Abstract
Plasma-soluble CD30 (sCD30) is the result of proteolytic splicing from the membrane-bound form of CD30, a putative marker of type 2 cytokine-producing cells. We measured sCD30 levels in children with tuberculosis, a disease characterized by prominent type 1 lymphocyte cytokine responses. We postulated that disease severity and nutritional status would alter cytokine responses and therefore sCD30 levels. Samples from South African children enrolled prospectively at the time of diagnosis of tuberculosis were analyzed. (Patients were originally enrolled in a randomized, double-blind placebo-controlled study of the effects of oral vitamin A supplementation on prognosis of tuberculosis.) Plasma samples collected at the time of diagnosis and 6 and 12 weeks later (during antituberculosis therapy) were analyzed. sCD30 levels were measured by enzyme immunoassay. The 91 children included in the study demonstrated high levels of sCD30 at diagnosis (median, 98 U/liter; range, 11 to 1,569 U/liter). Although there was a trend toward higher sCD30 levels in more severe disease (e.g., culture-positive disease or miliary disease), this was not statistically significant. Significantly higher sCD30 levels were demonstrated in the presence of nutritional compromise: the sCD30 level was higher in patients with a weight below the third percentile for age, in those with clinical signs of kwashiorkor, and in those with a low hemoglobin content. There was minimal change in the sCD30 level after 12 weeks of therapy, even though patients improved clinically. However, changes in sCD30 after 12 weeks differed significantly when 46 patients (51%) who received vitamin A were compared with those who had received a placebo. Vitamin A-supplemented children demonstrated a mean (± standard error of the mean) decrease in sCD30 by a factor of 0.99 ± 0.02 over 12 weeks, whereas a factor increase of 1.05 ± 0.02 was demonstrated in the placebo group (P = 0.02). We conclude that children with tuberculosis had high sCD30 levels, which may reflect the presence of a type 2 cytokine response. Nutritional compromise was associated with higher sCD30 levels. Vitamin A therapy resulted in modulation of sCD30 levels over time.
It is estimated that one-third of the world’s population is infected with Mycobacterium tuberculosis (6). Although only 10% of infected patients will develop clinical disease, approximately 3 million deaths were directly attributed to tuberculosis worldwide in 1990 (14). Four hundred fifty thousand of the people who died were children younger than 15 years of age (14).
The immunopathogenesis of tuberculosis remains incompletely understood. Host responses to M. tuberculosis in children, who virtually invariably manifest with the primary form of tuberculosis, may differ from those in adults, whose disease most commonly results from reactivation of dormant infection. Although most studies to date have focused on adults, a better understanding of immune responses in children may be particularly important for developing novel primary vaccination and immunotherapeutic strategies.
Type 1 cytokine responses are thought to be central in protective immunity against tuberculosis in adults (1). In contrast, type 2 cytokine responses may represent deleterious immunity to intracellular bacteria such as M. tuberculosis, Mycobacterium leprae, and Leishmania major (1, 17, 21). The role of type 2 responses in tuberculosis is not clear. Surcel et al. have demonstrated increased numbers of interleukin 4 (IL-4)-producing cells in tuberculosis patients compared with numbers in tuberculin-sensitized healthy controls in an ELISPOT assay (22). There was no difference in gamma interferon (IFN-γ)-producing cells (reflecting a type 1 response) between the two groups. Sanchez et al. demonstrated increased IL-4 production by purified protein derivative-stimulated peripheral blood mononuclear cells (PBMC) when patients were compared with healthy tuberculin reactors (19). Zhang et al. failed to document increased type 2 responses in PBMC (24). Instead, patients with clinical disease had depressed type 1 responses compared with infected persons without clinical disease, suggesting that depressed type 1 responses rather than enhanced type 2 responses may characterize ineffective immunity to M. tuberculosis. It is possible therefore that immunity to M. tuberculosis is analogous to that to M. leprae, in which patients with milder disease (tuberculoid leprosy) may manifest type 1 responses whereas more severe disease (lepromatous leprosy) is characterized by type 2 responses (21).
