Abstract
Heavy minerals (HMs) are used in many high-tech applications (e.g. nuclear reactors, photovoltaic cells, electronics, green, and nano- and space technology), and thus global demand is increasing day by day. This review article is focused on the global distribution, genesis, economic geology, exploration and exploitation, demand (i.e. past, present, and future status of annual global production, consumption, and price), applications (geological and industrial uses), and major environmental issues mostly related to the HM sand industry. Heavy mineral deposits are distributed in more than 45 countries. Major HM deposits are located in Australia, Asia, and Africa, as secondary coastal placers bordering the Indian Ocean. Onshore and offshore deposits in the Americas, Europe, and other countries also contribute to the global HM market. Heavy mineral deposits are categorised as primary (magmatic, hydrothermal, metamorphic) or secondary (weathered, eroded, and transported sediments) deposits. Titanium, zirconium, and rare earth element (REEs) bearing minerals are key industrial commodities in the current global market. The heavy mineral industry has experienced healthy growth in unit price and global production due to increased demand generated by rapidly expanding economies such as those of China and India. Global production of zircon, ilmenite, and rutile has gradually increased over the last few decades. Global apparent consumption of ilmenite declined slightly from 1970 to 1995, in part due to introduction of stringent regulatory measures and government environmental policies in Europe and North America, as the main consumers of HMs at present. Mining and utilisation planning following the United Nations Sustainable Development Goals are highly appropriate for the sustainability of the HM industry, and to overcome ecological challenges, health issues, and social resistance towards HM exploitation. Finally, we forecast changes in production and price of three HMs (ilmenite, rutile, and zircon) for the decade from 2020 to 2030, assuming there are no disturbances in production due to external factors such as the Covid-19 global pandemic or unfavourable geopolitical interventions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12517-022-10874-0.
Keywords: Ilmenite, Rutile, Zircon, REE bearing heavy minerals, Heavy mineral supply drivers, Heavy mineral demand
Introduction
Heavy minerals (HMs) are valuable commodities for modern-day industrial applications. These minerals are denser than the common rock forming minerals such as quartz and feldspars. Boswell (1916) described HMs as “the portion of density greater than 2.8 g/cm3 which are of the greatest interest, beauty, and value from a stratigraphic point of view”. The term “heavy” refers to their high specific gravity (i.e. greater than 2.85 g/cm3 or denser than bromoform, or denser than non-toxic sodium polytungstate at 2.90 g/cm3) relative to light minerals such as quartz, feldspar, and Ca–Mg-bearing minerals (Mange and Maurer 1992; De Meijer 1998; Dill 1998, 2010; Andò 2020). For example, ilmenite is essential for manufacture of paints and aircraft, while zircon is required in the control rods of atomic reactors and in the production of advanced ceramics. Moreover, tantalite and cassiterite are essential raw materials for manufacture of transistors and tin cans. Heavy minerals are represented by a wide spectrum of mineral species, including silicates, sulphates, sulphides, oxides, and phosphates (Table 1).
Table 1.
Properties of selected heavy minerals
| Heavy mineral | Chemistry | Specific gravity (SG)/gcm−3 | Hardness (Mohs) | Magnetic susceptibility | Electrical conductivity | Radioactivity |
|---|---|---|---|---|---|---|
| Amphibole | X0-1Y2C5T8O22 1) | 2.4–3.6 | 5.0–6.0 | High | High | No |
| Andalusite | Al2SiO5 | 3.13–3.17 | 6.5–7.0 | Low | Low | No |
| Apatite | Ca5(PO4)3(F,Cl,OH) | 3.16–3.22 | 5.00 | Low | Low | No |
| Arsenopyrite | Fe3+AsS | 6.10 | 5.5–6 | Semi | Semi | No |
| Baddeleyite/caldesite | ZrO2 | 5.75 | 6.50 | Low | Low | No |
| Barite | BaSO4 | 4.50 | 3.00 | Low | Low | No |
| Biotite | K(Fe2+,Mg)2(Al,Fe3+,Mg)([Si,Al]Si2O10)(OH,F)2 | 2.8–3.4 | 2.5–3 | Low | Low | No |
| Carnotite | K2(UO2)2(VO4)2.3H2O | 4.10 | 2.00 | Low | Low | Yes |
| Cassiterite | SnO2 | 6.8–7.0 | 6.0–7.0 | Low | High | No |
| Chromite | Fe2+Cr2O | 4.5–4.8 | 5.50 | Semi | High | No |
| Chloritoid | (Fe2+,Mg,Mn)2(Al,Fe3+)(OH)4Al3O2(SiO4)2 | 3.46–3.80 | 6.50 | Low | Low | Low |
| Columbite | (Mg,Mn,Fe2+)Nb2O6 | 5.3–7.3 | 6.00 | Semi | High | No |
| Corundum | Al2O3 | 3.95–4.10 | 9.00 | Low | Low | No |
| Diamond | C | 3.52 | 10.00 | Low | Low | No |
| Epidote | X2Y3(Si2O7)(SiO4)O(OH) 2) | 3.38–3.49 | 6.0–7.0 | Low | Low | No |
| Fluorite | CaF2 | 3.18–3.56 | 4.00 | Low | High | No |
| Garnet | X3Y2Si3O12 3) | 3.9–4.2 | 7.5–8.5 | Semi | Low | No |
| Geothite | FeO(OH) or α-Fe3+O(OH) | 4.27–4.29 | 5–5.5 | Semi | High | No |
| Gold | Au | 15–19.3 | 2.5–3 | Low | High | No |
| Ilmenite | FeTiO3 | 4.5–5.0 | 5.0–5.5 | High | High | No |
| Kyanite | Al2OSiO4 | 3.56–3.67 | 4.5–7.0 | Low | Low | No |
| Leucoxene | Vary (Fe2O3.nTiO2) | 3.6–4.3 | Vary | Semi | Semi | No |
| Limonite | (Fe,O,OH,H2O) | 2.7–4.3 | 4–5.5 | Semi | Semi | No |
| Magnetite | Fe2+Fe23+O4 | 5.17–5.18 | 5.5–6.5 | High | High | No |
| Molybdenite | MoS2 | 4.62–4.73 | 1–1.5 | Semi | Semi | No |
| Monazite | (Ce,La,Th,Nd,Y)PO4 | 4.8–5.5 | 5.0–5.5 | Semi | Low | Yes |
| Muscovite | KAl2(AlSi3O10)(OH)2 | 2.8–3.1 | 2.50 | Semi | Low | No |
| Olivine | X2(SiO4) 4) | 3.3–4.4 | 6.5–7 | Low | Low | No |
| Palladium | Pd | 12.00 | 4.5–5 | Semi | High | No |
| Platinum | Pt | 21.45 | 3.50 | Semi | High | No |
| Pyrite | FeS2 | 4.8–5.0 | 6–6.5 | High | Low | No |
| Pyromorphite | Pb5(PO4)3Cl | 6.5–7.1 | 3.5–4 | Low | Semi | No |
| Pyroxene | XYSi2O6 5) | 3.2–4.9 | 5.0–6.0 | Low | Low | No |
| Rutile | TiO2 | 4.2–4.3 | 6.0–6.5 | Low | High | No |
| Scheelite | Ca(WO4) | 5.9–6.1 | 4.5–5 | Low | High | No |
| Siderite | FeCO3 | 3.87–3.96 | 3.75–4.25 | Semi | Semi | No |
| Sillimanite | Al2SiO5 | 3.24 | 6.0–7.0 | Low | Low | No |
| Sphalerite | ZnS | 3.9–4.1 | 3.5–4 | Low | Low | No |
| Sphene (titanite) | CaTiSiO5 6) | 3.48–3.6 | 5–5.5 | Low | Low | No |
| Spinel | MgAl2O4 | 3.58–3.61 (Zn rich spinel - 4.40) | 7.5–8.0 | Semi | Low | No |
| Spodumene | LiAlSi2O6 | 3.1–3.2 | 6.5–7 | Low | Low | No |
| Staurolite | Fe22+Al9Si4O23(OH) | 3.65–3.77 | 7.0–7.5 | Semi | Low | No |
| Stibnite | Sb2S3 | 4.5–4.63 | 2.00 | Low | Low | No |
| Tantalite | (Fe2+,Mg,Mn)Ta2O6 | 8+ | 6–6.5 | Semi | Semi | No |
| Thorianite | ThO2 | 9.70 | 6.5–7 | Low | Low | Yes |
| Thorite | Th(SiO4) 7) | 5.30 | 4.50 | Low | Low | Yes |
| Tourmaline | (Al,Fe,Li,Mg,Mn)3(Al,Cr, Fe,V)6(BO3)3(Si,Al,B)6O18(OH,F)4 | 3.06 | 7–7.5 | Low | Low | No |
| Uraninite | UO2 | 10.63–10.65 | 5.0–6.0 | Low | Low | Yes |
| Wolframite | WO4 | 7–7.5 | 4–4.5 | High | High | No |
| Xenotime | (Y,Yb)(PO4) 8) | 4.4–5.1 | 4.50 | High | Low | Yes * |
| Zircon | Zr(SiO4) 9) | 4.6–4.7 | 7.50 | Low | Low | Yes * |
1Where X is most commonly Na or K; Y is most commonly Na, Zn, Li, Ca, Mn, Fe2+, or Mg; C is most commonly Mg, Fe2+, Mn, Al, Fe3+, Ti, Zn, or Cr; T is most commonly Si, Al, or Ti; 2where X is most commonly Ca, Mg, Sr, Pb, or sometimes a REE; Y is most commonly Al, Fe, Mg, or V; 3where X is most commonly Fe, Mg, Ca, or Mn; Y is most commonly Al, Fe3+, or Cr; 4where X is most commonly Ca, Fe, Mn, Ni, or Mg; 5where X is most commonly Ca, Fe, Mg, Na, or Li; Y is most commonly Al or Fe; 6often contains minor amounts of Al, Fe3+, and F; 7may sometimes contain U in place of Th; 8may sometimes contain U and Th; 9may sometimes contain minor amounts of U, Th, Hf, Y/REE, and P; *depends on the amount of U and Th
Ilmenite, rutile, zircon, garnet, and monazite are the most common minerals found in HM deposits of commercial interest (Perks and Mudd 2019, 2020). Ilmenite (FeTiO3), leucoxene (altered ilmenite: Fe2O3.nTiO2), and rutile (TiO2) are critical titanium feedstock minerals, while zircon ((Zr,Hf)SiO4)) is an important source of zirconia (ZrO2), zirconium (Zr), and hafnium (Hf). These minerals are commonly referred to as valuable heavy minerals (VHMs) due to their potential usage in advanced technologies (Perks and Mudd 2019, 2020, 2021). Other HMs (Table 1) can also be referred to as valuable, depending on their economic interest, extractability, and market value. Heavy minerals and rare earth elements (REEs) are close associates. Heavy minerals such as monazite and xenotime are rich in REEs such as Ce, La, Nd, Y, and Yb (Perks and Mudd 2019, 2020; Dushyantha et al. 2020). Platinum group elements (PGEs) (e.g. platinum and palladium), diamond, gold, and corundum (e.g. sapphire and ruby) are also associated with HM placer deposits (Elhaddad 1996; Haldar 2018). These native elements and gem minerals are not the focus of our current study, except as stated in specific locations. In addition, this paper is mostly concerned with HM sands containing ilmenite, rutile, monazite, and zircon as principal and critical components.
In this review paper, we outline and evaluate the global distribution, genesis, economic geology, exploration and exploitation, and applications of HMs. We also present the cartography of global HM distribution, deposit type, and exploration targets based on the US Geological Survey (USGS) database. Finally, we evaluate and synthesise the past, present, and future status of global annual consumption, price variation, and demand of prominent HMs, and the present status of the global REE-bearing HM industry.
Global distribution of HMs
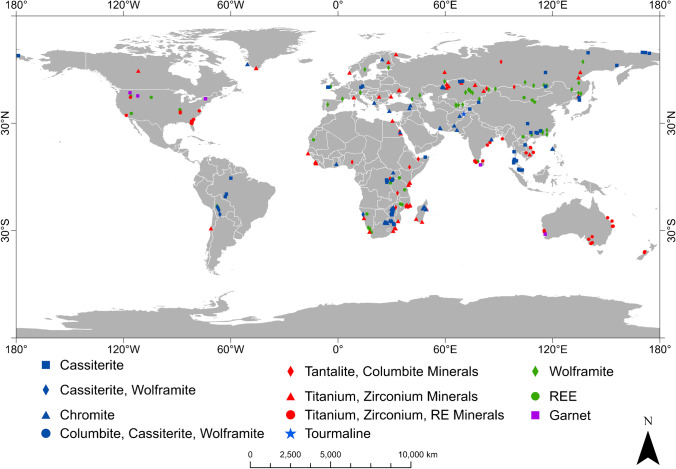
Significant heavy mineral deposits have been identified in around 45 countries (Fig. 1). However, Australia has dominated the global HM industry for many decades. Mudd and Jowitt (2016) estimated the 2015 global HM “resources” (excluding Australia) to be 564.4 million metric tonnes (Mt) of ilmenite, 24.7 Mt of rutile, and 39.2 Mt of zircon. The USGS estimated the global “reserves” in 2015 (excluding Australia; estimated Australian reserves are discussed in the “Major HM deposits bordering the Indian Ocean excluding Australia” section below) as 620 Mt of ilmenite, 32 Mt of rutile, and 27 Mt of zirconium dioxide (ZrO2, known as zirconia) (Perks and Mudd 2019; USGS 2020a). Quantitative estimates of common HMs by country are shown in Appendix 1.
Fig. 1.
Global distribution of major heavy mineral deposits and the main commodities in each occurrence (raw data from USGS 2020b)
The largest HM deposits are located along the coastal areas of countries bordering the Indian Ocean, including India, Sri Lanka, Indonesia, Malaysia, Western Australia, and the South African coastline (Hancox and Brandt 2000; Laxmi et al. 2013; Haldar 2018). Specific locations in the Eucla and Murray Basins in Australia, Eastern Australian coastline, the Indian and Sri Lankan coastlines, and Kwale and Richards Bay in South Africa are some of the best prospects for HM exploration and exploitation (Rozendaal et al. 2017; Amalan et al. 2018; Perks and Mudd 2019). The heavy mineral deposits in the coastal regions of the Indian Ocean are linked to three remarkable geological systems, namely the largest orogenic system on Earth (the Himalayan Range), the largest fluvio-deltaic system (the Bengal Delta), and the largest submarine fan system (the Bengal Deep Sea Fan) (Kuehl et al. 1989; Garzanti et al. 2004; Rahman et al. 2020). In particular, these systems have largely influenced formation of high-grade HM deposits along the coastlines of countries such as Sri Lanka, India, and Indonesia.
The eastern coast of Australia and the western coast of Africa (e.g. Grande Côte, Sierra Rutile, Namakwa, and Tormin) are also identified as potential areas for HM deposits (Rozendaal et al. 2017). The African continent has the potential to become an influential supplier of HMs (e.g. titanium feedstock and zircon) to the global market (Rozendaal et al. 2017). Several researchers have investigated potential HM deposits in offshore areas in the South Atlantic Ocean (Pilkey 1963; Gent et al. 2005). Coastal deposits in North and South America also contribute to supply of HMs to global industry (Carpenter and Carpenter 1991; Kasper-Zubillaga et al. 2008; Gonçalves and Braga 2019). HMs can be recovered as by-products from on-land oil sand deposits in Canada (Ciu et al. 2003; Kaminsky et al. 2008).
HMs and the contribution from Australia
Australia leads the global HM industry due to the availability of large deposits, both primary and secondary. For example, Australia has the world’s largest share of mineable resources of rutile and zircon, accounting for 52% and 53%, respectively. Australia is also rich in ilmenite, with the second largest resource in the world and a global share of 15%, whereas China leads with 31% (Geoscience Australia 2013). Mudd and Jowitt (2016) and Perks and Mudd (2019) estimated 2015 Australian HM “resources” of 435.2 Mt ilmenite, 60.7 Mt rutile, and 111.4 Mt zircon. These estimates included reserves. The USGS estimated 2015 Australian HMs reserves to be 201 Mt ilmenite, 27 Mt rutile, and 48 Mt of zirconium dioxide (interpreted as ZrO2), based on Geoscience Australia’s national mineral resources database (Perks and Mudd 2019; USGS 2020a). In addition, Australia accounts for 17 Mt of garnet within identifiable deposits (Perks and Mudd 2021).
The Murray and Eucla Basins, Australia
The Murray Basin is an intracratonic sedimentary basin of Cainozoic age. It extends across New South Wales, Victoria, and South Australia covering an approximate area of 300,000 km2 and it has become one of the leading HM suppliers (Roy et al. 2000; Perks and Mudd 2019; Poon et al. 2020). New prospects have recently been identified within the basin (Appendix 2). For example, Wimmera Industrial Minerals (WIM) 150 deposits (e.g. St Helens and Danube) contain a resource totalling 1.65 billion metric tonnes (Bt) of total HMs, comprising 31.4% ilmenite, 11.7% rutile, and 20.7% zircon (Klingner and Standing 2016). However, the economic importance of ilmenite production in low grade, fine-grained, sheet-like deposits in the Murray Basin (estimated volume 250 Mt) is limited due to contamination with chromium spinel (Perks and Mudd 2019). Separation of ilmenite from chromium spinel is extremely difficult due to their complex mineralogical properties such as the presence of impurity elements (e.g. Mn and Mg) and variation in the degree of ilmenite weathering (Bruckard et al. 2015). Ilmenite often contains intra-grain chromium (III) oxide known as chromia (Fisher-White et al. 2007), and so untreated low-grade ilmenite ores cannot be used in the sulphate route feedstock. However, either primary ilmenite concentrates (45–55 wt.% TiO2) or granulated slag fines (70–80 wt.% TiO2) are dissolved in 85–92% sulphuric acid at 150–180 °C to produce synthetic rutile. Freeman and Sparrow (1999) demonstrated a potential method for the removal of chromia from ilmenite in the Murray Basin. This hydrometallurgical process has not yet been commercialised in the Murray Basin (Perks and Mudd 2019).
