Abstract
The objective of this study was to evaluate the role of regional lymph node (LN) metastasis detected on preoperative CT and/or 18F-fluoro-2-deoxyglucose-positron emission tomography (FDG-PET) scans in the prediction of early tumor recurrence after curative surgical resection of pancreatic ductal adenocarcinoma (PDAC). This retrospective study included 137 patients who underwent upfront surgery with R0 resection of PDAC between 2013 and 2016. Regional LN metastasis was identified using two criteria: positive findings for regional LN metastasis on either preoperative CT or FDG-PET scans (LNOR), or on both preoperative CT and FDG-PET scans (LNAND). A total of 55 patients had early tumor recurrence within 12 months after curative resection. Univariable and multivariable Cox proportional hazard regression analysis showed that preoperative carbohydrate antigen 19–9 (CA19-9) levels, preoperative locally advanced status, and regional LN metastasis (both LNOR and LNAND criteria) were significant risk factors for early recurrence. Positive LNOR and LNAND showed significantly poorer recurrence-free survival compared to negative regional LN metastasis groups (p = 0.048 and p = 0.020, respectively). Compared with the LNAND criteria, the LNOR criteria provided higher sensitivity (22.4% vs. 15.5%, p = 0.046) and a higher negative predictive value (61.9% vs. 59.8%, p = 0.046). The LNOR definition provided more sensitive and accurate performance in diagnosing preoperative regional LN metastasis.
Subject terms: Cancer imaging, Pancreatic cancer
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is notorious for its poor prognosis. Although curative resection is desirable, many patients are diagnosed in an unresectable state1,2. Even in resectable or borderline resectable cases according to the National Comprehensive Cancer Network (NCCN) guidelines, the tumor recurrence rate is also high3–5. In addition, while R0 resection is one of the most important factors that influence prognosis, recurrences occur even in these cases6,7.
Regional LN metastasis have been reported in 14%–75% of patients with resectable PDAC8–10. Although regional LN metastasis is not a determining factor in assessing resectability per the NCCN guidelines, the NCCN guideline recommends considering neoadjuvant therapy in the presence of large regional LNs on preoperative imaging studies11. Regional LN metastasis indicates a high risk of disseminated disease and has been identified as a risk factor for early recurrence after surgical resection11–14. However, the impact of preoperatively diagnosed regional LN metastasis on early recurrence has been seldom reported with conflicting results15–17. As such, the clinical implications of regional LN metastasis identified on preoperative computed tomography (CT) or 18F-fluoro-2-deoxyglucose-positron emission tomography (FDG-PET) scans remain controversial2,4,18.
Indeed, conventional imaging modalities, including CT and FDG-PET scans, are often insufficient for the preoperative diagnosis of LN metastasis in PDAC patients19. CT studies have low sensitivity (7%–37%) with a more acceptable specificity (64–99%) in determining regional LN metastasis2,20–22. In addition, there are no established criteria for determining metastatic LNs on CT scans2. The advantages of FDG-PET scans include the capability to detect micro-metastases in small or normal-sized regional LNs, with slightly better performance (sensitivity: 30%–49%, specificity: 63%–93%) compared with CT scans20,23,24. However, evidence showing the performance of imaging studies in identifying regional LNs preoperatively are limited. Hence, collective or combined regional LN metastasis identification using CT and/or FDG-PET scans may help improve the diagnostic performance.
Therefore, this study aimed to evaluate the role of regional LN metastasis identified on preoperative CT and/or FDG-PET scans in predicting early tumor recurrence after curative surgical resection of PDAC.
Results
The baseline characteristics of the included patients are shown in Table 1. The median preoperative carbohydrate antigen 19–9 (CA19-9) level was 68.9 U/mL. Positive FDG uptake of the tumor was noted in 82.5% (113/137) of cases, with significant correlation with tumor size (Supplementary Table 1). Of all the tumors detected, 49.6% (68/137) were in the pancreatic head, and 82.5% (113/137) were classified as resectable according to preoperative imaging scans. PPPD (pylorus-preserving pancreaticoduodenectomy) was performed in 48.2% (66/137) of the patients. Regional LN metastasis was suggested in 13.9% (19/137) and 10.9% (15/137) of the patients according to the LNOR and LNAND definitions, respectively. Pathologic regional LN metastasis was confirmed in 42.3% (58/137) of the patients. Adjuvant therapy was administered in 73.7% (101/137) of the patients who received chemotherapy and/or concurrent chemoradiation therapy. Death occurred in 37.2% (51/137) of the patients, and recurrence was detected in 69.3% (95/137) of the patients. Among the 95 patients who developed recurrence, 57.9% (55/95) had early recurrence. The median overall survival (OS) was 34.1 months (interquartile range (IQR), 15.0–51.2 months), while the median recurrence-free survival (RFS) was 15.4 months (IQR, 6.6–43.4 months).
Table 1.
