Abstract
Introduction
Ruptured extragastrointestinal stromal tumor (EGIST) are rare; therefore, there are no standard guidelines for its treatment. Herein, we report the successful laparoscopic resection of a ruptured EGIST.
Presentation of case
The patient was a 59-year-old man, a Jehovah's Witness, who presented with sudden onset of left-sided abdominal pain. Contrast-enhanced computed tomography (CECT) performed from a previous hospital revealed intra-abdominal hemorrhage. Repeat CECT at our institution revealed extravasation and serum ascites. A hematoma was found anterior to the omentum, and a tumor was detected which did not have continuity with the surrounding organs of the gastrointestinal tract. Complete tumor resection via laparoscopic surgery was performed and the specimen was sent for histopathology, which revealed bundle-like proliferation of spindle-shaped cells. Immunohistochemical staining was completed, which was positive for KIT and CD34. Based on surgical and pathological findings, the final diagnosis was extragastrointestinal stromal tumor originating from the omentum.
Discussion
EGISTs have a similar morphology to that of gastrointestinal stromal tumors, but instead, arise outside the gastrointestinal tract. A significant differentiation and key to the diagnosis of EGIST is the absence of continuity with the gastrointestinal tract. The preferred treatment for EGIST is complete surgical resection, and the use of laparoscopy has not been well studied. Postoperative histopathological examination, along with immunohistochemical staining, aid confirmatory diagnosis.
Conclusion
Laparoscopic removal of EGISTs is a minimally invasive and potentially useful technique for the management of this tumor type.
Abbreviations: CECT, contrast-enhanced computed tomography; EGIST, extragastrointestinal stromal tumor; GIST, gastrointestinal stromal tumor; HPF, high-power field; ICC, interstitial cell of Cajal
Keywords: Extragastrointestinal stromal tumor, Gastrointestinal stromal tumor, Mesenchymal tumor, Laparoscopy, Case report
Highlights
-
•
Diagnosis and treatment of a spontaneously ruptured tumor is complicated.
-
•
Religion restrictions in management should be respected and followed.
-
•
First line of treatment of EGISTs is complete surgical resection.
-
•
Laparoscopic removal of EGISTs is minimally invasive and potentially useful.
1. Introduction
Mesenchymal tumors that arise outside the gastrointestinal tract and show histopathology and immunohistochemical staining similar to gastrointestinal stromal tumors (GIST) are called extragastrointestinal stromal tumors (EGIST), which are relatively rare [1]. The first line of treatment for EGIST is complete surgical resection; however, the indications for laparoscopic surgery in cases of ruptured EGISTs have not been fully discussed. We describe a case of successful laparoscopic resection of an EGIST originating from the omentum, which spontaneously ruptured and caused an intra-abdominal hemorrhage.
This work has been reported in line with the SCARE 2020 criteria [2].
2. Presentation of case
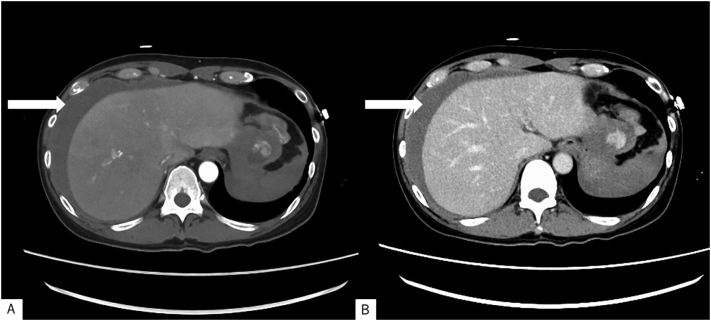
A 59-year-old man with an unremarkable past medical history visited the emergency room of another hospital due to severe left lateral abdominal pain. CECT revealed intra-abdominal hemorrhage. However, the patient was a Jehovah's Witness, for whom blood transfusion is contraindicated; hence, he was referred to our hospital for management. Upon arrival, the patient was conscious, with a Japan Coma Scale score of 0 and a Glasgow Coma Scale score of 15. Blood pressure was 109/78 mmHg, pulse rate was 78 beats/min, respiratory rate was 16 breaths/min, oxygen saturation was 98 % on room air, and body temperature was 36.9 °C. Physical examination revealed compression pain on the left lateral abdominal region, and blood tests revealed a Hb level of 11.2 g/d. CECT demonstrated an intraperitoneal mass (maximum diameter, 40 mm) between the stomach and spleen, with enhancement in the arterial phase and contrast spread in the delayed layer (Fig. 1A, B). There was also an accumulation of ascites fluid, which was suspected to be intra-abdominal hemorrhage, and no obvious free gas was seen in the abdominal cavity.
Fig. 1.
Contrast-enhanced computed tomography findings.
