 | Negative |
| 40 < S/P% ≤ 50 | Doubtful |
| 50 < S/P% ≤ 80 | Positive |
| S/P% 80 > 80 | Strong positive |
*The sample (S) to positive (P) was estimated using the following formula: S/P = ((OD Sample − ODNC)/(ODPC − ODNC) × 100).
Only ‘positive’ and ‘strong positive’ (Table 1) results were included for the calculation of seroprevalence.
Statistical analysis
From the seven selected districts of Punjab, 112 farms were sampled with herd sizes ranging between five and 60 animals. Descriptive statistical analysis was applied to determine the seroprevalence of herds for antibodies again C. burnetii. A herd was considered positive when a single animal was positive to the ELISA test.
All data collected through predesigned questionnaires was entered in an Excel file (Microsoft Excel 2016), which was then imported into open-source R software (version 3.2.3). Univariate analysis was conducted to study the association between coxiellosis seropositivity and thirteen independent variables (Table 4) included for odds ratio analysis. Of these thirteen variables, only seven variables were selected for initial multivariate analysis, using the selection criteria of p < 0.2 to check their contribution towards seropositivity. In the initial multivariate model, all variables with p > 0.05 were excluded sequentially and their effect on odds ratios and p-values of other predictors were noted. Finally, a multivariate model was developed with five variables that had proven to be significant predictors of Q fever seropositivity at p < 0.05.
Table 4.
Summary of the variables included in the univariable analysis to test for association with ELISA positive Q fever results.
| Variables | Categories | Positive/tested | Prevalence % (95% CI) | Odds ratio | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Keeping animals together | Yes | 51/216 | 23.61 (18.23–29.95) | 0.82 | 0.53–1.96 | 0.389 |
| No | 63/232 | 27.15 (21.64–33.44) | Ref | |||
| Acaricide use | Yes | 69/363 | 19.00 (15.17–23.50) | Ref | 2.91–7.93 | < 0.001* |
| No | 45/85 | 52.94 (41.86–63.74) | 4.79 | |||
| Tick presence | Yes | 70/179 | 39.10 (31.99–46.69) | 3.28 | 2.12–5.13 | < 0.001* |
| No | 44/269 | 16.35 (12.25–21.44) | Ref | |||
| Milk reduction during tick presence | Yes | 83/336 | 24.70 (20.25–29.73) | 0.85 | 0.53–1.40 | 0.531 |
| No | 31/112 | 27.67 (19.84–37.06) | Ref | |||
| Abortion occurred last year on the farm | Yes | 91/194 | 47.64 (39.76–54.17) | 8.87 | 5.39–15.10 | < 0.001* |
| No | 23/254 | 9.05 (5.94–13.44) | Ref | |||
| Quarantine facility | Yes | 41/152 | 26.97 (20.25–34.87) | Ref | 0.56–1.39 | 0.595 |
| No | 73/296 | 24.66 (19.94–30.05) | 0.886 | |||
| Type of floor | Sandy | 61/165 | 36.96 (29.69–44.86) | 2.54 | 1.60–3.90 | 2.540 |
| Concreted | 53/283 | 18.72 (14.45–23.87) | Ref | |||
| Presence of sheep and goats | Yes | 97/333 | 29.12 (24.36–34.38) | 2.36 | 1.37–4.29 | 0.002* |
| No | 17/115 | 14.78 (9.09–22.89) | Ref | |||
| Presence of breeding bull | Yes | 24/72 | 33.33 (22.92–45.53) | 1.58 | 0.91–2.71 | 0.095* |
| No | 90/376 | 23.93 (19.77–28.63) | Ref | |||
| General vaccination practices | Yes | 12/40 | 30.00 (17.08–46.71) | Ref | 0.38–1.64 | 0.489 |
| No | 102/408 | 25.00 (20.93–29.54) | 0.77 | |||
| Separate parturition area | Yes | 31/167 | 18.56 (13.13–25.47) | Ref | 1.16–2.96 | 0.010* |
| No | 83/281 | 29.53 (24.34–35.30) | 1.83 | |||
| Animal tested | Cow | 53/224 | 23.66 (18.36–29.87) | Ref | 0.54–1.27 | 0.386 |
| Buffalo | 61/224 | 27.23 (21.61–33.64) | 0.83 | |||
| Reproductive status of animal | Pregnant | 49/162 | 30.24 (23.41–38.03) | 1.47 | 0.95–2.27 | 0.080* |
| Non-pregnant | 65/286 | 22.72 (18.09–28.11) | Ref |
Ref reference category.
Significant values are in bold.
