Abstract
Background
Venous thromboembolism (VTE) is a severe complication of total knee arthroplasty (TKA). Cementation and the use of tourniquet during TKA have both been hypothesized to be risk factors of VTE. The purpose of our study was to determine if either of these surgical factors increases the risk of VTE in patients undergoing TKA.
Methods
A single-institution, retrospective study was conducted, consisting of 16,972 patients undergoing a primary or revision TKA from 2008 to 2020. Of the total, 1020 patients were excluded from the tourniquet analysis as tourniquet data were unavailable. Clinical records were consulted to identify demographics, surgical variables, and outcomes. Queries of clinical notes and phone-call logs were conducted to capture VTE events following discharge. Statistical analysis consisted of univariate analysis, regression analysis, and propensity score matching.
Results
Compared to patients who did not receive tourniquet, the patients with tourniquet did not demonstrate a significantly higher rate of VTE in the univariate analysis (1.00 vs 1.31, P = .132). Propensity score analysis also showed no difference between the 2 cohorts (1.10 vs 0.85, P = .306). Cemented patients similarly did not demonstrate an increased risk of VTE in either the univariate (1.26 vs 1.22, P = .895) or propensity score analysis (1.42 vs 1.26, P = .710) compared to cementless patients. Regression analysis, looking at the interaction between cement and tourniquet with VTE risk as the dependent variable, revealed neither to be risk factors for VTE (odds ratio 1.38, 95% confidence interval 0.63-3.08, P = .426).
Conclusions
In our cohort, neither tourniquet nor cement was a significant risk factor for VTE following TKA.
Keywords: Knee, Arthroplasty, Cement, Tourniquet, VTE
Introduction
Venous thromboembolism (VTE) is a significant reason for morbidity and cause for increased hospital costs following surgery. While VTE is considered a preventable cause of mortality, VTE is a common cause of readmissions, which are doubled for patients who develop VTE after surgery and are linked with possible future complications, including postthrombotic syndrome, cardiopulmonary sequela and recurrence, major bleeding associated with anticoagulation, and other adverse events [1]. Recognizing risk factors for VTE following surgery is important to mitigate this risk.
The risk of developing VTE is both surgical and patient-related [2]. Patients undergoing total knee arthroplasty (TKA) are considered at high risk of VTE because of the duration of surgery and limited mobility associated with the preoperative and postoperative conditions. Other potential surgical factors associated with an increased VTE risk are the use of tourniquet and polymethylmethacrylate bone cement. While suggested as risk factors for VTE, this topic remains controversial [2,3].
Patients who receive tourniquet during TKA have been hypothesized to be at an increased risk of VTE due to increased venous stasis, a major risk factor for VTE [4,5]. While the use of tourniquet improves visualization, lowers intraoperative blood loss, and provides a clear operative field, it has been also been suggested to be associated with an increased incidence of VTE [6,7]. Nevertheless, many studies have reported conflicting results, leading to low-grade recommendations [5,8,9]. Similar speculation exists regarding the use of cement and its association with VTE risk. The use of cement has been shown to influence patients’ hemodynamics and activate a hypercoagulable state [10]. Consequently, several studies have suggested the use of cement to be associated with an increased risk of VTE, but these concerns have yet to be fully considered [11,12].
While the influence of tourniquet and cement on VTE risk following TKA has been investigated, previous studies investigating these associations were not contemporary, were underpowered, and did not include many changes in practice during the last decade. The purpose of this study is to use institutional data to examine whether the use of cement or tourniquet during TKA is associated with a higher risk of VTE.
Material and methods
A retrospective, cohort study was conducted at a single institution following approval from the institutional review board. The cohort consisted of 16,972 patients undergoing primary or revision TKA from 2008 to 2020. Exclusion criteria were utilized in the tourniquet analysis for cases in which tourniquet use information was not accessible (n = 1020). Demographic data, health history, and patient comorbidities were collected from clinical notes and hospital records. These included variables such as age, body mass index, race, smoking status, alcohol intake, history of VTE, Charlson Comorbidity Index, American Society of Anesthesiologists classification, and surgical history. Patients with a significant history of VTE or hypercoagulable state were excluded from the study. Other surgical variables, including unilateral vs bilateral operations, anesthesia, tranexamic acid (TXA) administration, tourniquet use, and operative time, were recorded. It is important to note that our institution began administering TXA in 2012. The use of cement was also determined through the use of a prosthesis database of prospectively collected data, which outlines the implants used for the operation. Additionally, hospital records and clinic notes were utilized to identify in-hospital complications, specifically VTE events.
VTE events were defined as diagnosed symptomatic distal venous thromboembolism (DVT) or symptomatic pulmonary emboli (PE). DVT and PE were diagnosed based on ultrasound or computed tomography scan when possible. For those patients diagnosed at an outside institution, diagnosis relied on patient reporting or phone-call logs from the outside hospital. Additionally, keyword searches were conducted throughout patient-provider phone-call logs as well as clinical notes and dictations to capture VTE events up until 90 days following discharge. These keyword searches, assembled by trained orthopedic surgeons, looked to increase the yield of VTE events by identifying patients that were admitted to outside institutions for VTE. The searches consisted of identifying keywords associated with VTE events and utilizing a coding system to identify the presence of these words in the clinical notes, dictations, medical histories, and phone-call logs of all the patients who underwent TKA from 2008 to 2020. Each of the identified documents and patient records were manually screened to identify patients who experienced a VTE event following discharge.
