Abstract
We investigated the association between a wide range of comorbidities and COVID-19 in-hospital mortality and assessed the influence of multi morbidity on the risk of COVID-19-related death using a large, regional cohort of 6036 hospitalized patients. This retrospective cohort study was conducted using Patient Administration System Admissions and Discharges data. The International Classification of Diseases 10th edition (ICD-10) diagnosis codes were used to identify common comorbidities and the outcome measure. Individuals with lymphoma (odds ratio [OR], 2.78;95% CI,1.64–4.74), metastatic cancer (OR, 2.17; 95% CI,1.25–3.77), solid tumour without metastasis (OR, 1.67; 95% CI,1.16–2.41), liver disease (OR: 2.50, 95% CI,1.53–4.07), congestive heart failure (OR, 1.69; 95% CI,1.32–2.15), chronic obstructive pulmonary disease (OR, 1.43; 95% CI,1.18–1.72), obesity (OR, 5.28; 95% CI,2.92–9.52), renal disease (OR, 1.81; 95% CI,1.51–2.19), and dementia (OR, 1.44; 95% CI,1.17–1.76) were at increased risk of COVID-19 mortality. Asthma was associated with a lower risk of death compared to non-asthma controls (OR, 0.60; 95% CI,0.42–0.86). Individuals with two (OR, 1.79; 95% CI, 1.47–2.20; P < 0.001), and three or more comorbidities (OR, 1.80; 95% CI, 1.43–2.27; P < 0.001) were at increasingly higher risk of death when compared to those with no underlying conditions. Furthermore, multi morbidity patterns were analysed by identifying clusters of conditions in hospitalised COVID-19 patients using k-mode clustering, an unsupervised machine learning technique. Six patient clusters were identified, with recognisable co-occurrences of COVID-19 with different combinations of diseases, namely, cardiovascular (100%) and renal (15.6%) diseases in patient Cluster 1; mental and neurological disorders (100%) with metabolic and endocrine diseases (19.3%) in patient Cluster 2; respiratory (100%) and cardiovascular (15.0%) diseases in patient Cluster 3, cancer (5.9%) with genitourinary (9.0%) as well as metabolic and endocrine diseases (9.6%) in patient Cluster 4; metabolic and endocrine diseases (100%) and cardiovascular diseases (69.1%) in patient Cluster 5; mental and neurological disorders (100%) with cardiovascular diseases (100%) in patient Cluster 6. The highest mortality of 29.4% was reported in Cluster 6.
Subject terms: Risk factors, Diseases, Medical research
Introduction
The coronavirus disease 2019, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), continues to pose a major threat to public health worldwide. The clinical spectrum of COVID-19 ranges from an asymptomatic state or mild respiratory symptoms to severe viral pneumonia and acute respiratory distress syndrome1,2. Although severe illness from COVID-19 can occur in healthy individuals of any age, the risk of severe symptoms with rapid disease progression and death is significantly higher in older adults, with people over the age of 80 having 20-fold increased risk of COVID-19-related death than adults aged 50–59 years3. The severity of, and mortality due to, COVID-19 are higher in males than females4. The Open SAFELY analysis of 17,278,392 primary care records, including 10,926 COVID-19 deaths, showed that older people, males, and those living in deprived areas were at significantly higher risk of COVID-19-related death3. Furthermore, various pre-existing conditions, such as, cardiovascular disease4–8, diabetes4,5,7, hypertension4,7,8, respiratory diseases4,7,8, cancer4,9, chronic kidney disease5,10, obesity5,11,12 have been associated with increased risk of COVID-19 occurrence, the development of severe health conditions, and higher risk of death. In fact, reports showed that over 90% of individuals that died as a direct consequence of SARS‐CoV‐2 infection had at least one pre-existing condition13. Chen et al.14 reported that patients with severe or critical COVID-19 who died were on average 17 years older, more likely to be male, and more likely to have a comorbidity such as hypertension, diabetes, cardiovascular disease, or chronic lung disease. A study by the Chinese Center for Disease Control and Prevention (44,672 confirmed cases, 1,023 deaths) showed that several pre-existing medical conditions including cardiovascular disease, hypertension, diabetes, respiratory disease, and cancer, substantially increased the risk of COVID-19 adverse outcomes15. In the UK, a cross-sectional survey of 16,749 individuals hospitalized with COVID-19 demonstrated that patients with chronic cardiac, pulmonary, and kidney disease, cancer, dementia and obesity were at higher risk of death16. People with obesity who contracted SARS-CoV-2, not only required invasive mechanical ventilation more often but were also 48% more likely to die17,18.
Given the evidence suggesting that COVID-19 disproportionately impacts the elderly and people living with long-term conditions, more research into the factors associated with deaths due to COVID-19 involving different population groups and settings is needed to better inform the design of strategies to reduce harm to those at highest risk. Furthermore, there is a paucity of research on the impact of multi morbidity on the risk of a fatal outcome associated with COVID-19. In this study, we aimed to estimate the impact of multiple long-term conditions on COVID-19 in-hospital mortality and assess the influence of multi morbidity on the risk of COVID-19-related death using a large, regional cohort of hospitalized patients.
Results
Analytical population
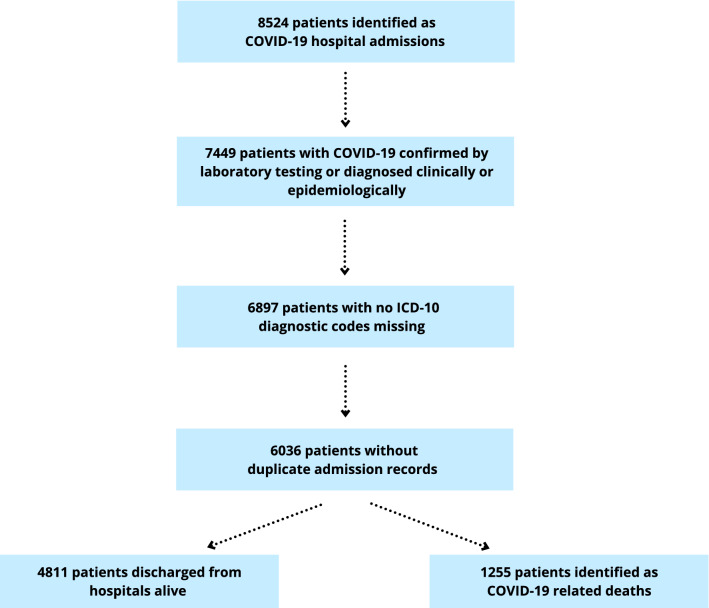
The dataset used in this study was based on the hospital identifying the admission as a COVID-19 admission and coding the method of admission accordingly. In effect, 8524 records identified as COVID-19 hospital admissions were initially considered for inclusion. Patients (n = 1075) were then excluded from the analysis if COVID-19 was not confirmed by laboratory testing or not diagnosed clinically or epidemiologically as reflected in the ICD-10 coding (note that the method of admission was not retrospectively amended). In addition, 552 patients with no ICD-10 codes were excluded. In effect, analyses were restricted to patients with ICD-10 diagnosis code for COVID-19, including primary or secondary diagnosis code of U07.1 and U07.2, with complete ICD-10 data on pre-existing health conditions. The ICD-10 diagnosis codes from multiple records of hospital admissions for a specific individual, including transfers between or within hospitals, were combined and the duplicate ICD-10 codes removed (n = 861). This resulted in a subset of 6036 (70.8%) hospitalized COVID-19 cases included in the analysis (Fig. 1). Out of 6036 patients, 5955 (98.7%) represented cases when COVID-19 was confirmed by laboratory testing (ICD-10 diagnosis code U07.1) while 81 (1.3%) corresponded to COVID-19 cases that were diagnosed clinically or epidemiologically since laboratory testing was inconclusive or not available (ICD-10 diagnosis code U07.2).
Figure 1.
Flow diagram indicating the selection of study participants.
Demographic and clinical characteristics of COVID-19 patients are shown in Table 1. The median (interquartile range, IQR) age of the 6036 patients was 72 (57–82) years. Patients that died were older (80 (73–86) years) than those that survived hospitalization (68 (54–80) years). Of those admitted to hospital with COVID-19, there was a higher proportion of males (3213, 53.2%) than females (2823, 46.8%), with men having a higher risk of COVID-19–associated mortality (711, 58%) compared to women (514, 42%) (P < 0.001). While 1713 patients (28.4%) had no previous comorbidities, 2010 patients (33.3%) had one, 1507 patients (25.0%) had two, and 806 patients (13.3%) had three or more comorbidities, with hypertension (22.7%), chronic obstructive pulmonary disease (19.8%), and diabetes (15.6%) being the most common conditions.