The Eucla Basin is an onshore-offshore basin on the southern Australian passive margin (Pownceby et al. 2008). It was a Cenozoic depocentre stretching from Western Australia to South Australia (i.e. the HM deposits of Eucla Basin are located at a paleoshore with no relation to the present coastline), over a distance of about 2000 km (Hou et al. 2011). This basin is highly prospective for HMs including ilmenite, rutile, and zircon (Hou et al. 2011), mainly along the Ooldea Range (Ambrosia, Jacinth, and Tripitaka deposits) (Pownceby et al. 2008).
Other HM deposits in Australia
Aside from the Murray and Eucla Basins, several larger strings of HM deposits occur along the eastern and western Australian coastlines (Pownceby et al. 2008; Bruckard et al. 2015). For example, major coastal and offshore HM deposits occur at Bayfield, Cataby (Jackson and Christiansen 1993), Coojarloo, Eneabba, Fraser Island, Stradbroke Island (Hughes 1990), and North Stradbroke Island (Towner 1992). Black sand concentrations containing zircon-rutile-ilmenite-monazite coastal placer deposits have been reported in Victoria (Baker 1945). Ginko, Mindaire (Komesaroff 2001), WIM150 (Hughes 1990), and Chetwynd (Komesaroff 2001) represent inland HM deposits.
Major HM deposits bordering the Indian Ocean excluding Australia
Many modern and paleo-placer deposits occur bordering the Indian Ocean, mainly in coastal and shallow marine environments (Herath 1980; Wickremeratne 1986; Mallik et al. 1987; Anitha et al. 2020). Coastal stretches towards the Central Indian Ocean show the dominant provenance of their HMs. Sediments derived from Deccan Trap soils are transported through the drainage basins of the Krishna, Godavari, and Cauvery Rivers in India (Amalan et al. 2018), and sediments carried from Himalayan sources are transported to the Bay of Bengal by the Ganges, Brahmaputra, and Irrawaddy Rivers (Chaudhri 1972; Kolla and Rao 1990; Behera 2003). Jayaraju (2004) examined the factors controlling the distribution of HMs along the southern tip of India and found that source rock composition, existing drainage pattern, topography, climate, and coastal processes play key roles in their formation and distribution. Similarly, the distribution of HM placer deposits along the eastern coast of Sri Lanka and India can be controlled by summer monsoon currents in the Indian Ocean (Shankar et al. 2002; Amalan et al. 2018; Subasinghe and Ratnayake 2021).
Prominent coastal HM occurrences in India include the Chavara (Jackson and Christiansen 1993), Nindakara, Manavalakurichi (Murali et al. 1983), Kudiraimozhi (Harben and Kužvart 1996), Chatrapur (Jackson and Christiansen 1993), and Vishakhapatnam deposits (Murali et al. 1983). Heavy mineral deposits are also distributed along the coastline of Tamil Nadu/South India and at Honnavar beach, central west coast of India (Mohan and Rajamanickam 1995; Hegde et al. 2006; Gandhi and Raja 2014). Almost all of these deposits have ilmenite, rutile, zircon, leucoxene, and monazite as the main HM assemblage.
Sri Lanka is also noted for its highly concentrated HM resources, such as those at the Pulmoddai deposit. This is the largest HM deposit in Sri Lanka (8 km long and 6 m thick) and is also the highest-grade HM deposit in the world, at a grade of some 80% (Appendix 2). Several other coastal HM placer deposits also occur in Sri Lanka (Herath 1980; Wickremeratne 1986; Amalan et al. 2018; Subasinghe et al. 2021). Most of these deposits occur along the eastern coast of Sri Lanka from Panama to Mulativu, at locations such as Verugal, Kokilai, and Thirukkovil (Subasinghe et al. 2021).
The Moiskal Island deposit in Bangladesh is characterised by titanium-zirconium and REE-bearing minerals. Kuakata beach sands and Cox’s Bazaar dunes in Bangladesh are also rich in HMs (Bari et al. 2011). Besides, several HM deposits have been identified in countries bordering the Indian Ocean such as the Gulf of Thailand (Charusiri et al. 1991) and Malaysia (Kamitani and Naito 1998). Heavy mineral deposits also occur on the southwestern coast of Korea and the Pacific Ocean (Lee et al. 1988), and less economic HM placers are found towards the Arabian Sea, adjacent to countries such as Pakistan (Choudry et al. 2010).
HMs in the African Continent
The African coastline has been a site of active HM production since the early nineteenth century. Some older mines such as Cape Morgan and Umgababa, which produced titanium-rich minerals, are no longer active due to environmental constraints imposed since the 1950s (Langton and Jackson 1961). The extensive African coastline currently offers significant exploration potential, as it possibly represents the largest depository of VHMs on Earth (Rozendaal et al. 2017). For example, Richards Bay of South Africa contains the largest string of HM deposits along the Indian Ocean coast, stretching between the southeast coast of South Africa and Mozambique. Seven HM deposits in this string have been developed into producing mines (Rozendaal et al. 2017), and around 30 deposits have been identified as marginally economic for production (Tyler and Minnitt 2004; Van Gosen et al. 2014).
The combined productions of old and new mines such as Grande Côte in Senegal and Kwale in Kenya have made the African continent one of the leading global producers of titanium feedstock and the second largest producer of zircon after Australia (Rozendaal et al. 2017). Inland HMs, REEs, gold, and diamonds also feature in Africa, as inland HM placers within river basins (Hancox and Brandt 2000; Tyler and Minnitt 2004). For example, Namibia has the richest known marine diamond deposits in the world and the main commodities exported from this nation are diamonds, uranium, and zinc.
Egypt is noted for its black sands along the Mediterranean Sea coast (Ramadan et al. 2012). The radioactivity of Egyptian black sands has been the focus of many researchers, as the occurrence of radiogenic HMs such as monazite, radioactive zircon, thorite, and uranothorite raises the level of radiation along the coastline (Abdel-Karim and El-Shafey 2012; El-Gamal and Saleh 2012). Hancox and Brandt (2000) also highlight the HM potential of Liberia and its ability to enter the global HM market, based on economic concentrations of ilmenite, rutile, zircon, garnet, and kyanite.
Genesis of HM occurrences
Heavy mineral deposits can be classified as either primary or secondary deposits (Fig. 2). Magmatic, hydrothermal, and/or metamorphic processes are important in the formation of primary deposits (Skinner and Barton Jr. 1973; Mange and Wright 2007; Dill 2010; Dushyantha et al. 2020), whereas weathering, erosion, and transport of sediments are important factors in formation of secondary deposits (Zahid and Barbeau Jr. 2010; Zhong et al. 2016). Heavy minerals occur in igneous and metamorphic rocks as primary or neometamorphic phases, although usually only in minor amounts (Mange and Wright 2007). These HMs subsequently accumulate in soils and sediments during weathering and are later concentrated along meandering rivers and on floodplains. Nevertheless, large-scale secondary deposits are mostly formed along coastal areas due to hydrodynamic and gravitational sorting (Mallik et al. 1987; Cascalho and Fradique 2007; Garzanti and Andò 2007a, b, 2019; Garzanti et al. 2008; Amalan et al. 2018). Secondary deposits are favoured over primary deposits due to their size, greater HM concentrations, and lower mining and processing costs. The geological settings of HM producing areas are mainly on previously or currently active margins, whereas the majority of coastal locations are on currently passive continental margins (Fig. 2).
Fig. 2.
Types of global heavy mineral deposits (primary or secondary) (raw data from USGS 2020b)
Primary HM deposits
Several theories have been introduced to explain models of ore genesis (e.g. Skinner and Barton Jr. 1973; Beane 1979; Groves and Bierlein 2007). Guan (2014) explained the genesis of primary titanium-bearing HM ores. Iron- and titanium-bearing HM deposits associated with phosphorous and vanadium are classified into four types, namely massif-type anorthosite, mafic-ultramafic layered intrusions, mafic layered intrusions, and alkaline ultramafic complexes (Dill 2010; Guan 2014). According to Perks and Mudd (2019), only massif-type anorthosite and mafic layered intrusions are currently the focus of titanium mineral production. Intrusive magmatism and island arc magmatism followed by orogenic compression also result in iron-titanium occurrences (Dill 2010). Lac-du-Pin-Rouge and Magpie (Canada), Sanford Lake (USA), Rødsand (Norway), and Smaalands-Taberg and Ulvö (Sweden) are examples of magmatically segregated titanium-rich HM deposits (Dill 2010). The Lac Tio deposit in Canada, the Tellnes deposit (about 300 Mt of ore with an average grade of 18% TiO2) in Norway, and the Damia deposits in China are examples of massif-type anorthosite deposits (Dill 2010; Perks and Mudd 2019). All of these deposits are currently producing ilmenite. The Panzhihua deposit (870 Mt of ore) in China is an example of a mafic-layered intrusion deposit (Dill 2010; Perks and Mudd 2019, 2021), in which ilmenite is a by-product of iron-ore mining. The Machedu deposit in the Tete Massif of Mozambique, the titanium-vanadium deposit at Gusevogorsk (Central Ural Mountains) in Russia, the rutile-rich Bamble-Arendal deposit (southern Norway), the Ilímaussaq deposit (Greenland), the Piampaludo-Savona deposit (western Ligurian Alps of Italy), the Lovozero Massif (near Murmansk, Russia), the San Benito occurrence (CA, USA) and the Powderhorn Complex (Cebolla Creek) in Colorado (USA) are other examples of primary titanium deposits (Dill 2010). About 40% of global ilmenite production is derived from primary deposits (Perks and Mudd 2021).
Although zirconium exists mainly (about 93%) in sedimentary deposits (Perks and Mudd 2021), zirconium can also occur in primary HM deposits associated with igneous and metamorphic rocks (Perks and Mudd 2019). For example, the Ilímaussaq deposit (Greenland) and Seiland Island zircon syenites (Norway) are primary occurrences of zirconium (Dill 2010). However, low concentrations have restricted mining of zirconium from primary deposits. The Toongi alkaline magmatic complex south of Dubbo New South Wales (Australia) has been investigated as part of a joint zirconium-niobium-REE project (Dill 2010). Zirconium is also derived from baddeleyite (Söderlund and Johansson 2002). Russia’s Kovdor baddeleyite-apatite-magnetite deposit is the only “hard-rock” (primary) zirconium mine currently in production (Perks and Mudd 2019).
Historic “Tatara” mines in Japan are also an example of primary sources of iron sand. Titanium magnetite and ferro-titanium iron ores in igneous rocks (e.g. granite and diorite) were generated as accessory phases in intrusions cooled at depth and were later released as a result of deep weathering, and mined by sluice-box processing of deep granitic grus. Tatara mines were located at numerous locations in Shimane, Tottori, Toyama, and Kyushu prefectures. TiO2 composition of these deposits range from 1.3 to 8.8%, and the concentrates were used for production of high-quality steels for Japanese traditional swords (Tani et al. 2014).
The genesis of primary HM deposits has been thoroughly debated. Magma differentiation, assimilation, magma mixing, immiscibility, solid-state remobilisation, or a combination of these processes mainly control the formation of primary HM deposits (Charlier et al. 2006 and references therein; Dill 2010; Guan 2014). Sorting of HMs can be simply explained by gravitational accumulation due to differing densities, such as ilmenite (4.8 gcm−3) from plagioclase (2.7 gcm−3). This mechanism can result in pure ilmenite ores under appropriate conditions (slowly cooling magma chamber) such as in the Lac Tio deposit (Charlier et al. 2006). Several investigations also report genesis of ore deposits related to hydrothermal origins such as magmatic-hydrothermal and migmatitic-hydrothermal precipitation (Skinner and Barton 1973; Jiang et al. 2004; Dill 2010).
Secondary HM deposits
In secondary HM deposits, fluvial, marine, and aeolian processes are important in naturally concentrating durable and chemically resistant HMs released from weathered parent rocks (Dill 2010; Rozendaal et al. 2017; Perks and Mudd 2019). The characteristics of these deposits are governed by multiple factors, including tectonics, coastal geomorphology, sea-level fluctuations, tides, climate, and the weathering resistance and provenance of parental rocks (Rozendaal et al. 2017). For example, beach/marine deposits are characterised by concentration of resistant minerals such as ilmenite, rutile, and zircon, particularly generated during the Pliocene and Holocene epochs (Rozendaal et al. 2017; Perks and Mudd 2019). Heavy minerals are transported and sorted into higher-grade accumulations at specific sites due to the influence of coastal geomorphology, and the energy and direction of oceanic currents (Komar 2007; Amalan et al. 2018).
Macdonald (1983) proposed a classification of placer deposits into eluvial placers, colluvial placers, fluvial placers, glacial placers, littoral placers (beach and spits), aeolian placers (coastal dunes and island dunes), and marine placers. Fluvial placers such as point bars and lag gravels, riffles, and potholes are globally important for the accumulation and mining of gold, platinoids, and gemstones. HMs such as monazite and xenotime are also found as fluvial placers in Horse Creek in Aiken Country, SC, and at Magang in Hubei Province, China. Ilmenite deposits are also found as mineable fluvial placers in Central VA, USA (Elsner 2010). Alluvial placers are also sources of HM sands, such as those found in Sierra Leone in West Africa (Cardarelli 2008), but commercially exploited deposits are rare. Around 60% of ilmenite and leucoxene, and 100% of rutile and zircon in the global HM market are produced from sedimentary sources (USGS 2020b).
Marine placer deposits can be further divided into strandline and offshore deposits based on dominant grain size, deposit geometry, grade, and volume (Hughes 1990). Strandline deposits are characterised by coarse-grained, linear, high-grade, and low volume accumulations, whereas offshore deposits are characterised by fine-grained, sheet-like, low-grade, and high-volume bodies. Offshore deposits are also known as WIM-style deposits (named after “Wimmera Industrial Minerals”) (Perks and Mudd 2019). These are one of the main HM-producing areas in Australia, with the first significant sand discovery being made in the early 1980s. Although the mechanisms associated with strandline deposits are well understood, those of WIM deposits are not yet well defined (Perks and Mudd 2019).
Historical overview of geochemistry in HM analyses
Single-mineral analysis is an often-used method to quantify and interpret the detailed characteristics of mineral deposits (Mange and Morton 2007). Single-mineral analysis is mainly divided into petrographic and geochemical studies. Classical petrographic methods such as QFL (quartz, feldspar, lithics) point counting of sandstones are fundamental tools to examine geological history (e.g. provenance, tectonic setting, transportation, degree of source weathering, sorting, depositional environment, and paleoclimatic variations). Proxies such as colour, shape, habit, and zoning patterns of individual minerals can be used in petrographic studies (Mange and Morton 2007). For example, several authors have used zircon and its zoning patterns of it as petrogenetic indicators to determine multiple geological records of igneous, metamorphic, and sedimentary rocks, and as a chronological indicator for age determination using parent isotopes (Corfu et al. 2003). Tourmaline and zircon are also used as provenance indicators based on their colour variations (Krynine 1946; Mange-Rajetzky 1995). Mineralogical studies using optical microscopy have limitations due to the fine-grained nature of many sandstones, post-depositional effects, and destruction of original detrital mineralogy. This technique requires highly skilled personnel, but is a cheap and useful method for obtaining high-quality optical observations (Lane et al. 2008; Andò et al. 2012). Consequently, modern rapid, highly precise, automated geochemical analytical methods are suitable for rapid processing of a large number of samples in regional studies.
More sophisticated instruments have been developed with recent technological advancements. For example, Luepke (1980) reported the earliest geochemical analyses for provenance studies using atomic absorption techniques. Detrital HMs such as garnets, chrome spinel, ilmenite, rutile, zircon, tourmaline, amphiboles, pyroxenes, and apatite are now frequently utilised in geochemically based studies (Mange and Morton 2007). Isotopic and radiogenic (e.g. fission track dating) analyses of zircons, apatite, monazite, and titanate are also used in provenance studies (Fedo et al. 2003; Carter 2007).
Many microbeam techniques have been applied to detrital HM studies. For example, single-grain electron microbeam analysis was used during the 1980s in numerous geochemically based studies, utilising minerals such as amphibole (Mange-Rajetzky and Oberhänsli 1982), clinopyroxene (Cawood 1983), tourmaline (Henry and Guidotti 1985), garnet (Morton 1985), zircon (Owen 1987), and chrome spinel (Press 1986). Laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) has enabled high-precision determinations of trace element contents and isotopic compositions of minerals such as garnet (Zack et al. 2011; Salama et al. 2016). The sensitive high-resolution ion microprobe (SHRIMP) is used for minerals showing variation in major element composition (e.g. zircon and apatite) (Hallsworth et al. 2000; Belousova et al. 2002; Morton and Yaxley 2007). Furthermore, electron probe micro-analysis (EPMA) has been used extensively to map individual mineral grains and determine their chemical compositions (Grigsby 1991; Shimizu et al. 2019).
Analytical techniques such as X-ray fluorescence (XRF), scanning electron microscopy (SEM) with energy/wavelength dispersive X-ray (EDX/WDX) detectors, X-ray diffraction (XRD) analysis for phase detection and semi-quantitative mineralogical analyses, and inductively coupled plasma mass spectrometry (ICP-MS) and/or optical emission spectrometry (OES) can be used for understanding geochemistry of HMs (Ludden et al. 1995; Roser and Coombs 2005; Dill 2007; Zahid and Barbeau, Jr. 2010; Salama et al. 2016; Mounteney et al. 2018). However, whole-rock geochemical compositions vary due to many independent variables, and thus results need to be interpreted in combination with other lines of evidence (e.g. geology, chronology, petrography, paleontology). In addition to these techniques, Raman spectroscopy has recently gained attention in HM studies. Loose sediments, grain mounts, and thin sections can be characterised with minimal sample preparation, and hence this technique is feasible and easy to apply. Furthermore, polymorphs of Ti-oxides (i.e. rutile, anatase and brookite) and other Ti-bearing minerals can be rapidly differentiated using Raman spectroscopy (Bersani et al. 2009; Andò and Garzanti 2013; Lünsdorf et al. 2019; Borromeo et al. 2022).
Common heavy minerals
Ilmenite (FeTiO3)
Ilmenite, a dull greyish black colour mineral (also referred to as ash gold or ferrous titanate), is one of the most important economic components in the HM industry (Pownceby 2005; Pownceby and Bourne 2006). Ilmenite is a major source of titanium. Ilmenites have a wide range of composition because of their formation either by direct crystallisation from a melt or as a result of oxidation-exsolution from titanomagnetite (Dill 2010). Its formula can thus be expressed as (Fe,Mg,Mn)x(Fe,Al)yTizO(x+1.5y+2z), since its structure can accommodate Fe2O3, MnO, MgO, and Al2O3 (Kucukkaragoz and Eric 2006).
