Abstract
Background
Although we had previously reported the cardiac and neurologic outcomes of Chinese and South Asian Ontarians in wave 1 of COVID-19, data on subsequent waves of COVID-19 remain unexamined. This is an extension study of this cohort in waves 2 and 3.
Methods
We identified adult Ontarians with a positive COVID-19 polymerase chain reaction test from January 1, 2020 to June 30, 2021, and they were classified as being Chinese or South Asian using a validated surname algorithm; we compared their outcomes of mortality, and cardiac and neurologic complications with those of the general population using multivariable logistic regression models.
Results
Compared to the general population (n = 439,977), the Chinese population (n = 15,208) was older (mean age 44.2 vs 40.6 years, P < 0.001) and the South Asian population (n = 46,333) was younger (39.2 years, P < 0.001). The Chinese population had a higher 30-day mortality (odds ratio [OR] 1.44; 95% confidence interval [CI] 1.28-1.61) and more hospitalization or emergency department visits (OR, 1.14; 95% CI, 1.09-1.28), with a trend toward a higher incidence of cardiac complications (OR, 1.03; 95% CI, 0.87-1.12) and neurologic complications (OR, 1.23; 95% CI, 0.96-1.58). South Asians had a lower 30-day mortality (OR, 0.88; 95% CI, 0.78-0.98) but a higher incidence of hospitalization or emergency department visits (OR, 1.17; 95% CI, 1.14-1.20) with a trend toward a lower incidence of cardiac complications (OR, 0.76; 95% CI, 0.67-0.87) and neurologic complications (OR, 0.89; 95% CI, 0.73-1.09). There was also a significant difference in these outcomes between wave 1, 2 and 3, with a greater mortality in all groups in waves 2 and 3.
Conclusions
Ethnicity continues to be an important determinant of mortality, cardiac and neurologic outcomes, and healthcare use among patients with COVID-19, requiring further studies to understand factors driving these differences.
Résumé
Contexte
Nous avons déjà présenté les issues cliniques cardiaques et neurologiques chez les Ontariens de descendance chinoise ou sud-asiatique pour la première vague de la pandémie de COVID-19, mais les données au sujet des vagues ultérieures n’avaient pas encore été analysées. Nous présentons ici une prolongation de cette étude de cohortes pour la seconde et la troisième vague de COVID-19.
Méthodologie
Notre analyse porte sur des adultes ontariens ayant obtenu un résultat positif à un test de COVID-19 par réaction en chaîne de la polymérase entre le 1er janvier 2020 et le 30 juin 2021. Un algorithme validé pour l’analyse des noms de famille a été utilisé pour isoler les sujets de descendance chinoise ou sud-asiatique, et leur taux de mortalité de même que les complications cardiaques et neurologiques ont été comparés à ceux de la population générale à l’aide de modèles de régression logistique multivariée.
Résultats
En comparaison de la population générale (n = 439 977), les personnes de descendance chinoise (n = 15 208) se sont révélées plus âgées (âge moyen de 44,2 ans contre 40,6 ans, P < 0,001), tandis que les personnes de descendance sud-asiatique (n = 46 333) étaient plus jeunes (39,2 ans, P < 0,001). Dans la population de descendance chinoise, le taux de mortalité après 30 jours était plus élevé (rapport de cotes [RC] de 1,44; intervalle de confiance [IC] à 95 % de 1,28 à 1,61), et davantage d’hospitalisations ou de consultations aux urgences sont survenues (RC de 1,14; IC à 95 % de 1,09 à 1,28). L’incidence de complications cardiaques (RC de 1,03; IC à 95 % de 0,87 à 1,12) et de complications neurologiques (RC de 1,23; IC à 95 % de 0,96 à 1,58) avait également tendance à être plus élevée. Chez les personnes de descendance sud-asiatique, le taux de mortalité après 30 jours était plus faible (RC de 0,88; IC à 95 % de 0,78 à 0,98), mais l’incidence d’hospitalisations ou de consultations aux urgences était plus élevée (RC de 1,17; IC à 95 % de 1,14 à 1,20). Elles présentaient également une tendance vers une plus faible incidence de complications cardiaques (RC de 0,76; IC à 95 % de 0,67 à 0,87) et de complications neurologiques (RC de 0,89; IC à 95 % de 0,73 à 1,09). Des différences significatives ont également été observées pour ces paramètres entre les vagues 1, 2 et 3 de la maladie, et le taux de mortalité était plus élevé pour tous les groupes des vagues 2 et 3.
Conclusions
L’origine ethnique demeure un déterminant important de la mortalité, des issues cliniques cardiaques et neurologiques ainsi que de l’utilisation des ressources en santé chez les patients atteints de la COVID-19. D’autres études sont toutefois nécessaires pour mieux comprendre les facteurs qui expliquent ces différences.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the global pandemic known as coronavirus disease (COVID-19).1,2 Differences in COVID-19 infection rates and adverse outcomes following infection across different ethnic groups have been described.3, 4, 5, 6, 7, 8, 9, 10 In the US, the overall mortality rate among African Americans with COVID-19 was significantly higher than that of White and Asian people.11
In Canada’s most populous province—Ontario—South Asians (29.6% of the total population) and Chinese people (19.4%) represent the largest visible minorities.12,13 We have previously described the cardiac and neurologic complications among Chinese and South Asian Ontarians during the first wave of the pandemic (between January 15, 2020 and July 17, 2020), identifying significant differences in baseline demographics and outcomes.3 Since then, 2 subsequent waves (wave 2: July 18, 2020 to March 4, 2021; wave 3: March 5, 2021 to June 30, 2021) of COVID-19 infection in Ontario have been observed, with variation in infection rates and outcomes based on various demographic and social factors. Thus, we conducted this extension study using similar methodology to evaluate mortality, cardiac and neurologic complications, and healthcare utilization in waves 1, 2, and 3 among Chinese and South Asian Ontarians, compared to the general population and compared to these outcomes across the 3 waves.
Methods
Data sources and study population
To identify the cohort, we used the Case and Contact Management System (CCM) database and selected adults (aged 18 to 105 years) who had a positive COVID polymerase chain reaction (PCR) test result between January 1, 2020 and June 30, 2021 (inclusive). We excluded patients who were not Ontario residents at the time of the COVID test. If a person had multiple positive tests within the study period, the first positive test date was chosen as the index date. Our methodology has been previously reported.3 In brief, data from the Registered Persons Database, the Canadian Institute for Health Information Discharge Abstract Database, the National Ambulatory Care Reporting System, and the Ontario Health Insurance Plan physician claims database were used to identify demographics and health conditions, health outcomes, and health service utilization. The Registered Persons Database provides demographic information about anyone who has ever received an Ontario health card number. The Canadian Insitute for Health Information Discharge Abstract Database contains patient-level data for hospitalizations. The National Ambulatory Care Reporting System captures information on patient visits to emergency departments (EDs). The Ontario Health Insurance Plan captures information regarding the services provided by practicing physicians in Ontario. These datasets were linked using unique encoded identifiers and analyzed at ICES. Long-term care status was ascertained using the Ontario Drug Benefit database and the Continuing Care Reporting System.