We proposed measurement of plasma-soluble CD30 (sCD30) in tuberculosis patients as a marker of type 2 cytokine responses. CD30 is a member of the tumor necrosis receptor family and is expressed on antigen-activated T cells, B cells and NK cells and possibly on macrophages (4, 7, 10). CD30–CD30-ligand interactions result in a variety of functions, including T-cell costimulation, upregulation of cell surface antigens (such as adhesion molecules), and enhanced cytokine secretion (4, 7, 10). Human CD30+ T-cell clones preferentially produce type 2 cytokines (5); however, type 1 cytokine-producing cells may also express CD30 in vivo (11). In mice, CD30 expression on activated CD4+ cells reflected the ability of these cells to respond to IL-4, a type 2 cytokine (15). Similarly, CD30-CD30-ligand interaction in mice resulted in IL-5 production (a type 2 cytokine) but not IFN-γ production (a type 1 cytokine) (2). Thus, although CD30 expression may not be an intrinsic marker of type 2 cytokine-producing cells, it might reflect the presence of type 2 cytokine-producing cells.
sCD30, an 85-kDa molecule, is the result of proteolytic splicing of the 120-kDa membrane-bound form (7). High levels of sCD30 have been demonstrated in diseases in which type 2 cytokine responses predominate, for example, in atopy, advanced human immunodeficiency virus (HIV) infection, measles, systemic lupus erythematosus, and Ommen’s syndrome (18). These data suggest that sCD30 concentration parallels CD30 expression and may be a plasma marker of type 2 cytokine profiles. Should sCD30 be a marker of type 2 cytokine response, its easy measurement in plasma could offer significant advantages over traditional in vitro cytokine-based assays, including measurement of mRNA, intracellular protein, or secreted protein.
We postulated that deleterious type 2 cytokine responses would be present in children with more extensive tuberculosis compared with patients with milder disease, resulting in higher sCD30 levels. We also postulated that nutritional compromise would modulate sCD30 levels. We measured sCD30 longitudinally over the course of therapy, postulating that sCD30 levels would return to normal as the children improved clinically. As all patients in this cohort were subjects in a separate study of the effect of oral vitamin A therapy on prognosis in childhood tuberculosis (12), we also investigated effects of this immunomodulator on longitudinal changes in sCD30.
MATERIALS AND METHODS
Study design.
Children presenting with pulmonary tuberculosis to the Red Cross War Memorial Children’s Hospital in Cape Town, South Africa, were enrolled prospectively. Pulmonary tuberculosis was diagnosed on the basis of environmental exposure characteristics, clinical symptomatology, physical findings, chest roentgenography, and microbiologic investigations for detection and isolation of M. tuberculosis. Only patients meeting World Health Organization criteria of “definite” and “probable” tuberculosis were enrolled (23). Chest roentgenographic findings were later redocumented after consensus by two study pediatricians and a pediatric radiologist, who were blinded at that time. Children infected with HIV were excluded.
Patients were randomly selected at a ratio of 1:1 by a computer-generated list to receive either vitamin A or an identical-appearing placebo at diagnosis in a separate double-blind study of the effects of this vitamin on clinical outcome (12). Vitamin A (200,000 IU per dose) was administered orally on days 0 and 1.
Patients were again assessed after 6 and 12 weeks of standard antituberculosis therapy. At all three evaluations, plasma was collected and frozen at −70°C for subsequent sCD30 determination. At all evaluations a complete blood count and leukocyte differential count (Coulter MACM, Miami, Fla.) and determination of serum electrolytes, total protein, albumin, transthyretin, bilirubin, liver enzymes, cholesterol (Cxynchron CX4; Beckmann, Munich, Germany), retinol (by spectrofluorometry; spectrofluorophotometer model RF-1501 [Shimadzu, Kyoto, Japan]), zinc (by atomic absorption spectrophotometry; Beckman model 1272M atomic absorptiometer) were also performed. All specimens were processed in the same manner.
