Abstract
The increasing demand for new bioactive compounds to combat the evolution of multi-drug resistance (MDR) requires research on microorganisms in different environments in order to identify new potent molecules. In this study, initial screening regarding the antimicrobial activity of 44 Actinomycetes isolates isolated from three soil samples from three different extremely cold sites in Morocco was carried out. Primary and secondary screening were performed against Candida albicans ATCC 60,193, Escherichia coli ATCC 25,922, Staphylococcus aureus ATCC 25,923, Bacillus cereus ATCC 14,579, other clinical MDR bacteria, and thirteen phytopathogenic fungi. Based on the results obtained, 11 active isolates were selected for further study. The 11microbial isolates were identified based on morphological and biochemical characters and their molecular identification was performed using 16S rRNA sequence homology. The UV–visible analysis of dichloromethane extracts of the five Streptomyces sp. Strains that showed high antimicrobial and antioxidant (ABTS 35.8% and DPPH 25.6%) activities revealed the absence of polyene molecules. GC–MS analysis of the dichloromethane extract of E23-4 as the most active strain revealed the presence of 21 volatile compounds including Pyrrolopyrazine (98%) and Benzeneacetic acid (90%). In conclusion, we studied the isolation of new Streptomyces strains to produce new compounds with antimicrobial and antioxidant activities in a cold and microbiologically unexplored region of Morocco. Furthermore, this study has demonstrated a significant (P < 0.0001) positive correlation between total phenolic and flavonoid contents and antioxidant capacity, paving the way for the further characterization of these Streptomyces sp. isolates for their optimal use for anticancer, antioxidant, and antimicrobial purposes.
Subject terms: Biochemistry, Immunology, Microbiology
Introduction
Emergent multi-drug resistant (MDR) bacteria including the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter) represent a global threat to human health and may lead to severe infections that are difficult to treat, resulting in prolonged illness and over times, increased risk of death1. Imbalance between the synthesis and accumulation of reactive oxygen species (ROS) in cells and tissues has been shown to cause oxidative stresses that can lead to cancer, cardiovascular, kidney, and neurological diseases2. In this context, many previous studies have been oriented towards the search for microorganisms in different environments in order to identify new potent molecules with a wide spectrum of activities3. Recently, natural molecules of microbial origin have become the main sources for the production of constantly used antibiotics4,5. In addition, it has been estimated that 70% of secondary metabolites are derived from microbial sources3,6. Soil contains many ecological niches, and their microbes produce various bio-molecules that are biologically active against a wide range of pathogens7. Also, more than 95% of the Actinomycetes strains were isolated from soil and Streptomyces sp. represent the dominant genus8. Streptomyces belonging to the Actinomyceaceae family and are recognized as potential producers of secondary metabolites, including antibiotic, anti-cancer, antioxidant, antimicrobial, and immunosuppressant agents9. Streptomyces are Gram-positive, filamentous, spore-forming bacteria that have a relatively large genomic (DNA) sequence with a high guanine and cytosine (G + C) content1,10. Streptomyces comprise a very important bacterial genus that contains over 800 species11 which are recognized worldwide as an inexhaustible source of antibiotics used in human therapy3,12,13. Antibiotics derived from the Streptomyces genus play a crucial role in the medical sector. In addition, it has been highlighted that this genus produces around 50% of therapeutically useful antibiotics14. It has also been shown that each Streptomyces strain has the capacity to produce more than 30 secondary metaboliteson average15.
The majority of Streptomyces are aerobic. However, when conditions are limited, Streptomyces produce aerial hyphae that can divide to produce spores which can withstand unfavorable conditions and are easily transferred to other environments and nutrient sources1. During this phase, Streptomyces produce some highly versatile natural products (NPs) that are not required for growth or/and reproduction, but help protect their host against pathogens1.
To adapt and survive in extremely cold or cryogenic environments, some microorganisms including Streptomyces can produce secondary metabolites of great interest for biotechnological applications16. Considering this, we oriented our research towards new bio-active molecules with a wide spectrum of activities extracted from Streptomyces sp. isolated in extremely cold environments. To the best of our knowledge, there is no information available regarding the biological and antimicrobial activities of Streptomyces strains isolated from extremely cold environments in Morocco. Therefore, the objectives of the present study were to: (1) isolate Streptomyces species from the soils of three different cold sites in the Fez-Meknes region, Morocco (Sebt Jahjouh El Hajeb, Ain Vittel Ifrane forest, and Azrou forest); (2) conduct a primary screening of Streptomyces isolates for their antimicrobial activity; (3) perform a morphological, biochemical, and physiological characterization, and molecular identification by 16 s rRNA gene sequencing of the 11isolates that showed the highest antimicrobial activity (non-pathogenic bacteria and fungi); (4) determine the antimicrobial activity of their organic extracts against some MDR pathogenic bacteria and phytopathogenic fungi; (5) determine the total phenolic and flavonoid compounds in the organic extracts of the five isolates showing the highest antimicrobial activity (pathogenic bacteria and fungi), and their antioxidant activity in vitro; (6) evaluate the toxicity of their organic extracts by UV–visible; (7) determine the volatile compound profile of the most active isolate (high antimicrobial and antioxidant activities) by gas chromatography-mass spectrometry (GC–MS) analysis.
Results
Physico-chemical analysis of soil samples
The analysis of the soil of site A showed that it is an alkaline (pH = 8.3), not salty (EC = 0.20 ds/m) soil. The texture diagram allowed us to classify it as a sandy-loam soil which is poor in clay (19.97%), has a normal amount of total nitrogen (0.15%), and is moderately rich in organic matter (3.48%). In addition, our findings revealed the presence of exchangeable cations such as K, Mg, Al, Ca, and Si. Mineral elements including O, Fe, and Si were present in the highest concentrations followed by Al, Ca, K, and Mg (Table 1 and Supplementary Fig. S1a). The soil at site B is an alkaline, sandy (90.01%), not salty soil. It is relatively clay- and silt-poor and has a normal content of total nitrogen. It is rich in organic matter and poor in mineral elements except for O, Ca, and Si which were present in high concentrations (Table 1 and Supplementary Fig. S1b). The soil of site C has a slightly alkaline pH, is not salty, and has a slightly elevated total nitrogen contents. It is rich in organic matter (7.61%) and moderately rich in mineral elements (K, Mg, Si, etc.); however, O, Si, and Fe were detected in high concentrations (Table 1 and Supplementary Fig. S1c). The data of the soil pH values in the three sites showed some differences among the different soil textures. The lowest value (pH = 7.29) was recorded in the sandy-silty texture (site A), while the highest (pH = 8.57) was recorded in the sandy texture. The highest values of organic carbon contents were recorded for sites with a sandy-silty texture (sites B and C).
Table 1.
Physico-chemical analysis of soil samples.
| Physico-chemical parameters | Site A | Site B | Site C |
|---|---|---|---|
| Textural soil types | Sandy-silty | Sandy | Sandy-silty |
| Clay (%) | 19.97 | 5.00 | 9.99 |
| Sandy (%) | 50.08 | 90.01 | 55.04 |
| Silt (%) | 29.95 | 4.99 | 34.97 |
| pH | 8.30 | 8.57 | 7.29 |
| EC (ds/m) | 0.20 | 0.30 | 0.15 |
| OM (%) | 3.48 | 5.65 | 7.61 |
| TN (%) | 0.15 | 0.24 | 0.33 |
| Mg (%) | 1.09 | 0.31 | 1.59 |
| Al (%) | 5.91 | 1.08 | 6.70 |
| K (%) | 1,59 | 2.79 | 1.39 |
| Ca (%) | 2,83 | 23.00 | 0.74 |
| S (%) | 0.11 | 0.03 | 0.01 |
| Cl (%) | 0.15 | 0.04 | – |
| P (%) | 0.31 | 0.00 | 0.36 |
| Fe (%) | 14.80 | 1.63 | 12.58 |
| Mn (%) | 0.13 | 0.04 | 0.20 |
| Cu (%) | 0.03 | – | 0.01 |
| Zn (%) | 0.01 | 0.01 | 0.03 |
Site A (Sebt Jahjouh El Hajeb); Site B (Forest of Ain Vittel Ifrane); Site C (Forest of Azrou);
(–) not detectable, EC electrical conductivity, OM organic matter, TN total nitrogen.
Isolation of Actinomycetes strains
A total of 44 phenotypically different presumptive Actinomycetes strains were isolated from soil samples collected in the three studied sites, including 11 strains (25%) from site A, 14 strains (31.82%) from site B, and 19 strains (43.18%) from site C (Table 2). The maximum number of isolates was recorded in the M2 medium (n = 20) followed by BEN (n = 11), GLM (n = 8), and GA (n = 4) (Table 2).
Table 2.
Total number of Streptomyces isolates obtained from the three studied sites (A, B and C) according to the culture media used.
| Collection site of soil samples | Number of Actinomycetes colonies in different isolation media (× 103 CFU/mL) | Total number of Streptomyces colonies (× 103CFU/mL) | Number of colonies with distinct morphological characteristics in each medium | Total number of isolates | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M2 | Ben | GLM | GA | M2 | Ben | GLM | GA | |||
| Site A | 80 | 9 | 16 | 12 | 117 | 4 | 2 | 4 | 1 | 11 |
| Site B | 60 | 26 | 14 | 4 | 104 | 7 | 4 | 2 | 1 | 14 |
| Site C | 45 | 12 | 14 | 14 | 85 | 9 | 4 | 3 | 2 | 18 |
| Total | 185 | 47 | 44 | 30 | 306 | 20 | 11 | 9 | 4 | 43 |
Site A (SebtJ ahjouh El Hajeb); Site B (Forest of Ain Vittel Ifrane); and Site C (Forest ofAzrou).
Screening of active Actinomycetes isolates
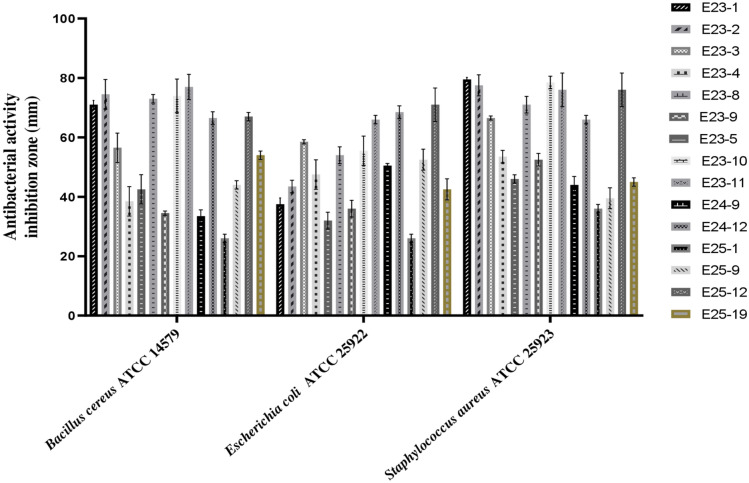
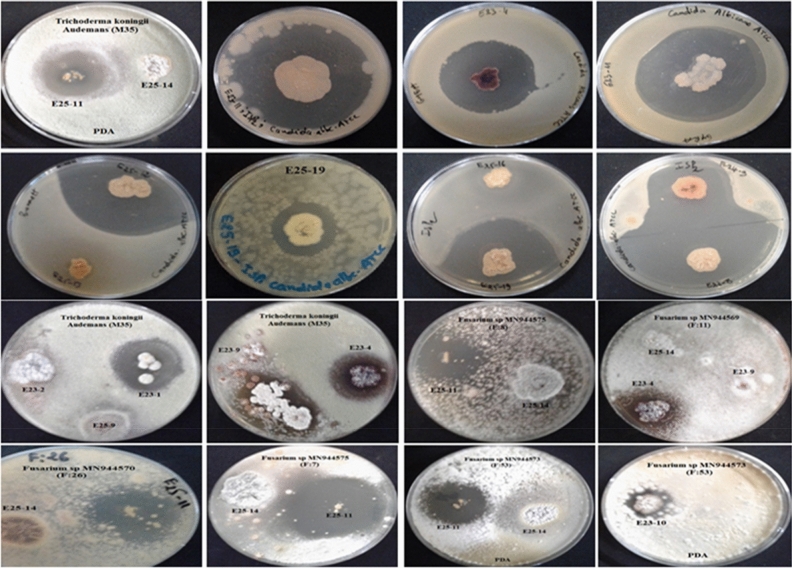
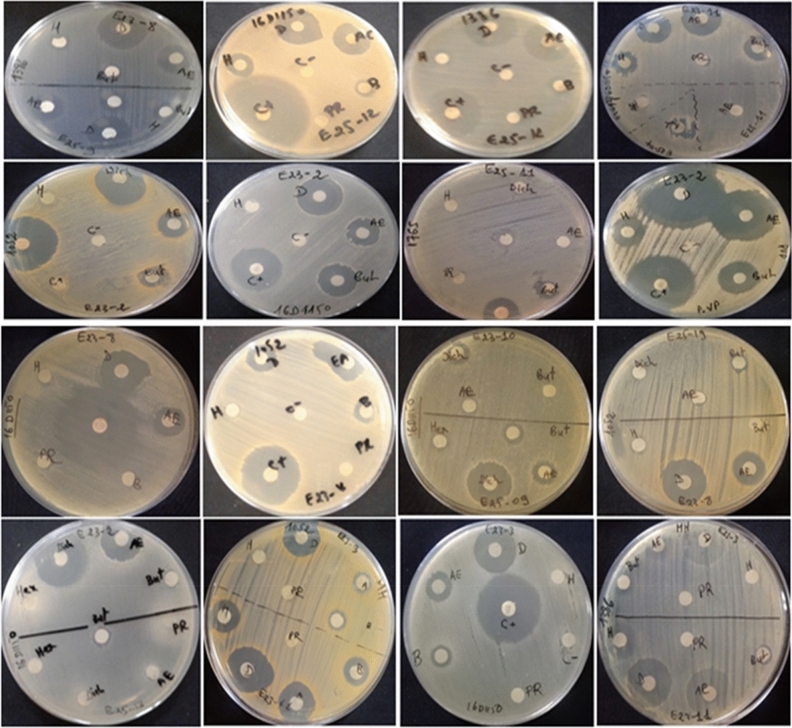
Among the 44 Actinomycetes isolates tested, only 16 isolates (37.21%) exhibited moderate to strong activity against Gram-positive bacteria (Bacillus cereusATCC 14,579, Staphylococcus aureus ATCC 25,923), Gram-negative bacteria (Escherichia coli ATCC 25,922) (Figs. 1 and 2), and all the fungal strains tested (Figs. 3, 4, and 5). These 16 bioactive isolates were subjected to secondary screening against some MDR pathogenic bacteria (Table 3, Fig. 6), as well as phyto-pathogenic fungi (Table 4, Fig. 7).The results of the primary and secondary screening showed that most of the isolates tested were more active against Gram-positive bacteria than Gram-negative bacteria (Figs. 1, 2 and 6, Table 3).