Statistical analysis consisted of descriptive statistics and univariate analysis. To isolate the role of cement in the risk of VTE in these patients, 2 separate propensity score matches were completed here focusing on cement and tourniquet as the dependent outcomes. The matches were performed in a 1:1 setting using cement vs no cement and tourniquet vs no tourniquet as the dependent outcomes. The variables used in the match included VTE, anticoagulation, sex, age, body mass index, Charlson Comorbidity Index, unilateral vs bilateral, and either the use of tourniquet or cement. In the cement match, year of surgery was also included. Following the matches, the data were broken by comparing cement to cementless and tourniquet to no tourniquet. Continuous data were presented as mean (standard deviation), and categorical data were presented as cell count (%). T-tests were used to calculate P values for continuous data, and chi-square testing was used to calculate P values for categorical data. Lastly, a bivariate logistic regression was ran looking at VTE as the dependent outcome and assessing the effects of cement and tourniquet. Significance was determined at P values <.05. All statistical analyses were done using R Studio (Version 3.6.3; Vienna, Austria).
Results
Tourniquet
The tourniquet was utilized in 70.6% (10,752/15,216) of the cases included in the analysis. There were several differences between the demographics and comorbidities between the patients for whom the tourniquet was utilized and for whom the tourniquet was omitted (Table 1). There were no significant differences in the incidence of VTE between the 2 groups (0.96 vs 1.28, P = .115). While in the hospital, patients with no tourniquet use were more likely to be administered TXA intraoperatively (66.1% vs 35.5%, P < .001) and receive a cementless implant (35.6% vs 10.2%, P < .001). Patients with tourniquet use were more likely to receive a blood transfusion (3.23% vs 4.61%, P < .001).
Table 1.
Univariate analysis of unmatched demographics and clinical variables comparing patients who received a cementless prosthesis to those who received a cemented prosthesis.
| Variables | Cementless |
Cemented |
P value |
|---|---|---|---|
| N = 3382 | N = 12803 | ||
| VTE anticoagulation | <.001 | ||
| Other | 890 (26.3%) | 5507 (43.0%) | |
| Aspirin | 2492 (73.7%) | 7296 (57.0%) | |
| Sex | .031 | ||
| Female | 1909 (56.4%) | 7492 (58.5%) | |
| Male | 1473 (43.6%) | 5311 (41.5%) | |
| Age | 64.4 (9.56) | 65.6 (9.51) | <.001 |
| Smoker | <.001 | ||
| No | 2229 (65.9%) | 7588 (59.3%) | |
| Yes | 1153 (34.1%) | 5215 (40.7%) | |
| Alcohol | .602 | ||
| No | 1675 (49.5%) | 6274 (49.0%) | |
| Yes | 1707 (50.5%) | 6529 (51.0%) | |
| BMI | 30.7 (5.22) | 31.2 (5.52) | <.001 |
| CHF | .180 | ||
| No | 2953 (98.0%) | 12223 (97.6%) | |
| Yes | 59 (1.96%) | 299 (2.39%) | |
| CPD | .017 | ||
| No | 2675 (88.8%) | 10917 (87.2%) | |
| Yes | 337 (11.2%) | 1605 (12.8%) | |
| CVD | .217 | ||
| No | 2974 (98.7%) | 12323 (98.4%) | |
| Yes | 38 (1.26%) | 199 (1.59%) | |
| Dementia | .902 | ||
| No | 3006 (99.8%) | 12493 (99.8%) | |
| Yes | 6 (0.20%) | 29 (0.23%) | |
| DM | .072 | ||
| No | 2842 (94.4%) | 11701 (93.4%) | |
| Yes | 170 (5.64%) | 821 (6.56%) | |
| HemiPara | 1.000 | ||
| No | 3011 (100.0%) | 12515 (99.9%) | |
| Yes | 1 (0.03%) | 7 (0.06%) | |
| Cancer | .941 | ||
| No | 2979 (98.9%) | 12376 (98.8%) | |
| Malignancy | 27 (0.90%) | 118 (0.94%) | |
| Metastatic | 6 (0.20%) | 28 (0.22%) | |
| MI | <.001 | ||
| No | 2933 (97.4%) | 11988 (95.7%) | |
| Yes | 79 (2.62%) | 534 (4.26%) | |
| MildLiver | .402 | ||
| No | 2979 (98.9%) | 12408 (99.1%) | |
| Yes | 33 (1.10%) | 114 (0.91%) | |
| ModSevereLiver | 1.000 | ||
| No | 3011 (100.0%) | 12515 (99.9%) | |
| Yes | 1 (0.03%) | 7 (0.06%) | |
| Peptic ulcer | .197 | ||
| No | 3004 (99.7%) | 12465 (99.5%) | |