Intraperitoneal mass (maximum diameter, 40 mm) between the stomach and spleen, with enhancement in the (A) arterial phase and contrast spread in the (B) delayed layer. Accumulation of ascites fluid (arrow) suspected as intra-abdominal hemorrhage, and no obvious free gas in the abdominal cavity.
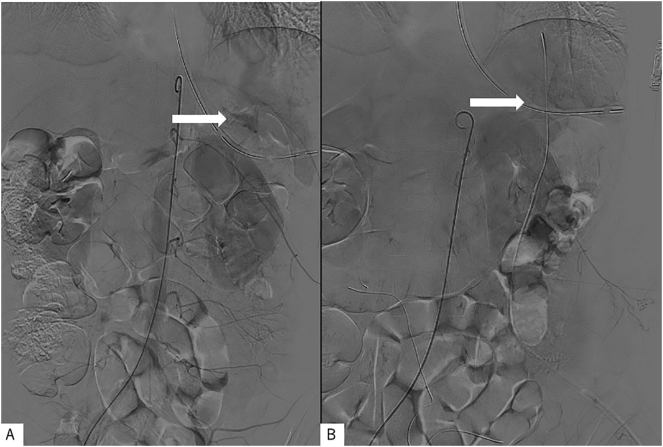
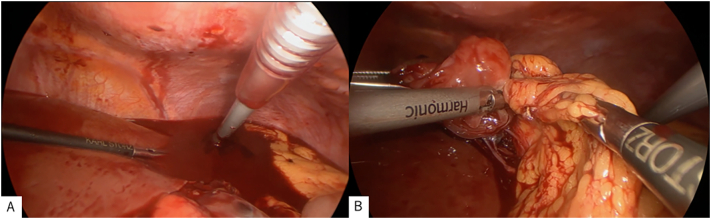
Based on the findings, the initial working diagnosis was intra-abdominal hemorrhage due to spontaneous rupture of the tumor; hence, endovascular treatment for hemorrhage control was performed, followed by laparoscopic surgery. Aortography revealed suspected hemorrhage (Fig. 2A). Endovascular embolization was attempted but the patient developed hypotension; thus, hemostasis was not achieved. A large amount of bloody ascites with clots was seen in the abdominal cavity, and a hematoma-covered mass (Fig. 3A), which was mobile, elastic, and soft, was found on the anterior surface of the omentum. The tumor was easily resected and detached from the omentum, as only sparse fibrous adhesions to the stomach were observed (Fig. 3B). Hemostasis was confirmed through a final aortography, and the operation was terminated (Fig. 2B). The operation time was 73 min, and blood loss was 730 mL. The gross appearance of the resected specimen showed no evidence of the original tumor, which was elastic, soft, and smooth with hemorrhage.
Fig. 2.
Aortogram findings.
(A) Before surgical tumor excision; (B) after surgical excision of the tumors.
Fig. 3.
Surgical findings.
(A) Intraperitoneal bleeding.
(B) Detachment of the tumor from the omentum.
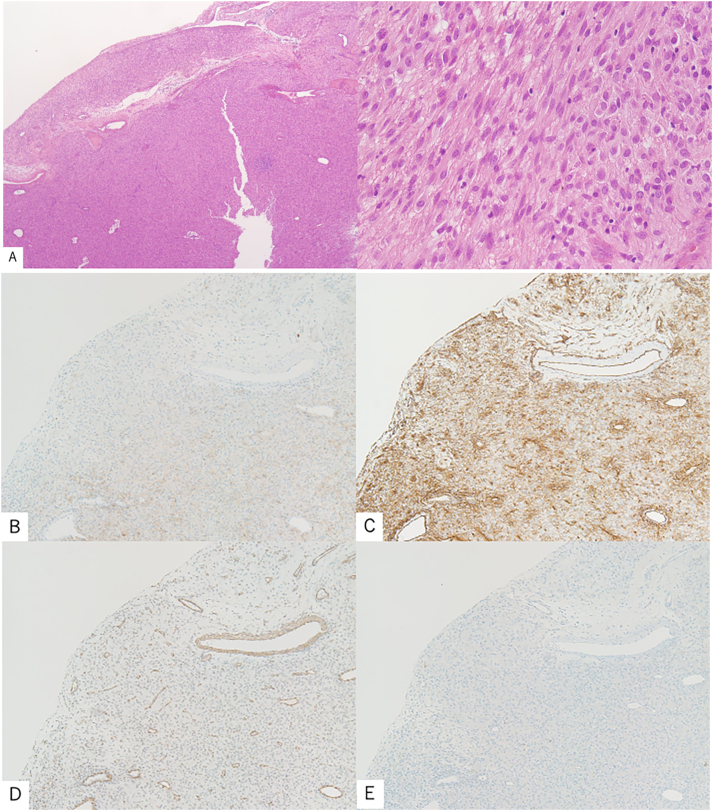
Histopathological findings consisted mainly of enlarged nuclei with increased chromatin and irregular bundles of spindle-shaped tumor cells with eosinophilic endoplasmic reticulum (ER) (Fig. 4A). The mitotic count was 0–2 cells/50 high-power field (HPF). Histological section immunoreactivity was positive for c-kit (Fig. 4B) and CD34 (Fig. 4C), and negative for SMA (Fig. 4D) and S100 (Fig. 4E). The clinical findings were consistent with EGIST. The tumor was ruptured and it had a mitotic count of 2/50 HPF; hence, it was classified as high-risk, according to the modified Fletcher classification [3], [4].
