Summary
The unreliability of commercial recombinant asprosin preparations and variability between asprosin detection assays have proven to be a bottleneck in experimental interpretation. This protocol describes the use of viral vectors and expression plasmid for overexpression and secretion of human asprosin to achieve sustained elevation of asprosin protein in mice and HEK293T cells without using recombinant proteins. This protocol also includes a sandwich ELISA using anti-asprosin monoclonal antibodies for detection of asprosin in media from cultured cells and in plasma of mice.
For complete details on the use and execution of this protocol, please refer to Duerrschmid et al. (2017), Mishra et al. (2021), and Mishra et al. (2022).
Subject areas: Metabolism, Molecular biology, Gene expression, Antibody, Neuroscience, Molecular/Chemical probes
Graphical abstract

Highlights
-
•
Protocol for overexpression and secretion of asprosin in mice and HEK293T cell culture
-
•
Injection of mice with adenoviral and AAV vector for in vivo asprosin overexpression
-
•
HEK293T cell transfection with expression plasmid for in vitro asprosin overexpression
-
•
ELISA protocol using anti-asprosin monoclonal antibodies for detection of asprosin
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The unreliability of commercial recombinant asprosin preparations and variability between asprosin detection assays have proven to be a bottleneck in experimental interpretation. This protocol describes the use of viral vectors and expression plasmid for overexpression and secretion of human asprosin to achieve sustained elevation of asprosin protein in mice and HEK293T cells without using recombinant proteins. This protocol also includes a sandwich ELISA using anti-asprosin monoclonal antibodies for detection of asprosin in media from cultured cells and in plasma of mice.
Before you begin
Asprosin, the C-terminal cleavage product of profibrillin-1 was recently identified as a fasting-induced glucogenic hormone that modulates hepatic glucose release, and as an orexigenic hormone that crosses the blood-brain barrier to directly activate appetite-promoting neurons in the hypothalamus (Romere et al., 2016; Duerrschmid et al., 2017). The protocol below describes the specific steps for the overexpression and secretion of human asprosin in adult mice and in HEK293T cells, using an asprosin encoding adenoviral vector, an adeno-associated viral vector and a mammalian expression plasmid. As opposed to acute effects of recombinant asprosin treatment, the use of the viral vector approach ensures chronic elevation of asprosin levels in the circulation. This also eschews the unreliability associated with the recombinant protein approach. Several studies have reported a high correlation of circulating asprosin levels with metabolic conditions such as obesity, diabetes mellitus type 1 and 2, and insulin resistance (Ovali and Bozgeyik, 2022). However, the enormous variability of reported blood asprosin levels illustrates the need for a reliable and sensitive ELISA detection method. This protocol describes a sandwich ELISA, using two anti-asprosin monoclonal antibodies for detection of overexpressed asprosin in immunoglobin and albumin cleared plasma of mice and in conditioned media from HEK293T cell culture.
Institutional permissions
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animals were handled according to approved institutional animal care and use committee (IACUC) protocols (#2018-0042) of the Case Western Reserve University. The protocol was approved by the Committee on the Ethics of Animal Experiments of Case Western Reserve University. Experimenters using this protocol will need to acquire permissions from the relevant institutions.
Preparation for culture of HEK293T cells
Timing: 48 h
-
1.Preparation of growth media.
-
a.Add 50 mL of fetal bovine serum (FBS) and 5 mL of penicillin-streptomycin solution to 445 mL of DMEM grown media (Dulbecco’s Modified Eagle Medium) under sterile conditions.
-
b.Bring this complete media (10% FBS and 1% Pen/Strep- DMEM) to 37°C temperature by placing it in 37°C water bath before use.
-
a.
-
2.HEK29T cell culture.
 CRITICAL: Perform all cell culture steps in sterile conditions in cell culture hood.
CRITICAL: Perform all cell culture steps in sterile conditions in cell culture hood.-
a.Plate 2 × 106 HEK293T cells (passage number range: 1–20) per 10 cm plate using sterile pipettes and add 10 mL of complete media to support the growth of cells.
-
b.Place the plate in 37°C incubator with humidified atmosphere of 5% CO2.
-
c.Examine the cell culture to monitor the cell health before doing the experiment.Note: Cell culture contamination detection kit (Thermo Fisher Scientific, Catalog # C7028) can be used for screening cultures for contamination by microorganisms. For this, the experimenter should follow the manufacturer’s protocol. (https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2Fmp07028.pdf).
-
d.Treat cells with plasmid after 48 h as detailed below or subculture the cells on reaching 80% or higher confluency for future experiments.
-
a.
Preparation for tail vein injection of adeno-associated viral vector in C57BL/6J mice
Timing: 5 min/mouse
-
3.Preparation of mice.
-
a.Ear tag and weigh mice before doing teil vein injection of adenoviral vector.
-
a.
Note: Begin acclimation of group-housed mice to dustless diet (cat#F0173. Bio-Serv) for a minimum of 3 days prior to the measurement of 24-hour food intake.
-
4.Viral vector preparation.
-
a.Dilute adeno-associated viral vectors (AAV8) with USP-grade sterile saline to reach a concentration of 66.6 × 108 GC per μL solution.
-
b.150 μL of this solution will be injected in the tail vein of mouse.Note: Mice injected with AAV8-empty (1 × 1012 GC/mouse) will serve as controls for experimental mice that will receive AAV8-IL2sp-6His-Asprosin (1 × 1012 GC/mouse) containing an N-terminal 6histidine-tagged human asprosin coding region preceded by an IL2 signal peptide, under control of an EF1 promoter.