Figure 1.
Primary screening (antibacterial activity) using the double layer method on ISP2 medium.
Figure 2.
Antibacerial activity of pure Actinomycetes isolates by the double layer method on ISP2 medium.
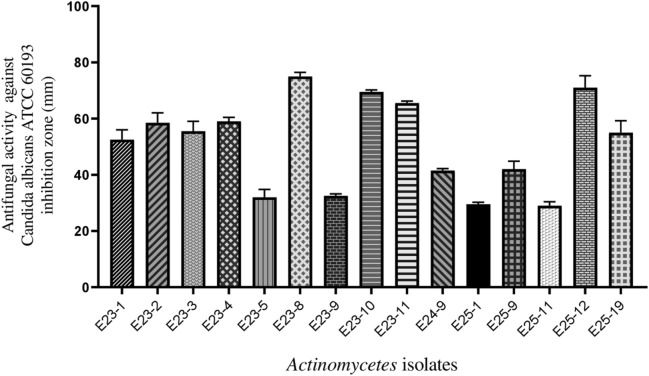
Figure 3.
Primary screening (antifungal activity) by the double layer method on ISP2 medium against Candida albicans ATCC 60,193.
Figure 4.
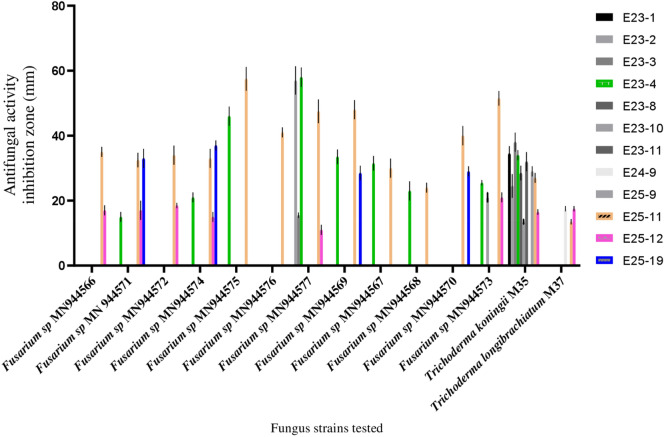
Primary screening (antifungal activity) by the double layer method on ISP2 medium against phytopathogenic fungi.
Figure 5.
Antifungal activity of Actinomycetes pure isolates by the double layer method on ISP2 medium.
Table 3.
Secondary screening (antibacterial activity) by disc diffusion method.
| Test strains | Dichloromethane extracts of bioactive isolates (inhibition zones in mm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E23-2 | E23-3 | E23-4 | E23-8 | E23-9 | E23-10 | E23-11 | E24-9 | E25-9 | E25-11 | E25-12 | PC | |
| Clinical Escherichia coli16D1150 | 22.5 ± 0.71 | 18 ± 2.12 | 22 ± 2.83 | 15.5 ± 0.71 | 21.5 ± 2.12 | 11.5 ± 0.71 | 15 ± 1.41 | 10.5 ± 0.72 | 19.5 ± 0.70 | 7 ± 0.0 | 23.5 ± 2.1 | 27 ± 1.41 |
| Clinical Proteus vulgaris 16C1737 | 25.5 ± 0.71 | – | 28 ± 1.41 | 22 ± 1.41 | 19 ± 1.41 | 22.5 ± 0.71 | 26.5 ± 2.12 | 17.5 ± 3.54 | 36.5 ± 2.12 | 14.5 ± 0.71 | 35.5 ± 2.12 | 35.5 ± 0.71 |
| Clinical Neisseria gonorhae16D1170 | – | – | – | 8 ± 0 | 8 ± 1.41 | – | – | – | 7.5 ± 0.71 | – | 7.5 ± 0.71 | 25.5 ± 2.12 |
| Escherichia coli ATCC 25,922 | 25.5 ± 0.71 | – | 25 ± 1.41 | 10 ± 0.0 | 13.5 ± 2.12 | 19 ± 1.41 | 20.5 ± 0.71 | 12.5 ± 0.71 | 21.5 ± 0.71 | 18 ± 1.41 | – | 25.5 ± 0.71 |
| Clinical Saphylococcus aureus 18k1052 | 22.5 ± 0.71 | 17.5 ± 0.7 | 19 ± 1.41 | 14.5 ± 0.71 | 8.5 ± 0.71 | 11 ± 2.83 | 18 ± 1.41 | 10.5 ± 0.72 | 16.5 ± 0.71 | – | 23 ± 2.83 | 24 ± 1.41 |
| Clinical Enterococcus faecalis18k1386 | 25 ± 1.41 | 18.5 ± 2.12 | 20.5 ± 0.71 | 14.5 ± 0.71 | 8.5 ± 0.71 | 10 ± 1.41 | 19 ± 1.41 | 7 ± 0.00 | 19.5 ± 0.71 | – | 25 ± 1.41 | 11 ± 1.41 |
| Staphylococcus aureus ATCC 25,923 | 24.5 ± 3.53 | – | 21.5 ± 0.71 | 13.5 ± 0.71 | 10.5 ± 0.71 | 16.5 ± 2.12 | 19.5 ± 0.71 | 13 ± 1.41 | 22.5 ± 0.71 | 17.5 ± 0.71 | 17.5 ± 0.71 | 27.5 ± 2.12 |
| Bacillus cereus ATCC 14,579 | 24 ± 1.41 | – | 23 ± 0.0 | 14.5 ± 0.71 | 10.5 ± 0.71 | 14.5 ± 0.71 | 21.5 ± 0.10 | 14 ± 0.0 | 22 ± 2.82 | 19.5 ± 4.95 | 19 ± 1.41 | 28 ± 4.24 |
(–) no inhibition zone, PC positive control (Streptomycine) and disc diameter = 6 mm.
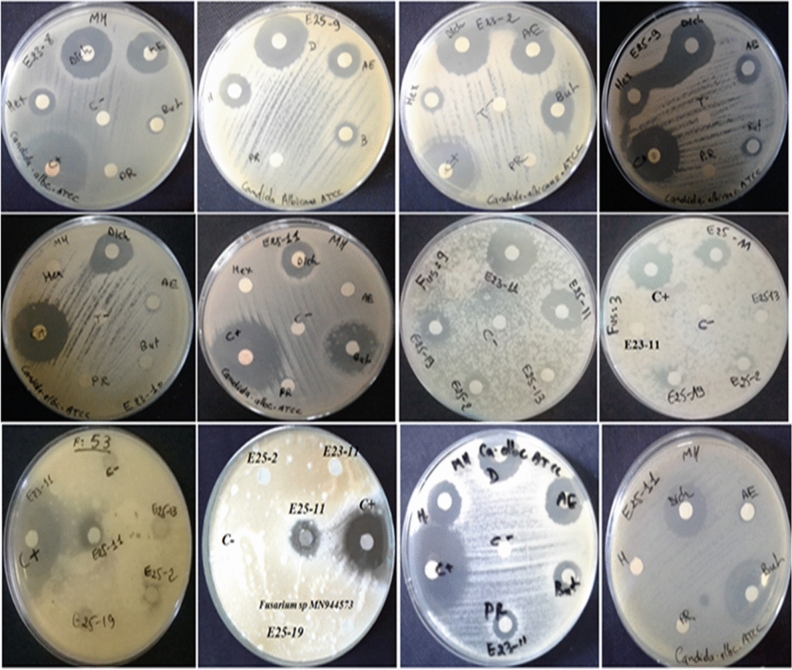
Figure 6.
Secondary screening (antibacterial activity) by the disc diffusion method on MH medium.
Table 4.
Secondary screening (antifungal activity) by disc diffusion method.
| Test strains | Dichloromethane extracts of active isolates (inhibition zone in mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E23-2 | E23-3 | E23-4 | E23-8 | E23-10 | E23-11 | E25-9 | E25-11 | E25-12 | PC | |
| Candida albicans ATTC 60,193 | 22.5 ± 0.71 | – | 21.5 ± 0.71 | 125 ± 0,71 | 17.5 ± 2.12 | 19.5 ± 0.71 | 22 ± 1.41 | 16.5 ± 2.12 | 20.5 ± 0.71 | 27 ± 2.83 |
| Fusarium sp. MN94457 | 18.5 ± 2.12 | 22 ± 2.82 | – | 13.5 ± 2.12 | – | 22 ± 1.41 | – | 19 ± 1.41 | – | 31.5 ± 0.71 |
| Fusarium sp. MN944571 | – | – | – | – | – | 20.5 ± 0.71 | – | 14.5 ± 0.71 | – | 19.5 ± 0.71 |
| Fusarium sp. MN944567 | 11 ± 1.41 | – | – | – | – | – | – | – | – | 28.5 ± 2.12 |
| Fusarium sp. MN944568 | – | – | 16 ± 2.82 | – | – | – | – | – | – | 27 ± 1.41 |
| Fusarium sp. MN944569 | – | 10.5 ± 0.71 | – | – | – | 14.5 ± 0.71 | – | 9.5 ± 0.71 | – | 27.5 ± 0.71 |
| Fusarium sp. MN944570 | – | 9.5 ± 0.71 | – | – | – | 19.5 ± 2.12 | – | – | – | 26.5 ± 0.71 |
| Trichoderma koningii (M35) | – | – | – | – | – | 21 ± 1.41 | – | 16.5 ± 2.12 | – | 16.5 ± 2.12 |
(–) no inhibition zone, PC positive control (Cycloheximide) and disc diameter = 6 mm.
Figure 7.
Secondary screening (antifungal activity) by the disc diffusion method on MH medium.
Production kinetics of secondary metabolites
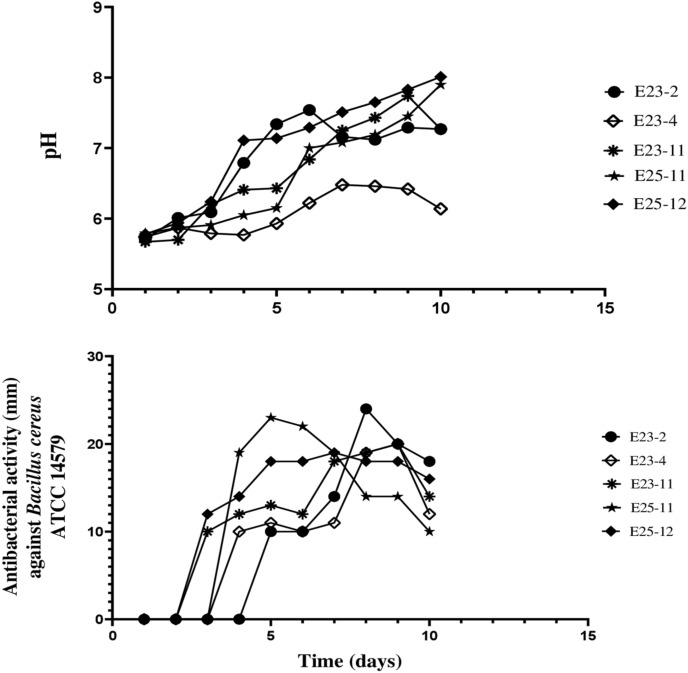
The antibiotic production kinetics and pH evolution performed by the well diffusion method on the ISP2 liquid medium are shown in Fig. 8 and Supplementary Fig. S2. It was noticed that the antibacterial activities started two days after incubation for the E25-12 and E23-11 strains, three days after incubation for the E25-11 and E23-4 strains, and after four days for the E23-2 strain. During the incubation period, the kinetics of pH evolution showed a slight variation between 5.67 and 8.01.
Figure 8.
Kinetics of pH evolution and production of antibiotics against Bacillus cereus ATCC 14,579.