| Yes | 8 (0.27%) | 57 (0.46%) | |
| PVD | 1.000 | ||
| No | 2966 (98.5%) | 12331 (98.5%) | |
| Yes | 46 (1.53%) | 191 (1.53%) | |
| Renal | <.001 | ||
| No | 2954 (98.1%) | 12112 (96.7%) | |
| Yes | 58 (1.93%) | 410 (3.27%) | |
| Rheumatic | .019 | ||
| No | 2919 (96.9%) | 12018 (96.0%) | |
| Yes | 93 (3.09%) | 504 (4.02%) | |
| CCI | 0.39 (0.84) | 0.51 (0.96) | <.001 |
| Number of joints | <.001 | ||
| Unilateral | 3217 (95.1%) | 11668 (91.1%) | |
| Bilateral | 165 (4.88%) | 1135 (8.87%) | |
| Type of surgery | <.001 | ||
| Primary | 3049 (90.2%) | 11196 (87.4%) | |
| Revision | 333 (9.85%) | 1607 (12.6%) | |
| OP time | 80.6 (36.1) | 82.9 (31.9) | <.001 |
| Tourniquet | <.001 | ||
| No | 1589 (59.2%) | 2875 (22.9%) | |
| Yes | 1095 (40.8%) | 9657 (77.1%) | |
| TXA | <.001 | ||
| No | 1332 (40.8%) | 7152 (57.2%) | |
| Yes | 1931 (59.2%) | 5362 (42.8%) | |
| Blood transfusion | .794 | ||
| No | 3227 (95.4%) | 12232 (95.5%) | |
| Yes | 155 (4.58%) | 571 (4.46%) | |
| Any VTE | .727 | ||
| No | 3339 (98.7%) | 12652 (98.8%) | |
| Yes | 43 (1.27%) | 151 (1.18%) | |
| Any DVT | .701 | ||
| No | 3361 (99.4%) | 12733 (99.5%) | |
| Yes | 21 (0.62%) | 70 (0.55%) | |
| Any PE | .735 | ||
| No | 3356 (99.2%) | 12714 (99.3%) | |
| Yes | 26 (0.77%) | 89 (0.70%) |
BMI, body mass index; CCI, Charlson Comorbidity Index; CHF, congestive heart failure; CPD, chronic pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; MI, myocardial infarction; PVD, peripheral vascular disease; OP, operative; HemiPara, hemiparesis; MildLiver, mild liver disease; ModSevereLiver, moderate-severe liver disease..
Following 1:1 propensity score matching, there was no significant difference in total VTE rate between the 2 groups (1.02 vs 0.69, P = .141, Table 2). Operative time and the use of cement remained significantly lower in the no-tourniquet group, while the administration of TXA continued to be significantly higher. Regression analysis with VTE as the dependent outcome also determined tourniquet to not be a significant risk factor for VTE (odds ratio [OR] 1.34, 95% confidence interval [CI] 0.96-1.91, P = .098, Table 5).
Table 2.
Univariate analysis of 1:1 propensity-score-matched demographics and clinical variables comparing patients who received a cementless prosthesis to those who received a cemented prosthesis.
| Variables | Cementless |
Cemented |
P value |
|---|---|---|---|
| N = 2382 | N = 2382 | ||
| Date of surgery (y) | 2015 (2.70) | 2015 (2.73) | .411 |
| VTE anticoagulation | .537 | ||
| Other | 543 (22.8%) | 562 (23.6%) | |
| Aspirin | 1839 (77.2%) | 1820 (76.4%) | |
| Sex | .861 | ||
| Female | 1367 (57.4%) | 1360 (57.1%) | |
| Male | 1015 (42.6%) | 1022 (42.9%) | |
| Age | 64.7 (9.06) | 64.8 (9.30) | .670 |
| BMI | 30.8 (5.23) | 31.0 (5.18) | .281 |
| CCI | 0.41 (0.85) | 0.42 (0.82) | .597 |
| Number of joints | .300 | ||
| Unilateral | 2280 (95.7%) | 2264 (95.0%) | |
| Bilateral | 102 (4.28%) | 118 (4.95%) | |
| Type of surgery | .921 | ||
| Primary | 2157 (90.6%) | 2154 (90.4%) | |
| Revision | 225 (9.45%) | 228 (9.57%) | |
| OP time | 81.8 (37.9) | 85.1 (33.3) | <.001 |
| Tourniquet | .861 | ||
| No | 1313 (55.1%) | 1320 (55.4%) | |
| Yes | 1069 (44.9%) | 1062 (44.6%) | |
| TXA | .457 | ||
| No | 981 (42.5%) | 968 (41.4%) | |
| Yes | 1325 (57.5%) | 1369 (58.6%) | |
| Blood transfusion | .076 | ||
| No | 2265 (95.1%) | 2291 (96.2%) | |
| Yes | 117 (4.91%) | 91 (3.82%) | |
| Any VTE | .065 | ||
| No | 2350 (98.7%) | 2364 (99.2%) | |
| Yes | 32 (1.34%) | 18 (0.76%) | |
| Any DVT | .343 | ||
| No | 2365 (99.3%) | 2371 (99.5%) | |
| Yes | 17 (0.71%) | 11 (0.46%) | |
| Any PE | .044 | ||
| No | 2361 (99.1%) | 2373 (99.6%) | |
| Yes | 21 (0.88%) | 9 (0.38%) |
Variables included in the match are bolded.
BMI, body mass index; CCI, Charlson Comorbidity Index; OP, operative.
Table 5.