Ilmenite has been used as a fingerprinting tool in provenance studies, as detrital ilmenite retains the chemical signatures of mafic and felsic igneous rocks (Basu and Molinaroli 1991; Grigsby 1991). Schroeder et al. (2002) provide evidence of morphological changes and chemical alteration (dissolution, fracturing, solid-state transformation, anodic oxidation) of ilmenite during weathering. Nowicki et al. (2007) investigated the importance of ilmenite as a pathfinding mineral in diamond exploration. The economic importance of ilmenite depends on its grain size (i.e. increase in particle size decreases the dissolution of Ti and Fe in HCl and H2SO4), which is also an important factor during refining and chemical treatment in mineral processing (Fouda et al. 2010).
Primary ilmenite deposits are found in Canada, Norway, and China (Mudd and Jowitt 2016), whereas the Murray and Eucla Basins in Australia and the Pulmoddai deposit in Sri Lanka are examples of secondary ilmenite deposits (Amalan et al. 2018; Subasinghe et al. 2021).
Leucoxene (altered ilmenite: Fe2O3.nTiO2)
Leucoxene or altered ilmenite (referred to as a mixture of pseudorutile and rutile) is also a source of titanium (Frost et al. 1983). The colour of leucoxene is variable, from black, dark brown, light brown, light grey, reddish, orange, and yellow, through to white (Abdel-Karim et al. 2017). However, the inherent colour (commonly yellow or white) of leucoxene depends on the degree of alteration and TiO2 content with little or no Fe (Temple 1966).
The process of ilmenite alteration is known as leucoxinisation (Abdel-Karim et al. 2017), the degree of which increases the impurity levels by increasing SiO2 and Al2O3 contents (Hugo and Cornell 1991). The name “leucoxene” is used for the end product of the alteration process. Consequently, leucoxinisation affects the overall grade and magnetic susceptibility (Frost et al.,1983). Richards Bay in South Africa and coastlines bordering the Indian Ocean are prominent sites for production of leucoxene sands.
Rutile (TiO2)
Rutile is the most important titanium ore used in the production of pigments and titanium metal. Rutile occurs in a range of colours, usually in shades of deep or brownish red. Rutile may also occur greenish, bluish, or violet in the presence of impurities such as niobium (Nb) and chromium (Cr). Titanium dioxide has three natural occurring polymorphic forms, namely rutile, anatase, and brookite (in the decreasing order of geological abundance) (Dill 2010; Meinhold 2010). All these three polymorphs have a theoretical composition of 100% TiO2 (Dill 2010), although it is limited to ~ 95% in natural occurrence. Titanium dioxide also has two high-pressure forms known as srilankite (TiO2-II) and TiO2-III (Ren et al. 2000; Hanaor and Sorrell 2011). Sri Lanka, India, Australia, and Sierra Leone are significant producers of natural rutile, but reserves have decreased rapidly with recent rise in demand. Meinhold (2010) broadly discusses the applications and importance of rutile in earth science. For example, rutile is an ultra-stable mineral occurring in both ancient and recent sediments (Mange and Morton 2007) and originates from both magmatic and metamorphic parageneses. Consequently, rutile can also be used as a provenance indicator (Zack et al. 2004).
Zircon ((Zr,Hf)SiO4)
Zircon is an accessory mineral in both igneous and metamorphic rocks. Zircon is extremely resistant to weathering and abrasion, and hence is common in sedimentary rocks and in alluvial and beach placer deposits (Dryden and Dryden 1946; Armstrong-Altrin et al. 2015). Zircon occurs in a range of colours, such as colourless, red, brown, yellow, grey, pink, and green (Abdel-Karim et al. 2017). Hafnium (Hf) and zirconium (Zr) are geochemically similar, and Hf can replace Zr in the zircon crystal lattice (Abdel-Karim et al. 2017). The amounts of Hf, Y, Nb, and Ta within the crystal lattice vary between zircon species. Zircons with higher Hf contents and those with higher Y, Nb, and Ta are called alvites and naegites, respectively (Abdel-Karim et al. 2017). Zircon is also considered to be a gemstone (Jain et al. 2001; Dill 2010).
Zircons are sometimes enriched in REEs (Taylor and McLennan 1985; McLennan 1989) and are used as provenance indicators (Owen 1987; Hoskin and Ireland 2000; Hartmann and Santos 2004, and many others). Zircon was first reported from placers in 1895, and its first economic extraction dates back to 1922, from beach placers in northeast FL, USA (Elsner 2010). China, Australia, and South Africa are major producers of zircon, while Kenya, Senegal, Mozambique, Ukraine, India, Sri Lanka, and Indonesia also produce considerable amounts for the global market.
Baddeleyite (ZrO2) is found as an accessory zirconium mineral in many mafic and other silica-undersaturated plutonic rocks and dykes (Söderlund and Johansson 2002). Baddeleyite is mined at economic scale only at the primary deposit of Kovodor, Russia (Perks and Mudd 2019), and placer deposits in Brazil and Sri Lanka (Elsner 2010).
Monazite ((Ce,La,Th,Nd,Y)PO4) and xenotime ((Y,Yb)(PO4))
Monazite is the second largest source (accounting for 13% overall) of REEs after bastnaesite (83%) (Boulanger et al. 2019). Monazites are found in igneous and metamorphic rocks, and as complex ores associated with iron oxides, aluminosilicates, and apatite (Ferron et al. 1991; Chang et al. 1998; Wall 2014). However, monazite is commonly concentrated as secondary beach deposits due to its resistance to chemical weathering (Long et al. 2012). Monazite placer deposits occur in Australia, Brazil, China, India, Sri Lanka, Malaysia, South Africa, and Thailand (Dushyantha et al. 2020).
Monazite contains both uranium and thorium (Ferron et al. 1991) and is a radioactive mineral due mainly to its thorium content (O’Driscoll 1991; Mudd and Jowitt 2016; Dushyantha et al. 2020). Monazite is also a major source of cerium and light REEs due to the presence of 10–40% La2O3, 4–12% ThO2, 20–30% Ce2O3, and considerable Nd, Pr, and Sm (Thompson et al. 2012). Monazite usually occurs as honey yellow, yellowish-brown, reddish-brown, greenish-brown, grey, or colourless grains with a resinous and vitreous lustre (Abdel-Karim et al. 2017).
Xenotime is an yttrium phosphate mineral (O’Driscoll 1991) and is a potential target for rare earth oxides (REOs) and heavy REEs (Dushyantha et al. 2020). Around 55–60% of REOs are associated with xenotime (Jaireth et al. 2014; Mudd and Jowitt 2016). Xenotime is recovered as a by-product from monazite, zircon, rutile, ilmenite, and garnet placer deposits (Cheng et al. 1994) and from tin placer mining (Van Gosen et al. 2014). South Indian coasts are prominent producers of by-product xenotime from placer mining.
Sillimanite group (andalusite, kyanite, sillimanite) (Al2SiO5)
Andalusite, kyanite, and sillimanite are three sillimanite group minerals (polymorphs of Al2SiO5) (Perks and Mudd 2019). These group of minerals naturally occur in three coloured varieties (brown, blue, and yellow). The term “sillimanite minerals” is generally used for these three natural polymorphs in the industrial mineral trade in Anglo-Saxon countries (Ihlen 2000). Kyanite and andalusite are the terms commonly used in the USA and the former Soviet republics, respectively. Sillimanite minerals are important raw materials in the production of high-alumina refractory materials (Lepezin et al. 2010). About 90% of sillimanite minerals are used in the fabrication of refractories (Elsner 2010; Nyoka et al. 2013). South Africa, USA, France, and India are the main commercial producers of sillimanite minerals.
Garnet ((Ca,Mg,Fe,Mn)3(Al,Fe,Mn,V,Cr)2(SiO4)3)
The generalised chemical formula of garnet is X3Y2(SiO4)3, where X can be Ca2+, Mg2+, Fe2+, or Mn2+, and Y can be Al3+, Fe3+, Mn3+, V3+, Ti3+, or Cr3+ (Mange and Morton 2007; Grew et al. 2013; Abdel-Karim et al. 2017). The physical properties of garnets such as almandine (Fe3Al2Si3O12), spessartine (Mn3Al2Si3O12), pyrope (Mg3Al2Si3O12), and grossular (Ca3Al2Si3O12) are determined by the broad spectrum of garnet composition. For example, iron and manganese-rich garnets have higher specific gravity and hardness and possess red shading (Abdel-Karim et al. 2017). Garnets are usually red colour, but can also occur in a wide range of colours such as orange, pink, green, honey brown, and black colours. In addition, Garzanti et al. (2021) developed a methodology using Raman spectroscopy to identify the molar composition of each garnet grain as percentages of the five most frequent end members (almandine, pyrope, spessartine, andradite, and grossular).
Garnet is widely used as a provenance indicator due to its unique characteristics such as occurrence in all rock types, preservation of information during metamorphism, a wide spectrum of major element composition, stability during burial diagenesis and weathering, and resistance to abrasion (Takeuchi 1994; Von Eynatten and Gaupp 1999; Baxter et al. 2013). Garnet was regarded as a gem mineral until 1878. Garnet has been used as an abrasive since 1980, when Barton Mines Corporation started mining garnets for industrial applications. Unfortunately, only a few commercial sources of garnets remain in Australia, India, South Africa, and the USA (O’Driscoll 2010; Perks and Mudd 2021). Nevertheless, unknown quantities of garnets occur in countries such as China and Sri Lanka. The extent of these deposits is not well documented (USGS 2019; Subasinghe et al. 2021).
Staurolite (Fe22+Al9Si4O23(OH))
Staurolite has properties similar to garnet and typically yellow. Potential applications for staurolite include use as sandblasting agents, media for water filtration, water jet cutting, and glass grinding. Staurolite is found associated with other HMs in India and in parts of GA and VA in the USA (Perks and Mudd 2019).
Cassiterite (SnO2)
Cassiterite (tin oxide) is the most important source of tin. The colour of cassiterite is highly variable, ranging from black, brownish-black, reddish-brown, red, yellow, grey, and white, through to colourless (Abdel-Karim et al. 2017). Cassiterite is associated with placer deposits of gold, copper, lead, galena, bismuthinite, silver, and platinum group elements (PGEs) (Abdel-Karim et al. 2017). Cornwall and Devon in the UK was a main producer of cassiterite (tin) in the eighteenth and nineteenth centuries with multiple mines (Livingstone 1970). Primary tin mining in placer deposits of Cornwall ended just before the beginning of the twenty-first century mainly due to the collapse of International Tin Council in 1986. Current demand for cassiterite is now met by other countries, including Malaysia, Indonesia, Thailand, Nigeria, Myanmar, and China.
Magnetite (Fe3O4)
Magnetite is one of the main minerals exploited for iron extraction. Magnetite has a high magnetic susceptibility, as its name suggests. Colour is variable, from dull black to brownish-black (Abdel-Karim et al. 2017). Magnetite is a major component of black sands in volcanic regions such as Hawaii and New Zealand and is an accessory mineral associated mainly with igneous provinces (e.g. Tonkolili, Sierra Leone, and South Australian deposits).
Chrome spinel ((Mg,Fe2+)(Cr,Al,Fe3+)2O4)
Chrome spinel is found as an accessory mineral in ultramafic to mafic rocks and is more stable chemically than other minerals occurring in ultramafic rocks (Mange and Morton 2007). The colour of chrome spinel varies between shades of brown and black, and dark black grains of chrome spinel from ophiolitic sources show reddish rims. Chrome spinel is a useful fingerprint phase for provenance studies in tectonically active areas (Lee 1999; Lenaz et al. 2000). The chemistry of detrital chrome spinel is also a crucial alluvial tracer for diamond exploration in kimberlite terrains (Mange and Morton 2007). In addition, chrome spinel is used as an indicator to evaluate ore quality of Ti mineral deposits, where high Cr contents reflect lower quality (Pownceby 2005; Pownceby and Bourne 2006).
Apatite (Ca5(PO4)3(OH,F,Cl))
Apatite is common in igneous and metamorphic rocks (Chang et al. 1998). Concentration of apatite in sandstones as secondary deposits is controlled by the degree of weathering (Morton and Hallsworth 1999). Trace elements including Sr, Y, Mn, U, Th, and REEs can substitute for Ca in igneous apatite (Ayers and Watson 1991; Belousova et al. 2002). The enrichment of light REEs in apatite depends on the degree of fractionation and the REE content in the parent materials (Belousova et al. 2002; Mange and Morton 2007). For example, REE enrichment is observed in the apatite-bearing carbonatite deposit at Eppawala, Sri Lanka (Pitawala et al. 2003). Apatite ores are usually associated with igneous bodies, such as in the Kiruna apatite-magnetite deposit in Sweden and the Kola Peninsula nepheline-apatite deposit in Russia. The major practical significance of apatite is as the use of fertilizer. Apatites are also considered a gem variety mostly with transparent to translucent hues of green. Gem quality apatite is usually mined in Brazil, Burma, Madagascar, and Mexico.
Tourmaline
Tourmaline is a complex borosilicate mineral with a spectrum of compositions (Mange and Morton 2007). Tourmalines can occur in a variety of colours such as green, blue, yellow, shades of pink to red, colour zoned, black, and colourless. The formula of tourmaline can be represented as XY3(T6O18)(BO3)3V3W, where X can be filled by Na, Ca, or K, but is largely vacant. The Y site can be occupied by Li+, Mg2+, Fe2+, Mn2+, Al3+, Cr3+, V3+, Fe3+, or Ti4+, whereas T can be filled by Si, Al, and possibly B (boron). The V sites can be occupied by OH− and O2−, and W by F−, OH−, and O2− (Hawthorne and Henry 1999). Tourmaline is resistant in diagenesis and weathering (Morton and Hallsworth 1999, 2007), and hence is valuable in provenance studies (Henry and Guidotti 1985; Jiang et al. 1999; Li et al. 2004). Tourmaline is also a gem variety (Sun et al. 2019).
Amphiboles
Amphiboles are a complex group of minerals found in both igneous and metamorphic rocks. They crystallise over a wide spectrum of P-T conditions, and thus show a broad range of chemical and physical properties (Leake et al. 1997). The chemical composition of amphiboles can be expressed as A0-1B2C5T8O22(OH,F,Cl)2, where A can be Na or K; B can be Na, Zn, Li, Ca, Mn, Fe2+, or Mg; C can be Mg, Fe2+, Mn, Al, Fe3+, Ti, Zn, or Cr; and T can be Si, Al, or Ti. Amphiboles are usually dark in colour, but can range from colourless to white, green, brown, blue, and to black. Sodic amphiboles (blue amphiboles) are favoured in provenance studies because their paragenesis indicates high-pressure/low-temperature origin and exhumed subduction complexes (Schäfer et al. 1997; Mange and Morton 2007).
Chloritoid ((Fe2+,Mg,Mn)2(Al,Fe3+)(OH)4Al3O2(SiO4)2)
Chloritoid is an index mineral in metamorphic paragenesis. It is usually found in dark grey or black with greenish hues. For example, Al–Fe chloritoid with Mn is typically associated with regionally metamorphosed metapelites and high-pressure rocks with elevated Mg contents. Accordingly, detrital chloritoid can be used as a provenance indicator (Lonergan and Mange-Rajetzky 1994; Von Eynatten and Gaupp 1999).
Epidote group
Epidote group minerals are often found in regional metamorphosed rocks, but also occur in igneous and sedimentary rocks (Mange and Morton 2007). Epidote group minerals include zoisite, clinozoisite, epidote, piemonite, and allanite. These minerals can occur in a range of green shades and black. These epidote minerals are more stable during burial diagenesis than phases such as calcic amphiboles, andalusite, and sillimanite (Morton and Hallsworth 2007).
Pyroxene
Pyroxene is a common ferromagnesium rock-forming mineral in igneous and metamorphic rocks and is usually dark green to dark brown or black. Pyroxene is rather unstable during weathering and diagenesis, and consequently its survival in sedimentary records is a good indication of derivation from relatively proximal basic volcanic or plutonic sources (Mange and Morton 2007).
Exploration and exploitation of HMs
Geological concepts and methods are fundamental for the exploration and exploitation of HMs. Combinations of geological, geophysical, geochemical, and simulations (computer models) are applied in the investigation of HM deposits. Each HM deposit requires a distinct approach. However, exploration can be broadly divided into reconnaissance, detailed follow-up, and evaluation stages.
Reconnaissance exploration aims at rapid and low-cost sorting of prospective and non-prospective parts of an area. Interpretation of geophysical maps/aero geophysical investigations, satellite images and aerial photographs, and geochemical sampling of stream sediments and other regional geochemical surveys can be utilized in reconnaissance exploration. Secondly, detailed follow-up exploration is carried out where prospective areas are examined more closely to appraise mineral potential. Detailed geological mapping, geochemical and ground-based geophysical investigations, shallow trenching, and drilling can be carried out during detailed follow-up exploration (Macdonald et al. 1997; Van Gosen et al. 2014). For example, geophysical methods such as high-resolution ground and aeromagnetic surveys can be used to locate ilmenite and magnetite deposits (Rozendaal et al. 2017). Finally, at the evaluation stage, comprehensive data allow the final decision to develop a mine or to defer development. The evaluation of a successful exploration project generally concludes with compilation of reserve estimation and feasibility analysis.
Mining of HMs
HM mining in primary deposits
Primary HM deposits can be mined either open cast or underground, depending on the depth. Mining of bedrock/primary HM deposits requires considerably more energy than placer mining (e.g. Lac Tio deposit in Allard Lake, Canada, and Tellnes deposit in Norway) (Cardarelli 2008; Perks and Mudd 2019, 2021).
HM mining in secondary deposits
Secondary HM deposits can be mined by dredging (mining of material through suction or mechanical dredging from the bottom of a water body), dry mining (collection of material using front-end loaders or excavators), or hydraulic mining (with the aid of high-pressure jets). Major efforts have been made in the last two decades to develop mining methods for near-shore secondary HM deposits (e.g. monazite). Mining of placer deposits in lakes or coastal areas is done largely by dredges, with on-board storage of tailings after ore processing, and discharge of excess water and tailings back into the environment or in backfilling (Shalini et al. 2020).