Results
Seroprevalence of coxiellosis
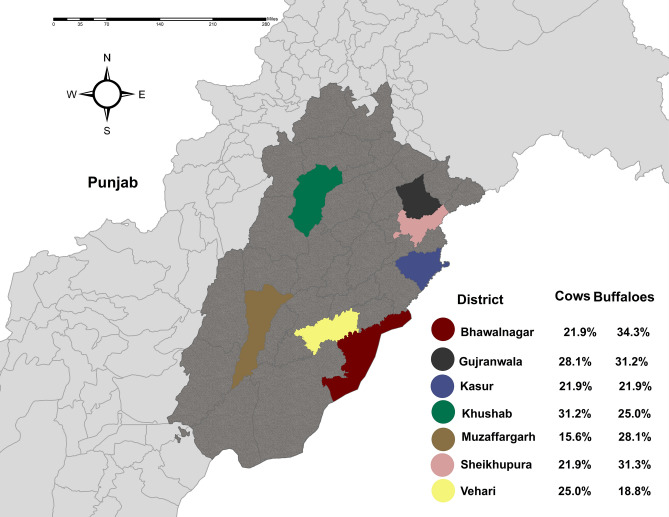
From the seven studied districts of Punjab, Pakistan, the farm level seroprevalence of coxiellosis (i.e. the percentage of farms with at least one positive case) was recorded as being 58.92% (66/112, 95% CI 49.22–68.01), with animal-based seroprevalence (i.e. the percentage of seropositive animals) being 25.44% (114/448, 95% CI 21.52–29.79). Coxiellosis in buffaloes was numerically higher at 27.23% (61/224, 95% CI 21.61–33.64) than in cattle at 23.66% (53/224, 95% CI 18.36–29.87), with the numerically highest prevalence (29.68%, 10/32, 95% CI 19.24–42.58) recorded in the district of Gujranwala, and the lowest prevalence (21.87%, 7/32, 95% CI 12.8–34.28) seen in the districts of Kasur, Muzaffargarh and Vehari. At the farm level, the numerically highest seroprevalence (81.25%, 13/16, 95% CI 53.69–95.02) was recorded in the district of Khushab and the lowest seroprevalence (31.25%, 5/16, 95% CI 12.13–58.51) in the district of Vehari (Fig. 1) (Tables 2, 3).
Figure 1.
Seroprevalence of Q fever in large ruminants in seven districts of Punjab, Pakistan.
Table 2.
Animal level seroprevalence of Q fever in large ruminants in Punjab, Pakistan.
| Districts | Positive/tested (cow) | Positive/tested (Buffalo) | Total positive/tested | Prevalence (%) | 95% CI |
|---|---|---|---|---|---|
| Kasur | 7/32 | 7/32 | 14/64 | 21.87 | 12.80–34.28 |
| Sheikhupura | 7/32 | 10/32 | 17/64 | 26.56 | 16.65–39.31 |
| Gujranwala | 9/32 | 10/32 | 19/64 | 29.68 | 19.24–42.58 |
| Muzaffargarh | 5/32 | 9/32 | 14/64 | 21.87 | 12.80–34.28 |
| Vehari | 8/32 | 6/32 | 14/64 | 21.87 | 12.80–34.28 |
| Khushab | 10/32 | 8/32 | 18/64 | 28.12 | 17.92–40.95 |
| Bahawalnagar | 7/32 | 11/32 | 18/64 | 28.12 | 17.92–40.95 |
| Total | 53/224 | 61/224 | 114/448 | 25.45 | 21.52–29.79 |
Table 3.
Farm level seroprevalence of Q fever in ruminant farms in Punjab, Pakistan.
| Districts | Positive/tested | Prevalence (%) | 95% CI | p-value |
|---|---|---|---|---|
| Kasur | 9/16 | 56.25 | 30.55–79.24 | 0.80 |
| Sheikhupura | 11/16 | 68.75 | 41.48–87.88 | 0.21 |
| Gujranwala | 10/16 | 62.50 | 35.87–83.71 | 0.45 |
| Muzaffargarh | 8/16 | 50.00 | 27.99–72.00 | 1.00 |
| Vehari | 5/16 | 31.25 | 12.13–58.51 | 0.21 |
| Khushab | 13/16 | 81.25 | 53.69–95.02 | 0.024 |
| Bahawalnagar | 10/16 | 62.50 | 35.87–83.71 | 0.45 |
| Total | 66/112 | 58.92 | 49.22–68.01 | 0.11 |
Odds ratio analysis
The univariate analysis conducted indicated significant associations between seropositivity of Q fever with no acaricide use (OR 4.79, CI 95% 2.91–7.93, p < 0.001) and tick presence (OR 3.28, CI 95% 2.12–5.13, p < 0.001). The presence of sheep and goats (OR 2.36, CI 95% 1.37–4.29, p = 0.002), abortion history from the preceding year at the farm (OR 8.87, CI 95% 5.39–15.10, p < 0.001), and absence of a separate parturition area (OR 1.83, CI 95% 1.16–2.96, p = 0.010) were also significantly associated with seropositivity of Q fever. Other factors, including keeping a mixed herd (OR 0.82, CI 95% 0.53–1.96, p = 0.389), milk reduction during tick presence (OR 0.85, CI 95% 0.53–1.40, p = 0.531), presence of a quarantine facility (OR 0.886 CI 95%: 0.56–1.39, p = 0.595) and sandy floor (OR 2.54, CI 95% 1.60–3.90, p = 2.540), vaccination practices (for Foot and Mouth Disease (FMD), hemorrhagic septicemia (HS), black quarter (BQ), brucellosis, theileriosis, and anthrax, as recommended by the Livestock Department of Punjab, Pakistan) (OR 0.77, CI 95% 0.38–1.64, p = 0.489), presence of a breeding bull (OR 1.58, CI 95% 0.91–2.71, p = 0.095) and reproductive status (OR 1.47, CI 95% 0.95–2.27, p = 0.080) were not significantly associated with Q fever seropositivity in univariate analysis (Table 4).
All seven of the variables having p-value less than 0.2 in univariate analysis were included in an initial multivariate model, where all variables with p > 0.05 were then excluded sequentially and their effect on odds ratios and p-values of other predictors noted. In the first step, for example, ‘presence of breeding bull’ was excluded, and the effect of this action on the odds ratio and p-values of the other factors noted, with this approach then repeated to next exclude ‘reproductive status of the animal’. The end result of this process was a final model containing five predictors that had proven to be significant predictors of Q fever seropositivity at p < 0.05, these being; no acaricide use (OR 5.61; 95% CI 2.97–10.94), presence of ticks (OR 3.23; 95% CI 1.87–5.69), abortion history in the preceding year at the farm (OR 14.96; 95% CI 8.09–29.34), presence of sheep and goats (OR 2.47; 95% CI 1.20–5.35), and absence of a separate parturition area (OR 3.17; 95% CI 1.76–5.86) (Table 5).
