Abstract
Cowdria ruminantium is the etiologic agent of heartwater, a disease causing major economic loss in ruminants in sub-Saharan Africa and the Caribbean. Development of a serodiagnostic test is essential for determining the carrier status of animals from regions where heartwater is endemic, but most available tests give false-positive reactions with sera against related Erhlichia species. Current approaches rely on molecular methods to define proteins and epitopes that may allow specific diagnosis. Two major antigenic proteins (MAPs), MAP1 and MAP2, have been examined for their use as antigens in the serodiagnosis of heartwater. The objectives of this study were (i) to determine if MAP2 is conserved among five geographically divergent strains of C. ruminantium and (ii) to determine if MAP2 homologs are present in Ehrlichia canis, the causative agent of canine ehrlichiosis, and Ehrlichia chaffeensis, the organism responsible for human monocytic ehrlichiosis. These two agents are closely related to C. ruminantium. The map2 gene from four strains of C. ruminantium was cloned, sequenced, and compared with the previously reported map2 gene from the Crystal Springs strain. Only 10 nucleic acid differences between the strains were identified, and they translate to only 3 amino acid changes, indicating that MAP2 is highly conserved. Genes encoding MAP2 homologs from E. canis and E. chaffeensis also were cloned and sequenced. Amino acid analysis of MAP2 homologs of E. chaffeensis and E. canis with MAP2 of C. ruminantium revealed 83.4 and 84.4% identities, respectively. Further analysis of MAP2 and its homologs revealed that the whole protein lacks specificity for heartwater diagnosis. The development of epitope-specific assays using this sequence information may produce diagnostic tests suitable for C. ruminantium and also other related rickettsiae.
Heartwater, or cowdriosis, a rickettsial disease of domestic and wild ruminants, is caused by the etiologic agent Cowdria ruminantium, which is transmitted by ticks belonging to the genus Amblyomma. Heartwater is responsible for mortality levels as high as 90% in susceptible ruminants and has been reported throughout sub-Saharan Africa and on several Caribbean islands (22). Animals surviving the disease may have persistent infections; thus, they are capable of serving as reservoirs for transmission to ticks (1).
Major antigenic protein 1 (MAP1) of C. ruminantium was found to be antigenically conserved in nine strains of C. ruminantium from Africa and the Caribbean (12) and was used in a competitive enzyme-linked immunosorbent assay (ELISA) using monoclonal antibodies (13). However, further studies revealed that MAP1 (complete, recombinant, or truncated) also reacts with sera from ruminants from heartwater-free areas (14, 15, 18) and with several members of the genus Ehrlichia (10). Furthermore, significant variability in nucleotide sequences of MAP1 among four African and two Caribbean strains ranged from 0.6 to 14.0% (20). These nucleic acid differences translated to amino acid substitutions, deletions, or insertions at three hypervariable regions of the gene (20). Demonstration of homology in MAP1 coding sequences between C. ruminantium and Ehrlichia spp. and divergence in hypervariable regions between strains suggest that MAP1 may not be a suitable candidate for specific serodiagnosis of heartwater. Thus, current serological tests for cowdriosis lack specificity, i.e., false-positive sera react with the whole C. ruminantium organism and the reported immunoreactive MAP1 (8).
The gene encoding another major antigenic protein (MAP2) was isolated, cloned, and expressed in Escherichia coli (17). Sera obtained from cattle, sheep, and goats infected with C. ruminantium reacted with this recombinant MAP2 (17). MAP2 is 55.5% identical at the amino acid level to major surface protein 5 (MSP5; 19 kDa) (17, 25) of Anaplasma marginale. A competitive ELISA using a monoclonal antibody against MSP5 has been evaluated and shown to be sensitive and specific for the diagnosis of Anaplasma infections in acutely or persistently infected cattle (16).
In this study, we examine the potential value of MAP2 as an antigen for the serodiagnosis of heartwater. First, we report the cloning and sequencing of map2 genes from several C. ruminantium strains of differing geographic origins. Second, we determine if, as with MAP1, false-positive sera react with MAP2. Finally, we determine whether MAP2-like proteins are present in Ehrlichia canis and Ehrlichia chaffeensis (causative agent of human monocytic ehrlichiosis), which are closely related to C. ruminantium according to 16S rRNA studies (6, 24), to evaluate whether epitope-specific tests are required for diagnosis of these rickettsiae.
MATERIALS AND METHODS
Origins of C. ruminantium strains, E. canis, and E. chaffeensis.