Regression analysis focusing on VTE risk as the dependent outcome with cement and tourniquet by themselves as the primary variable.
| Variable | Estimate | P value | Odds ratio | Lower 95 | Upper 95 |
|---|---|---|---|---|---|
| Cement | −0.08 | .662 | 0.93 | 0.67 | 1.32 |
| Tourniquet | 0.29 | .098 | 1.34 | 0.96 | 1.91 |
Cement
Of the 16,185 cases in our cohort, 3382 (21.0%) received a cementless knee implant. There were significant differences in the patient demographics and comorbidities between the cemented and cementless patients (Table 3). There was no difference in the rate of blood transfusions (4.58% vs 4.46%, P = .794) and the incidence of VTE events, including both DVT and PE, between the cementless and cemented groups (1.27 vs 1.18, P = .727). Univariate analysis showed cementless patients to be associated with significantly lower operative times (80.6 ± 36.1 vs 82.9 ± 31.9, P < .001) and that the tourniquet is less likely to be used in the cementless cases (40.8% vs 77.1%, P < .001). Aspirin was also significantly more likely to be given to patients who received a cementless TKA for VTE prophylaxis (73.7% vs 57.0%).
Table 3.
Univariate analysis of unmatched demographics and clinical variables comparing patients who received tourniquet to those patients who received no tourniquet.
| Variables | No tourniquet |
Tourniquet |
P value |
|---|---|---|---|
| N = 4464 | N = 10752 | ||
| VTE anticoagulation | <.001 | ||
| Other | 769 (17.2%) | 5406 (50.3%) | |
| Aspirin | 3695 (82.8%) | 5346 (49.7%) | |
| Sex | .005 | ||
| Female | 2527 (56.6%) | 6352 (59.1%) | |
| Male | 1937 (43.4%) | 4400 (40.9%) | |
| Age | 65.6 (8.70) | 65.3 (9.70) | .192 |
| Smoker | <.001 | ||
| No | 3145 (70.5%) | 6014 (55.9%) | |
| Yes | 1319 (29.5%) | 4738 (44.1%) | |
| Alcohol | .004 | ||
| No | 2272 (50.9%) | 5195 (48.3%) | |
| Yes | 2192 (49.1%) | 5557 (51.7%) | |
| BMI | 30.8 (4.98) | 31.3 (5.64) | <.001 |
| CHF | <.001 | ||
| No | 3871 (98.8%) | 10458 (97.4%) | |
| Yes | 49 (1.25%) | 282 (2.63%) | |
| CPD | <.001 | ||
| No | 3541 (90.3%) | 9275 (86.4%) | |
| Yes | 379 (9.67%) | 1465 (13.6%) | |
| CVD | <.001 | ||
| No | 3886 (99.1%) | 10561 (98.3%) | |
| Yes | 34 (0.87%) | 179 (1.67%) | |
| Dementia | .996 | ||
| No | 3912 (99.8%) | 10720 (99.8%) | |
| Yes | 8 (0.20%) | 20 (0.19%) | |
| DM combined | <.001 | ||
| No | 3592 (91.6%) | 10119 (94.2%) | |
| Yes | 328 (8.37%) | 621 (5.78%) | |
| HemiPara | .352 | ||
| No | 3920 (100%) | 10734 (99.9%) | |
| Yes | 0 (0.00%) | 6 (0.06%) | |
| Cancer | .205 | ||
| No | 3885 (99.1%) | 10606 (98.8%) | |
| Malignancy | 29 (0.74%) | 111 (1.03%) | |
| Metastatic | 6 (0.15%) | 23 (0.21%) | |
| MI | <.001 | ||
| No | 3830 (97.7%) | 10240 (95.3%) | |
| Yes | 90 (2.30%) | 500 (4.66%) | |
| MildLiver | .003 | ||
| No | 3867 (98.6%) | 10655 (99.2%) | |
| Yes | 53 (1.35%) | 85 (0.79%) | |
| ModSevereLiver | .201 | ||
| No | 3920 (100%) | 10733 (99.9%) | |
| Yes | 0 (0.00%) | 7 (0.07%) | |
| Peptic ulcer | .921 | ||
| No | 3904 (99.6%) | 10693 (99.6%) | |
| Yes | 16 (0.41%) | 47 (0.44%) | |
| PVD | .422 | ||
| No | 3856 (98.4%) | 10586 (98.6%) | |
| Yes | 64 (1.63%) | 154 (1.43%) | |
| Renal | <.001 | ||
| No | 3846 (98.1%) | 10370 (96.6%) | |
| Yes | 74 (1.89%) | 370 (3.45%) | |
| Rheumatic | .124 | ||
| No | 3784 (96.5%) | 10306 (96.0%) | |
| Yes | 136 (3.47%) | 434 (4.04%) | |
| CCI | 0.38 (0.78) | 0.52 (0.98) | <.001 |
| Number of joints | <.001 | ||
| Unilateral | 4427 (99.2%) | 9573 (89.0%) | |
| Bilateral | 37 (0.83%) | 1179 (11.0%) | |
| Type of surgery | <.001 | ||
| Primary | 4318 (96.7%) | 9117 (84.8%) | |
| Revision | 146 (3.27%) | 1635 (15.2%) | |
| OP time | 83.1 (35.5) | 82.3 (31.6) | .100 |
| TXA | <.001 | ||
| No | 1501 (33.9%) | 6731 (64.5%) | |
| Yes | 2932 (66.1%) | 3704 (35.5%) | |
| Cement | <.001 | ||
| No | 1589 (35.6%) | 1095 (10.2%) | |
| Yes | 2875 (64.4%) | 9657 (89.8%) | |
| Blood transfusion | <.001 | ||
| No | 4320 (96.8%) | 10256 (95.4%) | |
| Yes | 144 (3.23%) | 496 (4.61%) | |
| Any VTE | .115 | ||
| No | 4421 (99.0%) | 10614 (98.7%) | |
| Yes | 43 (0.96%) | 138 (1.28%) | |
| Any DVT | .781 | ||
| No | 4438 (99.4%) | 10695 (99.5%) | |
| Yes | 26 (0.58%) | 57 (0.53%) | |
| Any PE | .058 | ||
| No | 4441 (99.5%) | 10664 (99.2%) | |
| Yes | 23 (0.52%) | 88 (0.82%) |
BMI, body mass index; CCI, Charlson Comorbidity Index; CHF, congestive heart failure; CPD, chronic pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; MI, myocardial infarction; PVD, peripheral vascular disease; OP, operative; HemiPara, hemiparesis; MildLiver, mild liver disease; ModSevereLiver, moderate-severe liver disease..