Table 5.
Final multivariate model including significant predictors of Q fever seropositivity.
| Predictors | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Acaricide use (no) | 5.61 | 2.97–10.94 | < 0.001 |
| Tick presence (yes) | 3.23 | 1.87–5.69 | < 0.001 |
| Abortion in the preceding year (yes) | 14.96 | 8.09–29.34 | < 0.001 |
| Presence of sheep and goats (yes) | 2.47 | 1.20–5.35 | 0.017 |
| Separate parturition area (no) | 3.17 | 1.76–5.86 | < 0.001 |
Tick identification
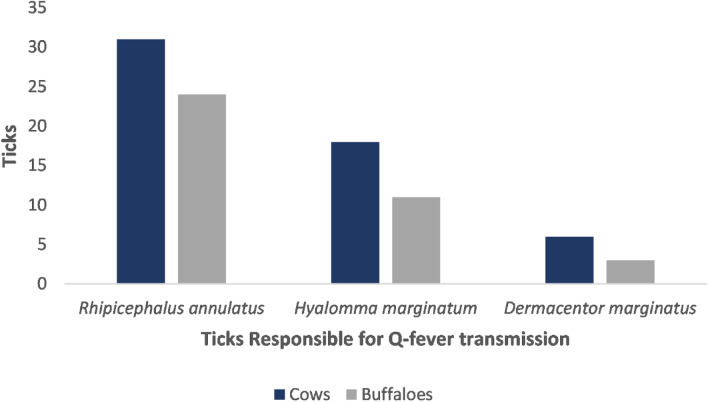
All tick samples were morphologically identified. Almost 20.7% (93/448) of animals included in the study were infested with one or more of Rhipicephalus annulatus, Dermacentor marginatus and Hyalomma marginatum, all of which have been reported as major vector of Q fever, and the presence of which supports that these ticks might carry this pathogen and be responsible for its transmission (Fig. 2). Almost 19.3% (86/448) of animals were infected with other tick species (Hyalomma scupense, and Hyalomma truncatum) which are not known to be responsible for Q fever transmission, but which may be associated with other tick-borne diseases (Table 6). Conversely, 60.0% (269/448) of animals were not infected with ticks at the time of sampling. A Chi-square test found a significant association (χ2 = 61.95, p < 0.001) between the presence of tick species previously reported as responsible for Q fever transmission (i.e. Rhipicephalus annulatus, Dermacentor marginatus and Hyalomma marginatum) and ELISA positive results for Q fever in animals (Table 7).
Figure 2.
Ticks responsible for Q fever.
Table 6.
Identified tick species from cattle and buffaloes.
Table 7.
Association of tick species with seroprevalence of Q fever with χ2 test.
| Variable | Positive | Negative | Odds ratio (95% CI) | p-value |
|---|---|---|---|---|
| Animals with ticks responsible for Q fever transmission | 53 | 40 | 5.38 (4.65–6.43) | < 0.001 |
| Animals with tick are not responsible for Q fever transmission | 17 | 69 | 0.19 (0.11–0.24) | |
| Animals with no ticks | 44 | 225 | 0.15 (0.9–0.18) |
Discussion
This is the first study to estimate the seroprevalence of Q fever in cattle and buffaloes in the selected seven districts of Punjab, Pakistan, revealing an overall seroprevalence of 25.45% (95% CI: 21.52–29.79) in the bovine population. In cattle, seroprevalence was lower (23.66%) than in buffaloes (27.23%), in agreement with earlier studies in Punjab, India, where respective seroprevalences of 23.2% and 24.1% were recorded72. On a global scale a slightly lower seroprevalence in cattle has been reported (20%)12, though with figures varying widely between countries and being higher than recorded in the current work in many cases (24% in Canada, 39% in the Netherlands, 40% in Germany, 46% in Japan and 82% in the USA)73–76. Lower seroprevalence among Pakistan’s cattle and buffaloes is suggested in a 2019 study by Rashid et al., with only 6.1% of animals testing positive for Q fever across 11 dairy farms77. This notably low figure probably resulted from this work only encompassing institutional holdings (managed by government authorities), where animal management would have been delivered by qualified veterinarians. This is in contrast to the current study, which surveyed non-institutional, commercial operations and small-holder farms to provide a better indication of seroprevalence across the sector as a whole. Nevertheless, that this variation in seroprevalence might also be due to sampling different geographical areas under varying environmental conditions78, 79 cannot be discounted. Variation in Q fever seroprevalence between bovines and additional animal species in Pakistan could also be expected based on the research elsewhere. Hussain et al., for example, detected anti-C. burnetii antibodies in 288 of 920 camels sampled in Pakistan (31.3%, 95% CI 28.3–34.4%)57, suggesting higher seroprevalence in this species than in the cattle/buffalo population sampled in the current study (25.45%, 95% CI 21.52–29.79). Work has also been done in this region to suggest that C. burnetii is widespread outside of its living hosts, being detectable by DNA analysis in 47 of 2,425 soil samples taken by Shabbir et al.1, though with soils testing positive at a notably lower rate than animals (i.e. 1.94%, 95% CI ± 0.55, versus the higher figures noted above for cattle, buffaloes and camels).