Fig. 4.
Microscopic findings of the resected specimen.
(A) Tumor composition of increased spindle cells (Hematoxylin and Eosin staining). Immunoreactivity in histological sections positive for (B) c-kit and (C) CD34; negative for (D) SMA and (E) S-100 proteins.
The patient had a good postoperative course with no progression of anemia, and was discharged from the hospital on the 14th day of hospitalization. The patient was treated with imatinib 400 mg/day to prevent recurrence. One year after surgery, follow-up CT showed no apparent recurrence, and the patient is currently under outpatient observation.
3. Discussion
GISTs are mesenchymal tumors that originate from the interstitial cells of Cajal (ICCs), which are composed of spindle-shaped or epithelioid tumor cells. The majority of these lesions have mutations in the c-kit or platelet-derived growth factor receptor alpha (PDGFRA) gene [5]. ICCs, which act as pacemakers for gastrointestinal peristalsis, are densely located in the muscularis propria of the gastrointestinal tract, especially around ganglion cells of the intermuscular plexus. Therefore, most GISTs originate from the intrinsic muscularis propria of the gastrointestinal tract, where ICCs are distributed; however, 5 % of tumors with a morphology very similar to that of GISTs arise outside the gastrointestinal tract and are termed EGISTs [6], [7].
Sakurai et al. [8] demonstrated that ICC-like c-kit-positive bipolar cells are also present in the omentum, suggesting that GISTs can also occur outside the gastrointestinal tract. Since no clear diagnostic criteria for EGIST have been established, there is concern that some of these reported cases may not be EGISTs but could be metastases and extramural GISTs [9].
The diagnosis of EGIST requires the following two conditions: (1) no continuity with the gastrointestinal tract and (2) exclusion of the possibility of metastases from other GISTs. If the patient has a history of GIST, it is difficult to rule out EGIST even if there is no continuity with the gastrointestinal tract, and the diagnosis of metastasis should be made first. The most important issue in the diagnosis is the differentiation between GIST with extramural growth and a true EGIST; hence, evaluation of the continuity between the tumor and the gastrointestinal tract is indicated.
The first step is to make a gross diagnosis based on surgical findings. Tumors reported as EGIST are often relatively large, and there are many cases in which the tumor was found to have strong adhesion with the surrounding intestinal tract, causing difficult detachment; thus, a combined resection was performed. It is difficult to diagnose an intra-abdominal tumor as an extra-intestinal primary tumor, although a presumptive diagnosis can be made preoperatively and intraoperatively. Continuity with the digestive tract must be ruled out by pathological examination of the resected specimen; however, there is no absolute indicator for evaluating the continuity between the tumor and the gastrointestinal tract, even in pathological diagnosis.
At present, there are no established treatment guidelines or prognostic guidelines for EGIST, and decisions are generally made in accordance with those for GIST. Furthermore, indications for laparoscopic resection of EGIST have not been fully discussed. The principle of resection is to secure a safe margin without damaging the false capsule, and the choice of treatment for EGIST is complete surgical resection, similar to that for GIST. In this case, interventional radiology was first performed for hemostasis but was quickly converted to laparoscopy when the patient developed hypotension intraoperatively. A hematoma was found at the attachment of the omentum to the stomach, and resection was performed distal to the hematoma. The hematoma and stomach showed sparse fibrous adhesions, and the tumor was easily resected without damaging the stomach. However, other tumors were scattered; hence, complete resection of all detected tumors was performed.
This was a case of a spontaneously ruptured tumor that was not initially detected by upper and lower gastrointestinal endoscopy. Although no obvious intraoperative traffic in the gastrointestinal tract was observed, it was difficult to accurately determine whether the tumor was a primary extraintestinal lesion. It was necessary to rule out continuity with the gastrointestinal tract by pathological examination of the resected specimen. However, it was difficult to make an accurate determination because the tumor was not in its original form. Furthermore, in postoperative pathological examinations, in addition to the fact that most of the findings were similar to those of GIST, such as bunchy proliferation of spindle-shaped cells, EGIST was the final diagnosis based on the characteristic immunohistological and intraoperative findings.
