Abstract
To predict the maturity of bananas, the present study used non-destructive methods to analyze changes in the sweetness and color of the stalks, middles, and tips of bananas during ripening. The results indicated that the respective maturation of these three segments did not occur simultaneously, as indicated by the differential enzyme activity and gene expression levels recorded in these segments. A principal component analysis and cluster plots were used to review the classification of banana maturity, highlighting that banana maturation can be divided into six stages. Two distinct maturity prediction algorithms were established using random forest, artificial neural network, and support vector machines, and they also indicated that dividing the maturity of bananas into six stages was adequate. These findings contribute to the development of quality evaluation and of a rapid grading system for processing, which improves the quality and sale of banana fruits and the related processed products.
Keywords: Banana, Maturity, Segments, Prediction
Graphical abstract
Highlights
-
•
Sweetness and color during ripening were assessed along banana fingers.
-
•
A new maturity prediction model was established for bananas.
-
•
Banana maturity was divided in six stages.
-
•
The theoretical basis for developing a maturity grading detection device was set.
1. Introduction
Banana (Musa spp.) is one of the most popular fruits worldwide. It is a typical climacteric fruit, with a respiratory peak during post-harvest ripening, which leads to fruit softening and even rotting, hence limiting its shelf life (Clendennen and May, 1997; Jiang et al., 1999). Notably, a delayed harvest may reduce shelf life and increase fruit susceptibility to diseases, which negatively influences manufacturers and retailers, while harvesting too early causes poor consumption quality, leading to complaints from consumers (Xie et al., 2018). Depending on the maturity degree of the fruit, bananas can be processed into banana chips, banana liqueur, banana jam, or banana flour (Gamlath, 2010; Suntharalingam and Ravindran, 1993). Thus, it is essential to determine and accurately predict banana maturity for appropriate subsequent utilization (Laylieam and Kosittrakun, 2010). In the food industry, determining ripeness is very important to obtain good quality fruit. Saragih et al. (2021) used computer vision and other techniques to classify the maturity of ambarella fruit based on color characteristics. Nnodim et al. (2021) established a finite element model to develop a tactile sensor to distinguish mango fruits with different maturity, by measuring the elastic modulus of mango and using mechanical ANSYS parametric Design Language (APDL) software. Studies on banana ripeness are scarce, so it is necessary to study banana maturity.
Banana maturity has generally been determined by visual inspection based on the color of banana peels (Gomes et al., 2013). However, this method is subjective and, hence, characterized by a low accuracy. So there are many ways to objectively judge the ripeness of bananas. Zhang et al. (2018) investigated the ripening process of banana with the biological indexes such as starch content, soluble solid content, sugar content, and hardness, which was based on biochemical or physicochemical properties and computer vision-based methods. Mendoza and Aguilera (2004) implemented a computer vision system for identifying banana ripening stages based on information about banana color, the development of brown spots, and image texture. Adebayo et al. (2016) extracted optical properties such as absorption, scattering reduction, and effective attenuation coefficient of bananas from backscattering images of five different wavelengths, and explored the potential of using these optical properties to predict banana fruit maturity and quality attributes. Therefore, it is inaccurate to predict banana maturity based on the color of the peels and it is of great significance to establish the prediction model of banana maturity.
Fruit quality and maturity have generally been evaluated based on size, color, and texture (Sanaeifar et al., 2016). Wan et al. (2021) proposed an automatic prediction system according to the color characteristics of strawberries and tomatoes to determine the maturity or ripening stage of fruits. Chepiński et al. (2019) judged the optimal harvest time by measuring the internal quality (hardness, extract content) and fruit size of cherry fruit, which also contributed to the prediction of cherry ripening from another perspective. The decrease in firmness and increase in sweetness during fruits ripening is mainly caused by the degradation of cell wall components such as pectin, cellulose, and starch. For example, α-amylase (AL), β-amylase, and invertase (IN) are involved in starch degradation, while polygalacturonase (PG), xyloglucan endotransglycosylase (XET), and pectate lyase (PL) regulate cell wall degradation (Loay et al., 2020; Shan et al., 2020). Furthermore, during fruit ripening, the color of the skin will change significantly. For example, the color of banana peels changes from green to yellow as a consequence of chlorophyll degradation (Maduwanthi and Marapana, 2017), and color transformation is related to chlorophyll degradation caused by MaSGR, MaPAO, MaACS, and MaACO genes (Yang et al., 2009) which ultimately leads to fruit ripening (Wu et al., 2019). It has previously been reported that the proteins involved in chlorophyll degradation are different from those that degrade the cell wall and starch (Lina et al., 2016), which indicates that changes in sweetness might not be consistent with changes in color during ripening, as exemplified by green-ripe bananas. Sweetness and color are internal and external characteristics, respectively, related to the maturity of bananas (Xie et al., 2018), so there may be mistakes in evaluating banana maturity based on apparent color. Therefore, modeling and prediction of banana maturity are necessary, and they are important for the evaluation of banana quality.
Nondestructive methods to evaluate fruit quality have received increasing attention. Nondestructive technology can be used to determine the sweetness of bananas and the color of their peels during ripening, thus contributing to the development of a new method for evaluating and predicting the stage of maturity of bananas more accurately (Jaiswal et al., 2014). In turn, this could improve the quality and sale of the bananas and the related deep-processing products. Furthermore, several studies have evaluated the variations in the quality of different parts of various fruits, such as the citrus (Srivastava and Sadistap, 2021), Kiwifruit (Du et al., 2019), fuyu persimmon (Suzuki et al., 2011) and mango (Rungpichayapichet et al., 2016); however, to our knowledge, no studies have analyzed whether the maturity of different banana segments is consistent along the length of banana fingers.
The objective of the present study was to evaluate the differences between the sweetness and color of three segments along the length of banana fingers during ripening. In addition, the sweetness and the color of these segments were evaluated to develop a comprehensive prediction method for banana maturity. This study may contribute to the development of a maturity grading detection device that enables rapid detection of the quality of bananas.
2. Materials and methods
2.1. Material and treatments
Bananas (Musa spp. AAA group) in the green-ripe stage were obtained from an orchard in Foshan, China. They were brought to the laboratory within a day, and then individual banana fingers with similar sizes were separated. Each finger was divided into three segments along the length: the stalk, middle, and tip of the banana (Fig. 1). Bananas were frozen in liquid nitrogen and stored at −80 °C until they were analyzed.