Baseline characteristics of the study population.
| Variables | Values |
|---|---|
| Age* | 64.9 ± 10.0 |
| Male | 79 (57.7%) |
| BMI (kg/m2)* | 23.0 ± 2.8 |
| Preoperative CA19-9 (U/mL)† | 68.9 (19.6–284.8) |
| Head location | 68 (49.6%) |
| Tumor size† | 25.0 (18.0–31.0) |
| Positive uptake of tumor on FDG-PET scan | 113 (82.5%) |
| LNOR | 19 (13.9%) |
| LNAND | 15 (10.9%) |
| NCCN resectability | |
| Resectable | 113 (82.5%) |
| Borderline resectable | 14 (10.2%) |
| Locally advanced | 10 (7.3%) |
| Type of surgery | |
| PPPD | 66 (48.2%) |
| Distal pancreatectomy | 63 (46.0%) |
| Total pancreatectomy | 8 (5.8%) |
| Pathologic T stage (pT)‡ | |
| T1 | 10 (7.3%) |
| T2 | 27 (19.8%) |
| T3 | 99 (72.3%) |
| T4 | 1 (0.7%) |
| Pathologic LN metastasis (pN) ‡ | 58 (42.3%) |
| Adjuvant therapy | 101 (73.7%) |
| Recurrence | 95 (69.3%) |
| Early recurrence | 55 (57.9%) |
| Location of recurrence | |
| Locoregional recurrence | 31 (22.6%) |
| Distant recurrence | 64 (46.7%) |
| Death | 51 (37.2%) |
| Overall survival (months)† | 34.1 (15.0–51.2) |
| Recurrence-free survival (months)† | 15.4 (6.6–43.4) |
Data are presented as numbers (%) unless otherwise indicated. *Data are presented as mean ± standard deviation. †Data are presented as median (1st quartile–3rd quartile). ‡TNM staging according to the 8th edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM staging system. BMI, body mass index; CA19-9, carbohydrate antigen 19-9; FDG-PET, 18F-fluoro-2-deoxyglucose-positron emission tomography; LNOR, regional LN metastasis on preoperative CT or FDG-PET scans; LNAND, regional LN metastasis on preoperative CT and FDG-PET scans; NCCN, National Comprehensive Cancer Network; PPPD, pylorus-preserving pancreaticoduodenectomy.
Preoperative characteristics according to recurrence group
The differences in preoperative risk factors between the early recurrence group and the no or late recurrence group are shown in Table 2. Significant differences between the two recurrence groups were observed in the NCCN resectability (p = 0.040) and regional LN metastasis by both LNOR and LNAND definitions (p = 0.014 and p = 0.012, respectively). There were significantly more deaths in the early recurrence group (65.5% (36/55) vs. 18.3% (15/82)) compared to the no or late recurrence group. The early recurrence group showed shorter OS than the no or late recurrence group (median 13.6 months vs. 43.8 months) (Fig. 1). On comparison of early recurrence and late recurrence groups, LNOR occurred more frequently in the early recurrence group than in the late recurrence group (23.6% (13/55) vs. 5.0% (2/40), p = 0.030) (Supplementary Table 2. Death also occurred more frequently in the early recurrence group than in the late recurrence group (65.5% (36/55) vs. 27.5% (11/40), p = 0.001), with significantly shorter OS (median 13.6 months vs. 38.4 months, p < 0.001).
Table 2.
Comparison of preoperative characteristics according to the status of tumor recurrence.
| Variables | No or late recurrence (n = 82) | Early recurrence (n = 55) | p-value |
|---|---|---|---|
| Age* | 65.4 ± 9.3 | 64.0 ± 11.1 | 0.445 |
| Male | 36 (43.9%) | 22 (40.0%) | 0.782 |
| BMI (kg/m2)* | 23.2 ± 2.7 | 22.8 ± 2.9 | 0.420 |
| Preoperative CA19-9 (U/mL)† | 56.5 (16.6–232.5) | 108.2 (22.8–329.6) | 0.238 |
| Head location | 36 (43.9%) | 32 (58.2%) | 0.143 |
| Tumor size† | 24.5 (17.0–32.0) | 25.0 (20.0–30.0) | 0.871 |
| Positive uptake of tumor on FDG-PET scan | 65 (79.3%) | 48 (87.3%) | 0.362 |
| LNOR | 6 (7.3%) | 13 (23.6%) | 0.014 |
| LNAND | 4 (4.9%) | 11 (20.0%) | 0.012 |
| NCCN resectability | 0.040 | ||
| Resectable | 73 (89.0%) | 40 (72.7%) | |
| Borderline resectable | 6 (7.3%) | 8 (14.5%) | |
| Locally advanced | 3 (3.7%) | 7 (12.7%) | |
| Death | 15 (18.3%) | 36 (65.5%) | < 0.001 |
| Overall survival (months)† | 43.8 (32.3–57.2) | 13.6 (9.6–21.4) | < 0.001 |
Data are presented as numbers (%) with the p-values of Fisher’s exact test unless otherwise indicated. *Data are presented as mean ± standard deviation, with the p-value of Student’s t-test. †Data are presented as median (1st quartile–3rd quartile), with the p-value of the Mann–Whitney U test. BMI, body mass index; CA19-9, carbohydrate antigen 19–9; FDG-PET, 18F-fluoro-2-deoxyglucose-positron emission tomography; LNOR, regional LN metastasis on preoperative CT or FDG-PET scans; LNAND, regional LN metastasis on preoperative CT and FDG-PET scans; NCCN, National Comprehensive Cancer Network.