A 1:1 propensity score matching revealed no difference in total VTE risk between the cemented and cementless groups (1.34 vs 0.76, P = .065, Table 4). However, following the match, patients who underwent a cementless TKA were more likely to have a postoperative PE (0.88% vs 0.38%, P = .044), and patients who underwent a cemented TKA were more likely to undergo a shorter surgery (81.8 ± 37.9 minutes vs 85.1 ± 33.3 minutes, P < .001). There were no additional significant differences between the 2 matched groups in patient demographics, comorbidities, or clinical variables. Additionally, regression analysis with VTE as the dependent outcome revealed cement to not be a significant risk factor for VTE (OR 0.93, 95% CI 0.67-1.32, P = .662, Table 5).
Table 4.
Univariate analysis of 1:1 propensity-score-matched demographics and clinical variables comparing patients who received tourniquet to those who received no tourniquet.
| Variables | No tourniquet |
Tourniquet |
P value |
|---|---|---|---|
| N = 3917 | N = 3917 | ||
| VTE anticoagulation | .168 | ||
| Other | 590 (15.1%) | 546 (13.9%) | |
| Aspirin | 3327 (84.9%) | 3371 (86.1%) | |
| Sex | .178 | ||
| Female | 2204 (56.3%) | 2264 (57.8%) | |
| Male | 1713 (43.7%) | 1653 (42.2%) | |
| Age | 65.6 (8.74) | 65.8 (9.42) | .257 |
| BMI | 30.8 (5.01) | 30.8 (5.28) | .991 |
| CCI | 0.38 (0.77) | 0.37 (0.74) | .800 |
| Number of joints | .196 | ||
| Unilateral | 3880 (99.1%) | 3867 (98.7%) | |
| Bilateral | 37 (0.94%) | 50 (1.28%) | |
| Type of surgery | .161 | ||
| Primary | 3777 (96.4%) | 3752 (95.8%) | |
| Revision | 140 (3.57%) | 165 (4.21%) | |
| OP time | 83.3 (34.4) | 78.4 (26.1) | .008 |
| TXA | <.001 | ||
| No | 1311 (33.7%) | 1745 (44.9%) | |
| Yes | 2577 (66.3%) | 2143 (55.1%) | |
| Blood transfusion | .573 | ||
| No | 3780 (96.5%) | 3790 (96.8%) | |
| Yes | 137 (3.50%) | 127 (3.24%) | |
| Any VTE | .141 | ||
| No | 3877 (99.0%) | 3890 (99.3%) | |
| Yes | 40 (1.02%) | 27 (0.69%) | |
| Any DVT | .018 | ||
| No | 3892 (99.4%) | 3907 (99.7%) | |
| Yes | 25 (0.64%) | 10 (0.26%) | |
| Any PE | .416 | ||
| No | 3895 (99.4%) | 3901 (99.6%) | |
| Yes | 22 (0.56%) | 16 (0.41%) |
Variables included in the match are bolded.
BMI, body mass index; CCI, Charlson Comorbidity Index; OP, operative.
