Abstract
In the last decade, hyaluronic acid (HA) has attracted an ever-growing interest in the biomedical engineering field as a biocompatible, biodegradable, and chemically versatile molecule. In fact, HA is a major component of the extracellular matrix (ECM) and is essential for the maintenance of cellular homeostasis and crosstalk. Innovative experimental strategies in vitro and in vivo using three-dimensional (3D) HA systems have been increasingly reported in studies of diseases, replacement of tissue and organ damage, repairing wounds, and encapsulating stem cells for tissue regeneration. The present work aims to give an overview and comparison of recent work carried out on HA systems showing advantages, limitations, and their complementarity, for a comprehensive characterization of their use. A special attention is paid to the use of HA in three important areas: cancer, diseases of the central nervous system (CNS), and tissue regeneration, discussing the most innovative experimental strategies. Finally, perspectives within and beyond these research fields are discussed.
Keywords: Hyaluronic acid, Hydrogel, Biomaterial, Tissue engineering, Cancer, Central nervous system, Regenerative medicine
Graphical abstract
1. Introduction
In the last decade, research worldwide has focused on biomaterials as a way to mimic human soft tissue and extracellular matrix (ECM) towards understanding the cellular crosstalk and their interaction within their microenvironment [[1], [2], [3]]. The ECM is composed of two main classes of macromolecules: polysaccharide chains belonging to the class of glycosaminoglycans (GAGs) and fibrous proteins. The first ones are usually found bound to proteins to form proteoglycans, which can be rich in sulfate groups, such as chondroitin sulfate, dermatan sulfate, heparan sulfate, and keratan sulfate, or without sulfate groups, such as hyaluronic acid (HA). The second ones include two groups: one with primarily structural function, such as collagen and elastin, and another with primarily adhesive function, such as fibronectin, laminins, entactin, and vitronectin [4]. Current experimental cell culture approaches involve two-dimensional (2D) systems such as polystyrene and glass, which do not represent an ideal physiological condition. In these systems, the achieved results are altered compared to those obtained in vivo, leading to the development of various 3D systems [5,6]. Thus, experimental methods exploring substrates which mimic the native ECM in a three-dimensional (3D) space are highly required [[7], [8], [9], [10], [11]]. Interactions with the microenvironment are essential for the study of cellular response [[12], [13], [14], [15], [16], [17]]. Careful evaluation of the physical and chemical characteristics of the polymer used for the 3D system realization is of high demand to avoid alterations of cellular responses. Crucial properties such as biocompatibility, environmental responsiveness, biodegradability with controllable degradation rate and a highly porous 3D structure, allow bioadhesion, cell penetration, cell interaction and cell proliferation [18]. Thus, the choice of the most suitable biomaterial depends on their appropriate biological and physicochemical properties (biocompatibility, biodegradability, porosity, structure, stiffness, and manufacturing technologies) and applications. To date, both natural and synthetic materials are used to realize 3D cell systems [[19], [20], [21]]. Natural materials (i.e., collagen, gelatin, chitosan, HA, heparin alginate, silk and decellularized tissue matrices) are inherently biocompatible exhibiting good cellular interactions, low immunogenicity, and mild antigenic properties, but show poor mechanical properties, high production costs, and low reproducibility [[22], [23], [24], [25]]. On the other hand, synthetic polymers (i.e. polyglycolic acid -PGA, polylactic acid – PLA, polycaprolactone – PCL, polyurethane- PU, polytetrafluoroethylene -PTFE), are a class of hydrophobic materials with tightly controlled physico-chemical properties, high mechanical strength, slow degradation rate, low cellular affinity, weak cellular response, but high reproducibility and reduced costs. Also, their synthesis can produce toxic by-products for cells.
Among the natural polymers selected for the realization of 3D cellular systems, for dermatological formulations [26] and regenerative medicine [[27], [28], [29], [30]] the most popular biomaterial used is hyaluronic acid (HA) [31]. HA is a molecule easy to customize chemically and mechanically, with high-water retention and viscoelastic properties [32] and an indispensable component of the ECM [33,34]. HA has been shown to be involved in cell proliferation, cell migration, angiogenesis, fetal development, and tissue function. It is directly released from the cell surface by the action of an enzymatic complex localized in the plasma membrane, called hyaluronan synthases (HAS), binding to several cell surface membrane proteins present on different cell types and regulating inflammatory processes by interaction with Toll-like receptor (TLR) 4 and TLR2, protecting epithelial cells from tissue damage [35]. HA is inherently hydrophilic with polar and apolar segments in the polymer structure, which allow to chemically interact with different chemical agents [36]. Its structural, physical, physicochemical and degradable properties as well as its biological properties mainly depend on its molecular weight (MW), which in turn depends on the source (i.e. the MW of animal materials can equal up to 20,000 kDa; bacterial hyaluronic acid between 1000 and 4000 kDa) [37,38]. High MW hyaluronic acid (>500 kDa) commonly presents anti-angiogenic activity, and the increase in MW aids to the strengthening of the 3D polymer network as well as increasing solution viscosity and viscoelasticity [39]. Moreover, if HA at high MW is not promptly replaced, pathologic inflammatory conditions can compromise the strength of HA scaffolds, exposing the cells to permanent damage [40,41]. This is cause of pathological conditions [42,43]. HAs with low MWs (from 0.4 to 4.0 kDa) show anti-apoptotic properties, whereas MWs between 6 and 20 kDa exhibit immunostimulatory, angiogenic, and phlogistic activities.
In general, HA has also shown to maintain the homeostasis of the environment and to clean the extracellular environment from damaging free radicals [[44], [45], [46]]. For example, Xu et al. demonstrated that the modified hyaluronic acid methacrylate (HAMA) hydrogel with phenylboronic acid (PBA), compared with glucose-reactive HAMA-PBA/catechin (HMPC) hydrogels is more able to scavenge intracellular reactive oxygen species and protect NIH/3T3 fibroblast cells from oxidative stress damage, increasing superoxide dismutase activity, stabilizing the glutathione/oxidized glutathione ratio, and reducing malondialdehyde content [47].
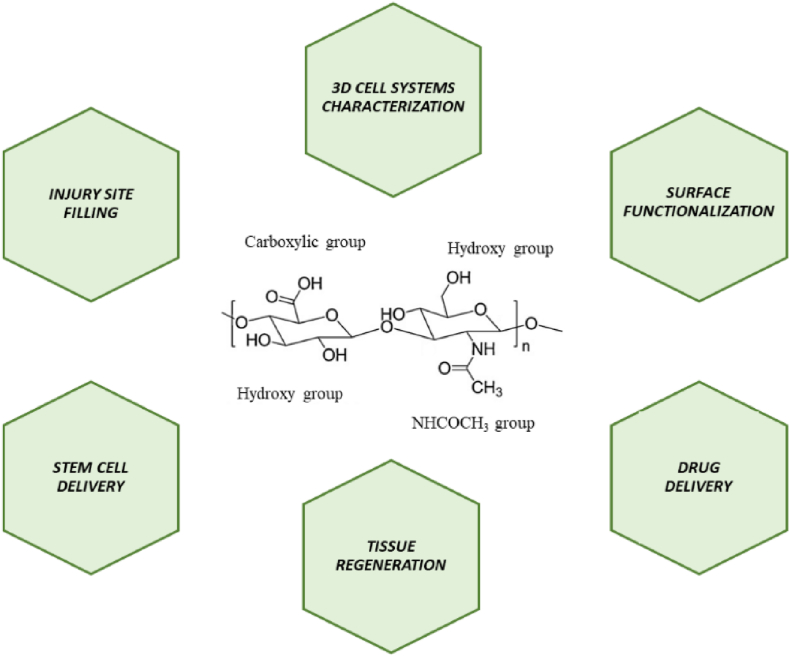
The realization of a HA system with high biocompatibility, and which degradation products do not induce inflammation or oxidative stress still represents an open challenge in terms of experimental reproducibility. First of all, the surface must allow cell adhesion and support cell growth. The porosity of the hydrogel must promote cell motility and interaction, and this is related to the phenotype of the studied cell. Furthermore, the porosity is essential to allow the nutrients exchange. Finally, these properties (i.e. mechanical and structural) must be tailored for the intended application and microenvironment into which the scaffold will be located [48]. Herein, we aim to give a critical overview of all the major application fields of the HA hydrogels and the main experimental techniques applied (Fig. 1).
Fig. 1.
Schematic representation of the chemical structure of HA and target sites for chemical modification as well as the most important biomedical approaches used.
2. Hyaluronic acid hydrogels fabrication
The main goal in the hydrogel realization for cell cultures is to obtain a biocompatible system, preferably aqueous based so that no solvents could compromise cell survival and biochemical cell response. HA is highly soluble, easily degradable in vivo, and has very poor mechanical properties. In fact, HA is not able to form a physical gel alone, requiring chemical modifications, covalent crosslinking, and gelling agents to obtain a solid hydrogel as well as to improve properties, including stiffness, viscosity, solubility, degradation, and biological properties. Chemical modification involves three functional groups: the carboxyl group, the hydroxyl group, and the N-acetyl group [49]. The various chemical modification methods to obtain HA derivatives are detailed in various reviews [[50], [51], [52], [53]]. In brief, chemical modifications of HA regarding the carboxyl group, include amidation, Ugi condensation, esterification, and oxidation [[54], [55], [56], [57], [58], [59]]. Using hydroxyl groups, it is possible to induce the formation of ethers, hemiacetals, carbamates and promote esterification processes [54,[60], [61], [62]]. Finally, the N-acetyl group can be modified by deacetylation/amidation [50,52,63] (Table 1).
Table 1.
Chemical modification of HA hydrogel [50].
| HA hydrogel group modifications | Reaction type | Reagents |
|---|---|---|
| -COOH | Amidation | carbodiimides |
| 2-chloro-1-methylpyridinium iodide | ||
| 2-chloro-dimethoxy-1,3,5-triazine | ||
| 1,1′-carbonyldiimidazole | ||
| Ugi condensation | ||
| Esterification | alkyl halides | |
| diazomethane | ||
| tosylate | ||
| epoxides | ||
| -OH | Ether formation | epoxides |
| divinyl sulfone | ||
| ethylene sulfide | ||
| Hemiacetal formation | glutaraldehyde | |
| Esterification | methacrylic anhydride | |
| acyl-chloride activated carboxylate | ||
| alkyl succinic anhydrides | ||
| Carbamate formation | cyanogen bromide | |
| Oxidation | epoxides | |
| -NHCOCH3 | Deacetylation/amidation | hydrazine sulfate |
Subject to the type of bond formed between the polymer chains from which they derive, the hydrogels can be classified as “reversible or physical” and “permanent or chemical” [64]. Physical hydrogels are obtained from weak interactions such as H-bonding, Van der Waals interactions, hydrophobic forces, or molecular entanglements. These hydrogels are easily degraded by temperature or pH changes. Chemical hydrogels generally present a gel structure obtained by covalent bonds generated by crosslinking polymers with radiations, chemical crosslinkers, polyfunctional compounds, or free radical generating compounds (Table 2). Moreover, they show improved chemical, mechanical, and thermal stability respect to physical hydrogels.
Table 2.