Significant values are in [bold].
Figure 1.

Overall survival (OS) of pancreatic ductal adenocarcinoma (PDAC) patients according to the recurrence group. The Kaplan-Meier curve showed that the early recurrence group had significantly poorer OS than the no or late recurrence groups.
Preoperative risk factors for early recurrence
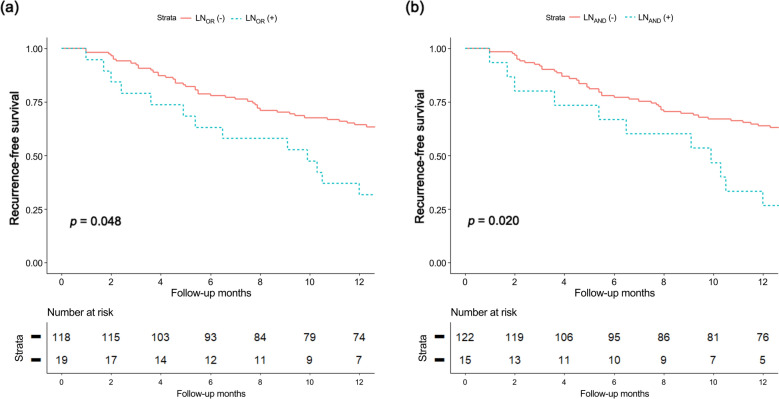
On univariable Cox proportional hazard analysis of early recurrence, preoperative CA19-9 level (× 100 U/mL), preoperative locally advanced status, LNOR, and LNAND were significant risk factors. On multivariable Cox proportional hazard analysis using either LNOR or LNAND definitions, regional LN metastasis by any definition was a significant risk factor for early recurrence, along with preoperative CA19-9 levels and preoperative locally advanced status (Table 3). Positive regional LN metastasis according to the LNOR (Fig. 2a) and LNAND (Fig. 2b) definitions showed significantly poorer RFS (p = 0.048 and p = 0.020, respectively). A representative case is shown in Fig. 3.
Table 3.
Univariable and multivariable Cox proportional hazard analyses between preoperative characteristics and early recurrence.
| Variables | Univariable | Multivariable with LNOR | Multivariable with LNAND | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age | 0.989 | 0.963–1.016 | 0.417 | – | – | – | – | – | – |
| Male sex | 1.180 | 0.688–2.025 | 0.547 | – | – | – | – | – | – |
| BMI (kg/m2) | 0.953 | 0.864–1.051 | 0.338 | – | – | – | – | – | – |
| Preoperative CA19-9 (× 100 U/mL) | 1.008 | 1.002–1.015 | 0.012 | 1.009 | 1.002–1.016 | 0.010 | 1.011 | 1.004–1.017 | 0.003 |
| Tumor size | 0.996 | 0.981–1.011 | 0.563 | – | – | – | – | – | – |
| Head location | 1.523 | 0.891–2.604 | 0.124 | – | – | – | – | – | – |
| Positive uptake of tumor on FDG-PET scan | 1.385 | 0.543 – 3.529 | 0.495 | ||||||
| NCCN resectability | 0.020 | < 0.001 | 0.023 | ||||||
| Resectable | 1 | Reference | 1 | Reference | 1 | Reference | |||
| Borderline resectable | 1.748 | 0.817–3.737 | 0.150 | 1.551 | 0.705–3.413 | 0.275 | 1.382 | 0.732–2.612 | 0.319 |
| Locally advanced (unresectable) | 2.797 | 1.249–6.262 | 0.012 | 2.445 | 1.073–5.571 | 0.033 | 2.649 | 1.275–5.507 | 0.009 |
| LNOR | 2.382 | 1.278–4.442 | 0.006 | 2.027 | 1.057–3.885 | 0.033 | – | – | – |
| LNAND | 2.494 | 1.287–4.835 | 0.007 | – | – | – | 2.086 | 1.054–4.125 | 0.035 |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; CA19-9, carbohydrate antigen 19–9; FDG-PET, 18F-fluoro-2-deoxyglucose-positron emission tomography; LNOR, regional LN metastasis on preoperative CT or FDG-PET scans; LNAND, regional LN metastasis on preoperative CT and FDG-PET scans; NCCN, National Comprehensive Cancer Network.