Fig. 1.
Distribution diagram of stem, middle and tip of banana.
2.2. Stages of maturity
According to the reported method (Maduwanthi and Marapana, 2017), the process of banana ripening was divided into seven stages, in which the color of banana peels varied from totally green (i.e., RS1: days 1–4), green with yellow traces (i.e., RS2: days 5–6), more green than yellow (i.e., RS3: days 7–8), more yellow than green (i.e., RS4: days 9–10), yellow with green edges (i.e., RS5: days 11–12), completely yellow (i.e., RS6: days 13–15), and yellow with brown spots (i.e., RS7: after day 15).
2.3. Measurement of color parameters
The color of banana peels was measured using a colorimeter (Minolta Chroma Meter CR 400; Konica Minolta, Tokyo, Japan). The nose cone of the colorimeter was positioned directly at the surface of the bananas, avoiding any leaking of the light projected by the colorimeter. The color parameters were measured in three places on each segment of the banana during ripening (i.e., days 1–21), ensuring that each part of the banana peel was uniform. Thereafter, the color values of each segment of the banana peel were averaged.
2.4. Analysis of sweetness
The sugar content of the bananas was measured with a non-destructive Brix meter (Brix Meter N-1; Kyoto Electronics Manufacturing, Kyoto, Japan) by pressing the Brix meter on the surface of each banana and ensuring that the cushion on the Brix meter was fitted well to the surface of the banana. The sweetness was analyzed in three places on each banana segment during ripening (i.e., days 1–21), and the average values of each segment were recorded.
2.5. Extraction and assay of the activity of starch and cell wall degradation enzymes
The activities of AL, IN, and PG were determined with 3,5-dinitrosalicylic acid using assay kits (BC0615, BC0575, and BC2665, respectively; Solarbio, Beijing, China), following the manufacturer's instructions. The activities of these enzymes were calculated based on the protein concentration (i.e., U/mg protein).
2.6. RNA isolation and gene expression analysis
Frozen banana pulps and peels were powdered with a mortar and pestle in the presence of liquid nitrogen. The total RNA was isolated using the RNAprep Pure Plant Kit (DP441; Tiangen, Beijing, China) according to the instructions. A quantitative real-time PCR was conducted to assess the transcript levels of MaSGR, MaPaO, MaACS, and MaACO in banana peels and those of MaPL2, MaXET5, and MaBAM7 in banana pulp (Zhai et al., 2020). The expression levels of MaXET, MaPL2, and MaBAM7 were calculated relative to MaRPS2 expression (i.e., ribosomal protein 2), and the expression levels of MaACO, MaACS, MaSGR, and MaPaO were calculated relative to MaACT1 expression using the 2−ΔΔCt method (Chen et al., 2011). Primers are listed in Table 1.
Table 1.
Primer sequences of target and reference genes.
|
Assay |
Primer sequence |
|
|---|---|---|
| Forward primer (5′-3′) | Reverse primer (5′-3′) | |
| MaBAM7 | GCCGACGACAGCATTGACCT | CAGCCATCTTCGAGTTCTTG |
| MaPL2 | GGCTCCACTGCCATTACG | GTGCGTGTAGTCATTGTTTACC |
| MaXET5 | TCGCCTTCTACACATCCAAC | CTCCAGTCCTTCCCTCTCAC |
| MaPaO | TTCAACGGTCCTGTCCAAA | TCACGCAAAGCATAAGCCAC |
| MaSGR | GCAATGGTGTGGGTCTACT | TCCGTTCGCAAGCGTTCT |
| MaACS | TAGCGCTGAGGAGGATCGAG | CCGCTGTCGATGTTTCAGGT |
| MaACO | AGGTCTATCCGAGGTTCGTG | TAACGCGAAGTACAGCGACA |
| MaRPS2 | TAGGGATTCCGACGATTTGTTT | TAGCGTCATCATTGGCTGGGA |
| MaACT1 | TGGTATGGAAGCCGCTGGTA | TCTGCTGGAATGTGCTGAGG |
2.7. Statistical analysis and models
Two maturity prediction systems were established with the random forest (RF), support vector machine (SVM), and artificial neural network (NNET) methods. One prediction algorithm only used the average color values of the whole banana during ripening to develop the model to predict seven stages of maturity, according to earlier studies, while the other prediction system included both the color and sweetness values of the three banana segments to predict six stages of maturity, then compared the accuracy and kappa values of the two different maturity prediction models. All models were developed in R 3.2.6 with the caret package. Data are expressed as the mean ± standard deviation of three replicates. Significant differences between means were calculated using Duncan's multiple range test in SPSS 17.0 (IBM, Armonk, NY, USA). A p-value below 0.05 was considered statistically significant.
3. Results and discussion
This study determined the changes in three segments of banana during ripening and used the sweetness and color of three banana segments comprehensively to develop a maturity prediction model.
3.1. Changes in the whole banana during ripening
3.1.1. Sweetness and color values
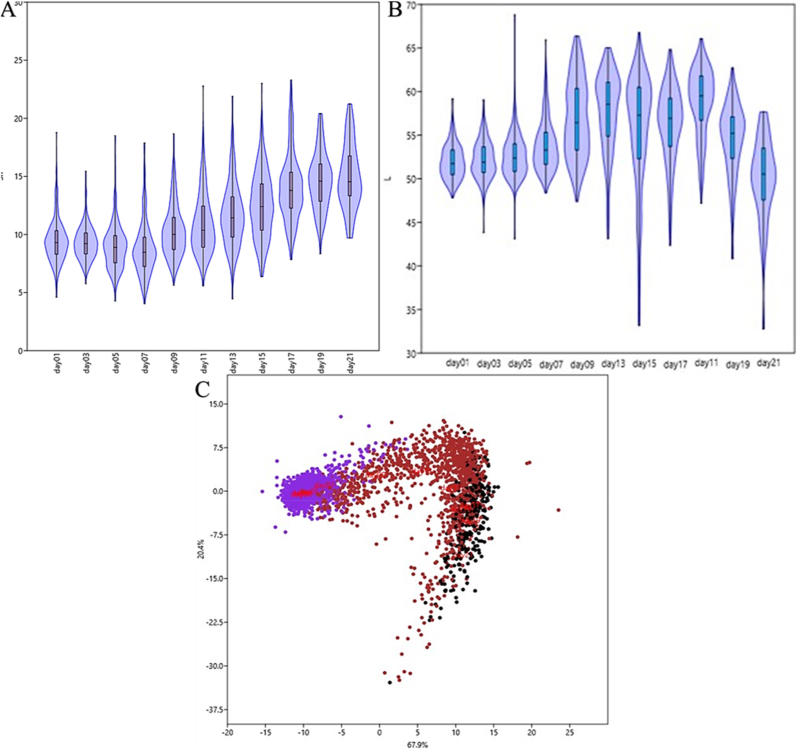
Changes in the sweetness of bananas during ripening are displayed in Fig. 2A. The sweetness substantially increased from RS4 to RS7, indicating that starch hydrolyzed to produce soluble solids during this period, which increased the sugar content of banana (Kouassi et al., 2020). Notably, there was no obvious change in sweetness after RS7. We hypothesize that the enzyme activity associated with starch degradation in fruit can only maintain the current rate, but not increase the rate of decomposition. The changes in the ‘L’ value of bananas during ripening are shown in Fig. 2B. The ‘L’ value increased at RS4, but it began to decrease upon reaching RS6. The color of the peel changes from green to yellow during ripening. The color of the banana peel became completely yellow because of the cell wall and starch degradation, and a decrease in synthetic chlorophyll (Yang et al., 2009).