Written informed parental consent was obtained for participation in the study, and the study protocol was approved by the Research and Ethics Committee of the University of Cape Town.
sCD30 assay.
sCD30 was determined by a second-generation sandwich enzyme-linked immunosorbent assay (DAKO Corp., Glostrup, Denmark), as described previously (16). Briefly, prediluted sCD30 calibrators, a curve control, and individual specimens were added with peroxidase-conjugated mouse anti-CD30 antibody to microtiter wells precoated with another anti-CD30 monoclonal antibody. After 2 h of incubation, unbound material was washed away and a chromogenic substrate was added to the wells. The reaction was stopped, and the absorbance at 450 nm was measured. A standard curve was obtained from sCD30 calibrators, while sCD30 concentrations in plasma samples were determined by interpolation from the curve. When results above the value of the highest sCD30 calibrator were obtained, tests were repeated with dilutions to bring values within the linear section of the standard curve. The coefficient of variation of the assay was 9%, as estimated by duplicate determinations of two specimens at five separate runs.
Statistical considerations.
Weight measurements were documented as z scores to account for sex and age. Correlation of sCD30 with other continuous variables was determined by Spearman coefficients. Because there was a strong correlation between age and sCD30 level, all further statistical analyses were corrected for age. Differences in sCD30 levels in various tuberculosis disease categories (see Table 2) and nutritional categories (see Table 3) at baseline were determined by analysis of variance (ANOVA) of mean log10-transformed sCD30 values. Multiple linear regression, using log10-transformed values when not normally distributed, was utilized to determine the differential effects of various disease and nutritional categories and of age on statistically significant ANOVA results and Spearman correlations. Differences between sCD30 levels at baseline and those at subsequent evaluations were determined by paired t tests of log10-transformed sCD30 values. Analysis was performed with SPSS software (version 6.1; SPSS Inc., Chicago, Ill.).
TABLE 2.
Effect of disease severity on sCD30 level at presentation in 91 children with pulmonary tuberculosis
| Milder disease | n | sCD30 (U/liter) | More severe disease | n | sCD30 (U/liter) | Pa | |
|---|---|---|---|---|---|---|---|
| Primary complex disease | 32 | 111 (67–201)b | More advanced disease | 59 | 93 (64–154) | 0.28 | |
| Pleural effusionc | 28 | 88 (64–138) | No pleural effusion | 63 | 116 (69–166) | 0.35 | |
| Culture-negative disease | 59 | 98 (67–166) | Culture-positive disease | 32 | 98 (51–154) | 0.31 | |
| Pulmonary disease only | 72 | 93 (61–154) | Extrapulmonary disease | 19 | 136 (88–216) | 0.09 | |
| No miliary disease | 85 | 96 (66–154) | Miliary disease | 6 | 188 (89–216) | 0.21 |
Significance of difference between log10 sCD30 level for each group, linear regression analysis, corrected for age.
Median (interquartile range).
Presence of pleural effusion in primary tuberculosis usually represents mild disease; however, pleural effusions in his cohort also represented complications of progressive primary tuberculosis, i.e., more severe disease.
TABLE 3.
Effect of nutritional status on sCD30 level at presentation in 91 children with pulmonary tuberculosis
| Nutritional compromise | n | sCD30 (U/liter) | Less compromised | n | sCD30 (U/liter) | Pa | |
|---|---|---|---|---|---|---|---|
| Kwashiorkor | 10 | 170 (143–216)b | No kwashiorkor | 81 | 93 (64–153) | 0.07 | |
| Weight z score <−2 | 30 | 140 (87–179) | Weight z score ≥−2 | 61 | 93 (63–150) | 0.05 | |
| Albumin <3.5 g/dl | 62 | 102 (64–166) | Albumin ≥3.5 g/dl | 29 | 106 (74–154) | 0.96 | |
| Prealbumin <0.11 g/ml | 47 | 114 (66–165) | Prealbumin ≥0.11 g/ml | 44 | 86 (57–153) | 0.50 | |
| Hemoglobin <10 g/dl | 31 | 150 (88–206) | Hemoglobin ≥10 g/dl | 60 | 88 (57–124) | 0.04 | |
| Vitamin A <20 μg/dl | 57 | 114 (66–165) | Vitamin A ≥20 μg/dl | 34 | 86 (66–154) | 0.72 |
Significance of difference between log10 sCD30 level for each group, linear regression analysis, corrected for age.
Median (interquartile range).
RESULTS
Patient characteristics.