Significant values are in [bold].
Figure 2.
Recurrence-free survival (RFS) according to the status of regional lymph node (LN) metastasis on preoperative images. The positive regional LN metastasis group according to both (a) LNOR and (b) LNAND criteria showed significantly lower RFS than the no regional LN metastasis group.
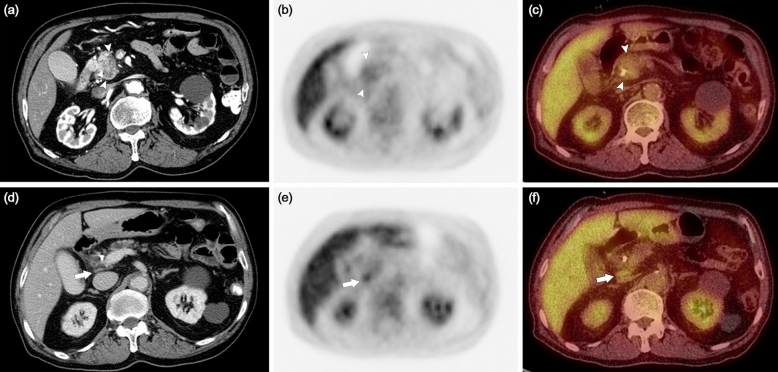
Figure 3.
Contrast-enhanced dynamic computed tomography (CT) and 18F-fluoro-2-deoxyglucose-positron emission tomography (FDG-PET) scans of a 76-year old male patient diagnosed with pancreatic head cancer. A 2.7 cm sized hypoattenuating mass (arrowheads) is noted on the arterial phase of the CT (a), which shows significant FDG uptake on the FDG-PET (b) and FDG-PET/CT scans (c). A small lymph node (LN) was noted in the portocaval space (arrow) on the venous phase of the CT (d), with significant FDG uptake on the FDG-PET (e) and FDG-PET/CT scans (f). The patient was categorized as positive regional LN metastasis by the LNOR definition, but negative regional LN metastasis by the LNAND definition. After pylorus-preserving pancreaticoduodenectomy and regional LN dissection, regional LN metastasis was confirmed. Early local recurrence was identified on CT and FDG-PET scans performed 10.5 months after surgery. Patient expired 15.9 months after surgery.
Regional LN metastasis by LNOR and LNAND definitions
Regional LN metastasis according to the LNOR and LNAND definitions was compared to the pathologic regional LN metastasis as the reference standard (Table 4). Compared with the LNAND criteria, the LNOR definition provided a significantly higher sensitivity (22.4% vs. 15.5%, p = 0.046) and negative predictive value (NPV; 61.9% vs. 59.8%, p = 0.046). The LNOR definition also provided a higher positive predictive value (PPV; 68.4% vs. 60.0%, p = 0.122) and accuracy (62.8% vs. 59.9%, p = 0.134), but without statistical significance. No difference was observed in the specificity between the two definitions (92.4%).
Table 4.
Diagnostic performance of regional LN metastasis by LNOR and LNAND definitions.
| Sensitivity | Specificity | PPV | NPV | Accuracy (95% confidence interval) | ||
|---|---|---|---|---|---|---|
| LNOR | 0.224 | 0.924 | 0.684 | 0.619 | 0.628 (0.541–0.709) | |
| LNAND | 0.155 | 0.924 | 0.600 | 0.598 | 0.599 (0.511–0.681) | |
| p-value | 0.046* | 1.000* | 0.122† | 0.046† | 0.134* | |
PPV, positive predictive value; NPV, negative predictive value; LNOR, regional LN metastasis on preoperative CT or FDG-PET (18F-fluoro-2-deoxyglucose-positron emission tomography) scans; LNAND, regional LN metastasis on preoperative CT and FDG-PET scans; *McNemar’s test was used. †Generalised estimating equation was used.
Discussion
This study showed that preoperative CA19-9 level, NCCN resectability, and regional LN metastasis can be used to identify patients at risk of developing early tumor recurrence after undergoing surgical resection. Regional LN metastasis according to the LNOR and LNAND definitions was a significant risk factor for early recurrence, with the LNOR definition showing higher sensitivity than the LNAND definition.
PDAC patients have a high incidence of regional LN metastasis, with a positivity rate of up to 80% in those with resectable PDAC25. In this study, regional LN metastasis detected on preoperative imaging was a significant risk factor for early tumor recurrence, which is consistent with previous reports13,15. However, the preoperative identification of regional LN metastasis on imaging studies remains challenging due to the lack of consensus on the diagnostic criteria and overall low sensitivity20,21,23,26. Our results showed that both the LNOR and LNAND definitions provided sensitivity, specificity, PPV, NPV, and accuracy which fell within the range of previous reports2,27–30. The LNOR definition provided more sensitive and accurate performance in predicting regional LN metastasis by identifying LNs with any signs of positive metastasis on either preoperative CT or FDG-PET scans. Considering that the aim of preoperative imaging studies may be in discerning those patients who will benefit from curative surgery, the more sensitive LNOR definition may be more appropriate in the preoperative setting to help guide the course of treatment. In addition, performing both CT and FDG-PET may be necessary in correctly identifying regional LN metastasis with high diagnostic accuracy, helping to better predict the risk of early tumor recurrence after curative resection. Further efforts to improve the diagnostic performance for preoperative regional LN metastasis are necessary.