HM processing and extraction
Concentration of ore from hard rock ore material
Standard mineral processing practices of primary HM deposits can be summarized as follows. Ore is crushed in jaw crushers or cone crushers and sized through grizzly. The crushed ore materials are then transferred to a beneficiation plant, where roasting, calcination, and/or chemical treatments are carried out to isolate the required component of the ore. The partially processed materials are then subjected to magnetic, electrostatic, or flotation techniques for concentration. The resulting HM concentrates are then beneficiated and smelted or leached to extract the valuable components (Gilman 2008; Perks and Mudd 2019).
Concentration of ore from heavy mineral sands
Wet sands can be used directly as feedstock in wet concentrators. Dry HM sands are washed and pumped into a wet-mill concentrator (Perks and Mudd 2019). Figure 3 illustrates the wet and dry mill processes of HM sands. For example, screens, Reichert cones, Humphrey spirals, and hydrocyclones are used to separate coarse material (rock fragments, wood, shells) and light minerals such as quartz and feldspar (Falconer 1970). The resulting HM concentrates vary according to the grade of the placer deposit (Cardarelli 2008). The tailings (gangue material) are then returned for site rehabilitation/backfilling.
Fig. 3.
Process of a typical wet concentration flow sheet for heavy minerals and b dry mill process for heavy minerals. Output from the wet process is taken as the feedstock for the dry mill process (modified after Perks and Mudd 2019)
The concentrate of the wet process is the feedstock for the dry mill, and the tailings are used in backfilling (Fig. 3). After wet processing (e.g. Jigging or Wilfley table), the concentrate is dewatered and then directed to processes such as dense media separation, magnetic separation, or electrostatic separation (Rejith and Sundararajam 2017; Perks and Mudd 2019). In the dry mill, valuable minerals are separated from non-valuable minerals (mostly quartz) using physical properties (Table 1). In addition, Pownceby et al. (2015) demonstrated the use of flotation for HM separation, and Freeman and Sparrow (1999) suggested ultra-sonic techniques to replace high-intensity magnetic separation. The processing of HM sands uses comparatively fewer chemicals such as floatation aids and is thus more environmentally friendly than other mineral industries (Gilman 2008).
Extraction
Metal extraction is usually initiated in the beneficiation plants, normally at the mine site to avoid the cost of transportation (Jordens et al. 2013). Although coarse-grained ore minerals (i.e. from primary deposits) can sometimes be separated by handpicking (usually high labour costs) or using optical ore sorting, most HM ores are fine grained (i.e. from secondary deposits) and must be well ground before separation. The crushed HMs can be separated based on the differing physical properties of ores and gangue minerals (Jordens et al. 2013; Perks and Mudd 2019). Density, magnetic, and electrical conductivity of minerals are the physical properties (Table 1) commonly used in HM processing. After concentrating the desired component, HMs are smelted or leached to isolate the targeted metals, elements, and/or oxides. Separation of metal (or the valuable component) from the concentrate can usually be done using pyrometallurgy or hydrometallurgy (e.g. solvent extraction-electrowinning) methods. For example, ilmenite ores were first smelted in NJ, USA, in the late nineteenth century, and a plant began producing titanium alloys in 1906 (Morley 1981). The first titanium white pigment was produced 2 years later to replace toxic Pb and Zn white paint pigments (Brooks 2000). Many methodologies have since been introduced for the conversion of low-grade titanium ores into synthetic rutile via chemical, physical, physicochemical, and thermochemical routes (Zhang et al. 2011; Nguyen and Lee 2018; Wijewardhana and Ratnayake 2021; Wijewardhana et al. 2021).
Applications of HMs
Geological uses
Geochemical signatures of HMs are broadly used for tracing the geological history of sedimentary terranes or examining geological processes (e.g. Bhatia and Crook 1986; Roser and Korsch 1999; Lacassie et al. 2004; Ortiz and Roser 2006; Cao et al. 2015). Therefore, understanding whole-rock geochemistry is important for geological applications. For example, HMs have been used to examine lithological source/provenance (Dill 1998; Roser and Korsch 1999), tectonic setting (Kamikubo and Takeuchi 2011), diagenesis (Morton and Hallsworth 2007), and source area weathering (Andò et al. 2012; Roy and Roser 2013). Hydrodynamic properties of HMs have been used to identify sediment transportation patterns and shoreline changes on the nearshore continental shelf (Frihy and Komar 1993; Garzanti and Andò 2019). Heavy minerals are also used to investigate paleo-tsunami deposits, as concentration decreases upwards in tsunamigenic units, whereas storm sediments feature cyclic concentrations and laminations (Costa et al. 2017). Furthermore, HMs can be used to reconstruct ice-sheet drainage patterns and river activity under present glaciers (Passchier 2007; Weckwerth and Chabowski 2013). Consequently, HMs are useful tools in paleoclimatic studies and environmental sciences.
Industrial uses
Titanium-based HMs
Titanium dioxide (TiO2) shows several remarkable properties such as photo-catalysis, high resistance to UV degradation, thermal stability, non-toxicity, chemical inertness, high refractive index, good reflectivity causing luminosity, and whiteness (Liu et al. 2005; Pihosh et al. 2009). Figure 4 shows the applications of titanium minerals after several processing stages. Although leucoxene and ilmenite are rich in titanium, there are no direct applications for these mineral phases, and hence they are mostly processed to produce TiO2 pigments. Accordingly, about 95% of titanium mineral (ilmenite, rutile, and leucoxene) production is used for manufacture of TiO2 pigment. These pigments are used in paints and varnishes, rubber, plastics, linoleum, artificial fibres, paper, glass, printing inks, and ceramics (Elsner 2010). In addition, TiO2 pigments have advanced applications in environmental, green energy, and nanotechnological fields, such as in purification filters, gas sensors, photovoltaic cells (solar cells), and electro-ceramics (Wang and Lin 2010; Tian-Hui et al. 2012; Bai et al. 2014).
Fig. 4.
Structural overview of titanium minerals: applications of titanium products after separation, beneficiation, and pigment production (modified after Elsner 2010)
Approximately 5% of the titanium minerals extracted are used to produce titanium metal, mainly through the Kroll process (Elsner 2010; Zhang et al. 2011). Titanium metal is characterised by low apparent density (4.507 gcm−3), resistance to corrosion, high mechanical strength, good thermal conductivity, high melting point, high electrical resistivity, small expansion coefficient, and non-toxicity. These properties are essential for fabrication of most of the accessories in aircraft (both fixed wing and rotary wing) and spacecraft, biomedical engineering (e.g. prostheses and implants), chemical engineering technologies, insulators for batteries, and magnets (Elsner 2010).
Zircon
About 54% of the world’s production of zircon is utilised in the ceramic industry, 14% in the foundry industry, and around 11–14% in the refractory industry (Zircon Industry Association 2020). For example, zircon is used to enhance opacity in traditional ceramics and to improve the abrasion resistance of glazed surfaces (Elsner 2010). Processed zircon is used in the control rods of atomic reactors. Advanced ceramics fabricated using zircon have characteristic properties such as high temperature and chemical stability, hardness, and corrosion resistance. Zirconia (ZrO2) ceramics can thus be utilised in items such as precision ball valves, pump seals, bearings, stamping, extrusion and drawing dies, thermal insulators, and bushings (Elsner 2010; Zircon Industry Association 2020).
Zircon also has several military applications, such as use in ammunition and explosives (Elsner 2010). Airbags, flash bulbs in photography, and oxygen sensors in petrol engines also utilise material from processed zircon sands. Zirconia (ZrO2) has dielectric and piezoelectric properties that allow the fabrication of special electro-ceramics which are used in a wide range of automotive, aerospace, and telecommunication applications, and in items like solar cells, casting alloys for turbine blades, and biomedical implants (Banerjee 1998; Gediga et al. 2019; Zircon Industry Association 2020).
REE-bearing HMs (monazite and xenotime)
Rare earth elements are referred to as the vitamins of material science (Eggert et al. 2016). Irrespective of the quantity, REEs found in heavy minerals such as monazite and xenotime can be used to alter material properties (Eggert et al. 2016; Dushyantha et al. 2020). For example, REEs increase the strength of permanent magnets in high-efficiency motors. In addition, REE-bearing HMs are used in batteries in electronic devices and vehicles, industrial processes as catalysts, and in light-emitting diodes (LEDs) (Eggert et al. 2016). Judge et al. (2017) emphasised the use of REEs in solar panels and wind turbines. Furthermore, REEs are important in agricultural operations (e.g. as feed additives for a variety of farm animals) (Pang et al. 2002; Abdelnour et al. 2019) and in military and defence applications (e.g. ammunition and smart bombs) (Massari and Ruberti 2013). Monazite and xenotime contain U and Th in their crystal lattices, and hence these radioactive minerals can also be used as alternative energy sources (Stegen 2015).
Sillimanite group HMs
Sillimanite group minerals can be used as spark plug insulators and linings of smelting and electric furnaces due to properties such as their high resistance to abrasion and slag erosion, average conductivity, high-pressure resistance, small coefficients of expansion, and excellence as refractory materials (Elsner 2010).
Garnet
Garnet sand is a good abrasive (Elsner 2010), and thus ~ 50–55% of global garnet sand production is utilised in sandblasting, and for related purposes such as removal of salt, algae, and rust from ships, and in the petrochemical industry for pipeline, tank, and boiler cleaning (Muttashar et al. 2018). Garnet can also be used as a water filtration medium and in jet cutting (Elsner 2010). In addition, garnets are valued as semi-precious stones in the gem industry (Nouri et al. 2021).
Other HMs
Other HMs also have important and advanced applications. For example, chromite is used in the steel and refractory industries. Similarly, magnetite is one of the major components of the iron ore raw material used in the steel industry. Magnetite is also utilised as a filling material in water filtration, as a dense medium for radiation shielding, as a heavy media separation, as an additive in fertilizers, and as an abrasive in sandblasting (Elsner 2010; Kulal and Badalamoole 2020). Cassiterite is mainly used for the extraction of tin metal (Angadi et al. 2015), and scheelite and wolframite are sources of tungsten metal (Kuzmin and Purans 2001). Columbite-tantalite is used to produce niobium and tantalum metals, which are essential components in the fabrication of vehicle parts (e.g. sensors in engines), camera lenses, colour screens (e.g. LED screens), ceramic capacitors, electronic devices, and medical implants (Bencharit et al. 2014; Schulz et al. 2017). Heavy minerals have also been used in forensic sciences during criminal/murder investigations by fingerprinting origins of soils by analysis of their HM assemblages (Isphording 2007; Palenik 2007). Moreover, HMs are also used in neoichonological research in tracking locomotion made by vertebrates using natural selective mineral sorting, imaging small-scale structures, their darker colour, and storm debris (Buynevich 2011).
Supply drivers and the demand for HMs
Past and present situations
Exploration targets have been progressively identified for the sustainability of the HMs industry (Fig. 5). In particular, coastal areas bordering the Indian Ocean have potential HM deposits in addition to those already discovered and developed. These promising targets and new deposits would create a surplus of HMs and hence disturb the existing market balance, as supply and demand mainly control the market price (Hugo 2015; Iluka Resources 2015; Subasinghe and Ratnayake 2022). Figure 6 shows the price variation of three prominent HMs. Heavy mineral sand products were traditionally sold on the basis of long-term contracts, often referred to as “legacy contracts.” This contractual setting leads to extended periods of relative price stability and only moderate price growth (Fig. 6). Declining grades, increasing operational costs, and adverse currency movements are the major driving forces controlling the price of raw materials. Regardless of these factors, deposits previously abandoned due to economic constraints or even due to non-feasibility may be mined subsequently with improvements in technology and increased demand (Mudd 2010; Meinert et al. 2016). Low returns act as a disincentive for new investment, and the industry is still largely reliant on mining provinces which have been operating for several decades. Avoiding “legacy contracts” thus provides opportunities to increase HM prices. For example, Iluka Resources is an Australian-based resources, exploration, and project development company. It is the largest supplier of zircon, titanium dioxide-derived rutile, and synthetic rutile to the global mineral market. Iluka Resources had quit legacy contracts by 2010, triggering an increase in HM prices (Fig. 6). The use of legacy contracts by other industry participants reportedly ended by the end of 2014, and prices thus stabilised again (Fig. 6).
Fig. 5.
Global distribution of significant heavy mineral prospects (raw data from USGS 2020b)
Fig. 6.

Unit prices of selected HM sands (ilmenite, rutile, and zircon) from 1950 to 2015, and forecast prices through to 2030 (raw data from US Bureau of Mines and the USGS Minerals Yearbook, and Mineral Commodity Summaries). A time-series analysis was carried out using Minitab 17 statistical software to forecast the future prices of these commodities
The prices of HMs also returned to normal around 2011 due to an oversupply of HMs from two new, large deposits at Kwale in Kenya and Grande Côte in Senegal (Rozendaal et al. 2017). In addition, several other multi-billion tonne HM deposits along the east coast of Africa (e.g. Corridor Sands, Mutamba, Tigen, Roode Heuwel, and Alexandria-Rosetta-El Burullus) are in the development stage. In this case, global HM sand prices are likely to fall once production begins at these deposits (Rozendaal et al. 2017). In addition, waste generation and associated waste disposal and production costs are lower for high-purity feedstock. For example, natural rutile feedstock yields less waste during pigment production compared to use of ilmenite or leucoxene feedstock (Perks and Mudd 2019). Conversely, low-grade feedstock produces greater volumes of environmentally unfriendly by-products during processing (Ward et al. 1989; Robinson et al. 1997). Accordingly, the price of HMs also depends on the types and purity of the feedstock (Perks and Mudd 2019). However, since these HMs are not traded on exchanges such as the London Metal Exchange (LME), costs have an influence on the negotiation of long-term contracts.
Zircon has become an irreplaceable mineral which has a solid market position (Gilman 2008). However, a small number of countries (Australia, some African countries, India, and Sri Lanka) produce most of the raw zircon for the global market (Perks and Mudd 2019; Subasinghe et al. 2021). Some relatively small suppliers (e.g. South Africa and Mozambique) can increase their supply, and the lack of communication (e.g. missing opportunities to sign contracts and later supply of raw materials directly to the market) from lesser or unknown suppliers (e.g. Pakistan and Nigeria) can also influence the price of HMs in the global market (Perks and Mudd 2019, 2020). Moreover, most developing countries with HM resources have various challenges and limitations for production and processing (e.g. Subasinghe et al. 2021). Geopolitics and government policies also influence demand and the price of HMs. Furthermore, high demand from specific sectors such as from the automobile, aircraft, and nuclear plant industries also influences sales.
Although China has dominated the rare earth industry with monopolistic policies, their contribution to HM industry is still comparatively low. For example, China limited its RE exports in 2010 to minimise environmental degradation and to build a strategic stockpile of REEs (Hurst 2010). This triggered a global spike in RE heavy mineral prices and created anxiety among worldwide RE stakeholders of a future shortage of raw material (Eggert et al. 2016; Dushyantha et al. 2020). Figure 7 shows the price variation of a few rare earth oxides (REOs) from 2001 to 2016. The period from 2010 to 2013 with a spike in prices is known as the “Rare Earth Crisis”. This arose from the export policies adopted by China (Dushyantha et al. 2020). For example, Australia formerly exported monazite primarily for the extraction of REOs, but with the rise of China’s REO industry, this became uneconomic from the mid-1990s (Mudd and Jowitt 2016). New mines and abandoned mines (e.g. Mountain Pass deposits in the USA) outside China became the focus in an effort to overcome RE supply shortages. China subsequently expanded its RE production and relaxed export controls by 2013. The price of several REEs then decreased (Fig. 7) as a result of additional production (Mancheri et al. 2019). Nonetheless, China still dominates the RE industry, even at lower prices of REEs. In this regard, economists and mining consultants predict that with the growth of their economy, China will gradually take control of the HM industry in the near future (Rodin 2015).
Fig. 7.

Unit prices for selected rare-earth oxides (REOs) from 2001 to 2016 (modified after Elsner 2010; Eggert et al. 2016)
Global annual supply/production (Fig. 8) of ilmenite from 1950 to 2015 greatly exceeds that of zircon and rutile (natural and synthetic) (USGS 2020b). Comparatively, the prices of zircon and rutile are higher than that of ilmenite (Fig. 6). Ilmenite is becoming the dominant mineral by value in Australia, but globally zircon is becoming more important. Global rutile production is low, mainly due to its scarcity. Production of zircon sand is historically cyclical, and periods of shortage occur due to under-supply, as zircon is often extracted as a by-product in mineral sand mining for titanium minerals (Perks and Mudd 2019, 2020, 2021). Gilman (2008) noted that a number of new operations and projects for zircon extraction have been developed since 2004, for usage in control rods of nuclear power plants.
Fig. 8.

Global annual production of zircon, ilmenite, and rutile (raw data from Bureau of Mines and the USGS Minerals Yearbook, and the Mineral Commodity Summaries). A time-series decomposition analysis was carried out using Minitab 17 statistical software to forecast production through to 2030
Annual global apparent consumption of ilmenite (1950–1995) and rutile and zircon (1950–2005) (Fig. 9) shows that titanium mineral consumption (ilmenite and rutile) greatly exceeds than of zircon. Much of the ilmenite produced is consumed for rutile production, and a portion of the HMs is stored as strategic stockpiles. Therefore, these minerals are not consumed at a rate similar to production, and the global HM consumption is not at 100% capacity (Subasinghe et al. 2021). Flattening of global apparent consumption of ilmenite between 1970 and 1995 (Fig. 9) is related to stringent regulatory measures and government policies put forward by organisations such as the Environmental Protection Agency (EPA) and Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH) in North America and Europe, both of which are leading HM consumers. These expanded regulatory measures were primarily due to the toxic waste generated during the processing of HMs such as ilmenite and leucoxene. In addition, TiO2 is coming under pressure in the European Union (EU), as for example, the use of TiO2 in food is forbidden from mid-2022.
Fig. 9.