Of the 110 patients initially enrolled, 19 were subsequently excluded from analysis, because the final diagnosis was not tuberculosis (n = 8), the patient was HIV positive (n = 2), or follow-up data were regarded as inadequate (n = 9). Results from 91 patients were therefore included in the final analysis. Seventy-six patients (84%) attended the 6-week follow-up visit, and 81 (89%) attended the 12-week follow-up visit. The median age of patients was 39 months (range, 3 to 171 months); the female-to-male ratio was 0.9.
The clinical severity of tuberculosis ranged widely, from 32 cases (35%) of primary complex disease to 19 patients (21%) with extrapulmonary disease in addition to pulmonary tuberculosis. Thirty-two patients (35%) had positive mycobacterial cultures.
Generally poor nutritional status at diagnosis was demonstrated by a mean (± standard error of the mean [SEM]) weight z score of −1.42 ± 0.14 (normal mean, 0; normal range, −2 to 2). Clinical features of kwashiorkor (protein energy malnutrition) were present in 10 patients (11%), and serum albumin was below the lower limit of normal (<3.5 g/dl) in 62 patients (68%), while 57 patients (63%) had low vitamin A levels (<20 μg/dl).
Forty-six patients (51%) received vitamin A therapy at presentation, while 45 patients (49%) received a placebo. There were no differences in disease or nutritional characteristics between the two treatment groups (Table 1).
TABLE 1.
Baseline characteristicsa
| Characteristic | Vitamin A therapy (n = 46) | Placebo therapy (n = 45) |
|---|---|---|
| Median age (range) | 46 mo (5–171) | 36 mo (3–152) |
| Male/female ratio | 0.8 | 1.8 |
| Weight z score | −1.47 ± 1.48 | −1.38 ± 1.18 |
| Kwashiorkorb | 7 (15%) | 3 (7%) |
| Extrapulmonary tuberculosis | 9 (20%) | 10 (22%) |
| Vitamin A (retinol, μg/dl) | 17.5 ± 10.8 | 19.1 ± 10.2 |
| Transthyretin (mg/dl) | 0.11 ± 0.04 | 0.11 ± 0.05 |
| Albumin (g/dl) | 3.03 ± 0.60 | 3.17 ± 0.61 |
| Hemoglobin (g/dl) | 10.1 ± 1.7 | 10.2 ± 1.6 |
| Roentgenographic classificationc | ||
| Primary complex disease | 16 (35%) | 16 (36%) |
| Progressive primary disease | 27 (59%) | 29 (64%) |
| Miliary disease | 4 (9%) | 2 (4%) |
| Post-primary disease | 3 (7%) | 0 (0%) |
| M. tuberculosis culture positive | 19 (41%) | 13 (29%) |
Unless otherwise specified, results are presented either as numbers of patients, with percentages in parentheses, or as mean values with standard deviations. There were no differences between vitamin A- and placebo-treated groups.
Kwashiorkor was defined as weight below 60% of the expected mean weight for age, plus edema with or without skin changes suggestive of protein malnutrition.
Roentgenographic classification: primary complex disease, segmental parenchymal lesion and/or intrathoracic lymphadenopathy; progressive primary disease, any complication of primary complex disease, e.g., airway compression of lymph nodes, endobronchial spread with parenchymal involvement, or hematogenous spread; post-primary disease, adult-type, or reactivation, disease.
sCD30 levels at diagnosis.
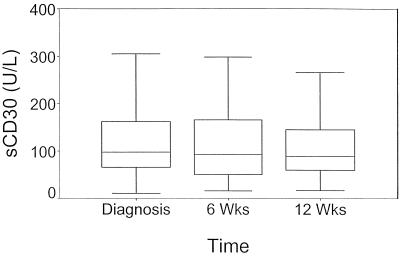
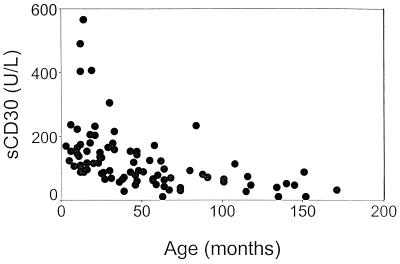
The median sCD30 level at baseline was 98 U/liter (range, 11 to 1,569 U/liter) (Fig. 1). There was a strong negative correlation between sCD30 level and age (r = −0.6; P < 0.001): younger children had higher sCD30 levels (Fig. 2). We corrected all subsequent data analysis for age.