Cultural, micro-morphological, biochemical and physiological characteristics of isolates
Among the 16 bioactive isolates, 11 showed strong antimicrobial activity and were selected for taxonomic, physiological, and biochemical studies. The results are presented in Table 5 (as well as in Supplementary Figs. S3 and S4, and Supplementary Table S1). Actinomycetes isolates grew very well on all tested media (ISP1, ISP2, ISP4, ISP5 and GYEA). Melanin pigment production, characterized by a dark brown coloration, was observed on the ISP9 base medium complemented by carbonaceous substrates (glucose, melezitose, manitol, trehalose, cellobiose, sucrose, raffinose, xylose, melibiose, mannose, fructose, galactose, maltose, and ribose).
Table 5.
Cultural, micro-morphological, biochemical and physiological characteristics of Actinomycetes isolates.
| Characteristics | E23-2 | E23-3 | E23-4 | E23-8 | E23-9 | E23-10 | E23-11 | E24-9 | E25-9 | E25-11 | E25-12 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assimilation | |||||||||||
| Ribose | − | + | + | − | − | − | − | 2 + | + | − | + |
| Melezitose | + | 3 + | − | − | − | − | + | − | − | − | − |
| Manitol | 3 + | 3 + | 3 + | 3 + | − | 2 + | 3 + | − | 3 + | 3 + | 3 + |
| Trehalose | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + |
| Cellobiose | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + |
| Sucrose | 3 + | 3 + | 3 + | 3 + | 3 + | 2 + | 3 + | 3 + | 3 + | − | 3 + |
| Raffinose | 3 + | 3 + | 3 + | 3 + | 3 + | 2 + | 3 + | − | 3 + | 3 + | 3 + |
| Xylose | 3 + | 3 + | + | 3 + | + | 3 + | 2 + | 3 + | 3 + | 3 + | 3 + |
| Melibiose | 3 + | 3 + | 3 + | 3 + | − | + | 3 + | − | 3 + | 3 + | 3 + |
| Mannose | 3 + | 3 + | 3 + | 3 + | 3 + | 2 + | 3 + | 3 + | 3 + | 3 + | 3 + |
| Fructose | 3 + | 2 + | 3 + | 3 + | 3 + | 2 + | 3 + | + | 3 + | 3 + | 3 + |
| Galactose | 3 + | 3 + | 3 + | + | 3 + | 3 + | 3 + | + | 3 + | 3 + | 3 + |
| Maltose | 3 + | 2 + | 2 + | 2 + | 2 + | + | 3 + | 3 + | 2 + | 3 + | 3 + |
| Glucose | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + |
| Growth on | |||||||||||
| ISP1 | 3 + | 3 + | 2 + | 3 + | 3 + | 2 + | 2 + | 3 + | 2 + | 3 + | 3 + |
| ISP2 | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 2 + | 3 + | 3 + | 3 + | 3 + |
| ISP4 | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 2 + | 3 + | 2 + | 3 + | 3 + |
| ISP5 | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 2 + | 3 + | 3 + | 3 + | 3 + |
| ISP7 | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | + | 3 + | 2 + | 3 + | 3 + |
| GYEA | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 2 + | 3 + | 3 + |
| pH tolerance | |||||||||||
| 4,63 | − | − | − | − | − | − | − | − | − | − | − |
| 5,33 | 2 + | + | + | + | + | 2 + | + | + | + | 2 + | + |
| 6,41 | 2 + | 3 + | 3 + | 3 + | 2 + | 2 + | 2 + | + | 2 + | 3 + | 2 + |
| 7,31 | 3 + | 3 + | 2 + | 3 + | 3 + | 3 + | 2 + | 2 + | 3 + | 3 + | 3 + |
| 8,28 | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + |
| 9,27 | 3 + | 2 + | 2 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 2 + | 3 + |
| 10,03 | 2 + | 2 + | + | 3 + | + | 2 + | 3 + | 3 + | + | 2 + | 3 + |
| NaCl tolerance | |||||||||||
| 1% | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + |
| 2% | 3 + | 3 + | 3 + | 3 + | 2 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + |
| 3% | 3 + | 3 + | 2 + | 3 + | + | 3 + | 3 + | 2 + | 3 + | 3 + | 3 + |
| 4% | 3 + | 3 + | 2 + | 2 + | + | 3 + | 2 + | + | 3 + | 3 + | 2 + |
| 5% | 2 + | 3 + | + | + | + | 2 + | 2 + | + | 2 + | 3 + | 2 + |
| 7% | 2 + | + | − | + | − | 3 + | + | − | − | − | + |
| 10% | − | − | − | − | − | − | − | − | − | − | − |
| Growth on | |||||||||||
| 4 °C | − | − | − | − | − | − | − | − | − | − | − |
| 28 °C | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + | 3 + |
| 37 °C | 3 + | 3 + | + | 2 + | 2 + | 2 + | 2 + | 3 + | + | 3 + | 2 + |
| 46 °C | − | − | − | − | − | − | − | − | − | − | − |
(−) no growth; (+) low growth; (2+) intermediate growth; (3) good growth.
Hierarchical cluster analysis
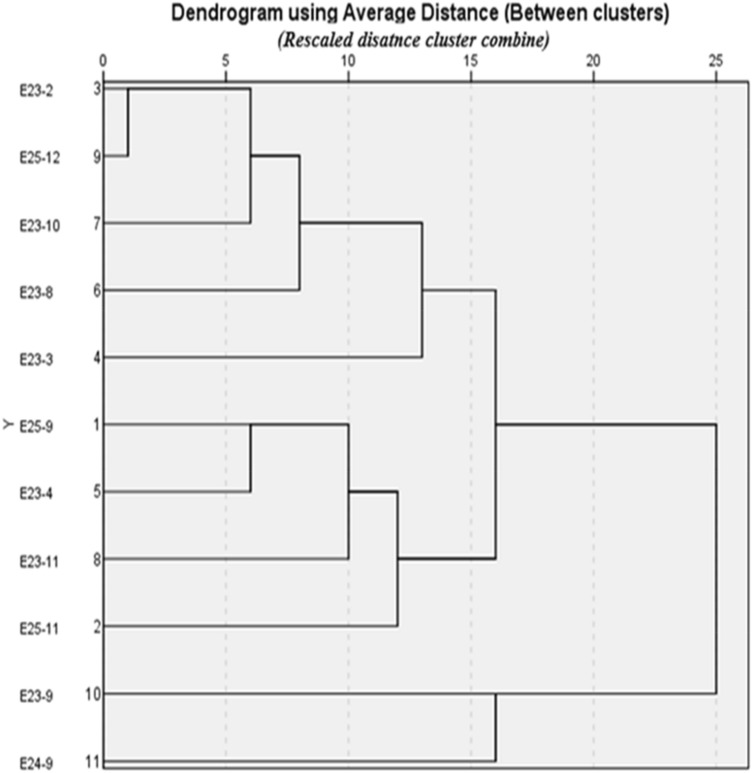
The 11 strains that showed strong antimicrobial activity in the secondary screening were subjected to a hierarchical cluster analysis based on 43 macroscopic, microscopic, biochemical, and physiological characters. A dendrogram based on the average relationship among groups was created using SPSS version 23 software (IBM SPSS statistics 23). The dendrogram divided the active strains of Actinomycetes into two main clusters (Fig. 9). Cluster 1 includes nine strains (E23-2, E23-3, E23-4, E23-8, E23-10, E23-11, E25-9, E2511, and E25-12), while the E23-9 and E24-9 strains formed the second cluster.
Figure 9.
Dendrogram showing the evolutionary relationship of the Actinomycetes isolates.
Molecular identification and phylogenetic analysis of bioactive strains
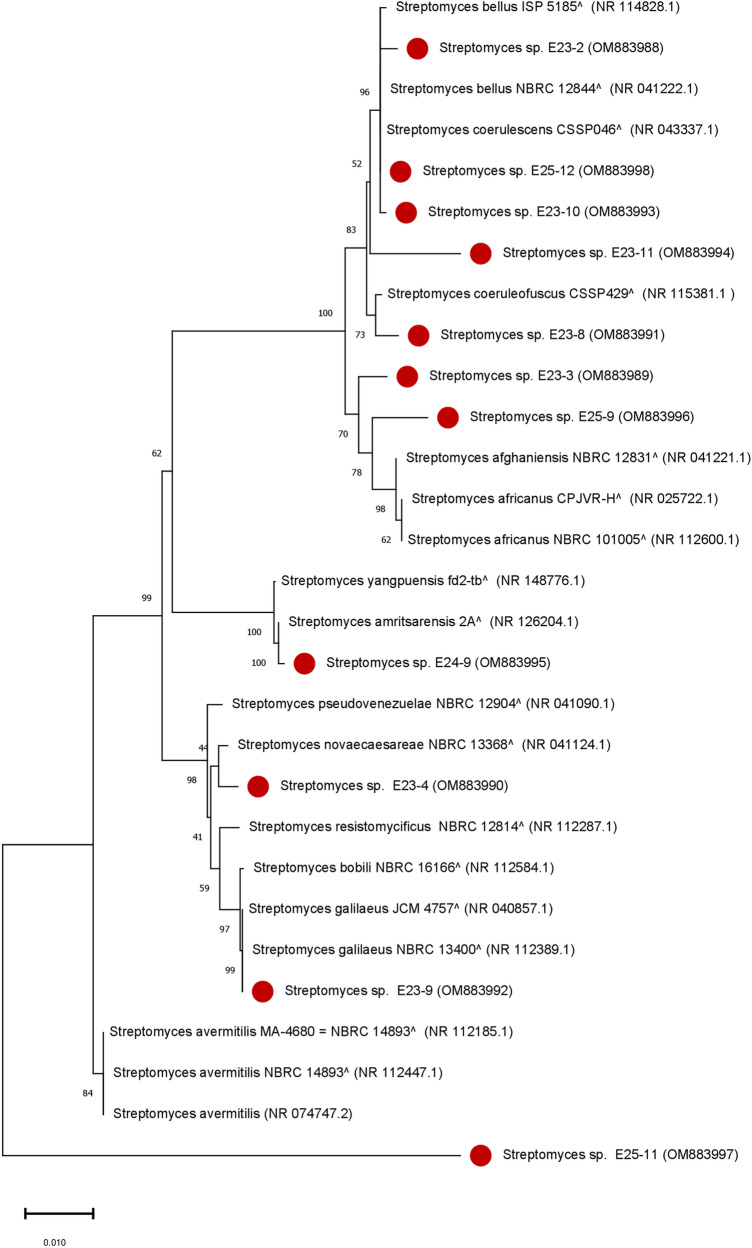
The 11 active Actinomycetes isolates were selected for further characterization using the 16S ribosomal RNA homology sequence study17. The Basic Local Alignment Search Tool (BLAST N) program was used to find the closest correspondence in the database of non redundant reference rRNA sequences (refseq_rna). The results indicate that all the isolates tested belong to the genus Streptomyces (100%) (Fig. 10). Six isolates were identified up to a species level while the other 5 isolates were identified up to a genus level (Supplementary Table S1). The 5 isolates (E23-3, E23-9, E23-10, E24-9, and E25-12) exhibited a very high percentage of similarity at the species level (> 99%). Isolate E23-3 showed a 99.06% similarity with Streptomyces africanus NBRC 101005 T (NR_112600.1), E23-9 was 99.28% similar to Streptomyces galilaeus NBRC 13400 T (NR_112389.1), E24-9 showed a 99.63% similarity with Streptomyces amritsarensis 2AT (NR_126204.1), while isolates E23-10 and E25-12 were both closely similar to the Streptomyces bellus strain NBRC 12844 T (NR_041222.1) (Supplementary Table S2) but differed morphologically.
Figure 10.
Phylogenetic tree based on 16S rRNA gene sequences of Actinomycetes isolates.The phylogenetic tree based on the 16S rRNA gene was established by statistical method (Neighbour-Joining Tree test), showing the evolutionary relationships among the 11 Actinomycetes isolates marked in red circle and their closest known taxa. Each branch in the tree is associated with a bootstrap value (a percentage ranging from 0 to 100%) which indicates the number of times it has been found during the repetitions and thus determines its credibility, only the values (> 40%) are displayed. The bar (0.010), represents the number of substitutions per nucleotide position (1% of divergence between sequences). GenBank accession numbers are shown in parentheses.
Total phenolic and flavonoid contents of crude extracts
The results of the total phenolic and flavonoid contents in the dichloromethane extracts of Streptomyces isolates are summarized in Table 6. The results revealed a significant difference (P < 0.05) among the tested isolates. The E23-11, E25-11, and E23-4 isolates exhibited the highest phenolic contents among the isolates. Concerning flavonoids, the isolate E23-4 contained the highest flavonoid contents.
Table 6.
Total phenolic and flavonoid contents of dicholoromethane extract of Actinomycetes.
| Dichloromethane extracts | Total phenol’s contents (mg GAE/mg extract) | Total flavanoids contents (mg QE/mg extract) |
|---|---|---|
| E23-2 | 0.36 ± 0.012C | 0.028 ± 0.005C |
| E23-4 | 0.42 ± 0.004B | 0.044 ± 0.001A |
| E23-11 | 0.64 ± 0.008A | 0.031 ± 0.002B |
| E25-11 | 0.49 ± 0.016B | 0.035 ± 0.002B |
| E25-12 | 0.10 ± 0.001D | 0.032 ± 0.005B |
Values expressed are means ± SD (n = 3).
In each column, averaged means within followed by the same letters are not significantly different according to one-way (ANOVA) using Tukey's multiple comparisons test, P < 0.05.