Table 1.
Demographic and clinical characteristics of patients.
| Discharged (n = 4811) | Died (n = 1225) | P value | |
|---|---|---|---|
| Age, median (IQR) | 68 (54–80) | 80 (73–86) | < 0.001 |
| Sex, n (%) | |||
| Female | 2309 (48.0) | 514 (42.0) | < 0.001 |
| Male | 2502 (52.0) | 711 (58.0) | |
| Congestive heart failure, n (%) | |||
| No | 4599 (95.6) | 1095 (89.4) | < 0.001 |
| Yes | 212 (4.4) | 130 (10.6) | |
| Cardiac arrhythmias, n (%) | |||
| No | 4253 (88.4) | 1030 (84.0) | < 0.001 |
| Yes | 558 (11.6) | 195 (16.0) | |
| Chronic obstructive pulmonary disease, n (%) | |||
| No | 3885 (80.8) | 958 (78.2) | 0.049 |
| Yes | 926 (19.2) | 267 (21.8) | |
| Diabetes, n (%) | |||
| No | 4086 (84.9) | 1007 (82.2) | 0.02 |
| Yes | 725 (15.1) | 218 (17.8) | |
| Hypertension, n (%) | |||
| No | 3738 (77.7) | 927 (75.7) | 0.14 |
| Yes | 1073 (22.3) | 298 (24.3) | |
| Hypothyroidism, n (%) | |||
| No | 4629 (96.2) | 1188 (97.0) | 0.23 |
| Yes | 182 (3.8) | 37 (3.0) | |
| Renal disease, n (%) | |||
| No | 4402 (91.5) | 985 (80.4) | < 0.001 |
| Yes | 409 (8.5) | 240 (19.6) | |
| Liver disease, n (%) | |||
| No | 4740 (98.5) | 1198 (97.8) | 0.08 |
| Yes | 71 (1.5) | 27 (2.2) | |
| Lymphoma, n (%) | |||
| No | 4771 (99.2) | 1198 (97.8) | < 0.001 |
| Yes | 40 (0.8) | 27 (2.2) | |
| Metastatic cancer, n (%) | |||
| No | 4766 (99.1) | 1192 (97.3) | < 0.001 |
| Yes | 45 (0.9) | 33 (2.7) | |
| Solid tumour without metastasis, n (%) | |||
| No | 4700 (97.7) | 1155 (94.3) | < 0.001 |
| Yes | 111 (2.3) | 70 (5.7) | |
| Rheumatoid arthritis/collagen vascular diseases, n (%) | |||
| No | 4727 (98.3) | 1203 (98.2) | 0.9 |
| Yes | 84 (1.7) | 22 (1.8) | |
| Obesity, n (%) | |||
| No | 4763 (99.0) | 1204 (98.3) | 0.049 |
| Yes | 48 (1.0) | 21 (1.7) | |
| Asthma, n (%) | |||
| No | 4403 (91.5) | 1173 (95.8) | < 0.001 |
| Yes | 408 (8.5) | 52 (4.2) | |
| Depression, n (%) | |||
| No | 4583 (95.3) | 1205 (98.4) | < 0.001 |
| Yes | 228 (4.7) | 20 (1.6) | |
| Dementia, n (%) | |||
| No | 4467 (92.9) | 1038 (84.7) | < 0.001 |
| Yes | 344 (7.1) | 187 (15.3) | |
| Neurological disorders, n (%) | |||
| No | 4557 (94.7) | 1153 (94.1) | |
| Yes | 254 (5.3) | 72 (5.9) | |
Statistically significant P < 0.05 values are in bold.
Comorbidities and in-hospital mortality in patients with COVID-19
An overview of associations between different comorbidities examined in this study and in-hospital mortality in patients with COVID-19 are shown in Table 2. Only 15.3% (187/1225) of patients who died in hospital from COVID-19 had no documented long-term conditions. Out of patients who were discharged, 31.7% (1526/4811) had no record of pre-existing long-term conditions. However, it is worth noting that patients with no record of pre-existing long-term conditions who died were significantly older (median (IQR): 81 (72–86) years) than those who were discharged (58 (44–72) years) (P < 0.05). Age was the factor most strongly associated with higher risk of COVID-19–associated mortality with a clear large gradient seen with increasing age (e.g., OR, 3.62; 95% CI, 1.83–7.71; P < 0.001 in patients aged 50–59 years; OR, 8.98; 95% CI, 4.69–17.24; P < 0.001 in patients aged 60–69 years; and OR, 14.53; 95% CI, 7.62–27.69; P < 0.001 in patients 70–79 years compared with those aged 40 to 49 years). The largest mortality risk was observed in patients aged ≥ 80 years compared with those aged 40–49 years (OR, 23.77; 95% CI, 12.48–45.27; P < 0.001). No significant difference in risk of COVID-19-related death was found between the age groups 0–40 years and 40–49 years (OR, 0.60; 95% CI, 0.20–1.77, P = 0.36). The risk of death was significantly higher in men (OR, 1.41; 95% CI, 1.22–1.62; P < 0.001). Furthermore, individuals with obesity (OR, 5.28; 95% CI, 2.92–9.52; P < 0.001), lymphoma (OR, 2.78; 95% CI, 1.64–4.74; P < 0.001), metastatic cancer (OR, 2.17; 95% CI, 1.25–3.77; P = 0.006), solid tumour without metastasis (OR, 1.67; 95% CI, 1.16–2.41; P = 0.006), liver disease (OR: 2.50, 95% CI, 1.53–4.07; P < 0.001), congestive heart failure (OR, 1.69; 95% CI, 1.32–2.15; P < 0.001), chronic obstructive pulmonary disease (OR, 1.43; 95% CI, 1.18–1.72; P < 0.001), renal disease (OR, 1.81; 95% CI, 1.51–2.19; P < 0.001), dementia (OR, 1.44; 95% CI, 1.17–1.76; P < 0.001) were at increased risk of in-hospital mortality. People with asthma were significantly less likely to die due to COVID-19 (OR, 0.60; 95% CI, 0.42–0.86; P = 0.005). No significant associations were observed between cardiac arrhythmias, diabetes, hypertension, hypothyroidism, rheumatoid arthritis/collagen vascular diseases, depression, neurological disorders, and risk of death (Table 2).
Table 2.
Multivariable logistic regression analysis for factors associated with COVID-19-related death.
| Characteristic | Level | Number of deaths, n (%) | Adjusted odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Age | 40–49 | 10 (0.8) | reference | – |
| 0–39 | 5 (0.4) | 0.60 (0.20,1.77) | 0.36 | |
| 50–59 | 60 (4.9) | 3.62 (1.83,7.17) | < 0.001 | |
| 60–69 | 176 (14.4) | 8.98 (4.68,17.24) | < 0.001 | |
| 70–79 | 370 (30.2) | 14.53 (7.62,27.69) | < 0.001 | |
| ≥ 80 | 604 (49.3) | 23.77 (12.48,45.27) | < 0.001 | |
| Sex | Male | 711 (58.0) | 1.41 (1.22,1.62) | < 0.001 |
| Congestive heart failure | Yes | 130 (10.6) | 1.69 (1.32,2.15) | < 0.001 |
| Cardiac arrhythmias | Yes | 195 (16.0) | 0.92 (0.76,1.11) | 0.38 |
| COPD | Yes | 267 (21.8) | 1.43 (1.18,1.72) | < 0.001 |
| Diabetes | Yes | 218 (17.8) | 1.13 (0.95,1.36) | 0.17 |
| Hypertension | Yes | 298 (24.3) | 0.94 (0.80,1.10) | 0.43 |
| Hypothyroidism | Yes | 37 (3.0) | 0.91 (0.62,1.34) | 0.65 |
| Renal disease | Yes | 240 (19.6) | 1.81 (1.51,2.19) | < 0.001 |
| Liver disease | Yes | 27 (2.2) | 2.50 (1.53,4.07) | < 0.001 |
| Lymphoma | Yes | 27 (2.2) | 2.78 (1.64,4.74) | < 0.001 |
| Metastatic cancer | Yes | 33 (2.7) | 2.17 (1.25,3.77) | 0.006 |
| Solid tumour without metastasis | Yes | 70 (5.7) | 1.67 (1.16,2.41) | 0.006 |
| Rheumatoid arthritis/ collagen vascular diseases | Yes | 22 (1.8) | 1.08 (0.66,1.79) | 0.78 |
| Obesity | Yes | 21 (1.7) | 5.28 (2.92,9.52) | < 0.001 |
| Asthma | Yes | 52 (4.2) | 0.60 (0.42,0.86) | 0.005 |
| Depression | Yes | 20 (1.6) | 0.62 (0.37,1.00) | 0.05 |
| Dementia | Yes | 187 (15.3) | 1.44 (1.17,1.76) | < 0.001 |
| Neurological disorders | Yes | 72 (5.9) | 1.20 (0.90,1.61) | 0.21 |
Statistically significant P < 0.05 values are in bold.