Despite its relatively low diagnostic performance, the preoperative diagnosis of LN metastasis based on CT or FDG-PET is a significant risk factor for early recurrence. Significant differences were observed in the RFS according to the presence of regional LN metastasis. Moreover, PDAC with high-risk factors but without distant metastasis at initial diagnosis is still considered as a systemic disease, even in resectable cases, suggesting the necessity of neoadjuvant therapy in patients at an increased risk of early tumor recurrence11,31. Our results suggest that positive regional LN metastasis on preoperative CT or FDG-PET could serve as a practical guide for determining the appropriate treatment.
The preoperative CA19-9 level was a significant factor for early tumor recurrence in our study. Previous studies have also identified CA19-9 level as a useful predictor of early tumor recurrence within 6 months or 12 months after curative resection, poor OS and RFS4,32–34. However, although any elevation in the CA19-9 level is considered as a poor prognostic factor, there is no consensus on the cutoff of CA19-9 level4,33–38. A dichotomous analysis of the CA19-9 cut-off values for a higher risk of early recurrence has yielded a wide range of values, from 50 U/mL to 529 U/mL4,32–34. Others showed that the analysis of CA19-9 level as continuous variable better reflects the proportional risk of early recurrence, suggesting that dichotomisation of CA19-9 level diminishes its predictive power34,39. In this study, we used the preoperative CA19-9 value divided by 100 which may provide easier calculation than the previously used log values, thereby facilitating the prediction of early tumor recurrence in the clinical setting34,39.
NCCN resectability is a widely accepted and utilised prognostic risk factor for tumor recurrence and OS13,39. In our study, even the locally advanced group successfully underwent R0 resection, but showed significantly lower RFS compared to the resectable group. Therefore, these patients may be at higher risk for early tumor recurrence despite undergoing with R0 resection and may benefit from other treatment methods40,41. On the contrary, the RFS of the borderline resectable group was not significantly different from that of both resectable and locally advanced groups, which may indicate the heterogeneity of the borderline resectable group in terms of early tumor recurrence. Therefore, the borderline resectable group may be better classified using additional factors in addition to the tumor-vessel relationship, such as regional LN status and CA19-9 level as suggested by the recent consensus guidelines42.
Our study has several limitations. First, its retrospective nature included inherent bias. Second, patients with Lewis-negative pancreatic cancer was not accounted for in this study. Third, preoperatively suspected regional LN metastasis was not pathologically confirmed in a node-by-node manner, leading to difficulties in assessing the diagnostic accuracy of LN metastasis identified on preoperative scans. In addition, N1 or N2 was not divided based on the number of positive LNs, as suggested by the 8th American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM staging system.
In conclusion, preoperative LN metastasis detected on CT or FDG-PET scans, along with elevated serum CA19-9 levels, and locally advanced status were significant risk factors for predicting early recurrence of PDAC after surgery. The LNOR definition provided more sensitive and accurate performance in diagnosing preoperative regional LN metastasis.
Methods
This retrospective study was approved by the Institutional Review Board at Yonsei University College of Medicine, and was conducted in accordance with the Declaration of Helsinki as revised in 2013. Informed consent was obtained from all patients enrolled in this study. The requirement for written informed consent was waived owing to the retrospective nature of the study.
Study population
The institutional cohort of the PDAC registry from a tertiary hospital (Yonsei University Medical Center, Seoul, Korea) was retrospectively searched to collect consecutive data of patients with pathologically confirmed PDAC between January 2013 and December 2016 (Fig. 4). A total of 1,159 patients were identified. Patients diagnosed with PDAC who underwent curative surgical resection at our institution were included in the study. Exclusion criteria included 1) disseminated disease at diagnosis, 2) no surgical resection, 3) R1 or R2 resection, 4) administration of neoadjuvant therapy (either chemotherapy or concurrent chemoradiation therapy), and 5) no preoperative FDG-PET scans. A total of 137 patients who underwent upfront surgery resulting in R0 resection were included in the final analysis.
Figure 4.
Flow diagram of patients included in this study. From an existing cohort of patients with pathologically proven pancreatic adenocarcinoma from 2013 to 2016, a total of 137 patients who underwent upfront surgery and successful R0 resection were eligible for the final analysis. FDG-PET, 18F-fluoro-2-deoxyglucose-positron emission tomography.