Trends in cement and tourniquet use
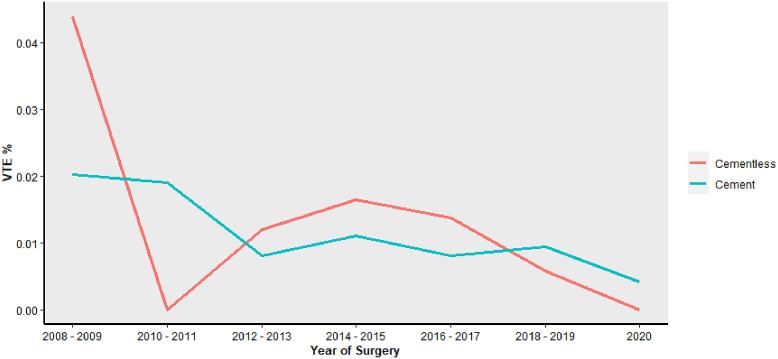
While the use of tourniquet has declined recently, cementless prostheses have been adopted for TKA procedures. There has been an increase in not only cementless and no-tourniquet procedures but also procedures that use neither tourniquet nor cement (Supplementary Table 1). However, the risk of VTE following TKA has remained relatively stable over the course of 2008-2020 in patients receiving tourniquet or no tourniquet (Fig. 1). Additionally, the rate is similar between the cementless and cemented cohorts across the same timeline (Fig. 2). Importantly, an analysis comparing the rate of VTE, DVT, and PE across the 4 groups identified no significant differences. (Supplementary Table 1). Regression analysis focusing on VTE risk in these cohorts demonstrated neither cement (OR 0.93, 95% CI 0.67-1.32, P = .662) nor tourniquet (OR 1.34, 95% CI 0.96-1.91, P = .098) to significantly increase the risk of VTE (Table 5). Additionally, further regression analysis, which analyzed the risk of VTE through the interaction between tourniquet and cement, determined no significant increase in the risk of postoperative VTE events (OR 1.59, 95% CI 0.71-3.68, P = .269, Table 6).
Figure 1.
The trends in the rate of VTE from 2008 to 2020 in patients who received tourniquet (blue) and those who did not receive tourniquet (red).
Figure 2.
The trends in the rate of VTE from 2008 to 2020 in patients who received a cemented prosthesis (blue) and those who received a cementless prosthesis (red).
Table 6.
Regression analysis focusing on VTE risk as the dependent outcome with cement interacted with tourniquet.
| Variable | Estimate | P value | Odds ratio | Lower 95 | Upper 95 |
|---|---|---|---|---|---|
| Cement | −0.46 | .136 | 0.63 | 0.35 | 1.17 |
| Tourniquet | 0.02 | .964 | 1.02 | 0.50 | 2.01 |
| Cement interacted with Tourniquet | 0.46 | .269 | 1.59 | 0.71 | 3.68 |
Discussion
As one of the most severe complications of joint replacement surgery, VTE is studied thoroughly to better understand ways to prevent its occurrence and protect patients. Cementation and the use of tourniquet during TKA have both been identified as potential risk factors for developing VTE. Due to their conventional use, it is important to determine if they are increasing the risk of VTE for patients. To our knowledge, our study is the largest, single-institution retrospective study to analyze the association of tourniquet use and cementation with the risk of VTE in patients undergoing TKA. The findings of our study determined that the use of cement and tourniquet did not increase the risk of developing VTE following TKA.
Several studies have analyzed the use of tourniquet and its potential role in increasing the risk of VTE in patients undergoing TKA. The use of tourniquet has been associated with several complications, including postoperative swelling, pain, muscular damage, and importantly, thromboembolic risk [6,13,14]. However, these have demonstrated conflicting results and continued debate on the topic. Our findings revealed the use of tourniquet to impart no significant increase in the risk of VTE for patients undergoing TKA. These findings are similar to those presented in the meta-analysis of randomized, controlled trials (RCTs) by Li et al., which determined that patients treated with a tourniquet were not at a significantly higher risk of developing DVT [8]. Additionally, RCTs on patients undergoing TKA with or without tourniquet by Fukuda et al. and Harvey et al. demonstrated no difference in DVT risk [5,15]. However, studies presented by Tai et al. and Mori et al. reported an increased risk in patients treated with a tourniquet [14,16]. These conflicting results highlight the need to continue research on the potential VTE risk associated with the use of tourniquet. While our findings from a large, single-institution database demonstrate that the use of tourniquet does not increase the risk of VTE, we suggest surgeons vigilantly monitor for postoperative VTE following TKA.
Cementation of the prosthesis during TKA is also a controversial topic as it has been hypothesized to increase the risk of VTE [10,17,18]. Cement has been reported to activate the clotting cascade during the operation and, as a result, increase the VTE risk. While this topic has been previously studied in the literature, our study utilized a large, contemporary single-institution database, which was able to account for changes in clinical practice over the past decade. Our study determined cement fixation to not be a significant risk factor for VTE compared to patients with cementless prostheses. These findings correlate with several studies that have compared cemented to cementless patients undergoing TKA [11,12,[19], [20], [21]]. The large meta-analysis of Liu et al. involving 26 studies, which compared the rates of DVT between cemented and cementless TKA cases, reported no significant difference between the 2 groups [19]. Additionally, the RCT by Clarke et al., which randomized patients to receive either a cemented or cementless prosthesis, demonstrated no significant increase in the VTE incidence in the cemented group [20]. While several studies have identified cement as a significant risk factor for VTE [11,12], our findings correlate with other reports that the use of cement does not increase the risk of VTE.