-
c.Dilute Adenoviral vector (Ad5) with USP-grade sterile saline to reach a concentration of 24 × 106 pfu per μL solution.
-
d.150 μL of this solution will be injected in the tail vein of mouse.Note: Mice injected with Ad5-empty (3.6 × 109 pfu/mouse) served as controls for experimental mice that received Ad5-hFBN1 viral vector (3.6 × 109 pfu/mouse) containing the human FBN1 coding region under control of a CMV promoter.
-
a.
Preparation for ELISA detection of overexpressed asprosin
Timing: 3 h
-
5.Specimen collection and storage.
-
a.Plasma: Collect plasma using EDTA as an anticoagulant.
-
i.Centrifuge blood samples for 15 min at 3,000 rpm on a fixed-angle rotors that holds tubes at a stable angle (typically 45°) relative to the axis of rotation at 4°C.
-
ii.Store plasma samples at −20°C or −80°C until IgG and albumin removal as describe in steps below.
 CRITICAL: IgG and albumin removal is critical for the success of asprosin detection in ELISA.Note: Use proteome purify mouse serum protein immunodepletion resins (R&D Systems, MIDR002-020) as per the manufacturer’s protocol for removal of IgG and albumin from 10 μL aliquot of plasma. (https://resources.rndsystems.com/pdfs/datasheets/midr002.pdf?v=20220916&_ga=2.184636265.1951199203.1663339320-332755903.1663339320&_gac=1.216404580.1663340189.Cj0KCQjwvZCZBhCiARIsAPXbajucBuwUCG_NXfqe9g5qhlMyxaBGwCrW_rVpkDN2cNVLkklQp9XkdBAaAq3bEALw_wcB). Protocol is described briefly below.
CRITICAL: IgG and albumin removal is critical for the success of asprosin detection in ELISA.Note: Use proteome purify mouse serum protein immunodepletion resins (R&D Systems, MIDR002-020) as per the manufacturer’s protocol for removal of IgG and albumin from 10 μL aliquot of plasma. (https://resources.rndsystems.com/pdfs/datasheets/midr002.pdf?v=20220916&_ga=2.184636265.1951199203.1663339320-332755903.1663339320&_gac=1.216404580.1663340189.Cj0KCQjwvZCZBhCiARIsAPXbajucBuwUCG_NXfqe9g5qhlMyxaBGwCrW_rVpkDN2cNVLkklQp9XkdBAaAq3bEALw_wcB). Protocol is described briefly below. -
iii.Add 10 μL of plasma to a test tube containing 1.0 mL of the suspended immunodepletion resin.
 CRITICAL: It is important to mix the immunodepletion resins well by inverting the tube several times prior to pipetting to keep the solution homogenous.
CRITICAL: It is important to mix the immunodepletion resins well by inverting the tube several times prior to pipetting to keep the solution homogenous. -
iv.Place the tube on a rotary shaker at high speed and mix for 60 min.Note: The mixing speed should be sufficient to keep the immunodepletion resins in suspension.
-
v.Pipette 500 μL of the immunodepletion resin mixture of each sample into the upper chamber of Spin-X filter units. Two Spin-X Filter Units per sample will be required for this step.
-
vi.Centrifuge for 2 min at 1,000–2,000 × g in the microcentrifuge tube. Combine the two filtrates of each sample. The volume of the combined filtrates will be approximately 400–500 μL.
-
vii.Discard the used immunodepletion resin and subject the filtrate to concentration procedure.
-
viii.Use Vivaspin 500 (VS0131) columns for concentration of purified plasma filtrates. Apply 400–500 μL of filtrate to the columns and spin the column at 12,000 g until the samples are concentrated to 40–50 μL.
-
ix.Detect asprosin in this concentrated sample using the ELISA protocol detailed below.
-
i.
-
b.Cell culture conditioned media.
-
i.Centrifuge conditioned media from HEK293T cell culture at 1,000 × g for 5 min to remove debris.
-
ii.Transfer supernatant to a fresh tube.
-
iii.Add (1×) proteinase inhibitor.
-
iv.Store samples at −20°C or −80°C or assay immediately.
-
i.
-
a.
-
6.ELISA Reagent preparation.
-
a.Blocking buffer:
-
i.Warm 10× blocking buffer (SeraCare, Catalog# 5140-0006; 10% Bovine serum albumin in phosphate buffer, storage condition: 4°C) at 37°C briefly and mix until crystals dissolve.
-
ii.Make 1× solution with MilliQ water and add 0.5% tween 20.Note: Add 10 mL of blocking buffer to 90 mL of MilliQ water and add 500 μL of Tween 20. Preparing fresh blocking buffer for each ELISA is recommended.Alternatives: Phosphate buffer with 1% BSA (pH 7.4) can be prepared by adding 20.214 g of Sodium Phosphate Dibasic Heptahydrate, 3.394 g of Sodium Phosphate Monobasic Monohydrate and 10 g of Bovine serum albumin to 800 mL distilled water, followed by adjusting pH to 7.4 using HCl or NaOH and adding water until the final volume is 1 L. Working blocking solution (phosphate buffer with 1% BSA, 0.5% tween 20) can be prepared by adding 5 mL of tween 20 to the 1 L of phosphate buffer.
-
i.
-
b.Wash buffer:
-
i.If wash buffer contains visible crystals, warm 20× wash buffer (SeraCare, Catalog#5150-001, imidazole-buffered saline and Tween 20; storage condition: 4°C) at 37°C briefly and mix until dissolved.