Medical record and image review
The PDAC cohort was compiled by two radiologists after independently reviewing the preoperative CT and FDG-PET scans, which were carried out as routine preoperative work-up at our institution. The two readers were blinded to the clinical and pathologic information of the patients. From this cohort, results of the CT and FDG-PET scans performed within one month prior to surgery, age, sex, and serum CA19-9 level of the eligible patients were obtained. The tumor location was categorised as head (including head, uncinate process, and neck) or body/tail. The tumor size was categorised as 20 mm and smaller in size, or larger than 20mm adapted from the 8th AJCC/UICC TNM staging system43. The presence or absence of the main pancreatic duct or bile duct dilatation were recorded. Tumor resectability was evaluated based on the NCCN guidelines (version 2.2021)11. Clinical T stage was classified according to the 8th edition of the AJCC/UICC TNM staging system43. The regional LNs for PDAC in the pancreatic head were found in the supra- or infra-pyloric areas, along the common hepatic artery, along the hepatoduodenal ligament, around the celiac artery, retropancreatic area, and peripancreatic area44. The regional LNs for PDAC at body or tail location included LNs along the common hepatic artery, hepatoduodenal ligament, splenic hilum, and retropancreatic, peripancreatic areas, and the mesenteric root areas44. Regional LN metastasis on contrast-enhanced CT was identified when LNs showed increased size (short axis larger than 10 mm) or necrosis45. Positive FDG uptake was defined as increased FDG accumulation compared with the surrounding tissues that was not related to normal physiologic uptake. LNOR was defined as a regional LN metastasis identified on contrast-enhanced CT or FDG-PET scans, while LNAND was defined as a regional LN metastasis identified on contrast-enhanced CT findings and FDG-PET scans20,46. Pancreatic CT was performed as recommended by NCCN guidelines, and detailed protocol of FDG-PET scan are summarized (Supplementary Methods 1 and 2)11,47.
Postoperative follow-up and diagnosis of tumor recurrence
Postoperative surveillance included CA19-9 measurement, contrast-enhanced abdomen-pelvic CT, FDG-PET, and chest CT scans. Tumor recurrence was diagnosed using imaging studies. In patients where imaging findings were inconclusive, tumor recurrence was confirmed by pathologic diagnosis. Early recurrence was defined as tumor recurrence within 12 months after surgery14. OS was defined as the period from the date of diagnosis to the date of death or last follow-up. RFS was defined as the period from the date of surgery to the date of tumor recurrence or last follow-up. Patients were routinely followed up using CT, serum CA19-9 and CEA levels at intervals of two or three months until at least 12 months after surgery. Post-operative adjuvant therapy (chemotherapy with or without radiation therapy) was administered at the clinician’s discretion, according to the pathologic findings.
Statistical analysis
All analyses were performed using a commercial software (R version 4.2.1, R Foundation for Statistical Computing, Vienna, Austria)48. Continuous variables between the recurrence groups were compared using Student’s t-test for parametric values and Mann-Whitney U-test for non-parametric variables. The Fisher’s exact test was used to compare the frequencies of variables between the two recurrence groups. Spearman’s correlation was used to analyse the correlation between tumor size, positive FDG uptake of the tumor, and positive FDG uptake of regional LNs. Kaplan-Meier survival analyses with log-rank tests were used to determine the OS and RFS. Cox proportional hazard regression analysis was used to determine the preoperative risk factors for early tumor recurrence. Considering the wide range of CA19-9 values (range, 0.1–20,000 U/mL), the preoperative CA19-9 level divided by 100 (× 100 U/mL) was utilized to facilitate data manipulation. The diagnostic performances of LNOR and LNAND definitions were determined based on the pathologically confirmed metastatic regional LNs as a reference standard on a per-patient basis using confusion matrix analyses. The performances of LNOR and LNAND were compared using the McNemar’s test to determine the sensitivity and specificity, and the generalised estimating equation for PPV and NPV. A p-value of < 0.05 was considered significant.
Supplementary Information
Author contributions
M.S.P. had full access to all the data in the study and was responsible for maintaining the integrity of the data and the accuracy of the data analysis. J.K.Y. contributed to the study concept and design, data acquisition, data analysis and interpretation, and manuscript drafting. K.H.H. contributed to the analysis and interpretation of data and critical revision of the manuscript. S.S.K., M.J.K., H.S.L., B.S.M., S.H.H., M.Y., and H.K.H. contributed to the data acquisition and critical revision of the manuscript for important intellectual content.
Funding
The authors state that this work has not received any funding.
Data availability
The datasets generated or analysed during the study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-22126-y.