Exposure
We classified patients as being Chinese or South Asian using a validated surname algorithm with high specificity as reported by Shah et al. in 2010.14 We compared Chinese and South Asian people to the general Ontario population (those not classified as Chinese or South Asian). The cohort was stratified into 3 waves: wave 1 was from January 15, 2020 to July 17, 2020; wave 2 was from July 18, 2020 to March 4, 2021; and wave 3 was from March 5, 2021 to June 30, 2021.
Outcomes
The primary outcomes at 30 days following a positive PCR test were as follows: death, composite cardiac outcome (myocardial infarction, heart failure, arrhythmia, atrial fibrillation/flutter, myocarditis, pulmonary embolism/deep vein thrombosis); composite neurologic outcome (hemorrhagic stroke, ischemic stroke, seizure, meningitis, encephalitis, encephalopathy, and Parkinson’s Disease); and healthcare utilization (hospitalization, ED visit, intensive care unit [ICU] admission, use of extracorporeal membrane oxygenation, use of mechanical ventilation). Codes are listed in Supplemental Table S1. Outcomes were measured at the individual patient level. The final follow-up date was July 31, 2021.
Statistical analysis
Baseline and outcome characteristics were compared among the Chinese, South Asian, and general populations. For continuous variables, descriptive statistics included mean values with standard deviation, and median values with interquartile range; the P values were calculated using 1-way analysis of variance for means, and the Kruskal-Wallis test for medians. Categorical variables were described using proportions, and P values from a χ2 test were provided.
We reported crude and age- and sex-standardized rates of outcomes. We used the Ontario population from the year 2020 as the reference population. We used multivariable logistic regression to determine whether ethnicity was associated with our outcomes of interest. A separate logistic regression model was built for each binary outcome, with ethnicity as the main exposure categorical variable, and the general population as the reference group. We adjusted for age, sex, income quintile, long-term care residence, and Charlson Index in the 90 days prior to a positive COVID test. We reported odds ratios (ORs) and 95% confidence intervals (CIs) comparing Chinese people and South Asian people to the general population. Using stratified analyses, we compared the outcomes across the 3 waves.
Sensitivity analyses
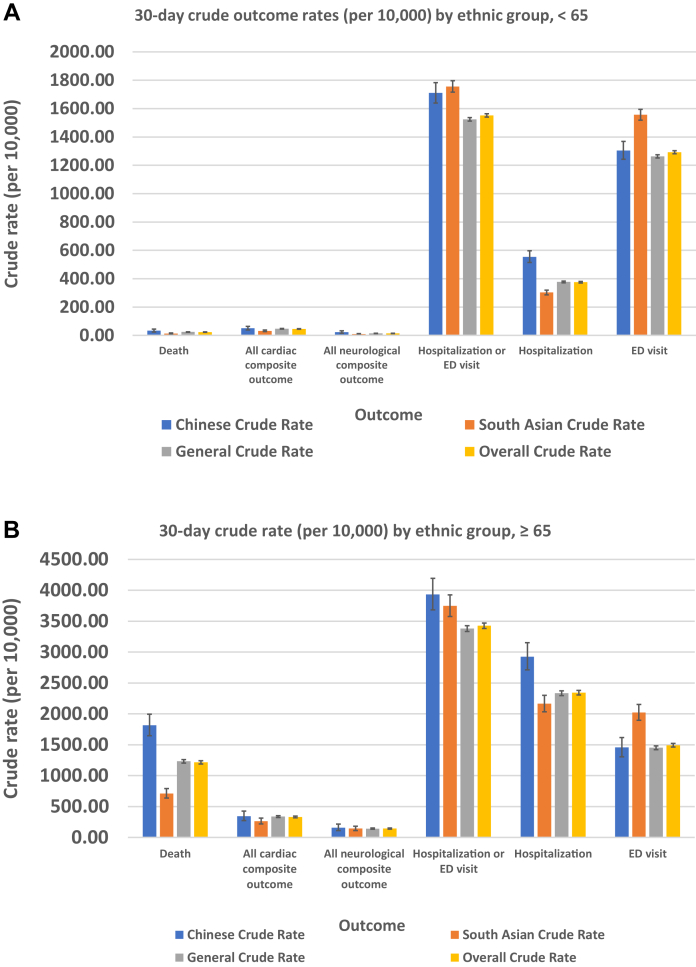
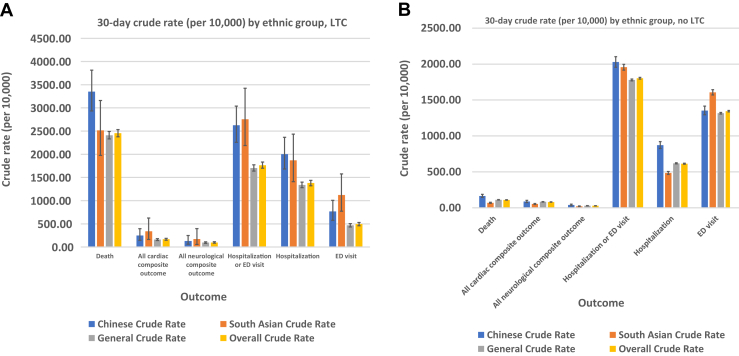
Recognizing that age is an important predictor of outcomes, we conducted sensitivity analyses, stratifying the cohort and limiting the analyses to those aged < 65 years and those aged ≥ 65 years (Figure 1). We also evaluated the variation in outcomes among those who did reside vs did not reside in a long-term care facility (Figure 2).
Figure 1.
30-day outcome crude rates, by ethnic group, for patients aged (A) < 65 years and (B) ≥ 65 years. ED, emergency department.
Figure 2.
30-day outcome crude rates, by ethnic group, for patients (A) in long-term care (LTC) and (B) not in LTC. ED, emergency department.
Results
All patients in our analyses had a positive PCR test for COVID-19. Compared to the general population (n = 439,977), the Chinese population (n = 25,208) was older (mean age 44.2 vs 40.6 years, P < 0.001), whereas the South Asian population (n = 46,333) was younger (mean age 39.2 years, P < 0.001). The mean age decreased significantly from wave 1 to wave 3: Chinese population (54.3, 45.9, and 40.4 years, respectively); South Asian population (43.9, 39.8, and 37.7 years, respectively); and general population (52.9, 41.6, and 37.4 years, respectively) (P < 0.001). The majority of COVID-19 cases in Ontario for all 3 cohorts were from wave 2 and wave 3: Chinese population, 93.3%; South Asian population, 95.1%; and general population, 92.7%. Further differences in baseline characteristics are described in Table 1.
Table 1.