Ticks are considered major reservoirs of C. burnetii and are responsible for the transmission of coxiellosis to domestic and wild animals80, 81. The multiplication of C. burnetii in the mid-gut of infected ticks has been demonstrated, with the bacteria being present throughout the entire life of the tick and transovarial transmission to the next generation progeny being a possibility82. The findings of the current study support an association between ticks and coxiellosis, with the latter being three times more likely to occur where the former is found. Moreover, farms that did not implement the use of acaricides were five times more likely to be positive for coxiellosis according to our final multivariate model.
Various studies from numerous countries around the globe have found ticks to be positive for coxiellosis56, 83–86, with Duron et al. reporting that over 40 tick species can serve as vectors of Q fever81. In Pakistan, coxiellosis has been reported in tick pools collected from sheep and goats with seroprevalences of 31.0% and 7.7%, respectively23. We found that Rhipicephalus annulatus, Dermacentor marginatus and Hyalomma marginatum were the major tick species found on those farms that were seropositive for coxiellosis, with work elsewhere using molecular techniques to confirm that these species can vector coxiellosis63, 68, this being consistent with our results. According to Browne et al., acaricide use reduces tick population feeding on cattle87, which is also in accordance with our finding that a lack of acaricide use on the farm can increase the chances of Q fever.
The current study reported that a history of on-farm abortions in livestock poses 14× greater odds of seropositivity for Q fever. Similar results have been reported elsewhere, and can be explained by the link to reproductive disorders often seen with Q fever; for example, seropositivity of C. burnetii has been reported as significantly associated with reproductive disorders in livestock from France88, the Netherlands73, Hungary89, Germany90, and Japan91. According to a study conducted in Italy, the distribution of seropositivity in cows and abortion of calves was linked, with peak abortions observed in seasons of higher tick prevalence in the study area92.
The current study also demonstrated, in the final multivariate model, that farms having separate parturition areas were three times less likely to have coxiellosis. Another study conducted in Egypt reported that presence of abortion and parturition material can contribute significantly to transmission of Q fever93. Reproductive disorders, abortion, and parturition material should therefore be managed carefully to reduce the odds of coxiellosis spread, and to this end a separate parturition area on the farm can be recommended.
Presence of sheep and goats also presents a risk of coxiellosis according to the current study, where these animals can also harbor C. burnetii. A study conducted in Iran demonstrated a relatively high seroprevalence of Q fever in small ruminants with a history of abortion94. Nevertheless, the seroprevalence of coxiellosis in ovines can vary significantly between countries, with past research suggesting figures of 20% in France, 3.5% in the Netherlands, 8.7% in Germany and 56.9% in Bulgaria95. Caprine seroprevalence may similarly vary, having been reported as 7.8% in the Netherlands, 2.5% in Germany, and 40% in Bulgaria95. Such studies confirm that Q fever is commonly prevalent in small ruminants. In the case of Pakistan, recent research has reported 15.6% and 15% seroprevalence in sheep and goats respectively56, explaining links between the presence of these animals and the occurrence of coxiellosis in larger livestock in the current study.
Conclusions
This is the first study to explore the seroprevalence and associated risk factors of coxiellosis in large ruminants in the seven selected districts sampled in Punjab, Pakistan. This work provides baseline data and valuable insight into the major contributing factors that drive seropositivity of coxiellosis in large ruminants in this region. Based on these findings it can be recommended that abortions in herds should not be neglected, with proper screening undertaken to evaluate the cause. This, and other measures, should reduce the burden of coxiellosis, and other livestock diseases similarly associated with reproductive disorders. Tick management, achieved through acaricide use or other means, can also play a vital role in management, as can implementing hygienic measures that can reduce Q fever contamination and spread, and minimize movement of C. burnetii from small to large ruminants.
Supplementary Information
Acknowledgements
The authors are thankful to all authors whose articles are included in this study. They are thankful Md PEAR Hossain for their valuable contribution and help. They are also thankful to all those veterinarians who helped us with data collection. They appreciate those farmers who have shown willingness in data provision and sample collection.
Author contributions
S.H. conceived the study, gathered and entered data, performed the statistical analysis and drafted the initial version of the manuscript. S.H., O.S. and A.H. revised the initial manuscript. F.M.Y.H., U.A., J.Z., B.S., A.R., and O.S. provided intellectual inputs, O.S., J.L., A.R., D.G., and A.C.C. critically revised the manuscript to create the final version. All authors read and approved the final manuscript.
Funding
Olivier Sparagano is Principal Investigator of an internal research fund of the Department of Infectious Diseases and Public Health of the City University of Hong Kong (Project Number 9380108).
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sabir Hussain, Email: sahussain8-c@my.cityu.edu.hk.
Olivier Sparagano, Email: olivier.sparagano@cityu.edu.hk.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-21405-y.