Our study is not without limitations. As a retrospective study, the data rely on clinical notes and reports that are subject to inaccuracies. Although operative reports, which detail the use of tourniquet and cement, along with prosthesis databases are accurate, errors may still exist. Unfortunately, we were unable to include year of surgery in the match for tourniquet use. However, by analyzing the use of tourniquet by year, we saw that physicians are beginning to use tourniquet less frequently. Additionally, it is difficult to identity VTE events that occur following discharge. One of the major limitations was that we included patients who were diagnosed with VTE at an outside institution. Without access to the outside hospital’s records, we were unable to confirm the diagnostic practices in this cohort and relied on either patient reporting or phone-call logs from the outside institution. However, by including these patients, we were able to increase the capture rate and sample size. Thus, we were able to make more substantial conclusions. Although thorough keyword searches of clinical notes, dictations, and patient-provider phone-call logs were employed, these rely on the patient to report these events to the surgeon. As a result, there may have been VTE events that were not captured and influence the results. Anytime the results of no significant difference are being reported, concerns are raised regarding type 2 error. However, by including all TKA cases from our single institution from 2008 to 2020 and accounting for changes in clinical practice, the large, homogenous cohort should reduce the likelihood of a type 2 error. These changes in practice over the last 20 years include the administration of TXA, the adoption of aspirin as the preferred postoperative anticoagulation, and increases in operations with cementless implants and no tourniquet. These are critical changes in clinical care that were considered in the matching system to avoid confounding. Lastly, we observed several significant differences between the cohorts regarding baseline characteristics and identified some important variables that we were not able to control for, such as tourniquet duration. While we are unable to determine why the surgeons chose to use the tourniquet or cement vs going cementless for each patient, we utilized propensity score matching to account for differences in the baseline characteristics.
Conclusions
The findings of our study demonstrated that the use of cement and tourniquet seem to be safe measures during TKA and do not result in an increased risk of VTE. While it is important for surgeons to weigh the risks and benefits of the use of cement and tourniquet preoperatively, these treatments do not seem to be significant risk factors for VTE.
Conflicts of interest
Y. Fillingham receives royalties from Exactech, Inc. and Medacta; is a paid consultant for Exactech, Inc.; Johnson & Johnson, and Medacta; has stock or stock options in Parvizi Surgical Innovations; receives royalties from Elsevier; and is a board member in American Academy of Orthopaedic Surgeons and American Association of Hip and Knee Surgeons. All other authors declare no potential conflicts of interest.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2022.08.020.
Appendix
Supplementary Table 1.
Analysis comparing the changes in TKA procedures from 2008 to 2020 and the VTE rates associated with the different procedures.
| Variables | Tourniquet and cement (p1) |
No tourniquet and cement (p2) |
Tourniquet and no cement (p3) |
No tourniquet and no cement (p4) |
p Overall | p1 vs p2 | p1 vs p3 | p1 vs p4 | p2 vs p3 | p2 vs p4 | p3 vs p4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 10229 | N = 2965 | N = 1145 | N = 1613 | ||||||||
| DOS (y) | 0.000 | 0.000 | <0.001 | 0.000 | <0.001 | <0.001 | <0.001 | ||||
| 2008 | 1032 (10.2%) | 46 (1.55%) | 91 (8.10%) | 2 (0.12%) | |||||||
| 2009 | 1118 (11.0%) | 31 (1.05%) | 16 (1.42%) | 5 (0.31%) | |||||||
| 2010 | 1121 (11.0%) | 39 (1.32%) | 14 (1.25%) | 0 (0.00%) | |||||||
| 2011 | 1065 (10.5%) | 44 (1.48%) | 76 (6.77%) | 9 (0.56%) | |||||||
| 2012 | 811 (7.99%) | 16 (0.54%) | 182 (16.2%) | 11 (0.68%) | |||||||
| 2013 | 770 (7.59%) | 125 (4.22%) | 167 (14.9%) | 141 (8.75%) | |||||||
| 2014 | 946 (9.32%) | 219 (7.39%) | 46 (4.10%) | 144 (8.94%) | |||||||
| 2015 | 698 (6.88%) | 392 (13.2%) | 120 (10.7%) | 235 (14.6%) | |||||||
| 2016 | 546 (5.38%) | 484 (16.3%) | 189 (16.8%) | 275 (17.1%) | |||||||
| 2017 | 734 (7.24%) | 577 (19.5%) | 72 (6.41%) | 188 (11.7%) | |||||||
| 2018 | 552 (5.44%) | 391 (13.2%) | 77 (6.86%) | 327 (20.3%) | |||||||
| 2019 | 462 (4.55%) | 411 (13.9%) | 58 (5.16%) | 232 (14.4%) | |||||||
| 2020 | 290 (2.86%) | 188 (6.34%) | 15 (1.34%) | 42 (2.61%) | |||||||
| Any VTE | 0.359 | 0.643 | 0.891 | 0.891 | 0.686 | 0.891 | 0.891 | ||||
| No | 10096 (98.7%) | 2938 (99.1%) | 1129 (98.6%) | 1594 (98.8%) | |||||||
| Yes | 133 (1.30%) | 27 (0.91%) | 16 (1.40%) | 19 (1.18%) | |||||||
| Any DVT | 0.226 | 0.735 | 0.429 | 0.429 | 0.429 | 0.429 | 0.429 | ||||
| No | 10166 (99.4%) | 2949 (99.5%) | 1142 (99.7%) | 1599 (99.1%) | |||||||
| Yes | 63 (0.62%) | 16 (0.54%) | 3 (0.26%) | 14 (0.87%) | |||||||
| Any PE | 0.195 | 0.426 | 0.426 | 0.426 | 0.426 | 1.000 | 0.426 | ||||
| No | 10146 (99.2%) | 2948 (99.4%) | 1132 (98.9%) | 1604 (99.4%) | |||||||
| Yes | 83 (0.81%) | 17 (0.57%) | 13 (1.14%) | 9 (0.56%) |
DOS, date of surgery.