Global annual apparent consumption of ilmenite, rutile, and zircon (raw data from US Bureau of Mines and the USGS Minerals Yearbook, and Mineral Commodity Summaries)
Predictions
Heavy mineral industries have experienced healthy growth rates in unit price and global production due to growing demand for HMs. Global production of zircon, ilmenite, and rutile (natural and synthetic) has gradually risen over the last few decades. A time series decomposition analysis suggests global production of ilmenite and rutile is expected to experience compound annual growth rates (CAGRs) of 1.27% and l.20%, respectively, over the forecast period of 2020–2030 (Fig. 8). Although global production of zircon is expected to witness a CAGR of 1.40% over the same period, individual companies may have different growth rates for these commodities. For example, Iluka Resources leads the global zirconium market with over 25% of the total production and has an estimated CAGR of 6.25% over the forecast period of 2019–2024, greater than other HM producers such as Rio Tinto, Tronox Holdings (CAGR of 6.0%), and Eramet (Report Linker 2019; Fortune Business Insights 2021).
Zircon consumption can be enhanced mainly due to the growth in foundries and refractories, increased construction of nuclear power stations in Asia-Pacific (e.g. China is the fastest-growing consumer of nuclear energy, with 12 nuclear reactors under construction, and more about to be started), and escalating usage of zirconium in surface coatings (Xiao and Jiang 2018; Report Linker 2019; World Nuclear Association 2020).
Rapidly growing economies such as those in China and India will strengthen the CAGR of global production of HMs over the forecast period. The Middle East, Africa, and Central and South America will also contribute, as will the leading HM consumers of Europe and North America. Consequently, the prices of ilmenite, rutile (natural and synthetic), and zircon are expected to witness healthy global CAGRs of 1.43%, 1.39%, and 1.62%, respectively, during the decade from 2020 to 2030 (Fig. 6). In addition, prices of these minerals may also fluctuate due to the influence of currency movements. In this study, these estimates were made assuming no disruptions such as the Covid-19 global pandemic and unfavourable geopolitical situations to the global HM market during the forecast period.
Environmental impacts, health issues, and remediation measures
Environmental impacts
Geological, geochemical, and geophysical explorations have limited impacts on the environment, as they are usually confined to access roads/survey lines and to sampling. Some activities such as core drilling have some environmental impact due to the larger-scale access required and the use of organic fluids in drilling muds. However, most environmental impacts are associated with the mining and processing of the HMs. The impacts caused during the initial stages of mining (e.g. deforestation and dust generation) are generally unavoidable. Beach sand mining operations (placer mining) are often carried out in very sensitive coastal areas and hence the damage on coastal ecosystems must be minimised. Development of sophisticated mining methods and tools can reduce the impacts on the environment (Lubke and Avis 1999). In this regard, monitoring of environmental impacts is carried out by numerous regulatory/protection agencies during the mining and processing stages.
Heavy mineral mines exploit non-renewable resources. Carbon emission during mining and processing of HMs is another environmental problem that contributes to global warming and climate change (Haque et al. 2014; Mehmood 2018). Pyrometallurgical methods are more environmentally friendly than hydrometallurgical methods (Kaniki and Tumba 2019), as the latter make extensive use of chemical reagents during the extraction and processing of HMs and REEs (De Baar et al. 1983; Ali 2014). Consequently, environmentally unfriendly by-products such as iron (III) chloride (Robinson et al. 1997) and acidic iron (II) sulphate (Ward et al. 1989) can be released during HM processing (Snyders et al. 2005). In addition, both pigment and intermediate processing (e.g. slagging) during titanium mineral processing emit large amounts of CO2 (Adams 2003). Mine-to-pigment routes differ from mineral to mineral (Perks and Mudd 2019), but in terms of greenhouse gas emissions, the differences are much narrower. In this regard, present hydrometallurgical processes should be replaced with environmentally friendly microbial methods. Apart from deliberate release of waste, sudden accidents such as tailings dam failures, collapse of tailings dams due to seismic activity, and exposure of waste material to rainwater can be identified as severe environmental problems in the HM industry (Mudd 2021). In the global context, remediation requirements are implemented as parts of law for active HM mines, although many mines were abandoned before these laws were put in place. Besides, deforestation, dust generation, groundwater contamination, and hazards (e.g. tailing dam failures, release of toxic effluents) can directly impose problems on the public. Any such impacts on the public are likely to cause increased pressure and objections towards the consenting of mining and processing of HMs.
Health issues
Toxicological examinations on REE-induced health issues are scarce. However, evidence of health issues (e.g. iatrogenic, acute kidney failures, nephrogenic systematic fibrosis) associated with the HM industry (especially for HMs containing REOs) has been reported in the literature (Chien et al. 2011; Bernstein et al. 2012). The oxidation state of iron in goethite and haematite is 3+, while magnetite has a combination of Fe2+ and Fe3+ oxidation states. The reaction rate of H2O2 with Fe2+ sites is significantly greater than that with Fe3+ sites (Entwistle et al. 2019). This oxidative stress can thus result in a variety of negative human outcomes in the iron and steel industry, including DNA damage, chronic lung inflammation (Øvrevik et al. 2015), and cardiovascular disease (Kelly and Fussell 2017). Moreover, the use of TiO2 in food has been declared no longer safe and thus its use in food processing will be ceased from the mid-2022.
Remediation measures
Environmental impacts related to the mining of HMs are virtually unavoidable, although reclamation and rehabilitation of mining ventures enable the negative outcomes to be minimised (The United Nations: Sustainable Development Goals 2018; Brooks 2000). The tailings (silica and trash minerals) generated at processing and beneficiation plants are currently either stockpiled or transported back to the mined-out pits for rehabilitation (Brooks 2000). Rehabilitation and restoration projects such as forestry (FL, USA), brushing (Western Australia), and row-crop cultivating (VA and NC, USA) on degraded environments are presently given great emphasis in ecology (Environmental Protection Agency 1995).
Radioactive and chemical waste issues are also associated with the extraction of REOs from monazite and xenotime. Environmental impacts related to HM processing can be addressed with good engineering approaches and with solutions such as extensive environmental and radiological baseline surveys, rigorous monitoring, and transparent public reporting (Mudd and Jowitt 2016). In this regard, various green energy solutions have been put forward to minimise environmentally unfriendly emissions during HM processing (Perks and Mudd 2019). For example, the Rio Tinto deposit (a stockwork, massive sulphide, and gossan hydrothermal deposit) in Canada uses hydroelectric power and generates 0.71 tonnes of CO2 per tonne of TiO2 slag. In comparison, most of the South African mines use coal as their source of energy and therefore generate 2.17 tonnes of CO2 per tonne of TiO2 slag (Adams 2003).
The heavy mineral industry should focus strictly on the problems associated with mining and mineral processing to follow the Sustainable Development Goals (SDGs) developed by the United Nations. These goals focus on good health and well-being, clean water and sanitation, affordable and clean energy, responsible consumption and production, climate action, life below water, and life on land for securing the Earth for future generations (The United Nations: Sustainable Development Goals 2018). In addition, substitution and recycling are strongly recommended to minimise the environmental impacts due to mining and processing of HMs. Hence, everyone has individual responsibilities for the betterment of this planet.
Conclusions
Countries bordering the Indian Ocean, including the Australian and African coasts, are the main producers of the world’s HMs. Most of the placer deposits found in or along these coasts commonly contain concentrated ilmenite, leucoxene, rutile, zircon, garnet, monazite, and sillimanite. Several inland HM production mines in China, Russia, Europe, and the Americas also cater to the present demands of the global HM market. Heavy minerals play a major role in advanced technology, research and development, and green energy applications. Present HM resources are sufficient to meet current global demand, except when short-term geospatial issues (e.g. conflicts between countries, terrorism, natural hazards) intervene. However, demand for HMs has grown over the past two decades due to the advancement of new technology and a move away from coal and oil-based energy sources. Global awareness should be heightened to minimise wastage, ensure sustainable utilisation, and promote recycling wherever possible to avoid spiking of prices of these non-renewable HMs (including REE-bearing HMs) resources.
The global production of valuable HMs such as ilmenite, rutile (natural and synthetic), and especially zircon is expected to grow healthily over the forecast period of 2020–2030, despite turbulences such as the Covid-19 global pandemic. The prices of HMs should also increase over the forecast period, mainly due to growth of the Chinese and Indian economies. Consequently, new prospects must be explored to investigate their potential for future production. In addition, governments and relevant regulatory bodies should implement and establish United Nations Sustainable Development Goals (SDGs) in the HM industry by introducing environmentally friendly processing methods.
Supplementary Information
Currently known global reserve estimates of placer heavy mineral deposits (sources: Elsner 2010; Geoscience Australia 2013; Perks and Mudd 2019, 2020, 2021). The definitions of resource (i.e., reasonable prospects for eventual economic extraction) and reserve (i.e., the economically mineable part of a resource) are based on Meinert et al. (2016). (XLSX 19.9 kb)
Acknowledgements
We are grateful to Editor-in-Chief Prof. Abdullah M. Al-Amri and reviewers Prof. Emanuela Schingaro and Prof. Sergio Andò for their constructive comments and suggestions on a previous version of this manuscript.
Funding
Accelerating Higher Education Expansion and Development (AHEAD) Development Oriented Research (DOR) grant funded by World Bank.
Availability of data and materials
See appendix.
Code availability
Not applicable.
Declarations
Conflicts of interest
The authors declare that they have no competing interests.
References
- Abdel-Karim AAM, El-Shafey AHM. Mineralogy and chemical distribution study of placer cassiterite and some associated new recorded minerals, east Rosetta, Egypt. Arab J Geosci. 2012;5:807–816. doi: 10.1007/s12517-011-0282-y. [DOI] [Google Scholar]
- Abdel-Karim AAM, Moustafa AI, El-Afandy AH, Barakat MG. Mineralogy, chemical characteristics and upgrading of beach ilmenite of the top meter of black sand deposits of the Kafr Al-Sheikh Governate, Northern Egypt. Acta Geol Sin. 2017;91:1326–1338. doi: 10.1111/1755-6724.13364. [DOI] [Google Scholar]
- Abdelnour SA, Abd El-Hack MEA, Khafaga AF, Noreldin AE, Arif M, Chaudhry MT, Losacco C, Abdeen A, Abdel-Daim MM. Impacts of rare earth elements on animal health and production: highlights of cerium and lanthanum. Sci Total Environ. 2019;672:1021–1032. doi: 10.1016/j.scitotenv.2019.02.270. [DOI] [PubMed] [Google Scholar]
- Adams R (2003) Plenty of TiO2 feedstock to talk about at Miami & Melbourne. Foc Pigment 1–4
- Ali SH. Social and environmental impact of the rare earth industries. Resources. 2014;3:123–134. doi: 10.3390/resources3010123. [DOI] [Google Scholar]
- Amalan K, Ratnayake AS, Ratnayake NP, Weththasinghe SM, Dushyantha N, Lakmali N, Premasiri R. Influence of nearshore sediment dynamics on the distribution of heavy minerals placer deposits in Sri Lanka. Environ Earth Sci. 2018;77:737. doi: 10.1007/s12665-018-7914-4. [DOI] [Google Scholar]
- Andò S. Gravimetric separation of heavy minerals in sediments and rocks. Minerals. 2020;10:273. doi: 10.3390/min10030273. [DOI] [Google Scholar]
- Andò S, Garzanti E. Raman spectroscopy in heavy mineral studies. In: Scott RA, Smyth HR, Morton AC, Richardson N, editors. Sediment provenance studies in hydrocarbon exploration and production. 386. London: Geological Society London Special Publications; 2013. pp. 395–412. [Google Scholar]
- Andò S, Garzanti E, Padoan M, Limonta M. Corrosion of heavy minerals during weathering and diagenesis: a catalog for optical analysis. Sediment Geol. 2012;280:165–178. doi: 10.1016/j.sedgeo.2012.03.023. [DOI] [Google Scholar]
- Angadi SI, Sreenivas T, Jeon HS, Baek SH, Mishra BK. A review of cassiterite beneficiation fundamentals and plant practices. Miner Eng. 2015;70:178–200. doi: 10.1016/j.mineng.2014.09.009. [DOI] [Google Scholar]
- Anitha JK, Joseph S, Rejith RG, Sundararajan M. Monazite chemistry and its distribution along the coast of Neendakara–Kayamkulam belt, Kerala, India. SN Appl Sci. 2020;2:1–18. doi: 10.1007/s42452-020-2594-6. [DOI] [Google Scholar]
- Armstrong-Altrin JS, Machain-Castillo ML, Rosales-Hoz L, Carranza-Edwards A, Sanchez-Cabeza JA, Ruíz-Fernández AC. Provenance and depositional history of continental slope sediments in the Southwestern Gulf of Mexico unraveled by geochemical analysis. Cont Shelf Res. 2015;95:15–26. doi: 10.1016/j.csr.2015.01.003. [DOI] [Google Scholar]
- Ayers JC, Watson EB. Solubility of apatite, monazite, zircon, and rutile in supercritical aqueous fluids with implications for subduction zone geochemistry. Philos Trans R Soc A. 1991;335:365–375. doi: 10.1098/rsta.1991.0052. [DOI] [Google Scholar]
- Bai Y, Mora-Sero I, De Angelis F, Bisquert J, Wang P. Titanium dioxide nanomaterials for photovoltaic applications. Chem Rev. 2014;114:10095–10130. doi: 10.1021/cr400606n. [DOI] [PubMed] [Google Scholar]
- Baker G. Heavy black sands on some Victorian beaches. J Sediment Res. 1945;15:11–19. doi: 10.1306/D4269206-2B26-11D7-8648000102C1865D. [DOI] [Google Scholar]
- Banerjee G. Beach sand minerals: a new material resource for glass and ceramics. Bull Mater Sci. 1998;21:349–354. doi: 10.1007/BF02744965. [DOI] [Google Scholar]
- Bari Z, Rajib M, Ameen SM. Heavy mineral assemblages of the beach sands of Kuakata, southern Bangladesh. Jahangirnagar Univ J Sci. 2011;34:143–158. [Google Scholar]
- Basu A, Molinaroli E. Reliability and application of detrital opaque Fe-Ti oxide minerals in provenance determination. J Geol Soc Lond. 1991;57:55–65. doi: 10.1144/GSL.SP.1991.057.01.06. [DOI] [Google Scholar]
- Baxter EF, Caddick MJ, Ague JJ. Garnet: common mineral, uncommonly useful. Elements. 2013;9:415–419. doi: 10.2113/gselements.9.6.415. [DOI] [Google Scholar]
- Beane RE. Genesis of metalliferous ore deposits. Rev Geophys. 1979;17:903–912. doi: 10.1029/RG017i004p00903. [DOI] [Google Scholar]
- Behera P. Heavy minerals in beach sands of Gopalpur and Paradeep along Orissa coastline, east coast of India. Indian J Mar Sci. 2003;32:172–174. [Google Scholar]
- Belousova EA, Griffin WL, O’Reilly SY, Fisher NI. Igneous zircon: trace element composition as an indicator of source rock type. Contrib Mineral Petrol. 2002;143:602–622. doi: 10.1007/s00410-002-0364-7. [DOI] [Google Scholar]
- Bencharit S, Byrd WC, Altarawneh S, Hosseini B, Leong A, Reside G, Morelli T, Offenbacher S. Development and applications of porous tantalum trabecular metal-enhanced titanium dental implants. Clin Implant Dent R. 2014;16:817–826. doi: 10.1111/cid.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein EJ, Schmidt-Lauber C, Kay J. Nephrogenic systemic fibrosis: a systemic fibrosing disease resulting from gadolinium exposure. Best Pract Res Clin Rheumatol. 2012;26:489–503. doi: 10.1016/j.berh.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Bersani D, Andò S, Vignola P, Moltifiori G, Marino IG, Lottici PP, Diella V. Micro-Raman spectroscopy as a routine tool for garnet analysis. Spectrochim Acta A Mol Biomol. 2009;73:484–491. doi: 10.1016/j.saa.2008.11.033. [DOI] [PubMed] [Google Scholar]
- Bhatia MR, Crook KA. Trace element characteristics of greywackes and tectonic setting discrimination of sedimentary basins. Contrib Mineral Petrol. 1986;92:181–193. doi: 10.1007/BF00375292. [DOI] [Google Scholar]
- Borromeo L, Andò S, Bersani D, Garzanti E, Gentile P, Mantovani L, Tribaudino M. Detrital orthopyroxene as a tracer of geodynamic setting: a Raman and SEM-EDS provenance study. Chem Geol. 2022;596:120809. doi: 10.1016/j.chemgeo.2022.120809. [DOI] [Google Scholar]
- Boswell PGH. The application of petrological and quantitative methods to stratigraphy. Geol Mag. 1916;3:105–111. doi: 10.1017/S001675680019137X. [DOI] [Google Scholar]
- Boulanger JF, Bazin C, Turgeon K. Effect of depressants and temperature on bastnaesite and monazite flotation separation from a Canadian rare earth element (REE) ore. Minerals. 2019;9:225. doi: 10.3390/min9040225. [DOI] [Google Scholar]
- Brooks DR. Reclamation of land disturbed by mining of heavy minerals. Reclam Lands Disturb Min. 2000;41:725–754. doi: 10.2134/agronmonogr41.c29. [DOI] [Google Scholar]
- Bruckard WJ, Pownceby MI, Smith LK, Sparrow GJ. Review of processing conditions for Murray Basin ilmenite concentrates. Miner Process Extr Metall Rev. 2015;124:47–63. doi: 10.1179/1743285514Y.0000000083. [DOI] [Google Scholar]
- Buynevich IV. Heavy minerals add weight to neoichonological research. Palaios. 2011;26:1–4. doi: 10.2110/palo.2011.S01. [DOI] [Google Scholar]
- Cao L, Jiang T, Wang Z, Zhang Y, Sun H. Provenance of Upper Miocene sediments in the Yinggehai and Qiongdongnan basins, north-western South China Sea; evidence from REE, heavy minerals and zircon U-Pb ages. Mar Geol. 2015;361:136–146. doi: 10.1016/j.margeo.2015.01.007. [DOI] [Google Scholar]
- Cardarelli FO. Materials Handbook. 2. London: Springer; 2008. [Google Scholar]
- Carpenter RH, Carpenter SF. Heavy mineral deposits in the upper coastal plain of North Carolina and Virginia. Econ Geol. 1991;86:1657–1671. doi: 10.2113/gsecongeo.86.8.1657. [DOI] [Google Scholar]
- Carter A (2007) Heavy minerals and detrital fission-track thermochronology. In: Mange MA, Wright DT (eds), Heavy minerals in use. Developments in Sedimentology 58, pp. 851–868. 10.1016/S0070-4571(07)58033-7
- Cascalho J, Fradique C, (2007). The sources and hydraulic sorting of heavy minerals on the northern Portuguese continental margin. in: Mange MA, Wright DT (Eds.), Heavy minerals in use. Developments in Sedimentology 58, pp. 75–110. 10.1016/S0070-4571(07)58003-9.