Main models, advantages, and disadvantages of HA hydrogel [50].
| HA hydrogel models | Bond interactions | Advantage | Disadvantage |
|---|---|---|---|
| Physical or reversible hydrogel | H-bonding | Soft gel | Not stable |
| Van der Waals interactions | Nontoxic | Low mechanical resistance | |
| Hydrophobic forces | Different pure size | ||
| Molecular entanglements | |||
| Chemical or permanent hydrogel | Covalent bonds | Soft gel | Could be toxic |
| Controlled pore size |
2.1. Physical hydrogels
Hydrogels produced using non-covalent bonds have been subject of great interest in the biological field. The advantage of a physical hydrogel is to obtain a system with flexible properties, and responsive to factors such as light, temperature, and pH [65,66]. It represents a mechanically and chemically weaker hydrogel, but at the same time, its high degradability may facilitate the study of cellular systems as it becomes easier to collect the sample for molecular biology techniques [67]. A self-degradable system can also facilitate self-healing processes in regenerative medicine [68]. Physical hydrogels polymerization is triggered by electrostatic, hydrophilic and hydrophobic interactions, as well as a structural complementarity between two molecules called “host” and “guest” [69]. One of the most widely used host molecules for HA are cyclodextrins [70], which are characterized by hydrophobic motifs and therefore show a high affinity for hydrophobic hosts. Among these, adamantane has been reported to form an HA with the guest β-cyclodextrin [70]. By varying host and guest concentrations and molar ratio showed to change the crosslink density and structure of these HA. Another host/guest pair is represented by β-cyclodextrin and azobenzenes [71]. Modulation of the hydrogel properties were reported to be obtained using light, by inducing the isomerization of azobenzene which, in turn, affected the binding affinity between host/guest molecules, the network connectivity, release capabilities and the stiffness of the hydrogel. Rowland et al. functionalized HA hydrogels with Cucurbitaceae uril and cysteine-phenylalanine as the host/guest pair [72] enabling the hydrogel to appropriately remodel to the surrounding environment. A further approach was successfully attempted by Montanari and colleagues, with HA functionalized with an amphiphilic property via self-assembly of macromers following hydrophobic chemical derivatization of HA with cholesterol moieties. In particular, their nanohydrogels (NHs) were an effective transport system for alginate lyase, which resulted effective for the treatment of biofilm-associated infections [73]. Physically crosslinked HA-based hydrogels can be also obtained through ionic interactions using block copolymers of PEG and cationic poly(2-aminoethyl methacrylate [74], DNA hybridization obtaining thiolated oligo DNA modified HA [75], p–p interactions to form HA-benzoyl cysteine derivative [76], as well as molecular recognition through collagen binding peptide grafted HA, to achieve collagen-HA hydrogels [77]. Although physical hydrogels are characterized by simple preparation and versatility in regulating parameters like temperature or pH, and lack of residual or unreacted agents such as initiators or catalysts, they are characterized by poor reproducibility and stability of the system and lack of control of molecular interactions.
2.2. Chemical hydrogels
Hydrogels obtained by chemical crosslinking show stronger mechanical, chemical, and thermal stability. These hydrogels exhibit higher strength and a more physically, than chemically, stable structure. The downside is represented by the use of metal catalysts, photoinitiators, and low reaction yields which affect the biocompatibility of the cell microenvironment, the biodegradability of the materials, and limit the transport of nutrients and metabolites [78]. The approaches used for chemical crosslinking processes are various, such as condensation reactions, enzymatic cross-linking, disulfide crosslinking, click chemistry [79], polymerization [53]. These reactions can also be carried out directly using divinyl sulfone, glutaraldehyde, carbodiimide, and bisepoxide. However, these processes are complex and associated with the formation of compounds with a high toxicity rate [[80], [81], [82], [83]]. Specifically, chemical modification through Schiff base, Michael addition reactions and photocrosslinking of the hydroxyl and carboxyl groups lead to methacrylated, acrylated and thiolated HA derivatives, which maintain the biocompatibility and biodegradability of native HA [52,84].
Commonly, methacrylated HA, or acrylated HA are obtained through the Michael addition reaction, which is generally characterized by reactions of nucleophiles (Michael donor, e.g. thiol groups) with α,β-unsaturated carbonyls (Michael acceptor, such as maleimide, acrylate, acrylamide or vinylsulphone) and occurs rapidly under mild reaction conditions with a high degree of chemical selectivity [85,86]. thiolated HAs are obtained using a HA derivative as the nucleophile (the donor), instead of a cross-linker.
Photopolymerization involves the use water-soluble photoinitiators such as Irgacure 2959 or Lithium Acylphosphinate (LAP) and exposure with UV irradiation to trigger a propagation step with free radical reactions, which ends with the formation of covalent bonds between two propagating HA chains or between a propagating HA chain and a radical [50,87,88]. In this case the HA chemical structure is modified through methacrylatetion with Methacrylic anhydride or glycidyl methacrylate with the photoinitiator. Photo-crosslinkable HAs present advantages such as uniform mechanical and physical properties, and high reproducibility, faster gelation times and are typically employed to encapsulate the cells inside the hydrogel or as 2D substrates [89,90].
A further technique, which does not involve modification of the HA chains, as in Michael addition reaction or photocrosslinking, is carbodiimide chemistry. Carbodiimide compounds such as cyanamide or 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDAC or EDC), react with carboxylic acid groups to produce a reactive bond with primary amine functional groups [82,[91], [92], [93]]. Cross-linking with EDAC is more exploited as it occurs in physiological-like conditions (pH 7.4, 37 °C), although its maximum efficiency can be achieved with a slightly acidic pH (4.5) [94,95]. This method has allowed to obtain cytocompatible HA-based hydrogels for a variety of biomedical applications, including studying cancer cell behaviors in vitro [96]. A further efficient strategy for hydrogel synthesis is represented by the Diels-Alder (DA) reaction, which occurs without any catalysts or byproducts [97]. This reaction occurs between an electron-rich diene (e.g. furan) and an electron-deficient dienophile (e.g. maleimide).
3. 2D vs 3D
For many years the 2D monolayer culture has been the most widely used system for the study of cellular response to exogenous stimuli. However, 2D in vitro studies do not always display correspondence with clinical results. A 3D microenvironment enables a multidirectional interaction and geometric confinement with neighbouring cells, stiffness and matrix components respect to a 2D substrate [5,6]. In particular, changes in cell function within confined 3D environments have been hypothesized to be regulated through YAP-mediated mechanotransduction mechanisms showing that cells in 3D experienced lower mechanical stresses compared to 2D. 3D confinement can influence also growth, development, and homeostatic profiles of tissues [98]. Also, 3D environments have been shown to modulate mesenchymal stem cells (MSC) differentiation and proliferation respect to 2D [99]. Differences in the metabolic response between 2D and 3D cell cultures has been highlighted in different studies. For example, Wang et al. used photocrosslinked HA methacrylate (HAMA), to show that the degree of methacrylatetion can modulate the hydrogel microstructure, mechanical performance, and liquid absorption and degradation capabilities. A methacrylatetion around 60% exhibited a better porous structure for the breast cancer cell line (MCF-7) used. Furthermore, the authors observed a proliferative and aggregative capacities increase in the 3D system with higher expression levels of VEGF, bFGF, and interleukin-8 (IL-8), compared to the 2D monolayer culture [100]. The authors also demonstrated that implantation of a 3D system composed of HA hydrogels in 4–5-week-old BALB/c nude mice can promote tumor growth compared to implantation of a 2D system, showing that their 3D system is a more representative model of the proliferative capabilities of a tumor mass.
Ananthanarayanan and co-workers compared invasiveness of cells on different formulations of methacrylated HA prepared on glass-bottom 6-well plates (2D) and cells encapsulated in the hydrogel during gelation with varying HA weight percent and crosslinking density, also in response to change of stiffnesses and ligand (RGD peptide) density [101]. They observed a distinct mode of motility within their hydrogels (in 3D respect to 2D), remarkably similar to brain slice cultures, showing that their HA-RGD hydrogels were able to recapitulate key morphological features of the in vivo phenotype.
Seidlits et al. developed a hydrogel realized by Michael-type addition between 4-arm polyethylene glycol-maleimide (PEG-Mal) and thiolated HA associated with several peptides to increase cell adhesion, such as RGD, IKVAV and YIGSR peptides, analyzing the differentiation process between a 2D and a 3D culture. Increased differentiation to oligodendrocytes and neurons was observed in the 3D culture, with reduced differentiation into activated astrocytes compared to the 2D culture. They also considered that the presence of high molecular weight HA (∼700 kDa) in the 3D cultures may contribute to prevent complete maturation. Moreover, the differentiation process in 3D culture was more efficient in hydrogels containing RGD peptide than in hydrogels containing YIGSR and CYS. The same increase was observed in cell viability. The authors also found no change in cPARP protein levels, indicative of apoptotic activation, and no reduction in Ki67 levels in the differentiation step of the 3D culture, indicative of a blocking of the proliferation process corresponding to the differentiation step [102].
Although molecular biological studies are easier and faster in 2D, we argue for a greater focus on fundamental processes that orchestrate the cellular behavior in 3D. Studies based on 3D environments would aid in the fabrication of functional tissue-engineering constructs and represent a viable alternative to animal studies.
4. Hyaluronic acid hydrogels in cancer
For decades the complex mechanisms of tumorigenesis, angiogenesis, invasion and metastasis of cancer disease has relied on two-dimensional (2D) monolayer cell culture platforms and/or in vivo animal models (xenografts). As above mentioned, HA-based hydrogels are ideal to realize a more biocompatible and representative system of a physiological condition [103] and have been a focal point for studying invasive tumors especially for brain tumors and brain metastatic breast cancer cells, given the abundant presence of HA in the brain ECM [104]. For instance, Ananthanarayanan's group observed a correlation among hydrogel stiffness, cell spreading and RGD peptide density [101]. Their results confirmed previous studies [105], showing that HA-RGD hydrogels within the range of stiffness of brain tissues were invaded by glioma tumor spheroids and that the invasiveness and colonization of tumor cell lines in their hydrogels were correlated with the expression of hyaluronidase [106] and matrix degradation [107]. thiolated HA and combined thiolated and acrylated HA have been used to investigate drug delivery systems [108] or for drug screening applications [109,110]. A composite hydrogel made of various collagen and concentrations of thiolated HA, showed that varying the HA concentration influenced encapsulated patient derived OSU-2 glioblastoma cells' morphology and migration in vitro. Specifically, lower concentrations of HA induced a more spindle-like morphology and increased cell migration [111]. Thiol-modified HA were used by Rehfeldt and co-workers to observe changes in the cytoskeleton structure with fewer vinculin adhesions in 2D than in 3D, demonstrating the presence focal adhesions on the cell apex only in the 3D structure [112]. HA hydrogels fabricated via thiol Michael addition reaction were used by Narkhede et al. to study the behavior of brain metastatic breast cancer cells (i.e., MDA-MB-231Br cells) in vitro [113]. They observed increased adhesion, spreading, proliferation and migration of cells cultured on top of these hydrogels which varied with the hydrogel stiffness. Moreover, they showed that softer HA hydrogels promoted a dormant phenotype whereas stiffer triggered a proliferative one [114]. Acrylated HA hydrogels were used to study cell proliferation and invasion of HT1080 fibrosarcoma cells [115]. The authors studied the impact of matrix stiffness (soft, medium, and stiff HA hydrogels) on encapsulated HT1080 cell fate in different oxygen levels (atmospheric, hypoxic and severely hypoxic) by producing hydrogels with different concentration of matrix metalloproteinase (MMP) sensitive peptide crosslinkers. HT1080 cells adapted better to hypoxia with no correlation noted with matrix stiffness. In addition, their results showed that endothelial sprouting and invasion were affected by varying stiffness and oxygen tension in the presence of HT1080 cells. Increasing endothelial sprouting and invasion for all hydrogels was reported under hypoxia conditions and was accompanied by vascular endothelial growth factor (VEGF) as well as angiopoietin-1 (ANG-1) upregulation. However, under atmospheric condition, a negative correlation was observed between endothelial sprouting and invasion with matrix stiffness. Naked et al. also used HA hydrogels to encapsulate glioblastoma cells (U87, and patient derived D456 cells) [116] within the liquid hydrogel precursor solution before polymerization. They observed that the cells can generate spheres, with an increase in cell sphere size in both cell lines in absence of serum, in particular for D456 cells. They also demonstrated that glioblastoma cells displayed an intrinsic stem cell characteristic and this peculiarity is confirmed by higher expression levels of markers such as Nestin, SOX2, and CD113 [117]. This data is also validated in 3D HA hydrogel compared to the 2D monolayer culture and the liquid suspension. Furthermore, Leite et al. showed that encapsulation of human glioblastoma cell lines (U-87MG, SNB-19 and UP-007) increased viability and cell proliferation respect to 2D [118].
The degree of methacrylatetion of HA also plays a key role in cell behavior as higher degrees of methacrylatetion leads to higher values of elastic and loss modulus. For example, Xu et al. showed that varying the mole percentage of grafted glycidyl methacrylate varied the tumorigenic activity and cell adhesion of MCF-7 breast cancer cells seeded in the hydrogel disks [119]. Photocrosslinkable HA were employed in combination with GelMA to study the effect of different chain lengths of the HA network on glioblastoma invasion. A number of studies reported that the absence of HA matrix bound in cell seeded hydrogels, induced increased cell migration [120,121]. Moreover, inducing hypoxia in the hydrogels increased invasiveness of the cells respect to normoxic conditions [122]. In another study, Pedron et al. incorporated cells within the liquid hydrogel precursor solution of methacrylated HA into GelMA or 4 arm-PEG hydrogels via photo-crosslinking, showing an increase of proliferation in the absence of HA in GelMA hydrogels. Further, HA incorporation also promoted clustering of cells in both hydrogels tested [123].