Observations, n = 6036, including 1225 COVID-19 deaths.
CI Confidence Interval; COPD Chronic obstructive pulmonary disease.
Increasing number of comorbidities was associated with a greater COVID-19 in-hospital mortality risk. Individuals with one pre-existing health condition had an adjusted OR for in-hospital death of 1.44 (95% CI, 1.18–1.75; P < 0.001), compared to those with no pre-existing health conditions (Table 3). Furthermore, the risk of death was increasingly higher in patients with two (OR, 1.79; 95% CI, 1.47–2.20; P < 0.001), and three or more comorbidities (OR, 1.80; 95% CI, 1.43–2.27; P < 0.001). Analyses stratified by sex showed a similar trend, i.e., both men and women with multiple pre-existing health conditions had increased odds of death when compared to those with no pre-existing health conditions (Table 3). However, the interaction test showed that the effect of the number of pre-existing conditions on the risk of COVID-19-related death did not differ significantly between males and females.
Table 3.
Number of pre-existing health conditions and risk of COVID-19-related death.
| Pre-existing health conditions | All | Males | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number, n (%) | Number of deaths, n (%) | Adjusted odds ratio (95% CIa) | P value | Total number, n (%) | Number of deaths, n (%) | Adjusted odds ratio (95% CIb) | P value | Total number, n (%) | Number of deaths, n (%) | Adjusted odds ratio (95% CIb) | P value | |
| 0 | 1713 (28.4) | 187 (15.3) | Reference | 913 (28.4) | 111 (15.6) | Reference | 800 (28.3) | 76 (14.8) | Reference | |||
| 1 | 2010 (33.3) | 430 (35.1) | 1.44 (1.18,1.75) | < 0.001 | 1104 (34.4) | 245 (34.5) | 1.34 (1.04,1.74) | 0.03 | 906 (32.1) | 185 (35.9) | 1.56 (1.15,2.11) | 0.004 |
| 2 | 1507 (25.0) | 394 (32.2) | 1.79 (1.47,2.20) | < 0.001 | 826 (25.7) | 239 (33.6) | 1.84 (1.41,2.40) | < 0.001 | 681 (24.1) | 155 (30.2) | 1.74 (1.27,2.38) | < 0.001 |
| 3 or more | 806 (13.4) | 214 (17.4) | 1.80 (1.43,2.27) | < 0.001 | 370 (11.5) | 116 (16.3) | 2.00 (1.46,2.75) | < 0.001 | 436 (15.5) | 98 (19.1) | 1.63 (1.16,2.29) | 0.005 |
aAdjusted odds ratios: adjusted for age and sex.
bAdjusted odds ratios: adjusted for age.
CI Confidence Interval; COPD Chronic obstructive pulmonary disease.
Significant values are in bold.
Multimorbidity clusters
Out of 6,036 hospitalised patients, 2313 (38.3%) had ≥ 2 conditions. The optimal k-modes clustering solution yielded 6 patient groups. The number of patients in each group ranged from 648 (Cluster 2) to 2293 (Cluster 3). The identified patient clusters had significant differences in mortality, ranging from 15.3% (Cluster 4) to 29.4% (Cluster 6). Median (IQR) age ranged from 63 (49–78) (Cluster 4) to 82 (74–86) (Cluster 6). Table 4 describes the prevalence of diseases in each patient cluster. Within each patient cluster, disease groups with the highest prevalence were highlighted in bold while other less common conditions with a higher prevalence than in other clusters were underlined. Cluster 1 was characterised by cardiovascular (100.0%), genitourinary (15.6%), and respiratory diseases (9.9%). Cluster 2 included a high percentage of patients with mental and neurological disorders (100%), metabolic and endocrine diseases (19.3%), and genitourinary diseases (9.4%). It was also Cluster 2 that had the highest percentage of patients with digestive diseases (2.5%). Note that digestive disease group included only patients with liver disease. Cluster 3 was characterized by respiratory (100%), cardiovascular (15.0%), and metabolic and endocrine diseases (12.7%) while Cluster 5 corresponded to the patient group with a high prevalence of metabolic & endocrine (100%), cardiovascular (69.1%), and genitourinary diseases (15.0%). The highest percentage of patients with cancer (5.9%) was found in Cluster 5. Finally, Cluster 6, with the highest mortality, was characterized by cardiovascular diseases (100.0%), and mental and neurological disorders (100%). Other featured conditions in this group were respiratory diseases (15.7%). This cluster had also the highest median (IQR) age of 82 (74–86). Several conditions were present in multiple patient clusters; however, their prevalence significantly differ.
Table 4.
Multimorbidity clusters in a population of hospitalised patients with COVID-19.
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | Cluster 6 | |
|---|---|---|---|---|---|---|
| Number of patients, n (%) | 1225 (20.3) | 648 (10.7) | 902 (14.9) | 2293 (38.1) | 682 (11.3) | 286 (4.7) |
| Age, median (IQR) | 77 (67–85) | 75 (59–83) | 70 (59–79) | 63 (49–78) | 72 (61–82) | 82 (74–86) |
| Disease group | ||||||
| Autoimmune (%) | 2.1 | 1.2 | 1.7 | 1.8 | 2.0 | 1.0 |
| Metabolic & endocrine (%) | 0.0 | 19.3 | 12.7 | 9.6 | 100.0 | 8.0 |
| Respiratory (%) | 9.9 | 8.6 | 100.0 | 0.0 | 10.1 | 15.7 |
| Cardiovascular (%) | 100.0 | 0.0 | 15.0 | 0.0 | 69.1 | 100.0 |
| Mental & neurological (%) | 0.0 | 100.0 | 5.5 | 0.0 | 4.4 | 100.0 |
| Neoplasms (%) | 4.2 | 2.5 | 4.3 | 5.9 | 2.3 | 3.5 |
| Digestive (%) | 1.6 | 2.5 | 1.0 | 1.7 | 2.2 | 0.0 |
| Genitourinary (%) | 15.6 | 9.4 | 6.0 | 9.0 | 15.0 | 12.2 |
| Mortality (%) | 23.8 | 23.5 | 22.0 | 15.3 | 21.8 | 29.4 |
Values in bold correspond to three conditions with the highest prevalence within patient groups (clusters of conditions). Underlined values represent other, less common conditions that have a higher prevalence than in other groups.
Sensitivity analysis
The results of two sensitivity analyses, based on identifying COVID-19 related admissions with ICD-10 code diagnosis: (1) including only primary or secondary diagnosis code of U07.1 (Table 5) and (2) including U07.1 or U07.2 as the primary code (Table 6), were consistent with those of the primary analysis. The magnitude and significance of associations between potential risk factors and the outcome measure remained robust under different assumptions. The difference in results between the primary and sensitivity analyses was observed only for neurological disorders, i.e., neurological disorders were found to be associated with increased risk of COVID-19-related deaths in sensitivity analysis in which COVID-19 deaths were defined as COVID-19 related admissions with ICD-10 diagnosis code U07.1 or U07.2 as the primary code for clinical diagnosis (OR, 1.45; 95% CI, 1.04–2.01; P = 0.03).
Table 5.