Baseline characteristics of patients with positive COVID test, by ethnic group, in Ontario: waves 1, 2, and 3
| Characteristic | Chinese population | South Asian population | General population | Overall | P | P |
|---|---|---|---|---|---|---|
| n = 15,208 | n = 46,333 | n = 439,977 | N = 501,518 | Chinese vs general | South Asian vs general | |
| Age, y, mean (SD) | 44.15 ± 21.20 | 39.19 ± 18.87 | 40.63 ± 21.37 | 40.60 ± 21.16 | < 0.001 | < 0.001 |
| Age, y, median (IQR) | 43 (29–58) | 37 (26–53) | 39 (24–56) | 39 (24-55) | < 0.001 | < 0.001 |
| Age groups, y | < 0.001 | < 0.001 | ||||
| ≤ 19 | 1766 (11.6) | 6454 (13.9) | 74,096 (16.8) | 82,316 (16.4%) | ||
| 20–39 | 5010 (32.9) | 18,523 (40.0) | 150,481 (34.2) | 174,014 (34.7%) | ||
| 40–59 | 5008 (32.9) | 14,204 (30.7) | 130,576 (29.7) | 149,788 (29.9%) | ||
| 60–79 | 2414 (15.9) | 6150 (13.3) | 62,009 (14.1) | 70,573 (14.1%) | ||
| ≥ 80 | 1010 (6.6) | 1002 (2.2) | 22,815 (5.2) | 24,827 (5.0%) | ||
| Sex | 0.93 | < .001 | ||||
| Female | 7741 (50.9) | 21,684 (46.8) | 223,793 (50.9) | 253,218 (50.5%) | ||
| Male | 7467 (49.1) | 24,649 (53.2) | 216,184 (49.1) | 248,300 (49.5%) | ||
| Income quintile | < 0.001 | < 0.001 | ||||
| Missing | 51 (0.3) | 66 (0.1) | 1396 (0.3) | 1513 (0.3%) | ||
| 1 (lowest) | 3882 (25.5) | 8073 (17.4) | 112,051 (25.5) | 124,006 (24.7%) | ||
| 2 | 3876 (25.5) | 12,177 (26.3) | 91,786 (20.9) | 107,839 (21.5%) | ||
| 3 | 2673 (17.6) | 13,988 (30.2) | 90,032 (20.5) | 106,693 (21.3%) | ||
| 4 | 2666 (17.5) | 7808 (16.9) | 79,019 (18.0) | 89,493 (17.8%) | ||
| 5 (highest) | 2060 (13.5) | 4221 (9.1) | 65,693 (14.9) | 71,974 (14.4%) | ||
| Residence | < 0.001 | < 0.001 | ||||
| Missing | 51 (0.3) | 65 (0.1) | 1205 (0.3) | 1321 (0.3%) | ||
| Rural | 58 (0.4) | 247 (0.5) | 19,580 (4.5) | 19,885 (4.0%) | ||
| Urban | 15,099 (99.3) | 46,021 (99.3) | 419,192 (95.3) | 480,312 (95.8%) | ||
| Asthma | 1781 (11.7) | 7279 (15.7) | 72,043 (16.4) | 81,103 (16.2%) | < 0.001 | < 0.001 |
| Diabetes | 1943 (12.8) | 8010 (17.3) | 56,669 (12.9) | 66,622 (13.3%) | 0.707 | < 0.001 |
| Hypertension | 3241 (21.3) | 9691 (20.9) | 93,533 (21.3) | 106,465 (21.2%) | 0.876 | 0.086 |
| Heart failure | 284 (1.9) | 639 (1.4) | 11,057 (2.5) | 11,980 (2.4%) | < 0.001 | < 0.001 |
| COPD | 205 (1.3) | 320 (0.7) | 8674 (2.0) | 9199 (1.8%) | < 0.001 | < 0.001 |
| Dementia | 631 (4.1) | 446 (1.0) | 16,319 (3.7) | 17,396 (3.5%) | 0.005 | < 0.001 |
| LTC | 689 (4.5) | 294 (0.6) | 14,578 (3.3) | 15,561 (3.1%) | < 0.001 | < 0.001 |
| LTC time of test | 337 (2.2) | 120 (0.3) | 5646 (1.3) | 6103 (1.2%) | < 0.001 | < 0.001 |
| Hospitalization in previous 5 y | 2985 (19.6) | 7591 (16.4) | 97,427 (22.1) | 108,003 (21.5%) | < 0.001 | < 0.001 |
| Chronic kidney disease | 426 (2.8) | 1127 (2.4) | 12,847 (2.9) | 14,400 (2.9%) | 0.392 | < 0.001 |
| Charlson comorbidity index (past 5 y from index) | ||||||
| Acute myocardial infarction | 76 (0.5) | 333 (0.7) | 3459 (0.8) | 3868 (0.8%) | < 0.001 | 0.116 |
| Congestive heart failure | 143 (0.9) | 311 (0.7) | 5696 (1.3) | 6150 (1.2%) | < 0.001 | < 0.001 |
| Peripheral vascular disease | 49 (0.3) | 40 (0.1) | 1631 (0.4) | 1720 (0.3%) | 0.332 | < 0.001 |
| Cerebrovascular disease | 171 (1.1) | 210 (0.5) | 4189 (1.0) | 4570 (0.9%) | 0.032 | < 0.001 |
| Dementia | 280 (1.8) | 193 (0.4) | 7006 (1.6) | 7479 (1.5%) | 0.016 | < 0.001 |
| COPD or other respiratory diseases | 98 (0.6) | 223 (0.5) | 5119 (1.2) | 5440 (1.1%) | < 0.001 | < 0.001 |
| Rheumatic-like diseases | 17 (0.1) | 37 (0.1) | 641 (0.1) | 695 (0.1%) | 0.279 | < .001 |
| Ulcers of the digestive system | 63 (0.4) | 58 (0.1) | 1223 (0.3) | 1344 (0.3%) | 0.002 | < .001 |
| Liver disease—mild | 41 (0.3) | 57 (0.1) | 1096 (0.2) | 1194 (0.2%) | 0.619 | < .001 |
| Diabetes with no chronic complications | 236 (1.6) | 591 (1.3) | 6086 (1.4) | 6913 (1.4%) | 0.081 | 0.058 |
| Diabetes with chronic complications | 290 (1.9) | 794 (1.7) | 9760 (2.2) | 10,844 (2.2%) | 0.01 | < 0.001 |
| Hemiplegia or paraplegia | 56 (0.4) | 56 (0.1) | 1267 (0.3) | 1379 (0.3%) | 0.071 | < 0.001 |
| Renal (kidney) disease | 94 (0.6) | 181 (0.4) | 3078 (0.7) | 3353 (0.7%) | 0.235 | < 0.001 |
| Cancer (no secondary found) | 138 (0.9) | 234 (0.5) | 4352 (1.0) | 4724 (0.9%) | 0.316 | < 0.001 |
| Liver disease—moderate or severe | 14 (0.1) | 38 (0.1) | 451 (0.1) | 503 (0.1%) | 0.692 | 0.186 |
| Cancer (metastatic—secondary) | 55 (0.4) | 56 (0.1) | 1316 (0.3) | 1427 (0.3%) | 0.166 | < 0.001 |
| HIV / AIDS∗ | 0 (0.0) | 3 (0.0) | 118 (0.0) | 121 (0.0%) | 0.043 | 0.008 |
| Mean ± SD | 0.18 ± 0.78 | 0.11 ± 0.60 | 0.19 ± 0.81 | 0.18 ± 0.79 | 0.1 | < 0.001 |
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0-0) | 0.049 | < 0.001 |
| Charlson category | ||||||
| 0 | 14,101 (92.7) | 44,135 (95.3) | 406,041 (92.3) | 464,277 (92.6%) | 0.141 | < 0.001 |
| 1 | 421 (2.8) | 865 (1.9) | 12,991 (3.0) | 14,277 (2.8%) | ||
| ≥ 2 | 686 (4.5) | 1333 (2.9) | 20,945 (4.8) | 22,964 (4.6%) | ||
| Wave (χ2 test) | < 0.001 | < 0.001 | ||||
| 1 | 1024 (6.7) | 2274 (4.9) | 32,147 (7.3) | 35,445 (7.1%) | ||
| 2 | 7810 (51.4) | 25,648 (55.4) | 216,165 (49.1) | 249,623 (49.8%) | ||
| 3 | 6374 (41.9) | 18,411 (39.7) | 191,665 (43.6) | 216,450 (43.2%) | ||
Values are n (%), unless otherwise indicated. Waves: 1 = January 15, 2020 to July 17, 2020; 2 = July 18, 2020 to March 4, 2021; 3 = March 5, 2021 to June 30, 2021 (end of cohort selection).