References
- 1.Shabbir MZ, et al. Evidence of Coxiella burnetii in Punjab province, Pakistan. Acta Trop. 2016;163:61–69. doi: 10.1016/j.actatropica.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Million M, Raoult D. Recent advances in the study of Q fever epidemiology, diagnosis and management. J. Infect. 2015;71:S2–S9. doi: 10.1016/j.jinf.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Musso D, Broult J, Parola P, Raoult D, Fournier P-E. Absence of antibodies to Rickettsia spp., Bartonella spp., Ehrlichia spp. and Coxiella burnetii in Tahiti, French Polynesia. BMC Infect. Dis. 2014;14:1–4. doi: 10.1186/1471-2334-14-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan MM, Bertagna P. The geographical distribution of Q fever. Bull. World Health Organ. 1955;13:829. [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz R, et al. Outbreaks of abortions by Coxiella burnetii in small ruminant flocks and a longitudinal serological approach on archived bulk tank milk suggest Q fever emergence in Central Portugal. Transbound. Emerg. Dis. 2018;65:972–975. doi: 10.1111/tbed.12913. [DOI] [PubMed] [Google Scholar]
- 6.Cardinale E, Esnault O, Beral M, Naze F, Michault A. Emergence of Coxiella burnetii in ruminants on Reunion Island? Prevalence and risk factors. PLoS Negl. Trop. Dis. 2014;8:e3055. doi: 10.1371/journal.pntd.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellini C, et al. Q fever outbreak in the terraced vineyards of Lavaux, Switzerland. New Microbes New Infect. 2014;2:93–99. doi: 10.1002/nmi2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Santi VP, et al. Q fever epidemic in Cayenne, French Guiana, epidemiologically linked to three-toed sloth. Comp. Immunol. Microbiol. Infect. Dis. 2018;56:34–38. doi: 10.1016/j.cimid.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Kampschreur LM, et al. Chronic Q fever in the Netherlands 5 years after the start of the Q fever epidemic: Results from the Dutch chronic Q fever database. J. Clin. Microbiol. 2014;52:1637–1643. doi: 10.1128/JCM.03221-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoker M, Marmion B. The spread of Q fever from animals to man: The natural history of a rickettsial disease. Bull. World Health Organ. 1955;13:781. [PMC free article] [PubMed] [Google Scholar]
- 11.Guatteo R, Beaudeau F, Joly A, Seegers H. Coxiella burnetii shedding by dairy cows. Vet. Res. 2007;38:849–860. doi: 10.1051/vetres:2007038. [DOI] [PubMed] [Google Scholar]
- 12.Guatteo R, Seegers H, Taurel A-F, Joly A, Beaudeau F. Prevalence of Coxiella burnetii infection in domestic ruminants: A critical review. Vet. Microbiol. 2011;149:1–16. doi: 10.1016/j.vetmic.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Arricau-Bouvery N, Rodolakis A. Is Q fever an emerging or re-emerging zoonosis? Vet. Res. 2005;36:327–349. doi: 10.1051/vetres:2005010. [DOI] [PubMed] [Google Scholar]
- 14.Berri M, Crochet D, Santiago S, Rodolakis A. Spread of Coxiella burnetii infection in a flock of sheep after an episode of Q fever. Vet. Rec. 2005;157:737–740. doi: 10.1136/vr.157.23.737. [DOI] [PubMed] [Google Scholar]
- 15.Berri M, Rousset E, Champion J, Russo P, Rodolakis A. Goats may experience reproductive failures and shed Coxiella burnetii at two successive parturitions after a Q fever infection. Res. Vet. Sci. 2007;83:47–52. doi: 10.1016/j.rvsc.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Muskens J, Van Maanen C, Mars M. Dairy cows with metritis: Coxiella burnetii test results in uterine, blood and bulk milk samples. Vet. Microbiol. 2011;147:186–189. doi: 10.1016/j.vetmic.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 17.Agerholm JS. Coxiella burnetii associated reproductive disorders in domestic animals—A critical review. Acta Vet. Scand. 2013;55:1–11. doi: 10.1186/1751-0147-55-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz-Fons F, et al. Seroepidemiological study of Q fever in domestic ruminants in semi-extensive grazing systems. BMC Vet. Res. 2010;6:1–6. doi: 10.1186/1746-6148-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennerman E, Rousset E, Gölcü E, Dufour P. Seroprevalence of Q fever (coxiellosis) in sheep from the Southern Marmara Region, Turkey. Comp. Immunol. Microbiol. Infect. Dis. 2010;33:37–45. doi: 10.1016/j.cimid.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 20.García-Pérez A, et al. Investigation of Coxiella burnetii occurrence in dairy sheep flocks by bulk-tank milk analysis and antibody level determination. J. Dairy Sci. 2009;92:1581–1584. doi: 10.3168/jds.2008-1672. [DOI] [PubMed] [Google Scholar]
- 21.Heinzen RA, Hackstadt T, Samuel JE. Developmental biology of Coxiella burnetii. Trends Microbiol. 1999;7:149–154. doi: 10.1016/S0966-842X(99)01475-4. [DOI] [PubMed] [Google Scholar]
- 22.Berri M, Souriau A, Crosby M, Rodolakis A. Shedding of Coxiella burnetii in ewes in two pregnancies following an episode of Coxiella abortion in a sheep flock. Vet. Microbiol. 2002;85:55–60. doi: 10.1016/S0378-1135(01)00480-1. [DOI] [PubMed] [Google Scholar]
- 23.Ullah Q, Jamil H, Qureshi ZI, Saqib M, Neubauer H. Sero-epidemiology of Q fever (Coxiellosis) in small ruminants kept at government livestock farms of Punjab, Pakistan. Pak. J. Zool. 2019;51:140. [Google Scholar]