Sensitivity analysis for the association between pre-existing health condition and in-hospital mortality in patients with COVID-19.
| Characteristic | Level | Number of deaths, n (%) | Adjusted odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Age | 40–49 | 10 (0.8) | reference | – |
| 0–39 | 5 (0.4) | 0.60 (0.20,1.77) | 0.35 | |
| 50–59 | 59 (4.9) | 3.54 (1.79,7.02) | < 0.001 | |
| 60–69 | 174 (14.4) | 8.73 (4.54,16.77) | < 0.001 | |
| 70–79 | 366 (30.2) | 14.24 (7.47,27.17) | < 0.001 | |
| ≥ 80 | 598 (49.3) | 23.12 (12.13,44.04) | < 0.001 | |
| Sex | Male | 704 (58.1) | 1.40 (1.22,1.62) | < 0.001 |
| Congestive heart failure | Yes | 127 (10.5) | 1.70 (1.33,2.18) | < 0.001 |
| Cardiac arrhythmias | Yes | 191 (15.8) | 0.92 (0.76,1.12) | 0.4 |
| COPD | Yes | 262 (21.6) | 1.42 (1.18,1.71) | < 0.001 |
| Diabetes | Yes | 217 (17.9) | 1.14 (0.95,1.37) | 0.16 |
| Hypertension | Yes | 296 (24.4) | 0.94 (0.80,1.10) | 0.44 |
| Hypothyroidism | Yes | 37 (3.0) | 0.91 (0.62,1.34) | 0.64 |
| Renal disease | Yes | 238 (19.6) | 1.84 (1.52,2.21) | < 0.001 |
| Liver disease | Yes | 27 (2.2) | 2.46 (1.51,4.00) | < 0.001 |
| Lymphoma | Yes | 27 (2.2) | 2.78 (1.63,4.74) | < 0.001 |
| Metastatic cancer | Yes | 33 (2.7) | 2.18 (1.25,3.79) | 0.006 |
| Solid tumour without metastasis | Yes | 70 (5.8) | 1.69 (1.17,2.45) | 0.005 |
| Rheumatoid arthritis/ collagen vascular diseases | Yes | 21 (1.7) | 1.01 (0.61,1.69) | 0.96 |
| Obesity | Yes | 21 (1.7) | 5.27 (2.92,9.51) | < 0.001 |
| Asthma | Yes | 51 (4.2) | 0.59 (0.42,0.85) | 0.004 |
| Depression | Yes | 20 (1.7) | 0.63 (0.37,1.00) | 0.05 |
| Dementia | Yes | 185 (15.3) | 1.43 (1.17,1.76) | < 0.001 |
| Neurological disorders | Yes | 72 (5.9) | 1.20 (0.90,1.61) | 0.22 |
COVID-19-related deaths were defined as COVID-19 related admissions with ICD-10 code diagnosis, including only primary or secondary diagnosis code of U07.1.
Statistically significant P < 0.05 values are in bold.
Observations, n = 5955 including 1212 COVID-19 deaths.
CI Confidence Interval.
Table 6.
Sensitivity analysis for the association between pre-existing health conditions and in-hospital mortality in patients with COVID-19.
| Characteristic | Level | Number of deaths, n (%) | Adjusted odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Age | 40–49 | 9 (0.8) | reference | – |
| 0–39 | 5 (0.4) | 0.93 (0.31,2.82) | 0.90 | |
| 50–59 | 51 (4.7) | 3.41 (1.65,7.02) | < 0.001 | |
| 60–69 | 163 (15.0) | 9.19 (4.62,18.27) | < 0.001 | |
| 70–79 | 325 (29.8) | 15.21 (7.7,30.06) | < 0.001 | |
| ≥ 80 | 537 (49.3) | 27.1 (13.72,53.54) | < 0.001 | |
| Sex | Male | 633 (58.1) | 1.32 (1.14,1.54) | < 0.001 |
| Congestive heart failure | Yes | 112 (10.3) | 1.75 (1.33,2.3) | < 0.001 |
| Cardiac arrhythmias | Yes | 175 (16.1) | 0.97 (0.78,1.19) | 0.75 |
| COPD | Yes | 254 (23.3) | 1.39 (1.14,1.69) | 0.001 |
| Diabetes | Yes | 200 (18.3) | 1.16 (0.96,1.41) | 0.13 |
| Hypertension | Yes | 268 (24.6) | 1.01 (0.85,1.2) | 0.88 |
| Hypothyroidism | Yes | 32 (2.9) | 0.87 (0.57,1.32) | 0.50 |
| Renal disease | Yes | 220 (20.2) | 1.82 (1.49,2.22) | < 0.001 |
| Liver disease | Yes | 19 (1.7) | 2.47 (1.36,4.49) | 0.003 |
| Lymphoma | Yes | 24 (2.2) | 3.39 (1.86,6.2) | < 0.001 |
| Metastatic cancer | Yes | 27 (2.5) | 2.11 (1.11,4.02) | 0.02 |
| Solid tumour without metastasis | Yes | 59 (5.4) | 2.08 (1.36,3.17) | < 0.001 |
| Rheumatoid arthritis/ collagen vascular diseases | Yes | 21 (1.9) | 1.2 (0.71,2.04) | 0.50 |
| Obesity | Yes | 20 (1.8) | 5.06 (2.77,9.26) | < 0.001 |
| Asthma | Yes | 50 (4.6) | 0.61 (0.42,0.88) | 0.008 |
| Depression | Yes | 20 (1.8) | 0.64 (0.39,1.05) | 0.08 |
| Dementia | Yes | 169 (15.5) | 1.52 (1.22,1.9) | < 0.001 |
| Neurological disorders | Yes | 61 (5.6) | 1.45 (1.04,2.01) | 0.03 |
COVID-19 deaths were defined as COVID-19 related admissions with ICD-10 diagnosis code U07.1 or U07.2 as the primary code for clinical diagnosis.
Statistically significant P < 0.05 values are in bold.
Observations, n = 4960 including 1090 COVID-19 deaths.
CI Confidence Interval; COPD Chronic obstructive pulmonary disease.
Compared with people with no underlying conditions, the risk of death was significantly higher for those with one or more comorbidities (OR, 1.63; 95% CI, 1.36–1.94; P < 0.001) (Table S2 in Supplementary Material). Moreover, the risk of death was increasingly higher in patients with two and three or more comorbidities (Table S3 and S4 in Supplementary Material). These results strengthened the credibility of our main findings by highlighting the significant association between underlying health conditions and in-hospital mortality in individuals with COVID-19.
Since the primary analysis indicated that asthma was associated with a decreased risk of COVID-19-related death, we also performed a third sensitivity analysis to compare the risk of death from COVID-19 in people with asthma compared to those with no documented comorbidities. Our results showed no difference in risk of death from COVID-19 in people with asthma compared to patients with no record of underlying long-term conditions (OR, 1.22; 95% CI, 0.86–1.75; P = 0.27), suggesting that the presence of other comorbidities may have an impact on the significance of results of the primary analysis. We intend to investigate the extents of multimorbidity in patients with asthma as well as the relationship between asthma medications and different multimorbidity patterns in future work.
Discussion
Early recognition of high-risk and critically ill patients has become a priority in improving treatment and reducing mortality among patients who contracted SARS-CoV-22. In this study, we examined the impact of multiple long-term conditions on the in-hospital mortality in individuals with COVID-19 to determine risk factors for COVID-19-related deaths. Our findings can help improve the effectiveness of management of COVID-19 patients and contribute to further development of policies for prevention and response to COVID-19 and its critical outcomes. Using a large, well-documented, regional cohort of hospitalised COVID-19 patients, we found that pre-existing health conditions, including obesity, liver disease, renal disease, metastatic cancer, solid tumour without metastasis, lymphoma, congestive heart failure, chronic obstructive pulmonary disease, and dementia are clinical risk factors associated with COVID-19 mortality, with chronic obstructive pulmonary disease, renal disease, and dementia being the most prevalent among those that died.