COPD, chronic obstructive pulmonary disease; HIV/AIDS, human immunodeficiency virus/acquired immune deficiency syndrome; IQR, interquartile range; LTC, long-term care; SD, standard deviation.
Very low number of cases.
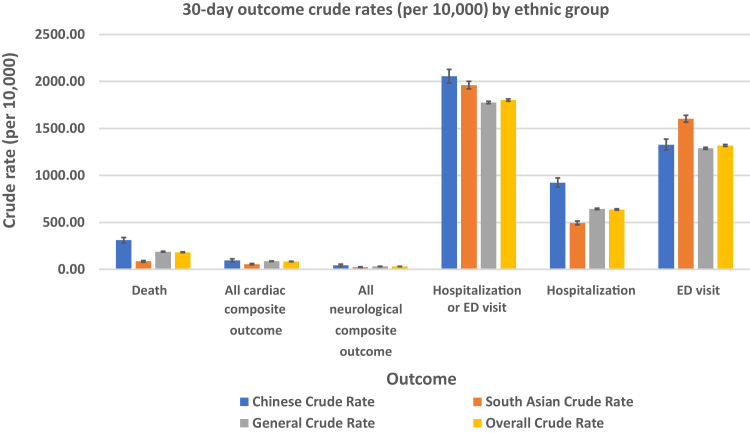
Table 2 shows the crude rates of each outcome of interest. Compared to the rate in the general population, 30-day mortality was higher in the Chinese population (3.1 vs 1.9%, P < 0.001) and lower in the South Asian population (0.9 vs 1.9%, P < 0.001). Compared to that in the general population, the incidence of cardiac complications was similar in the Chinese population (1.0% vs 0.9%) and lower in the South Asian population (0.6% vs 0.9%, P < 0.001), whereas the incidence of neurologic complications was similar in the Chinese population (0.4% vs 0.3%) and the South Asian population (0.2% vs 0.3%; Fig. 3). Healthcare utilization was more frequent in both the Chinese and South Asian populations, compared to that in the general population (Table 2).
Table 2.
Crude rates of 30-day mortality, cardiac and neurologic complications, and healthcare use of patients with COVID-19 in Ontario
| Outcome |
Chinese patients |
South Asian patients |
General population of patients |
P |
P |
|---|---|---|---|---|---|
| N = 15,208 | N = 46,333 | N = 439,977 | Chinese vs general | South Asian vs general | |
| Death | 471 (3.1) | 394 (0.9) | 8280 (1.9) | < 0.001 | < 0.001 |
| All cardiac composite outcome | 145 (1.0) | 257 (0.6) | 3789 (0.9) | 0.227 | < 0.001 |
| AMI | 11 (0.1) | 29 (0.1) | 237 (0.1) | 0.337 | 0.445 |
| HF | 20 (0.1) | 52 (0.1) | 684 (0.2) | 0.46 | 0.023 |
| Arrhythmia—excluding atrial fibrillation and flutter | 43 (0.3) | 67 (0.1) | 919 (0.2) | 0.051 | 0.003 |
| Atrial fibrillation and flutter | 52 (0.3) | 54 (0.1) | 1174 (0.3) | 0.079 | < 0.001 |
| Myocarditis | 1– 5∗ | 1–5∗ | 12–16∗ | 0.56 | 0.609 |
| DVT/PE | 43 (0.3) | 82 (0.2) | 1183 (0.3) | 0.746 | < 0.001 |
| All neurological composite outcome | 66 (0.4) | 106 (0.2) | 1385 (0.3) | 0.01 | 0.001 |
| Stroke | 19 (0.1) | 29 (0.1) | 367 (0.1) | 0.084 | 0.135 |
| Ischemic stroke | 12 (0.1) | 20 (0.0) | 290 (0.1) | 0.541 | 0.065 |
| Hemorrhagic stroke | 7 (0.0) | 10 (0.0) | 85 (0.0) | 0.023 | 0.74 |
| Seizure | 4–8∗ | 6–10∗ | 165 (0.0) | 0.772 | 0.05 |
| Meningitis | 0 (0.0) | 0 (0.0) | 1–5∗ | 0.71 | 0.516 |
| Encephalitis | 0 (0.0) | 0 (0.0) | 1–5∗ | 0.853 | 0.746 |
| Encephalopathy | 33 (0.2) | 50 (0.1) | 712 (0.2) | 0.098 | 0.005 |
| Viral meningitis not specified elsewhere | 0 (0.0) | 0 (0.0) | 1–5∗ | 0.853 | 0.746 |
| GBS | 0 (0.0) | 0 (0.0) | 9 (0.0) | 0.577 | 0.33 |
| Inflammatory myositis/myalgia | 0 (0.0) | 0 (0.0) | 0 (0.0) | n/a | n/a |
| Parkinson's Disease | 10 (0.1) | 20 (0.0) | 169 (0.0) | 0.095 | 0.621 |
| Health services | |||||
| Hospitalization or ED visit | 3126 (20.6) | 9086 (19.6) | 78,166 (17.8) | < 0.001 | < 0.001 |
| Hospitalization | 1404 (9.2) | 2288 (4.9) | 28,300 (6.4) | < 0.001 | < 0.001 |
| ED visit | 2019 (13.3) | 7430 (16.0) | 56,685 (12.9) | 0.156 | < 0.001 |
| ICU admission | 414 (2.7) | 613 (1.3) | 7315 (1.7) | < 0.001 | < 0.001 |
| ECMO | 6 (0.0) | 22 (0.0) | 139 (0.0) | 0.593 | 0.074 |
| Mechanical ventilation | 224 (1.5) | 358 (0.8) | 3908 (0.9) | < 0.001 | 0.011 |
Values are n (%), unless otherwise indicated.
