Abstract
Introduction and importance
Ewing's sarcoma is an aggressive malignancy primarily affecting skeletal system in children and young adults.
Case presentation
We report an unusual case of Ewing sarcoma in a 14-year-old boy with clinical and radiological features of rapid onset metachronous skeletal metastasis (within 4 weeks of diagnosis).
Clinical discussion
Although the deterioration of symptoms was very rapid, it is unusual to note that in the presence of such widespread metastatic disease the lungs per se remained uninvolved.
Conclusion
We describe a unique case of metastatic Ewing's sarcoma who showed rapid systemic disease progression with widespread skeletal metastases (and CNS involvement) but without any evidence of pulmonary involvement.
Keywords: Ewing, Sarcoma, Presentation, Atypical
Highlights
-
•
Ewing sarcoma is considered aggressive malignancy primarily affecting skeletal system.
-
•
It is usually very slowly progressive in nature (years).
-
•
This case presentation showed aggressiveness behavior with very unpredictable outcomes short period from diagnosis to metastasis.
-
•
Up to our knowledge, this atypical and rapid disease progression (within a month of diagnosis) was not previously reported in literature.
1. Introduction and importance
“Ewing family of tumors” is a term used to describe a group of tumors sharing same neoplastic entity, genetic features and immunohistochemically staining [1]. These tumors represent skeletal Ewing sarcoma, extra skeletal Ewing sarcoma, primitive neuroectodermal tumors and Ewing sarcoma originating in the chest wall (Askin sarcoma) [1]. Ewing sarcoma can emerge from bones such as pelvis, femur, tibia and ribs. In addition, it can arise from soft tissue as well like thoracic wall, gluteal muscle, pleural cavities and cervical muscles [2]. The proportion of Ewing sarcoma cases that are associated with chromosomal translocation t(11;22)(q24;12) that creates fusion between 5′ segment of EWS gene with 3′ segment of ETS family gene FLI-1, resulting EWS-FLI-1 fusion protein that acts as an erratic transcriptional activator [3]. Patients with Ewing sarcoma can present with localized disease. However, they can also present with metastatic disease in about 30 % of cases [4]. Cases presenting with pulmonary metastasis have a better prognosis than those presenting with bone marrow metastasis [4]. Clinical presentation of Ewing sarcoma differs among age groups according to variable factors including sex, stage, and tumor size [5]. It has been noted that, older patients, above 15 years of age, had more frequent presentation with metastasis than patient younger than 9 years of age [5]. Moreover, patients younger than 9 years of age presented more frequently with smaller size tumors than children older than 10 years of age and above [5] Standardized treatment for Ewing family of tumors including local treatment and systematic chemotherapy has been shown to improve long term results [6]. This applied only to children and young adult patients below the age of 40 years, as patients above 40 years show a decreased incidence of ESFT which is targeted in this treatment protocol, this is the reason why they were excluded from the treatment protocol [6]. Aggressive local treatment protocol with aggressive neo-adjuvant and adjuvant chemotherapy has been mentioned in literature, with remission rate around 65 % in patients with local disease [7]. Multiple factors associated with poor response to this treatment method including large tumor size (more than 100 cm), elevated lactic dehydrogenase level, older age, involvement of axial skeleton, and poor response to chemotherapy [7].
We report a case of a 14 years old boy biopsied and diagnosed with Ewing sarcoma involving the left proximal tibia and synchronous skeletal metastases (skip lesion ipsilateral distal femur) at presentation. Over the ensuing 4 weeks he developed aggressive, unusual, multiple skeletal metastasis without any evidence of pulmonary involvement however.
2. Case report
14 years old male medically free, referred from local hospital as a case of left knee swelling. He had normal development and was delivered at full term through normal spontaneous vaginal delivery with not significant family nor drug history. Four months back from presentation, he started to have left knee pain which was gradual in onset, intermittent, at first it was localized then diffuse involving the thigh and leg, progressing over time, aggravated by walking and relieved at the beginning with rest but now has pain even at rest. Not associated with fever or history of trauma or recent infection. Negative history of weight loss and other constitutional symptoms. Later on, he developed headache, it was intermittent and progressing over time. On examination, the boy looked healthy, thin with normal color. Local examination revealed swelling over the left proximal leg without any discoloration or sinuses. No hotness could be appreciated but mild tenderness over the knee joint line. He was able to flex the knee up to 100° with discomfort. Extension lag of about 15° was noted. Distal neurovascular was intact. All investigations ordered for the patient including local CT scan, local MRI, CT chest, CT and MRI brain.