In vitro antioxidant activities
All dichloromethane extracts of Streptomyces tested revealed a lower DPPH free radical scavenging activity than ascorbic acid (P < 0.01) (Table 7), while no significant difference was recorded among the extracts (P > 0.01). Regarding ABTS free radical scavenging activity, the dichloromethane extracts of the tested Streptomyces strains exhibited ABTS cationic radical scavenging activity in the range of 29–35% inhibition (P < 0.0001) (Table 7). The E23-4 strain extract exhibited the highest activity (35.79%), followed by the E23-2 (33.8%) and E25-11 (32.67%) strains. However, no significant difference was recorded between the E23-11 (29.45%) and E25-12 (29.31%) strains. The highest antioxidant activity (76.38%) was recorded in trolox (positive control) (Table 7).
Table 7.
Antioxidant activity of isolates dichloromethane extracts in different antioxidant assays (ABTSand DPPH).
| Actinomycetes isolates | Antioxidant activity | |
|---|---|---|
| ABTS radical Scavenging activity (%) | DPPH radical Scavenging activity (%) | |
| E23-2 | 33.81 ± 3.12B**** | 21.17 ± 3.18B** |
| E23-4 | 35.79 ± 0.22B**** | 25.55 ± 0.27B *** |
| E23-11 | 29.45 ± 2.06C**** | 21.88 ± 4.19B*** |
| E25-11 | 32.67 ± 0.36B**** | 24.45 ± 2.37B** |
| E25-12 | 29.31 ± 0.71C**** | 19.95 ± 1.09B*** |
| Trolox | 66.67 ± 0.74A | ND |
| Ascorbic Acid | ND | 76.38 ± 1.82A |
Values expressed are means ± SD (n = 3).
In each column, averaged means within followed by the same letters are not significantly different according to one-way (ANOVA) using Tukey's multiple comparisons test, P < 0.05.
ND not determined.
**P < 0.01, ***P < 0.001, ****P < 0.0001 indicates statistically significant difference.
Correlation of total phenolic and flavonoid contents with antioxidant activities
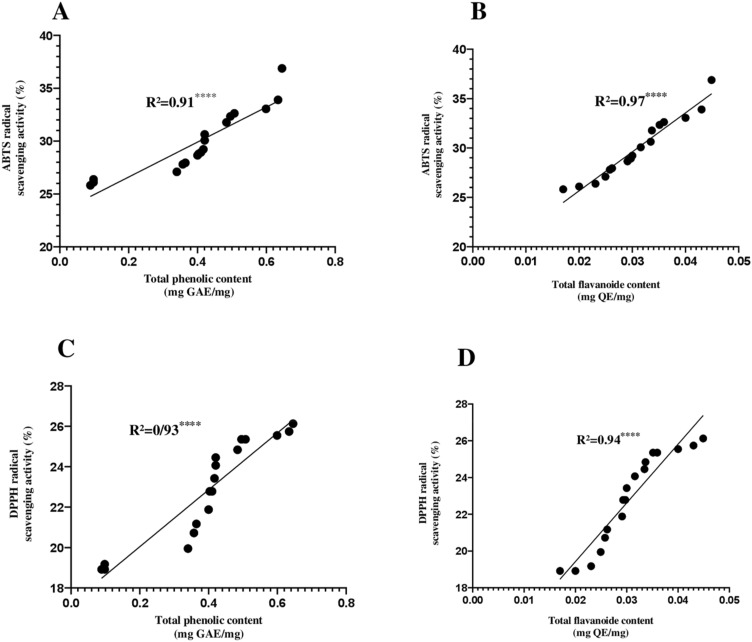
The results revealed a highly significant positive correlation (P < 0.0001) between the total phenolic and flavonoid contents and the antioxidant capacity, analyzed by two different assays (ABTS and DPPH). The highest correlation was observed in the ABTS radical scavenging activity (R2 = 0.94) (Fig. 11A,B), followed by DPPH radical scavenging activity (R2 = 0.93) (Fig. 11C,D).
Figure 11.
Pearson correlation graphics. (A) Pearson correlation between ABTS and total phenolic content, (B) Pearson correlation between ABTS and flavonoids content, (C) Pearson correlation between DPPH and total phenolic content, and (D) Pearson correlation between DPPH and flavonoids content. “****” (P < 0.0001) highly significant between tests.
UV–visible spectrum analysis of extracts
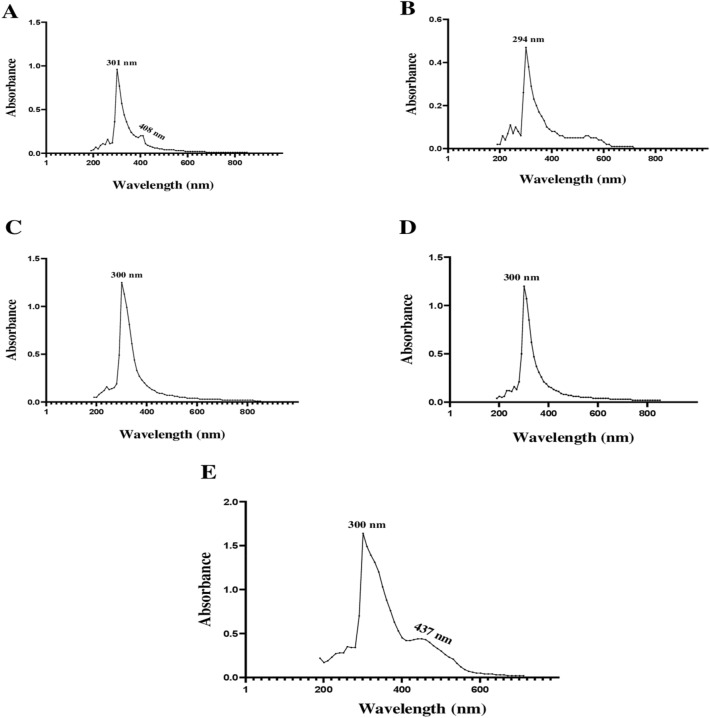
The absorption spectra of dichloromethane extracts obtained from the five most active Streptomyces strains tested did not show the three characteristic absorption peaks of polyenes. Two absorption peaks were obtained at 301 and 408 nm for the E23-2 strain. Only one peak was obtained at 294 nm for the E23-4 strain. Moreover, one peak was obtained at 300 nm for the E23-11 and E25-11 strains, and two peaks were obtained at 300 and 437 nm for the E25-12 strain (Fig. 12).
Figure 12.
UV–visible spectra for the five active crude dichloromethane extracts. (A) Brut extract of Streptomyces sp. E23-2 OM883988, (B) brut extract of Streptomyces sp. E23-4 OM883990, (C) brut extract of Streptomyces sp. E23-11 OM883994, (D) brut extract of Streptomyces sp. E25-11 OM883997, (E) brut extract of Streptomyces bellus E25-12 OM883998.
Identification of bioactive volatile compounds by GC–MS
The GC–MS analysis of the dichloromethane extract of the E23-4 OM883990 strain which showed a very strong antimicrobial and antioxidant activity compared to the other strains tested allowed us to identify a total of 21 volatile compounds whose elution lasted between 9.197 and 49.381 min. Among these identified compounds, twelve were recognized for their antimicrobial, antifungal, antioxidant, antitumor, and other biological activities (Table 8, Supplementary Figs. S5 and S6). Diethyl trisulfide, Indole-3-carboxylic acid,5-methoxy-2-methyl-1-(3-methylphenyl)-ethyl ester,disulfide, dimethyl, l-Leucine, N-cyclopropylcarbonyl-, pentadecylester; 1,2-Benzisothiazol-3-amine,TBDMS derivative, Pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro-3-(phenylmethyl), and benzeneacetic acid were the main compounds identified from the dichloromethane extract of the E23-4 OM883990 strain.
Table 8.
21 volatile compounds identified by GC–MS from dichloromethane crude extract of Streptomyces sp. E23-4 OM883990 strain. RT: Retention time; M.W.: Molecular Weight.
| RT (time) | Area (%) | M.W. (g/mol) | Molecular formula | Coompound name | Reported bioactivity |
|---|---|---|---|---|---|
| 9.197 | 3.37 | 94.20 | C2H6S2 | Disulfide, dimethyl | Antioxidant, Antifungal, Analgesic effect94–96 |
| 9.366 | 0.28 | 85.13 | C3H3NS | 1,3-thiazole | Antibacterial activity97, anti-inflammatory activity98 , antitumor and cytotoxic activity99 |
| 9.467 | 1.25 | 94.10 | C6H5OH | Phenol | Antimicrobial activity, antibacterial activity, and antioxidant activity100 |
| 9.580 | 0.30 | 112.08 | C4H4N2O2 | Maleic hydrazide | Anti-tumorigenic effect101 |
| 10.267 | 0.18 | 126.11 | C6H6O3 | Methyl 2-furoate | Antifungal102 |
| 10.583 | 0.67 | 126.11 | C6H6O3 | Maltol | Antioxidant, Anti-inflammatory, and Antitumor103 |
| 10.955 | 0.76 | 144.12 | C6H8O4 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | Antioxidant104 |
| 12.746 | 0.80 | 136.14 | C8H8O2 | Benzeneacetic acid | Antifungal, Antimicrobial, and Antioxidant70–72 |
| 19.767 | 0.40 | 222.24 | C12H14O4 | Diethyl Phthalate | Antimicrobial, Antifungal105 |
| 24.962 | 1.57 | 283.36 | C15H25NO4 | l-Proline, N-allyloxycarbonyl-, isohexyl ester | Not yet reported |
| 26.866 | 3.22 | 409.6 | C25H47NO3 | l-Leucine, N-cyclopropylcarbonyl-, pentadecyl ester | Antibacterial106 |
| 27.328 | 6.21 | 154.3 | C4H10S3 | Diethyl trisulphide | Antimicrobial62 |
| 27.553 | 1.83 | 451.68 | C27H49NO4 | l-Proline, N-allyloxycarbonyl-, octadecyl ester | Not yet reported |
| 34.968 | 2.77 | 244.29 | C14H16N2O2 | Pyrrolo [1, 2-a] pyrazine-1, 4-dione, hexahydro-3-(phenylmethyl) | Antifungal, Antioxidant, and Anibacterial65–67 |
| 47.307 | 3.54 | 323.41 | C18H17N3OS | Acetamide, N-[4-[2-[(3-methylphenyl) amino]-4-thiazolyl]phenyl]- | Anticancer activity107 |
| 47.454 | 4.76 | 323.39 | C20H21NO3 | Indole-3-carboxylic acid, 5-methoxy-2-methyl-1-(3-methylphenyl)-, ethyl ester | Anticonvulsant, Antioxidant, Anticancer, Antibacterial and Anti-inflammatory Antitubercular, Antimalarial Resistance mediator against necrotrophic pathogens 63,64 |
| 47.961 | 0.22 | 222.40 | C13H22OSi | Thymol, TMS derivative | Anibacterial, Antifungal, Antioxidant, Anticancer24,108,109 |
| 48.524 | 0.36 | 264.46 | C13H20N2SSi | 1, 2-Benzisothiazol-3-amine, TBDMS derivative | Antifungal, Antioxidant and Anti-proliferative68,69 |
| 48.829 | 0.36 | 222.40 | C13H22OSi | Thymol, TMS derivative | Anibacterial, Antifungal, Antioxidant, Anticancer24,108,109 |
| 48.975 | 0.21 | 222.47 | C12H22Si2 | 1,2-Bis (trimethylsilyl) benzene | Antimicrobial, Antioxidant, Anibacterial110 |
| 49.381 | 0.44 | 207.27 | C15H13N | Benzo[h]quinoline, 2,4-dimethyl- | anti-inflammatory111, antioxidant112, anti-HIV113, antifungal114, treatment of neurodegenerative diseases115, antitubercular116, and anticancer117 |
Discussion
Physico-chemical analysis of soil samples
Several studies have reported that the analysis of soil physico-chemical properties such as texture, pH, salinity, mineral elements, organic matter contents, and total nitrogen could influence soil microbial communities, especially Streptomyces spp., which are the most abundant microorganisms identified in soil18,19. To understand the impact of ecological conditions on the distribution of Streptomyces in different soils, a statistical analysis was performed to determine the correlation between each physico-chemical parameter of the soil and the total number of Streptomyces isolated. A correlation between these parameters and the total number of Streptomyces (TNS) was recorded. There was a significant positive correlation between the TNS and organic matter (r = 1.00**, P < 0.01), as well as between TNS and total nitrogen (r = 1.00**, P < 0.01). In addition, there was a significant negative correlation between TNS and sulfur (S) (r = − 1.00**, P < 0.01), and between TNS and Cl (r = − 1.00**, P < 0.01) (Supplementary Table S3). Similar results were previously reported by Dhanasekaran et al.20. Moreover, our results were coherent with those of Ghanem et al.21, which showed a significant positive correlation between total nitrogen, organic matter, and the TNS isolated, while the correlation between pH, temperature, and dissolved phosphates and the TNS isolated revealed non-significant negative values. It can be concluded that although Actinomycetes are ubiquitous, their population dynamics are often influenced by the available nutrients and the physico-chemical conditions of the ecosystem.