- 24.Bernard H, et al. High seroprevalence of Coxiella burnetii antibodies in veterinarians associated with cattle obstetrics, Bavaria, 2009. Vector-Borne Zoonotic Dis. 2012;12:552–557. doi: 10.1089/vbz.2011.0879. [DOI] [PubMed] [Google Scholar]
- 25.Groten T, et al. Who is at risk of occupational Q fever: New insights from a multi-profession cross-sectional study. BMJ Open. 2020;10:e030088. doi: 10.1136/bmjopen-2019-030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angelakis E, Raoult D. Q fever. Vet. Microbiol. 2010;140:297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Grist N. The persistence of Q-fever infection in a dairy herd. Vet. Rec. 1959;71:839–841. [Google Scholar]
- 28.Tissot-Dupont H, Amadei M-A, Nezri M, Raoult D. Wind in November, Q fever in December. Emerg. Infect. Dis. 2004;10:1264. doi: 10.3201/eid1007.030724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fishbein DB, Raoult D. A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am. J. Trop. Med. Hyg. 1992;47:35–40. doi: 10.4269/ajtmh.1992.47.35. [DOI] [PubMed] [Google Scholar]
- 30.Kruszewska D, Tylewska-Wierzbanowska S. Isolation of Coxiella burnetii from bull semen. Res. Vet. Sci. 1997;62:299–300. doi: 10.1016/S0034-5288(97)90210-1. [DOI] [PubMed] [Google Scholar]
- 31.Milazzo A, et al. Sexually transmitted Q fever. Clin. Infect. Dis. 2001;33:399–402. doi: 10.1086/321878. [DOI] [PubMed] [Google Scholar]
- 32.Mori M, Mertens K, Cutler SJ, Santos AS. Critical aspects for detection of Coxiella burnetii. Vector-Borne Zoonotic Dis. 2017;17:33–41. doi: 10.1089/vbz.2016.1958. [DOI] [PubMed] [Google Scholar]
- 33.Sidi-Boumedine K, et al. Development of harmonised schemes for the monitoring and reporting of Q-fever in animals in the European Union. EFSA Support. Publ. 2010;7:48E. [Google Scholar]
- 34.Paul S, Toft N, Agerholm JS, Christoffersen A-B, Agger JF. Bayesian estimation of sensitivity and specificity of Coxiella burnetii antibody ELISA tests in bovine blood and milk. Prev. Vet. Med. 2013;109:258–263. doi: 10.1016/j.prevetmed.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Horigan MW, Bell MM, Pollard TR, Sayers AR, Pritchard GC. Q fever diagnosis in domestic ruminants: Comparison between complement fixation and commercial enzyme-linked immunosorbent assays. J. Vet. Diagn. Investig. 2011;23:924–931. doi: 10.1177/1040638711416971. [DOI] [PubMed] [Google Scholar]
- 36.Hogerwerf L, et al. Test and cull of high risk Coxiella burnetii infected pregnant dairy goats is not feasible due to poor test performance. Vet. J. 2014;200:343–345. doi: 10.1016/j.tvjl.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Wood C, et al. Validation of an indirect immunofluorescence assay (IFA) for the detection of IgG antibodies against Coxiella burnetii in bovine serum. Prev. Vet. Med. 2019;169:104698. doi: 10.1016/j.prevetmed.2019.104698. [DOI] [PubMed] [Google Scholar]
- 38.Slaba K, Skultety L, Toman R. Efficiency of various serological techniques for diagnosing Coxiella burnetii infection. Acta Virol. 2005;49:123–127. [PubMed] [Google Scholar]
- 39.Kantsø B, Svendsen CB, Jørgensen CS, Krogfelt KA. Comparison of two commercially available ELISA antibody test kits for detection of human antibodies against Coxiella burnetii. Scand. J. Infect. Dis. 2012;44:489–494. doi: 10.3109/00365548.2012.664777. [DOI] [PubMed] [Google Scholar]
- 40.Meekelenkamp J, Schneeberger P, Wever P, Leenders A. Comparison of ELISA and indirect immunofluorescent antibody assay detecting Coxiella burnetii IgM phase II for the diagnosis of acute Q fever. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:1267–1270. doi: 10.1007/s10096-011-1438-0. [DOI] [PubMed] [Google Scholar]
- 41.Medić S, et al. Q fever outbreak in the village of Noćaj, Srem county, Vojvodina province, Serbia, January to February 2012. Eurosurveillance. 2012;17:20143. doi: 10.2807/ese.17.15.20143-en. [DOI] [PubMed] [Google Scholar]
- 42.Herremans T, et al. Comparison of the performance of IFA, CFA, and ELISA assays for the serodiagnosis of acute Q fever by quality assessment. Diagn. Microbiol. Infect. Dis. 2013;75:16–21. doi: 10.1016/j.diagmicrobio.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Stephen S, et al. Unreliability of three commercial Coxiella burnetii phase II IgM ELISA kits for the seroscreening of acute Q fever in human cases. Indian J. Med. Res. 2017;146:386. doi: 10.4103/ijmr.IJMR_1815_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La Scola B, Raoult D. Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J. Clin. Microbiol. 1996;34:2270–2274. doi: 10.1128/jcm.34.9.2270-2274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musso D, Raoult D. Serological cross-reactions between Coxiella burnetii and Legionella micdadei. Clin. Diagn. Lab. Immunol. 1997;4:208–212. doi: 10.1128/cdli.4.2.208-212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lukáčová M, Melničáková J, Kazar J. Cross-reactivity between Coxiella burnetii and chlamydiae. Folia Microbiol. 1999;44:579–584. doi: 10.1007/BF02816263. [DOI] [PubMed] [Google Scholar]