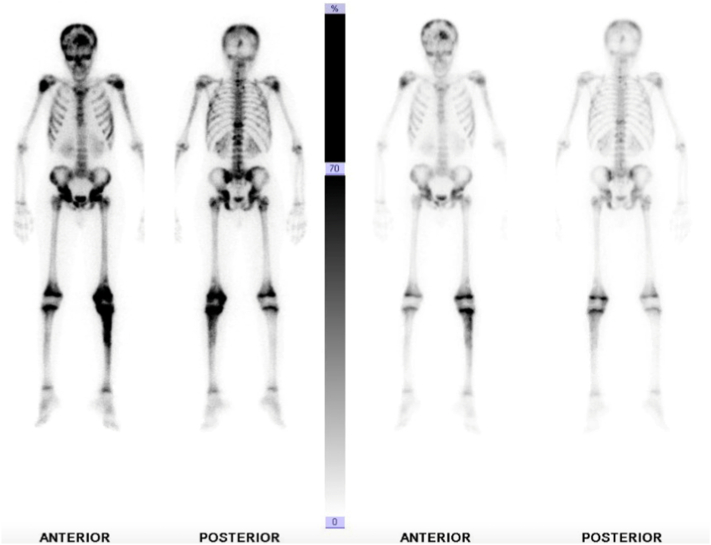
He underwent CT guided biopsy in outside hospital which turned out to be Ewing sarcoma. We requested the slides and the block for confirmation in our lab, which also revealed the same pathology results. X-rays showed ill-defined mixed density lytic lesion with aggressive periosteal reaction in the form of codman triangle and cortical breakthrough in proximal tibia, and also ill-defined lesion in the distal femur likely representing skip lesion (Fig. 1, Fig. 2). His chest CT was negative for any pulmonary metastases. Over the next month he developed diffuse skeletal metastases. Beyond the left distal femur and proximal tibial lesions associated with a soft tissue component on the technetium scan, increased tracer uptake was noted involving the skull, multilevel whole spine, bilateral ribs, multiple pelvic bones, bilateral proximal humerii and bilateral proximal femur (Fig. 3). CT and MRI of brain and spine was showing diffuse permeative bony lesions with multiple areas of soft tissue components along the left frontal lobe and right fronto-parietal convexity, there was also diffuse total spine involvement by the sarcoma including posterior elements predominantly at T1–2, T12 region. Mild compression deformities were seen at T8, T9, T11–12 and L5, dorsal epidural disease was seen at T12 which was abutting the conus medullaris. Minimal dorsal epidural disease was also noted at C7 to T2 without any cord compression.
Fig. 1.