AMI, acute myocardial infarction; DVT, deep vein thrombosis; ECMO, extracorporeal membrane oxygenation; ED, emergency department; GBS, Guillain Barre syndrome; HF, heart failure; ICU, intensive care unit; n/a, not applicable; PE, pulmonary embolus.
Small number of reported cases.
Figure 3.
30-day outcome crude rates (per 10,000), by ethnic group. ED, emergency department.
In multivariable logistic regression analyses, the odds ratio (OR) of 30-day mortality was higher in the Chinese population (1.44; 95% confidence interval [CI] 1.28-1.61) and lower in the South Asian population (OR, 0.88; 95% CI, 0.78-0.98) compared to that in the general population (Table 3). The OR for cardiac complications was similar between the Chinese and the general populations (OR, 1.03; 95% CI, 0.87-1.22) and lower in the South Asian population (OR, 0.76; 95% CI, 0.67-0.87). Neurologic complications were numerically more frequent in the Chinese population than in the general population (OR, 1.23; 95% CI, 0.96-1.58) and were less likely in the South Asian population (OR, 0.88; 95% CI, 0.72-1.08), but the confidence intervals included null values. The OR for hospitalization or ED visit was higher in the Chinese population (OR, 1.13; 95% CI, 1.09-1.18) and the South Asian population (OR, 1.17; 95% CI, 1.14-1.20) compared to that in the general population (Table 3).
Table 3.
Results of multivariable logistic regression analyses evaluating outcomes in patients with COVID-19 infection in Ontario
| Outcome |
Chinese vs general population |
South Asian vs general population |
|---|---|---|
| Adjusted∗ OR (95% CI) | Adjusted∗ OR (95% CI) | |
| 30-day mortality | 1.44 (1.28–1.61) | 0.88 (0.78–0.98) |
| All cardiac complications | 1.03 (0.87–1.12) | 0.76 (0.67–0.87) |
| All neurologic complications | 1.23 (0.96–1.58) | 0.89 (0.73–1.09) |
| All hospitalization or ED visits | 1.14 (1.09–1.28) | 1.17 (1.14–1.20) |
CI, confidence interval; ED, emergency department; OR, odds ratio.
Adjusted Odds Ratio (aOR).
Among those aged < 65 years, the variation in mortality rate was no longer observed, but both South Asian and Chinese patients had higher rates of healthcare utilization than the general population (Fig. 1A). For those aged ≥ 65 years, mortality rates, cardiac and neurologic complication rates, and ICU admission rates were all increased significantly compared to the rates among those aged < 65 years (Fig. 1B). The mortality rate, and the cardiac and neurologic complication rates were all increased significantly for those residing in long-term care facilities (Fig. 2A). Among those not residing in long-term care facilities, we found a higher rate of healthcare utilization, except hospitalization, among both ethnic groups compared to that in the general population (Fig. 2B).
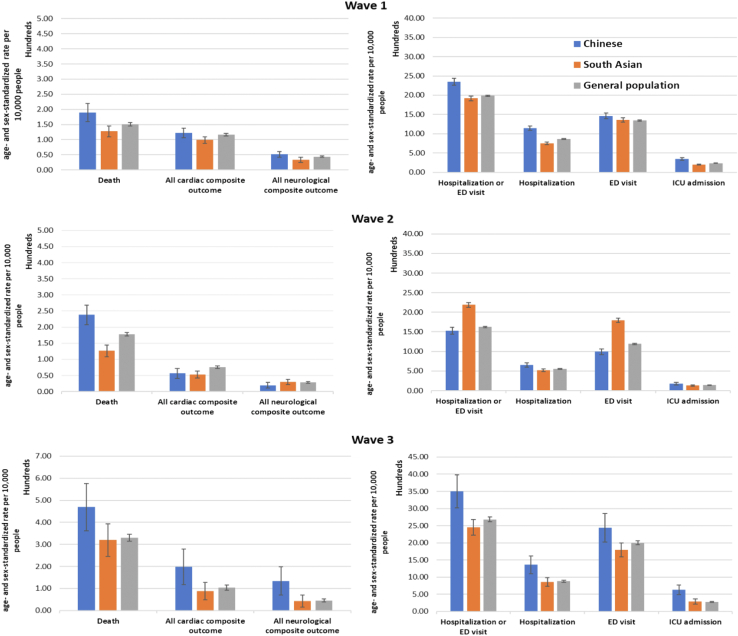
Variations across the 3 waves
Figure 4 shows the variation in age- and sex-standardized rates of our outcomes of interest across the 3 waves for all 3 groups. In contrast to wave 1, higher age- and sex-standardized rates of mortality, cardiac and neurologic outcomes, and healthcare use were observed in all 3 groups. Across all waves, Chinese patients were found to have the highest rates of mortality and cardiac and neurologic complications, whereas South Asian patients had the lowest rates. In contrast, healthcare use by the South Asian population was highest during wave 2, whereas that of Chinese patients was highest in wave 3.
Figure 4.
Age- and sex-standardized (per 10,000 people) rates (with 95% confidence intervals as vertical lines) of COVID-19-related outcomes among various ethnic groups in Ontario during waves 1, 2, and 3. ED, emergency department; ICU, intensive care unit.
Interpretation
We found variations in COVID-19-related outcomes among the Chinese and South Asian populations in Ontario, with Chinese patients being older and South Asian patients being younger than the general population. Despite adjusting for baseline differences in age, income, and comorbidities, Chinese patients with COVID-19 had a higher OR of COVID-19-related mortality and healthcare utilization, and the South Asian population had a lower OR of mortality, but a higher OR of healthcare utilization compared to those in the general population (Table 3).
The younger age at the time of COVID-19 infection among the South Asian population could be, in part, attributed to a larger proportion of the South Asian population being frontline healthcare workers or working in distribution centers or other at-risk workplaces, compared to that in the general population.2,13 Other factors driving these differences could include variation in vaccination rates or the use of masks or other public health measures by different age and ethnic groups. Future work should evaluate the impact of these factors on the observed differences and across different jurisdictions in Canada.
The higher mortality rate in Chinese patients with COVID-19, despite adjusting for baseline differences, remains unexplained (Table 3). A greater proportion of Chinese patients in our cohort resided in a long-term care facility compared to that of the general population (2.2% vs 1.3%), with the latter being associated with higher COVID-19 mortality (Table 1). Yet, in our sensitivity analyses, the higher odds of mortality among Chinese patients persisted when we studied outcomes of those not residing in a long-term care facility. A higher proportion of the Chinese population had dementia compared to that of the general population in our cohort, which could be one explanation. In contrast, the South Asian population was younger at the time of COVID-19, yet in adjusted analyses, COVID-19-related mortality was lower in this population. The reasons for this finding remain unclear, and further work, including information on vaccination status and its timing, is required to understand the implications of these findings.
COVID-19-related healthcare use was higher among the South Asian population in wave 2, and it was higher for the Chinese population in wave 3; this finding was driven mostly by a higher rate in ED visits in both these groups. Despite relatively similar baseline comorbidity status, the higher ICU admission rate among Chinese patients, especially in wave 3, may suggest that a greater number of ED visits was due to greater COVID-19 severity in this population, but the lack of a similar increase in ICU admission rates for South Asian patients in wave 2 could suggest that the greater number of ED visits by the South Asian population may be due to lack of access to primary care physicians or use of the ED for routine medical care.