The total number of isolated Streptomyces was higher in sites C and B than site A, reflecting the richness of these two sites (B and C) in organic matter. Lee and Hwang22 reported that organic matter contents in soil can also be an important environmental indicator influencing the colonization of Actinomycetes. Another study reported that the number of Actinomycetes increases with organic matter contents in soil23.These results were confirmed by this study, in which a significant positive correlation was found between TNS and organic matter (Supplementary Table S3). Furthermore, it has been reported that soil pH provides selective pressure for bacterial growth24,25. Unlike fungi which prefer acidic and humid circumstances, the majority of Actinomycetes have been shown to grow best under slightly alkaline conditions26. Moreover, Lee and Hwang22 reported that the majority of soil Actinomycetes are neutrophilic. They grow in a pH range between 7 and 11, with optimal growth at neutral or slightly alkaline pH values (between 7 and 8)27. Indeed, they are particularly sensitive to acidity28. Consequently, the average pH of our samples was 8.05, which means that pH of the tested soils was slightly alkaline and did not prevent the growth of Actinomycetes.
Pretreatment of soil samples and isolation of Actinomycetes strains
The soil samples were pretreated with calcium bicarbonate (CaCO3) to reduce the fungal flora and increase the number of probable Actinomycetes29. According to a previous study27, drying soil samples for 7–21 days at ambient temperature can reduce the soil microbial flora because fast-growing bacterial colonies inhibit the growth of other bacteria, including Actinomycetes. Hence, in order to isolate Actinomycetes, the number of these bacteria (fast-growing bacteria) must be reduced. Moreover, the addition of an antimicrobial agent to culture media has been shown to be effective in removing contaminating microorganisms and thus facilitating the growth of slow-growing Actinomycetes30.
Isolation was performed on four culture media chosen according to previous studies30–33. Compared to the BEN, GLM, and GA media, the M2 medium appeared to be the best for isolating Actinomycetes. This result can be explained by the presence of macromolecules (starch and casein) in this medium, which are catabolized by the majority of Actinomycetes and make the medium (M2) favorable to the growth of these bacteria34. In addition, this medium contains trace elements that are essential for bacterial growth34. The GA medium was the least effective in isolating Actinomycetes, suggesting that the main component of this medium (L-asparagine) may not provide an adequate nitrogen source for Actinomycetes isolated in the Fez-Meknes region.
Screening of active Actinomycetes isolates
The results of the primary and secondary screening revealed that the majority of the tested Actinomycetes isolates were more active against Gram-positive bacteria than Gram-negative bacteria. This difference could be explained by the higher complexity of the cell walls of Gram-negative bacteria compared to those of Gram-positive bacteria. The cell walls of Gram-positive bacteria contain lipopolysaccharides (LPS) as their main structural component, which are anchored in the phospholipid layer, making the wall impermeable to lipophilic solutes35,36. In contrast, the walls of Gram-positive bacteria mainly consist of peptidoglycan, which does not provide an effective permeability barrier, making Gram-positive bacteria susceptible to metabolites29,37. All the tested clinical bacteria were multi-resistant to antibiotics (Supplementary Table S4), but none of these were resistant to the organic extracts of the tested Actinomycetes isolates. This suggests that these Actinomycetes isolates secrete different antibiotics than those to which the bacteria are resistant.
Morphological identification of bioactive strains
The production of melanoid pigments is a very important characteristic for Streptomyces classification38. According to Dastager et al.39, some Actinomycetes can produce dark brown substances in culture media commonly called melanoid pigments. Vinarov et al.40 considered that the compounds of this melanoid pigment are irregular polymers which are dark brown in color and formed by many microorganisms via fermentative oxidation. Melanoid pigmentshave a broad spectrum of biological activities serving radioprotective41, antioxidant42, antimicrobial43, and antitumor44 functions. Actinomycetes strains grow at a pH ranging from 5.33 to 10.03 with an optimum growth at pH 8.28, but they can not grow in acidic media (pH < 5). Hence, it was seen that the Actinomycetes isolates tested here tended to grow better under neutral or slightly alkaline pH conditions. Most Actinomycetes species are mesophilic with optimal growth temperatures between 25 and 30 °C8. The most favorable temperature range for the growth of the tested Actinomycetes strains was between 28 and 37 °C. None of the Actinomycetes strains tested could grow at 4 °C and 46 °C. These results agree well with those obtained by Singh et al.45. All Actinomycetes strains tolerate NaCl concentrations from 1 to 5%, without any growth beyond 7% (Table 5). These results suggest that the selected Actinomycetes strains had the ability to resist NaCl concentrations up to 70 g/L (7%). Tian et al.43 concluded that saline or hyper-saline environments require special consideration because they could provide new avenues for the discovery of new natural and industrially useful molecules.
Molecular identification and phylogenetic analysis of bioactive strains
The results of the molecular identification based on the 16S ribosomal RNA homology are in agreement with previous studies and confirm that Streptomyces are the predominant genus in soils compared to the other Actinomycetes genera46,47. In fact, Kitouni et al.47 reported that 93% of Actinomycetes active strains belong to the Streptomyces genus.
In this study, three strains (E23-11, E25-9, and E25-11) were found to have a similarity of at least 98.7% with the closest known strains, which implies that they could be new taxa48. These results were confirmed with other databases (EzBioCloud) and yielded the same similarities with the same closest taxa (90.24% for E25-11, 97.25% for E23-11, and 98.20% for E25-9). The phylogenetic tree analysis of the selected strains showed that the proximity or distance among these strains were independent of the profile of macroscopic, microscopic, biochemical, and physiological characters. For example, phylogenetically, the E23-4 and E24-9 strains were quite similar, but considerable differences were found between their macroscopic, physiological, and biochemical characteristics, as well as antimicrobial activity. The dendrogram revealed that these two strains were distinct. It was recalled that the E23-4 strain was isolated from the Sebt Jahjouh El Hajeb site, and the second strain (E24-9) was isolated from the Ain Vittel Ifrane forest site, and therefore, the difference in natural habitats (origin of isolation of the strains) could be the cause of this independence. These results agree with those reported by Sengupta et al.49.
In vitro antioxidant activities
The uncontrolled production of oxygen-free radicals causes oxidative stress, which has been identified as a major cause of health problems such as cancer and other diseases50. Antioxidants can reduce the accumulation of free radicals in the body, protecting it from oxidative damage51. The Streptomyces bacteria genus has been recognized as one of the major sources of natural antioxidant compounds52–55. However, evidence suggests that the use of a single test may not be sufficient to determine the antioxidant activity of extracts56. Therefore, two in vitro assays were performed (ABTS and DPPH). The results of DPPH and ABTS free radical scavenging activities revealed a strong antioxidant capacity of the Streptomyces strains tested, which may suggest that these strains may be able to produce one or more antioxidants that could be useful to prevent oxidative stress. At a concentration of 1 mg/mL, the dichloromethane extract of the E23-4 OM883990 strain showed the highest free radical scavenging activity for both ABTS and DPPH assays. Using the same concentration, Tan et al.57 obtained DPPH (12.03%) and ABTS (27%) for a Streptomyces sp. MUM212 strain dichloromethane extract from Malaysia. Previous studies have shown that the Streptomyces genus can produce natural products such as phenols, flavonoids, steroids, and other compounds that are known for their antioxidant activity55,56,58. Phenolic compounds are known for their antioxidant activity as well as other bioactivities such as anti-inflammatory, antimicrobial, and anti-allergic properties59,60.The statistical analysis in this study supports this hypothesis since a strong correlation was found between antioxidant activity and total phenolic and flavonoid contents.
Detection of polyene molecules by UV–vis
The analysis of the spectra of the active dichloromethane extracts of the five Streptomyces sp. strains showed high antimicrobial activity indicating that they do not contain molecules of a polyene nature, which are characterized by three characteristic absorption maxima in the UV–visible between 291 and 405 nm61. This result is very interesting, as polyene molecules are usually discarded in research programs on new bioactive molecules due to their toxicity. It was demonstrated that antifungal molecules of a polyene nature interact with cholesterol since the latter has a structure close to that of ergosterol, the main sterol of fungal cells. This may explain the toxicity of this type of antifungal molecules57.
Identification of bioactive volatile compounds by GC–MS
In this study, the GC–MS analysis of the dichloromethane extract of the E23-4 OM883990 strain revealed the presence of 21volatile compounds with seven majors ones including: diethyl trisulfide, which was found to have a high retention time as well as antibacterial and antifungal activities62; indole derivatives (Indole-3-carboxylic acid, 5-methoxy-2-methyl-1-(3-methylphenyl)-, ethyl ester), which exhibit various biological activities namely anticonvulsant, antioxidant, anticancer, antibacterial, anti-inflammatory, antitubercular, and antimalarial activities63,64; pyrrolizidine derivatives (Pyrrolo [1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)), which are natural heterocyclic compounds known to exhibit antifungal, antioxidant, and antibacterial activities65–67; 1,2-Benzisothiazol-3-amine, a TBDMS derivative which is one of the tert-butyldimethylsilyl (TBDMS) derivatives that are considered an antifungal, antioxidant, and antiproliferative bio-molecule68,69; and benzeneacetic acid, which showed antifungal activity against phytopathogens70–72. It is possible that all compounds recorded in the tested E23-4 strain dichloromethane extract were responsible for the diversity of biological activities exhibited by this extract. In addition, the highest antimicrobial and antioxidant activities exhibited by the E23-4 strain may have been caused by the synergistic effect of the secondary metabolites present in the E23-4 dichloromethane extract. Our results are in agreement with those found by Das et al.47, who showed that the ethyl acetate extract of the Streptomyces sp. strain EA-PWS52 consists of bioactive molecules such as benzene acetic acid, pyrrolizidine derivatives, heterocyclic compounds, and other compounds that can exhibit antibacterial, antifungal, and antioxidant activities30.
Conclusions
In conclusion, the Streptomyces genus remains an excellent source for the production of natural bioactive compounds, since two thirds of all the new bioactive compounds discovered from Actinobacteria between 2015 and 2019 were obtained from the Streptomyces genus14. This study describes the isolation of Actinomycetes strains, particularly the Streptomyces genus, from an extremely cold and microbiologically unexplored terrestrial environment of northwestern Morocco. The Streptomyces sp. E23-2 OM883988, E23-4 OM883990, E23-11OM883994, E25-11OM883997 and Streptomyce sbellus E25-12 OM883998 strains showed remarkable antimicrobial activity against MDR strains and phytopathogenic fungi. These strains can also scavenge several free radicals, including DPPH and ABTS radicals. The phenolic and flavonoid compounds present in their extracts could be the main constituents responsible for their antioxidant properties. The different bioactive compounds identified using GC–MS in the E23-4 dichloromethane extract of Streptomyces sp.OM883990 have a wide spectrum of pharmacological activities and can be used to treat bacterial infections multi-resistant, and to counteract diseases caused by oxidative stress such as cancer, cardiovascular diseases, renal diseases, and neurological diseases. These identified bioactive compounds require further studies regarding their mechanisms of action at the intracellular level. In addition, future directions would be to identify and isolate the most interesting compounds in terms of antimicrobial, insecticidal, antioxidant, and antitumor screening using Fourier Transform Infrared Spectroscopy (FTIR), High Performance Liquid Chromatography (HPLC), High Resolution Mass Spectrometry (HRMS), and reconfirming the structure using Nuclear Magnetic Resonance (NMR). Also, further studies regarding the pathways involved in the antimicrobial and antioxidant properties of the identified compounds are needed.
Materials and methods
Collection of soil samples
The soil samples were collected during February and early March, 2019 in the Northwest of three different habitats in Morocco including Sebt Jahjouh El Hajeb (GPS: 33° 41′ 16″ N, 5° 22′ 15″ W), forest Ain Vittel Ifrane (GPS: 31° 42′ 07″ N, 6° 20′ 57″ W), and forest of Azrou (GPS: 33° 26′ 28″ N 5° 13′ 22″ W) (Supplementary Fig. S7). In order to avoid similarity in Acctinomycete species, the soil samples were collected at five different points within a 400 m2 zone for each habitat49. For each point, the top five centimeters of soil was removed using a sterile spatula, and 150 to 200 g of soil from the underlying layer was collected, and placed in stomacher sachets, then mixed, and homogenized to generate a heterogeneous sample. The samples were transported aseptically to the laboratory, and conserved at 4 °C until use.
Physico-chemical analysis of soil samples
The pH of each soil sample was determined using a pH meter (OCRISON, micro-pH 2000). Electrical conductivity (EC) was determined by a conductivity meter (sevenGoTM), and minerals including C, O, Mg, Si, Fe, K, and Ca were analyzed by scanning electron microscope (SEM) (JEOL: JSM-IT500HR) while other minerals (Zn, Mn, Cl, Al, P, Cu, and S) were analyzed by energy dispersive X-ray fluorescence method (Epsilon 3XLE from PANalytical, France). The organic matter (OM), and soil texture were analyzed according to the methods described by Jackson73.
Pretreatment of soil samples, and isolation of Actinomycetes strains
In order to increase the number of Actinomycetes, the soil samples were pretreated using two methods: Drying method49, and the enrichment method47. Isolation was performed by the serial dilution method74. Four media were used to isolate Actinomycetes: (1) M231, (2) GA31, (3) GLM30,32, and (4) Bennett33. Each medium was mixed after sterilization and cooling with 50 mg/L actidione (cycloheximide) to inhibit fungal growth. Plates (90 mm diameter) were incubated at 28 °C and monitored daily during three weeks to check for Actinomycetes growth. Actinomycetes colonies recognized by their macroscopic and microscopic aspects (Supplementary Fig. S8) were subcultured on ISP2 medium by the streak method in order to obtain pure cultures, and then were short-term stored in inclined tubes at 4 °C, and in 20% glycerol at − 20 °C for long-term storage75.