Three strains of C. ruminantium from Zimbabwe (Crystal Springs, Highway, and Palm River [4]), one strain from Sudan, Um Banein (11), and the Antigua strain (3) from the Caribbean were grown in bovine aortic endothelial cells (2, 4). E. canis (Oklahoma strain) and E. chaffeensis (Arkansas isolate) were grown in the canine macrophage cell line DH82 in minimum essential medium containing 12.5% fetal bovine serum and 200 mM l-glutamine (7). Organisms were harvested from the culture following the complete lysis of host cells, and rickettsial genomic DNA was isolated as previously described (5, 20).
Amplification of the map2 genes of C. ruminantium strains of different geographic origins and of the map2-like genes of E. chaffeensis and E. canis.
Primers AB249 and AB251, corresponding to flanking regions of the map2 gene (open reading frame [ORF] 1) of C. ruminantium clone pF5.2 (17), were synthesized. Primers for PCR amplification of E. canis and E. chaffeensis were selected by using information from one of three sources: (i) sequences determined after alignment of conserved regions of the map2 gene of C. ruminantium and the msp5 gene of A. marginale, (ii) sequences obtained from newly sequenced regions of the map2-like genes of E. canis or E. chaffeensis, and (iii) primers developed to sequence the pF5.2 clone of the Crystal Springs strain of C. ruminantium (17). Primers (Table 1) were synthesized by the Oligonucleotide Synthesis Laboratory (Interdisciplinary Center for Biotechnology Research, University of Florida, Gainesville) or National Biosciences, Inc. (Plymouth, Minn.). These primers were used in a PCR assay to amplify DNA of the strains of C. ruminantium, E. canis, and E. chaffeensis. Briefly, target DNA (1 ng) was amplified in a mixture of 0.4 mM deoxynucleoside triphosphates, 0.5 μM (each) primer, 20 mM MgCl2, 100 mM KCl, 200 mM Tris-HCl (pH 8.2), 60 mM (NH4)2SO4, 1% Triton X-100, 100 μg of nuclease-free bovine serum albumin/ml, and 2.5 U of native Pfu DNA polymerase. PCR assays, for the amplification of map2 and map2-like genes of C. ruminantium, E. canis, and E. chaffeensis, were performed at 94°C for 5 min, followed by 30 cycles of varying denaturing, annealing, and extension temperatures and times (Table 2). A final extension step at 72°C was performed for 10 min in all reactions. The amplicons were analyzed by gel electrophoresis on a 0.8% agarose gel in 1× TBE buffer (89 mM Tris, 89 mM boric acid, 2 mM disodium EDTA). PCR products were purified by using the QIAquick Spin PCR purification kit (Qiagen, Inc., Chatsworth, Calif.) as described by the manufacturer. The DNA was eluted in 50 μl of 10 mM Tris-HCl, pH 8.3.
TABLE 1.
Primers designed or selected for amplification of the genes encoding the MAP2 homologs of E. canis and E. chaffeensis
| Primers | Nucleic acid sequencea (5′ to 3′) |
|---|---|
| AB249 | AAACTCTAATTTTATACA |
| AB251 | AAAATAAGACTAAAAGAAAC |
| AB282 | GTSTTYATAACTRTTGATCC |
| AB284 | GARAAYCTMCCAAAYTCTTC |
| AB346 | CCTGTTAACATTTGAATTCTTG |
| AB347 | GCATTTGAATTCTAGGATC |
| AB146 | CAGGGAAGCTTGCAATTTTTTTGGGATATTC |
| F13 | GTAATTACTCATACACTAA |
| F17 | GAGCACATCATGAAGGCTATC |
| AB602 | GGTACATTATTGCAGAATGG |
| AB608 | TACNNAGNTNNNNCNNNCNNT |
| AB620 | AAACTACTGAACAGGTAATT |
| AB623 | CGTATTTGTTGTGCGCTGG |
| AB624 | CGTATTTGCTGTGCATTTAC |
M = A or C; N = A, C, G, or T; R = A or G; S = G or C; Y = C or T.
TABLE 2.
Primer combinations and optimal temperatures for amplification of the genes encoding the MAP2 homologs of E. canis and E. chaffeensis
| Primer combination | Denaturing
|
Annealing
|
Extension
|
|||
|---|---|---|---|---|---|---|
| Time (min) | Temp (°C) | Time (min) | Temp (°C) | Time (min) | Temp (°C) | |
| AB249-AB251 | 1 | 40 | 1 | 72 | 1.5 | 94 |
| AB282-AB284 | 1 | 40 | 1 | 72 | 1.5 | 94 |
| AB346-F17 | 1.5 | 37 | 1.5 | 72 | 1.5 | 94 |
| AB347-AB146 | 1.5 | 37 | 1.5 | 72 | 1.5 | 94 |
| AB602-AB608 | 1 | 40 | 1 | 72 | 1.5 | 94 |
| AB620-AB623 | 1 | 47 | 1 | 72 | 1.5 | 94 |
| AB620-AB624 | 1 | 47 | 1 | 72 | 1.5 | 94 |
Cloning and restriction enzyme analysis.