- 47.Graham JV, Baden L, Tsiodras S, Karchmer AW. Q fever endocarditis associated with extensive serological cross-reactivity. Clin. Infect. Dis. 2000;30:609–610. doi: 10.1086/313701. [DOI] [PubMed] [Google Scholar]
- 48.Edouard S, et al. Low antibodies titer and serological cross-reaction between Coxiella burnetii and Legionella pneumophila challenge the diagnosis of mediastinitis, an emerging Q fever clinical entity. Infection. 2017;45:911–915. doi: 10.1007/s15010-017-1048-6. [DOI] [PubMed] [Google Scholar]
- 49.Rehman A, Jingdong L, Chandio AA, Hussain I. Livestock production and population census in Pakistan: Determining their relationship with agricultural GDP using econometric analysis. Inf. Process. Agric. 2017;4:168–177. [Google Scholar]
- 50.Khan MJ, Abbas A, Naeem M, Ayaz MM, Akhter S. Current issues and future prospects of dairy sector in Pakistan. Sci. Tech. Dev. 2013;32:126–139. [Google Scholar]
- 51.Go P. Economic Survey of Pakistan. Economic Advisor's Wing Ministry of Finance; 2019. [Google Scholar]
- 52.Hussain S, et al. Knowledge, attitude, and practices associated with brucellosis among livestock owners and its public health impact in Punjab, Pakistan. Biologia. 2021;76:2921–2929. doi: 10.1007/s11756-021-00765-2. [DOI] [Google Scholar]
- 53.Yousaf R, et al. Seroprevalence and molecular detection of brucellosis in hospitalized patients in Lahore Hospitals, Pakistan. Infect. Dis. Rep. 2021;13:166–172. doi: 10.3390/idr13010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jamali MK, Yousaf A, Sarki I, Babar A, Sharna SN. Assessments of prevalence of brucellosis in camels through the contrast of serological assessments in South Punjab, Pakistan. Am. J. Zool. 2021;4:65–68. [Google Scholar]
- 55.Iqbal MZ, et al. Molecular identification of Coxiella burnetii, and incidence and risk factors of coxiellosis in bovines of Punjab, Pakistan. Pak. J. Zool. 2021;54:20210121170109. [Google Scholar]
- 56.Ullah Q, et al. Serological and molecular investigation of Coxiella burnetii in small ruminants and ticks in Punjab, Pakistan. Int. J. Environ. Res. Public Health. 2019;16:4271. doi: 10.3390/ijerph16214271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hussain S, et al. Seroprevalence and molecular evidence of Coxiella burnetii in dromedary camels of Pakistan. Front. Vet. Sci. 2022;9:908479. doi: 10.3389/fvets.2022.908479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cameron A. Survey Toolbox for Livestock Diseases, a Practical Manual and Software Package for Active Surveillance in Developing Countries, ACIAR Monograph 54. Australian Centre for International Agricultural Research; 1999. [Google Scholar]
- 59.Thrusfield M. Veterinary Epidemiology. Wiley; 2018. [Google Scholar]
- 60.Walker AR. Ticks of Domestic Animals in Africa: A Guide to Identification of Species. Bioscience Reports Edinburgh; 2003. [Google Scholar]
- 61.Walker A, Matthews J, Preston P. The development of electronic keys for the identification of ticks. Int. J. Trop. Insect Sci. 2005;25:2–5. doi: 10.1079/IJT200546. [DOI] [Google Scholar]
- 62.Rehman A, et al. Distribution of ticks infesting ruminants and risk factors associated with high tick prevalence in livestock farms in the semi-arid and arid agro-ecological zones of Pakistan. Parasit. Vectors. 2017;10:1–15. doi: 10.1186/s13071-017-2138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chisu V, Foxi C, Mannu R, Satta G, Masala G. A five-year survey of tick species and identification of tick-borne bacteria in Sardinia, Italy. Ticks Tick-Borne Dis. 2018;9:678–681. doi: 10.1016/j.ttbdis.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Mancini F, et al. Detection of tick-borne pathogens in ticks collected in the suburban area of Monte Romano, Lazio Region, Central Italy. Ann. dell'Istit. Superiore di Sanita. 2019;55:143–150. doi: 10.4415/ANN_19_02_06. [DOI] [PubMed] [Google Scholar]
- 65.Toma L, et al. Detection of microbial agents in ticks collected from migratory birds in central Italy. Vector-Borne Zoonotic Dis. 2014;14:199–205. doi: 10.1089/vbz.2013.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mancini F, et al. Prevalence of tick-borne pathogens in an urban park in Rome, Italy. Ann. Agric. Environ. Med. 2014;21:723. doi: 10.5604/12321966.1129922. [DOI] [PubMed] [Google Scholar]
- 67.Toledo A, et al. Detection of Coxiella burnetii in ticks collected from Central Spain. Vector-Borne Zoonotic Dis. 2009;9:465–468. doi: 10.1089/vbz.2008.0070. [DOI] [PubMed] [Google Scholar]
- 68.Špitalská E, Kocianová E. Detection of Coxiella burnetii in ticks collected in Slovakia and Hungary. Eur. J. Epidemiol. 2003;18:263–266. doi: 10.1023/A:1023330222657. [DOI] [PubMed] [Google Scholar]
- 69.Bonnet S, et al. Prevalence of tick-borne pathogens in adult Dermacentor spp. ticks from nine collection sites in France. Vector-borne Zoonotic Dis. 2013;13:226–236. doi: 10.1089/vbz.2011.0933. [DOI] [PubMed] [Google Scholar]