Our results are consistent with several studies4,5,7,19–22. Chronic obstructive pulmonary disease (COPD) was found to increase the odds of death by nearly threefold in a large meta-analysis of 30 studies that examined the vital status of COVID-19 patients with COPD19. Substantial mortality rates in COVID-19 patients with COPD were also observed by other studies20,21. It has been suggested that the association between COPD and risk of poor outcomes in COVID-19 might be related to the fact that the innate and acquired antiviral immune responses in individuals with COPD are impaired, leading to delayed virus clearance19. Dementia was identified as a major risk factor for death in COVID‐19 cases22,23. Wang et al.22 showed that the odds of COVID-19-related death in patients with dementia doubled when compared to individuals without dementia, with the highest mortality risk in adults with vascular dementia (OR, 3.17; 95% CI, 2.97–3.37, P < 0.001). Some evidence suggested that elevated risk of neurological complications from COVID-19 in people with dementia might be caused by the pre-existing brain pathology24. For example, the breakdown of the blood–brain barrier, i.e., a defence mechanism against disease-causing pathogens, in patients with Alzheimer’s disease and vascular dementia, can increase the ability of bacterial, fungal, and viral pathogens to access the brain more easily and this in turn may have an effect on the severity of COVID-19 and associated fatal outcomes25,26. A number of studies investigated the impact of chronic diseases and health conditions on risk of COVID-19-related death through multivariate analyses4,5,7–10,27; however, some of them were based on a small sample size, included a limited list of underlying medical conditions or focused on the impact of a specific medical condition on COVID-19 mortality adjusted for demographic and/or socioeconomic characteristics7,9,10. Moreover, previous evidence on the relationship between multi morbidity and COVID-19-related death was limited4,5. A UK prospective observational study of 20,133 patients who were hospitalised with COVID-19 showed that the risk of death was higher for patients with dementia, chronic pulmonary disease, kidney disease, cardiac disease, liver disease, malignancy, and obesity5. Another study based on 10,926 COVID-19-related deaths reported similar results: individuals with COVID-19 and underlying kidney disease, liver disease, cardiovascular disease, chronic respiratory diseases, obesity, and recent history of haematological malignancy or other cancers had a greater risk of dying27. Cancer, possibly due to its ability to cause immunodeficiency inherently or through medication, was identified as a major risk factor for COVID-19-related deaths in several studies4,5,27. In individuals with heart failure and kidney disease, both the SARS-CoV-2 infection and the immune response to the viral infection could destabilize pre-existing conditions, leading to the development of acute cardiac28 or kidney29 injuries and hence, increase the risk of a fatal outcome associated with COVID-1930. People with obesity have been previously characterized by systemic low-grade inflammation, impaired immune response to infections, and higher susceptibility and mortality associated with infections17,31,32. These factors may all lead to a greater mortality risk in those who contracted SARS-CoV-2. Several studies emerging from different countries identified obesity as an independent risk factor for hospitalisation and death due to COVID-1911,12,18,31,32, with a BMI ≥ 35 kg/m2 radically increasing the mortality risk33.
In our study, asthma diagnosis was present among 7.6% of hospitalized patients with COVID-19, which is lower than the 9.8% prevalence of asthma in Northern Ireland34. At the same time, we found asthma to be associated with a lower risk of COVID-19-related death in the full sample, although the results of our sensitivity analysis showed no difference in risk of COVID-19-related death in patients with asthma compared to individuals with no documented comorbidities. This finding supports the mixed evidence on the role of asthma in influencing COVID-19-related outcomes. The Open SAFELY study identified asthma as a significant risk factor of death in patients with COVID-19 and indicated that patients on inhaled corticosteroids have the greatest risk3. In vitro studies have suggested that corticosteroids use can result in impaired antiviral innate immune responses35,36 and delayed virus clearance37, and this in turn can potentially lead to more severe outcomes in individuals who contracted SARS-CoV-2; however, this hypothesis has to be further tested. Several studies found no statistically significant difference in mortality risk by asthma status38,39. For example, the prospective case–control study based on the UK Biobank data showed that asthma did not significantly increase the odds of COVID-19 mortality38. More recent work however indicated that people with asthma were in fact less likely to die due to COVID-1940. Interestingly, the risk of severe clinical outcomes of COVID-19 was lower in people with allergic asthma41. Akenroye et al.42 suggested that some asthma medications, such as mepolizumab, reslizumab, and benralizumab, may enhance immune responses to viral infections and potentially decrease susceptibility to additional lung injury from diseases such as COVID-19. The association between asthma and COVID-19 mortality could also differ by the degree of asthma severity3. Given that our sensitivity analysis showed no difference in risk of COVID-19-related death in patients with asthma compared to individuals with no documented comorbidities, but our primary results indicated a lower risk of COVID-19-related death in patients with asthma compared to non-asthma controls, further analysis is required to evaluate the impact of differing patterns and extents of multimorbidity in patients with asthma (e.g. cardiometabolic multimorbidity) and the role of different asthma medications when examining COVID-19-related outcomes.
We confirmed that COVID-19-related mortality increased with older age. In particular, patients in age groups ≥ 50 years old had higher odds of COVID-19-related death when compared with those aged 40 to 49 years. Higher COVID-19 mortality among older adults has been known since early in the pandemic and has been described in detail3,27,43. The analysis based on the US epidemiologic data demonstrated that the overall COVID-19 case-fatality rate among individuals infected with SARS-CoV-19 was highest in those aged ≥ 85 years (range 10–27%), followed by those aged 65–84 years (3–11%), aged 55–64 years (1–3%), and aged < 55 years (< 1%)43. Greater risk of death due to COVID-19 in older adults is likely related to their declining immune defences, however other hypotheses have also been suggested44.
Finally, we demonstrated that multimorbidity is an important clinical characteristic to consider in the context of the COVID19 pandemic. Our findings showing the higher COVID-19 in-hospital mortality risk in people with multiple underlying conditions are consistent with other published data45. A cross-sectional, multicenter, observational study of Italian COVID-19 population found that increasing multimorbidity, measured by the Charlson Comorbidity Index, was strongly associated with COVID-19-related death46. Kim et al.47, in their study of 2,491 COVID-19 patients, reported that individuals with 3 or more underlying conditions had a 1.8 times higher risk of in-hospital mortality than patients with no underlying conditions. Multimorbidity was also reported to be a predictor of the risk of COVID-19 infection in a large UK Biobank cohort of 428,199 participants; however, the authors did not report on the relationship between the co-existence of multiple underlying conditions and risk of death48.
We also analysed multimorbidity patterns by identifying clusters of conditions in hospitalised COVID-19 patients using an unsupervised machine learning technique (k-mode clustering). Our study revealed recognisable co-occurrences of COVID-19 with different combinations of diseases, with a potentially causal link or underlying mechanism, including cardiovascular diseases, respiratory diseases, mental and neurological disorders, metabolic and endocrine diseases, and renal diseases. For example, Cluster 1 characterized by the group of individuals with cardiovascular diseases had also a high percentage of cases with COPD. Previous studies suggested that the systemic inflammatory response associated with COPD may act as a possible mechanism that links COPD with increased risk for cardiovascular diseases49. Furthermore, it was demonstrated that COPD is associated with increased carotid intimal medial thickness (CIMT) and that among those with COPD, CIMT is linked to higher cardiovascular mortality50. Evidence also supports the association between renal disease and cognitive impairment (Cluster 2); however, the mechanisms underlying this association are not completely elucidated51. Although, direct impact of uremic toxins has been proposed as a potential cause of cognitive decline, studies showed that dialysis prescription did not reverse symptoms of cognitive impairment52. Co-occurrence of other conditions, such as, (i) metabolic syndrome (defined by the presence of metabolic abnormalities including obesity, glucose intolerance, and elevated blood pressure), cardiovascular disease, and liver disease (Cluster 2, 3 and 5)53,54; (ii) mental disorders and heart disease (Cluster 6)55, and (iii) cancer with obesity and diabetes (Cluster 4)56 has also been broadly documented and shown to be associated with high mortality. Therefore, the identification of these multi morbidity patterns among hospitalized individuals with COVID-19 can help identify opportunities to target patient-centred care towards people with high-risk ages and a specific combination of health conditions, leading to improved clinical outcomes. Note that we acknowledge that a presence of a specific chronic disease or a combination of chronic diseases in our analytic sample may have acted as an effect modifier of COVID-19 death but could also be associated with COVID-19 death via the existence of another common cause i.e., other clinical characteristics or socio-economic factors not included in our study.