- Cawood PA. Modal composition of detrital clinopyroxene geochemistry of lithic sandstones from the New England Fold Belt (east Australia): a Palaeozoic forearc terrane. Geol Soc Am Bull. 1983;94:1199–1214. doi: 10.1130/0016-7606(1983)94<1199:MCADCG>2.0.CO;2. [DOI] [Google Scholar]
- Chang LL, Howie RA, Zussman J. Rock-forming minerals. 2. London: Geological Society of London; 1998. p. 383. [Google Scholar]
- Charlier B, Duchesne JC, van der Auwera J. Magma chamber processes in the Tellnes ilmenite deposit (Rogaland Anorthosite Province, SW Norway) and the formation of Fe–Ti ores in massif-type anorthosites. Chem Geol. 2006;234:264–290. doi: 10.1016/j.chemgeo.2006.05.007. [DOI] [Google Scholar]
- Charusiri P, Khantaprab C, Pisutha-Arnond V, Yumuang S. Classification of rare-earth element (REE) deposits in Thailand - a genetic approach. Mater Sci Forum. 1991;70–72:105–124. doi: 10.4028/www.scientific.net/msf.70-72.105. [DOI] [Google Scholar]
- Chaudhri RS. Heavy minerals from the Siwalik formations of the north-western Himalayas. Sediment Geol. 1972;8:77–82. doi: 10.1016/0037-0738(72)90042-5. [DOI] [Google Scholar]
- Cheng TW, Partridge AC, Tran T, Wong PLM. The surface properties and flotation behavior of xenotime. Miner Eng. 1994;7:1085–1098. doi: 10.1016/0892-6875(94)90001-9. [DOI] [Google Scholar]
- Chien CC, Wang HY, Wang JJ, Kan WC, Chien TW, Lin CY, Su SB. Risk of acute kidney injury after exposure to gadolinium-based contrast in patients with renal impairment. Ren Fail. 2011;33:758–764. doi: 10.3109/0886022X.2011.599911. [DOI] [PubMed] [Google Scholar]
- Choudry MAF, Nurgis Y, Hussain A, Abbai HN. Distribution and percentage of heavy minerals along Makran coastline of Pakistan. Am J Sci Res. 2010;11:86–91. [Google Scholar]
- Ciu Z, Liu Q, Etsell TH, Oxenford J, Coward J. Heavy minerals in the Athabasca oil sands tailings – potential and recovery process. Can Metall Q. 2003;42:383–392. doi: 10.1179/cmq.2003.42.4.383. [DOI] [Google Scholar]
- Corfu F, Hanchar JM, Hoskin PWO, Kinny P. Atlas of zircon textures. Rev Mineral Geochem. 2003;53:469–500. doi: 10.2113/0530469. [DOI] [Google Scholar]
- Costa PJM, Gelfenbaum G, Dawson S, La Selle S, Milne F, Cascalho J, Ponte Lira C, Andrade C, Freitas MC, Jaffe B. The application of microtextural and heavy mineral analysis to discriminate between storm and tsunami deposits. J Geol Soc Lond. 2017;456:167–190. doi: 10.1144/SP456.7. [DOI] [Google Scholar]
- De Baar HJW, Bacon MP, Brewer PG. Rare-earth distributions with a positive Ce anomaly in the Western North Atlantic Ocean. Nature. 1983;301:324–327. doi: 10.1038/301324a0. [DOI] [Google Scholar]
- De Meijer RJ. Heavy minerals: from ‘Edelstein’ to Einstein. J Geochem Explor. 1998;62:81–103. doi: 10.1016/S0375-6742(97)00073-3. [DOI] [Google Scholar]
- Dill HG. A review of heavy minerals in clastic sediments with case studies from the alluvial-fan through the nearshore-marine environments. Earth-Sci Rev. 1998;45:103–132. doi: 10.1016/S0012-8252(98)00030-0. [DOI] [Google Scholar]
- Dill HG. Grain morphology of heavy minerals from marine and continental placer deposits, with special reference to Fe–Ti oxides. Sediment Geol. 2007;198:1–27. doi: 10.1016/j.sedgeo.2006.11.002. [DOI] [Google Scholar]
- Dill HG. The “chessboard” classification scheme of mineral deposits: Mineralogy and geology from aluminium to zirconium. Earth-Sci Rev. 2010;100:1–420. doi: 10.1016/j.earscirev.2009.10.011. [DOI] [Google Scholar]
- Dryden L, Dryden C. Comparative rates of weathering of some common heavy minerals. J Sediment Petrol. 1946;16:91–96. doi: 10.1306/D426926A-2B26-11D7-8648000102C1865D. [DOI] [Google Scholar]
- Dushyantha N, Batapola N, Ilankoon IMSK, Rohitha S, Premasiri R, Abeysinghe B, Ratnayake N, Dissanayake K. The story of rare earth elements (REEs): occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol Rev. 2020;122:103521. doi: 10.1016/j.oregeorev.2020.103521. [DOI] [Google Scholar]
- Eggert R, Wadia C, Anderson C, Bauer D, Fields F, Meinert L, Taylor P. Rare earths: market disruption, innovation, and global supply chains. Annu Rev Environ Resour. 2016;41:199–222. doi: 10.1146/annurev-environ-110615-085700. [DOI] [Google Scholar]
- El-Gamal AA, Saleh IH. Radiological and mineralogical investigation of accretion and erosion coastal sediments in Nile Delta region, Egypt. J Oceanogr Mar Sci. 2012;3:41–55. doi: 10.5897/JOMS12.001. [DOI] [Google Scholar]
- Elhaddad MA. The first occurrence of platinum group minerals (PGM) in a chromite deposit in the Eastern Desert, Egypt. Miner Depos. 1996;31:439–445. doi: 10.1007/BF00189191. [DOI] [Google Scholar]
- Elsner H (2010) Heavy minerals of economic importance. Germany: Bundesanstalt für Geowissenschaften und Rohstoffe (BGR). Federal Institute for Geosciences and Natural Resources
- Entwistle JA, Hursthouse AS, Reis PAM, Stewart AG. Metalliferous mine dust: human health impacts and potential determinants of disease in mining communities. Curr Pollut Rep. 2019;5:67–83. doi: 10.1007/s40726-019-00108-5. [DOI] [Google Scholar]
- Environmental Protection Agency . Series on: Environmental Management in Mining. Canberra: Australian Federal Environment Department; 1995. Rehabilitation and revegetation: best practice; p. 36. [Google Scholar]
- Falconer A (1970) Gravity separation: old technique/new methods. Phys Separ Sci Eng 12
- Fedo CM, Sircombe KN, Rainbird RH. Detrital zircon analysis of the sedimentary record. Rev Mineral Geochem. 2003;53:277–303. doi: 10.2113/0530277. [DOI] [Google Scholar]
- Ferron CJ, Bulatovic SM, Salter RS. Beneficiation of rare earth oxide minerals. Mater Sci Forum. 1991;70:251–270. doi: 10.4028/www.scientific.net/MSF.70-72.251. [DOI] [Google Scholar]
- Fisher-White MJ, Freeman DE, Grey IE, Lanyon MR, Pownceby MI, Sparrow GJ. Removal of chrome spinels from Murray Basin ilmenites by low temperature roasting. Miner Process Extr Metall Rev. 2007;116:123–132. doi: 10.1179/174328507X163841. [DOI] [Google Scholar]
- Fortune Business Insights (2021) Titanium dioxide (TiO2) market to rise at 6.0% CAGR till 2027; flourishing automotive and construction industries to augment growth, Available at: https://www.fortunebusinessinsights.com/press-release/global-titanium-dioxide-tio2-market-10491 (Accessed on March 2021)
- Fouda MF, Amin RS, Saleh HI, Labib AA, Mousa HA. Preparation and characterization of nanosized titania prepared from beach black sands broad on the Mediterranean Sea coast in Egypt via reaction with acids. Aust J Basic Appl Sci. 2010;4:4540–4553. [Google Scholar]
- Freeman D, Sparrow G. Ultrasonic processing of mineral sands. Murray Basin Mineral Sands Conference. Mildura: Australian Institute of Geoscientists; 1999. [Google Scholar]
- Frihy OE, Komar PD. Long-term shoreline changes and the concentration of heavy minerals in beach sands of the Nile Delta, Egypt. Mar Geol. 1993;115:253–261. doi: 10.1016/0025-3227(93)90054-Y. [DOI] [Google Scholar]
- Frost MT, Grey IE, Harrowfield IR, Mason K. The dependence of alumina and silica contents on the extent of alteration of weathered ilmenites from Western Australia. Mineral Mag. 1983;47:201–208. doi: 10.1180/minmag.1983.047.343.10. [DOI] [Google Scholar]
- Gandhi MS, Raja M. Heavy mineral distribution and geochemical studies of coastal sediments between Besant Nagar and Marakkanam, Tamil Nadu, India. J Radiat Res. 2014;7:256–268. doi: 10.1016/j.jrras.2014.06.002. [DOI] [Google Scholar]
- Garzanti E, Andò S. Plate tectonics and heavy mineral suites of modern sands. In: Mange M, Wright D, editors. Heavy minerals in use, developments in sedimentology 58. Amsterdam: Elsevier; 2007. pp. 741–763. [Google Scholar]
- Garzanti E, Andò S. Heavy-mineral concentration in modern sands: Implications for provenance interpretation. In: Mange M, Wright D, editors. Heavy minerals in use, developments in sedimentology 58. Amsterdam: Elsevier; 2007. pp. 517–545. [Google Scholar]
- Garzanti E, Andò S. Heavy minerals for junior woodchucks. Minerals. 2019;9:148. doi: 10.3390/min9030148. [DOI] [Google Scholar]
- Garzanti E, Vezzoli G, Ando S, France-Lanord C, Singh SK, Foster G. Sand petrology and focused erosion in collision orogens: the Brahmaputra case. Earth Planet Sci Lett. 2004;220:157–174. doi: 10.1016/S0012-821X(04)00035-4. [DOI] [Google Scholar]
- Garzanti E, Andò S, Vezzoli G. Settling equivalence of detrital minerals and grain-size dependence of sediment composition. Earth Planet Sci Lett. 2008;273:138–151. doi: 10.1016/j.epsl.2008.06.020. [DOI] [Google Scholar]
- Garzanti E, Barbarano M, Andò S, Lenzi M, Deng K, Yang S. Provenance of Neogene sandstones in western Taiwan traced with garnet geochemistry and zircon geochronology. Basin Res. 2021;33:2069–2088. doi: 10.1111/bre.12548. [DOI] [Google Scholar]
- Gediga J, Morfino A, Finkbeiner M, Schulz M, Harlow K. Life cycle assessment of zircon sand. Int J Life Cycle Assess. 2019;24:1976–1984. doi: 10.1007/s11367-019-01619-5. [DOI] [Google Scholar]
- Gent RM, Alvarez MM, Iglesias JMGJ, Álvarez TJ. Offshore occurrences of heavy-mineral placers, Northwest Galicia, Spain. Mar Georesour Geotec. 2005;23:39–59. doi: 10.1080/10641190590959939. [DOI] [Google Scholar]
- Geoscience Australia . Australia’s identified mineral resources. Canberra: geoscience Australia; 2013. [Google Scholar]
- Gilman SK. Zirconium. Min Eng (Colorado) 2008;57:62–63. [Google Scholar]
- Gonçalves CC, Braga PFA. Heavy mineral sands in Brazil: deposits, characteristics, and exploration potential of selected areas. Minerals. 2019;9:176. doi: 10.3390/min9030176. [DOI] [Google Scholar]
- Grew ES, Locock AJ, Mills SJ, Galuskina IO, Galuskin EV, Hålenius U. Nomenclature of the garnet supergroup. Am Mineral. 2013;98:785–811. doi: 10.2138/am.2013.4201. [DOI] [Google Scholar]
- Grigsby JD. Chemical fingerprinting in detrital ilmenite: a viable alternative in provenance research? J Sediment Res. 1991;62:331–337. doi: 10.1306/D42678F7-2B26-11D7-8648000102C1865D. [DOI] [Google Scholar]
- Groves DI, Bierlein FP. Geodynamic settings of mineral deposit systems. J Geol Soc. 2007;164:19–30. doi: 10.1144/0016-76492006-065. [DOI] [Google Scholar]
- Guan JX. Petrogenesis of the panzhihua-type gabbroic layered intrusions and associated Fe-Ti-V oxide deposits: insights from mineral chemistry and numerical modelling (Doctoral dissertation) Tasmania: University of Tasmania; 2014. [Google Scholar]
- Haldar SK. Mineral exploration: principles and applications. 2. Amsterdam: Elsevier; 2018. [Google Scholar]
- Hallsworth CR, Morton AC, Claoue-Long JC, Fanning CM. Carboniferous sand provenance in the Pennine Basin, UK: constraints from heavy mineral and detrital zircon age data. Sediment Geol. 2000;137:147–185. doi: 10.1016/S0037-0738(00)00153-6. [DOI] [Google Scholar]
- Hanaor DAH, Sorrell CC. Review of the anatase to rutile phase transformation. J Mater Sci. 2011;46:855–874. doi: 10.1007/s10853-010-5113-0. [DOI] [Google Scholar]
- Hancox PJ, Brandt D. An overview of the heavy mineral potential of Liberia. J South Afr Inst Min Metall. 2000;100:29–34. [Google Scholar]
- Haque N, Hughes A, Lim S, Vernon C. Rare earth elements: overview of mining, mineralogy, uses, sustainability and environmental impact. Resources. 2014;3:614–635. doi: 10.3390/resources3040614. [DOI] [Google Scholar]
- Harben PW, Kužvart M. Industrial minerals: a global geology. London; Industrial Minerals Information Ltd; 1996. p. 462. [Google Scholar]
- Hartmann LA, Santos JOS. Predominance of high Th/U magmatic zircon in Brazilian Shield sandstones. Geology. 2004;32:73–76. doi: 10.1130/G20007.1. [DOI] [Google Scholar]
- Hawthorne FC, Henry DJ. Classification of the minerals of the tourmaline group. Eur J Mineral. 1999;11:201–215. doi: 10.1127/ejm/11/2/0201. [DOI] [Google Scholar]
- Hegde VS, Shalini G, Kanchanagouri DG. Provenance of heavy minerals with special reference to ilmenite of the Honnavar beach, central west coast of India. Curr Sci. 2006;91:644–648. [Google Scholar]
- Henry DJ, Guidotti CV. Tourmaline as a petrogenetic indicator mineral: an example from the staurolite-grade metapelites of NW Maine. Am Mineral. 1985;70:1–15. [Google Scholar]
- Herath JW. Economic Bulletin No. 2. 2nd revised ed. Colombo: Geological Survey Department; 1980. Mineral resources of Sri Lanka. [Google Scholar]
- Hoskin PWO, Ireland TR. Rare earth element chemistry of zircon and its use as a provenance indicator. Geology. 2000;28:627–630. doi: 10.1130/0091-7613(2000)28<627:REECOZ>2.0.CO;2. [DOI] [Google Scholar]
- Hou B, Keeling J, Reid A, Fairclough M, Warland I, Belousova E, Frakes L, Hocking R. Heavy mineral sands in the Eucla Basin, southern Australia: deposition and province-scale prospectivity. Econ Geol. 2011;106:687–712. doi: 10.2113/econgeo.106.4.687. [DOI] [Google Scholar]
- Hughes FE. Geology of the mineral deposits of Australia and Papua New Guinea: (in two volumes) Parkville, Vic: Australasian Inst Min Metall. 1990;1:828. [Google Scholar]
- Hugo V. Challenges facing the mineral sand industry – a review from Iluka Resources. Perth Western: Iluka Resources Ltd; 2015. p. 31. [Google Scholar]
- Hugo VE, Cornell DH. Altered ilmenites in Holocene dunes from Zululand, South Africa: petrographic evidence for multistage alteration. South Afr J Geol. 1991;94:365–378. [Google Scholar]
- Hurst C. China’s rare earth elements industry: what can the west learn? Washington DC: Institute for the Analysis of Global Security; 2010. [Google Scholar]
- Ihlen PM. Utilisation of sillimanite minerals, their geology, and potential occurrences in Norway – an overview. Norg Geol Unders Bull. 2000;436:113–128. [Google Scholar]
- Iluka Resources (2015) Annual report 2014. Iluka 168
- Isphording WC (2007) Forensic use of heavy minerals in civil and criminal investigations. In: Mange MA, Wright DT (Eds.), Heavy Minerals in Use. Developments in Sedimentology 58, pp. 963–982. 10.1016/S0070-4571(07)58038-6.