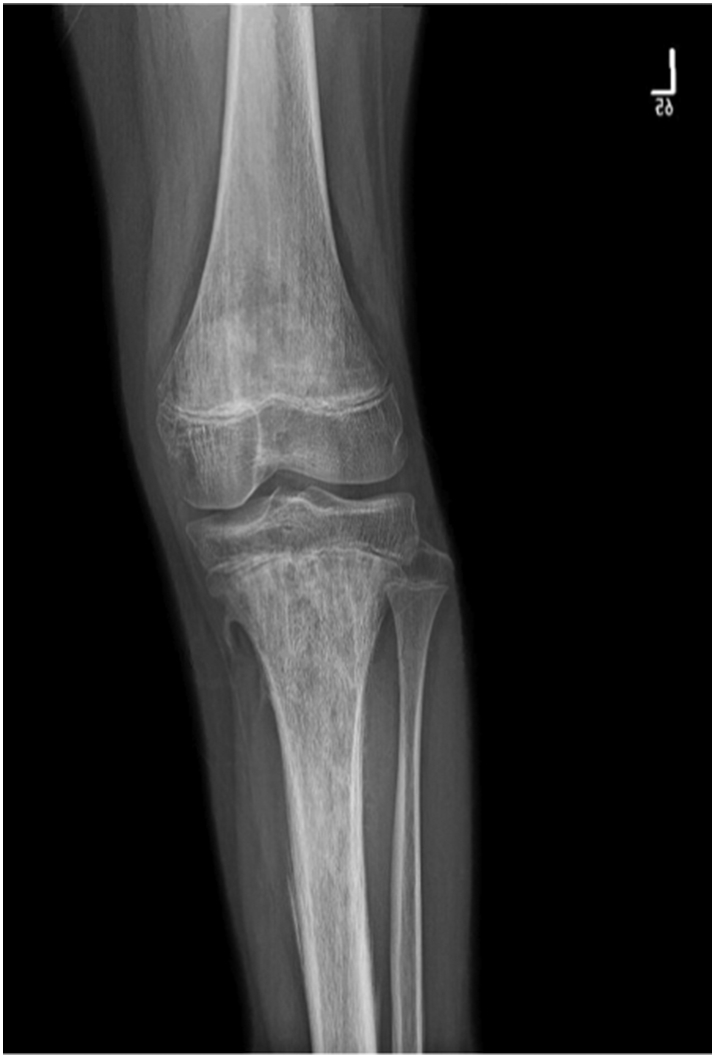
Anteroposterior view of left knee.
Fig. 2.
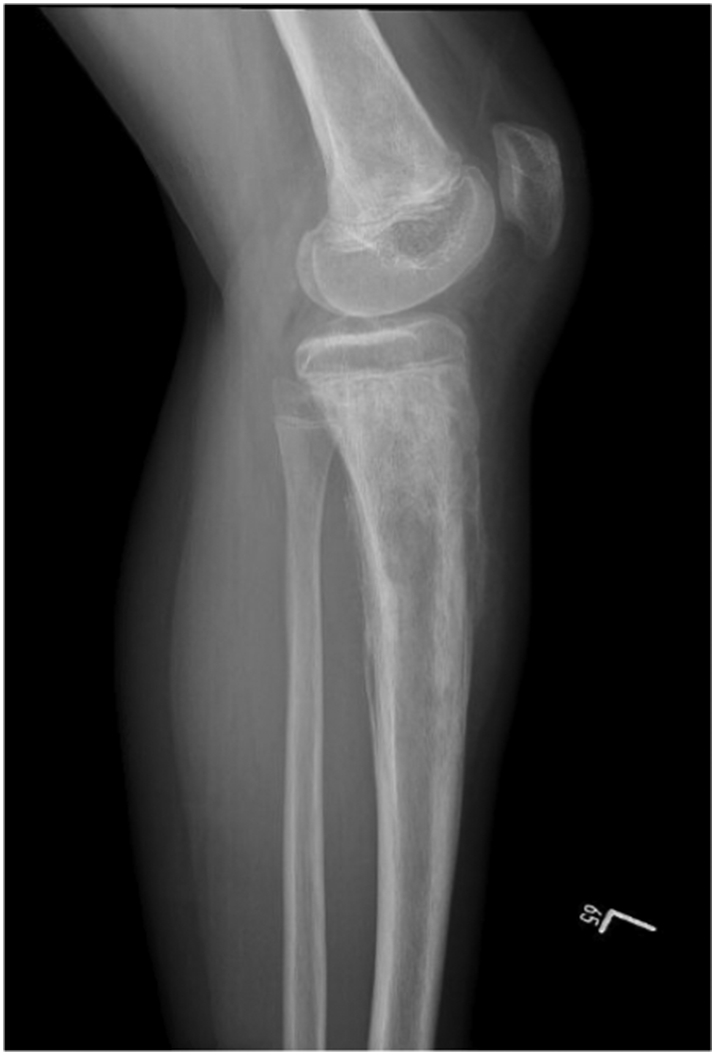
Lateral view of left knee.
Fig. 3.
Bone scan.
It was however very unusual to note that in the presence of such widespread metastatic disease his lungs per se remained uninvolved.
The patient presented in July 2020 and post workup was commenced on VAIA chemotherapy regimen but it was evident that treatment here will be palliative and within 6 months the patient was unfortunately deceased.