- 70.Varela-Castro L, et al. On the possible role of ticks in the eco-epidemiology of Coxiella burnetii in a Mediterranean ecosystem. Ticks Tick-Borne Dis. 2018;9:687–694. doi: 10.1016/j.ttbdis.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 71.Körner S, Makert GR, Ulbert S, Pfeffer M, Mertens-Scholz K. The prevalence of Coxiella burnetii in hard ticks in europe and their role in q fever transmission revisited—A systematic review. Front. Vet. Sci. 2021;8:655715. doi: 10.3389/fvets.2021.655715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sodhi S, Joshi D, Sharma D, Baxi K. Seroprevalence of brucellosis and Q fever in dairy animals. Zentralbl. Veterinarmed. B. 1980;27:683–685. doi: 10.1111/j.1439-0450.1980.tb01733.x. [DOI] [PubMed] [Google Scholar]
- 73.Houwers D, Richardus J. Infections with Coxiella burnetii in man and animals in The Netherlands. Med. Microbiol. Infect. Dis. Virol. Parasitol. 1987;267:30–36. doi: 10.1016/s0176-6724(87)80183-9. [DOI] [PubMed] [Google Scholar]
- 74.Htwe K. Seroepidemiology of Coxiella burnetii in domestic and companion animals in Japan. Vet. Rec. 1992;131:490. doi: 10.1136/vr.131.21.490. [DOI] [PubMed] [Google Scholar]
- 75.Řeháček J, et al. Studies of the prevalence of Coxiella burnetii, the agent of Q fever, in the foothills of the southern Bavarian Forest, Germany. Zentralblatt Bakteriol. 1993;278:132–138. doi: 10.1016/S0934-8840(11)80287-2. [DOI] [PubMed] [Google Scholar]
- 76.Hatchette T, Campbell N, Whitney H, Hudson R, Marrie TJ. Seroprevalence of Coxiella burnetii in selected populations of domestic ruminants in Newfoundland. Can. Vet. J. 2002;43:363. [PMC free article] [PubMed] [Google Scholar]
- 77.Rashid I, Saqib M, Ahmad T, Sajid MS. Sero-prevalence and associated risk factors of Q fever in cattle and buffaloes managed at institutional dairy farms. Pak. Vet. J. 2019;39:221. doi: 10.29261/pakvetj/2019.029. [DOI] [Google Scholar]
- 78.Paul S, Agger JF, Markussen B, Christoffersen A-B, Agerholm JS. Factors associated with Coxiella burnetii antibody positivity in Danish dairy cows. Prev. Vet. Med. 2012;107:57–64. doi: 10.1016/j.prevetmed.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 79.Rizzo F, et al. Q fever seroprevalence and risk factors in sheep and goats in northwest Italy. Prev. Vet. Med. 2016;130:10–17. doi: 10.1016/j.prevetmed.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 80.Norlander L. Q fever epidemiology and pathogenesis. Microbes Infect. 2000;2:417–424. doi: 10.1016/S1286-4579(00)00325-7. [DOI] [PubMed] [Google Scholar]
- 81.Duron O, Sidi-Boumedine K, Rousset E, Moutailler S, Jourdain E. The importance of ticks in Q fever transmission: What has (and has not) been demonstrated? Trends Parasitol. 2015;31:536–552. doi: 10.1016/j.pt.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 82.Hussain, S., Saqib, M. & Ashfaq, K. First molecular evidence of Coxiella burnetii in ticks collected from dromedary camels in Punjab, Pakistan (2021).
- 83.Cooper A, Stephens J, Ketheesan N, Govan B. Detection of Coxiella burnetii DNA in wildlife and ticks in northern Queensland, Australia. Vector-Borne Zoonotic Dis. 2013;13:12–16. doi: 10.1089/vbz.2011.0853. [DOI] [PubMed] [Google Scholar]
- 84.Woollen N, Daniels E, Yeary T, Leipold H, Phillips R. Chlamydial infection and perinatal mortality in a swine herd. J. Am. Vet. Med. Assoc. 1990;197:600–601. [PubMed] [Google Scholar]
- 85.Noda AA, Rodríguez I, Miranda J, Contreras V, Mattar S. First molecular evidence of Coxiella burnetii infecting ticks in Cuba. Ticks Tick-Borne Dis. 2016;7:68–70. doi: 10.1016/j.ttbdis.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 86.Śmieja M, Orzechowski J, Stolarski MS. TIE: An ability test of emotional intelligence. PLoS ONE. 2014;9:e103484. doi: 10.1371/journal.pone.0103484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Browne A, et al. Serosurvey of Coxiella burnetii (Q fever) in dromedary camels (Camelus dromedarius) in Laikipia County, Kenya. Zoonoses Public Health. 2017;64:543–549. doi: 10.1111/zph.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Durand, M. L'infection Bovine par L'agent de la Fievre q en 1977 (1978).
- 89.Rady M, Glavits R, Nagy G. Demonstration in Hungary of Q fever associated with abortions in cattle and sheep. Acta Vet. Hung. 1985;33:169. [PubMed] [Google Scholar]
- 90.Reusch C, Frost J, Lohrbach W, Wachendörfer G. Comparative studies of guinea pig and mouse tests for the detection of Coxiella burnetii–and a study of Q fever distribution in south and central Hessen. Dtsch. Tierarztl. Wochenschr. 1984;91:47–52. [PubMed] [Google Scholar]
- 91.To H, et al. Prevalence of Coxiella burnetii infection in dairy cattle with reproductive disorders. J. Vet. Med. Sci. 1998;60:859–861. doi: 10.1292/jvms.60.859. [DOI] [PubMed] [Google Scholar]
- 92.Cabassi CS, et al. Association between Coxiella burnetii seropositivity and abortion in dairy cattle of Northern Italy. Microbiol.-Q. J. Microbiol. Sci. 2006;29:211–214. [PubMed] [Google Scholar]
- 93.Rodolakis A. Q fever in dairy animals. Ann. N. Y. Acad. Sci. 2009;1166:90–93. doi: 10.1111/j.1749-6632.2009.04511.x. [DOI] [PubMed] [Google Scholar]
- 94.Asadi J, Kafi M, Khalili M. Seroprevalence of Q fever in sheep and goat flocks with a history of abortion in Iran between 2011 and 2012. Vet. Ital. 2013;49:163–168. [PubMed] [Google Scholar]
- 95.Georgiev M, et al. Q fever in humans and farm animals in four European countries, 1982 to 2010. Eurosurveillance. 2013;18:20407. doi: 10.2807/ese.18.08.20407-en. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).