- Jackson WD, Christiansen G (1993) International Strategic Minerals Inventory Summary Report-rare-earth oxides (No. 930). US Government Printing Office
- Jain JC, Neal CR, Hanchar JM. Problems associated with the determination of rare earth elements of a “gem” quality zircon by inductively coupled plasma-mass spectrometry. Geostand Newslett. 2001;25:229–237. doi: 10.1111/j.1751-908X.2001.tb00598.x. [DOI] [Google Scholar]
- Jaireth S, Hoatson DM, Miezitis Y. Geological setting and resources of the major rare-earth-element deposits in Australia. Ore Geol Rev. 2014;62:72–128. doi: 10.1016/j.oregeorev.2014.02.008. [DOI] [Google Scholar]
- Jayaraju N. Controls on formation and distribution of heavy minerals along southern tip of India. J Ind Geophy Union. 2004;8:191–194. [Google Scholar]
- Jiang SY, Yang JH, Palmer MR. Chemical compositions of tourmaline from the Inch conglomerate formation, Dingle Peninsula, SW Ireland and their implication in provenance analysis. Chem Erde-Geochem. 1999;59:123–133. [Google Scholar]
- Jiang SY, Yu JM, Lu JJ. Trace and rare-earth element geochemistry in tourmaline and cassiterite from the Yunlong tin deposit, Yunnan, China: Implication for migmatitic–hydrothermal fluid evolution and ore genesis. Chem Geol. 2004;209:193–213. doi: 10.1016/j.chemgeo.2004.04.021. [DOI] [Google Scholar]
- Jordens A, Cheng YP, Waters KE. A review of the beneficiation of rare earth element bearing minerals. Miner Eng. 2013;41:97–114. doi: 10.1016/j.mineng.2012.10.017. [DOI] [Google Scholar]
- Judge WD, Xiao ZW, Kipouros GJ. Application of rare earths for higher efficiencies in energy conversion. In: Kim H, Alam S, Neelameggham N, Oosterhof H, Ouchi T, Guan X, editors. Rare Metal Technology. The Minerals, Metals & Materials Series. Cham: Springer; 2017. [Google Scholar]
- Kamikubo H, Takeuchi M. Detrital heavy minerals from Lower Jurassic clastic rocks in the Joetsu area, central Japan: Paleo-Mesozoic tectonics in the East Asian continental margin constrained by limited chloritoid occurrences in Japan. Island Arc. 2011;20:221–247. doi: 10.1111/j.1440-1738.2011.00762.x. [DOI] [Google Scholar]
- Kaminsky HAW, Etsell TH, Ivey DG, Omotoso O. Characterisation of heavy minerals in the Athabasca oil sands. Miner Eng. 2008;21:264–271. doi: 10.1016/j.mineng.2007.09.011. [DOI] [Google Scholar]
- Kamitani M, Naito K (1998) Mineral resource map of Asia: Metal Mining Agency of Japan, scale 1: 35,000,000. Available: www.mmaj.go.jp/mric_web/deposit/index.htm (Accessed July 2020)
- Kaniki AT, Tumba K. Management of mineral processing tailings and metallurgical slags of the Congolese copper belt: Environmental stakes and perspectives. J Clean Prod. 2019;210:1406–1413. doi: 10.1016/j.jclepro.2018.11.131. [DOI] [Google Scholar]
- Kasper-Zubillaga JJ, Carranza-Edwards, Morton-Bermea O. Heavy minerals and rare earth elements in coastal and inland dune sands of El Vizcaino desert, Baja California Peninsula, Mexico. Mar Georesour Geotechnol. 2008;26:172–188. doi: 10.1080/10641190802258932. [DOI] [Google Scholar]
- Kelly FJ, Fussell JC. Role of oxidative stress in cardiovascular disease outcomes following exposure to ambient air pollution. Free Radic Biol Med. 2017;110:345–367. doi: 10.1016/j.freeradbiomed.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Klingner D, Standing CA. WIM150 mineral sand deposit, Murray Basin, Australia: geology and mineral resources. Appl Earth Sci. 2016;125:121–127. doi: 10.1080/03717453.2016.1199505. [DOI] [Google Scholar]
- Kolla V, Rao NM. Sedimentary sources in the surface and near-surface sediments of the Bay of Bengal. Geo-Mar Lett. 1990;10:129–135. doi: 10.1007/BF02085927. [DOI] [Google Scholar]
- Komar PD (2007) The entrainment, transport and sorting of heavy minerals by waves and currents. in: Mange MA, Wright DT (Eds.), Heavy Minerals in Use. Developments in Sedimentology 58, pp. 3–48. 10.1016/S0070-4571(07)58001-5
- Komesaroff M. Murray Basin mineral sands - players, progress and plans. Indus Miner. 2001;410:51–53. [Google Scholar]
- Krynine PD. The tourmaline group in sediments. J Geol. 1946;54:65–87. doi: 10.1086/625323. [DOI] [Google Scholar]
- Kucukkaragoz CS, Eric RH. Solid state reduction of a natural ilmenite. Miner Eng. 2006;19:334–337. doi: 10.1016/j.mineng.2005.09.015. [DOI] [Google Scholar]
- Kuehl SA, Hariu TM, Moore WS. Shelf sedimentation of the Ganges- Brahmaputra River system: evidence for sediment bypassing to the Bengal fan. Geology. 1989;179:1132–1135. doi: 10.1130/0091-7613(1989)017<1132:SSOTGB>2.3.CO;2. [DOI] [Google Scholar]
- Kulal P, Badalamoole V. Efficient removal of dyes and heavy metal ions from waste water using Gum ghatti-graft-poly (4-acryloylmorpholine) hydrogel incorporated with magnetite nanoparticles. J Environ Chem Eng. 2020;8:104207. doi: 10.1016/j.jece.2020.104207. [DOI] [Google Scholar]
- Kuzmin A, Purans J. Local atomic and electronic structure of tungsten ions in AWO4 crystals of scheelite and wolframite types. Radiat Meas. 2001;33:583–586. doi: 10.1016/S1350-4487(01)00063-4. [DOI] [Google Scholar]
- Lacassie JP, Roser B, Del Solar JR, Hervé F. Discovering geochemical patterns using self-organizing neural networks: a new perspective for sedimentary provenance analysis. Sediment Geol. 2004;165:175–191. doi: 10.1016/j.sedgeo.2003.12.001. [DOI] [Google Scholar]
- Lane GR, Martin C, Pirard E. Techniques and applications for predictive metallurgy and ore characterisation using optical image analysis. Miner Eng. 2008;21:568–577. doi: 10.1016/j.mineng.2007.11.009. [DOI] [Google Scholar]
- Langton G, Jackson EJ. Transactions of the 7th Commonwealth Mining and Metallurgical Congress. Johannesburg: SAIMM; 1961. Recovery of ilmenite, rutile and zircon at Umgababa; pp. 1072–1091. [Google Scholar]
- Laxmi T, Srikant SS, Rao DS, Rao RB. Beneficiation studies on recovery and in-depth characterization of ilmenite from red sediments of badlands topography of Ganjam District, Odisha, India. Int J Min Sci Technol. 2013;23:725–731. doi: 10.1016/j.ijmst.2013.08.017. [DOI] [Google Scholar]
- Leake BE, Woolley AR, Arps CES, Birch WD, Gilbert MC, Grice JD, Hawthorne FC, Kato A, Kisch HJ, Krivovichev VG, Linthout K, Laird J, Mandarino J, Maresch WV, Nickel EH, Rock NMS, Schumacher JC, Smith DC, Stephenson NCN, Ungaretti L, Whittaker EJW, Youzhi G. Nomenclature of amphiboles: report of the subcommittee on amphiboles of the International Mineralogical Association Commission on new minerals and mineral names. Min Mag. 1997;61:295–310. doi: 10.1180/minmag.1997.061.405.13. [DOI] [Google Scholar]
- Lee YI. Geotectonic significance of detrital chromian spinel: a review. Geosci J. 1999;3:23. doi: 10.1007/BF02910231. [DOI] [Google Scholar]
- Lee HJ, Jeong KS, Han SJ, Bahk KS. Heavy minerals indicative of Holocene transgression in the southeastern Yellow Sea. Cont Shelf Res. 1988;8:255–266. doi: 10.1016/0278-4343(88)90032-5. [DOI] [Google Scholar]
- Lenaz D, Kamenetsky VS, Crawford AJ, Princivalle F. Melt inclusions in detrital spinel from the SE Alps (Italy–Slovenia): a new approach to provenance studies of sedimentary basins. Contrib Mineral Petrol. 2000;139:748–758. doi: 10.1007/s004100000170. [DOI] [Google Scholar]
- Lepezin GG, Kargopolov SA, Zhirakovskii VY. Sillimanite group minerals: a new promising raw material for the Russian aluminium industry. Russ Geol Geophys. 2010;51:1247–1256. doi: 10.1016/j.rgg.2010.11.004. [DOI] [Google Scholar]
- Li R, Li S, Jin F, Wan Y, Zhang S. Provenance of Carboniferous sedimentary rocks in the northern margin of Dabie Mountains, central China and the tectonic significance: constraints from trace elements, mineral chemistry and SHRIMP dating of zircons. Sediment Geol. 2004;166:245–264. doi: 10.1016/j.sedgeo.2003.12.009. [DOI] [Google Scholar]
- Liu B, Zhao X, Zhao Q, Li C, He X. The effect of O2 partial pressure on the structure and photocatalytic property of TiO2 films prepared by sputtering. Mater Chem Phys. 2005;90:207–212. doi: 10.1016/j.matchemphys.2004.10.038. [DOI] [Google Scholar]
- Livingstone A (1970) Park Jr. and MacDiarmid RA Ore Deposits. San Francisco and London (WH Freeman and Co.) 1964. Mineral Mag 37(289):634–635
- Lonergan L, Mange-Rajetzky MA. Evidence for the internal zone unroofing from foreland basin sediments, Betic Cordillera, SE Spain. J Geol Soc. 1994;151:515–529. doi: 10.1144/gsjgs.151.3.0515. [DOI] [Google Scholar]
- Long KR, Van Gosen BS, Foley, .K, Cordier D, (2012). The principal rare earth elements deposits of the United States: a summary of domestic deposits and a global perspective. In: Non-Renewable Resource Issues, 131–155.
- Lubke RA, Avis AM. A review of the concepts and application of rehabilitation following heavy mineral dune mining. Mar Pollut Bull. 1999;37:546–557. doi: 10.1016/S0025-326X(99)00138-1. [DOI] [Google Scholar]
- Ludden JN, Feng R, Gauther G, Stix J, Shi L, Francis D, Machado N, Wu G. Applications of LAM-ICP-MS analysis to minerals. Can Mineral. 1995;33:419–434. [Google Scholar]
- Luepke G. Opaque minerals as aids in distinguishing between source and sorting effects on beach-sand mineralogy in southwestern Oregon. J Sediment Res. 1980;50:489–496. doi: 10.1306/212F7A36-2B24-11D7-8648000102C1865D. [DOI] [Google Scholar]
- Lünsdorf NK, Kalies J, Ahlers P, Dunkl I, von Eynatten H. Semi-automated heavy-mineral analysis by Raman spectroscopy. Minerals. 2019;9:385. doi: 10.3390/min9070385. [DOI] [Google Scholar]
- Macdonald EH. Alluvial mining. The geology, technology and economics of placers. London: Springer; 1983. [Google Scholar]
- Macdonald WG, Rozendaal A, De Meijer RJ. Radiometric characteristics of heavy mineral deposits along the west coast of South Africa. Mineral Deposita. 1997;32:371–381. doi: 10.1007/s001260050103. [DOI] [Google Scholar]
- Mallik TK, Vasudevan V, Verghese PA, Machado T. The black sand placer deposits of Kerala beach, southwest India. Mar Geol. 1987;77:129–150. doi: 10.1016/0025-3227(87)90088-0. [DOI] [Google Scholar]
- Mancheri NA, Sprecher B, Bailey G, Ge J, Tukker A. Effect of Chinese policies on rare earth supply chain resilience. Resour Conserv Recycl. 2019;142:101–112. doi: 10.1016/j.resconrec.2018.11.017. [DOI] [Google Scholar]
- Mange MA, Maurer HFW. Heavy minerals in colour. London: Chapman and Hall; 1992. p. 147. [Google Scholar]
- Mange MA, Morton AC (2007) Geochemistry of heavy minerals. In: Mange MA. Wright, D.T. (Eds.), Heavy Minerals in Use. Developments in Sedimentology 58:345–391. 10.1016/S0070-4571(07)58013-1
- Mange MA, Wright DT (2007) High-resolution heavy mineral analysis (HRHMA): a brief summary. In: Mange M A & Wright DT (eds) Heavy Minerals in Use. Elsevier, Amsterdam, Developments in Sedimentology 58, pp. 433–436. 10.1016/S0070-4571(07)58016-7
- Mange-Rajetzky MA. Subdivision and correlation of monotonous sandstone sequences using high resolution heavy mineral analysis, a case study: the Triassic of the Central Graben. J Geol Soc Lond. 1995;89:23–30. doi: 10.1144/GSL.SP.1995.089.01.03. [DOI] [Google Scholar]
- Mange-Rajetzky MA, Oberhänsli R. Detrital lawsonite and blue sodic amphibole in the Molasse of Savoy, France and their significance in assessing Alpine evolution. Schweiz Mineral Petrogr. 1982;62:415–436. [Google Scholar]
- Massari S, Ruberti M. Rare earth elements as critical raw materials: focus on international markets and future strategies. Res Policy. 2013;38:36–43. doi: 10.1016/j.resourpol.2012.07.001. [DOI] [Google Scholar]
- McLennan SM (1989) Rare earth elements in sedimentary rocks: influence of provenance and sedimentary processes. In: Lipin BR and McKay GA (eds) Geochemistry and Mineralogy of Rare Earth Elements, pp. 169–200. 10.1515/9781501509032-010
- Mehmood M. Rare earth elements-a review. J Ecol Nat Resour. 2018;2:1–6. [Google Scholar]
- Meinert LD, Robinson GR, Nassar NT. Mineral resources: Reserves, peak production and the future. Resources. 2016;5:14. doi: 10.3390/resources5010014. [DOI] [Google Scholar]
- Meinhold G. Rutile and its applications in earth sciences. Earth-Sci Rev. 2010;102:1–28. doi: 10.1016/j.earscirev.2010.06.001. [DOI] [Google Scholar]
- Mohan PM, Rajamanickam GV. Distribution of heavy minerals in Parangipettai (Porto Novo) beach, Tamil Nadu. J Geol Soc India. 1995;46:401–408. [Google Scholar]
- Morley IW. Black sands: a history of the mineral sand mining industry in eastern Australia. St. Lucia: University of Queensland Press; 1981. [Google Scholar]
- Morton AC. A new approach to provenance studies: electron microprobe analysis of detrital garnets from Middle Jurassic sandstones of the northern North Sea. Sedimentology. 1985;32:553–566. doi: 10.1111/j.1365-3091.1985.tb00470.x. [DOI] [Google Scholar]
- Morton AC, Hallsworth CR. Processes controlling the composition of heavy mineral assemblages in sandstones. Sediment Geol. 1999;124:3–29. doi: 10.1016/S0037-0738(98)00118-3. [DOI] [Google Scholar]
- Morton AC, Hallsworth CR (2007) Stability of detrital heavy minerals during burial diagenesis. in: Mange, M.A., Wright, D.T. (Eds.), Heavy Minerals in Use. Developments in Sedimentology 58:215–245. 10.1016/S0070-4571(07)58007-6
- Morton AC, Yaxley G. Detrital apatite geochemistry and its application in provenance studies. Geol Soc Am Bull. 2007;420:319–344. [Google Scholar]
- Mounteney I, Burton AK, Farrant AR, Watts MJ, Kemp SJ, Cook JM. Heavy mineral analysis by ICP-AES a tool to aid sediment provenancing. J Geochem Explor. 2018;184:1–10. doi: 10.1016/j.gexplo.2017.10.007. [DOI] [Google Scholar]
- Mudd GM. The environmental sustainability of mining in Australia: key mega-trends and looming constraints. Res Policy. 2010;35:98–115. doi: 10.1016/j.resourpol.2009.12.001. [DOI] [Google Scholar]
- Mudd GM. Encyclopedia of Geology. 2nd ed, 5. London: Academia Press; 2021. The resources cycle: key sustainability issues for the mining of metals and minerals; pp. 607–620. [Google Scholar]
- Mudd GM, Jowitt SM. Rare earth elements from heavy mineral sands: assessing the potential of a forgotten resource. Appl Earth Sci. 2016;125:107–113. doi: 10.1080/03717453.2016.1194955. [DOI] [Google Scholar]
- Murali AV, Parthasarathy R, Mahadevan TM, Das MS. Trace element characteristics, REE patterns and partition coefficients of zircons from different geological environments–a case study on Indian zircons. Geochim Cosmochim Acta. 1983;47:2047–2052. doi: 10.1016/0016-7037(83)90220-X. [DOI] [Google Scholar]
- Muttashar HL, Ali NB, Ariffin MAM, Hussin MW. Microstructures and physical properties of waste garnets as a promising construction material. Case Stud Constr Mater. 2018;8:87–96. doi: 10.1016/j.cscm.2017.12.001. [DOI] [Google Scholar]
- Nguyen TH, Lee MS. A review on the recovery of titanium dioxide from ilmenite ores by direct leaching technologies. Miner Process Extr Metall Rev. 2018;40:231–247. doi: 10.1080/08827508.2018.1502668. [DOI] [Google Scholar]
- Nouri F, Stern RJ, Azizi H (2021) A review of garnet deposits in western and southern Iran. Int Geol Rev 1–28. 10.1080/00206814.2020.1838335
- Nowicki TE, Moore RO, Gurney JJ, Baumgartner MC (2007) Diamonds and associated heavy minerals in kimberlite: a review of key concepts and applications. in: Mange MA, Wright DT (Eds.), Heavy Minerals in Use. Developments in Sedimentology 58:1235–1267. 10.1016/S0070-4571(07)58046-5
- Nyoka M, Brazier D, Courtney T, Parry RA. Merits of using andalusite-based refractories compared to bauxite-based refractories. J South Afr Inst Min Metall. 2013;113:647–650. [Google Scholar]
- O’Driscoll M. An overview of rare earth minerals supply and applications. Mater Sci Forum. 1991;70:409–420. doi: 10.4028/www.scientific.net/MSF.70-72.409. [DOI] [Google Scholar]
- O’Driscoll M (2010) End user focus: scratching the surface. Industrial Minerals. Available at: https://www.indmin.com/Article/2698282/AluminaBauxite-Features/End-User-Focus-Scratching-the-surface.html (Accessed on March 2021)
- Ortiz E, Roser BP. Major and trace element provenance signatures in stream sediments from the Kando river, San’in district, southwest Japan. Island Arc. 2006;15:223–238. doi: 10.1111/j.1440-1738.2006.00523.x. [DOI] [Google Scholar]