The strengths of our study include the large, regional cohort of hospitalised COVID-19 patients, providing high statistical power to investigate associations between different risk factors and COVID-19 in-hospital mortality. The use of ICD-10 diagnosis codes assigned by medical professionals working in all hospitals throughout Northern Ireland meant that comprehensive information on a wide range of comorbidities were available. Our results remained robust in a number of sensitivity analyses and reinforced previous findings of a higher risk of COVID-19-related death associated with obesity12, liver disease57, renal disease5, metastatic cancer58, congestive heart failure59, and COPD19. Furthermore, we investigated the associations between several conditions for which little data exist regarding risk for in-hospital mortality in patients with COVID-19, such as hypothyroidism, solid tumour without metastasis, lymphoma, dementia, and other neurological disorders. We also added to the, so far inconclusive, evidence on the role of asthma in influencing COVID-19-related outcomes. Finally, given a paucity of research on the impact of multi morbidity on the risk of COVID-19 in-hospital mortality, we examined the association between increasing multi morbidity, multi morbidity patterns, and COVID-19-related death. To our knowledge, this is the first study to characterise patterns of multi morbidity in a hospitalised population with COVID 19 using an unsupervised machine learning approach.
The interpretation of our results should be made considering several limitations. First, due to unavailability of ICD-10 diagnosis codes, we were unable to consider the records of 552 patients with COVID-19, admitted to hospital in the period from March 1, 2020, to January 31, 2021. Furthermore, we have only used the comorbidity data collected in the studied period. Therefore, it is possible we missed some comorbidities by not including information reported at prior admissions. Second, the underlying cause of death was allocated based on the hospital records of ICD-10 diagnostic coding and discharge status, not death certificate; this might have led not only to the underestimation of the real magnitude of mortality due to COVID-19 but also potential misclassification of deaths from other causes. To assess the extent to which these inaccuracies may have affected our estimates, a similar analysis should be performed in the future using death certificate–based ICD-10 diagnosis codes. Third, since the ICD-10 diagnosis codes were assigned by medical professionals working in different hospitals, they may not have captured intended disease concepts with complete consistency. Fourth, our analysis is cross-sectional, meaning that both the independent variables and the outcome were collected simultaneously. Although the central element of the cross-sectional design is the lack of the temporal information required to describe the evolution of the underlying dynamics, in case of our study, the temporal link between the outcome and independent variables can be cautiously assumed since the presence of underlying health conditions most likely preceded the outcome studied (i.e., fatal/non-fatal hospitalization due to COVID-19). Fifth, the impact of inpatient treatment or procedures performed during hospitalisation as well as the information on the duration of the long-term conditions on patient outcomes was not considered in this study. In addition, unavailability of data on clinical parameters such as oxygen and ventilation treatment limited the opportunity of a more comprehensive analysis including multiple levels of severity as the outcome. Sixth, the purpose of using the clustering approach was for this method to potentially become part of the pipeline for discovering the various multi morbidity profiles that are associated with mortality due to COVID-19. Extensions to this research may include a more robust look at the effects of the hyper parameters and different analytic samples on the associations between different disease groups and COVID-19-related deaths. Furthermore, the results of the clustering algorithm should be further validated by domain experts to determine its clinical utility. It is important to highlight that this analysis was performed among a cohort of individuals hospitalized with COVID-19 and may not be generalizable to those non-hospitalized. The lack of data on non-hospitalized individuals with SARS-CoV-2 infection limited our ability to examine utilization of our algorithm outside of the hospitalized patients. However, our population represented a diverse cohort with a variety of comorbidities and is representative of high-risk individuals across Northern Ireland. Seventh, as mentioned above, our analytic sample does not represent a true random selection from the population since it is solely based on hospitalised patients, that died or did not die during the admission episode following COVID-19 diagnosis. This non-randomness of sample selection may have had an impact on our study results. Older adults, those obese, and with pre-existing medical conditions are more susceptible to adverse COVID-19 outcomes, while COVID-19 severity likely influences hospitalisation60. As such, characteristics related to our sample inclusion, also relate to the hypothesised risk factors and the outcome of interest and hence, investigating these factors within hospitalised patients may have introduced the possibility of collider bias. In future work, the likelihood and extent of collider bias associated with sample selection could be evaluated by comparing means, variances, and distributions of variables in the sample of individuals with COVID-19 that have been hospitalized with those in a representative sample of the NI population61. This could not be evaluated in the current data set. Finally, our study may be subject to other potential sources of bias. For example, selection bias could have been introduced by not including non-hospitalized individuals living in long-term care or assisted living facilities that suffered from severe COVID-19 symptoms and subsequently died due to COVID-1962. Bias due to omission of a confounder from the model (unmeasured confounder) is also a possibility. Several demographic factors (e.g., race/ethnicity, disability status, socio-economic status), as well as structural factors (e.g., literacy) were not included in the analysis63–66. Moreover, we did not control for the effect of concurrently prescribed medications such as antipsychotics, proton pump inhibitors, antihistamines, and opioid analgesics that were previously shown to increase the risk of adverse outcomes among patients with COVID-1967. The association between certain drug classes, polypharmacy, and the risk of COVID-19 mortality should be addressed in future work. In addition, further analysis on host-specific genetic factors and their relationships with severe manifestations of COVID-19, in particular, in individuals with no underlying health conditions that died as a direct consequence of SARS‐CoV‐2 infection, could allow us to better understand relationships between environmental risk factors and severe outcomes associated with COVID-19 and identify potential targets for therapeutic development.
This study estimated the impact of the broad spectrum of comorbidities on COVID-19 in-hospital mortality using a large, regional cohort of hospitalized patients. We found that individuals with lymphoma, metastatic cancer, solid tumour without metastasis, liver disease, congestive heart failure, chronic obstructive pulmonary disease, obesity, renal disease, and dementia were at significantly increased risk for COVID-19-related death. In addition, we showed that the presence of multiple coexisting health conditions further increased odds of death. Given that effective clinical management of patients with multimorbidity is therefore a critical step towards their survival from COVID-19, multidisciplinary clinical teams should prepare comprehensive care plans that can be streamlined and meet the dynamic needs of such patients. Future work should investigate the associations between different patterns of multimorbidity and COVID-19 mortality to better characterize those individuals who would benefit from enhanced preventive measures.
Methods
Study design and study population
This retrospective cohort study was conducted using the Patient Administration System (PAS) data from Northern Ireland, covering the period from March 1, 2020, to January 31, 2021. The PAS data includes patient level data on hospital admissions, transfers, and discharges for all NHS Trusts in Northern Ireland. In total, 8524 records identified as COVID-19 hospital admissions (using PAS admission codes) were initially selected for analysis (Fig. 1). We then excluded n = 1075 patients that were not clinically, epidemiologically, or laboratory-confirmed COVID-19 cases as indicated by COVID-19-related ICD-10 diagnostic codes of U07.1 and U07.2, leaving 7449 patients eligible for analysis. Next, we excluded n = 552 patients for whom ICD-10 codes of U07.1 and U07.2 were not recorded. In effect, analyses were restricted to n = 6897 patients with ICD-10 codes for COVID-19, including the primary or secondary diagnosis code of U07.1 and U07.2, with complete ICD-10 data on underlying conditions. Finally, we combined the ICD-10 diagnosis codes from multiple hospitalizations due to COVID-19, including transfers between or within hospitals, and removed the duplicate ICD-10 codes (n = 861). Note that the discharge code of the most recent hospital stay was used for patients with multiple records of hospital admissions. This resulted in a subset of 6036 hospitalized COVID-19 cases included in the analysis. Specifically, we compared characteristics of COVID-19 patients that were discharged from hospitals (n = 4811) with those that died in hospital during the admission episode following COVID-19 diagnosis (n = 1225). Figure 1 shows the stages of the selection process.
Outcomes
The outcome used in our analysis were hospital discharge and in-hospital COVID-19-related death, defined on the basis of a discharge status and the International Classification of Diseases (version 10; ICD-10) code diagnosis, of U07.1 (“COVID-19, virus identified”) or U07.2 (“COVID-19, virus not identified”), recorded as either a primary or secondary diagnosis to ensure the inclusion of all people who had COVID-19 in hospital68. Specifically, U07.1 code was assigned when COVID-19 was confirmed by laboratory testing irrespective of severity of clinical signs or symptoms while U07.2 code was used when COVID-19 was diagnosed clinically or epidemiologically but laboratory testing was inconclusive or not available. For patients with multiple records of hospital admissions identified as COVID-19 hospital admissions, including those transferred between or within hospitals, the discharge code of the most recent hospital stay, was considered the outcome measure. Therefore, the same person was not included more than once in the analytic sample while all in-hospital COVID-19-related deaths were counted. The presence of specific comorbidities in patients with multiple records was established by combining the ICD-10 diagnosis codes from all visits and removing the duplicate codes.