Cardiac and neurologic complications
In the current study, a decline in deaths, as well as in cardiac complications, occured from wave 1 to waves 2 and 3, from 2.6% to 0.7% and 1.0% (P < 0.001), respectively. Data from 40 healthcare systems participating in a large healthcare network indicate that the risk for cardiac complications,15 including myocarditis16 and pericarditis, was significantly higher after SARS-CoV-2 infection than after mRNA COVID-19 vaccination for both sexes in all age groups.17 These findings therefore support vaccination among all at-risk populations, including the Chinese population and the South Asian population.
We found that a history of myocardial infarction, heart failure, and chronic obstructive pulmonary disease18 or other respiratory diseases was less frequent in the Chinese population, compared to the general population. The recently Cardiac Complications in Patients With SARS Corona Virus 2 (COVID-19) Registry (CAPACITY-COVID) registry and the Lean European Open Survey on SARS-CoV-2 (LEOSS) study demonstrated that significant heterogeneity exists in the intensity of association between the types of heart disease and in-hospital mortality. Of all patients with heart disease, those with heart failure are at greatest risk of death when hospitalized with COVID-19.19, 20, 21, 22 On the other hand, serious cardiac complications are rare during hospitalization.23, 24, 25
The risk of COVID-19 may be higher in patients with chronic heart failure,26,27 in part due to advanced age and the presence of multiple comorbidities, as was evident in this study. These patients with COVID-19 also have a significantly higher risk of adverse outcomes.18,28, 29, 30, 31, 32, 33, 34, 35, 36 These findings indicate that guideline-directed medical therapy should be continued in patients with heart failure, regardless of their COVID-19 status.37 In a comparison of COVID-19 patients treated in the ICU vs those not treated in the ICU in Shenzhen, China,38 troponin elevation,39 ventricular wall thickening, pulmonary hypertension, and cardiac complications including myocardial injury, arrhythmia,40 and heart failure, were found to be more common in ICU patients with COVID-19. Cardiac injury in COVID-19 patients may be related more to the systemic response after infection rather than direct damage to the heart by coronavirus, at least in the Chinese population.
Neurologic complications of COVID-1941, 42, 43, 44, 45, 46, 47 can be divided into 2 major categories: de novo neurologic complications as a direct result of COVID-19 infections, or exacerbation of preexisting neurologic conditions when patients were infected by the SARS-CoV-2 virus.48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 In our current study, the Chinese patients did not differ significantly in the prevalence of premorbid conditions, compared to the general population. This finding suggests that the excessive neurologic complication rate among the Chinese patients could be due to a direct effect of COVID-19 infection rather than exacerbation of their preexisting neurologic conditions and their having a higher mean age. Among all the neurologic complications, encephalopathy was the most common, accounting for 6.4% of hospitalized patients and 14.1% of Chinese patients admitted to ICU, but this incidence was not statistically different compared to that in the general population (Table 1). Encephalopathy in COVID-19 patients could be due to a combination of etiologic factors—hypoxemia secondary to respiratory failure, toxic and metabolic factors secondary to acute illness, and as recently recognized, inflammatory brain diseases.30,42 Encephalopathy of admitted COVID-19 patients in a large cohort study was associated with 5.5 times increased risk of death (OR 4.01- 7.57, P < 0.001).47 In our current study, very few cases of hemorrhagic stroke,63,64 ischemic stroke,10,65, 66, 67 seizures, and Parkinson’s disease occurred among the Chinese population, but this low incidence could be due to the relatively small number of reported cases in this cohort. The estimated incidence of stroke as a complication of COVID-19 varies between 2.5% and 5%, according to various recent publications.64,68, 69, 70, 71 Ongoing clinical-pathologic studies, including examining the angiotensin converting enzyme receptor (ACER-2), are required to determine whether the SARS-CoV-2 virus directly invades neural tissues of the central and peripheral nervous system.11,68,72 Such future studies would be vital in explaining why differences occur in neurologic and cardiac complications among various ethnic groups.73
Limitations
Although using a surname algorithm to identify those who are Chinese or South Asian has a high specificity—99.7% for both ethnicities—it has a much lower sensitivity of 50.4% for the South Asian population and 80.2% for the Chinese population.14 Since we used only 30-day all-cause mortality rates, and cardiac and neurologic complication rates, the current study is not able to capture “long-haul” COVID-19 patients and their eventual outcomes.36,48,74,75 In addition, the vaccination status of this cohort had not been ascertained, although it is a very important determinant of the risk of infection and its complications.76,77 A mass COVID-19 vaccination effort began in Ontario in December, 2020, and vaccination status data from that point onward were not available for this study. The datasets from the Canadian Institute for Health Information are finalized on an annual basis, and hospitalization datasets may be incomplete. A majority of the hospitals report on a monthly basis, but a few institutions may report on an annual basis. The number of hospitalizations may be an underestimate of the true volume.
Conclusions
In this very large, population-based retrospective cohort study in Ontario, being of Chinese or South Asian ethnicity, based on a surname algorithm, was found to be independently associated with mortality and healthcare use among Ontarians with COVID-19 infection in waves 1, 2, and 3. These findings are relevant to healthcare authorities across Canada when dealing with subsequent waves of this pandemic.77,78 Additionally, our findings have implications for healthcare policymakers regarding how limited resources and vaccinations should be allocated for at-risk populations.14,39,79
Funding Sources
This study was supported by the Ontario Ministry of Health-Ontario Health Data Platform (OHDP): Project TRIM # 2021 3155 006 000-OHDP; the research fund, Department of Medicine, University of Toronto: fund # 472617/FC 100089/CC 10895; and the Research Committee, Chinese Canadian Heart and Brain Association (CCHABA). This study was supported by the Ontario Health Data Platform (OHDP), a Province of Ontario initiative to support Ontario’s ongoing response to COVID-19 and its related impacts. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC).
Disclosures
The authors have no conflicts of interest to disclose. Dr Joseph Y. Chu is the Chair of Research of the Chinese Canadian Heart and Brain Association (CCHABA).
Acknowledgements
Parts of this material are based on data and/ or information compiled and provided by CIHI. However, the analyses, conclusions, opinions, and statements expressed in the material are those of the authors, and not necessarily those of CIHI. We acknowledge the administrative support by Ms. Daniella Barron of ICES.
Footnotes
Ethics Statement: This research has adhered to the REB of University of Toronto ethical guidelines.