- Øvrevik J, Refsnes M, Låg M, Holme JA, Schwarze PE. Activation of pro-inflammatory responses in cells of the airway mucosa by particulate matter: oxidant- and non-oxidant mediated triggering mechanisms. Biomolecules. 2015;5:1399–1440. doi: 10.3390/biom5031399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MR. Hafnium content of detrital zircons, a new tool for provenance study. J Sediment Res. 1987;57:824–830. doi: 10.1306/212F8C74-2B24-11D7-8648000102C1865D. [DOI] [Google Scholar]
- Palenik S (2007) Heavy minerals in forensic science. in: Mange MA Wright DT (Eds.), Heavy Minerals in Use. Developments in Sedimentology 58, pp. 937–961. 10.1016/S0070-4571(07)58037-4
- Pang X, Li D, Peng A. Application of rare-earth elements in the agriculture of China and its environmental behaviour in soil. Environ Sci Pollut Res. 2002;9:143. doi: 10.1007/BF02987462. [DOI] [PubMed] [Google Scholar]
- Passchier S (2007) The use of heavy minerals in the reconstruction of ice-sheet drainage patterns: An example from the edge of the east Antarctic ice sheet. In: Mange MA, Wright DT (Eds.), Heavy Minerals in Use. Developments in Sedimentology 58, pp. 677–699. 10.1016/S0070-4571(07)58027-1
- Perks C, Mudd G. Titanium, zirconium resources and production: a state of the art literature review. Ore Geol Rev. 2019;107:629–646. doi: 10.1016/j.oregeorev.2019.02.025. [DOI] [Google Scholar]
- Perks C, Mudd G (2020) A detailed assessment of global Zr and Ti production. Miner Econ:1–26. 10.1007/s13563-020-00240-5
- Perks C, Mudd G (2021) Soft rocks, hard rocks: the world’s resources and reserves of Ti and Zr and associated critical minerals. Int Geol Rev:1–22. 10.1080/00206814.2021.1904294
- Pihosh Y, Goto M, Kasahara A, Tosa M. Photocatalytic property of TiO2 thin films sputtered-deposited on unheated substrates. Appl Surf Sci. 2009;256:937–942. doi: 10.1016/j.apsusc.2009.05.155. [DOI] [Google Scholar]
- Pilkey OH. Heavy minerals of the U.S. south Atlantic continental shelf and slope. Geol Soc Am Bull. 1963;74:641–648. doi: 10.1130/0016-7606(1963)74[641:HMOTUS]2.0.CO;2. [DOI] [Google Scholar]
- Pitawala A, Schidlowski M, Dahanayake K, Hofmeister W. Geochemical and petrological characteristics of Eppawala phosphate deposits, Sri Lanka. Mineral Deposita. 2003;38:505–515. doi: 10.1007/s00126-002-0327-y. [DOI] [Google Scholar]
- Poon P, Graham IT, Liepa EAC, Cohen DR, Pringle IJ, Burkett DA, Privat K. Mineral distribution and provenance of heavy minerals sands (zircon, ilmenite, rutile) deposits from the NW Murray Basin, far western NSW, Australia. Aust J Earth Sci. 2020;67:575–590. doi: 10.1080/08120099.2020.1703813. [DOI] [Google Scholar]
- Pownceby M. Compositional and textural variation in detrital chrome spinels from the Murray Basin, southeastern Australia. Min Mag. 2005;69:191–204. doi: 10.1180/0026461056920246. [DOI] [Google Scholar]
- Pownceby M, Bourne P. Detrital chrome-spinel grains in heavy-mineral sand deposits from southeast Africa. Min Mag. 2006;70:51–64. doi: 10.1180/0026461067010312. [DOI] [Google Scholar]
- Pownceby MI, Sparrow GJ, Fisher-White MJ. Mineralogical characterization of Eucla Basin ilmenite concentrates - first results from a new global resource. Miner Eng. 2008;21:587–597. doi: 10.1016/j.mineng.2007.11.011. [DOI] [Google Scholar]
- Pownceby MI, Sparrow GJ, Aral H, Smith LK, Bruckard WJ. Recovery and processing of zircon from Murray Basin mineral sand deposits. Miner Process Extr Metall Rev. 2015;124:240–253. doi: 10.1179/1743285515Y.0000000016. [DOI] [Google Scholar]
- Press S. Detrital spinels from alpine-type source rocks in Middle Devonian sediments of the Rhenish Massif. Geol Rundsch. 1986;75:333–340. doi: 10.1007/BF01820615. [DOI] [Google Scholar]
- Rahman MJJ, Pownceby MI, Rana MS. Occurrence and distribution of heavy minerals in sand deposits of the Jamuna River, Bangladesh. Ore Geol Rev. 2020;116:103273. doi: 10.1016/j.oregeorev.2019.103273. [DOI] [Google Scholar]
- Ramadan F, Zalamah A, Saad AM. Provenance of heavy minerals in some occurrences of placer deposits along tip of Egyptian Mediterranean coastal plain. J Appl Res. 2012;8:5322–5332. [Google Scholar]
- Rejith RG, Sundararajam M. Combined magnetic, electrostatic, and gravity separation techniques for recovering strategic heavy minerals from beach sands. Mar Georesour Geotechnol. 2017;36:959–965. doi: 10.1080/1064119X.2017.1403523. [DOI] [Google Scholar]
- Ren R, Yang Z, Shaw LL. Polymorphic transformation and powder characteristics of TiO2 during high energy milling. J Mater Sci. 2000;35:6015–6026. doi: 10.1023/A:1026751017284. [DOI] [Google Scholar]
- Report Linker (2019) Zirconium market – growth, trends, and forecast (2019-2024). Available: https://www.globenewswire.com/news-release/2019/10/08/1926813/0/en/The-global-zirconium-market-is-consolidated-with-the-top-five-companies-accounting-for-around-70-of-the-global-consumption-in-2018.html (Accessed October 2020)
- Robinson M, Clamp F, Mobbs DB, Pearse RV. In: Advances in extractive metallurgy. Jones MJ, editor. London: The institution of Mining and Metallurgy; 1997. pp. 89–96. [Google Scholar]
- Rodin J (2015) Heavy minerals industry growth mostly influenced by China. [Online]. Available: https://www.miningweekly.com/article/heavy-minerals-industry-growth-mostly-influenced-by-china-2015-03-13 (Accessed July 2020)
- Roser BP, Coombs DS. Geochemistry of the Willsher Group, southeast Otago, New Zealand, and comparison with the Murihiku and Dun Mountain-Maitai Terranes. New Zeal J Geol Geophys. 2005;48:415–434. doi: 10.1080/00288306.2005.9515123. [DOI] [Google Scholar]
- Roser BP, Korsch RJ. Geochemical characterization, evolution and source of a Mesozoic accretionary wedge: the Torlesse terrane, New Zealand. Geol Mag. 1999;136:493–512. doi: 10.1017/S0016756899003003. [DOI] [Google Scholar]
- Roy DK, Roser BP. Geochemical evolution of the Tertiary succession of the NW shelf, Bengal basin, Bangladesh: Implications for provenance, paleoweathering and Himalayan erosion. J Asian Earth Sci. 2013;78:248–262. doi: 10.1016/j.jseaes.2013.04.045. [DOI] [Google Scholar]
- Roy PS, Whitehouse J, Cowell PJ, Oakes G. Mineral sands occurrences in the Murray Basin, southeastern Australia. Econ Geol. 2000;95:1107–1128. doi: 10.2113/gsecongeo.95.5.1107. [DOI] [Google Scholar]
- Rozendaal A, Philander C, Heyn R. The coastal heavy mineral sand deposits of Africa. South. Afr J Geol. 2017;120:133–152. doi: 10.25131/gssajg.120.1.133. [DOI] [Google Scholar]
- Salama W, Anand RR, Verrall M. Mineral exploration and basement mapping in areas of deep transported cover using heavy minerals and paleoredox fronts, Yilgarn Craton, Western Australia. Ore Geol Rev. 2016;72:485–509. doi: 10.1016/j.oregeorev.2015.07.014. [DOI] [Google Scholar]
- Schäfer J, Neuroth H, Ahrendt H, Dörr W. Accretion and exhumation at a Variscan active margin, recorded in the Saxothuringian flysch. Geol Rundsch. 1997;86:599–611. doi: 10.1007/s005310050166. [DOI] [Google Scholar]
- Schroeder PA, Le Govan JF, Roden MF. Weathering of ilmenite from granite and chlorite schist in the Georgia Piedmont. Am Mineral. 2002;87:1616–1625. doi: 10.2138/am-2002-11-1211. [DOI] [Google Scholar]
- Schulz KJ, Piatak NM, Papp JF (2017) Niobium and tantalum, Schulz KJ, De Young JH, Jr, Seal RR II, and Bradley DC, eds., Critical mineral resources of the United States-Economic and environmental geology and prospects for future supply: U.S.Geological Survey Professional Paper 1802, p. M1–M34, 10.3133/pp1802M
- Shalini G, Hegde VS, Soumya M, Korkoppa MM. Provenance and implications of heavy minerals in the beach sands of the India’s central west coast. J Coast Res. 2020;36:353–361. doi: 10.2112/JCOASTRES-D-19-00046.1. [DOI] [Google Scholar]
- Shankar D, Vinayachandran PN, Unnikrishnan AS. The monsoon currents in the north Indian Ocean. Prog Oceanogr. 2002;52:63–120. doi: 10.1016/S0079-6611(02)00024-1. [DOI] [Google Scholar]
- Shimizu M, Sano N, Ueki T, Komatsu T, Yasue KI, Niwa M. Provenance identification based on EPMA analyses of heavy minerals: case study of the Toki sand and gravel formation, central Japan. Island Arc. 2019;28:12295. doi: 10.1111/iar.12295. [DOI] [Google Scholar]
- Skinner BJ, Barton PB., Jr Genesis of mineral deposits. Annu Rev Earth Planet Sci. 1973;1:183–211. doi: 10.1146/annurev.ea.01.050173.001151. [DOI] [Google Scholar]
- Snyders E, Potgieter JH, Nel JT. The upgrading of an inferior grade zircon to superior opacifier for sanitary ware and glazes. J South Afr Inst Min Metall. 2005;105:459–463. [Google Scholar]
- Söderlund U, Johansson L. A simple way to extract baddeleyite (ZrO2) Geochem Geophys Geosyst. 2002;3:2. doi: 10.1029/2001GC000212. [DOI] [Google Scholar]
- Stegen KS. Heavy rare earths, permanent magnets, and renewable energies: an imminent crisis. Energy Policy. 2015;79:1–8. doi: 10.1016/j.enpol.2014.12.015. [DOI] [Google Scholar]
- Subasinghe HCS, Ratnayake AS. Processing of ilmenite into synthetic rutile using ball milling induced sulphurisation and carbothermic reduction. Miner Eng. 2021;173:107197. doi: 10.1016/j.mineng.2021.107197. [DOI] [Google Scholar]
- Subasinghe HCS, Ratnayake AS (2022) General review of titanium ores in exploitation: present status and forecast. Comun Geol 109:21–31. 10.34637/aab4-mk81
- Subasinghe HCS, Ratnayake AS, Sameera KAG (2021) State-of-the-art and perspectives in the heavy mineral industry of Sri Lanka. Miner Econ:1–13. 10.1007/s13563-021-00274-3
- Sun Z, Palke AC, Breeding CM, Dutrow BL. A new method for determining gem tourmaline species by LA-ICP-MS. Gems Gemol. 2019;55:1. doi: 10.5741/GEMS.55.1.2. [DOI] [Google Scholar]
- Takeuchi M. Changes in garnet chemistry show a progressive denudation of the source areas for Permian-Jurassic sandstones, southern Kitakami Terrane, Japan. Sediment Geol. 1994;93:85–105. doi: 10.1016/0037-0738(94)90030-2. [DOI] [Google Scholar]
- Tani H, Inazumi T, Terashima K. Mineralogical study of iron sand with different metallurgical characteristic to smelting with use of Japanese classic iron-making furnace “Tatara”. ISIJ Int. 2014;54:1044–1050. doi: 10.2355/isijinternational.54.1044. [DOI] [Google Scholar]
- Taylor SR, McLennan SM. The continental crust: its composition and evolution. Oxford: Blackwell; 1985. p. 312. [Google Scholar]
- Temple AK. Alteration of ilmenite. Econ Geol. 1966;61:695–714. doi: 10.2113/gsecongeo.61.4.695. [DOI] [Google Scholar]
- The United Nations: Sustainable Development Goals (2018) Available: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (Accessed July 2020)
- Thompson W, Lombard A, Santiago E, Singh A. In Proceedings of the 10th International Congress for Applied Mineralogy (ICAM) Berlin: Springer; 2012. Mineralogical studies in assisting beneficiation of rare earth element minerals from carbonatite deposits; pp. 665–672. [Google Scholar]
- Tian-Hui Z, Ling-Yu P, Su-Ling Z, Zheng X, Qian W, Chao K. Application of TiO2 with different structures in solar cells. Chin Phys B. 2012;21:118401. doi: 10.1088/1674-1056/21/11/118401. [DOI] [Google Scholar]
- Towner RR (1992) International strategic minerals inventory summary report; zirconium (No. 930-L). US Dept. of the Interior, Geological Survey. 10.3133/cir930L
- Tyler RM, Minnitt RCA. A review of the sub-Saharan heavy mineral sands deposits: implication for new projects in southern Africa. J South Afr Inst Min Metall. 2004;104:89–99. [Google Scholar]
- USGS . Mineral commodity summaries: garnet (industrial) Reston: US Geological Survey (USGS); 2019. [Google Scholar]
- USGS . Mineral commodity summaries: titanium mineral concentrates. Reston: US Geological Survey (USGS); 2020. [Google Scholar]
- USGS (2020b) Mineral resources online spatial data. [Online]. Available: https://mrdata.usgs.gov (Accessed May 2020)
- Van Gosen BS, Fey DL, Shah AK, Verplanck PL, Hoefen TM (2014) Deposit model for heavy-mineral sands in coastal environments: Chapter L in Mineral deposit models for resource assessment (No. 2010-5070-L). US Geological Survey. 10.3133/sir20105070L
- Von Eynatten H, Gaupp R. Provenance of Cretaceous synorogenic sandstones in the Eastern Alps: constraints from framework petrography, heavy mineral analysis and mineral chemistry. Sediment Geol. 1999;124:81–111. doi: 10.1016/S0037-0738(98)00122-5. [DOI] [Google Scholar]
- Wall F (2014) Rare earth elements. Critical metals Handbook. 312–339. 10.1002/9781118755341.ch13
- Wang J, Lin Z. Dye-sensitized TiO2 nanotube solar cells with markedly enhanced performance via rational surface engineering. Chem Mater. 2010;22:579–584. doi: 10.1021/cm903164k. [DOI] [Google Scholar]
- Ward CB, Muir DM, Ritchie IM. Recovery of by-product iron oxides from the upgrading of ilmenite to synthetic rutile. Extr Metall. 1989;89:1019–1040. [Google Scholar]
- Weckwerth P, Chabowski M. Heavy minerals as a tool to reconstruct river activity during the Weichselian glaciation (Toruń Basin, Poland) Geologos. 2013;19:25–46. doi: 10.2478/logos-2013-0003. [DOI] [Google Scholar]
- Wickremeratne WS. Preliminary studies on the offshore occurrences of monazite-bearing heavy-mineral placers, southwestern Sri Lanka. Mar Geol. 1986;72:1–9. doi: 10.1016/0025-3227(86)90095-2. [DOI] [Google Scholar]
- Wijewardhana TDU, Ratnayake AS. Applicability of carbothermic reduction for upgrading Sri Lankan ilmenite ores: towards converting ilmenite into synthetic rutile by mechanical activation. Bull Natl Res Cent. 2021;45:1–10. doi: 10.1186/s42269-021-00608-9. [DOI] [Google Scholar]
- Wijewardhana TDU, Subasinghe HCS, Ratnayake AS. Value addition to ilmenite using carbonized waste coconut shells: a mechanochemical approach aided with powdered seashells as a rate raiser. Mining Metall Explor. 2021;38:1573–1587. doi: 10.1007/s42461-021-00420-z. [DOI] [Google Scholar]
- World Nuclear Association (2020) Nuclear power in China. Available: https://www.world-nuclear.org/information-library/country-profiles/countries-a-f/china-nuclear power.aspx#:~:text=Nuclear%20Power%20in%20China&text=Mainland%20China%20has%2048%20nuclear,a%20closed%20nuclear%20fuel%20cycle (Accessed October 2020)
- Xiao XJ, Jiang KJ. China’s nuclear power under the global 1.5°C target: preliminary feasibility study and prospects. Adv Clim Chang Res. 2018;9:138–143. doi: 10.1016/j.accre.2018.05.002. [DOI] [Google Scholar]
- Zack TV, Von Eynatten H, Kronz A. Rutile geochemistry and its potential use in quantitative provenance studies. Sediment Geol. 2004;171:37–58. doi: 10.1016/j.sedgeo.2004.05.009. [DOI] [Google Scholar]
- Zack T, Stockli DF, Luvizotto GL, Barth MG, Belousova E, Wolfe MR, Hinton RW. In situ U-Pb rutile dating by LA-ICP-MS: 208Pb correction and prospects for geological applications. Contrib Mineral Petrol. 2011;162:515–530. doi: 10.1007/s00410-011-0609-4. [DOI] [Google Scholar]
- Zahid KM, Barbeau DL., Jr Provenance of eastern Magallanes foreland basin sediments: heavy mineral analysis reveals Paleogene tectonic unroofing of the Fuegian Andes hinterland. Sediment Geol. 2010;229:64–74. doi: 10.1016/j.sedgeo.2010.06.006. [DOI] [Google Scholar]
- Zhang W, Zhu Z, Cheng CY. A literature review of titanium metallurgical processes. Hydrometallurgy. 2011;108:177–188. doi: 10.1016/j.hydromet.2011.04.005. [DOI] [Google Scholar]
- Zhong L, Li G, Yan W, Xia B, Feng Y, Miao L, Zhao J. Using zircon U-Pb ages to constrain the provenance and transport of heavy minerals within the north western shelf of the South China Sea. J Asian Earth Sci. 2016;134:176–190. doi: 10.1016/j.jseaes.2016.11.019. [DOI] [Google Scholar]
- Zircon Industry Association (2020) Zircon Industry Association, 24 Old Street, Mayfair, London, UK. [Online]. Available: https://www.zircon-association.org/emerging-research-and-development.html (Accessed July 2020)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Currently known global reserve estimates of placer heavy mineral deposits (sources: Elsner 2010; Geoscience Australia 2013; Perks and Mudd 2019, 2020, 2021). The definitions of resource (i.e., reasonable prospects for eventual economic extraction) and reserve (i.e., the economically mineable part of a resource) are based on Meinert et al. (2016). (XLSX 19.9 kb)
Data Availability Statement
See appendix.
Not applicable.