Fig. 2.
Changes in the sweetness and color of the whole banana during ripening. (A) Variations in sweetness; (B) Variations in the ‘L’ value; (C) Principal component analysis of the sweetness and color values. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.1.2. Principal component analysis (PCA)
Based on a previous study, PCA coupled clustering analysis was conducted to determine fruit quality via data dimension reduction for several quality indexes related to the appearance and taste of fruits. The PCA was used to determine whether there was a relationship between the sweetness and color of bananas during ripening, and it highlighted that both the sweetness and color values were indicators of the maturity of bananas. A PCA was conducted to analyze the relationship between the sweetness and color values of bananas during ripening, and the results indicated a linear relationship between these two factors (Fig. 2C). Similarly, Milosevic and Milosevic (2013) conducted a PCA analysis of European apricot germplasm fruit quality, soluble solid content, maturity index, and sweetness index, and found that there was a high correlation between peel color and kernel taste characteristics.
3.2. Changes in the sweetness and color values of the three banana segments during ripening
3.2.1. Sweetness
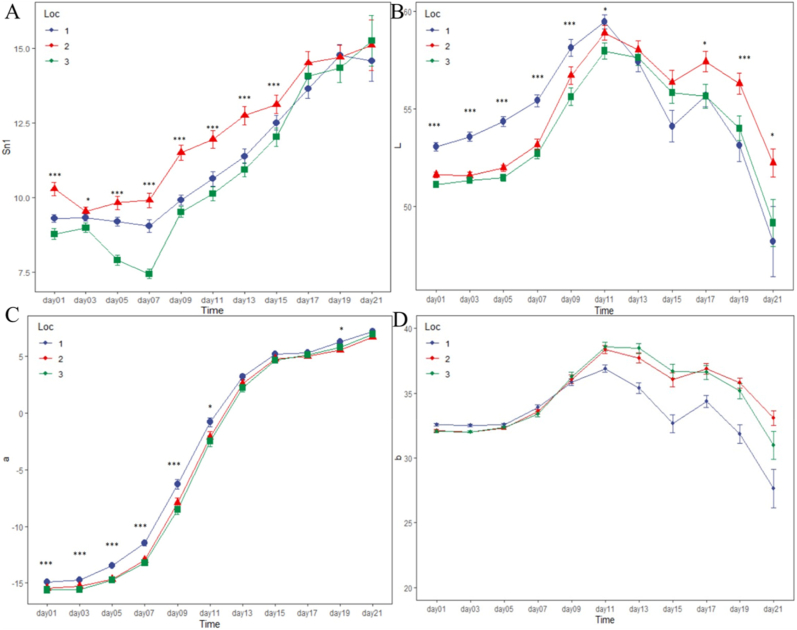
Although the changes in sweetness differed between the three banana segments during ripening (Fig. 3A), they were similar throughout the whole banana (Fig. 2A). There were significant differences (p < 0.05) between the sweetness of the middle and of both ends of the banana until reaching RS7. That is, the sweetness of the banana middles was higher than that of the tips and stalks of the bananas during the ripening process, which indicated that the cell walls and starch of the middle of the bananas degraded the fastest.
Fig. 3.
Changes in the sweetness and color of each banana segment during ripening. (A) Variations in sweetness; (B) Variations in the ‘L’ value; (C) Variations in the ‘a’ value; (D) Variations in the ‘b’ value. The statistical significance was set at p < 0.05; *p < 0.05; ***p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2.2. Color values
The ‘L’ values of the three segments of the banana peels increased from RS1 to RS5. They then began to decrease at RS6 (Fig. 3B), which is similar to the changes in the ‘L’ value observed with the whole banana peels (Fig. 2B). The ‘L’ value of each segment indicated the fastest growth appeared between RS3 and RS4. Furthermore, there were significant differences (p < 0.05) in the ‘L’ values between the stalks and the other segments of the banana peels; that is, the ‘L’ value of the stalks was higher than that of the other segments until reaching RS5, while the ‘L’ values of all the segments of the banana peels were almost equal at RS6. The ‘a’ value continually increased throughout the ripening process (Fig. 3C), and there were significant differences between the ‘a’ value of the stalks and that of the other segments of the banana peels until RS5. There were significant differences (p < 0.05) between the ‘b’ value of the stalks and that of the other segments of banana peels before RS3 and after RS5 (Fig. 3D). The ‘b’ value of the stalk segments decreased faster than that of the other segments, and the banana stalks displayed brown spots before the other segments of banana peels did. Overall, the color of both ends of the banana peels changed to yellow first, which may indicate that chlorophyll was degraded earlier in the stalk and the tip than in the middle of the banana peel.
Furthermore, the respective changes in the sweetness and color of the stalks, middles, and tips of the bananas, which illustrate the maturity along the length of the fruit, differed over the same period. That is, the changes in sweetness and color were not synchronized nor identical between the three banana segments during ripening. Notably, the sweetness of bananas constantly increases during the ripening process, hence causing the pulp texture to continually soften (Maduwanthi and Marapana, 2017). Therefore, the firmness of the three banana segments may vary. Finally, the color of both ends of the banana peels became yellow and displayed brown spots earlier than the middle part did, which also highlighted that we should comprehensively evaluate the quality of bananas, that is, based on both the sweetness and color.