See page 902 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2022.06.008
Supplementary Material
References
- 1.Public Health Ontario COVID-19 in Ontario—a focus on diversity: January 15 to May 14, 2020. 2020 https://www.publichealthontario.ca Available at: [Google Scholar]
- 2.Subedi R., Greenberg L., Turcotte M. COVID-19 mortality rates in Canada’s ethno-cultural neighborhoods. https://www150.statcan.gc.ca/n1/en/catalogue/45280001202000100079 Available at:
- 3.Chu J.Y., Kaliwal Y., Koh M., et al. COVID-19 and its cardiac and neurological complications among Ontario visible minorities. Can J Neurol Sci. 2022;49:504–513. doi: 10.1017/cjn.2021.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien K., St-Jean M., Wood P., et al. COVID-19 death comorbidities in Canada. https://www150.statcan.gc.ca/n1/pub/45-28-0001/2020001/article/00087-eng.htm Available at:
- 5.Suleyman G., Fadel R.A., Malette K., et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.12270. e2012270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper M.W., Napoles A.M., Perez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.APM Research Lab The color of coronavirus: COVID-19 deaths by race and ethnicity in the U.S. https://repository.gheli.harvard.edu/repository/13941/ Available at:
- 8.Haynes N., Cooper L.A., Albert M.A. At the heart of the matter: unmasking and addressing COVID-19’s toll on diverse populations. Circulation. 2020;142:105–107. doi: 10.1161/CIRCULATIONAHA.120.048126. [DOI] [PubMed] [Google Scholar]
- 9.City and County of San Francisco. COVID-19 cases and deaths. Available at: https://sf.gov/data/covid-19-cases-and-deaths. Accessed September 15, 2021.
- 10.Warrior L., Kim C.Y., Burdick D.J., et al. Leading with inclusion during the COVID-19 pandemic. Neurology. 2020;95:537–542. doi: 10.1212/WNL.0000000000010641. [DOI] [PubMed] [Google Scholar]
- 11.US Centers for Disease Control and Prevention. COVID-19 in Racial and Ethnic Minority Groups. Available at: https://stacks.cdc.gov/view/cdc/89820/cdc_89820_DS1.pdf. Accessed September 15, 2021.
- 12.Statistics Canada. StatCan Census Profile 2016: Ontario (Province) and Canada. Available at: https://www12.statcan.gc.ca/census- recensement/2016/dp- pd/prof/index.cfm lang=e. Accessed September 25, 2021.
- 13.Statistics Canada Impacts on immigrants and people designated as visible minorities. https://www150.statcan.gc.ca/n1/pub/11-631-x/2020004/s6-eng.htm Available at:
- 14.Shah B.R., Chiu M., Amin S., et al. Surname lists to identify South Asian and Chinese ethnicity from secondary data in Ontario, Canada: a validation study. BMC Med Res Methodol. 2010;10:42. doi: 10.1186/1471-2288-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang W.-T., Toh H.S., Liao C.-T., et al. Cardiac involvement of COVID-19: a comprehensive review. Am J Med Sci. 2021;361:14–22. doi: 10.1016/j.amjms.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawakami R., Sakamoto A., Kawai K., et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis. JACC review topic of the week. J Am Coll Cardiol. 2021;77:314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Block J.P., Boehmer T.K., Forrest C.B., et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination - PCORnet, United States, January 2021. MMWR Morb Mortal Wkly Rep. January 2022;2022(71):517–523. doi: 10.15585/mmwr.mm7114e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilkes A., Ashworth M., Schofield P., et al. Does COPD risk vary by ethnicity? A retrospective cross-sectional study. Int J COPD. 2016;11:739–746. doi: 10.2147/COPD.S96391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez-Garcia J., Lee S., Gupta A., et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. 2020;76:2334–2348. doi: 10.1016/j.jacc.2020.09.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehra M.R., Ruschitzka F. COVID-19 illness and heart failure: a missing link? JACC Heart Fail. 2020;8:512–514. doi: 10.1016/j.jchf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt A.S., Jering K.S., Vaduganathan M., et al. Clinical outcomes in patients with heart failure hospitalized with COVID-19. JACC Heart Fail. 2021;9:65–73. doi: 10.1016/j.jchf.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freaney P.M., Shah S.J., Khan S.S. COVID-19 and heart failure with preserved ejection fraction. JAMA. 2020;324:1499–1500. doi: 10.1001/jama.2020.17445. [DOI] [PubMed] [Google Scholar]
- 23.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The CAPACITY-COVID Collaborative Consortium and LEOSS Study Group CAPACITY-COVID: a European Registry to determine the role of cardiovascular disease in the COVID-19 pandemic. Eur Heart J. 2022;43:1104–1120. doi: 10.1093/eurheartj/ehaa280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canadian Cardiovascular Society Guidance from the CCS COVID-19 rapid response team and CCS affiliate organizations: Is it COVID-19 or is it heart failure? Management of ambulatory heart failure patients. https://ccs.ca/app/uploads/2021/02/COVID-or-HF-RRT.pdf Available at:
- 27.Panjrath G., Krepp J. COVID-19 and heart failure: harsh reality of pre-existing conditions. J Am Coll Cardiol. 2020;76:349–351. doi: 10.1016/j.jacc.2020.09.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giustino G., Pinney S., Lala A., et al. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia. Focus Seminar. J Am Coll Cardiol. 2020;76:2011–2023. doi: 10.1016/j.jacc.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia S., Dehghani P., Grines C., et al. Initial findings from the North American COVID-19 myocardial infarction registry. J Am Coll Cardiol. 2021;77:1994–2003. doi: 10.1016/j.jacc.2021.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remsik J., Wilcox J.A., Babady N.E., et al. Inflammatory leptomeningeal cytokines mediate COVID-19 neurological symptoms in cancer patients. Cancer Cell. 2021;39:276–283. doi: 10.1016/j.ccell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahrami H., Kronmal R., Bluemke D.A., et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung M.K., Zidar D.A., Bristow M.R., et al. COVID-19 and cardiovascular disease from bench to bedside. Circ Res. 2021;128:1214–1236. doi: 10.1161/CIRCRESAHA.121.317997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Task Force for the management of COVID-19 of the European Society of Cardiology. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 1—epidemiology, pathophysiology, and diagnosis. Eur Heart J. 2022;43:1033–1058. doi: 10.1093/eurheartj/ehab696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Task Force for the management of COVID-19 of the European Society of Cardiology. ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 2—care pathways, treatment, and follow-up. Eur Heart J. 2022;43:1059–1103. doi: 10.1093/eurheartj/ehab697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim S.L., Woo K.L., Lim E., et al. Impact of COVID-19 on health-related quality of life in patients with cardiovascular disease: a multi-ethnic Asian study. Health Qual Life Outcomes. 2020 doi: 10.1186/s12955-020-01640-5. 18:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbasi J. The COVID heart—one year after SARS-CoV-2 infection, patients have an array of increased cardiovascular risks. JAMA. 2022;327:1113–1114. doi: 10.1001/jama.2022.2411. [DOI] [PubMed] [Google Scholar]
- 37.Roifman I., Arora R.C., Bewick D., et al. Cardiovascular care delivery during the second wave of COVID-19 in Canada. Can J Cardiol. 2021;37:790–793. doi: 10.1016/j.cjca.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J.H., Wu W.-B., Qu J.-X., et al. Cardiac manifestations of COVID-19 in Shenzhen, China. Infection. 2020;48:861–870. doi: 10.1007/s15010-020-01473-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tersalvi G., Vicenzi M., Calabretta D., et al. Elevated troponin in patients with Coronavirus Disease 2019 (COVID-19): possible mechanisms. J Card Fail. 2020;26:470–475. doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kochi A.N., Tagliari A.P., Forleo G.B., et al. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nepal G., Rehrig J.H., Shretha G.S., et al. Neurological manifestations of COVID-19: a systematic review. Crit Care. 2020;24:421. doi: 10.1186/s13054-020-03121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellul M.A., Benjamin L., Singh B., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D., Yin Y., Hu C., et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24:188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tu H., Tu S., Gao S., et al. The epidemiological and clinical features of COVID-19 and lessons from this global infectious public health event. J Infect. 2020;81:1–9. doi: 10.1016/j.jinf.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qureshi A., Abd-Allah F., Alsenani F., et al. Management of acute ischemic stroke in patients with COVID-19 infection: report of an international panel. Int J Stroke. 2020;15:540–554. doi: 10.1177/1747493020923234. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y., Li W., Wang D., et al. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc Neurol. 2020;5:177–179. doi: 10.1136/svn-2020-000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chou S. Neurological complications of COVID-19. Paper presented at: 73rd Annual Meeting–American Academy of Neurology: hot topics: neuro-COVID plenary session. April 20, 2021, Virtual conference.