3. Clinical discussion
Ewing sarcoma presentation can differ according to the site of involvement, but as a common presentation, it includes local symptoms and fever [8]. As mentioned in the recent update by Jones and Barr, that localized pain and fever were a common initial presentation for Ewing sarcoma [8]. Similarly, to what they reported, our patient had an initial complain of localized pain without fever in the left knee. Interestingly, they reported that primary location of the tumor has a significant importance in regards to the prognosis, and they localized the pelvis to be the worst location. In contrast, after clinical and radiological examination of our patient, we found that the patient had a proximal tibia Ewing sarcoma with skip lesion in the ipsilateral femur. With extremely rapid progression of the tumor involving the pelvis and other axial and appendicular skeleton within a very short period of time. Also, Ng et al. [9], stressing that the pelvic primary location of Ewing sarcoma has worse prognosis than extremities, again opposing our findings in the case, with the proximal tibia as primary location with an impressively fast metastasis to the pelvis within 2–4 weeks of presentation. In addition, K. Choi and his associates [1], commented that localized pain and swelling were common initial presentation for patients with Ewing sarcoma. However, they also pointed out pathologic fracture, constitutional symptoms and palpable painless mass are common as well.
Letson et al. [10] and Simon and Finn [11] are two separate studies that both agreed that the typical symptom for primary malignant tumor is a continued, dull aching pain which is more severe at night and during rest. In the presented case, the character of the pain is almost the same and it was progressive over time until reaching a point that it became typical as mention in a primary malignant tumor. We believe that the aggressiveness of rapid progression of Ewing sarcoma in our case is the cause of a bit later typical presentation. This reflects how fast Ewing sarcoma spread in our reported case. Although, PET CT scan considered to be done, but unfortunately patient was too sick and not tolerating nuclear imaging. Furthermore, Vlasak and Sim [12] Reported that around 70 % of Ewing sarcoma patients presented with a palpable mass at the time of diagnosis. Moreover, Björn and Torulf Widhe [13], reaffirmed this point as they found all the Ewing sarcoma cases were presented to them to have palpable mass at presentation. In addition, they noted that doctor's delay in diagnosing these tumors is significantly short. They concluded that the presentation of palpable mass is crucial finding that suggest malignancy. In our reported case, the boy presented with palpable mass that was noted by local examination initially at the proximal tibia with rapid progression of swelling to involve the distal femur within 2–3 weeks, indicating the fast spread of the disease.
An article from Taiwan reported 50 cases diagnosed with Ewing sarcoma, they found 42 % (21 patients) of them were metastatic at the initial presentation [14]. 47.6 % of them reported brain metastasis and more than 42 % reported lung metastasis [14]. As noted in their study the brain metastasis cases exceeded the lung metastasis which indicates the aggressiveness of the disease. Another article from the United States demonstrated that 25 % of the Ewing sarcoma cases were metastatic at the time of diagnosis [15]. Another article from Japan declared that 16.9 % (41 cases of 224) of Ewing sarcoma cases were metastatic at the time of diagnosis [16]. Although, none of them commented on the nature of progression nor the time from the presentation to the development of metastasis. It is obvious in our reported case that the rapid progression of the disease is unusual.
In the article reported by Faris Shweikeh and his associates [17], they stated that the mean interval of Ewing sarcoma cases from the time of initial presentation to the time of brain metastasis is ranging from 20 to 30 months, although can be before 24 months in some and in other tumors may be longer (more than 36 months) like liposarcoma and chondrosarcoma. Our reported case is unusually short.
Eung Yeop Kim et al. [18], they reported a 21 years old female with Ewing sarcoma of the intradural confirmed by histopathology and immunohistochemistry first diagnosed on 1996 post-surgical resection, radiation and chemotherapy with evidence of recurrence depicted on 2004 when she complained of headache then diagnosed with brain metastasis. This case indicates very slow progressing lesion it takes years while in our case there was aggressiveness behavior.