-
ii.Make 1× using MilliQ water.Note: Dilute 20 mL of 20× wash buffer concentrate into deionized or distilled water to yield 400 mL of 1× wash buffer.Alternatives: Imidazole wash buffer containing 50 mM sodium dihydrogen phosphate, 300 mM sodium chloride, and 20 mM imidazole with 0.5% Tween-20, pH 8.0 can be prepared in lab or bought from other commercial vendors such as Bioplus buffers and agents (catalog # 42320002-1) or Bioworld (catalog # 42320002-1).
-
i.
-
c.Capture antibody:
-
i.Briefly spin the vial of anti-asprosin mouse monoclonal antibody before use (Storage condition: −20°C).
-
ii.Prepare 1 ng/μL solution of antibody using 1× blocking buffer.Note: For 50 wells, make 5 mL of 1 ng/μL solution by adding 5 μL of 1 μg/μL capture antibody to 4,995 μL of 1× blocking buffer. Pipette up and down to mix gently. We recommend preparing fresh capture antibody solution for each ELISA run.
-
i.
-
d.Detection antibody:
-
i.Briefly spin the vial of anti-asprosin rabbit monoclonal antibody before use (Storage condition: −20°C).
-
ii.Prepare 1 ng/μL solution of antibody using 1× blocking buffer.Note: for 50 wells, make 5 mL of 1 ng/μL solution by adding 5 μL of 1 μg/μL detection antibody to 4,995 μL of 1× blocking buffer. Pipette up and down to mix gently. We recommend preparing fresh detection antibody solution for each ELISA run.
-
i.
-
e.HRP-tagged secondary antibody:
-
i.Briefly spin the vial of HRP-anti-rabbit secondary antibody before use (Storage condition: 4°C).
-
ii.Prepare 1: 10,000 dilution using 1× blocking buffer.Note: For 10 mL preparation, add 1 μL to 10 mL of blocking buffer. Pipette up and down to mix gently. We recommend preparing fresh secondary antibody solution for each ELISA run.
-
i.
-
f.TMB Substrate: 3,3′, 5, 5' – Ready to use tetramethyl benzidine substrate: SeraCare, Catalog # 5120-0077 (Storage condition: 4°C). Bring to room temperature (18°C–22°C) before use.
-
g.TMB Stop solution: Ready to use (Storage condition: 4°C, SeraCare, Catalog# 5150-0021; 0.16 M sulfuric acid). Bring to room temperature (18°C–22°C) before use.
-
h.Standard preparation:
-
i.Prepare asprosin standards (20 nM, 5 nM, 1.25 nM, 0.3125 nM, 0.078 nM, 0.0195 nM, 0.00488 nM) using blocking buffer.
-
ii.Label 8 tubes as 1–8. Tube 1 to 8 denote serial dilution of asprosin, from 20 nM, 5 nM, 1.25 nM, 0.3125 nM, 0.078 nM, 0.0195 nM, 0.00488 nM and blank.
-
iii.Add 999 μL of blocking buffer to tube 1, and 750 μL of blocking buffer to tubes 2–8.
-
iv.Add 1 μL of 20 μM stock of mammalian asprosin to tube 1 and pipette up and down to mix the solution. This tube corresponds to the 20 nM asprosin standard.
-
v.Add 250 μL of standard 1 to tube 2 and mix well to make 1 mL of 5 nM asprosin. Continue with serial dilution to prepare asprosin standards.
-
vi.Use blocking buffer as blank.Note: We recommend preparing fresh asprosin standards for each ELISA run.
-
i.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse Anti-Asprosin antibody (working concentration: 1 ng/μL) | Chopra lab | N/A |
| Rabbit Anti-Asprosin antibody (working concentration: 1 ng/μL) | Chopra lab | N/A |
| HRP-anti-Rabbit antibody (working dilution: 1:10000) | GE Healthcare | NA934-1ML |
| Chemicals, peptides, and recombinant proteins | ||
| DMEM | Cytvia | SH30285.01 |
| Penicillin-Streptomycin Solution | Sigma-Aldrich | P4458-100ML |
| Fetal Bovine Serum | Gemini Bio-Products | 100-106 |
| Recombinant human Asprosin | BioLegend | 761902 |
| TMB substrate Solution | SeraCare | 5120-0077 |
| TMB Stop Solution | SeraCare | 5150-0021 |
| Protease inhibitor | Thermo Fisher Scientific | 78429 |
| Wash solution concentrate | SeraCare | 5150-0011 |
| VivaFree columns | Vivaproducts | VFR01H22 |
| Trypsin-EDTA | Fisher Scientific | 25-200-056 |
| Fugene HD Transfection Reagent | Promega | E2311 |
| Coating solution concentrate | SeraCare | 5150-0014 |
| Blocking Buffer | SeraCare | 5140-0006 |
| Wash buffer solution (alternative) | Bioplus | 42320002-1 |
| Wash buffer solution (alternative) | Bioworld | 42320002-1 |
| Sodium phosphate dibasic heptahydrate | Sigma-Aldrich | S9390-100G |
| Sodium phosphate monobasic monohydrate | Sigma-Aldrich | S9638-25G |
| Bovine Serum Albumin | Sigma-Aldrich | A9430-100G |
| Tween 20 | MilliporeSigma | P1379 |
| USP-grade sterile saline | Baxter | 2F7123 |
| Imidazole | Sigma-Aldrich | I5513-5G |
| Sodium dihydrogen phosphate | MilliporeSigma | 1063700050 |
| Sarstedt Inc Microvette CB300 EDTA | Fisher Scientific | NC9141704 |
| Critical commercial assays | ||
| Cell culture contamination detection kit | Thermo Fisher Scientific | C7028 |
| Proteome Purify Mouse Serum Protein Immunodepletion Resin | R&D Systems | MIDR002-020 |
| Experimental models: Cell lines | ||
| HEK293T cells | ATCC | CRL-3216 |