Amplicons were concentrated to 20 μl in a Speed Vac concentrator (Savant Instruments, Inc., Farmingdale, N.Y.) and ligated to EcoRV-digested pBluescript SK(+) (Stratagene, La Jolla, Calif.) at 16°C in the presence of T4 DNA ligase at an insert/vector molar ratio of 8:1. The recombinant plasmid was used to transform competent E. coli XL1-Blue cells, and transformants were grown on Luria-Bertani agar plates in the presence of ampicillin (50 μg/ml), 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (50 mg/ml), and isopropyl-β-d-thiogalactopyranoside (IPTG) (0.2 mg/ml). Positive colonies were selected, inoculated into terrific broth (21) containing ampicillin (250 μg/ml), and incubated overnight at 37°C with vigorous shaking. Plasmid DNA from positive colonies was extracted by the boiling prep method (9). DNA was reconstituted in Tris-EDTA buffer (pH 8.0) containing 20 μg of DNase-free RNase/ml and was analyzed on a 0.8% agarose gel. Recombinant clones containing the map2 genes of different strains of C. ruminantium were digested with seven restriction enzymes (AccI, HincII, EcoRV, EcoRI, XbaI, BamHI, and HindIII) to compare their various sizes to those obtained for the reported Crystal Springs strain (GenBank accession no. G289922). The resulting restriction enzyme-digested DNA was analyzed on a 0.8% agarose gel.
DNA sequencing and sequence data analysis.
The DNA insert in pBluescript SK(+) was sequenced as double-stranded DNA by using Sequenase (United States Biochemical) as recommended by the manufacturer. T3 and T7 plasmid-specific promoter primers were used in initial reactions, and then new oligonucleotides were synthesized based on the sequences obtained (“primer walking”). Insert DNA was completely sequenced on both strands. DNA sequences were analyzed with the PCGENE (Intelligenetics) and Genetics Computer Group (University of Wisconsin) programs. Database searches were performed with the BLAST program of the National Center for Biotechnology Information (Bethesda, Md.).
C. ruminantium recombinant MAP2 production and purification.
The 627-bp ORF encoding MAP2 of C. ruminantium was subcloned into the expression vector pFLAG (International Biotechnologies, Inc., New Haven, Conn.) and transformed into E. coli for expression of recombinant protein (17). An E. coli colony expressing MAP2 was selected and expanded in L broth (1% tryptone–0.5% yeast extract–1% NaCl), induced with 0.1 mM IPTG, and purified by monoclonal antibody affinity chromatography, as described by the manufacturer of the pFLAG vector system. The protein concentration was determined, and the size and purity were analyzed on a 12% acrylamide gel. The antigenicity of the protein was analyzed by using an indirect ELISA and hyperimmune sheep serum.
Antisera.
Positive sera were collected from sheep born and reared under Amblyomma-free conditions in Zimbabwe and experimentally infected with the Welgevonden strain of C. ruminantium (17). False-positive sera were obtained from sheep (numbers 056, 060, 062, and 066) born and raised in regions of Zimbabwe where neither Amblyomma ticks nor the disease has ever been observed. Animals were confirmed free of infection by tick pickup, PCR, and challenge (18). Negative sera were obtained from sheep born and reared under Amblyomma-free conditions in Zimbabwe. Normal and anti-E. canis dog sera (obtained from the Veterinary Medical Teaching Hospital at the University of Florida) were confirmed positive or negative by an indirect fluorescent antibody test.
Indirect ELISA.
ELISA plates were coated by using isolated recombinant MAP2 (4 μg/ml) in 0.14 M NaCl–20 mM Na2HPO4–3 mM KH2PO4 (pH 7.2) containing 0.02% (wt/vol) sodium azide (phosphate-buffered saline [PBS]-azide) at 50 μl per well and incubated overnight at 4°C. After five washes with buffer containing PBS-azide and 0.05% (vol/vol) Tween-20, the wells were blocked for 60 min at room temperature with 1.0% (wt/vol) bovine serum albumin in PBS-azide. The plates were washed five times and incubated for 60 min with sera or supernatant diluted in PBS-azide (50 μl per well). The plates were washed (five times) and incubated at room temperature for a further 60 min in the presence of rabbit anti-species-specific immunoglobulin G conjugated to alkaline phosphatase (1:1,000; 50 μl per well). After another five washes, the substrate, p-nitrophenylphosphate (1 mg/ml; Sigma), in 0.16 M NaHCO3–0.14 M Na2CO3–0.02 M MgCl2 (pH 9.6) was added at 50 μl per well to each well and incubated for 30 min at room temperature. Absorbance was read at 405 nm.