3.3. Enzyme activity in the three banana segments during ripening
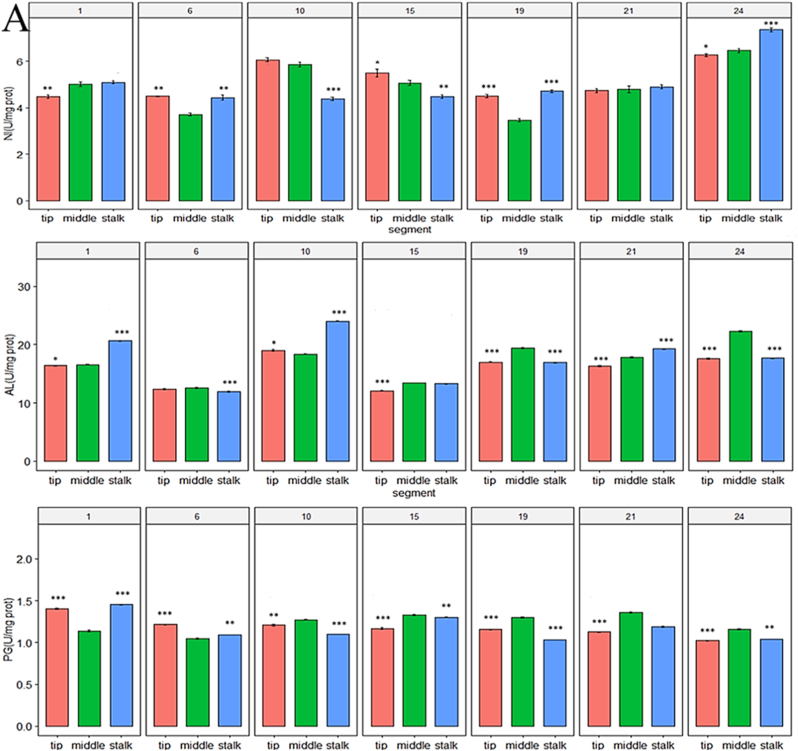
The activities of AL, IN, and PG in the stalks, middles, and tips of the bananas are presented in Fig. 4A. During ripening, the activity of IN in the middles of the bananas was lower than that in the tips and stalks of the bananas, whereas the activities of AL and PG in the middles were higher than those in both ends of the bananas (p < 0.05 for all). That is, the activities of the AL, IN, and PG enzymes, which are related to the sweetness of bananas, highlighted that the fruit maturity was not consistent between each of the three banana segments over the same period.
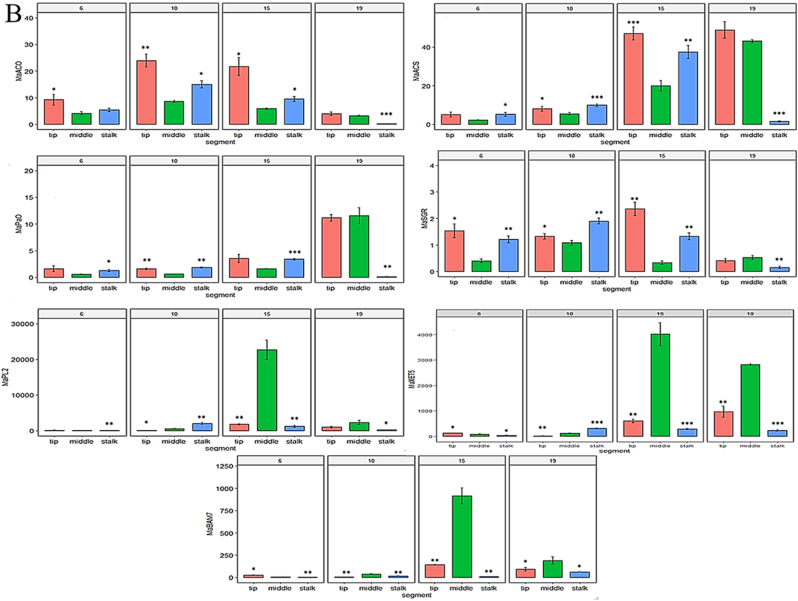
Fig. 4.
Enzyme activity and gene expression levels in each banana segment during ripening. (A) Activity of invertase (IN), α-amylase (AL), and polygalacturonase (PG) in three banana segments during ripening; (B) Expression levels of the MaACO, MaACS, MaPaO, MaSGR, MaPL2, MaXET5, and MaBAM7 genes in the stalks, middles, and tips of bananas during ripening. Expression levels were determined via real-time quantitative PCR. The statistical significance was set at p < 0.05; *p < 0.05; ***p < 0.001.
The enzyme activity of AL, IN, and PG in the middle of the bananas was higher than that in both ends, which was consistent with the results of a previous study (Mao and Kinsella, 1981). This explained why the sweetness of the middles of the bananas was the highest, and it further suggested that fruit maturity differs between the banana segments during ripening. Moreover, this highlighted that banana middles are suitable for processing into products that require high sweetness.
3.4. Gene expression in the three banana segments during ripening
The expression levels of MaACO and MaACS in both ends of the bananas were higher than those in the middles (p < 0.05), which indicated that the stalks and tips ripened earlier than the middle parts (Fig. 4B). Likewise, the expression levels of MaSGR and MaPaO in the stalks and tips of the bananas were significantly higher than those in the middles of the bananas (p < 0.05), which highlighted that chlorophyll degraded first, and quickly, in both ends of the bananas (Fig. 4B). In contrast, the expression levels of MaXET5, MaPL2, and MaBAM7 in the middles of the bananas were significantly higher than those in both ends during ripening (p < 0.05), which suggested that the sweetness of the banana middle was greater than that of the other segments (Fig. 4B).
The expression of MaXET5, MaPL2, and MaBAM7 was related to the degradation of starch and of the cell wall, and it regulates (Xiao et al., 2018) changes in sweetness (Shan et al., 2020), which was illustrated by the differences between the middles and both ends of the bananas. The expression levels in the middles of the bananas were higher than those in both ends, which further indicated that the sweetness was the highest in the middle.