Photocrosslinkable HA were recently used by Ahn and colleagues to observe similarities and differences of expression of chondrogenic markers and signaling pathway proteins of human adipose-derived stem cells (hASCs) between 2D tissue culture plates and 3D microenvironments of cells encapsulated in methacrylated hyaluronic acid hydrogels [124]. The authors observed that 3D microenvironments increased chondrogenic differentiation efficiency through amplification of the p38 pathway.
Dual crosslinking of 3D HA-based hydrogels with tunable elastic modulus were also reported [125,126]. For instance, Skardal et al. fabricated HA-based hydrogels with varying stiffness, dynamically tuning the crosslinker with linear PEGDA and 4-arm PEGDA [119]. They reported that encapsulated cells with aberrant EGFR expression increased their proliferation in stiffer environments showing that targeting the tumor microenvironment extracellular matrix rather than cells, might aid to slow or prevent the progression of GBM tumors. Jin et al. suggested that only HAase expressing glioma cells showed an increased invasiveness and colonization of the hydrogels after seeding, and were able to degrade HA hydrogels, fabricated via carbodiimide chemistry [127]. Analogously, David et al. used wells containing a similar HA hydrogel, as 2D microenvironments by seeding the cells on top, to show a correlation among cancer cell lines expressing CD44 and invasiveness. Their studies showed that secretion of HA was correlated with a decreased invasiveness [128]. On the other hand, cell lines that secreted more HAse yielded to a higher number of colonies consistent with previous observations. Another work reported by David et al. utilized these types of hydrogels to investigate the influence of collagen types (type I, III, IV, V) on cancer cell invasiveness in vitro, with collagen types I and III which better supported invasion compared to other types [129].
Among the most attractive features of HA is its potential application to nanomaterials for active targeting, having a strong affinity for cell surface receptors, (i.e., CD44). Pedrosa and colleagues reported in vitro and in vivo targetableity of HA nanogel obtained by grafting a thiolated hydrophobic chain in the polysaccharide backbone [130]. By using a non-small cancer lung cell, (namely A549) which expresses high levels of CD44 receptors, they reported higher cellular uptake which they inferred to be mediated by HyA receptors and probably by CD44 receptors being overexpressed in these cells. Moreover in vivo studies, conducted in tumor bearing mice induced with subcutaneous A549 cells tumor, showed selective targeting towards tumor tissue, which was associated to the passive accumulation through EPR effect and active targeting by HyA receptor affinity.
Thus, all these studies, summarized in Table 3, demonstrate that although 2D environment studies have been easier and strategic for investigation of the cellular signaling pathways underlying responses to biochemical and physical cues, these responses are dissimilar respect to 3D microenvironments and consequently it remains crucial the need to use 3D models to better understand and investigate substrates that recapitulate an in vivo situation.
Table 3.
Applied HA hydrogels scaffolding technologies in Cancer disease.
| Chemistry | Models | Scaffold Application | Biological Assessment | Properties Assessment | Biological Assay | Ref. |
|---|---|---|---|---|---|---|
| HA-methacrylate | 2D/3D | Brain matrix-mimetic platform | 373-MG human GBM cells U87-MG human GBM cells Rat C6 glioma cells |
Swelling Elastic modulus Gel microstructure (SEM) |
Cell viability | [101] |
| HA-methacrylate | 2D/3D | Inhibition of cell migration | 373-MG human GBM cells U87-MG human GBM cells |
Time-lapse Microscopy Analysis of Cell Migration Cell Morphological Analyses |
[105] | |
| HA-acrylate HA-reactive thiols |
2D/3D | ECM-mimetic platform | PCa human prostate adenocarcinoma cells | Swelling Rheological behavior Degradation |
Cell Viability Cell proliferation Cell Morphological Analyses Cell-cell adhesion Expression of angiogenic factors |
[108] |
| HA-RGD | 3D | ECM-mimetic platform Alkylating chemotherapies drugs treatment |
GBM cell lines derived from patients | Cell proliferation Expression of BCL-2 family factors Cell Morphological Analyses |
[110] | |
| HA-methacrylate-RGD | 2D | 3D Matrix stiffness Drug resistance |
MDA-MB-231 breast cancer cells | Swelling Elastic Modulus Mesh Size |
Cell proliferation Cell Morphological Analyses |
[113,114] |
| HA-methacrylate | 2D/3D | ECM-mimetic platform | Patient-derived D456 cells U87-MG human GBM cells |
Cell proliferation Apoptosis Assay Cell Morphological Analyses Stemness markers expression |
[116] |
5. Hyaluronic acid hydrogels in the neurodegenerative diseases
CNS diseases usually cause irreversible damage with increased difficulties in healing, regeneration, and physiological restoration functions, due to the inflammatory response of astrocytes, microglia, and other glial cells. Stroke, neurological disorders, traumatic brain injury (TBI), spinal cord injury (SCI), and demyelinating diseases may cause extensive damage with permanent loss of motor skills and cognitive impairment [[131], [132], [133]]. The early stages of nerve tissue damage are associated with cell death in the affected area, neuronal cell contusion, axonal shearing, and blood vessel damage. Currently, HAs have proven to be an excellent candidate as 3D systems able to promote tissue regeneration. [134,135], playing an important role in CNS, such as in cell migration, proliferation, differentiation, and other cell behaviors, as summarized in Table 4 [136,137]. Indeed, the CD44 receptor, present on the glial surface and neuronal cells, binds HA molecules influencing the behavior of neuronal cells both in physiological and pathological conditions by activating Src family kinases (SFKs) and the focal adhesion kinase cascade (FAK) [138,139]. For example, Ballios and co-workers [140] developed an injectable, biodegradable and light-sensitive blend of hyaluronan and methylcellulose (HAMC) hydrogel. The HAMC showed increased cell survival (through presence of HA) and distribution of cells (through presence of MC) of both retinal stem cells (RSCs) and neural stem and progenitor cells (NSCs). The pro-survival mechanism of HAMC was attributed to the interaction of the CD44 receptor with HA. Pharmacological transient disruption of the retinal outer limiting membrane, combined with HAMC delivery, resulted in enhanced rod survival and visual function. Nih et al. realized “microporous annealed particle” or MAP hydrogels through a droplet microfluidic device characterized by two different channels. HA-Ac solution, pre-reacted with K, Q, and RGD peptides, was flowed into one channel, while matrix metalloproteinase (MMP)-sensitive cross-linker flowed into the second channel. In fact, MMP is an important protein involved in tissue remodeling and cell migration and invasion (Fig. 5). The authors observed that injecting a porous MAP hydrogel solution into the ischemic cavity significantly reduced the inflammatory response compared to the non-porous hydrogel. Furthermore, the porosity of the MAP hydrogel favored the migration of NPCs to the stroke site [141].
Table 4.
Applied HA hydrogels scaffolding technologies in the Neurodegenerative diseases.
| Chemistry | Models | Scaffold Application | Biological Assessment | Properties Assessment | Biological Assay | Ref. |
|---|---|---|---|---|---|---|
| NorHA | 3D | Matrix stiffness | OPCs oligodendrocyte progenitor cells | Swelling Rheological behavior |
Cell viability Cell proliferation Mitochondrial Metabolic Activity ATP/DNA quantification Cell Morphological Analyses |
[142] |
| liposome-HA | 2D/3D | Inhibition of cell migration | Rat BMSCs Old male Wistar Han rats |
Gel microstructure (SEM) Thermal properties Rheological behavior Protein quantification |
Cell viability Cell-surface markers DNA quantification Rats health parameters Efficacy in EAE rats |
[148] |
| HA-RGD HA-fibrinogen |
3D | Injection of BMSCs into the CNS | Schwann cells | Swelling Degradation Gel microstructure (SEM) Mechanical compression |
Cell proliferation Cell alignment and circularity ECM proteins expression DNA repair capacity |
[149] |
| Gelatin-HA Gelatin-HA-polydopamine |
3D | Nerve tissue engineering | Retinal progenitor cells | Gel microstructure (SEM) Swelling Rheological behavior Degradation Mechanical compression |
Cell viability Inflammatory factors expression Apoptotic factors expression Adhesion factors expression Retinal neuronal differentiation |
[150] |
| HA-microporous annealed particle (MAP) | 3D | ECM-mimetic platform | NPCs neural progenitor cells | Rheological behavior | Ki67, DCX, NPCs, GFAP, Iba1 protein levels | [141] |
| HA | 3D | Brain tissue repair after stroke | Neural stem cells hESC-NS obtained from H6 hESC line Adult male wild-type Wistar rats |
Cell viability FluoroMyelin staining CAM, NESTIN, SOX2 and GFAP protein levels hPDGFRα, OPCs and hTUJ1 protein levels Cavity and glial scar analyses Behavioral analyses Inducing oligodendrocyte differentiation |
[151] |
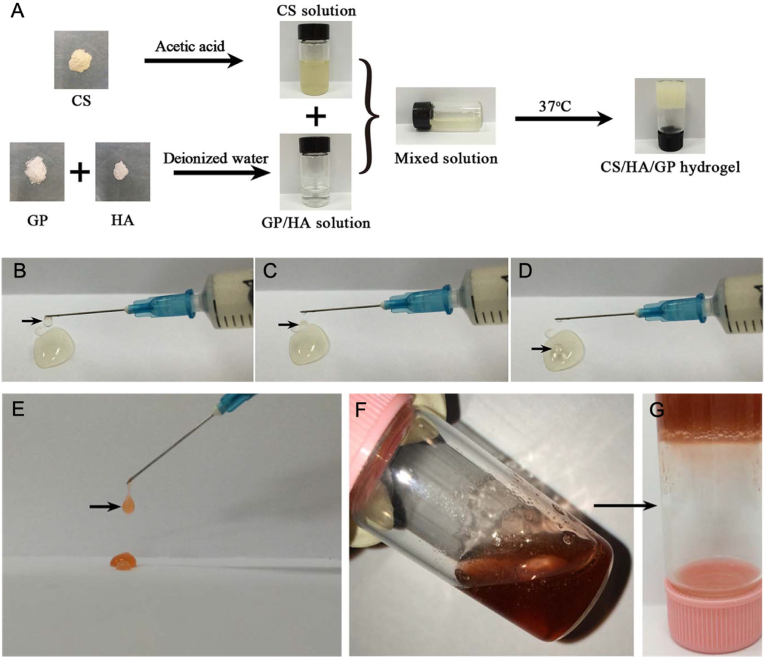
Fig. 5.
Schematization of the preparation of the hydrogel where A) the precursor solution of CS, GP, and HA, their solution at a low temperature of 4 °C, and the formed hydrogel at 37 °C are shown. (B–D) Photographs showing how the precursor solution was ejected to form droplets using a syringe. E) Representative photograph showing how the hydrogel conjugated with DOX precursor solution was ejected to form droplets using a syringe, (F) Image showing the hydrogel conjugated with DOX precursor solution at a temperature of 4 °C which at 37 °C (G) formed the hydrogel. Reprinted and modified with permission from Ref. [175].
In demyelinating diseases, there are still no available experimental protocols able to deliver remyelination solutions. This has motivated many researchers to investigate new strategies to drive cellular bioactivity, specifically of oligodendrocytes, whose main function is to regenerate myelin. To this purpose, Unal et al. studied the effects of the mechanical properties on oligodendrocyte progenitor cells (OPCs) encapsulated in Nor-amine (5-norbornene-2-methylamine) HA hydrogels (NorHA) [142]. NorHAs were formed by crosslinking norbornene groups with a dithiol crosslinker (DTT) through thiophene mediated radical addition using a LAP photoinitiator that absorbs strongly at 365 nm. The stiffness of the NorHA hydrogel reflected the characteristics of physiological tissues (200–2000 Pa). The authors observed that the hydrogel pore size decreased increasing the stiffness showing that cell proliferation and survival were closely related to low substrate stiffness. Furthermore, low stiffness hydrogel enhanced larger cell spheroid formation.