References
- 1.Diehl S, Lehmann K, Sadick M, Lachmann R, Georgi MJR. Pancreatic cancer: value of dual-phase helical CT in assessing resectability. Radiology. 1998;206:373–378. doi: 10.1148/radiology.206.2.9457188. [DOI] [PubMed] [Google Scholar]
- 2.Roche CJ, et al. CT and pathologic assessment of prospective nodal staging in patients with ductal adenocarcinoma of the head of the pancreas. Am. J. Roentgenol. 2003;180:475–480. doi: 10.2214/ajr.180.2.1800475. [DOI] [PubMed] [Google Scholar]
- 3.Assifi MM, et al. Neoadjuvant therapy in pancreatic adenocarcinoma: a meta-analysis of phase II trials. Surgery. 2011;150:466–473. doi: 10.1016/j.surg.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishio K, et al. Preoperative predictors for early recurrence of resectable pancreatic cancer. World J. Surg. Oncol. 2017;15:1–10. doi: 10.1186/s12957-016-1078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Geus, S. Neoadjuvant therapy versus upfront surgical strategies in localized pancreatic cancer: a Markov decision analysis. Emerg. Mol. Biomark. Treat. Strateg. Resectable Pancreatic Cancer, 99 (2021). [DOI] [PubMed]
- 6.Wagner M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. J. Br. Surg. 2004;91:586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 7.Fischer R, et al. Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy. Saudi J. Gastroenterol. 2012;18:118. doi: 10.4103/1319-3767.93815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakao A, et al. Lymph node metastases in carcinoma of the head of the pancreas region. J. Br. Surg. 1995;82:399–402. doi: 10.1002/bjs.1800820340. [DOI] [PubMed] [Google Scholar]
- 9.Bluemke DA, et al. Potentially resectable pancreatic adenocarcinoma: spiral CT assessment with surgical and pathologic correlation. Radiology. 1995;197:381–385. doi: 10.1148/radiology.197.2.7480681. [DOI] [PubMed] [Google Scholar]
- 10.Coley S, Strickland N, Walker J, Williamson R. Spiral CT and the pre-operative assessment of pancreatic adenocarcinoma. Clin. Radiol. 1997;52:24–30. doi: 10.1016/S0009-9260(97)80301-7. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines In Oncology: Pancreatic Adenocarcinoma, version 2.2021). https://www.nccn.org/guidelines (2021).
- 12.Liao W-C, et al. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14:1095–1103. doi: 10.1016/S1470-2045(13)70388-7. [DOI] [PubMed] [Google Scholar]
- 13.Sugawara T, et al. Prediction of early recurrence of pancreatic ductal adenocarcinoma after resection. PLoS ONE. 2021;16:e0249885. doi: 10.1371/journal.pone.0249885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groot, V. P. et al. Defining and predicting early recurrence in 957 patients with resected pancreatic ductal adenocarcinoma. Ann. Surg. (2019). [DOI] [PMC free article] [PubMed]
- 15.La Torre M, et al. Is a preoperative assessment of the early recurrence of pancreatic cancer possible after complete surgical resection? Gut. Liver. 2014;8:102. doi: 10.5009/gnl.2014.8.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee D-H, et al. Recent treatment patterns and survival outcomes in pancreatic cancer according to clinical stage based on single-center large-cohort data. Ann. Hepato-Biliary-Pancreat. Surg. 2018;22:386–396. doi: 10.14701/ahbps.2018.22.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu D, et al. Quantitative definitions of pain, CA19-9, and tumor size as high-risk features of resectable pancreatic cancer: a single-center retrospective cohort study. Gland Surg. 2021;10:770. doi: 10.21037/gs-20-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto I, et al. Proposed preoperative risk factors for early recurrence in patients with resectable pancreatic ductal adenocarcinoma after surgical resection: a multi-center retrospective study. Pancreatology. 2015;15:674–680. doi: 10.1016/j.pan.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Chen J-Q, Liu J-L, Qin X-G, Huang YJWJOGW. FDG-PET in diagnosis, staging and prognosis of pancreatic carcinoma: a meta-analysis. World J. Gastroenterol. 2013;19:4808. doi: 10.3748/wjg.v19.i29.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahani DV, Bonaffini PA, Catalano OA, Guimaraes AR, Blake MA. State-of-the-art PET/CT of the pancreas: Current role and emerging indications. Radiographics. 2012;32:1133–1158. doi: 10.1148/rg.324115143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin J, et al. Lymph node size and its association with nodal metastasis in ductal adenocarcinoma of the pancreas. J. Pathol. Transl. Med. 2020;54:387. doi: 10.4132/jptm.2020.06.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loch FN, et al. Accuracy of various criteria for lymph node staging in ductal adenocarcinoma of the pancreatic head by computed tomography and magnetic resonance imaging. World J. Surg. Oncol. 2020;18:1–10. doi: 10.1186/s12957-020-01951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould MK, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non–small-cell lung cancer: a meta-analysis. Ann. Intern. Med. 2003;139:879–892. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto I, et al. 18-Fluorodeoxyglucose positron emission tomography does not aid in diagnosis of pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol. 2013;11:712–718. doi: 10.1016/j.cgh.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 25.Erdem S, et al. Role of lymphadenectomy in resectable pancreatic cancer. Langenbeck's Arch. Surg. 2020;405:43–54. doi: 10.1007/s00423-020-01980-2. [DOI] [PubMed] [Google Scholar]