Björn and Widhe [13], mentioned a very critical point in regard to the duration between initial symptoms for the patients and the time of diagnosis. They analyzed 47 records of patients with diagnosis of Ewing sarcoma, comparing the symptoms at the initial visit with symptoms at the admission to the trauma center. They mention that trauma incident was a cause for emergency visit and they found 77 % of patients with Ewing sarcoma had an x ray during their visit to trauma center, found to have abnormal radiograph and referred accordingly to an oncology center.
Jones and Barr [8] added that the survival rate in Ewing sarcoma patient is mainly affected by multiple factors including; tumor size, tumor location, distant metastasis and response to chemotherapy [19].
4. Conclusion
We describe a unique case of metastatic Ewing's sarcoma who showed rapid systemic disease progression with widespread skeletal metastases (and CNS involvement) but without any evidence of pulmonary involvement. This atypical and rapid disease progression (within a month of diagnosis) we feel has not previously been reported in the orthopedic literature.
Written informed consent was obtained from the patient's parent for publication of this case report and accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal on request.
Consent
Written informed consent was obtained from the patient's parent for publication of this case report and accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal on request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Ethical approval
The study was approved by institutional review board in accordance with the national committee of bio ethics guidelines.
Funding
None.
Guarantor
Abdullah AlMarshad.
Research registration number
Not required.
CRediT authorship contribution statement
Pant Rajeev contributes with writing the paper, data analysis and supervision.
Abdullah Al-Marshad contributes the paper with writing the paper and data analysis.
Ibrahim AlMazrua contributes the paper with supervision, data analysis and interpretation.
Farah AlMulla contributes the paper with data collection, data analysis and interpretation.
Mohammed Alaboud contributes the paper with supervision, data analysis and interpretation.
Omar Alrifaie contributes the paper with supervision, data analysis and interpretation.
Mahmoud Shaheen contributes the paper with supervision, data analysis and interpretation.
Declaration of competing interest
None.
Footnotes
There are no patient details (name or medical record number), or institution name included in the figures.
We Thank Dr. Farah AlMulla for her invaluable support, encouragements and many discussions in this case report in addition to data collection.
References
- 1.Choi E.Y.K., Gardner J.M., Lucas D.R., McHugh J.B., Patel R.M. Ewing sarcoma. Semin. Diagn. Pathol. 2014;31:39–47. doi: 10.1053/j.semdp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Grünewald T.G.P., Cidre-Aranaz F., Surdez D., Tomazou E.M., De Álava E., Kovar H., Sorensen P.H., Delattre O., Dirksen U. Ewing sarcoma. Nat. Rev. Dis. Primers. 2018 doi: 10.1038/s41572-018-0003-x. [DOI] [PubMed] [Google Scholar]
- 3.Riggi N., Stamenkovic I. The biology of Ewing sarcoma. Cancer Lett. 2007;254:1–10. doi: 10.1016/j.canlet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Ladenstein R., Pötschger U., Le Deley M.C., Whelan J., Paulussen M., Oberlin O., Van Den Berg H., Dirksen U., Hjorth L., Michon J., Lewis I., Craft A., Jürgens H. Primary disseminated multifocal Ewing sarcoma: results of the euro-EWING 99 trial. J. Clin. Oncol. 2010;28:3284–3291. doi: 10.1200/JCO.2009.22.9864. [DOI] [PubMed] [Google Scholar]
- 5.Worch J., Ranft A., DuBois S.G., Paulussen M., Juergens H., Dirksen U. Age dependency of primary tumor sites and metastases in patients with Ewing sarcoma. Pediatr. Blood Cancer. 2018;65 doi: 10.1002/pbc.27251. [DOI] [PubMed] [Google Scholar]