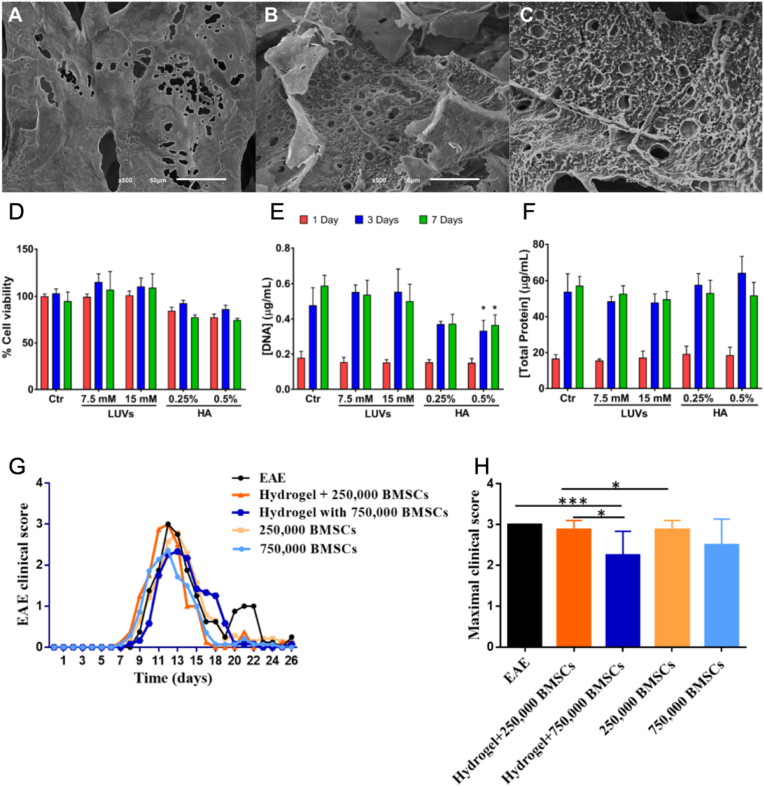
Many studies in neurodegenerative diseases are currently focused on stem cells as a way to restore brain functions [143,144]. Several HA-based hydrogel crosslinked with liposomes have been developed [[145], [146], [147]]. For instance, Ferreira et al. designed a hydrogel composed of phospholipids using HA as a cellular carrier [148]. His work aimed to study multiple sclerosis (SM) by evaluating the biocompatibility of the hydrogel in vitro, using encapsulated bone marrow mesenchymal stem cell (BMSC), and in vivo by injecting hydrogel containing BMSCs into the intracerebroventricular area of rats with autoimmune encephalomyelitis (EAE). The study was carried out on different hydrogels composed of 1% HA or by 1% HA and 7.5 mM or 15 mM of liposomes. In vitro cell viability analysis confirmed the biocompatibility of the system, even though HA alone induced a slight cell viability reduction. No substantial differences were observed in vivo between experimental rat groups in terms of locomotor and behavioral performance, but a reduction in symptoms was observed after about two weeks using carrier hydrogels (Fig. 2).
Fig. 2.
A-C) Representative SEM micrographs of hydrogels composed of 1% HA (A) and with 7.5 mM (B) or 15 mM (C) of liposomes. Scale bar 50 μm. D-F) Analysis of BMSCs at different liposomes concentrations (7.5 and 15 mM) and HA; 0.25 and 0.5%): D) cell viability, E) cell proliferation and F) total protein synthesis after 1, 3, and 7 days of culture. G-H) In vivo experiments reporting cumulative disease disability (G) and maximum clinical score () after ICV injection of the hydrogel incorporating BMSC cells in experimental autoimmune encephalomyelitis (EAE) rats compared to the control group (EAE animals without treatment) and the hydrogel incorporating BMSC cells. Reprinted and modified with permission from Ref. [148].
Hydrogels of alginate, fibrin, HA, and/or RGD peptide have been realized to generate 3D tissue scaffolds containing living Schwann cells for potential nerve tissue engineering applications. Ning and colleagues observed high cell viability, proliferation, expression of cellular proteins (DRG neurites, S100, laminin) and oriented Schwann cells and neurites, encapsulated on the bioprinted hydrogels, showing great potential to support regeneration of peripheral nerves after injury [149]. Gelatin-HA (Gel-HA) hydrogels formed through a Michael-type addition reaction with or without the introduction of mussel-inspired polydopamine (PDA), were also used for biomaterial-mediated retinal progenitor cell (RPC)-based transplantation therapy. The authors conducted studies in 2D (seeding cells on the hydrogels) and 3D (encapsulating the cells) and reported how Gel-HA hydrogel promoted RPCs proliferation while Gel-HA-PDA directed RPCs to preferentially differentiate toward retinal neurons via activation of a5β1-phosphatidylinositol-3-kinase (PI3K) pathway [150].
6. Hyaluronic acid hydrogels in tissue regeneration
Tissue regeneration represents an appealing solution for the repair of organs damaged by trauma, infection, or disease. Elasticity and traction resistance properties are essential to produce artificial tissues that may replace epidermis, vessels, or bone components. Furthermore, these bioengineered tissues aim not only to be replacement systems but also to be systems that can promote cell proliferation and differentiation, restoring ECM homeostasis [152]. In terms of tissue regeneration, HA has shown to be very promising, as reported in Table 5 [153]. Burdick's group reported photocrosslinkable NorHA with human platelet lysate (PL) [153,154]. They showed that assembling microgels to produce injectable granular hydrogels, featured embedded fibrillar networks which resembled the hierarchical architecture of ECM. Their formulation demonstrated tunable chemical and physical properties with improved in vitro culture of MSCs under serum-free conditions, and shear-thinning and self-healing properties, enabling 3D printing. NorHA hydrogels, reinforced with polycaprolactone (PCL) microfibers produced via melt-electrowetting [155] were also reported in relation to studies of the chondrogenesis of encapsulated MSCs with the variation of the mechanical properties of the construct. The authors showed that by varying both the macromer concentration and crosslinker concentration, modified the rigidity of the NorHA hydrogels alone, with softer (more loosely crosslinked) hydrogels improving cell viability and supporting neocartilage formation in vitro. Addition of PCL meshes improved the mechanical properties of the hydrogels, without reducing the ability of cells to synthesize and distribute ECM. Ex-vivo integration of these composites showed excellent tissue integration within explanted cartilage rings relative to acellular controls, to support neocartilage formation.
Table 5.
Applied HA hydrogels scaffolding technologies in Tissue regeneration.
| Chemistry | Scaffold Application | Biological Assessment | Properties Assessment | Biological Assay | Ref. |
|---|---|---|---|---|---|
| HA (NorHA) with human platelet lysate (PL) | ECM-mimetic platform | MSCs human mesenchymal stromal cells | Degradation Mechanical compression Rheological behavior Jammed microgel morphology |
C FITC-dextran incubation Cell Morphological Analyses |
[154] |
| HA (NorHA) with human platelet lysate (PL) | Neocartilage formation | MSCs human mesenchymal stromal cells | Mechanical compression MicroCT | Cell viability Cchondrogenic gene expression Neocartilage formation analyses |
[155] |
| HA-methacrylate | Articular cartilage repair | jbMSCs juvenile bovine mesenchymal stem cells Male juvenile Yucatan minipigs |
Scaffold microstructure (SEM) Degradation Mechanical properties |
DNA quantification Micro-computed tomography (μCT) Type II collagen staining MSC Infiltration analyses SDF-1α and TGF-β3 release |
[156] |
| HA-methacrylate | Bone regeneration | PBMCs rabbit peripheral blood mononuclear cells | Swelling X-ray diffraction (XRD) Scaffold microstructure (SEM) Mechanical properties |
Cell viability Histomorphometric assessment |
[160] |
| HA-methacrylate Gelatin-HA |
Cartilage tissue engineering | Chondrocyte-Spheroids | Rheological behavior Mechanical properties Scaffold microstructure (SEM) |
Cell viability Ki-67, Col II, and Col X protein levels Col2A1, Sox-9, Col10A1, and HIF-1a gene expressions |
[161] |
| HA-methacrylate | Diabetic wound repair | Human foreskin fibroblasts | Nuclear magnetic resonance (NMR) Swelling Mechanical properties Rheological behavior Lap shear t/burst pressure tests Protein quantification |
Cell proliferation Analysis of Cell Migration TNF-α and IFN-γ gene expression for Macrophage polarization F4/80 + CD206+DAPI + protein levels |
[46] |
Electrospun cell-free fibrous hyaluronic acid (HA) scaffold were also developed to specifically enhance cartilage repair [156]. The authors showed that using a combined release effect of stromal cell-derived factor 1 alpha (SDF-1α) and a transforming growth factor beta 3 (TGF-β3) improved cell recruitment and matrix deposition by mesenchymal progenitor cells, seeded on the scaffolds. However in vivo SDF-1α release caused inhibition of neo-cartilage tissue regeneration.
Ren et al. reported on how the modulation of molecular weight of HA modified by the maleimide group (HA-MAL) for cartilage regeneration can influence stemness properties of encapsulated BMSCs [157]. They observed that differences in the MW affected the stiffness of their hydrogels. In particular, they showed that a high MW HA backbone showed to influence stiffness and degradation rate of hydrogels as well as to induce BMSCs differentiation into cartilage by activating TRPV4 channels and elevated expression of type II collagen and SOX9, due also to the presence of physical entanglements and physical cross-linking points between molecules. Low MW HA hydrogels instead maintained stemness properties without promoting cell differentiation by activating the Wnt/β-catenin pathway, as the mechanical strength of the hydrogel was not enough to promote opening the TRPV4 channel in the BMSC plasma membrane.
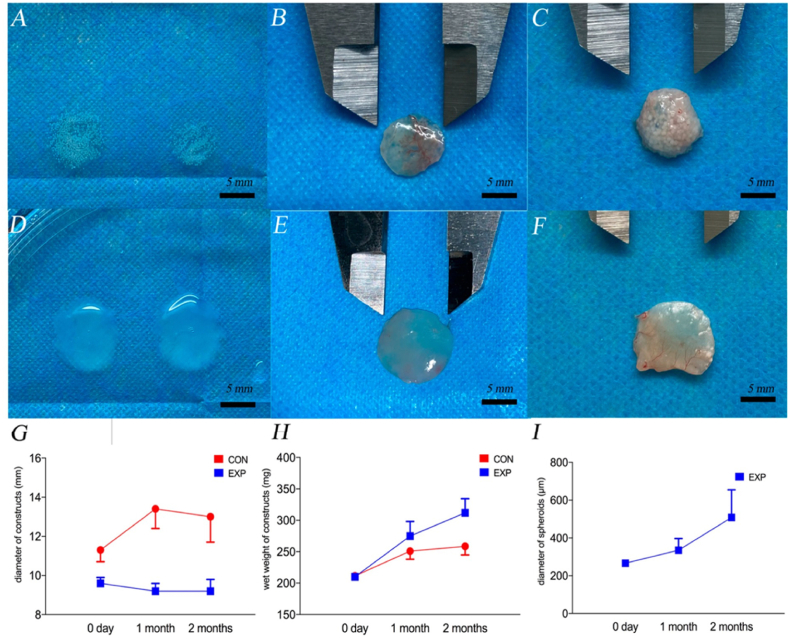
A recent work carried out by Zhang et al. reported different types of HA for bone regeneration applications [158]. The authors realized four HA hydrogels: I HA hydrogel with no additions; II HA hydrogel with nano-hydroxyapatite; III HA hydrogel with chitosan; and IV HA hydrogel with nano-hydroxyapatite and chitosan [159]. The photoinitiating system was driven by riboflavin, dimethylaminoethyl methacrylate (DMAEMA), and diphenyliodonium chloride. Nano-hydroxyapatite and chitosan increased the mechanical properties three-fold and the osteogenic potential compared with the control group. Cell viability assays were performed on rabbit peripheral blood mononuclear cells (PBMCs) seeded on the scaffolds. HA-chitosan blends (Group III) showed the highest cell viability. In vivo, these hydrogels were used to repair a surgical defect in the distal femoral head of a New Zealand white rabbit model. After eight weeks, the combination of HA with chitosan and nano-hydroxyapatite showed the highest regeneration capacity. However, these hydrogels present higher mechanical properties, due to the addition of nano-hydroxyapatite and chitosan and the use bioactive materials such as nano-graphene oxide, requires still testing of the osteogenic potential [160]. Studies performed on gelatin methacrylate (GelMA) and HAMA demonstrated how these systems support proliferation and cell survival of chondrocyte spheroids compared to a normal cell suspension. Fabrication of hydrogel constructs embedding spheroids showed extensive fusion between the spheres after two months compared to the construct made with dispersed cells (Fig. 3). The proliferative abilities of the spheroids were confirmed by increased Ki67 expression, whereas a positive COL-X level indicated that fewer chondrocytes were de-differentiated [161].