FIG. 1.
sCD30 levels at diagnosis and after 6 and 12 weeks of therapy in 91 children with tuberculosis. There were no differences in sCD30 levels between evaluations.
FIG. 2.
A strong negative correlation between sCD30 level and age was demonstrated in 91 children with tuberculosis (r = −0.6, P < 0.001).
Patients with additional extrapulmonary disease tended to have higher sCD30 levels (P = 0.09, corrected for age) than patients with pulmonary tuberculosis alone (Table 2). Similarly, a hemoglobin level below 10 g/dl, a weight z score of <−2 (which equals weight below the third percentile for age), and clinical signs of kwashiorkor were associated with higher sCD30 levels (P = 0.04, P = 0.05, and P = 0.07, respectively) (Table 3). When corrected for age, a negative correlation between both plasma albumin and cholesterol with sCD30 was demonstrated (P < 0.05).
To determine which factors were most strongly associated with sCD30 levels, we performed linear regression analysis. When (i) age, (ii) presence of extrapulmonary disease, (iii) presence of a hemoglobin level <10 g/dl, and (iv) presence of kwashiorkor and of a weight z score of <−2 were included in regression analysis, only age correlated significantly with sCD30 level (P < 0.001). When (i) presence of malnutrition and (ii) presence of extrapulmonary disease or (i) presence of a hemoglobin level <10 g/dl and (ii) presence of extrapulmonary disease were included in regression analysis, the nutritional variable was more significantly correlated with sCD30 level (data not shown). These results indicate that it was likely that patients with extrapulmonary disease had higher sCD30 levels because they were younger and because they tended to have a poorer nutritional status.
Change in sCD30 levels during therapy.
Median sCD30 levels did not change significantly from baseline (98 U/liter; interquartile range, 66 to 165 U/liter) to 6 weeks (93 U/liter; range, 51 to 177 U/liter) or to 12 weeks (89 U/liter; range, 60 to 145 U/liter) (Fig. 1). No significant changes in individual patients’ sCD30 levels could be demonstrated from baseline to 12 weeks (P = 0.62).
Median sCD30 levels in the vitamin A-treated group decreased from 96 U/liter (interquartile range, 70 to 180 U/liter) at diagnosis to 73 U/liter (range, 57 to 138 U/liter) at 12 weeks, whereas median values remained unchanged in the placebo-treated group at these time points (105 U/liter [range, 64 to 166 U/liter] versus 106 U/liter [range, 71 to 166 U/liter]). On an individual patient basis, vitamin A therapy was associated with a mean (± SEM) factor decrease of 0.99 ± 0.02 in sCD30 level from diagnosis to 12 weeks, whereas there was a mean factor increase of 1.05 ± 0.02 in the placebo therapy group (difference: P = 0.02, corrected for age). Similar changes in the sCD30 level in the vitamin A- and placebo-treated groups were demonstrated after 6 weeks of therapy (data not shown).
Within different tuberculosis disease categories and nutritional categories (as listed in Tables 2 and 3), no significant change in sCD30 level from baseline to follow-up evaluations was demonstrated (data not shown).
DISCUSSION
We determined sCD30 levels in a well-characterized cohort of children with tuberculosis. Although we did not have a control group of healthy children, sCD30 levels were significantly higher than those previously reported in healthy adults or newborns by use of the same assay. Levels in healthy adults ranged from 4.6 ± 0.4 U/liter (8) to 9.9 ± 3 U/liter (9) (mean ± SEM). Levels of sCD30 in newborns were 5.68 ± 3.94 U/liter (3). Our patients’ sCD30 levels were also higher than those levels reported in adults with asymptomatic HIV infection (52 ± 40.9 U/liter, mean ± standard deviation) (16) and in adults with hepatitis B infection (mean, 28 U/liter) (8).
A strong negative correlation between sCD30 level and age was demonstrated (Fig. 2). It is not known whether healthy children have higher sCD30 levels at younger ages. Alternatively, high sCD30 levels in our study may reflect the presence of type 2 cytokine responses in children with tuberculosis.