- 26.Megibow AJ, et al. Pancreatic adenocarcinoma: CT versus MR imaging in the evaluation of resectability–report of the Radiology Diagnostic Oncology Group. Radiology. 1995;195:327–332. doi: 10.1148/radiology.195.2.7724748. [DOI] [PubMed] [Google Scholar]
- 27.Rösch T, et al. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography: Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology. 1992;102:188–199. doi: 10.1016/0016-5085(92)91800-J. [DOI] [PubMed] [Google Scholar]
- 28.Palazzo L, et al. Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma. Endoscopy. 1993;25:143–150. doi: 10.1055/s-2007-1010273. [DOI] [PubMed] [Google Scholar]
- 29.Zeman RK, et al. TNM staging of pancreatic carcinoma using helical CT. AJR Am. J. Roentgenol. 1997;169:459–464. doi: 10.2214/ajr.169.2.9242754. [DOI] [PubMed] [Google Scholar]
- 30.Midwinter M, et al. Correlation between spiral computed tomography, endoscopic ultrasonography and findings at operation in pancreatic and ampullary tumours. Br. J. Surg. 1999;86:189–193. doi: 10.1046/j.1365-2168.1999.01042.x. [DOI] [PubMed] [Google Scholar]
- 31.Honselmann, K. C. et al. Timing but not patterns of recurrence are different between node-negative and node-positive resected pancreatic cancer. Ann. Surg. (2019). [DOI] [PMC free article] [PubMed]
- 32.Kang CM, et al. The use of adjusted preoperative CA 19–9 to predict the recurrence of resectable pancreatic cancer. J. Surg. Res. 2007;140:31–35. doi: 10.1016/j.jss.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Sugiura T, et al. Serum CA19-9 is a significant predictor among preoperative parameters for early recurrence after resection of pancreatic adenocarcinoma. J. Gastrointest. Surg. 2012;16:977–985. doi: 10.1007/s11605-012-1859-9. [DOI] [PubMed] [Google Scholar]
- 34.Kowalchuk RO, et al. Predicting adverse pathologic features and clinical outcomes of resectable pancreas cancer with preoperative CA 19–9. Front. Oncol. 2021;11:1645. doi: 10.3389/fonc.2021.651119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montgomery RC, et al. Prediction of recurrence and survival by post-resection CA 19–9 values in patients with adenocarcinoma of the pancreas. Ann. Surg. Oncol. 1997;4:551–556. doi: 10.1007/BF02305535. [DOI] [PubMed] [Google Scholar]
- 36.Barton JG, et al. Predictive and prognostic value of CA 19–9 in resected pancreatic adenocarcinoma. J. Gastrointest. Surg. 2009;13:2050. doi: 10.1007/s11605-009-0849-z. [DOI] [PubMed] [Google Scholar]
- 37.Hallemeier CL, et al. Preoperative CA 19–9 level is an important prognostic factor in patients with pancreatic adenocarcinoma treated with surgical resection and adjuvant concurrent chemoradiotherapy. Am. J. Clin. Oncol. 2011;34:567–572. doi: 10.1097/COC.0b013e3181f946fc. [DOI] [PubMed] [Google Scholar]
- 38.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest. Oncol. 2012;3:105. doi: 10.3978/j.issn.2078-6891.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim N, et al. Predictive nomogram for early recurrence after pancreatectomy in resectable pancreatic cancer: Risk classification using preoperative clinicopathologic factors. Cancers. 2020;12:137. doi: 10.3390/cancers12010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrone CR, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann. Surg. 2015;261:12. doi: 10.1097/SLA.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tempero MA, et al. Pancreatic adenocarcinoma, version 22021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2021;19:439–457. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 42.Isaji S, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2–11. doi: 10.1016/j.pan.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Cancer., A. J. C. O. AJCC Cancer Staging Manual. 8 edn XVII, 1032 (Springer International Publishing, Berlin, 2017).
- 44.Society, J. P. General rules for the study of pancreatic cancer (Kanehara Shuppan Tokyo, 1993).
- 45.Elsholtz FH, et al. Introducing the Node Reporting and Data System 1.0 (Node-RADS): a concept for standardized assessment of lymph nodes in cancer. Eur. Radiol. 2021;31:6116–6124. doi: 10.1007/s00330-020-07572-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soriano A, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: Prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Off. J. Am. Coll. Gastroenterol. ACG. 2004;99:492–501. doi: 10.1111/j.1572-0241.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 47.Park M, et al. Metabolic characteristics of solid pseudopapillary neoplasms of the pancreas: Their relationships with high intensity 18F-FDG PET images. Oncotarget. 2018;9:12009. doi: 10.18632/oncotarget.23846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated or analysed during the study are available from the corresponding author on reasonable request.