Fig. 3.
Representative views of the spheroid-laden construct before implantation EXP (A), 1 month after implantation (B), and 2 months after implantation (C). View of the cell-laden construct before implantation CON (D), 1 month after implantation (E), and 2 months after implantation (F). Analysis of (G) the changes in the diameter of constructs, (H) the wet weight of constructs, and (I) the diameter of chondro-spheroids. The blue spots and lines represent the EXP group, and the red spots and lines represent the CON group. Reprinted with permission from Ref. [161].
Methacrylated hyaluronic acid was bioprinted by Poldevaart et al. [162], showing intrinsic osteogenicity in relation to the gel concentration, suitable primary cell survival and exceptional spontaneous osteogenic differentiation in vitro.
Injectable forms of hydrogel for bone tissue regeneration using MC3T3 bone cells were designed by Noh et al. The developed hydrogels based on HA, hydroxyethyl acrylate (HEA) and gelatin-methacrylate (HA-g-pHEA-gelatin) displayed excellent biocompatibility and good printability. The bone cells encapsulated in the bioink showed good viability [163].
Human tissue-engineered skin substitutes based on HA has also been investigated for treatment of severe burn patients and commercialized [164] being easy to handle and of human (skin) origin [165,166]. Recently, Sánchez et al. reported a preclinical in vivo study using a fibrin–hyaluronic acid (HA-Skin), which exhibited improved values of cutaneous homeostasis and epidermal barrier function respect to healthy mouse skin showing to be a valid alternative to an autograft (the gold standard treatment) [167]. Dong et al. engineered a hydrogel composed of poly(ethylene glycol) diacrylate (HB-PEGDA) and thiol-functionalized HA (HA-SH), with or without RGD peptide to promote cell adhesion. Adipose-derived stem cells were encapsulated in a PEG-HA-RGD hydrogel. In the PEG-HA hydrogels, cells appeared with a sphere morphology with minimal vinculin secretion, whereas they spread and increased vinculin expression in the hydrogels with RGD peptide. RGD peptide promotes cell spreading, metabolic activity and proliferation, without altering protein levels of stemness markers such as SOX-2, Oct-4 and Kif-4 [168].
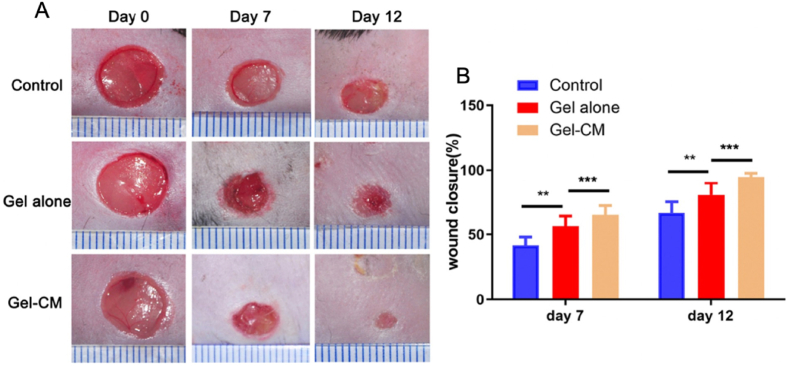
HA hydrogels have also been used to encapsulate stem cells to promote wound healing. In a recent study, Zhang et al. engineered an HA-based hydrogel grafted with methacrylic anhydride and N- (2-aminoethyl)-4- [4-(hydroxymethyl)-2-methoxy-5-nitrophenoxy]-butanamide (NB) groups to encapsulate a conditioned freeze-dried amnio-derived medium (AM-CM). The hypothesis advanced by the authors was based on the possibility of creating bonds between o-nitrosobenzaldehyde groups and amine groups on the tissue surface. The hydrogel showed excellent mechanical properties, high elasticity, favorable biocompatibility, and sustained release of AM-CM. Hydrogel biocompatibility was validated in vitro by cell viability assays performed on human umbilical vein endothelial cells (HUVECs). HAMA hydrogel conjugated with AM-CM also showed the ability to promote macrophage polarization toward an M2 phenotype with increased CD206 marker expression. In vivo tests were performed on db/db mice. Wounds on the back of the db/db mice were realized. The authors observed an increase in regeneration of the damaged tissues in the presence of AM-CM-containing hydrogels compared to hydrogels without AM-CM. They also observed an increase in CD31 levels that was correlated with an increase in angiogenesis (Fig. 4) [46].
Fig. 4.
A) In vivo representative images of the achievement of the hydrogel conjugated with AM-CM (Gel-CM) in promoting wound healing in db/db mice compaing a control group, a hydrogel alone group (Gel alone), and a Gel-CM group, observed at day 0, day 7, and day 12 post wounding. B) Quantitative analysis of wound closure rate of the three groups at different time points show the markedly greater wound closure rate in the Gel-CM group respect to the others which increased after 12 days. Reprinted with permission from Ref. [46].
7. HA in drug delivery
The development of fast and accurate targeting drug delivery systems as an alternative to conventional drug formulations has grown steadily over the years, mostly to address challenges associated with inadequate local drug availability and delivery sites [169]. Hydrogels as drug delivery systems (as summarized in Table 6) represent an excellent material due to a number of significant advantages such as a high-water content (which aids to easily encapsulate hydrophilic drugs), reduced risk of drug denaturation and aggregation upon exposure to organic solvents and the presence of a crosslinked network which aids to avoid permeation of proteins which could cause premature degradation of bioactive therapeutics [170]. For instance, HA and derivatives used as drug carriers, have shown to contribute to drug thickening, to react with other drugs to form conjugates, to sustain release, and improve drug targeting. In particular, injectable hydrogels have been developed as a promising and successful system due to the advantage to minimize invasiveness into target sites and the ability to be used for irregularly shaped sites [171]. For example, Loebel et al. [172], validated an injectable and printable hydrogel as a therapeutic agent delivery system, which exhibited shear-thinning and self-healing behavior. They first synthesized β-CD-modified hyaluronic acid (HA-g-β-CD) and adamantine-modified HA (HA-g-AD), which were mixed to form a gel through host and guest interaction, and which was tested in vivo. To prove cell delivery, the authors employed endothelial progenitor cells (EPCs) encapsulated within these hydrogel and injected into infarcted myocardial tissue of male adult Wistar rats, showing extended retention time, improved vascularization, and reduced scar tissue formation. Subsequent methacrylatetion of the hydrogel increased the mechanical properties of the constructs. Moreover thiol-modified fluorophores conjugated with peptides aided in monitoring degradation of the hydrogels subcutaneously injected into mice. Kharkar et al. [173], developed a hydrogel realized by Michael-type reaction between thiol groups and maleimide groups using aryl-thiol functionalized PEG (PEG-4-MPA, or PEG-4-PD-MPA that is a photodegradable PEG-4-MPA) and maleimide functionalized PEG or heparin (PEG-2-MI or Heparin-MI). During hydrogel formation, they entrapped proteins (i.e., growth factors including fibroblast growth factor FGF-2), cytokines, and immunomodulatory agents via heparin-associated receptor-ligand interactions which were controllably released by external stimuli of light and reducing environment. The authors showed to be able to successfully maintain the bioactivity of primary human aortic adventitial fibroblasts (AF) cells as well as proliferated the AF cells by protein release.
Table 6.
Applied HA hydrogels scaffolding technologies in Drug Delivery.
| Chemistry | Scaffold Application | Biological Assessment | Properties Assessment | Biological Assay | Ref. |
|---|---|---|---|---|---|
| HA-methacrylate HA-β-cyclodextrin MeHA-β-cyclodextrin HA-1-adamantane aceti MeHA-1-adamantane aceti |
Hydrogel protocols for cell and drug encapsulation | MSCs human mesenchyme-mal stromal cells | Fluorescent derivatization Rheological behavior Protein quantification Biomolecule release |
Cell viability | [172] |
| HA-adipic dihydrazide (ADH) | Formulation to attract glioma cells Anticancer drug delivery |
U87-MG human GBM cells | Nuclear magnetic resonance (NMR) Rheological behavior In vitro drug release |
Cell viability Boyden chamber for chemotactic invasion Cloning rings for invasion assay CD44 and MMP9 protein levels |
[174] |
| HA-chitosan/β-glycerophosphate (CS/HA/GP) | Hydrogels for pH sensitive drug release | Hela cancer cells | Rheological behavior Fourier transform infrared (FTIR) Scaffold microstructure (SEM) In vitro drug release Thermal sensitivity and injectability |
CD44 protein levels | [175] |
| S-NSA/A-HA S-NSA/A-HA/cisplatin (CDDP) CS-NSA/A-HA/doxorubicin (DOX) CS-NSA/A-HA/CDDP/DOX |
Hydrogel for the pH-responsive Co-delivery | A549 human lung cancer cells | Drug loading efficiency (DLE) Drug encapsulation efficiency (DEE) In vitro drug release FT-IR spectra Zeta potential values of hydrogel |
Cell viability Cell Morphological Analyses |
[180] |
| HA-bisphosphonate HA-BP-metal-organic framework (MOF) HA-BP·MOF@DOX |
Metal-organic framework (MOF) as a carrier | CT-26 colon carcinoma cells | Nuclear magnetic resonance (NMR) Scaffold microstructure (SEM) |
Cell viability Caspase-3 protein levels In vivo antitumor efficacy by analysis of tumor size Histological analysis |
[188] |
| HA-dopamine (DA) HA-3-(4-hydroxyphenyl) propionic acid (HPA) HA-DA-HPA |
Injectable hydrogel for neural regeneration | Human GFP-MSC cells Human iPSC-derived NSCs |
Fourier transform infrared (FTIR) Nuclear magnetic resonance (NMR) Rheological behavior Scaffold microstructure (SEM) |
Cell viability Pax6, Nestin and βIII-Tubulin gene expression |
[189] |
Kasapidou et al. developed a matrix of HA-based hydrogel with different cross-linking density adipic acid dihydrazide containing a soluble chemoattractant, able to attract glioma cells, and a chemotherapeutic agent [174]. The authors observed that the use of a small human urotensin II (hUII) peptide chemokine can attract U87MG cells. In addition, the combination with the chemotherapeutic agent DOX also demonstrated a significant cytotoxic effect by reducing cell viability to 80% after 48 h.
Zhang et al. fabricated injectable solutions based on chitosan (CS)/HA/β-sodium glycerophosphate (GP), able to gel at body temperature and acidic pH due to the hydrogen bonds that HA carboxyl groups form with protonated amino groups of chitosan [175]. The acidic pH is a characteristic condition of the tumor mass [176]. Furthermore, mixing the chemotherapeutic doxorubicin (DOX) did not alter the physic-chemical characteristics of the hydrogel system, allowing its complete solubilization, and representing a system with a powerful attraction to cancer cells, in this case, a HELA cell line (Fig. 5).
HAs have also been conjugated to the surface of Pluronic® F-127 micelle nanoparticles (NPs) with a suberoylanilide hydroxamic acid (SAHA), which has been approved in clinical trials for cancer therapies and for the treatment of lymphoma and myelodysplastic syndromes [177]. In particular, Edwards and colleagues adopted this approach as HA acts as a ligand for the CD44 receptor and to obtain a rapid SAHA delivery system, showing that their NPs containing these drugs were most efficient in 3D tumor models of endometrial cancers.