Primary screening of Actinomycetes isolates for antimicrobial activity
In order to select the best medium for antibiotics production, the antimicrobial activity of Actinomycetes pure isolates was tested by the double-layer method76 using four Agar media (ISP1, ISP2, GYEA and Bennett) against different microorganisms: Escherichia coli ATCC 25,922, Staphylococcus aureus ATCC 25,923, Bacillus ceureus ATCC 14,579 (non pathogenic bacteria), Candida albicans ATCC 60,193 (pathogenic fungi) (these microoganisms were collected from the Institut Pasteur Casablanca Morocco), Fusarium sp. MN944566, Fusarium sp. MN944567, Fusarium sp. MN944568, Fusarium sp. MN944569, Fusarium sp. MN944570, Fusarium sp. MN944571, Fusarium sp. MN944572, Fusarium sp. MN944573, Fusarium sp. MN944574, Fusarium sp. MN944575, Fusarium sp. MN944576, and Fusarium sp. MN944577 (phytopathogenic fungi) (these phytopathogenic fungus were obtained from Laboratory of Agro-Alimentary and Health, Faculty of Sciences and Techniques, Hassan First University of Settat Moroco), and 2 other fungi, Trichoderma longibrachiatum M37 and Trichoderma koningii M35, which cause invasive pulmonary and peritoneal infections respectively77,78. The antimicrobial activity was examined by measuring the zones of inhibition in mm. The phytopathogenic fungi (Fusarium sp.) were isolated from the mango tree Mangifera indica L. (infected at leaf level) native to the Indo-Burma region (senegal), is one of the oldest cultivated fruit trees in the world79.
Production kinetics of secondary metabolites
The kinetics of secondary metabolite production was monitored during 10 days on ISP2 medium selected from the primary screening test for the five Actinomycetes isolates that exhibited high antimicrobial activity using the disc diffusion method61,80.
Fermentation and extraction of secondary metabolites
The sixteen Actinomycetes isolates that showed significant antimicrobial activity in the primary screening were subjected to fermentation and subsequent extraction of secondary metabolites using organic solvents of increasing polarity. Indeed, Erlenmeyer flasks (500 mL) containing 100 mL of broth ISP2 culture medium chosen in the primary screening were inoculated with each active Actinomycetes isolate selected and incubated under constant agitation at 150 rpm under controlled conditions (28 °C).
In order to select the best solvent for extraction, the Actinomycetes cultures were centrifuged at 10,000 g for 20 min to remove the mycelial mass. One volume of supernatants was recovered and mixed vigorously in a separating funnel with the same volume of hexane. The aqueous phase obtained is then mixed with the same volume of dichloromethane and the same operations were done for the other organic solvents tested (ethyl acetate and butanol) by mixing in each time the aqueous phase obtained with the same volume of the next organic solvent tested. The organic extracts obtained were then evaporated at 45 °C. Finally the dry organic extracts obtained as well as the residual aqueous phases were solubilized in dimethylsulfoxide (DMSO) to calculate their concentrations74,81–84.
Evaluation of the antimicrobial activity of organic extracts
The antimicrobial activity of the obtained organic extracts was evaluated by the disc diffusion method described by Badji et al.76 against six phytopathogenic fungi (Fusarium sp. MN944568, Fusarium sp. MN944569, Fusarium sp. MN944570, Fusarium sp. MN944571, Fusarium sp. MN9445677) and Trichoderma longibrachiatum M37 which induces invasive pulmonary infection78, and five clinical MDR strains (Escherichia coli 16D1150, Enterococcus faecalis 18K1386, Staphylococcus aureus 18K1052, Proteus vulgaris 16C1737, and Neisseria gonorrhoeae 16D1170). The antibiotic resistance profile of the clinical MDR strains tested was verified against sixteen antibiotics (Supplementary Table S7). Concerning antifungal activity, the tested fungi were subcultured on PDA medium and incubated at 28 °C for 10 days. A fungal suspension was obtained by adding a part of the tested isolate culture (mysilia and conedia) to sterile distilled water. The optical density of each inoculum was adjusted by a spectrophotometer set at a wave length of 623 nm in order to obtain an optical density between 0.18 and 0.20 which corresponds to about 106 spore/mL85. A disc loaded with DMSO of a volume equal to that of the extract was used as a negative control. For the positive control, streptomycin and cycloheximide were used for antibacterial and antifungal activities, respectively. The diameter of the inhibition zones was measured after 24 h of incubation at 37 °C for bacteria and 48 h at 28 °C for fungi using foot caliper.
Cultural, micro-morphological, biochemical and physiological characteristics of isolates
The cultural characteristics such as intensity of growth, pigmentation of medium, colony aspect, and the presence of diffusible pigments in agar were observed on ISP (ISP-1, ISP-2, ISP-4, ISP-5) and GYEA media38. All these media were seeded using striation method38 for each isolate, and then the plates (90 mm in diameter) were incubated at 28 °C and monitored daily during 10 days. The micro-morphological characters of pure isolates were determined under the optical microscope (Olympus CX43RF) in the fresh state and after Gram staining. Physiological and biochemical characters of Actinomycetes isolates tested were evaluated according to previous studies17,81. They concern the production of melanoid pigments, the tolerance to different increasing concentrations of sodium chloride (NaCl) and pH values, the growth at different degrees of temperature, and the assimilation of carbohydrates. Based on the cultural, biochemical and physiological characteristics, a hierarchical classification of Actinomycetes groups was performed using specific software (IBM SPSS statistics 23). After the analysis, a dendrogram was generated using the average distance among groups.
Genomic DNA extraction, 16 rRNA gene sequencing and phylogenetic tree construction
The eleven Streptomyces sp. strains showed high antimicrobial activity were inoculated into 50 ml of ISP2 medium without Agar and subjected to constant agitation for 5–7 days until the turbidity of the medium was high. Cells were concentrated by centrifugation at 10,000 g for 20 min at 4 °C, and then congealed (without supernatant) in 2 mL eppendorfs tubes at − 20 °C until used86. DNA extraction was performed by an automated system (Mag Purix Bacterial DNA Extraction Kit) following the manufacturer's instructions. The DNA extracted from each isolate was quantified using a NanoDrop 8000 Spectrophotometer (thermo scientific) (Supplementary Fig. S9), and stocked at − 20 °C until use. All concentrations of extracted DNA samples were adjusted to100 ng/µL for the PCR reaction.
The 16S rRNA gene was amplified using the universal bacterial primer pairs Fd1 (5′-AGAGTTTGATCATGGCTCAG-3′) and rP2 (5′-ACGGTTACCTTGTTACGACTT-3′) to obtain an amplicon with a size of 1,500 bp with the reported conditions87. The amplified fragments were analyzed by electrophoresis. More precisely, 8 µL of PCR products were deposited on agarose gel (1%) in the presence of the 1 kb molecular weight marker (thermo scientific). The photo was visualized by the Gel-Documentation G-Box photo system (Supplementary Fig. S10).
The amplified products were purified using ExoSAP-IT PCR cleanup Kit (Applied Biosystems), and sequence reactions were performed by using a version 3.1 BigDye Terminator Cycle Sequencing Kit (Applied Biosystems). Sequencing products were purified using the BigDye® XTerminator™ Purification Kit (Applied Biosystems), and loaded onto an ABI 3130xL capillary sequencer (Applied Biosystems) according to the manufacturer’s instructions. The works of extraction, amplification and sequencing were carried out at the National Center of Scientific and Technical Research (CNRST, Rabat Morocco).
The assembly of the obtained sequences was performed using the version 5.15.0 DNA Baser Assembler software in order to generate the contigs, which have been recorded in FASTA form (.FASTA). Nucleotide sequences were aligned with MEGA X software88 using MUSCLE alignment. To designate the taxonomic status of the Actinomycetes isolates, the assembled and aligned sequences were compared with the database of non-redundant reference RNA sequences (refseq_rna) archived in the Genomic Data Bank, which is accessible on the internet at the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov), using the nucleotide search program BLAST. The phylogenetic tree was constructed with the same alignment software using the neighbor-joining tree method88.
Determination of total phenolic and flavonoid compounds
The determination of total phenolic compounds contained in the dichloromethane extracts of the five Streptomyces sp. strains showed high antimicrobial activity, was performed using the folin-ciocalteu method89. Gallic acid was used as standard. Quantification of flavonoid content in the same extracts was performed using the aluminum trichloride method90. Quercetin was used as standard.
In vitro antioxidant activities
The free radical scavenging activity (DPPH) of the dichloromethane extracts of the five Streptomyces sp. strains showed high antimicrobial activity was performed according to the method described by Blois91. The absorbance was measured at 517 nm using an Elisa microplate reader (2100-C, Optic Ivymen Systems, Spain). Ascorbic acid was used as a positive control. Concerning the free radical scavenging activity (ABTS), it was performed according to the method developed by Re et al92. Trolox was used as a positive control.
UV–visible analysis
The absorption spectra of the dichloromethane extracts of the five Streptomyces sp. strains showed high antimicrobial activity solubilized in DMSO were recorded between 190 and 850 nm using UV–vis scanning spectrophotometer (HACH Lange DR6000)92.
GC–MS analysis of E23-4 strain crude dichloromethane extract
The volatile compound profile of the crude dichloromethane extract of E23-4 strain OM883990 was characterized by gas chromatography (GC) (Agilent 7890A Series) coupled to mass spectrometry (MS) equipped with a multimode injector and a HP-5MS column with a dimension of 30 m × 0.250 mm × 0.25 μm at the Moroccan Foundation for Advanced Science, Innovation and Research (MAScIR) Institute. Briefly, four µL of the extract solubilized in an adequate volume of chloroform was injected into the column by 1:4 split mode using helium as carrier gas at 1.7 mL/min. The temperatures of the ion source and quadrupoles were 230 and 150 °C, respectively. The oven temperature program was started at 60 °C and finished at 360 °C. The compound's identification was performed by comparing the obtained mass spectra with the data available in NIST 2017 MS Library30,93.
Statistical analysis
All the experiments data were repeated three times and results were expressed as mean ± SD. Ordinary one-way ANOVA followed by Tukey's multiple comparisons test which was performed using GraphPad Prism 8.4.3 software (GraphPad software Inc., San Diego,CA,USA) to verify significant differences among groups in antioxidant activity tests and phenolic compound assays. Results were considered statistically significant when P ≤ 0.05. Pearson correlation analysis was performed using GraphPad Prism 8.4.3 software to determine the relationship between total Actinomycetes number and soil physico-chemical parameters as well as the relationship between phenolic compounds and antioxidant activity. Data measured in percentages were subjected to an arcsine transformation in order to approximate the normal distribution before analysis.
Supplementary Information
Author contributions
S.R., L.H., and A.K. conceived the experiments. S.R., A.R., and A.k. conducted the experiments. M.T., B.B., F.G., S.R., M.E., and A.K. analyzed the results. All authors reviewed the manuscript.
Data availability
All the data generated or analyzed during this study, including the nucleotide sequence data, are available as Supplementary Information files. Genome sequences of Streptomyces sp. E23-2, E23-3, E23-4, E23-8, E23-9, E23-10, E23-11, E24-9, E25-9, E25-11 and E25-12 have been deposited in National Centre for Biotechnology Information (NCBI) GenBank (https://www.ncbi.nlm.nih.gov/nucleotide/) under accession numbers of OM883988, OM883989, OM883990, OM883991, OM883992, OM883993, OM883994, OM883995, OM883996, OM883997 and OM883998.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-21644-z.