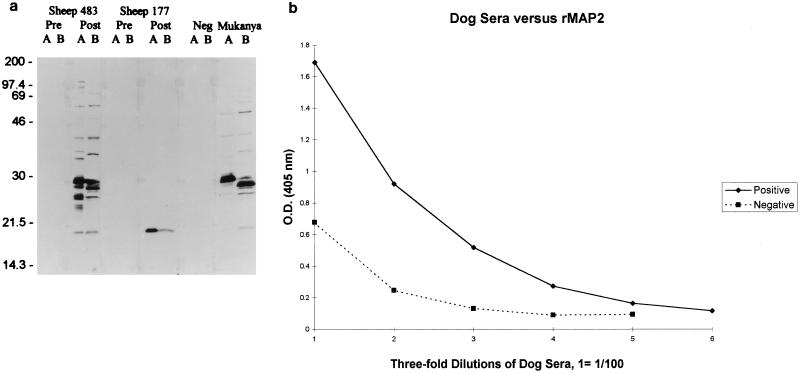
Immunoblot of C. ruminantium and E. canis antigens.
C. ruminantium (Fig. 5a, lanes A) or E. canis (Fig. 5a, lanes B) lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Membranes were blocked with Tris-buffered saline (TBS) (0.1 M Tris HCl–0.9% NaCl [pH 8.0])–0.25% gelatin, then reacted with an anti-C. ruminantium antiserum (from sheep 483; Crystal Springs strain), anti-recombinant MAP2 antiserum (from sheep 177), or anti-E. canis antiserum (dog Mukanya; E. canis Oklahoma). Membranes were washed three times in TBS containing 0.25% Tween 20, reacted with peroxidase-labeled protein G (Zymed) for 2 h, washed three times as described above, and developed by incubation with 4CN peroxidase substrate (Kirkegaard and Perry, Gaithersburg, Md.).
FIG. 5.
(a) Immunoblot demonstrating antigenic similarity between C. ruminantium and E. canis. Lysates of C. ruminantium (lanes A) or E. canis (lanes B) were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were reacted with anti-C. ruminantium antiserum (sheep 483), anti-rMAP2 antiserum (sheep 177), or anti-E. canis antiserum (Mukanya). (b) Indirect ELISA using dog sera. Serum from a dog infected with E. canis (positive) or from a healthy dog (negative) was tested in an indirect ELISA against recombinant MAP2.
Nucleotide sequence accession numbers. The nucleotide sequences of the map2 genes have been assigned the following GenBank accession numbers: Antigua map2 gene, AF117726; Highway map2 gene, AF117727; Palm River map2 gene, AF117728; Um Banein map2 gene, AF117729. The nucleotide sequences of the map2-like genes have been assigned the following GenBank accession numbers: E. canis map2-like gene, AF117730; E. chaffeensis map2-like gene, AF117731.
RESULTS
Analysis of the map2 genes of C. ruminantium.
Five geographically differing strains of C. ruminantium were examined in order to determine the conservation of MAP2. The map2 genes from the four strains were amplified with primers AB249 and AB251 (Table 1), resulting in an amplicon of approximately 0.74 kb (Fig. 1). The map2 genes from the strains were cloned by PCR cloning methods, and restriction enzyme analysis was performed and compared with that for the previously reported map2 gene of the Crystal Springs strain. Restriction map analysis of the map2 genes demonstrated similarities between Palm River, Um Banein, and Antigua and similarities between Crystal Springs and Highway (Fig. 2). EcoRV, XbaI, and HincII restriction sites were found in all strains analyzed. The AccI restriction site was observed in the Crystal Springs and Highway strains only. Likewise, the Palm River, Um Banein, and Antigua strains had identical restriction enzyme maps and contained an additional HincII site.
FIG. 1.
Analysis of PCR products of the five strains of C. ruminantium amplified by using primers AB249 and AB251. PCR amplicons of Antigua (lane A), Crystal Springs (lane C), Highway (lane H), Palm River (lane P), and Um Banein (lane U) are shown.
FIG. 2.
Restriction maps of the map2 genes from C. ruminantium strains. Maps are drawn left to right, 5′ to 3′ with respect to the MAP2 coding sequence. Restriction enzymes used for mapping are shown. The restriction map of the Crystal Springs strain map2 gene is presented for comparison.