HA nanoconjugated materials with pH-responsive properties have been formulated by conjugating the polymer with mesoporous silica NPs to carry anticancer DOX drugs. The ability of these injectable hydrogels to gel at acidic pH allowed injection of the drug directly into the tumor mass circumscribing its effect. Chen et al. demonstrated the biocompatibility of these biopolymers with healthy human embryonic kidney 293 cells, while by DOX transport they induced the death of breast cancer cells SKBR3 [178]. Issues in using hydrogels for drug delivery reside in the lack of precise control over drug release. In this regard, a detailed work on the hydrogels drug delivery capabilities has been recently carried out by Jo et al. The authors used a hydrogel derived from HA and diselenide based cross-linker. Hydrogels were rapidly formed via a Diels-Alder reaction between norbornene-functionalized HA (HA-Nb) and bifunctionalized diselenide with tetrazine (Tz), adding then DOX and indocyanine green (ICG). The release capacity of DOX in the presence and absence of ICG was studied under five different conditions: physiological environment (PBS, pH 7.4), acidic environment (PBS, pH 5), reducing microenvironment (10 mmol DTT), oxidizing environment (0.5% H2O2), and NIR-light irradiation (15 min). The data showed that ICG facilitates DOX delivery, and that drug release was almost zero in PBS pH 7.5. The pH-dependent properties promoted the protection of healthy cells. The antitumor activity of the system was also confirmed by cell viability assays performed on breast cancer cell line (BT-20) and n HEK-293 cells [179]. Also hydrogels developed from chitosan modified by nitrosalicylaldehyde and aldehyde HA (CS-NSA/A-HA) showed a very good ability to release antitumor drugs such as Cisplatin (CDDP) and DOX, with a significant reduction of A549 Human lung cancer cells cell viability in a range of concentrations between 6.25 and 100 μg/mL [180].
Surgical resection is the treatment of choice for breast cancer. However, post-operative recurrences are still very frequent and the lack of selectivity for tumor cells and the rapid drug clearance promote the occurrence of collateral effects [181,182]. Nanoparticle (NP)-based drug delivery systems represent one of the recent drug delivery strategies [183]. However, their low solubility in water, their susceptibility to pH changes and hypoxic conditions typical of a tumor environment, hampers their application [184,185]. Leng et al. optimized a system featuring paclitaxel (PTX) and epirubicin (EPB) nanoparticles, prepared by loading the drug into the tri-block copolymer Poly(e-caprolactone)-poly (ethylene glycol)-poly(e-caprolactone) (PCL-PEG-PCL or PCEC) and adding them in an injectable HA-hydrogel (PPNPs/EPB@HA-Gel). In vitro studies on 4T1 and MCF-7 proved inhibition of cell proliferation and migration, while in vivo studies in mice demonstrated the degradative capacity of HA-Gel in 9 days and a significant increase in cell apoptosis [186]. The same limitations of NPs have been encountered in the development of metal-organic frameworks (MOFs) for drug delivery [187]. However, the crosslinking between bisphosphonate-modified HA (HA-BP) and zinc, in combination with DOX (HA-BP MOF@DOX), showed pH-and ATP-responsive drug release and an efficient reduction of CT-26 cells viability [188].
Drug delivery through HA hydrogels is a topic of great interest also in the field of CNS diseases. A novel enzymatically crosslinked injectable hydrogel comprising HA, dopamine (DA), and 3-(4-hydroxyphenyl) propionic acid (HPA) was developed by Nguyen et al. The gelling process was completed thought hydrogen peroxide (H2O2) as an oxidant and horseradish peroxidase (HRP) as a catalyst. The innovative factor in these hydrogels was the encapsulation of the DA neurotransmitter able to restore dopaminergic neurons, which may represent a promising therapy for the treatment of PD. The synthesized hydrogels showed no toxicity under 0.75 μmol ml-1 of H2O2 [189]. HA-based hydrogels were also designed to reduce inflammatory processes triggered by traumatic brain injury (TBI) in order to counteract cell death resulting from chronic activation of microglia and macrophages. Jeong et al. studied the effect of dexamethasone-conjugated HA (HA-DXM) incorporated in e poly (ethylene) glycol-bis-(acryloyloxy acetate) (PEG-bis-AA) hydrogel. DX release was facilitated by the presence of hyaluronidase/esterase enzymes compared to the hydrogel solubilized exclusively in phosphate-buffered saline. The hydrogel, placed in the cranial cavity of Male Sprague Dawley rats with TBI showed reduced cranial cavity lesions and a significant decrease of proinflammatory cytokines expression, such as IL1β, TGFβ1, TNFα [190].
8. Conclusion
Over the last decades, the role of HA has increased exponentially, showing attractive and unique physical and biological properties. A mounting number of in vitro and, although fewer, in vivo studies highlight the therapeutic effects of HA and its versatile applications varying from tissue engineering, drug delivery to medical devices. However, further investigation of HA metabolism, fragmentation, mechanisms and associated downstream pathways are still required. Additional understanding of all these mechanisms with their biological responses and signaling pathways will guide the design of future hydrogels as well as laying the foundation to further clinical applications, and trials. An unexplored topic involves the missing link between HA characterization (i.e., size, length and localization) and the modulation of signals with different HA sizes, as well as the mechanistic roles of different molecular weight dependant HA in various ECM. Understanding these mechanisms may expand and advance the application of HA-based drug/formulation, oncological and neurodegenerative disease studies and tissue regeneration.
Physical hydrogels have been utilized in targeted therapies to promote controlled-prolonged release of drugs, but less to study cancer cell behaviors in vitro. Noteworthy, there is a dearth of protocols for the use of HA-based hydrogel systems as a vehicle for in situ drug delivery in the CNS. Benefits in using physical crosslinking are the absence of undesired residual or unreacted agents due to use of initiators or catalysts. However, the control of the molecular interactions represents still a challenge. Moreover, physical hydrogels are mechanically weaker in preserving themselves, for a long time, from changes in pH and temperature, when used in vivo. Chemical hydrogels are commonly exploited through mostly the Michael-addition reaction and photocrosslinking methods. These hydrogels have shown improved mechanical stability and properties thanks to the covalent bonds formed but are limited in responses to external cues. Properties such as its high biocompatibility, biodegradability, chemical versatility, gelation time, and byproducts of the crosslinking reaction are key aspects in choosing the appropriate method to obtain HA. For instance, in studies that involve cancer cell encapsulation, the gelation time represents a critical step to obtain 3D HA hydrogels.
Choosing the appropriate chemistry is crucial also in three dimensional (3D) bioprinting which recent progress, has shown promising advances using HA in the treatment of several disorders. However, HA alone fabricated via 3D printing can present weak printing properties and difficulties in extrusion of filaments, although improvement of rheological properties has been obtained by combining HA with other natural or synthetic polymers [[191], [192], [193]]. For example, recently, Wan and colleagues blended multicrosslinked HA bioprinted hydroges of maleated sodium hyaluronate with high substitution of the acrylate group and thiolated sodium hyaluronate showing good cytocompatibity and cell adhesion support [193]. Marchal's group reported a hybrid HA-based bioink mixed with a thermoplastic polymer (PLA) for chondrocyte growth and maintenance [194]. The PLA contributed to increase the mechanical properties of the bioink to support load forces exerted in native tissue. HA bioprinting has also been reported in pancreatic islet implantation in the study of diabetes [195], and in the fabrication of intelligent patches for the release of exosomes derived from human mesenchymal stem cells (hMSC-EXOs) in wound care. Controlled release of hMSC-EXOs showed to promote the proliferation and migratory capacities of both fibroblasts and endothelial cells by stimulating the expression of vascular endothelial growth factor to endothelial cells [196]. thiolated HA with methacrylated collagen has been used to bioprint tumor organoids for screening of different drugs [197]. These technically similar but functionally different approaches highlight not only the biocompatibility of HA, but, above all, they underline how its efficient degradability makes it an extremely versatile biopolymer. Moreover, different concentrations of HA can be bioprinted, preserving good printability. HA has been also bioprinted in mixtures, such as with GelMA, to produce artificial, biomimetic cartilage-like tissue. In fact, as cartilage tissue does not share the same content of HA in every area, the suspension of chondrocytes in mixtures with variable stiffness enables the creation of a modulable cartilage tissue [198]. 3D-printed HA matrices have contributed to identify an intrinsic relationship between adipocytes and breast cancer cells. Indeed, Horder et al. realized a 3D-printed breast cancer model consisting of a HA layer of human adipose-derived stromal cell (ASC) spheroids driven to differentiation into adipose microtissues, and a HA layer containing MDA-MB-231 cells. They observed a reduction in the lipid content induced by the tumor cells and a remodeling of the ECM within the adipose tissue, with increased expression of fibronectin, collagen I and collagen VI [199].
A deeper understanding should focus on how the physiochemical, mechanical and structural cues of the hydrogels regulate the phenotype, function and fate of cells. Moreover, crosslinking methods to lower cell damage arising for example from UV exposure such as click chemistry, should be explored. Further biomimetic 3D platforms could be obtained by adding other ECM components, such as fibronectin, collagens, or glycosaminoglycans in the hydrogels. In fact, as one of the main components of the ECM, HA can facilitate cellular homeostasis recovery and allow obtaining data more representative of different physiological conditions.
Often different approaches to study the same diseases make it difficult to achieve a common goal such as the identification of efficient therapy. HA-based hydrogels can allow also to decouple biophysical cues from biochemical cues in the cell microenvironment which will enable to probe cancer cell phenotype, study mechanisms of cancer progression, and response to treatment with the potential to identify therapeutic targets for different diseases. Moreover, respect to synthetic hydrogels, HA sustains probing of cell surface receptors typically overexpressed in different types of cancer cells, providing unique opportunities to study mechanisms of cancer progression, and response to treatment. The use of bioprinting and microfluidics could lead to potential innovative 3D in vitro models with HA that involves the implications of the immune system in cancer and in neurodegenerative diseases.
Despite significant progress, translation into the clinic applications of HA hydrogels is still inadequate. Unforeseen side effects and obstacles aside from incorrect administration hampers their applications, thus a systematic and comprehensive for material optimization is needed as well as a deeper understanding of the interactions between cells and matrix.
The HA versatility and the possibility to use it for the study of different pathologies make HA-based hydrogels the most promising and innovative approach for the coming decades.
Author contributions
Conceptualization: MG, OU, BC; Funding acquisition: BC, GG; Project administration, Resources, Supervision: BC; Roles/Writing - original draft: MG, BC; Writing - review & editing MG, OU, IEP, LM, BC.
Funding
The research leading to these results has received funding from AIRC under IG 2021 - ID. 26328 project – P.I. Cortese Barbara. The authors are also grateful to the “Tecnopolo per la medicina di precisione” (TecnoMed Puglia) - Regione Puglia: DGR n.2117 del November 21, 2018, CUP: B84I18000540002 and “Tecnopolo di Nanotecnologia e Fotonica per la medicina di precisione” (TECNOMED) - FISR/MIUR-CNR: delibera CIPE n.3449 del 7-08-2017, CUP: B83B17000010001.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Cortese Barbara reports financial support was provided by Airc Italian Foundation for Cancer Research. Gigli Giuseppe reports financial support was provided by Puglia Region.
Acknowledgments
We thank Irene Iacuitto, Giovanna Loffredo and Manuela Marchetti for practical administrative support. We also thank Domenico Sisto for useful technical support.
Data availability
No data was used for the research described in the article.