Covariates
We examined the following patient-level data for each admission: (i) demographics (age, sex); and (ii) ICD-10 diagnosis codes that were used to identify common comorbidities. The presence of specific comorbidities in patients with multiple records of hospital admissions, including those transferred between or within hospitals, was established by combining the ICD-10 diagnosis codes from all considered visits and removing the duplicate codes. Subsequently, the discharge code of the most recent hospital stay for a patient with multiple records of hospital admissions was considered the outcome measure. The ICD-10 coding criteria for all comorbidities were adapted from the Elixhauser comorbidity measure69. Accordingly, the following comorbidities were considered: congestive heart failure (I09.9, I11.0, I13.0, I13.2, I25.5, I42.0, I42.5–I42.9, I43.x, I50.x, P29.0), cardiac arrhythmias (I44.1–I44.3, I45.6, I45.9, I47.x–I49.x, R00.0, R00.1, R00.8, T82.1, Z45.0, Z95.0), chronic obstructive pulmonary disease (I27.8, I27.9, J40.x–J47.x, J60.x–J67.x, J68.4, J70.1, J70.3), diabetes including uncomplicated (E10.0, E10.1, E10.9, E11.0, E11.1, E11.9, E12.0, E12.1, E12.9, E13.0, E13.1, E13.9, E14.0, E14.1, E14.9) and complicated cases (E10.2–E10.8, E11.2–E11.8, E12.2–E12.8, E13.2–E13.8, E14.2–E14.8), hypertension (I10.x, I11.x–I13.x, I15.x), hypothyroidism (E00.x–E03.x, E89.0), renal disease (I12.0, I13.1, N03.2–N03.7, N05.2–N05.7, N18.x, N19.x, N25.0, Z49.0–Z49.2, Z94.0, Z99.2), liver disease (B18.x, I85.x, I86.4, I98.2, K70.x, K71.1, K71.3–K71.5, K71.7, K72.x–K74.x, K76.0, K76.2–K76.9, Z94.4), lymphoma (C81.x–C85.x, C88.x, C96.x, C90.0, C90.2), metastatic cancer (C77.x–C80.x), solid tumour without metastasis (C00.x–C26.x, C30.x–C34.x, C37.x–C41.x, C43.x, C45.x–C58.x, C60.x–C76.x, C97.x), rheumatoid arthritis/collagen vascular diseases (L94.0, L94.1, L94.3, M05.x, M06.x, M08.x, M12.0, M12.3, M30.x, M31.0–M31.3, M32.x–M35.x, M45.x, M46.1, M46.8, M46.9), obesity (E66.x), depression (F20.4, F31.3–F31.5, F32.x, F33.x, F34.1, F41.2, F43.2), dementia (F00.x–F03.x, F05.1, G30.x, G31.1), neurological disorders such as paralysis (G04.1, G11.4, G80.1, G80.2, G81.x, G82.x, G83.0–G83.4, G83.9) and other neurological disorders (G10.x–G13.x, G20.x–G22.x, G25.4, G25.5, G31.2, G31.8, G31.9, G32.x, G35.x–G37.x, G40.x, G41.x, G93.1, G93.4, R47.0, R56.x), and asthma (J.45).
Statistical analysis
Descriptive statistics were provided using median (interquartile range [IQR]) for continuous variables and counts and percentages for categorical data. The Shapiro–Wilk test was used to determine if the data deviates from a normal distribution. The Wilcoxon rank sum test was applied to compare groups of continuous data. Statistical differences between groups of categorical variables were established with the Fisher test. A two-sided P < 0.05 was considered statistically significant.
The association between patient characteristics and in-hospital mortality was examined using logistic regression models. Adjusted logistic regression analyses were presented as odds ratios (OR) with 95% confidence intervals (95% CI) and included age, sex, and pre-existing long-term conditions identified using ICD-10 coding rules. Statistical significance was determined using the Wald’s test. Non-linear dependence between age and the outcome measure was evaluated by comparing the generalised additive model with linear and spline terms for age against the model with only a linear term. The likelihood-ratio χ2 test indicated that linearity assumption was not satisfied (p < 0.001). We therefore grouped age into six categories (0–40, 40–50, 50–60, 60–70, 70–80 and ≥ 80 years) since odds ratios calculated for variables modelled as polynomials in the model, are not directly interpretable. Additionally, we provided the odds ratios adjusted for age parametrised as a 4-knot restricted cubic spline in the Supplementary Material (Table S1 in Supplementary Material). Furthermore, the non-linear relationship between age and the risk of death involving COVID-19 is shown in Fig.F1 in Supplementary Material.
We investigated the potential effect of having multiple pre-existing long-term conditions on the outcome measure (hospital discharge vs. in-hospital COVID-19-related death). The analysis was conducted for the total population and stratified by sex. Comorbidities were expressed as categorical variables from 0 to 2, then 3 or higher. The model including categories of comorbidities was adjusted for age and sex in the analysis including all patients and age only in the sex-stratified analysis, with age modelled as a polynomial in the logistic regression model.
We further investigated patterns of multimorbidity by performing a cluster analysis. First, we classified all considered health conditions into eight groups: (1) autoimmune diseases including rheumatoid arthritis and collagen vascular diseases; (2) metabolic and endocrine diseases including diabetes, hypothyroidism, and obesity; (3) respiratory diseases including chronic obstructive pulmonary disease and asthma; (4) cardiovascular diseases including hypertension, congestive heart failure, and cardiac arrhythmias; (5) mental and neurological disorders including depression, dementia, and neurological disorders; (6) neoplasms including lymphoma, metastatic cancer, and solid tumour without metastasis; (7) digestive system diseases including liver disease; and (8) genitourinary system diseases including renal diseases. To identify disease clusters within the analytic sample, we used the k-modes clustering approach, an unsupervised machine learning algorithm for grouping categorical data70. The method partitions the objects (patients) into a specified number of clusters such that the distance from objects to the assigned cluster modes is minimized. We used the Hamming distance to determine the dissimilarity of two objects. The optimal number of clusters was determined with the modified Elbow method using within-cluster differences71. Frequencies and percentages of disease groups were then calculated for each cluster. Descriptive statistics of age and in-hospital mortality in patients with COVID-19 were also provided for each cluster. All analyses were conducted using R Studio v.1.2.5033 operating R v.3.6.3.
Sensitivity analysis
Four sensitivity analyses were performed to validate the robustness of the main findings. First, COVID-19-related deaths were defined as COVID-19 related admissions with ICD-10 code diagnosis, including only primary or secondary diagnosis code of U07.1 which is likely to have a greater degree of specificity. Second, COVID-19-related deaths were defined as COVID-19 related admissions with ICD-10 diagnosis code U07.1 or U07.2 as the primary code for clinical diagnosis. Note that U07.1 code represents cases when COVID-19 was confirmed by laboratory testing while U07.2 code corresponds to COVID-19 cases that were diagnosed clinically or epidemiologically since laboratory testing was inconclusive or not available. Third, to assess the relative importance of underlying health conditions to adverse outcomes, we compared the risk of death from COVID-19 in individuals with comorbidity and those with no recorded comorbidity, adjusting for sex and age parametrised as a 4-knot restricted cubic spline. Finally, since asthma was found to be associated with a decreased risk of COVID-19-related death, we also performed a fourth sensitivity analysis to compare the risk of death from COVID-19 in people with asthma and those with no documented comorbidities. The model was adjusted for sex and age parametrised as a 4-knot restricted cubic spline.
Ethical considerations
The study and data collection were approved by the Office for Research Ethics Committees Northern Ireland (20/0062). We followed the ethical standards set by the Helsinki Declaration of 1964, as revised in 2013, and the research guidelines of the University of Ulster. The Office for Research Ethics Committees Northern Ireland considered the collection of routine data as evaluation of service and waived the need for written informed consent. The Office for Research Ethics Committees Northern Ireland approved the publication of results. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines72.
Supplementary Information
Acknowledgements
The authors wish to thank the Information Team, Performance Management Service Improvement Directorate, HSCB for assisting in the collation of the data.