- 6.Bacci G., Balladelli A., Forni C., Ferrari S., Longhi A., Bacchini P., Alberghini M., Fabbri N., Benassi M.S., Briccoli A., Picci P. Adjuvant and neoadjuvant chemotherapy for Ewing sarcoma family tumors in patients aged between 40 and 60: report of 35 cases and comparison of results with 586 younger patients treated with the same protocols in the same years. Cancer. 2007;109:780–786. doi: 10.1002/cncr.22456. [DOI] [PubMed] [Google Scholar]
- 7.Kolb E.A., Kushner B.H., Gorlick R., Laverdiere C., Healey J.H., LaQuaglia M.P., Huvos A.G., Qin J., Vu H.T., Wexler L., Wolden S., Meyers P.A. Long-term event-free survival after intensive chemotherapy for Ewing’s family of tumors in children and young adults. J. Clin. Oncol. 2003;21:3423–3430. doi: 10.1200/JCO.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Jones L.B., Barr J.S. Bone sarcomas: an update of the recent literature. current orthopaedicPractice. 2016;27:582–586. doi: 10.1097/BCO.0000000000000443. [DOI] [Google Scholar]
- 9.Ng V.Y., Jones R., Bompadre V., Louie P., Punt S., Conrad E.U. The effect of surgery with radiation on pelvic Ewing sarcoma survival. J. Surg. Oncol. 2015;112:861–865. doi: 10.1002/jso.24081. [DOI] [PubMed] [Google Scholar]
- 10.Letson G.D., Greenfield G.B., Heinrich S.D. Evaluation of the child with a bone or soft-tissue neoplasm. Orthop. Clin. N. Am. 1996;27:431–451. [PubMed] [Google Scholar]
- 11.Simon M.A., Finn H.A. Diagnostic strategy for bone and soft-tissue tumors. Instr. Course Lect. 1994 [PubMed] [Google Scholar]
- 12.Vlasak R., Sim F.H. Ewing’s sarcoma. Orthop. Clin. N. Am. 1996;27:591–603. [PubMed] [Google Scholar]
- 13.Lee C.Y., Yen C.C., Yen H.J., Shiau C.Y., Chao T.C., Wu P.K., Chen C.F., Chen P.C.H., Wu H.T.H., Chiou H.J., Chen C.C., Hung G.Y., Chen W.M. Outcomes of 50 patients with Ewing sarcoma family of tumors treated at a single institution in Taiwan. Medicine (United States) 2016;95 doi: 10.1097/MD.0000000000003830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esiashvili N., Goodman M., Marcus R.B. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: surveillance epidemiology and end results data. J. Pediatr. Hematol. Oncol. 2008;30:425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 15.Obata H., Ueda T., Kawai A., Ishii T., Ozaki T., Abe S., Tanaka K., Tsuchiya H., Matsumine A., Yabe H. Clinical outcome of patients with Ewing sarcoma family of tumors of bone in Japan: the Japanese musculoskeletal oncology group cooperative study. Cancer. 2007;109:767–775. doi: 10.1002/cncr.22481. [DOI] [PubMed] [Google Scholar]
- 16.Shweikeh F., Bukavina L., Saeed K., Sarkis R., Suneja A., Sweiss F., Drazin D. Brain metastasis in bone and soft tissue cancers: a review of incidence, interventions, and outcomes. Sarcoma. 2014;2014 doi: 10.1155/2014/475175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eung Y.K., Lee S.K., Dong J.K., Kim J., Lee K.S., Jung W., Dong I.K. Intracranial dural metastasis of Ewing’s sarcoma: a case report. Korean J. Radiol. 2008;9:76–79. doi: 10.3348/kjr.2008.9.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Widhe B., Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J. Bone Joint Surg. A. 2000;82:667–674. doi: 10.2106/00004623-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., Beamish A.J., Noureldin A., Rao A., Vasudevan B., Challacombe B., Perakath B., Kirshtein B., Ekser B., Pramesh C.S., Laskin D.M., Machado-Aranda D., Miguel D., Pagano D., Millham F.H., Roy G., Kadioglu H., Nixon I.J., Mukherjee I., McCaul J.A., Chi-Yong Ngu J., Albrecht J., Rivas J.G., Raveendran K., Derbyshire L., Ather M.H., Thorat M.A., Valmasoni M., Bashashati M., Chalkoo M., Teo N.Z., Raison N., Muensterer O.J., Bradley P.J., Goel P., Pai P.S., Afifi R.Y., Rosin R.D., Coppola R., Klappenbach R., Wynn R., de Wilde R.L., Surani S., Giordano S., Massarut S., Raja S.G., Basu S., Enam S.A., Manning T.G., Cross T., Karanth V.K.L., Kasivisvanathan V., Mei Z., The S.C.A.R.E. Guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84(2020):226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]