| Experimental models: Organisms/strains | ||
| 12-week-old male C57BL/6J Mus musculus | The Jackson Laboratory | RRID:IMSR_JAX:000664 |
| Recombinant DNA | ||
| Empty pcDNA3.1 plasmid | Creative Biogene | VET1452 |
| pcDNA3.1-IL2sp-6His-Asprosin plasmid | Chopra lab | N/A |
| AAV8-empty | Vector Biolabs | 7600 |
| AAV8-IL2sp-6His-Asprosin | Chopra lab | N/A |
| Ad5-CMV-Empty | BCM Gene Vector Core | CF-7214148-7 |
| Ad5-hFBN1 | BCM Gene Vector Core | N/A |
| Other | ||
| Dustless diet | Bio-Serv | F0173 |
Materials and equipment
Alternative Blocking buffer for ELISA
| Reagent | Final concentration | Amount |
|---|---|---|
| Sodium Phosphate Dibasic Heptahydrate | 0.075 M | 20.214 g |
| Sodium Phosphate Monobasic Monohydrate | 0.024 M | 3.394 g |
| Bovine serum albumin | 1% | 10 g |
| Tween 20 | 0.5% | 5 mL |
| ddH2O | N/A | Upto 1 L |
| Total | N/A | 1 L |
Store at room temperature (18°C–22°C) for up to one month.
CRITICAL: Adjust solution to final desired pH using HCl or NaOH before making up the volume to 1 L wit dd water.
Alternative wash buffer for ELISA
| Reagent | Final concentration | Amount |
|---|---|---|
| Sodium dihydrogen phosphate | 50 mM | 5.99 g |
| Sodium chloride | 300 mM | 17.53 g |
| Imidazole | 20 mM | 1.36 |
| Tween 20 | 0.5% | 5 mL |
| ddH2O | N/A | Upto 1 L |
| Total | N/A | 1 L |
Store at room temperature (18°C–22°C) for up to one month.
Step-by-step method details
Asprosin-expressing Ad5 and AAV8 vector transduction in mice
Timing: 15 min per mouse
This section describes the method for tail vein injection of asprosin coding- and empty-control adenoviral (Ad5) and Adeno-associated (serotype 8, AAV8) viral vectors in lean, male mice. This section further describes method for blood collection, food intake and body weight assessment of viral vector transduced mice.
-
1.
Prior to injection, warm animal (12-week-old C57BL/6J male mice) for 5–10 min to dilate the veins.
Note: Mice may be warmed by placing the animal in a commercially available warming box, or by using a warm water circulating pad (37°C) placed under the cage. Overhead heat lamp can also be used, however extra care must be taken to prevent overheating the animal (Resch et al., 2019; Yano et al., 2020).
-
2.
Position the tail on circulating heat pad with animal restrained on its side in a restrainer.
Note: Topical oil of wintergreen can be applied to the tail to aid in vasodilation (Hrapkiewicz and Medina, 2007).
CRITICAL: Orientation and location of the lateral tail vein must be ensured before performing injections. For a successful tail vein injection, it is critical to dilate the veins properly. Care should be taken to not overheat the tail, and to keep enough barrier paper towel layers between the heat pad and tail.
-
3.
Inject each mouse with 150 μL of AAV8 vector.
Note: Mice injected with AAV8-empty (1 × 1012 GC/mouse) will serve as control for experimental mice injected with AAV8-IL2sp-6His-Asprosin (1 × 1012 GC/mouse). AAV8 dilutions should be prepared as suggested in the section 4 of the protocol (Mishra et al., 2021, 2022).
-
4.
Ad5-hFBN1 (150 μL, 3.6 × 109 pfu/mouse) can also be used for asprosin overexpression in mice.
CRITICAL: It is critical to inject control mice with the same concentration of viral vectors as the experimental mice.
Note: Mice injected with Ad5-empty (3.6 × 109 pfu/mouse) served as controls for experimental mice that received Ad5-hFBN1 viral vector (3.6 × 109 pfu/mouse) containing the human FBN1 coding region under control of a CMV promoter. Ad5 dilutions should be prepared as suggested in the section 4 of the protocol (Mishra et al., 2021, 2022).
-
5.
Clean the site of injection with 70% ethanol and place the mouse back with its littermates in the parent mouse cage, as approved by the institution’s biosafety committee.
Note: The duration of the restraint should be kept to a minimum, and the equipment cleaned frequently to prevent pheromone-induced stress or cross contamination.
-
6.
Provide mice with food and water ad libitum throughout the experiment.
-
7.
Monitor changes in body weight and food intake.
Note: Dustless pellet diet can be used for manual measurement of food intake. For experiments using AAV8, body weight and food intake can be monitored once weekly for 15 weeks or longer, while for experiments using Ad5 vectors, body weight and food intake can be measured every fourth day for up to twenty-five days.
-
8.
Mice can be subjected to sub-mandibular bleeding or other institutional IACUC committee approved methods for blood collection and plasma extraction.
Note: Anesthesia is not required for submandibular bleeding.
-
9.
Subject plasma samples to proteome purification as outlined in section 5a, iii-vi in “before you begin” of this protocol.