References
- 1.Quinn GA, Banat AM, Abdelhameed AM, Banat IM. Streptomyces from traditional medicine: Sources of new innovations in antibiotic discovery. J. Med. Microbiol. 2020;69:1040–1048. doi: 10.1099/jmm.0.001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pizzino G, et al. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017;2017:1114. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudi Emerson de Lima Procópioa, Ingrid Reis da Silvaa, Mayra Kassawara Martinsa, João Lúcio de Azevedoa, J. M. de A. Antibiotics produced by Streptomyces. Brazilian J. Infect. Dis.16, 466–471 (2012). [DOI] [PubMed]
- 4.Liu X, et al. Bioprospecting microbial natural product libraries from the marine environment for drug discovery. J. Antibiot. (Tokyo) 2010;63:415–422. doi: 10.1038/ja.2010.56. [DOI] [PubMed] [Google Scholar]
- 5.Milshteyn A, Schneider JS, Brady SF. Mining the metabiome: Identifying novel natural products from microbial communities. Chem. Biol. 2014;21:1211–1223. doi: 10.1016/j.chembiol.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chassagne F, Cabanac G, Hubert G, David B, Marti G. The landscape of natural product diversity and their pharmacological relevance from a focus on the Dictionary of Natural Products ®. Phytochem. Rev. 2019;18:601–622. doi: 10.1007/s11101-019-09606-2. [DOI] [Google Scholar]
- 7.Ganesan P, et al. Antimicrobial activity of some Actinomycetes from Western Ghats of Tamil Nadu, India. Alexandria J. Med. 2017;53:101–110. doi: 10.1016/j.ajme.2016.03.004. [DOI] [Google Scholar]
- 8.Barka EA, et al. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016;80:1–43. doi: 10.1128/MMBR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka Y, Omura S. Metabolism and products of Actinomycetes-an introduction. Actinomycetologica. 1990;4:13–14. doi: 10.3209/saj.4_13. [DOI] [Google Scholar]
- 10.Barka EA, et al. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016;80:iii. doi: 10.1128/MMBR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Shaibani MM, et al. Biodiversity of secondary metabolites compounds isolated from phylum Actinobacteria and its therapeutic applications. Molecules. 2021;26:1–22. doi: 10.3390/molecules26154504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kekuda P, Onkarappa R, Jayanna N. Characterization and antibacterial activity of a glycoside antibiotic from Streptomyces variabilis PO-178. Sci. Technol. Arts Res. J. 2015;3:116. doi: 10.4314/star.v3i4.17. [DOI] [Google Scholar]
- 13.Labeda DP, et al. Phylogenetic study of the species within the family Streptomycetaceae. Antonie van Leeuwenhoek. Int. J. Gen. Mol. Microbiol. 2012;101:73–104. doi: 10.1007/s10482-011-9656-0. [DOI] [PubMed] [Google Scholar]
- 14.Jose PA, Maharshi A, Jha B. Actinobacteria in natural products research: Progress and prospects. Microbiol. Res. 2021;246:4417. doi: 10.1016/j.micres.2021.126708. [DOI] [PubMed] [Google Scholar]
- 15.LSooee N, et al. Thirty complete Streptomyces genome sequences for mining novel secondary metabolite biosynthetic gene clusters. Sci. Data. 2020;7:1–9. doi: 10.1038/s41597-020-0395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YM, Kim EH, Lee HK, Hong SG. Biodiversity and physiological characteristics of Antarctic and Arctic lichens-associated bacteria. World J. Microbiol. Biotechnol. 2014;30:2711–2721. doi: 10.1007/s11274-014-1695-z. [DOI] [PubMed] [Google Scholar]
- 17.Singh V, et al. Isolation, screening, and identification of novel isolates of Actinomycetes from India for antimicrobial applications. Front. Microbiol. 2016;7:6668. doi: 10.3389/fmicb.2016.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Kamali HH, Hassan HI, El-Kheir MSM. Effect of soil physico-chemical properties and plant species on bacterial diversity in semi-arid parts in Central Sudan. Part III: AL-Gaeli Region, Khartoun North. OALib. 2017;04:1–11. doi: 10.4236/oalib.1103337. [DOI] [Google Scholar]
- 19.Tecon R, Or D. Biophysical processes supporting the diversity of microbial life in soil. FEMS Microbiol. Rev. 2017;41:599–623. doi: 10.1093/femsre/fux039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhanasekaran D, Thajuddin N, Panneerselvam A. Distribution and ecobiology of antagonistic Streptomycetes from agriculture and coastal soil in Tamil Nadu, India. J. Cult. Collect. 2009;6:10–20. [Google Scholar]
- 21.Ghanem NB, Sabry SA, El-Sherif ZM, Abu El-Ela GA. Isolation and enumeration of marine Actinomycetes from seawater and sediments in Alexandria. J. Gen. Appl. Microbiol. 2000;46:105–111. doi: 10.2323/jgam.46.105. [DOI] [PubMed] [Google Scholar]
- 22.Lee JY, Hwang BK. Diversity of antifungal Actinomycetes in various vegetative soils of Korea. Can. J. Microbiol. 2002;48:407–417. doi: 10.1139/w02-025. [DOI] [PubMed] [Google Scholar]
- 23.Zanane C, Latrache H, Elfazazi K, Zahir H, Ellouali M. Isolation of Actinomycetes from different soils of Beni Amir Morocco. J. Mater. Environ. Sci. 2018;9:2994–3000. [Google Scholar]
- 24.Bååth E. Adaptation of soil bacterial communities to prevailing pH in different soils. FEMS Microbiol. Ecol. 1996;19:227–237. doi: 10.1016/0168-6496(96)00008-6. [DOI] [Google Scholar]
- 25.Rousk J, Brookes PC, Baath E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009;75:1589–1596. doi: 10.1128/AEM.02775-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewin GR, et al. Evolution and ecology of Actinobacteria and their bioenergy applications. Annu. Rev. Microbiol. 2016;70:235–254. doi: 10.1146/annurev-micro-102215-095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basilio A, et al. Patterns of antimicrobial activities from soil Actinomycetes isolated under different conditions of pH and salinity. J. Appl. Microbiol. 2003;95:814–823. doi: 10.1046/j.1365-2672.2003.02049.x. [DOI] [PubMed] [Google Scholar]
- 28.Davet, P. Vie microbienne du sol et production v{é}g{é}tale. (Editions Quae, 1996).
- 29.Ullah I, et al. Actinomycetes screening for bioactive potential isolated from the moist forest soils of Pakistan. Rec. Zool. Surv. Pakistan. 2012;13:10–13. [Google Scholar]
- 30.Das R, Romi W, Das R, Sharma HK, Thakur D. Antimicrobial potentiality of Actinobacteria isolated from two microbiologically unexplored forest ecosystems of Northeast India. BMC Microbiol. 2018;18:1–16. doi: 10.1186/s12866-018-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouaziz S, et al. Antifungal activity of Streptomyces sp. 14 strain isolated from Ouargla (Southeast of Algeria): Identification, production and characterization of the active substance. Int. J. Biosci. 2016;9:45–56. doi: 10.12692/ijb/9.5.45-56. [DOI] [Google Scholar]
- 32.Thakur D, Yadav A, Gogoi BK, Bora TC. Isolation and screening of Streptomyces in soil of protected forest areas from the states of Assam and Tripura, India, for antimicrobial metabolites. J. Mycol. Med. 2007;17:242–249. doi: 10.1016/j.mycmed.2007.08.001. [DOI] [Google Scholar]
- 33.Lee EJ, Hwang KY, Lee HS, Chung N. Characterization of a new Streptomyces sp. a1022 as a potential biocontrol agent. J. Appl. Biol. Chem. 2011;54:488–493. [Google Scholar]
- 34.Boughachiche F, Reghioua S, Zerizer H, Boulahrouf A. Activité antibactérienne d’espèces rares de Streptomyces contre des isolats cliniques multirésistants. Ann. Biol. Clin. (Paris) 2012;70:169–174. doi: 10.1684/abc.2012.0661. [DOI] [PubMed] [Google Scholar]
- 35.Zainal AZA, Abdul MN, Zainuddin Z, Chowdhury AJK. Selective isolation and antagonistic activity of Actinomycetes from mangrove forest of Pahang, Malaysia. Front. Life Sci. 2016;9:24–31. doi: 10.1080/21553769.2015.1051244. [DOI] [Google Scholar]
- 36.Naikpatil SV, Rathod JL. Selective isolation and antimicrobial activity of rare Actinomycetes from mangrove sediment of Karwar. J. Ecobiotechnology. 2011;3:48–53. [Google Scholar]
- 37.Fan L, Zheng J, Yang X, et al. The effect of natural air-dry time on Actinomycetes isolation from sample soil. J. Hainan Med. Coll. 2010;16:280–284. [Google Scholar]
- 38.Shirling EB, Gottlieb D, et al. Methods for characterization of. Strep tomyces. Sci. Rep. 1966;16:340. [Google Scholar]
- 39.Dastager SG, et al. Seperation, identification and analysis of pigment (melanin) production in Streptomyces. African J. Biotechnol. 2006;5:1131–1134. [Google Scholar]
- 40.Vinarov, A. U., Robysheva, Z. N., Sidorenko, T. E. & Dirina, E. N. Biotechnology of pigment melanin. In Proceedings of the 1st international congress Biotechnology-state of the art and prospects of development 96 (2002).
- 41.Dadachova E, et al. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS One. 2007;2:77. doi: 10.1371/journal.pone.0000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Goncalves RCR, Pombeiro-Sponchiado SR. Antioxidant activity of the melanin pigment extracted from Aspergillus nidulans. Biol. Pharm. Bull. 2005;28:1129–1131. doi: 10.1248/bpb.28.1129. [DOI] [PubMed] [Google Scholar]
- 43.Casadevall A, Rosas AL, Nosanchuk JD. Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol. 2000;3:354–358. doi: 10.1016/S1369-5274(00)00103-X. [DOI] [PubMed] [Google Scholar]
- 44.El-Obeid A, Al-Harbi S, Al-Jomah N, Hassib A. Herbal melanin modulates tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6) and vascular endothelial growth factor (VEGF) production. Phytomedicine. 2006;13:324–333. doi: 10.1016/j.phymed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Singh AP, Singh RB, Mishra S. Microbial and biochemical aspects of antibiotic producing microorganisms from soil samples of certain industrial area of india-an overview. Open Nutraceuticals J. 2012;5:107–112. doi: 10.2174/1876396001205010107. [DOI] [Google Scholar]
- 46.Williams ST, Vickers JC. Detection of Actinomycetes in the natural environment: Problems and perspectives. Biol. Actinomycetes. 1988;88:265–270. [Google Scholar]
- 47.Kitouni M, et al. Isolation of Actinomycetes producing bioactive substances from water, soil and tree bark samples of the north-east of Algeria. J. Mycol. Med. 2005;15:45–51. doi: 10.1016/j.mycmed.2004.12.005. [DOI] [Google Scholar]
- 48.Rossi-Tamisier M, Benamar S, Raoult D, Fournier PE. Cautionary tale of using 16s rRNA gene sequence similarity values in identification of human-associated bacterial species. Int. J. Syst. Evol. Microbiol. 2015;65:1929–1934. doi: 10.1099/ijs.0.000161. [DOI] [PubMed] [Google Scholar]
- 49.Sengupta S, Pramanik A, Ghosh A, Bhattacharyya M. Antimicrobial activities of Actinomycetes isolated from unexplored regions of Sundarbans mangrove ecosystem. BMC Microbiol. 2015;15:1–16. doi: 10.1186/s12866-015-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kunwar A, Priyadarsini K. Free radicals, oxidative stress and importance of antioxidants in human health. J. Med. Allied Sci. 2011;1:53–60. [Google Scholar]
- 51.Ser HL, et al. Streptomyces antioxidans sp. nov., a novel mangrove soil Actinobacterium with antioxidative and neuroprotective potentials. Front. Microbiol. 2016;7:1–14. doi: 10.3389/fmicb.2016.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kato S, et al. Studies on free radical scavenging substances from microorganisms I. Carazostatin, a new free radical scavenger produced by Streptomyces Chromofuscus DC 118. J. Antibiot. (Tokyo). 1989;42:1879–1881. doi: 10.7164/antibiotics.42.1879. [DOI] [PubMed] [Google Scholar]
- 53.Shin-ya K, Tanaka M, Furihata K, Hayakawa Y, Seto H. Structure of carquinostatin A, a new neuronal cell protecting substance produced by Streptomyces exfoliatus. Tetrahedron Lett. 1993;34:4943–4944. doi: 10.1016/S0040-4039(00)74052-4. [DOI] [Google Scholar]
- 54.Hosoya Y, et al. The structure of diphenazithionin, a novel antioxidant from Streptomyces griseus ISP 5236. Tetrahedron Lett. 1996;37:9227–9228. doi: 10.1016/S0040-4039(96)02190-9. [DOI] [Google Scholar]
- 55.Cheng C, et al. Ageloline A, new antioxidant and antichlamydial quinolone from the marine sponge-derived bacterium Streptomyces sp. SBT345. Tetrahedron Lett. 2016;57:2786–2789. doi: 10.1016/j.tetlet.2016.05.042. [DOI] [Google Scholar]
- 56.Law JWF, et al. Diversity of Streptomyces spp. from mangrove forest of Sarawak (Malaysia) and screening of their antioxidant and cytotoxic activities. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-51622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yilma S, et al. Large-conductance cholesterol-amphotericin B channels in reconstituted lipid bilayers. Biosens. Bioelectron. 2007;22:1359–1367. doi: 10.1016/j.bios.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Tan LTH, et al. Streptomyces sp. MUM212 as a source of antioxidants with radical scavenging and metal chelating properties. Front. Pharmacol. 2017;8:1–18. doi: 10.3389/fphar.2017.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- 61.Aouiche A, et al. Activité antimicrobienne de Streptomyces sp. PAL111 d’origine saharienne contre divers microorganismes cliniques et toxinogènes résistants aux antibiotiques. J. Mycol. Med. 2012;22:42–51. doi: 10.1016/j.mycmed.2011.12.077. [DOI] [PubMed] [Google Scholar]
- 62.Wook KJ, Eun HJ, Hun KS, Hang KK. Antimicrobial activity of alk(en)yl sulfides found in essential oils of garlic and onion. Food Sci. Biotechnol. 2004;13:235–239. [Google Scholar]
- 63.Kumar S, Ritika A. A brief review of the biological potential of indole derivatives. Futur. J. Pharm. Sci. 2020;6:7789. doi: 10.1186/s43094-020-00141-y. [DOI] [Google Scholar]
- 64.Gamir J, Pastor V, Cerezo M, Flors V. Identification of indole-3-carboxylic acid as mediator of priming against Plectosphaerella cucumerina. Plant Physiol. Biochem. 2012;61:169–179. doi: 10.1016/j.plaphy.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Raharja N, Widanarni I, Wahyudi AT. Marine sponge-associated bacteria as biocontrol agents of vibriosis on whiteleg shrimp caused by Vibrio parahaemolyticus. Biodiversitas. 2019;20:3164–3169. [Google Scholar]
- 66.Ser HL, et al. Presence of antioxidative agent, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- in newly isolated Streptomyces mangrovisoli sp. nov. Front. Microbiol. 2015;6:1–11. doi: 10.3389/fmicb.2015.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanjenbam P, Kannabiran K. VITPK9 isolated from the salt spring habitat of Manipur, India. Sci. Rep. 2016;10:265–270. [Google Scholar]
- 68.Ubaid JM, Hussein HM, Hameed IH. Determination of bioactive chemical composition of callosobruchus maculutus and investigation of its anti-fungal activity. Int. J. Pharmacogn. Phytochem. Res. 2016;8:1293–1299. [Google Scholar]
- 69.Maher T, et al. Optimization of ultrasound-assisted extraction of bioactive compounds from acacia seyal gum using response surface methodology and their chemical content identification by raman, FTIR, and GC-TOFMS. Antioxidants. 2021;10:555. doi: 10.3390/antiox10101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hwang BK, Lim SW, Kim BS, Lee JY, Moon SS. Isolation and in vivo and in vitro antifungal activity of phenylacetic acid and sodium phenylacetate from Streptomyces humidus. Appl. Environ. Microbiol. 2001;67:3739–3745. doi: 10.1128/AEM.67.8.3739-3745.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim Y, et al. Identification and antimicrobial activity of phenylacetic acid produced by bacillus licheniformis isolated from fermented Soybean, Chungkook-Jang. Curr. Microbiol. 2004;48:312–317. doi: 10.1007/s00284-003-4193-3. [DOI] [PubMed] [Google Scholar]
- 72.Nahar L, Russell WR, Middleton M, Shoeb M, Sarker SD. Antioxidant phenylacetic acid derivatives from the seeds of Ilex aquifolium. Acta Pharm. 2005;55(2):187–93. [PubMed] [Google Scholar]
- 73.Jackson, M. L. Soil chemical analysis prentice hall of India. Pvt. Ltd. New Delhi498, (1973).