References
- 1.Popova N.V., Jucker M. Cancers. 2022;14(1) doi: 10.3390/cancers14010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su M., et al. Tissue Cell. 2021;69 doi: 10.1016/j.tice.2020.101444. [DOI] [PubMed] [Google Scholar]
- 3.Shabani Z., et al. Int. J. Biol. Macromol. 2021;171:366. doi: 10.1016/j.ijbiomac.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Theocharis A.D., et al. Adv. Drug Deliv. Rev. 2016;97:4. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Colombo E., Cattaneo M.G. Int. J. Mol. Sci. 2021;22(4) doi: 10.3390/ijms22041633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nunes A.S., et al. Biotechnol. Bioeng. 2019;116(1):206. doi: 10.1002/bit.26845. [DOI] [PubMed] [Google Scholar]
- 7.Barbosa M.A.G., et al. Cancers. 2021;14(1) doi: 10.3390/cancers14010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barreiro Carpio M., et al. Front. Bioeng. Biotechnol. 2021:9. doi: 10.3389/fbioe.2021.773511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayvaz I., et al. J. Gastrointest. Cancer. 2021;52(4):1294. doi: 10.1007/s12029-021-00772-1. [DOI] [PubMed] [Google Scholar]
- 10.Flagelli A., et al. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.759982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiaee K., et al. Cells. 2021;10(12) doi: 10.3390/cells10123422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauhan D., et al. Oncology. 2022;100(6):354. doi: 10.1159/000524302. [DOI] [PubMed] [Google Scholar]
- 13.Huang J., et al. Semin. Cell Dev. Biol. 2022;(128):137. doi: 10.1016/j.semcdb.2022.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Al Halawani A., et al. Int. J. Mol. Sci. 2022;23(6) doi: 10.3390/ijms23063389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanes B., Rainero E. Cancers. 2022;14(6) doi: 10.3390/cancers14061433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed M.J., et al. Tissue Barriers. 2019;7(4) doi: 10.1080/21688370.2019.1651157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan Y., et al. Cells. 2019;8(3) doi: 10.3390/cells8030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radulescu D.M., et al. Polymers. 2022;14(4) doi: 10.3390/polym14040799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh M., et al. Adv. Exp. Med. Biol. 2019;1174:371. doi: 10.1007/978-981-13-9791-2_12. [DOI] [PubMed] [Google Scholar]
- 20.Hinderer S., et al. Adv. Drug Deliv. Rev. 2016;97:260. doi: 10.1016/j.addr.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Stowers R.S. 2021. Cells Tissues Organs; p. 1. [DOI] [PubMed] [Google Scholar]
- 22.Vazirzadeh M., et al. Stem Cell Rev Rep. 2022;18:2262–2278. doi: 10.1007/s12015-022-10362-8. [DOI] [PubMed] [Google Scholar]
- 23.Alven S., Aderibigbe B.A. Int. J. Mol. Sci. 2020;21(24) doi: 10.3390/ijms21249656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voci S., et al. Gels. 2021;7(2) doi: 10.3390/gels7020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almoshari Y. Gels. 2022;8(3) doi: 10.3390/gels8030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huynh A., Priefer R. Carbohydr. Res. 2020;489 doi: 10.1016/j.carres.2020.107950. [DOI] [PubMed] [Google Scholar]
- 27.Astudillo-Ortiz E., et al. Materials. 2021;14(23) doi: 10.3390/ma14237325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliva F., et al. Cells. 2021;10(11) doi: 10.3390/cells10113081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi W., et al. Biofabrication. 2022;14:014107. [Google Scholar]
- 30.Xu X., et al. Taiwan. J. Obstet. Gynecol. 2021;60(6):1031. doi: 10.1016/j.tjog.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Perez L.A., et al. Biomedicines. 2021;9(9) doi: 10.3390/biomedicines9091113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sikkema R., et al. Materials. 2021;14(17) doi: 10.3390/ma14174982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lapcık Jr, L., et al., (1998) 98 (8), 2663. [DOI] [PubMed]
- 34.Kotla N.G., et al. J. Contr. Release. 2021;336:598. doi: 10.1016/j.jconrel.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Jiang D., et al. Physiol. Rev. 2011;91(1):221. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khabarov V.N., et al. Biopharmaceuticals & Pharmaceutical Biotechnology. 2015:216. [Google Scholar]
- 37.Boeriu C.G., et al. Int. J. Carbohydr. Chem. 2013;2013 [Google Scholar]
- 38.Browne S., et al. ACS Biomater. Sci. Eng. 2020;6(2):1135. doi: 10.1021/acsbiomaterials.9b01419. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi Y., et al. Biorheology. 1994;31(3):235. doi: 10.3233/bir-1994-31302. [DOI] [PubMed] [Google Scholar]
- 40.Campo G.M., et al. Biochim. Biophys. Acta. 2011;1812(9):1170. doi: 10.1016/j.bbadis.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Chistyakov D.V., et al. Int. J. Mol. Sci. 2019;20(16) doi: 10.3390/ijms20163894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta R.C., et al. Front. Vet. Sci. 2019;6:192. doi: 10.3389/fvets.2019.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castillo F., et al. Clin. Oral Invest. 2021;25(8):4987. doi: 10.1007/s00784-021-03808-9. [DOI] [PubMed] [Google Scholar]
- 44.Itano N. J. Biochem. 2008;144(2):131. doi: 10.1093/jb/mvn046. [DOI] [PubMed] [Google Scholar]
- 45.Xiong J., et al. Macromol. Rapid Commun. 2022 [Google Scholar]
- 46.Zhang Y., et al. Carbohydr. Polym. 2022;276 doi: 10.1016/j.carbpol.2021.118761. [DOI] [PubMed] [Google Scholar]
- 47.Xu Z., et al. Acta Biomater. 2022;147:147. [Google Scholar]
- 48.Collins M.N., Birkinshaw C. Carbohydr. Polym. 2013;92(2):1262. doi: 10.1016/j.carbpol.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 49.Mero, A., and Campisi, M., (2014) 6 (2), 346.
- 50.Schanté C.E., et al. Carbohydr. Polym. 2011;85(3):469. [Google Scholar]
- 51.Hintze V., et al. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.830671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khunmanee S., et al. J. Tissue Eng. 2017;8 doi: 10.1177/2041731417726464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trombino S., et al. Pharmaceutics. 2019;11(8) doi: 10.3390/pharmaceutics11080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crescenzi V., et al. Biomacromolecules. 2003;4(4):1045. doi: 10.1021/bm0340669. [DOI] [PubMed] [Google Scholar]
- 55.de Nooy A.E., et al. Biomacromolecules. 2000;1(2):259. doi: 10.1021/bm005517h. [DOI] [PubMed] [Google Scholar]
- 56.Hirano K., et al. Carbohydr. Res. 2005;340(14):2297. doi: 10.1016/j.carres.2005.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jia X., et al. Biomaterials. 2004;25(19):4797. doi: 10.1016/j.biomaterials.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Maleki A., et al. Carbohydr. Res. 2007;342(18):2776. doi: 10.1016/j.carres.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 59.Nakajima N., Ikada Y. Bioconjugate Chem. 1995;6(1):123. doi: 10.1021/bc00031a015. [DOI] [PubMed] [Google Scholar]
- 60.Piron, E. T., Raymonde, (EP1303542A1).
- 61.Serban M.A., et al. Biomaterials. 2008;29(10):1388. doi: 10.1016/j.biomaterials.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pekař M.C.M. Carbohydr. Polym. 2009;76(3):443. [Google Scholar]
- 63.Prestwich G.D., Kuo J.W. Curr. Pharmaceut. Biotechnol. 2008;9(4):242. doi: 10.2174/138920108785161523. [DOI] [PubMed] [Google Scholar]
- 64.Ahmed E.M. J. Adv. Res. 2015;6(2):105. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu X., et al. Front. Chem. 2019;7:477. doi: 10.3389/fchem.2019.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hui E., et al. Biomacromolecules. 2019;20(11):4126. doi: 10.1021/acs.biomac.9b00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peerani E., et al. Methods Mol. Biol. 2019;2054:3. doi: 10.1007/978-1-4939-9769-5_1. [DOI] [PubMed] [Google Scholar]
- 68.Ruffini A., et al. Biomedicines. 2021;9(8) doi: 10.3390/biomedicines9080916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skopinska-Wisniewska J., et al. Int. J. Mol. Sci. 2021;22(14) doi: 10.3390/ijms22147402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodell C.B., et al. Biomacromolecules. 2013;14(11):4125. doi: 10.1021/bm401280z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosales A.M., et al. Bioconjugate Chem. 2018;29(4):905. doi: 10.1021/acs.bioconjchem.7b00802. [DOI] [PubMed] [Google Scholar]
- 72.Rowland M.J., et al. Biomacromolecules. 2015;16(8):2436. doi: 10.1021/acs.biomac.5b00680. [DOI] [PubMed] [Google Scholar]
- 73.Montanari E., et al. Nat. Biotechnol. 2017;37(Pt A):80. doi: 10.1016/j.nbt.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Cross D., et al. Macromol. Biosci. 2015;15(5):668. doi: 10.1002/mabi.201400491. [DOI] [PubMed] [Google Scholar]
- 75.Fujita S., et al. Adv. Polym. Technol. 2020;2020 [Google Scholar]
- 76.Palumbo F.S., et al. Acta Biomater. 2010;6(1):195. doi: 10.1016/j.actbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 77.Federico S., et al. Acta Biomater. 2016:38. doi: 10.1016/j.actbio.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 78.Appel E.A., et al. Chem. Soc. Rev. 2012;41(18):6195. doi: 10.1039/c2cs35264h. [DOI] [PubMed] [Google Scholar]
- 79.Kolb H.C., et al. Angew. Chem. 2001;40(11):2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 80.Andrade Del Olmo J., et al. Mater Sci Eng C Mater Biol Appl. 2021;125 doi: 10.1016/j.msec.2021.112102. [DOI] [PubMed] [Google Scholar]
- 81.Tang S., et al. Acta Biomater. 2016;43:292. doi: 10.1016/j.actbio.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 82.Tomihata K., Ikada Y. J. Biomed. Mater. Res. 1997;37(2):243. doi: 10.1002/(sici)1097-4636(199711)37:2<243::aid-jbm14>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 83.Wang W., et al. ACS Appl. Mater. Interfaces. 2021;13(47) doi: 10.1021/acsami.1c17267. [DOI] [PubMed] [Google Scholar]
- 84.Seidlits S.K., et al. Biomaterials. 2010;31(14):3930. doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 85.Yu L., Ding J. Chem. Soc. Rev. 2008;37(8):1473. doi: 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- 86.Ye B., et al. Mater. Lett. 2016;183:81. [Google Scholar]
- 87.Yue K., et al. Biomaterials. 2015;73:254. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schanté C.E., et al. Carbohydr. Polym. 2011;85(3):469. [Google Scholar]
- 89.Pedron S., et al. Adv. Mater. 2015;27(9):1567. doi: 10.1002/adma.201404896. [DOI] [PubMed] [Google Scholar]
- 90.Tang M., et al. Cell Res. 2020;30(10):833. doi: 10.1038/s41422-020-0338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sannino A., et al. Polymer. 2005;46(25) [Google Scholar]
- 92.Bulpitt, P., and Aeschlimann, D., (1999) 47 (2), 152. [DOI] [PubMed]
- 93.Tomihata K., Ikada Y. J. Biomed. Mater. Res. 1997;37(2):243. doi: 10.1002/(sici)1097-4636(199711)37:2<243::aid-jbm14>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 94.Park S.N., et al. Biomaterials. 2003;24(9):1631. doi: 10.1016/s0142-9612(02)00550-1. [DOI] [PubMed] [Google Scholar]
- 95.Kuo J.W., et al. Bioconjugate Chem. 1991;2(4):232. doi: 10.1021/bc00010a007. [DOI] [PubMed] [Google Scholar]
- 96.Hayward S.L., et al. Oncotarget. 2016;7(23) doi: 10.18632/oncotarget.8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gandini A. Prog. Polym. Sci. 2013;38:1. [Google Scholar]
- 98.Dudaryeva, O. Y., et al., (2021) 31 (52), 2104098.