-
10.
Detect overexpressed asprosin as outlined in the section ‘ELISA detection of asprosin’ below.
CRITICAL: It is critical to determine the appropriate amount of active/infectious Ad5 viral vector titers for successful in vivo overexpression of asprosin. If not enough virus is used, a significant overexpression of asprosin cannot be achieved. If too much is used, it will result in cytotoxicity or other undesired effects. It is advised to use the minimum Ad5 concentration that will result in 100% gene delivery. This optimal concentration differs dramatically between Ad5 preparations as the viral particles (vp) to pfu (plaque forming units) ratio differs for each Ad5 preparation. Optimal Ad5 concentrations should be established for each Ad5 preparation based on pilot in vitro and in vivo testing. Additionally, since tracking body weight and food intake is a reliable marker of successful asprosin overexpression, It is critical to use age-, sex- and weight-matched mice for the experiments.
HEK293T cell transfection with asprosin-expressing plasmid
Timing: 6 days
This section describes the method for collection of conditioned media from HEK293T cells transfected with human asprosin expressing mammalian expression plasmid (pcDNA3.1-IL2sp-6His-Asprosin) and empty-control plasmid (pcDNA3.1-control plasmid).
-
11.
Plate cells as detailed in section: preparation for culture of HEK293T cells of the before you begin part of this protocol.
-
12.
Treat HEK293T cells with plasmid once cells are estimated to be 70% confluent.
Note: It takes approximately 2 days (48 h) for cells to reach 70% confluency. Follow the Fugene HD transfection protocol for transfection of HEK293T cells with control and asprosin-overexpressing mammalian expression plasmids (https://www.promega.com/resources/protocols/technical-manuals/101/fugene-hd-transfection-reagent-protocol/).
-
13.
For each 10 cm plate, add 10 μg of plasmid to 1 mL of serum free media, and add 30 μL of FuGENE® HD transfection reagent in a microcentrifuge tube.
Note: For successful transfection of plasmid DNA into cultured cells, the ratio of 1:3 of FuGENE® HD Transfection Reagent:DNA is recommended.
-
14.
Incubate this solution at room temperature (18°C–22°C) for 15 min before adding it to the HEK293T cells.
Note: HEK293T cells transfected with pcDNA3.1-control plasmid will serve as control for HEK293T cells transfected with pcDNA3.1-IL2sp-6His-Asprosin. A successful transfection is critical for asprosin overexpression.
-
15.
Place the plate in 37°C incubator with humidified atmosphere of 5% CO2.
-
16.
24 h later, replace the media in 10 cm plate with 10 mL of fresh complete media.
-
17.
Leave the plate undisturbed in 37°C incubator with humidified atmosphere of 5% CO2 for the next 48 h.
-
18.
Collect conditioned media 72 h post transfection, and every 24 h following it if required.
-
19.
Add 10 μL of 100× Halt protease inhibitor per mL of the conditioned media and store it at 4°C for short duration, or at −20°C or −80°C for longer duration, until subjected to asprosin detection ELISA.
CRITICAL: A successful transfection is critical for asprosin overexpression in HEK293T cell culture. Care should be taken to not add concentrated DNA plasmid directly to the FuGENE® HD transfection reagent. This can lead to precipitation of the DNA. It is advised to first dilute DNA in serum free media before addition of the FuGENE® HD transfection reagent.
ELISA detection of asprosin
Timing: 48 h
This section describes a custom-built sandwich ELISA protocol for detection of overexpressed human asprosin in conditioned media from HEK293T cells transfected with asprosin-expressing plasmid. This protocol can also be used for detection of asprosin in plasma samples from mice transduced with human asprosin expressing and empty-control adenoviral (Ad5) and Adeno-associated (serotype 8, AAV8) viral vectors.
-
20.
Prepare reagents as outlined in step 6 of “before you begin” of this protocol.
Note: Bring all reagents and samples to room temperature (18°C–22°C) before use.
CRITICAL: It is recommended that all standards and samples be run at least in duplicate in the same ELISA plate.
-
21.
Select the number of wells required for running samples.
-
22.
Add 100 μL of (1 ng/μL) anti-asprosin mouse monoclonal antibody in the selected wells.
-
23.
Incubate the plate 16–18 h at 4°C.
-
24.
Add 250 μL of blocking solution and incubate the plate at room temperature (18°C–22°C) for 2 h.
-
25.
Discard the solution and wash 3 times with 1× wash solution.
Note: Wash by filling each well with wash buffer (350 μL) using a multi-channel Pipette or autowasher. Discard the wash buffer after 3–5 min incubation at room temperature (18°C–22°C).
CRITICAL: Complete removal of liquid at each step is essential to good performance. After the last wash, invert the plate and blot it against clean paper towels to remove any remaining wash buffer.
-
26.
For detection of asprosin in DMEM media from in vitro studies, add 100 μL of each standard and media samples in duplicates to the respective wells.
-
27.
For detection of asprosin from in vivo experiments, process 10 μL of plasma for proteome purification as indicated in steps 5a, iii-vi in “before you begin”.
-
28.
Concentrate the purified plasma filtrates to a volume of 40–50 μL as indicated in steps 5a, vii-viii in "before you begin"
-
29.
Add processed plasma to the respective wells and make up the volume to 100 μL with blocking buffer.
-
30.
Add 100 μL of blocking buffer to blank wells.
-
31.
Incubate the ELISA plate at 18°C–22°C for 3–4 h.
Alternatives: The plate can be incubated at 4°C overnight (16–18 h).