The expression levels of MaSGR and MaPaO were related to the degradation of chlorophyll, which regulates the color of bananas (Loay et al., 2020). The differences between these levels in both ends and in the middle of the bananas were consistent with the fact that both ends became yellow first. The expression levels were the highest in the tips, implying that the tip of the banana peel might be the first segment to change to yellow, which was inconsistent with a previous study (Jayanty et al., 2002). In addition, the results indicated that the stalk of the banana peel became yellow and displayed brown spots first.
The maximal MaACO expression occurred simultaneously in the three banana segments. Likewise, in another study, the occurrence of an ethylene peak in three segments was similar and related to the maturity of the fruit (Jayanty et al., 2002). Although, the expression of MaACO reached its maximum on the 10th day, the maximal expression of MaXET5, which is related to sweetness, and of MaPaO, which is associated with color values, occurred on the 15th and 19th days, respectively. This suggests that the changes in the sweetness of the banana pulp and in the color of the banana peel did not coincide, which further emphasizes that it is critical to study both the sweetness and the color of bananas to evaluate their quality.
3.5. Maturity classification of bananas via sweetness and color values
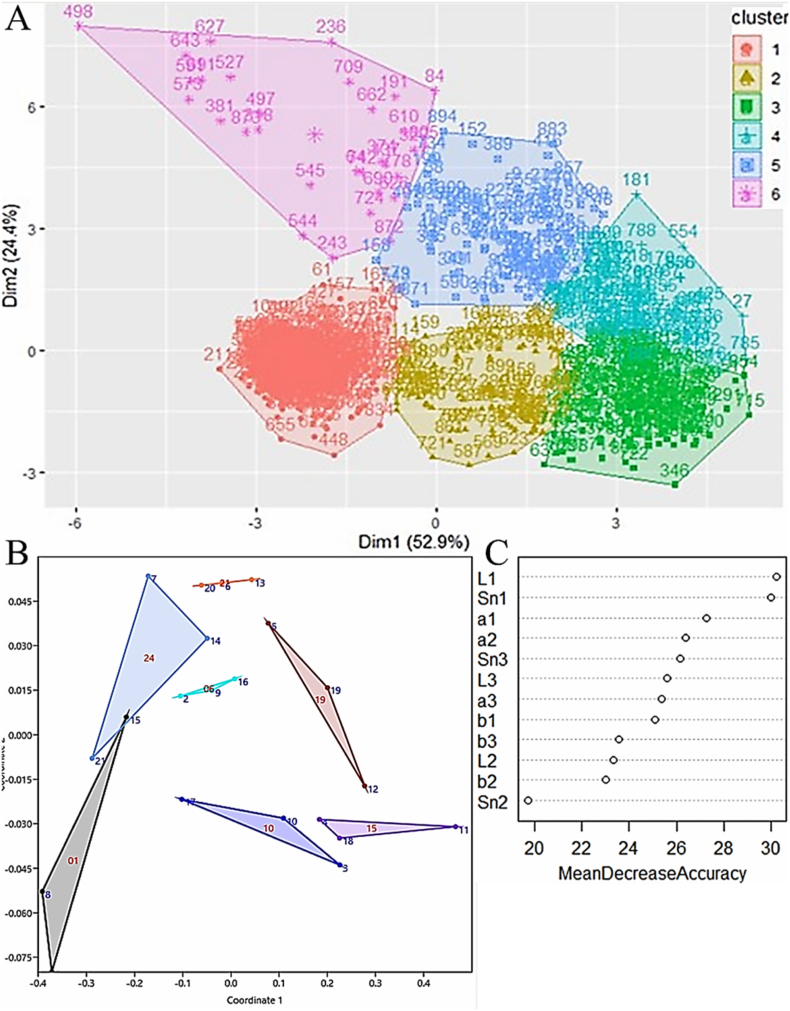
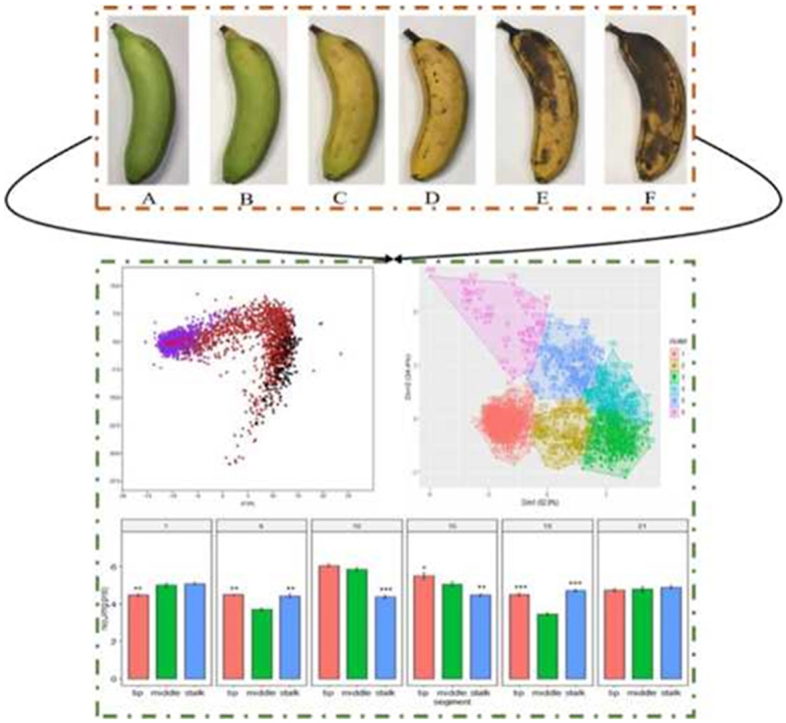
The sweetness and color values of the three banana segments were used to classify fruit maturity (Fig. 5). The automatic determination and prediction of banana maturity were based on the cluster plot of these two parameters (Fig. 5A). Banana maturity was divided into six stages based on the sweetness and color values of the three banana segments that were recorded during ripening, and the classification system thereby established fit 77.3% of the samples. The expression levels of MaXET5, MaPL2, MaBAM7, MaACO, MaPaO, and MaSGR in the three banana segments during ripening were also used to classify fruit maturity, in order to verify the relevance of that classification system (Fig. 5B). Moreover, on the 24th day, the bananas began to rot and smell distinct, and thus were not suitable for processing. Therefore, we divided the maturity of three segments of the banana into six stages (Fig. 6), and detailed changes in color and sweetness of each stage are as follows:
Fig. 5.