Cloned map2 genes were sequenced and compared with the known sequence of the Crystal Springs strain map2 gene (GenBank accession no. G289922) at both the nucleotide (Fig. 3) and amino acid (Fig. 4) levels. At both levels, Highway and Crystal Springs MAP2 sequences were identical. Palm River, Um Banein, and Antigua differed at nucleotides 111, 192, 319, 465, 483, 534, 553, and 594. Um Banein differed at nucleotide 170 from all other strains analyzed. Antigua differed at nucleotide 323 from all other strains analyzed. The DNA sequence changes at the AccI (5′-GTAGAC-3′) and HincII (5′-GTCAAC-3′) sites are in agreement with the restriction map data. Of the 10 nucleotide differences, 7 do not change the encoded amino acid, but changes at nucleotides 319, 323, and 553 lead to 3 amino acid substitutions. Serine (amino acid 107) and threonine (amino acid 185) of Crystal Springs and Highway were both replaced by alanine in Palm River, Um Banein, and Antigua. Antigua differs from all others at amino acid 108, where a glycine has been substituted for an aspartic acid. The map2 gene for all strains of C. ruminantium is 627 bases. Thus, MAP2 is highly conserved among these five geographically differing strains examined.
FIG. 3.
Comparison of the coding nucleotide sequences of MAP2 and MAP2 homologs of C. ruminantium strains and Ehrlichia species. The MAP2 coding sequences of the four strains of C. ruminantium and the two species of Ehrlichia were determined for both strands and were aligned with the MAP2 sequence from the Highway strain by using the Genetics Computer Group programs. The complete nucleotide sequence from the Highway strain is presented. The sequences for other strains and species are presented only when they differ from the Highway sequence. A dot indicates identity with the Highway sequence. The underlined area is the predicted ribosomal binding site of the revised MAP2 nucleic acid sequence of C. ruminantium.
FIG. 4.
Comparison of the MAP2 and MAP2-like coding sequences at the amino acid level. The nucleotide sequences for five strains of C. ruminantium and two Ehrlichia species were translated. The complete sequence for the Highway strain is presented. The sequences for other strains and the Ehrlichia species are presented only when they differ from the Highway sequence. A dot indicates identity with the Highway sequence.
Analysis of the map2-like genes of E. chaffeensis and E. canis.
The map2-like genes were amplified by using several sets of PCR primers (Tables 1 and 2), cloned, and sequenced. The nucleotide and translated amino acid sequences were compared with the sequences of the C. ruminantium strains (Fig. 3 and 4). When the amino acid sequences are aligned, Ehrlichia proteins begin 4 amino acids downstream from the starting methionine of the ORF of MAP2 of C. ruminantium (Fig. 4). Although MAP2-related polypeptides in E. chaffeensis and E. canis are highly homologous to C. ruminantium MAP2, they were distinguishable by numerous substitutions throughout the polypeptide chain. Overall, the MAP2 homologs of E. canis and E. chaffeensis were 83.4 and 84.4% identical, respectively, to MAP2 of C. ruminantium (Crystal Springs strain). These data show that MAP2 homologs are present in two Ehrlichia spp., E. canis and E. chaffeensis, but that there is variability throughout the nucleic acid and amino acid sequences that may allow the development of epitope-specific serodiagnostic assays.
Immunoassays demonstrating antigenic similarity between C. ruminantium and E. canis.
C. ruminantium antiserum (from sheep 483) recognized an approximately 21-kDa protein in C. ruminantium and E. canis (Fig. 5a). More specifically, anti-recombinant MAP2 serum (from sheep 177) recognized MAP2 in C. ruminantium (lane A) and a MAP2 homolog in E. canis (lane B). Anti-E. canis serum also recognized MAP2 of C. ruminantium and E. canis (Fig. 5). These data confirm the sequence data and show that a MAP2 homolog which shares epitopes with MAP2 of C. ruminantium is expressed in E. canis.
Indirect ELISA for heartwater diagnosis using MAP2.
Sera from sheep were tested to determine whether recombinant MAP2 is recognized by both anti-C. ruminantium and false-positive sera, as observed with MAP1 (18). Sera obtained from five animals experimentally infected with C. ruminantium tested positive at dilutions as low as 1:3,200 (Fig. 6). False-positive sera obtained from four animals from heartwater-free regions of Zimbabwe (18) reacted in a pattern similar to that of true-positive sera (Fig. 6). Immunoblot-negative sera (n = 3) consistently gave optical density values lower than the true-positive and false-positive sera at similar dilutions down to 1:12,800. Hence, an indirect ELISA using the complete recombinant MAP2 does not discriminate between true- and false-positive sera.