-
32.
Discard the solution and wash 3 times with 1× wash solution as described in step 25 above.
-
33.
Add 100 μL of (1 ng/μL) anti-asprosin rabbit monoclonal antibody to all wells and incubate the plate at room temperature for 4 h.
Alternatives: Incubate the plate at 4°C overnight (16–18 h).
-
34.
Discard the solution and wash 3 times with 1× wash solution, as described in step 25 above.
-
35.
Add 100 μL of HRP-labeled anti-Rabbit secondary antibody to each well and incubate the plate at room temperature (18°C–22°C) for 2 h.
-
36.
Discard the solution and wash 3 times with 1× wash solution, as described in step 25 above.
-
37.
Add 100 μL of substrate to each well. Cover wells and incubate for a maximum of 20 min at room temperature (18°C–22°C) in the dark with gentle shaking.
-
38.
Add 100 μL of Stop Solution to each well.
-
39.
Read absorbance at 450 nm immediately.
CRITICAL: DMEM media collected from cell culture may have some cell debris contamination. HEK293T cells also express FBN1 and asprosin (data not shown), thus it is advised to subject cell media to a quick spin (1,000 rpm, 5 min on a fixed-angle rotors at 4°C) before addition of media to the ELISA plate. An intense yellow color development on addition of substrate may lead to dark blue precipitates on addition of stop solution. An absorbance read from such samples will not be a true reflection of asprosin concentration. Therefore, color development on the ELISA plate should be monitored frequently after addition of substrate. It is also critical to read the ELISA plate immediately (within two minutes) of adding stop solution.
Expected outcomes
Media from HEK293T cells, collected 72 h post transfection with human asprosin expressing mammalian expression plasmid can be enriched with approximately 1.5–2.5 nM of human asprosin (Figure 1).
Figure 1.
In vitro overexpression of asprosin
Asprosin levels measured using ELISA in HEK293T cell conditioned media collected 72 h post transfection with 10 μg of pcDNA3.1 (empty vector control) or pcDNA3.1-IL2sp-6his-Asprosin. Asterisk (∗) indicate the range of alpha; ∗ p<0.05, ∗∗ p<0.01, ∗∗∗ p<0.001, and ∗∗∗∗ p<0.0001, as determined by student T-test. Data presented as mean ± SEM.
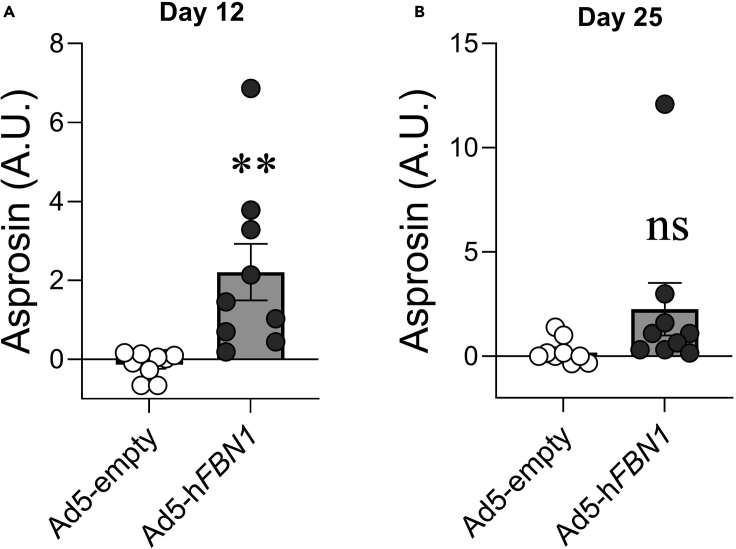
When compared to mice transduced with Ad5-empty and AAV8-empty control viral vectors, mice transduced with Ad5-FBN1and AAV8-IL2sp-6His-Asprosin are expected to exhibit hyperphagia and weight gain. An increased appetite and weight gain are reliable markers of a successful in vivo asprosin overexpression (Figure 2). Mice transduced with both viral vectors, Ad5-hFBN1 and AAV8-IL2sp-6His-Asprosin are expected to have higher detectable circulating human asprosin when compared with mice transduced with Ad5-empty and AAV8-empty viral vector (Figures 3 and 4), albeit with differences in timeline of the overexpression (Crystal, 2014; Zincarelli et al., 2008). For example, transduction with Ad5-hFBN1 viral vectors would show a transient over-expression of asprosin, as seen 12 days post-transduction in this experiment. Ad5 vectors produce a more accelerated peak expression, resulting in quicker increase in food intake and body weight (Figures 3A and 3B). However, owing to the target cell’s immune response to Ad5 vectors, this over-expression of asprosin is transient. Thus, with the use of Ad5-hFBN1, plasma asprosin levels can be expected to revert to the normal levels by day 25 post transduction (Figure 3B). In contrast, AAV8 vectors typically take up to 40–60 days to achieve peak protein expression in vivo (Crystal, 2014; Zincarelli et al., 2008). Thus, plasma samples from mice transduced with AAV8-IL2sp-6his-Asprosin before 40–60 days can be expected to not show an elevation in plasma asprosin levels (Figure 4A). Notably, around day 40–60, a 2–3 fold higher level of plasma asprosin and a concomitant increase in food intake and body weight can be expected in mice transduced with AAV8-IL2sp-6his-Asprosin (Figures 3C, 3D, and 4B).
Figure 2.