Classification of the maturity of bananas and the contribution of the stalk, middle, and tip of banana in maturity predictions. (A) Cluster analysis of the sweetness of the banana pulp and the color of the banana peel from the 1st to the 21st day of ripening; (B) Cluster analysis of the expression levels of the MaXET5, MaPL2, MaBAM7, MaACO, MaACS, MaPAO, and MaSGR genes from the 1st to the 24th day of ripening; (C) Ranking of the importance of each variable in relation with the maturity prediction of bananas. Banana segments are indicated by numbers; 1: stalk; 2: middle; 3: tip. Sn: sweetness. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
Appearance of the banana peel throughout the six maturity stages.
At the first stage of maturity (i.e., days 1–5), the sweetness of the middles of the bananas was higher than that of the stalks and tips (p < 0.05). The color of the banana peels was completely green in color due to the normal synthesis of chlorophyll (Fig. 6A). At the second stage of maturity (i.e., days 6–8), the sweetness of each banana segment increased rapidly, but the sweetness of the middles of the bananas remained significantly higher than that of the other segments (p < 0.05). The color change to yellow was the most obvious in the stalks of the banana peels, while the color of the middles and tips of the banana peels was green with yellow traces (Fig. 6B). At this stage, the starch begins to hydrolyze and chlorophyll synthesis is reduced, resulting in increased sugar content and a yellow peel color. At the third stage of maturity (i.e., days 9–12), the sweetness of the three banana segments continued to increase, but the sweetness of the middles of the bananas remained higher than that of the other segments. The color of the middles of the banana peels became yellow faster than that of the other segments, being almost entirely yellow, while the color of the stalks and the tips of the banana peels were more yellow than green (Fig. 6C). At the fourth stage of maturity (i.e., days 13–16), the sweetness of the banana tips increased faster than that of the other segments, but there were no significant differences between the sweetness of the three banana segments at the end of the fourth stage. The color of the three segments became completely yellow. Furthermore, brown spots appeared on the stalks of the banana peels (Fig. 6D). Fruit Browning on the outside due to the accumulation of free radicals during storage may be the cause of the brown spots on banana peels (Yang et al., 2010). At the fifth stage of maturity (i.e., days 17–18), there was no obvious increase in the sweetness of any banana segment. Moreover, there were no significant differences between the sweetness of the three segments at the end of the fifth stage. Brown spots appeared on the banana peels, and the stalks had more brown spots than the tips and middles (Fig. 6E). The sixth stage of maturity (i.e., after day 19) marked the end of the maturation of the bananas. The sweetness of the bananas did not change, and the color of the stalks and middles of the banana peels became almost completely brown (Fig. 6F). The study showed that the chlorophyll in the peel would decompose gradually due to the physiological activity of the sample, resulting in the change of fruit brightness value (Vazquez-Salinas and Lakshminarayana, 1985).
Based on our assessment of the sweetness and color of bananas during ripening, the shelf life of this fruit should occur from the third stage to the fifth stage, that is, from the 9th to the 17th day, and the sweetness of the three banana segments ranged between 10% and 14%, which was suitable for further processing.
3.6. Prediction of banana maturity
Two prediction algorithms for banana maturity were developed with SVM, RF, and NNET; one model was established based on the color and sweetness values of three banana segments to predict six stages of maturity (i.e., the predictions developed in this study), while the other model was based on the average color values of bananas to predict seven stages of maturity (i.e., the predictions established by previous studies).
For the two maturity prediction systems, mtry was of 2 in the final RF model. As for the maturity predictions with NNET, the selected optimal parameters were the same for both systems, namely size = 5 and decay = 0.1. With the SVM maturity predictions, the final values that were used for the two prediction algorithms were sigma = 0.176 and C = 1, and sigma = 0.18 and C = 1, respectively.
The accuracy and kappa values of the two prediction systems were compared (Table 2), and it was concluded that using the color and sweetness values of the three banana segments to predict fruit maturity was considerably more accurate than using the average color values of banana to predict maturity across seven stages. Additionally, the accuracy and kappa score of the maturity prediction with SVM were higher than those of the other models, which relied on the RF and NNET methods. Mishra et al. (2020) used portable visible near-infrared spectrometer to predict the hardness of mango in the ripening process by the iPLSR model, which realized the rapid non-destructive monitoring and control of the mango ripening process.
Table 2.
Accuracy and kappa of predictions.
| Methods | Predictions set up by previous studies |
Predictions set up in the present study |
||
|---|---|---|---|---|
| Accuracy | Kappa | Accuracy | Kappa | |
| SVM | 58% | 48% | 94% | 91% |
| RF | 69% | 63% | 93% | 90% |
| NNET | 62% | 54% | 79% | 70% |
Based on the cluster analysis, banana maturity can be reclassified into six stages. This differs from the maturity classification into seven stages that have been established in previous studies. Some retailers decide on the shelf-life of bananas based on the color of the banana peels, which is typically considered to range from yellow with green edges to yellow with some brown spots (Adebayo et al., 2016). However, green-ripe bananas are suitable for eating and processing (Loay et al., 2020). Therefore, it provided the basis for a new quality evaluation.
3.7. Importance of the three banana segments for predicting fruit maturity
The sweetness and color values of the stalks, middles, and tips of the bananas were compared using RF to determine their contribution to maturity prediction (Fig. 5C). The stalks of the bananas contributed the most to the prediction, which meant the decreased accuracy of the sweetness and color values was higher than that of the other segments. The second most important segment was the banana tip. Therefore, it was concluded that both ends of the bananas were more representative of fruit maturity than the banana middles.
Based on our results, quality evaluation of bananas using both the sweetness and color values is relevant, and it is indispensable to assess the characteristics of the different segments of a banana individually to predict its maturity. At the same time, there are also studies on establishing models for predicting maturity and shelf life by linking other fruit quality indicators. Razali et al. (2009) found a good relationship between the oil content of fresh fruit bunches and its image pixel value through research and established a mathematical model to determine the best harvest date of fresh fruit bunches. Rungpichayapichet et al. (2016) used the NIRS prediction model to study the effects of different harvest periods on mango quality after harvest, and established a model of mango maturity after harvest. However, there are few reports on the three banana segments. We found that the respective sweetness and color values of the three banana segments contributed distinctly to the maturity prediction. Whereas the importance of the different color values for the maturity prediction diverged. In the present study, the ‘L’ value of the banana stalk was the most relevant to predicting fruit maturity, which differed from a previous study in which the ‘a’ value was the most related to the textural profile of the fruit (Adebayo et al., 2016). Finally, the contribution of sweetness and color values to the maturity prediction differed, which highlighted that the changes in the sweetness and color of the bananas during ripening were not consistent, especially in the middle of the bananas.