The Miettinen classification [10] cannot be used to predict prognosis in this case because this classification is based on tumor localization in the gastrointestinal tract, but the Fletcher classification [11] uses tumor diameter and fission images. Ruptured cases were considered clinically malignant, according to the Fletcher classification. Therefore, in addition to close observation, postoperative adjuvant chemotherapy with imatinib, which is similar to that used for high-risk GISTs, may be an option. In previous reports on EGIST, imatinib was administered postoperatively in some cases. Although the observation period was insufficient, the patient remained recurrence-free for up to 12 months after surgery. To the best of our knowledge, there have been only four cases of ruptured EGIST, and this is the first case that was removed by single-stage laparoscopy [12], [13], [14], [15].
4. Conclusion
This was a case of a ruptured EGIST arising from the omentum that was successfully removed laparoscopically. Laparoscopic removal of EGISTs is a minimally invasive and potentially useful technique. However, it is a difficult procedure that requires advanced endoscopic surgical techniques; hence, it is recommended that the procedure be performed by a skilled endoscopic surgeon. Further investigation of its safety and curative efficacy is needed after accumulating more cases.
Consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Ethical approval has been exempted by our institution because this is a case report and no new studies or new techniques were carried out.
Author contribution
Takaaki Murata: Writing- Original draft preparation and performed the surgery.
Jun Kawachi: Writing- review & editing.
Takahiro Ishimori, Wataru Naitou, Yuto Igrashi, Yuma Suno: review.
All authors read and approved the final manuscript.
Registration of research studies
Not applicable.
Guarantor
The guarantor for this case report is Takaaki Murata.
Declaration of competing interest
All the authors certify that there is no conflict of interest regarding the material discussed in the manuscript.
Acknowledgments
Acknowledgements
None.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Miettinen M., Monihan J.M., Sarlomo-Rikala M., Kovatich A.J., Carr N.J., Emory T.S., et al. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am. J. Surg. Pathol. 1999;23:1109–1118. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 Guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 3.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 2008;39:1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Rutkowski P., Bylina E., Wozniak A., Nowecki Z.I., Osuch C., Matlok M., et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour – the impact of tumour rupture on patient outcomes. Eur. J. Surg. Oncol. 2011;37:890–896. doi: 10.1016/j.ejso.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Hirota S., Ohashi A., Nishida T., Isozaki K., Kinoshita K., Shinomura Y., et al. Gain-of- function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 2003;125:660–667. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 6.Reith J.D., Goldblum J.R., Lyles R.H., Weiss S.W. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod. Pathol. 2000;13:577–585. doi: 10.1038/modpathol.3880099. [DOI] [PubMed] [Google Scholar]
- 7.Miettinen M., Monihan J.M., Sarlomo-Rikala M., Kovatich A.J., Carr N.J., Emory T.S., et al. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am. J. Surg. Pathol. 1999;23:1109. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai S., Hishima T., Takazawa Y., Sano T., Nakajima T., Saito K., et al. Gastrointestinal stromal tumors and KIT-positive mesenchymal cells in the omentum. Pathol. Int. 2001;51:524–531. doi: 10.1046/j.1440-1827.2001.01224.x. [DOI] [PubMed] [Google Scholar]
- 9.Agaimy A., Wünsch P.H. Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically re-evaluate the concept of so-called extra gastrointestinal stromal tumours. Langenbeck's Arch. Surg. 2006;391:322–329. doi: 10.1007/s00423-005-0005-5. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M., Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin. Diagn. Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher C.D., Berman J.J., Corless C., Gorstein F., Lasota J., Longley B.J., et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum. Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 12.Seow-En I., Seow-Choen F., Lim T.K., Leow W.Q. Primary omental gastrointestinal stromal tumour (GIST) presenting with a large abdominal mass and spontaneous haemoperitoneum. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-205528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murayama Y., Yamamoto M., Iwasaki R., Miyazaki T., Saji Y., Doi Y., et al. Greater omentum gastrointestinal stromal tumor with PDGFRA-mutation and hemoperitoneum. World J. Gastrointest. Oncol. 2012;4:119–124. doi: 10.4251/wjgo.v4.i5.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Islam S., Hosein D., Bheem V., Harnarayan P. Primary greater omental GIST presenting with acute intra-abdominal haemorrhage. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-220254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kataoka M., Saitoh T., Kawashima K., Yazaki T., Sonoyama H., Okimoto E., et al. Primary extragastrointestinal stromal tumor of greater omentum with intraperitoneal bleeding. Intern. Med. 2021;60:3413–3419. doi: 10.2169/internalmedicine.6519-20. [DOI] [PMC free article] [PubMed] [Google Scholar]