- 48.Narth A. Neurologic complications of coronavirus infections. Neurology. 2020;94:809–810. doi: 10.1212/WNL.0000000000009455. [DOI] [PubMed] [Google Scholar]
- 49.Niazkar H.R., Zibaee B., Nasimi A., et al. The neurological manifestations of COVID-19: a review article. Neurol Sci. 2020;41:1667–1671. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Needham E.J., Chou S.H.-Y., Coles A.J., Menon D.K. Neurological implications of COVID-19 infections. Neurocrit Care. 2020;32:667–671. doi: 10.1007/s12028-020-00978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vinciguerra M., Greco E. Sras-CoV-2 and black population: ACE2 as shield or blade? Infect Genet Evol. 2020;84:104361. doi: 10.1016/j.meegid.2020.104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong W., Mu J., Guo J., et al. New onset neurologic events in people with COVID-19 in 3 regions in China. Neurology. 2020;95:e1479–e1487. doi: 10.1212/WNL.0000000000010034. [DOI] [PubMed] [Google Scholar]
- 53.Xie J., Wu W., Li S., et al. Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: a retrospective multicenter study. Intens Care Med. 2020;46:1863–1872. doi: 10.1007/s00134-020-06211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan D., Du T., Hong W., et al. Neurological complications and infection mechanism of SARS-CoV-2. Signal Transduct Target Ther. 2021;6:406. doi: 10.1038/s41392-021-00818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serrano-Castro P.J., Estvill-Torius G., Cabezudo-Garcia P., et al. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: a delayed pandemic? Neurologia (Engl Ed) 2020;35:245–251. doi: 10.1016/j.nrl.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsivgoulis G., Palaiodimou L., Zand R., et al. COVID-19 and cerebrovascular diseases: a comprehensive overview. Ther Adv Neurol Disord. 2020;13 doi: 10.1177/1756286420978004. 1756286420978004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orru G., Conversano C., Malloggi E., et al. Neurological complications of COVID-19 and possible neuroinvasion pathways: a systematic review. Int J Environ Res Public Health. 2020;17:6688. doi: 10.3390/ijerph17186688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shehata G.A., Lord K.C., Grudzinski M.C., et al. Neurological complications of COVID-19: underlying mechanisms and management. Int J Mol Sci. 2021;22:4081. doi: 10.3390/ijms22084081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharifian-Dorche M., Huot P., Osherov M., et al. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVD-19 pandemic. J Neurol Sci. 2020;417:117085. doi: 10.1016/j.jns.2020.117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whittaker A., Anson M., Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand. 2020;142:14–22. doi: 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maury A., Lyoubi A., Peiffer-Smadja N., et al. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: a narrative review for clinicians. Rev Neurol (Paris) 2021;177:51–64. doi: 10.1016/j.neurol.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van der Worp B., Sandset E.C., Caso V. Likely increase in the risk of death or disability from stroke during the COVID-19 pandemic. https://eso-stroke.org/likely-increase-in-the-risk-of-death-or-disability-from-stroke-during-the-covid-19-pandemic/ Available at:
- 64.Dhamoon M.S., Thaler A., Gururangan K., et al. Acute cerebrovascular events with COVID-19 infection. Stroke. 2021;52:48–56. doi: 10.1161/STROKEAHA.120.031668. [DOI] [PubMed] [Google Scholar]
- 65.Laurencin C., McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398–402. doi: 10.1007/s40615-020-00756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khosravani H., Rajendram P., Notario L., et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke. 2020;51:1891–1895. doi: 10.1161/STROKEAHA.120.029838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frontera J.A., Sabadia S., Lalchan R. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology. 2021;96:e575–e586. doi: 10.1212/WNL.0000000000010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L., Shen Y., Li M., et al. Clinical manifestations and evidence of neurological involvement in 2019 novel coronavirus SARSCoV-2: a systematic review and meta-analysis. J Neurol. 2020;267:2777–2789. doi: 10.1007/s00415-020-09974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382 doi: 10.1056/NEJMc2009787. e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Misra S., Kolappa K., Prasad M., et al. Frequency of neurologic manifestations in COVID-19: a systematic review and meta-analysis. Neurology. 2021;97:e2269–e2281. doi: 10.1212/WNL.0000000000012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kornowski R., Orvin K. The clinical challenge of ST-segment elevation myocardial infarction and COVID-19. J Am Coll Cardiol. 2021;77:2004–2006. doi: 10.1016/S0735-1097(21)03360-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solomon I.H., Normandin E., Bhattacharyya S., et al. Neuropathological Features of Covid-19. N Engl J Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Troxel A.B., Frontera J.A., Mendoza-Puccini C. The National Institutes of Health COVID-19 NeuroDatabank and NeuroBiobank: a national resource for learning, discovery, and progress. Front Neurol. 2021;11:615061. doi: 10.3389/fneur.2020.615061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katsoularis I., Fonseca-Rodriguez O., Farrington P., et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: nationwide self-controlled cases series and matched cohort study BMJ. 2022;376 doi: 10.1136/bmj-2021-069590. e069590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mao L., Wang M., Chen S., et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. https://www.medrxiv.org/content/10.1101/2020.02.22.20026500v1 Available at:
- 77.Bouzid D., Visseauz B., Kassasseya C., et al. Comparison of patients infected with delta versus omicron COVID-19 variants presenting to Paris emergency departments: a retrospective cohort study Ann Intern Med. 2022;175:831–837. doi: 10.7326/M22-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Public Health Ontario. Coronavirus Disease 2019 (COVID-19). Available at: https://www.publichealthontario.ca/en/Diseases-and-Conditions/Infectious-Diseases/Respiratory-Diseases/Novel-Coronavirus. Accessed September 15, 2021.
- 79.Wang C., Wang Z., Wang G., et al. COVID-19 in early 2021: current status and looking forward. Signal Transduct Target Ther. 2021;6:114. doi: 10.1038/s41392-021-00527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.