4. Conclusion
In the present study, the variations in the sweetness and color differed between the stalks, middles, and tips of the bananas; that is, the maturity level was inconsistent along the lengths of the bananas. Therefore, it is important to consider the sweetness and color of each banana segment to evaluate the quality of bananas. Furthermore, based on our comprehensive assessment of the sweetness and color values of three banana segments and the two prediction models that were evaluated, it is adequate to divide the maturity of bananas into six stages rather than seven stages, as previously reported.
The maturity of the stalks and tips of the bananas was more representative of the overall maturity than that of the middle parts. Notably, these results can be utilized to improve the method that is currently used to evaluate the quality and determine maturity of bananas. Overall, our study provides a basis for the effective development of rapid inspection and evaluation systems for the efficient sale and processing of bananas.
CRediT authorship contribution statement
Lukai Ma: Investigation, Methodology, Formal analysis, Data curation, Visualization, Writing - original draft. Churong Liang: Writing - review & editing, Investigation, Resources, Methodology. Yun Cui: Validation, Investigation, Resources, Supervision. Huiyan Du: Investigation, Methodology. Huifan Liu and Lixue Zhu: Validation, Writing - review & editing. Yuanshan Yu and Chuqiang Lu: Supervision, Validation. Soottawat Benjakul and Charles Brennan: Conceptualization, Project administration, Supervision, Writing - review & editing. Margaret Anne Brennan: Validation, Writing - review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Laboratory of Lingnan Modern Agriculture Project [NZ2021038]; the Key Area Research and Development Program of Guangdong Province [2019B020223003]; the Guangzhou Science, Technology and Innovation Commission [201804010480]; the Science and Technology Project of Heyuan [2019003]; and the Key Generic Technology System of the Agricultural Industry in Guangdong Province [2019KJ139].
Human and animals rights
The research did not involve any studies with human participants or animals performed by any of the authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank the Academy of Contemporary Agricultural Engineering Innovations of Zhongkai University of Agriculture and Engineering for providing the scientific research platform.
Editor name: Professor Aiqian Ye
Contributor Information
Huifan Liu, Email: lm_zkng@163.com.
Lixue Zhu, Email: zhulixue@zhku.edu.cn.
References
- Adebayo S.E., Hashim N., Abdan K., Hanafi M., Mollazade K. Prediction of quality attributes and ripeness classification of bananas using optical properties. Sci. Hortic. 2016;212:171–182. doi: 10.1016/j.scienta.2016.09.045. [DOI] [Google Scholar]
- Chen L., Zhong H.Y., Kuang J.F., Li J.G., Lu W.J., Chen J.Y. Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta. 2011;234(2):377–390. doi: 10.1007/s00425-011-1410-3. [DOI] [PubMed] [Google Scholar]
- Chepiński P., Ochmian I., Forczmański P. Sweet cherry skin colour measurement as an non-destructive indicator of fruit maturity. Acta Univ. Cibin. Ser. E Food Tech. 2019;23(2):157–166. doi: 10.2478/aucft-2019-0019. [DOI] [Google Scholar]
- Clendennen S.K., May G.D. Differential gene expression in ripening banana fruit. Plant Physiol. 1997;115(2):463–469. doi: 10.1104/pp.115.2.463. doi:doi.org/10.1104/pp.115.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du D., Wang J., Wang B., Zhu L., Hong X. Ripeness prediction of postharvest Kiwifruit using a MOS E-nose combined with chemometrics. Sensors. 2019;19(2):419. doi: 10.3390/s19020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlath S. Impact of ripening stages of banana flour on the quality of extruded products. Int. J. Food Sci. Technol. 2010;43(9):1541–1548. doi: 10.1111/j.1365-2621.2007.01574.x. [DOI] [Google Scholar]
- Gomes J.F.S., Vieira R.R., Leta F.R. Colorimetric indicator for classification of bananas during ripening. Sci. Hortic. 2013;150:201–205. doi: 10.1016/j.scienta.2012.11.014. [DOI] [Google Scholar]
- Jaiswal P., Jha S.N., Kaur P.P., Bhardwaj R., Singh A.K., Wadhawan V. Prediction of textural attributes using color values of banana (Musa sapientum) during ripening. J. Food Sci. Technol. 2014;51(6):1179–1184. doi: 10.1007/s13197-012-0614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanty, Song J., Rubinstein N.M., Beaudry A.C. Temporal relationship between ester biosynthesis and ripening events in bananas. J. Am. Soc. Horticul. Sci. Am. Soc. Horticult. Sci. 2002;127(6):998–1005. doi: 10.1046/j.1365-2591.2000.00269.x. [DOI] [Google Scholar]
- Jiang Y., C J.D., J M.A. Extension of the shelf life of banana fruit by 1-methylcyclopropene in combination with polyethylene bags. Postharvest Biol. Technol. 1999;16(2):187–193. doi: 10.1016/S0925-5214(99)00009-5. [DOI] [Google Scholar]
- Kouassi H.A., Assemand E.F., Gibert O., Maraval I., Ricci J., Thiemele D., Bugaud C. Textural and physicochemical predictors of sensory texture and sweetness of boiled plantain. Int. J. Food Sci. Technol. 2020;56(3):1160–1170. doi: 10.1111/ijfs.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laylieam S., Kosittrakun M. Effects of harvest maturity on banana quality. J. Food Qual. 2010;21(6):539–544. doi: 10.1111/j.1745-4557.1998.tb00543.x. [DOI] [Google Scholar]
- Lina D., Jun S., Charles F., Palmer L.C., Fillmore S., Zhang Z. Proteome changes in banana fruit peel tissue in response to ethylene and high-temperature treatments. Hortic. Res. 2016;3 doi: 10.1038/hortres.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loay A.A., Hamed S.E., El-Khateeb A.Y., Mohamed A.H. Implementation exogenous ATP on the starch degradation enzyme activities of ‘Grand Nain’ banana fruit during shelf life. Sci. Hortic. 2020;262 doi: 10.1016/j.scienta.2019.109021. [DOI] [Google Scholar]