- 74.Valan AM, Duraipandiyan V, Agastian P, Ignacimuthu S. In vitro antimicrobial activity of Streptomyces spp. ERI-3 isolated from Western Ghats rock soil (India) J. Mycol. Med. 2009;19:22–28. doi: 10.1016/j.mycmed.2008.12.002. [DOI] [Google Scholar]
- 75.Marimuthu S, et al. Antifungal activity of Streptomyces sp. SLR03 against tea fungal plant pathogen Pestalotiopsis theae. J. King Saud Univ. Sci. 2020;32:3258–3264. doi: 10.1016/j.jksus.2020.08.027. [DOI] [Google Scholar]
- 76.Badji B, Riba A, Mathieu F, Lebrihi A, Sabaou N. Activité antifongique d’une souche d'Actinomadura d'origine saharienne sur divers champignons pathogènes et toxinogènes. J. Mycol. Med. 2005;15:211–219. doi: 10.1016/j.mycmed.2005.07.001. [DOI] [Google Scholar]
- 77.Ragnaud JM, Marceau C, Roche-Bezian MC, Wone C. Infection péritonéale à Trichoderma koningii sur dialyse péritonéale continue ambulatoire. Médecine Mal. Infect. 1984;14:402–405. doi: 10.1016/S0399-077X(84)80067-0. [DOI] [Google Scholar]
- 78.Verrier T, et al. Infection pulmonaire invasive à Trichoderma longibrachiatum : À propos d’un cas. J. Mycol. Med. 2015;25:240. doi: 10.1016/j.mycmed.2015.06.056. [DOI] [Google Scholar]
- 79.Dieye C, et al. Characterization of Fusarium species associated with mango malformation disease in Southern Senegal. Int. J. Adv. Res. 2021;9:322–331. doi: 10.21474/IJAR01/12587. [DOI] [Google Scholar]
- 80.Badji B, Zitouni A, Mathieu F, Lebrihi A, Sabaou N. Antimicrobial compounds produced by Actinomadura sp AC104 isolated from an Algerian Saharan soil. Can. J. Microbiol. 2006;52:373–382. doi: 10.1139/w05-132. [DOI] [PubMed] [Google Scholar]
- 81.Juvonen, T. Milloin laskevan aortan aneurysmat pitäisi leikata? Duodecim vol. 114 (1998). [PubMed]
- 82.Tangjitjaroenkun J, Pluempanupat W, Tangchitcharoenkhul R, Yahayo W, Supabphol R. Antibacterial, antioxidant, cytotoxic effects and GC-MS analysis of mangrove-derived Streptomyces achromogenes TCH4 extract. Arch. Biol. Sci. 2021;73:223–235. doi: 10.2298/ABS210320017T. [DOI] [Google Scholar]
- 83.Bhat MP, Nayaka S, Kumar RS. A swamp forest Streptomyces sp. strain KF15 with broad spectrum antifungal activity against chilli pathogens exhibits anticancer activity on HeLa cells. Arch. Microbiol. 2022;204:1–17. doi: 10.1007/s00203-022-03147-7. [DOI] [PubMed] [Google Scholar]
- 84.Jinfeng EC, Mohamad RMI, Chai HK, Kok LH, Yoke KC. Analysis of chemical constituents, antimicrobial and anticancer activities of dichloromethane extracts of Sordariomycetes sp. endophytic fungi isolated from Strobilanthes crispus. World J. Microbiol. Biotechnol. 2017;33:5558. doi: 10.1007/s11274-016-2175-4. [DOI] [PubMed] [Google Scholar]
- 85.Bastide A, de Méo M, Andriantsoa M, Laget M, Duménil G. Isolement et sélection de souches d’Actinomycètes productrices de substances antifongiques de structure non-polyénique. MIRCEN J. Appl. Microbiol. Biotechnol. 1986;2:453–466. doi: 10.1007/BF00933368. [DOI] [Google Scholar]
- 86.Hei Y, et al. Antimicrobial activity and biosynthetic potential of cultivable Actinomycetes associated with Lichen symbiosis from Qinghai-Tibet Plateau. Microbiol. Res. 2021;244:126652. doi: 10.1016/j.micres.2020.126652. [DOI] [PubMed] [Google Scholar]
- 87.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. Weisburg. 1991;1991(173):697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bensadón S, Hervert-Hernández D, Sáyago-Ayerdi SG, Goñi I. By-products of opuntia ficus-indica as a source of antioxidant dietary fiber. Plant Foods Hum. Nutr. 2010;65:210–216. doi: 10.1007/s11130-010-0176-2. [DOI] [PubMed] [Google Scholar]
- 90.Bahorun T, et al. Oxygen species scavenging activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneimittelforschung. 1996;46:1086–1089. [PubMed] [Google Scholar]
- 91.Blois M. Antioxidant determinations by the use of a stable free radical. Nature. 1965;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 92.Re R, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 93.Chakraborty B, et al. Streptomyces filamentosus strain KS17 isolated from microbiologically unexplored marine ecosystems exhibited a broad spectrum of antimicrobial activity against human pathogens. Process Biochem. 2022;117:42–52. doi: 10.1016/j.procbio.2022.03.010. [DOI] [Google Scholar]
- 94.Pozsgai G, et al. Analgesic effect of dimethyl trisulfide in mice is mediated by TRPA1 and sst4 receptors. Nitric Oxide Biol. Chem. 2017;65:10–21. doi: 10.1016/j.niox.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 95.Tyagi S, Lee KJ, Shukla P, Chae JC. Dimethyl disulfide exerts antifungal activity against Sclerotinia minor by damaging its membrane and induces systemic resistance in host plants. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-63382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morales-López J, et al. Evaluation of antioxidant and hepatoprotective effects of white cabbage essential oil. Pharm. Biol. 2016;55:233–241. doi: 10.1080/13880209.2016.1258424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsuji K, Ishikawa H. Synthesis and anti-pseudomonal activity of new 2-isocephems with a dihydroxypyridone moiety at C-7. Bioorganic Med. Chem. Lett. 1994;4:1601–1606. doi: 10.1016/S0960-894X(01)80574-6. [DOI] [Google Scholar]
- 98.Sharma RN, Xavier FP, Vasu KK, Chaturvedi SC, Pancholi SS. Synthesis of 4-benzyl-1, 3-thiazole derivatives as potential anti-inflammatory agents: An analogue-based drug design approach. J. Enzyme Inhib. Med. Chem. 2009;24:890–897. doi: 10.1080/14756360802519558. [DOI] [PubMed] [Google Scholar]
- 99.Gürsoy E, Güzeldemirci NU. Synthesis and primary cytotoxicity evaluation of new imidazo[2,1-b]thiazole derivatives. Eur. J. Med. Chem. 2007;42:320–326. doi: 10.1016/j.ejmech.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 100.Floris B, Galloni P, Conte V, Sabuzi F. Tailored functionalization of natural phenols to improve biological activity. Biomolecules. 2021;11:4447. doi: 10.3390/biom11091325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Akin FJ. Anti-tumorigenic effect of maleic hydrazide on mouse skin. J. Agric. Food Chem. 1976;24:672–674. doi: 10.1021/jf60205a003. [DOI] [PubMed] [Google Scholar]
- 102.Hashim A, et al. Control of gray mold disease of tomato caused by botrytis cinerea using bacterial secondary metabolites. Malaysian Appl. Biol. 2020;49:89–97. doi: 10.55230/mabjournal.v49i5.1641. [DOI] [Google Scholar]
- 103.Han Y, et al. Maltol, a food flavoring agent, attenuates acute alcohol-induced oxidative damage in mice. Nutrients. 2015;7:682–696. doi: 10.3390/nu7010682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu X, Zhao M, Liu F, Zeng S, Hu J. Identification of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one as a strong antioxidant in glucose-histidine Maillard reaction products. Food Res. Int. 2013;51:397–403. doi: 10.1016/j.foodres.2012.12.044. [DOI] [Google Scholar]
- 105.Mangamuri U, et al. Bioactive metabolites produced by Streptomyces Cheonanensis VUK-A from Coringa mangrove sediments: Isolation, structure elucidation and bioactivity. 3 Biotech. 2016;6:1–8. doi: 10.1007/s13205-016-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumaran S, et al. Bioactive metabolites produced from Streptomyces enissocaesilis SSASC10 against fish pathogens. Biocatal. Agric. Biotechnol. 2020;29:101802. doi: 10.1016/j.bcab.2020.101802. [DOI] [Google Scholar]
- 107.Fallah-Tafti A, et al. Thiazolyl N-benzyl-substituted acetamide derivatives: Synthesis, Src kinase inhibitory and anticancer activities. Eur. J. Med. Chem. 2011;46:4853–4858. doi: 10.1016/j.ejmech.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 108.Marchese A, et al. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016;210:402–414. doi: 10.1016/j.foodchem.2016.04.111. [DOI] [PubMed] [Google Scholar]
- 109.Kang SH, et al. Anticancer effect of thymol on AGS human gastric carcinoma cells. J. Microbiol. Biotechnol. 2015;26:28–37. doi: 10.4014/jmb.1506.06073. [DOI] [PubMed] [Google Scholar]
- 110.Naveen M, Kavitha R. HPLC, GC–MS and FT-IR analysis of bioactive phytochemicals present in aqueous extract of leaf of Clerodendrum phlomidis: A further evidence for its medicinal diversity. Mater. Today Proc. 2021 doi: 10.1016/j.matpr.2021.05.414. [DOI] [Google Scholar]
- 111.Mukherjee S, Pal M. Quinolines: A new hope against inflammation. Drug Discov. Today. 2013;18:389–398. doi: 10.1016/j.drudis.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 112.Orhan PM, Tekiner B, Suzen S. Recent studies of antioxidant quinoline derivatives. Mini Rev. Med. Chem. 2013;13:365–372. doi: 10.2174/138955713804999793. [DOI] [PubMed] [Google Scholar]
- 113.Musiol R. Quinoline-based HIV integrase inhibitors. Curr. Pharm. Des. 2013;19:1835–1849. doi: 10.2174/1381612811319100008. [DOI] [PubMed] [Google Scholar]
- 114.Musiol R, Serda M, Hensel-Bielowka S, Polanski J. Quinoline-based antifungals. Curr. Med. Chem. 2010;17:1960–1973. doi: 10.2174/092986710791163966. [DOI] [PubMed] [Google Scholar]
- 115.Bongarzone S, Bolognesi ML. The concept of privileged structures in rational drug design: Focus on acridine and quinoline scaffolds in neurodegenerative and protozoan diseases. Expert Opin. Drug Discov. 2011;6:251–268. doi: 10.1517/17460441.2011.550914. [DOI] [PubMed] [Google Scholar]
- 116.Keri RS, Patil SA. Quinoline: A promising antitubercular target. Biomed. Pharmacother. 2014;68:1161–1175. doi: 10.1016/j.biopha.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 117.Afzal O, et al. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015;97:871–910. doi: 10.1016/j.ejmech.2014.07.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated or analyzed during this study, including the nucleotide sequence data, are available as Supplementary Information files. Genome sequences of Streptomyces sp. E23-2, E23-3, E23-4, E23-8, E23-9, E23-10, E23-11, E24-9, E25-9, E25-11 and E25-12 have been deposited in National Centre for Biotechnology Information (NCBI) GenBank (https://www.ncbi.nlm.nih.gov/nucleotide/) under accession numbers of OM883988, OM883989, OM883990, OM883991, OM883992, OM883993, OM883994, OM883995, OM883996, OM883997 and OM883998.