Appendix A. Supplementary data
References
- 1.Secemsky E.A., Rosenfield K., Kennedy K.F., Jaff M., Yeh R.W. High burden of 30-day readmissions after acute venous thromboembolism in the United States. J Am Heart Assoc. 2018;7:e009047. doi: 10.1161/JAHA.118.009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z., Shen B., Yang J., Zhou Z., Kang P., Pei F. Risk factors for venous thromboembolism of total hip arthroplasty and total knee arthroplasty: a systematic review of evidences in ten years. BMC Musculoskelet Disord. 2015;16:24. doi: 10.1186/s12891-015-0470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arcelus J.I., Kudrna J.C., Caprini J.A. Venous thromboembolism following major orthopedic surgery: what is the risk after discharge? Orthopedics. 2006;29:506–516. doi: 10.3928/01477447-20060601-16. [DOI] [PubMed] [Google Scholar]
- 4.Fisher W.D. Impact of venous thromboembolism on clinical management and therapy after hip and knee arthroplasty. Can J Surg. 2011;54:344–351. doi: 10.1503/cjs.007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuda A., Hasegawa M., Kato K., Shi D., Sudo A., Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee arthroplasty. Arch Orthop Trauma Surg. 2007;127:671–675. doi: 10.1007/s00402-006-0244-0. [DOI] [PubMed] [Google Scholar]
- 6.Klenerman L. Is a tourniquet really necessary for knee replacement? J Bone Joint Surg Br. 1995;77:174–175. [PubMed] [Google Scholar]
- 7.Liu D., Graham D., Gillies K., Gillies R.M. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26:207–213. doi: 10.5792/ksrr.2014.26.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X., Yin L., Chen Z.-Y., Zhu L., Wang H.L., Chen W., et al. The effect of tourniquet use in total knee arthroplasty: grading the evidence through an updated meta-analysis of randomized, controlled trials. Eur J Orthop Surg Traumatol. 2014;24:973–986. doi: 10.1007/s00590-013-1278-y. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy Deering E., Hu S.Y., Abdulkarim A. Does tourniquet use in TKA increase postoperative pain? A systematic review and meta-analysis. Clin Orthop Relat Res. 2019;477:547–558. doi: 10.1097/CORR.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi X., Zhang Y., Pan J., Ma L., Wang L., Wang J. Effect of bone cement implantation on haemodynamics in elderly patients and preventive measure in cemented hemiarthroplasty. Biomed Res Int. 2015;2015:568019. doi: 10.1155/2015/568019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hitos K., Fletcher J.P. Venous thromboembolism following primary total knee arthroplasty. Int Angiol. 2006;25:343–351. [PubMed] [Google Scholar]
- 12.Schiff R.L., Kahn S.R., Shrier I., Strulovitch C., Hammouda W., Cohen E., et al. Identifying orthopedic patients at high risk for venous thromboembolism despite thromboprophylaxis. Chest. 2005;128:3364–3371. doi: 10.1378/chest.128.5.3364. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W., Li N., Chen S., Tan Y., Al-Aidaros M., Chen L. The effects of a tourniquet used in total knee arthroplasty: a meta-analysis. J Orthop Surg Res. 2014;9:13. doi: 10.1186/1749-799X-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tai T.-W., Lin C.-J., Jou I.-M., Chang C.-W., Lai K.-A., Yang C.-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2011;19:1121–1130. doi: 10.1007/s00167-010-1342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey E.J., Leclerc J., Brooks C.E., Burke D.L. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12:291–296. doi: 10.1016/S0883-5403(97)90025-5. [DOI] [PubMed] [Google Scholar]
- 16.Mori N., Kimura S., Onodera T., Iwasaki N., Nakagawa I., Masuda T. Use of a pneumatic tourniquet in total knee arthroplasty increases the risk of distal deep vein thrombosis: a prospective, randomized study. Knee. 2016;23:887–889. doi: 10.1016/j.knee.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Dahl O.E., Aspelin T., Arnesen H., Seljeflot I., Kierulf P., Ruyter R., et al. Increased activation of coagulation and formation of late deep venous thrombosis following discontinuation of thromboprophylaxis after hip replacement surgery. Thromb Res. 1995;80:299–306. doi: 10.1016/0049-3848(95)00180-Y. [DOI] [PubMed] [Google Scholar]
- 18.Giercksky K.E., Bjørklid E., Prydz H., Renck H. Circulating tissue thromboplastin during hip surgery. Eur Surg Res. 1979;11:296–300. doi: 10.1159/000128078. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Zeng Y., Wu Y., Li M., Xie H., Shen B. A comprehensive comparison between cementless and cemented fixation in the total knee arthroplasty: an updated systematic review and meta-analysis. J Orthop Surg Res. 2021;16:176. doi: 10.1186/s13018-021-02299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke M.T., Green J.S., Harper W.M., Gregg P.J. Cement as a risk factor for deep-vein thrombosis. J Bone Joint Surg Br. 1998;80-B:611–613. doi: 10.1302/0301-620X.80B4.0800611. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y. The incidence of deep vein thrombosis after cementless and cemented knee replacement. J Bone Joint Surg Br. 1990;72-B:779–783. doi: 10.1302/0301-620X.72B5.2211755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.