- Maduwanthi T., Marapana R.A.U.J. Biochemical changes during ripening of banana: a review. Int. J. Food Sci. Nutr. 2017;2(5):166–169. [Google Scholar]
- Mao W.W., Kinsella J.E. Amylase activity in banana fruit: properties and changes in activity with ripening. J. Food Sci. 1981;46(5):1400–1403. doi: 10.1111/j.1365-2621.1981.tb04183.x. [DOI] [Google Scholar]
- Mendoza F., Aguilera J.M. Application of image analysis for classification of ripening bananas. J. Food Sci. 2004;69(9):E471–E477. doi:doi.org/10.1111/j.1365-2621.2004.tb09932.x. [Google Scholar]
- Milosevic T., Milosevic N. Segregation of apricot accessions on the basis of fruit quality attributes. Biosci. J. 2013;29(2):350–359. https://seer.ufu.br/index.php/biosciencejournal/article/view/14156 [Google Scholar]
- Mishra P., Woltering E., Harchioui N.E. Improved prediction of 'Kent' mango firmness during ripening by near-infrared spectroscopy supported by interval partial least square regression. Infrared Phys. Technol. 2020;110(8) doi: 10.1016/j.infrared.2020.103459. [DOI] [Google Scholar]
- Nnodim C.T., El-Bab A., Ikua B.W., Sila D.N. 2021. Design, Simulation, and Experimental Testing of a Tactile Sensor for Fruit Ripeness Detection: Transactions on Engineering Technologies. [Google Scholar]
- Razali M.H., Wan I., Ramli A.R., Sulaiman M.N., Harun M.H. Development of image based modeling for determination of oil content and days estimation for harvesting of fresh fruit bunches. Int. J. Food Eng. 2009;5(2):64–67. doi: 10.2202/1556-3758.1633. [DOI] [Google Scholar]
- Rungpichayapichet P., Mahayothee B., Nagle M., Khuwijitjaru P., Müller J. Robust NIRS models for non-destructive prediction of postharvest fruit ripeness and quality in mango. Postharvest Biol. Technol. 2016;111:31–40. doi: 10.1016/j.postharvbio.2015.07.006. [DOI] [Google Scholar]
- Sanaeifar A., Bakhshipour A., de la Guardia M. Prediction of banana quality indices from color features using support vector regression. Talanta. 2016;148:54–61. doi: 10.1016/j.talanta.2015.10.073. [DOI] [PubMed] [Google Scholar]
- Saragih R.E., Gloria D., Santoso A.J. Classification of ambarella fruit ripeness based on color feature extraction. ICIC Exp. Lett. 2021;15(9):1013–1020. doi: 10.24507/icicel.15.09.1013. [DOI] [Google Scholar]
- Shan W., Guo Y.F., Wei W., Chen J.Y., Lu W.J., Yuan D.B.…Kuang J.F. Banana MaBZR1/2 associate with MaMPK14 to modulate cell wall modifying genes during fruit ripening. Plant Cell Rep. 2020;39(1):35–46. doi: 10.1007/s00299-019-02471-5. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Sadistap S. Data fusion for fruit quality authentication: combining non-destructive sensing techniques to predict quality parameters of citrus cultivars. J. Food Meas. Char. 2021;16(1):344–365. doi: 10.1007/s11694-021-01165-5. [DOI] [Google Scholar]
- Suntharalingam S., Ravindran G. Physical and biochemical properties of green banana flour. Plant Foods Hum. Nutr. 1993;43(1):19. doi: 10.1007/BF01088092. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Niikawa T., Sakurai N. Evaluation of flesh texture and prediction of optimum ripeness in 'fuyu' persimmon after harvest. Hortic. Res. 2011;10(3):421–427. doi: 10.2503/hrj.10.421. [DOI] [Google Scholar]
- Vazquez-Salinas C., Lakshminarayana S. Compositional changes in mango fruit during ripening at different storage temperatures. J. Food Sci. 1985;50(6):1646–1648. doi: 10.1111/j.1365-2621.1985.tb10555.x. [DOI] [Google Scholar]
- Wan H.C., Sang K.K., Na M.H., Na I.S. Fruit ripeness prediction based on DNN feature induction from sparse dataset. Comput. Mater. Continua (CMC) 2021;(3) doi: 10.32604/CMC.2021.018758. [DOI] [Google Scholar]
- Wu Q., Ma Z.-z., Qin Y.-l., Li Y.-m., Huang B.-z., Zhang X.-l.…Pang X.-q. Imbalanced expression of stay-green 1 alleles in banana AAB/ABB cultivars prevents high-temperature-induced green ripening as in AAA Cavendish fruit. Postharvest Biol. Technol. 2019;158 doi: 10.1016/j.postharvbio.2019.110980. [DOI] [Google Scholar]
- Xiao Y.Y., Kuang J.F., Qi X.N., Ye Y.J., Wu Z.X., Chen J.Y., Lu W.J. A comprehensive investigation of starch degradation process and identification of a transcriptional activator MabHLH6 during banana fruit ripening. Plant Biotechnol. J. 2018;16(1):151–164. doi: 10.1111/pbi.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Chu B., He Y. Prediction of banana color and firmness using a novel wavelengths selection method of hyperspectral imaging. Food Chem. 2018;245:132–140. doi: 10.1016/j.foodchem.2017.10.079. [DOI] [PubMed] [Google Scholar]
- Yang X., Pang X., Xu L., Fang R., Huang X., Guan P., Zhang Z. Accumulation of soluble sugars in peel at high temperature leads to stay-green ripe banana fruit. J. Exp. Bot. 2009;60(14):4051–4062. doi: 10.1093/jxb/erp238. [DOI] [PubMed] [Google Scholar]
- Yang H., Zhou C., Wu F., Cheng J. Effect of nitric oxide on browning and lignification of peeled bamboo shoots. Postharvest Biol. Technol. 2010;57(1):72–76. doi: 10.1016/j.postharvbio.2010.02.004. [DOI] [Google Scholar]
- Zhai R., Ye S., Zhu G., Lu Y., Zhang X. Identification and integrated analysis of glyphosate stress-responsive microRNAs, lncRNAs, and mRNAs in rice using genome-wide high-throughput sequencing. BMC Genom. 2020;21(1) doi: 10.1186/s12864-020-6637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lian J., Fan M., Zheng Y. Deep indicator for fine-grained classification of banana's ripening stages. EURASIP J. Image and Video Proces. 2018;2018(1) doi: 10.1186/s13640-018-0284-8. [DOI] [Google Scholar]