- 99.Huebsch N., et al. Nat. Mater. 2010;9(6):518. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang J., et al. Colloids Surf. B Biointerfaces. 2022;209(Pt 2) [Google Scholar]
- 101.Ananthanarayanan B., et al. Biomaterials. 2011;32(31):7913. doi: 10.1016/j.biomaterials.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seidlits S.K., et al. J. Biomed. Mater. Res. 2019;107(4):704. doi: 10.1002/jbm.a.36603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim K., et al. Pharmaceutics. 2019;11(7):301. [Google Scholar]
- 104.Rao S.S., et al. Tissue Eng. B Rev. 2014;20(4):314. doi: 10.1089/ten.teb.2013.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rape A.D., Kumar S. Biomaterials. 2014;35(31):8846. doi: 10.1016/j.biomaterials.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.David L., et al. Matrix Biol. : J. Int. Soc. Mat. Biol. 2004;23(3):183. doi: 10.1016/j.matbio.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 107.Stern R. Semin. Cancer Biol. 2008;18(4):275. doi: 10.1016/j.semcancer.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 108.Xu X., et al. Biomaterials. 2012;33(35):9049. doi: 10.1016/j.biomaterials.2012.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xiao W., et al. Matrix Biol. : J. Int. Soc. Mat. Biol. 2020;85–86:128. [Google Scholar]
- 110.Xiao W., et al. Cancer Res. 2018;78(5):1358. doi: 10.1158/0008-5472.CAN-17-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rao S.S., et al. ACS Appl. Mater. Interfaces. 2013;5(19):9276. doi: 10.1021/am402097j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rehfeldt F., et al. Integrative biology : quantitative biosciences from nano to macro. 2012;4(4):422. doi: 10.1039/c2ib00150k. [DOI] [PubMed] [Google Scholar]
- 113.Narkhede A.A., et al. J. Biomed. Mater. Res. 2018;106(7):1832. doi: 10.1002/jbm.a.36379. [DOI] [PubMed] [Google Scholar]
- 114.Narkhede A.A., et al. Acta Biomater. 2020;107:65. doi: 10.1016/j.actbio.2020.02.039. [DOI] [PubMed] [Google Scholar]
- 115.Shen Y.-I., et al. Biomater. Sci. 2014;2(5):655. doi: 10.1039/C3BM60274E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakod P.S., et al. Biotechnol. Bioeng. 2020;117(2):511. doi: 10.1002/bit.27219. [DOI] [PubMed] [Google Scholar]
- 117.Tunici P., et al. Mol. Cancer. 2004;3:25. doi: 10.1186/1476-4598-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leite D.M., et al. Faseb. J. : official publication of the Federation of American Societies for Experimental Biology. 2020;34(1):1710. doi: 10.1096/fj.201901858RR. [DOI] [PubMed] [Google Scholar]
- 119.Xu W., et al. Acta Biomater. 2016;33:131. doi: 10.1016/j.actbio.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 120.Chen J.-W.E., et al. Frontiers Materials. 2018;5:39. doi: 10.3389/fmats.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen J.E., et al. Macromol. Biosci. 2017;17(8) doi: 10.1002/mabi.201700018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen J.E., et al. Biomater. Sci. 2018;6(4):854. doi: 10.1039/c7bm01195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pedron S., et al. Biomaterials. 2013;34(30):7408. doi: 10.1016/j.biomaterials.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 124.Ahn J., et al. NPG Asia Mater. 2022;14(1):46. [Google Scholar]
- 125.Skardal A., et al. Biotechnol. Bioeng. 2016;113(9):2020. doi: 10.1002/bit.25950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sivakumar H., et al. Gels. 2017;3(3):28. doi: 10.3390/gels3030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jin S.G., et al. J. Korean Neurosurg. Soc. 2009;46(5):472. doi: 10.3340/jkns.2009.46.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.David L., et al. Matrix Biol. : J. Int. Soc. Mat. Biol. 2004;23(3):183. doi: 10.1016/j.matbio.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 129.David L., et al. Cell Prolif. 2008;41(2):348. doi: 10.1111/j.1365-2184.2008.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pedrosa S.S., et al. Eur. J. Pharmaceut. Sci. 2017;104:102. doi: 10.1016/j.ejps.2017.03.045. [DOI] [PubMed] [Google Scholar]
- 131.Ng S.Y., Lee A.Y.W. Front. Cell. Neurosci. 2019;13:528. doi: 10.3389/fncel.2019.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu G., et al. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.792764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abu-Rub M., Miller R.H. Brain Sci. 2018;8(6) doi: 10.3390/brainsci8060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jensen G., et al. Cells. 2020;9(9) doi: 10.3390/cells9092113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kajtez J., et al. Neurochem. Int. 2021;147 doi: 10.1016/j.neuint.2021.105043. [DOI] [PubMed] [Google Scholar]
- 136.Zakusilo F.T., et al. Ageing Res. Rev. 2021;72 doi: 10.1016/j.arr.2021.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Song I., Dityatev A. Brain Res. Bull. 2018;136:101. doi: 10.1016/j.brainresbull.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 138.Peters A., Sherman L.S. Int. J. Mol. Sci. 2020;21(17) doi: 10.3390/ijms21175988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Skupien A., et al. J. Cell Sci. 2014;127(Pt 23):5038. doi: 10.1242/jcs.154542. [DOI] [PubMed] [Google Scholar]
- 140.Ballios B.G., et al. Stem Cell Rep. 2015;4(6):1031. doi: 10.1016/j.stemcr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nih L.R., et al. Adv. Mater. 2017;29(32) doi: 10.1002/adma.201606471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Unal D.B., et al. Biomacromolecules. 2020;21(12):4962. doi: 10.1021/acs.biomac.0c01164. [DOI] [PubMed] [Google Scholar]
- 143.Mirzaei S., et al. ACS Chem. Neurosci. 2021;12(22):4224. doi: 10.1021/acschemneuro.1c00484. [DOI] [PubMed] [Google Scholar]
- 144.Papa S., et al. Expet Opin. Biol. Ther. 2020;20(10):1203. doi: 10.1080/14712598.2020.1770725. [DOI] [PubMed] [Google Scholar]
- 145.Crommelin D.J.A., et al. J. Contr. Release. 2020;318:256. doi: 10.1016/j.jconrel.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 146.El Kechai N., et al. Int. J. Pharm. 2015;487(1–2):187. doi: 10.1016/j.ijpharm.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 147.Grijalvo S., et al. Biomater. Sci. 2016;4(4):555. doi: 10.1039/c5bm00481k. [DOI] [PubMed] [Google Scholar]
- 148.Ferreira H., et al. Life Sci. 2021;287 doi: 10.1016/j.lfs.2021.120108. [DOI] [PubMed] [Google Scholar]
- 149.Ning L., et al. Biofabrication. 2018;10(3) doi: 10.1088/1758-5090/aacd30. [DOI] [PubMed] [Google Scholar]
- 150.Tang Z., et al. Biomaterials. 2019;194:57. doi: 10.1016/j.biomaterials.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 151.Zarei-Kheirabadi M., et al. Stem Cell Res. Ther. 2019;10(1):380. doi: 10.1186/s13287-019-1448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cernencu A.I., et al. Biotechnol. Bioeng. 2021;119(3):762–783. doi: 10.1002/bit.28020. [DOI] [PubMed] [Google Scholar]
- 153.Burdick J.A., Prestwich G.D. Adv. Mater. 2011;23(12):H41. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mendes B.B., et al. Acta Biomater. 2021;119:101. doi: 10.1016/j.actbio.2020.10.040. [DOI] [PubMed] [Google Scholar]
- 155.Galarraga J.H., et al. Biofabrication. 2021;14(1) doi: 10.1088/1758-5090/ac3acb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Martin A.R., et al. Acta Biomater. 2021;126:170. doi: 10.1016/j.actbio.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ren Y., et al. ACS Appl. Bio Mater. 2021;4(3):2601. doi: 10.1021/acsabm.0c01591. [DOI] [PubMed] [Google Scholar]
- 158.Abid W.K., Al Mukhtar Y.H. J Taibah Univ Med Sci. 2019;14(1):14. doi: 10.1016/j.jtumed.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang X., et al. J. Mater. Sci. Mater. Med. 2012;23(8):1941. doi: 10.1007/s10856-012-4662-y. [DOI] [PubMed] [Google Scholar]
- 160.Abdul-Monem M.M., et al. J Taibah Univ Med Sci. 2021;16(4):529. doi: 10.1016/j.jtumed.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang G., et al. Gels. 2021;7(4) doi: 10.3390/gels7040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Poldervaart M.T., et al. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0177628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Noh I., et al. Biomater. Res. 2019;23:3. doi: 10.1186/s40824-018-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Greene J.J., Sidle D.M. Facial plastic surgery clinics of North America. 2015;23(4):423. doi: 10.1016/j.fsc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 165.Yang J.-A., et al. Biomaterials. 2012;33(25):5947. doi: 10.1016/j.biomaterials.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 166.Fallacara A., et al. Facial Plast. Surg. : FPS (Facial Plast. Surg.) 2017;33(1):87. doi: 10.1055/s-0036-1597685. [DOI] [PubMed] [Google Scholar]
- 167.Sierra-Sánchez Á., et al. J. Eur. Acad. Dermatol. Venereol. : JEADV. 2020;34(10):2414. doi: 10.1111/jdv.16342. [DOI] [PubMed] [Google Scholar]
- 168.Dong Y., et al. Acta Biomater. 2020;108:56. doi: 10.1016/j.actbio.2020.03.040. [DOI] [PubMed] [Google Scholar]
- 169.Li J., Mooney D.J. Nat. Rev. Mater. 2016;1(12) doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Su J., et al. Biomaterials. 2010;31(2):308. doi: 10.1016/j.biomaterials.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fu H., et al. J. Mater. Chem. B. 2021;9(48) doi: 10.1039/d1tb01914g. [DOI] [PubMed] [Google Scholar]
- 172.Loebel C., et al. Nat. Protoc. 2017;12(8):1521. doi: 10.1038/nprot.2017.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kharkar P.M., et al. Advanced healthcare materials. 2017;6(24) doi: 10.1002/adhm.201700713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kasapidou P.M., et al. Soft Matter. 2021;17(48) doi: 10.1039/d1sm01003d. [DOI] [PubMed] [Google Scholar]
- 175.Zhang W., et al. Carbohydr. Polym. 2018;186:82. doi: 10.1016/j.carbpol.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 176.Boedtkjer E., Pedersen S.F. Annu. Rev. Physiol. 2020;82:103. doi: 10.1146/annurev-physiol-021119-034627. [DOI] [PubMed] [Google Scholar]
- 177.Edwards, K., et al., (2021) 13 (16), 4032.
- 178.Chen X., Liu Z. Macromol. Rapid Commun. 2016;37(18):1533. doi: 10.1002/marc.201600261. [DOI] [PubMed] [Google Scholar]
- 179.Jo Y.J., et al. Carbohydr. Polym. 2022;286 doi: 10.1016/j.carbpol.2022.119303. [DOI] [PubMed] [Google Scholar]
- 180.Anirudhan T.S., et al. Int. J. Biol. Macromol. 2022;201:378. doi: 10.1016/j.ijbiomac.2022.01.022. [DOI] [PubMed] [Google Scholar]
- 181.Goldfarb Y., Ben-Eliyahu S. Breast Dis. 2006;26:99. doi: 10.3233/bd-2007-26109. [DOI] [PubMed] [Google Scholar]
- 182.Li Xilin, et al. Adv. Funct. Mater. 2020;30(45) doi: 10.1002/adfm.202005010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Garbayo E., et al. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(5):e1637. doi: 10.1002/wnan.1637. [DOI] [PubMed] [Google Scholar]
- 184.Owen Shawn C., C D.P.Y., Shoichet Molly S. Nano Today. 2012;7(1):53. [Google Scholar]
- 185.Kashkooli F.M., et al. Nanomed. 2022;17(10):695. doi: 10.2217/nnm-2021-0126. [DOI] [PubMed] [Google Scholar]
- 186.Leng Q., et al. Mater Sci Eng C Mater Biol Appl. 2021;129 doi: 10.1016/j.msec.2021.112390. [DOI] [PubMed] [Google Scholar]
- 187.Cao J., et al. Curr. Med. Chem. 2020;27(35):5949. doi: 10.2174/0929867326666190618152518. [DOI] [PubMed] [Google Scholar]
- 188.Zeng Y., et al. Acta Biomater. 2022;145:43–51. doi: 10.1016/j.actbio.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 189.Nguyen L.T.B., et al. Biomed. Mater. 2020;15(5) doi: 10.1088/1748-605X/ab8c43. [DOI] [PubMed] [Google Scholar]
- 190.Jeong D.U., et al. Biomed. Mater. 2021;16(3) doi: 10.1088/1748-605X/abc7f1. [DOI] [PubMed] [Google Scholar]
- 191.Fan Y., et al. Adv. Healthcare Mater. 2020;9(24) doi: 10.1002/adhm.202001410. [DOI] [PubMed] [Google Scholar]
- 192.Stichler S., et al. Biofabrication. 2017;9(4) doi: 10.1088/1758-5090/aa8cb7. [DOI] [PubMed] [Google Scholar]
- 193.Wan T., et al. ACS Appl. Bio Mater. 2022;5(1):334. doi: 10.1021/acsabm.1c01141. [DOI] [PubMed] [Google Scholar]
- 194.Antich C., et al. Acta Biomater. 2020;106:114. doi: 10.1016/j.actbio.2020.01.046. [DOI] [PubMed] [Google Scholar]
- 195.Wang D., et al. Acta Biomater. 2022:S1742. [Google Scholar]
- 196.Ferroni L., et al. Biomaterials Advances. 2022;139 doi: 10.1016/j.bioadv.2022.213000. [DOI] [PubMed] [Google Scholar]
- 197.Maloney E., et al. Micromachines. 2020;11(2) doi: 10.3390/mi11020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shopperly L.K., et al. J. Biomed. Mater. Res., Part B. 2022;110(10):2310. doi: 10.1002/jbm.b.35079. [DOI] [PubMed] [Google Scholar]
- 199.Horder H., et al. Cells. 2021;10(4) doi: 10.3390/cells10040803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.