We postulated that patients with more severe disease would have higher sCD30 levels, possibly indicating type 2 cytokine responses, than patients with milder forms of tuberculosis. More severely sick patients did tend to have elevated levels (Table 2); however, this trend was not invariably true. For example, no difference in sCD30 levels was found between patients with positive mycobacterial cultures (reflecting higher bacterial burdens and usually more severe childhood tuberculosis) and children with negative cultures (Table 2).
Compromised nutritional status tended to be associated with higher sCD30 levels (Table 3). Multivariate analysis demonstrated stronger independent associations between elevated sCD30 levels and markers of nutritional compromise, such as kwashiorkor, weight below the third percentile for age, and a low hemoglobin level, than between elevated sCD30 levels and markers of more severe tuberculosis. Additionally, there was a significant negative correlation between sCD30 level and plasma albumin and cholesterol levels, both of which are low in children with protein energy malnutrition. These findings suggest that sCD30 levels may be elevated in nutritionally compromised patients and that elevated sCD30 levels observed in children with more severe tuberculosis may therefore reflect poorer nutritional status. It is tempting to speculate that nutritional compromise may predispose a patient to a type 2 cytokine environment, which may contribute to host responses that are less effective, resulting in more severe disease.
Although we expected a decrease in sCD30 levels during the course of antituberculous therapy, no significant change was demonstrated, even though all patients improved clinically (Fig. 1).
Approximately half the patients received high-dose vitamin A supplementation at diagnosis in a separate study of the effect of this intervention on clinical outcome (12). The many immunomodulatory effects of vitamin A have been demonstrated both in vitro and in clinical situations (20). For example, significantly improved mortality and morbidity in children with severe measles were shown after supplementation with similarly large dosages of vitamin A (13). In our cohort, megadosages of vitamin A at diagnosis had no effect on clinical outcome (12) but were associated with a reduction in the median sCD30 level over time. However, on an individual patient basis, changes in sCD30 levels were small and are of doubtful significance.
In conclusion, high levels of sCD30 were demonstrated in our cohort of children with pulmonary tuberculosis. A strong negative correlation with age was found. sCD30 levels were still higher than those previously reported in healthy adults and newborns. Although there was a weak association between more severe disease and higher sCD30 levels, more severe nutritional compromise was significantly associated with higher sCD30 levels. Vitamin A therapy resulted in a change in sCD30 levels over time; however, the significance of this finding is not clear. Future correlation of PBMC cytokine production with sCD30 levels in children with tuberculosis will be helpful in determining whether sCD30 could function as a marker of the cytokine milieu during the infection.
REFERENCES
- 1.Barbes P F, Rom W N. Cytokine production in tuberculosis. In: Rom W N, Garay S M, editors. Tuberculosis. Boston, Mass: Little, Brown and Company; 1996. pp. 291–303. [Google Scholar]
- 2.Bowen M A, Lee R K, Miragliotta G, Nam S Y, Podack E R. Structure and expression of murine CD30 and its role in cytokine production. J Immunol. 1996;156:442–449. [PubMed] [Google Scholar]
- 3.Chilosi M, Facchetti F, Notarangelo L D, Romagnani S, Del Prete G, Almerigogna F, De Carli M, Pizzolo G. CD30 cell expression and abnormal soluble CD30 serum accumulation in Omenn’s syndrome: evidence for a T helper 2-mediated condition. Eur J Immunol. 1996;26:329–334. doi: 10.1002/eji.1830260209. [DOI] [PubMed] [Google Scholar]
- 4.De Bruin P C, Gruss H J, Van Der Valk P, Willemze R, Meijer C J. CD30 expression in normal and neoplastic lymphoid tissue: biological aspects and clinical implications. Leukemia. 1995;9:1620–1627. [PubMed] [Google Scholar]