FIG. 6.
Indirect ELISA using sheep sera. Sera obtained from animals experimentally infected with C. ruminantium (positive), sera obtained from animals from a heartwater-free region but reactive to C. ruminantium antigens in an immunoblot (false positive), and sera obtained from animals with no previous exposure and negative in an immunoblot to C. ruminantium (negative) were tested in an indirect ELISA against recombinant MAP2.
DISCUSSION
The isolation of genes encoding immunoreactive proteins of C. ruminantium may enable the development of diagnostic tests with improved sensitivity and specificity. The immunoreactive protein must be conserved among C. ruminantium strains and yet sufficiently different from the homologs present in related species. MAP1 has been tested in various assays (10, 13) and shown to cross-react with anti-Ehrlichia sera and with sera from known C. ruminantium-free animals (18). A truncated MAP1 protein, MAP1B, has improved specificity but still reacts with some anti-Ehrlichia sera (14, 15, 23). Recent data showing (i) the presence of three hypervariable regions in MAP1 in different strains of C. ruminantium (20), (ii) recognition of linear peptides representing these hypervariable regions (20) by sera from animals infected with C. ruminantium (19, 20), and (iii) the presence in E. canis and E. chaffeensis of MAP1 homologs which have sequences very similar to that of MAP1 of C. ruminantium, except in the hypervariable regions, suggest that MAP1 may not be an ideal diagnostic antigen.
MAP2 is an immunoreactive protein consistently recognized by sera from sheep, goats, and cattle infected with C. ruminantium (17). MAP2 is 55.5% identical to MSP5 of A. marginale. MSP5 is conserved in all the different isolates of A. marginale tested, and an epitope-specific assay using MSP5, which consistently identifies cattle both acutely and chronically infected with A. marginale, has been developed (16, 25). Examination of the MAP2 of five geographically diverse strains of C. ruminantium revealed a highly conserved protein with amino acid substitutions at only three positions (amino acid variability between 0 and 1.44%). This differs from the sequenced MAP1 of several strains, which was found to contain up to 10.0% variability in amino acid sequence (20). MAP2 meets two criteria for a suitable diagnostic antigen, immunoreactivity and conservation. However, further data showed reactivity between recombinant MAP2 and sera from known heartwater-free animals. Thus, like MAP1, MAP2 lacked specificity for a diagnostic test.
Because MAP2 reacted with false-positive sera, it was essential that we examine other closely related rickettsiae for the presence of MAP2 homologs in order to evaluate whether there is a basis for screening of anti-MAP2 monoclonal antibodies or the use of specific MAP2 regions to develop an epitope-specific assay. The existence of a MAP2 homolog in E. canis was demonstrated by immunoblotting total antigens of C. ruminantium and E. canis with an antiserum specific to recombinant MAP2.
The genes encoding MAP2 homologs in E. canis and E. chaffeensis were amplified and sequenced, and ORFs similar to the map2 gene were translated into amino acids. The resulting MAP2 homologs had amino acid similarities of 83.41% (for E. canis) and 84.39% (for E. chaffeensis) with MAP2 of C. ruminantium. MAP2 homologs in Ehrlichia species may hinder the development of adequate diagnosis for heartwater using MAP2. Although similarities exist between MAP2 homologs of C. ruminantium and Ehrlichia species, numerous sequence differences throughout the proteins were observed. The numerous substitutions found throughout MAP2 homologs in the different species suggest that it may be possible to define unique epitopes suitable for the diagnosis of either C. ruminantium or ehrlichial infections.
The initial discrepancy between the MAP2 homologs of C. ruminantium and Ehrlichia appears to be the first methionine. The second methionine of C. ruminantium (4 amino acids from the first methionine) aligns with the first methionine of the Ehrlichia species, suggesting that it is probably the initiator methionine in MAP2. This is further supported by alignment of MAP2 with MSP5 of A. marginale; MSP5 also begins at the second methionine of C. ruminantium. There is a predicted ribosomal binding site 5′ to the second methionine but 3′ to the first methionine (Fig. 3). Thus, it appears that the reported amino acid sequence of MAP2 (17) may be incorrect and should actually be shorter by 4 amino acids at the N terminus.