Viral overexpression of human asprosin results in increased appetite and body weight gain in lean mice
(A and B) cumulative food intake and body weight change were measured 15- and 18-days after 12-week-old, male, C57Bl/6 mice were tail-vein-injected with Ad5-empty or Ad5-hFBN1 (3.6 × 109 pfu/mouse) viral vector.
(C and D) Cumulative food intake and body weight change were measured 47 days and 60 days after 12-week-old, male, C57Bl/6 mice were tail-vein-injected with AAV8-empty or AAV8-IL2sp-6His-Asprosin (1 × 1012 GC/mouse) viral vector. Asterisk (∗) indicate the range of alpha; ∗ p<0.05, ∗∗ p<0.01, ∗∗∗ p<0.001, and ∗∗∗∗ p<0.0001, as determined by student T-test. Data presented as mean ± SEM.
Figure 3.
In vivo overexpression of Asprosin using adenoviral vector (Ad5)
(A and B) Human asprosin levels detected in plasma of Ad5-empty (control) and Ad5-hFBN1 injected C57BL/6J male mice, 12 days (A) and 25 days (B) after viral vector transduction. Asprosin detection signal is plotted relative to the average background signal detected in Ad5-empty injected mice. Asterisk (∗) indicate the range of alpha; ∗ p<0.05, ∗∗ p<0.01, ∗∗∗ p<0.001, and ∗∗∗∗ p<0.0001, and (ns) denotes the statistical non significance as determined by student T-test. Data presented as mean ± SEM.
Figure 4.
In vivo overexpression of Asprosin using adeno-associated viral vector 8
(A and B) Human asprosin levels detected in plasma of AAV8-empty (control) and AAV8-IL2sp-6His-Asprosin injected C57BL/6J male mice, 25 days (A) and 55 days (B) after adeno-associated viral vector transduction. Asprosin detection signal is plotted relative to the average background signal detected in AAV8-empty injected mice. Asterisk (∗) indicate the range of alpha; ∗ p<0.05, ∗∗ p<0.01, ∗∗∗ p<0.001, and ∗∗∗∗ p<0.0001, and (ns) denotes the statistical non significance as determined by student T-test. Data presented as mean ± SEM.
Limitations
The use of plasmids, Ad5 and AAV8 vectors in this protocol ensures a continuous overexpression of asprosin and cannot be used for study of physiological and cellular changes in response to acute asprosin treatment. Not all cell lines can be transfected with mammalian expression plasmids such as pcDNA3.1. Thus, the use of pcDNA3.1-IL2sp-6His-Asprosin plasmid for asprosin overexpression is restricted to easily transfectable cell lines such as HEK293T cells. In vivo asprosin overexpression is highly dependent on the titers of viral vectors and the efficiency of tail vein injections, thus accounting for variability in the timeline and magnitude of response. Using the appropriate amount of viral vector is very important for the outcome of experiments. If not enough viral vector is used, a significant increase in asprosin levels may not be achieved. If too much is used, it will result in cytotoxicity or other undesired effects. Viral particles (vp) to pfu (plaque forming units) ratio differs for each viral vector preparation. It is advised to check the efficiency of each new viral vector preparation in a pilot study. Additionally, as a physiological measure of successful asprosin overexpression, it is advised to corroborate changes in plasma asprosin levels with body weight and food intake increase in each experiment. Also, Ad5 vectors elicit and inflammatory and adaptive immune responses in mice and the asprosin overexpression with Ad5 vector is transient.
Troubleshooting
Problem 1
Asprosin signal not detected in plasma derived from AAV8-IL2sp-6His-Asprosin and Ad5-hFBN1 transduced mice (steps 3–8).
Potential solution
-
•
Given the variability in the efficiency of tail vein injection, track body weight and food intake in mice. An increase in body weight and food intake is a reliable marker of asprosin gain-of-function. If increase in body weight and food intake is not witnessed in the experiment, redo the experiment after testing the efficiency of viral vector preparations.
-
•
Higher volume of IgG and albumin depleted plasma samples can be used for detection of asprosin in ELISA.
-
•
Concentration of IgG and albumin depleted plasma samples can be performed with columns with hydrostat membrane. PES membrane columns are more prone to binding of proteins than Hydrostat membrane (Vivafree VFR01H22) that is designed for minimal interactions.
-
•
Asprosin Elisa troubleshooting can be done as outlined below.
Problem 2
Asprosin overexpression signal detected for less than 15 days in plasma derived from Ad5-hFBN1 transduced mice (steps 4–8).
Potential solution
There is high variability in transfection ability of adenoviral vectors, depending on the vp/mL (viral particles/ mL) and pfu/mL (plaque forming units/mL) of the Ad5 preparation. It is critical to determine the appropriate amount of active/infectious Ad5 viral vector titers for successful in vivo overexpression of asprosin. A less than optimal temporal overexpression of asprosin can be attributed to heightened cytotoxicity, and inflammatory responses in response to infection with high titer viral vector. On the other hand, if not enough virus is used, a significant overexpression of asprosin cannot be achieved. Optimal Ad5 concentrations needs to be established for each Ad5 preparation based on pilot in vitro and in vivo testing.
Problem 3
Unsuccessful transduction of HEK293T cells with asprosin-expressing plasmid (steps 11–14).
Potential solution
-
•
Adding Fugene HD transfection reagent to high concentration of plasmid can lead to DNA precipitation. Plasmid should first be diluted in serum free media, followed by the addition of transfection reagent.
-
•
Check the health of HEK293T cells every day. If excessive cell death is seen, increase the density of cells for the transfection step, or reduce the amount of DNA and transfection reagent used for transfection.