- 5.Del Prete G, De Carli M, Almerigogna F, Daniel C K, D’Elios M M, Zancuoghi G, Vinante F, Pizzolo G, Romagnani S. Preferential expression of CD30 by human CD4+ T cells producing Th2-like cytokines. FASEB J. 1995;9:81–86. [PubMed] [Google Scholar]
- 6.Enarson D A, Murray J F. Global epidemiology of tuberculosis. In: Rom W N, Garay S M, editors. Tuberculosis. Boston, Mass: Little, Brown and Company; 1996. pp. 57–75. [Google Scholar]
- 7.Falini B, Pileri S, Pizzolo G, Durkop H, Flenghi L, Stirpe F, Martelli M F, Stein H. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85:1–14. [PubMed] [Google Scholar]
- 8.Fattovich G, Vinante F, Giustina G, Morosato L, Alberti A, Ruol A, Pizzolo G. Serum levels of soluble CD30 in chronic hepatitis B virus infection. Clin Exp Immunol. 1995;103:105–110. doi: 10.1046/j.1365-2249.1996.915607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerli R, Muscat C, Bistoni O, Falini B, Tomassini C, Agea E, Tognellini R, Biagini P, Bertotto A. High levels of the soluble form of CD30 molecule in rheumatoid arthritis (RA) are expression of CD30+ T cell involvement in the inflamed joints. Clin Exp Immunol. 1995;102:547–550. doi: 10.1111/j.1365-2249.1995.tb03851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruss H J, Dower S K. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood. 1995;85:3378–3404. [PubMed] [Google Scholar]
- 11.Hamann D, Hilkens C M U, Grogan J L, Lens S M A, Kapsenberg M L, Yasdanbankhsh M, Van Lier R A W. CD30 expression does not discriminate between human Th1- and Th2-type T cells. J Immunol. 1996;156:1387–1391. [PubMed] [Google Scholar]
- 12.Hanekom W A, Potgieter S, Hughes E J, Malan H, Kessow G, Hussey G D. Vitamin A status and therapy in childhood tuberculosis. J Pediatr. 1997;131:925–927. doi: 10.1016/s0022-3476(97)70046-5. [DOI] [PubMed] [Google Scholar]
- 13.Hussey G D, Klein M. A randomized, controlled trial of vitamin A in children with severe measles. N Engl J Med. 1990;323:160–164. doi: 10.1056/NEJM199007193230304. [DOI] [PubMed] [Google Scholar]
- 14.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organisation. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T, Lee R K, Nam S Y, Al-Ramadi B K, Koni P A, Bottomly K, Podack E R, Flavell R A. Reciprocal regulation of CD30 expression on CD4+ T cells by IL-4 and IFN-γ. J Immunol. 1997;158:2090–2098. [PubMed] [Google Scholar]
- 16.Pizzolo G, Vinante F, Morosato L, Nadali G, Chilosi M, Gandini G, Sinicco A, Raiteri R, Semenzato G, Stein H, Perona G. High serum level of the soluble form of CD30 molecule in the early phase of HIV-1 infection as an independent predictor of progression to AIDS. AIDS. 1994;8:741–745. doi: 10.1097/00002030-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 18.Romagnani S, Del Prete G, Maggi E, Chilosi M, Caligaris-Cappio F, Pizzolo G. CD30 and type 2 T helper (Th2) responses. J Leukoc Biol. 1995;57:726–730. doi: 10.1002/jlb.57.5.726. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez F O, Rodriguez J I, Agudelo G, Garcia L F. Immune responsiveness and lymphokine production in patients with tuberculosis and healthy controls. Infect Immun. 1994;62:5673–5678. doi: 10.1128/iai.62.12.5673-5678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semba R D. Vitamin A, immunity and infection. Clin Infect Dis. 1994;14:489–499. doi: 10.1093/clinids/19.3.489. [DOI] [PubMed] [Google Scholar]
- 21.Sieling P A, Modlin R L. Regulation of cytokine patterns in leprosy. Ann N Y Acad Sci. 1994;730:42–52. doi: 10.1111/j.1749-6632.1994.tb44238.x. [DOI] [PubMed] [Google Scholar]
- 22.Surcel H M, Troye-Blomberg M, Paulie S, Andersson G, Moreno C, Pasvol G, Ivanyi J. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–176. [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. WHO tuberculosis programme framework for effective tuberculosis control, p. 179. WHO/TB/94. World Health Organization, Geneva, Switzerland.
- 24.Zhang M, Lin Y, Iyer D V, Gong J, Abrams J S, Barnes P F. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–3234. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]