Like MSP5 of A. marginale, therefore, MAP2 may be useful in the diagnosis of animals infected with C. ruminantium if a similarly unique epitope can be defined. MAP2 is highly conserved among geographically different isolates and is recognized by sera from C. ruminantium-infected sheep, goats, and cattle. However, MAP2 is recognized antigenically by anti-E. canis antiserum, and false-positive sera and MAP2 homologs were identified from E. canis and E. chaffeensis; thus MAP2 as a whole protein lacks specificity for heartwater diagnosis. From this study, it was determined that development of a serodiagnostic assay using MAP2 may require the use of truncated peptides or monoclonal antibodies specific to unique sequences of MAP2 of C. ruminantium. Further, the availability of the sequences of MAP2 homologs in E. chaffeensis and E. canis may aid in the development of specific diagnostic tests for these species.
ACKNOWLEDGMENTS
We thank Jacqueline E. Dawson and James G. Olsen of the Centers for Disease Control and Prevention for providing DNA from cultures of E. canis and E. chaffeensis; P. J. Kelly of the Faculty of Veterinary Science, University of Zimbabwe, for providing the dog anti-E. canis Mukanya sera; and A. Rick Alleman of the College of Veterinary Medicine, University of Florida, for providing us with normal and anti-E. canis dog sera. We appreciate the excellent assistance of Annie Moreland, Anna Lundgren, and Gillian Smith. In addition, we acknowledge the excellent services (oligonucleotide synthesis and DNA sequencing) provided by core facilities of the Interdisciplinary Center for Biotechnology Research, University of Florida.
This work received support from U.S. Agency of International Development (USAID) Cooperative Agreement LAG-1328-G-00-3030-00.
REFERENCES
- 1.Andrew H R, Norval R A. The carrier status of sheep, cattle and African buffalo recovered from heartwater. Vet Parasitol. 1989;34:261–266. doi: 10.1016/0304-4017(89)90056-3. [DOI] [PubMed] [Google Scholar]
- 2.Bezuidenhout J D, Paterson C L, Barnard B J. In vitro cultivation of Cowdria ruminantium. Onderstepoort J Vet Res. 1985;52:113–120. [PubMed] [Google Scholar]
- 3.Birnie E F, Burridge M J, Camus E, Barré N. Heartwater in the Caribbean: isolation of Cowdria ruminantium from Antigua. Vet Rec. 1985;116:121–123. doi: 10.1136/vr.116.5.121. [DOI] [PubMed] [Google Scholar]
- 4.Byrom B, Yunker C E. Improved culture conditions for Cowdria ruminantium (Rickettsiales), the agent of heartwater disease of domestic ruminants. Cytotechnology. 1990;4:285–290. doi: 10.1007/BF00563789. [DOI] [PubMed] [Google Scholar]
- 5.Chen S-M, Popov V L, Feng H M, Walker D H. Analysis and ultrastructural localization of Ehrlichia chaffeensis with monoclonal antibodies. Am J Trop Med Hyg. 1996;54:405–412. doi: 10.4269/ajtmh.1996.54.405. [DOI] [PubMed] [Google Scholar]
- 6.Dame J B, Mahan S M, Yowell C A. Phylogenetic relationship of Cowdria ruminantium, agent of heartwater, to Anaplasma marginale and other members of the order Rickettsiales determined on the basis of 16S rRNA sequence. Int J Syst Bacteriol. 1992;42:270–274. doi: 10.1099/00207713-42-2-270. [DOI] [PubMed] [Google Scholar]
- 7.Dawson J E, Stallknecht D E, Howerth E W, Warner C K, Biggie K, Davidson W R, Lockhart J M, Nettles V F, Olson J G, Childs J E. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J Clin Microbiol. 1994;32:2725–2728. doi: 10.1128/jcm.32.11.2725-2728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du Plessis J L, Camus E, Oberem P T, Malan L. Heartwater serology: some problems with the interpretation of results. Onderstepoort J Vet Res. 1987;54:327–329. [PubMed] [Google Scholar]
- 9.Holmes D S, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 10.Jongejan F, De Vries N, Nieuwenhuijs J, van Vliet V A, Wassink L A. The immunodominant 32-kilodalton protein of Cowdria ruminantium is conserved within the genus Ehrlichia. Rev Elev Med Vet Pays Trop. 1993;46:145–152. [PubMed] [Google Scholar]
- 11.Jongejan F, Morzaria S P, Shariff O A, Abdalla H M. Isolation and transmission of Cowdria ruminantium (causal agent of heartwater disease) in Blue Nile Province, Sudan. Vet Res Commun. 1984;8:141–145. doi: 10.1007/BF02214705. [DOI] [PubMed] [Google Scholar]