-
•
Use cell culture contamination detection kit (Thermo Fisher Scientific, Catalog # C7028) for screening cultures for contamination by microorganisms. For this, follow the manufacturer’s protocol (https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2Fmp07028.pdf).
-
•
Follow the troubleshooting guidelines as suggested in the Fugene HD transfection reagent manufacturer’s protocol (https://www.promega.com/-/media/files/resources/protocols/technical-manuals/101/fugene-hd-transfection-reagent.pdf?rev=005226306ffd4b06abc60af9b2d0ac5d&sc_lang=en).
Problem 4
Unsuccessful Asprosin detection in Sandwich ELISA (steps 37–39).
Potential solution
-
•
If a very high intensity signal is seen, use fresh TMB substrate solution which should be clear and colorless prior to addition to wells, and reduce the incubation time with TMB substrate. Ensure that the chemicals and glassware used is not contaminated. If the optical density values of samples exceed the detection range of standards, lower volume or diluted samples should be used for asprosin detection.
-
•
If a very low intensity signal is seen, ELISA should be rerun with more concentrated or higher volume of samples. Efficiency of viral vector and plasmid transduction should be tested as detailed above. Incubation at low temperatures can also affect binding efficiencies in ELISA. ELISA plates can be alternatively incubated at 37°C.
-
•
General ELISA troubleshooting tips can be followed, as recommended in Chapell et al. (2014); Gampala et al. (2019).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, [Dr. Atul R. Chopra, atul.chopra@case.edu].
Materials availability
Asprosin expressing plasmids and Ad5 and AAV8 vectors used in the study are available upon request from the lead contact.
Control plasmid (pcDNA3.1) and control adenoviral vector (Ad5-empty) and adeno-associated viral vector (AAV8-empty) are available from commercial vendors (pcDNA3.1: Creative Biogene, Cat#VET1452; Ad5-empty: Gene Vector Core, Baylor College of Medicine, Cat#100; AAV8-empty: Vector Biolabs, Cat#7600).
Rabbit anti-asprosin monoclonal antibody and mouse anti-asprosin monoclonal antibody used in the study is available upon request from the lead contact.
Acknowledgments
This work was supported by the NIDDK (DK102529, DK118290). We thank members of the Chopra lab for helpful suggestions and critical reading of the manuscript. We thank Zhiqiang An for rabbit monoclonal anti-asprosin antibody development. Summary figure created using Biorender.com.
Author contributions
Investigation, methodology, and writing, I.M.; conceptualization, resources, supervision, funding acquisition, and writing – review and editing, A.R.C.
Declaration of interests
A.R.C. has been awarded asprosin-related patents and is a co-founder, director, and officer of Vizigen, Inc., and Aceragen, Inc., and holds equity in both companies.
Data and code availability
The protocol includes all datasets generated during the study.
References
- Crystal R.G. Adenovirus: the first effective in vivo gene delivery vector. Hum. Gene Ther. 2014;25:3–11. doi: 10.1089/hum.2013.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerrschmid C., He Y., Wang C., Li C., Bournat J.C., Romere C., Saha P.K., Lee M.E., Phillips K.J., Jain M., et al. Asprosin is a centrally acting orexigenic hormone. Nat. Med. 2017;23:1444–1453. doi: 10.1038/nm.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampala S.S., Wulfkuhle B., Richey K.A. Detection of transgenic proteins by immunoassays. Methods Mol. Biol. 2019;1864:411–417. doi: 10.1007/978-1-4939-8778-8_25. [DOI] [PubMed] [Google Scholar]
- Hrapkiewicz K., Medina L. Second ed. Blackwell Publishing; 2007. Clinical Laboratory Animal Medicine. [Google Scholar]
- Mishra I., Duerrschmid C., Ku Z., He Y., Xie W., Silva E.S., Hoffman J., Xin W., Zhang N., Xu Y., et al. Asprosin-neutralizing antibodies as a treatment for metabolic syndrome. Elife. 2021;10:e63784. doi: 10.7554/eLife.63784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra I., Xie W.R., Bournat J.C., He Y., Wang C., Silva E.S., Liu H., Ku Z., Chen Y., Erokwu B.O., et al. Protein tyrosine phosphatase receptor δ serves as the orexigenic asprosin receptor. Cell Metabol. 2022;34:549–563.e8. doi: 10.1016/j.cmet.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovali M.A., Bozgeyik I. Asprosin, a C-terminal cleavage product of fibrillin 1 encoded by the FBN1 gene, in health and disease. Mol. Syndromol. 2022;13:175–183. doi: 10.1159/000520333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch M., Neels T., Tichy A., Palme R., Rülicke T. Impact assessment of tail-vein injection in mice using a modified anaesthesia induction chamber versus a common restrainer without anaesthesia. Lab. Anim. 2019;53:190–201. doi: 10.1177/0023677218786982. [DOI] [PubMed] [Google Scholar]
- Romere C., Duerrschmid C., Bournat J., Constable P., Jain M., Xia F., Saha P.K., Del Solar M., Zhu B., York B., et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell. 2016;165:566–579. doi: 10.1016/j.cell.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J., Lilly E.A., Noverr M.C., Fidel P.L. A contemporary warming/restraining device for efficient tail vein injections in a murine fungal sepsis model. J. Vis. Exp. 2020 doi: 10.3791/61961. [DOI] [PubMed] [Google Scholar]
- Zincarelli C., Soltys S., Rengo G., Rabinowitz J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The protocol includes all datasets generated during the study.