- 12.Jongejan F, Thielemans M J C, Briere C, Uilenberg G. Antigenic diversity of Cowdria ruminantium isolates determined by cross-immunity. Res Vet Sci. 1991;51:24–28. doi: 10.1016/0034-5288(91)90025-j. [DOI] [PubMed] [Google Scholar]
- 13.Jongejan F, Thielemans M J, De Groot M, Van Kooten P J S, van der Zeijst B A M. Competitive enzyme-linked immunosorbent assay for heartwater using monoclonal antibodies to a Cowdria ruminantium-specific 32-kilodalton protein. Vet Microbiol. 1991;28:199–211. doi: 10.1016/0378-1135(91)90093-u. [DOI] [PubMed] [Google Scholar]
- 14.Katz J B, Barbet A F, Mahan S M, Kumbula D, Lockhart J M, Keel M K, Dawson J E, Olson J G, Ewing S A. A recombinant antigen from the heartwater agent (Cowdria ruminantium) reactive with antibodies in some southeastern United States white-tailed deer (Odocoileus virginianus), but not cattle, sera. J Wildl Dis. 1996;32:424–430. doi: 10.7589/0090-3558-32.3.424. [DOI] [PubMed] [Google Scholar]
- 15.Katz J B, DeWald R, Dawson J E, Camus E, Martinez D, Mondry R. Development and evaluation of a recombinant antigen, monoclonal antibody-based competitive ELISA for heartwater serodiagnosis. J Vet Diagn Investig. 1997;9:130–135. doi: 10.1177/104063879700900204. [DOI] [PubMed] [Google Scholar]
- 16.Knowles D P, Torioni de Echaide S, Palmer G H, McGuire T C, Stiller D, McElwain T. Antibody against an Anaplasma marginale MSP5 epitope common to tick and erythrocyte stages identifies persistently infected cattle. J Clin Microbiol. 1996;34:2225–2230. doi: 10.1128/jcm.34.9.2225-2230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahan S M, McGuire T C, Semu S M, Bowie M V, Jongejan F, Rurangirwa F R, Barbet A F. Molecular cloning of a gene encoding the immunogenic 21 kDa protein of Cowdria ruminantium. Microbiology. 1994;140:2135–2142. doi: 10.1099/13500872-140-8-2135. [DOI] [PubMed] [Google Scholar]
- 18.Mahan S M, Tebele N, Mukwedeya D, Semu S, Nyathi C B, Wassink L A, Kelly P J, Peter T, Barbet A F. An immunoblotting diagnostic assay for heartwater based on the immunodominant 32-kilodalton protein of Cowdria ruminantium detects false positives in field sera. J Clin Microbiol. 1993;31:2729–2737. doi: 10.1128/jcm.31.10.2729-2737.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy G R, Sulsona C R, Barbet A F, Mahan S M, Burridge M J, Alleman A R. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichiae. Biochem Biophys Res Commun. 1998;247:636–643. doi: 10.1006/bbrc.1998.8844. [DOI] [PubMed] [Google Scholar]
- 20.Reddy G R, Sulsona C R, Harrison R H, Mahan S M, Burridge M J, Barbet A F. Sequence heterogeneity of the major antigenic protein 1 genes from Cowdria ruminantium isolates from different geographical areas. Clin Diagn Lab Immunol. 1996;3:417–422. doi: 10.1128/cdli.3.4.417-422.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tartof K D, Hobbs C A. Improved media for growing plasmid and cosmid clones. Bethesda Res Lab Focus. 1987;9:12. [Google Scholar]
- 22.Uilenberg G. Recent advances in the study of the vector role of ticks of the genus Amblyomma (Ixodidae) Rev Elev Med Vet Pays Trop. 1983;36:61–66. [PubMed] [Google Scholar]
- 23.van Vliet A H M, van der Zeijst B A M, Camus E, Mahan S M, Martinez D, Jongejan F. Use of a specific immunogenic region on the Cowdria ruminantium MAP1 protein in a serological assay. J Clin Microbiol. 1995;33:2405–2410. doi: 10.1128/jcm.33.9.2405-2410.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Vliet A H M, Jongejan F, van der Zeijst B A. Phylogenetic position of Cowdria ruminantium (Rickettsiales) determined by analysis of amplified 16S ribosomal DNA sequences. Int J Syst Bacteriol. 1992;42:494–498. doi: 10.1099/00207713-42-3-494. [DOI] [PubMed] [Google Scholar]
- 25.Visser E S, McGuire T C, Palmer G H, Davis W C, Shkap V, Pipano E, Knowles D P. The Anaplasma marginale msp5 gene encodes a 19-kilodalton protein conserved in all recognized Anaplasma species. Infect Immun. 1992;60:5139–5144. doi: 10.1128/iai.60.12.5139-5144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]