Author contributions
M.B., A.J.B., H.V.W. conceptualized the study. M.B. analysed the data. M.B., D.e.B., D.a.B., L.P., D.G., A.J.B. contributed to interpretation of the results. MB took the lead in writing the first draft. M.B., D.e.B., D.a.B., L.P., R.S., H.V.W., D.G., A.J.B. critically reviewed the manuscript. All authors approved the final manuscript.
Data availability
De-identified study data are available for access by accredited researchers in accordance with data sharing policies of HSCB Performance Management Service Improvement Directorate.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-20176-w.
References
- 1.Guan WJ, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun P, et al. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: A single arm meta-analysis. J. Med. Virol. 2020;92(6):612–617. doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson EJ, et al. Factors associated with COVID-19-related death using open safely. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng G, Yin M, Chen X, Zeng F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit. Care. 2020;24:1–3. doi: 10.1186/s13054-020-02902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Docherty AB, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: Prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae S, et al. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: A systematic review and meta-analysis. Heart. 2021;107(5):373–380. doi: 10.1136/heartjnl-2020-317901. [DOI] [PubMed] [Google Scholar]
- 7.Chen T, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng Y, et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: A propensity score-matched analysis. J. Hematol. Oncol. 2020;13(1):1–1. doi: 10.1186/s13045-020-00907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020;52(6):1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lighter J, et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin. Infect. Dis. 2020;71(15):896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietz W, Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity. 2020;28(6):1005. doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 13.National Records of Scotland. Deaths involving coronavirus (COVID-19) in Scotland. (2021).
- 14.Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 16.Docherty AB, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: Prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popkin BM, et al. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020;21(11):e13128. doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonnet A, LICORN. the Lille COVID‐19. Obesity Study Group et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28(1195):1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerayeli FV, et al. COPD and the risk of poor outcomes in COVID-19: A systematic review and meta-analysis. EClinicalMedicine. 2021;33:100789. doi: 10.1016/j.eclinm.2021.100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alqahtani JS, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: A rapid systematic review and meta-analysis. Plos One. 2020;15(5):e0233147. doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabbani G, et al. Pre-existing COPD is associated with an increased risk of mortality and severity in COVID-19: A rapid systematic review and meta-analysis. Expert Rev. Respir. Med. 2021;15(5):705–716. doi: 10.1080/17476348.2021.1866547. [DOI] [PubMed] [Google Scholar]
- 22.Wang Q, Davis PB, Gurney ME, Xu R. COVID-19 and dementia: Analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement. 2021;7(8):1297–1306. doi: 10.1002/alz.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tahira AC, Verjovski-Almeida S, Ferreira ST. Dementia is an age-independent risk factor for severity and death in COVID-19 inpatients. Alzheimers Dement. 2021;17(11):1818–1831. doi: 10.1002/alz.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo CL, et al. APOE e4 genotype predicts severe COVID-19 in the UK Biobank community cohort. J. Gerontol. A. 2020;75(11):2231–2232. doi: 10.1093/gerona/glaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweeney MD, Sagare AP, Zlokovic BV. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14(3):133. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueno M, et al. Blood-brain barrier damage in vascular dementia. Neuropathology. 2016;36(2):115–124. doi: 10.1111/neup.12262. [DOI] [PubMed] [Google Scholar]
- 27.Reilev M, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: A nationwide cohort. Int. J. Epidemiol. 2020;49(5):1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomasoni D, et al. COVID-19 and heart failure: From infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur. J. Heart Fail. 2020;22(6):957–66. doi: 10.1002/ejhf.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsch JS, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang R, Gui X, Zhang Y, Xiong Y. The role of essential organ-based comorbidities in the prognosis of COVID-19 infection patients. Expert Rev. Respir. Med. 2020;14(8):835–838. doi: 10.1080/17476348.2020.1761791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sattar N, McInnes IB, McMurray JJV. Obesity a risk factor for severe COVID-19 infection: Multiple potential mechanisms. Circulation. 2020;142(1):4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 32.Caussy C, Wallet F, Laville M, Disse E. Obesity is associated with severe forms of COVID-19. Obesity. 2020;28(7):1175. doi: 10.1002/oby.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rottoli M, et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? results from a single Italian centre. Eur. J. Endocrinol. 2020;183(4):389–397. doi: 10.1530/EJE-20-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asthma UK. Asthma facts and statistics. https://www.asthma.org.uk/about/media/facts-and-statistics/ (2017).
- 35.Davies JM, et al. Budesonide and formoterol reduce early innate anti-viral immune responses in vitro. Plos One. 2011;6(11):e27898. doi: 10.1371/journal.pone.0027898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson JL, et al. Reduced antiviral interferon production in poorly controlled asthma is associated with neutrophilic inflammation and high-dose inhaled corticosteroids. Chest. 2016;149(3):704–713. doi: 10.1016/j.chest.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Singanayagam A, et al. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nat. Commun. 2018;9(1):1–6. doi: 10.1038/s41467-018-04574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khawaja AP, et al. Associations with covid-19 hospitalisation amongst 406,793 adults: The UK Biobank prospective cohort study. MedRxiv. 2020 doi: 10.1101/2020.05.06.20092957. [DOI] [Google Scholar]
- 39.Sunjaya AP, Allida SM, Di Tanna GL, Jenkins CR. Asthma and COVID-19 risk: A systematic review and meta-analysis. Eur. Respir. J. 2022;59(3):2101209. doi: 10.1183/13993003.01209-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou H, Xu J, Li Y, Wang Y, Yang H. The association of asthma with COVID-19 mortality: An updated meta-analysis based on adjusted effect estimates. J. Allergy Clin. Immunol. Pract. 2021;9(11):3944–3968. doi: 10.1016/j.jaip.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Z, et al. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J. Allergy Clin. Immun. 2020;146(2):327–329. doi: 10.1016/j.jaci.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akenroye AT, Wood R, Keet C. Asthma, biologics, corticosteroids, and coronavirus disease 2019. Ann. Allergy Asthma Immunol. 2020;125(1):12–13. doi: 10.1016/j.anai.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen E, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet. Infect. Dis. 2020;20(9):e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging. 2020;12(10):9959. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández-Niño JA, Guerra-Gómez JA, Idrovo AJ. Multimorbidity patterns among COVID-19 deaths: Proposal for the construction of etiological models. Rev. Panam. Salud. Publica. 2020;44:166. doi: 10.26633/RPSP.2020.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iaccarino G, et al. Age and multimorbidity predict death among COVID-19 patients: Results of the SARS-RAS study of the italian society of hypertension. Hypertension. 2020;76(2):366–372. doi: 10.1161/HYPERTENSIONAHA.120.15324. [DOI] [PubMed] [Google Scholar]
- 47.Kim L, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET) Clin. Infect. Dis. 2021;72(9):e206–e214. doi: 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McQueenie R, et al. Multimorbidity, polypharmacy, and COVID-19 infection within the UK Biobank cohort. Plos One. 2020;15(8):e0238091. doi: 10.1371/journal.pone.0238091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sin DD, Man SP. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? the potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107(11):1514–1519. doi: 10.1161/01.CIR.0000056767.69054.B3. [DOI] [PubMed] [Google Scholar]
- 50.Maclay JD, MacNee W. Cardiovascular disease in COPD: Mechanisms. Chest. 2013;143(3):798–807. doi: 10.1378/chest.12-0938. [DOI] [PubMed] [Google Scholar]
- 51.Silva STD, Ribeiro RDCL, Rosa CDOB, Cotta RMM. Cognitive capacity in individuals with chronic kidney disease: Relation to demographic and clinical characteristics. Braz. J. Nephrol. 2014;36:163–170. doi: 10.5935/0101-2800.20140026. [DOI] [PubMed] [Google Scholar]
- 52.Radic J, et al. The possible impact of dialysis modality on cognitive function in chronic dialysis patients. Neth. J. Med. 2010;68(4):153–157. [PubMed] [Google Scholar]
- 53.Cheong KC, et al. Association of metabolic syndrome with risk of cardiovascular disease mortality and all-cause mortality among Malaysian adults: A retrospective cohort study. BMJ Open. 2021;11(8):e047849. doi: 10.1136/bmjopen-2020-047849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Käräjämäki AJ, et al. Non-alcoholic fatty liver disease with and without metabolic syndrome: Different long-term outcomes. Metabolism. 2017;66:55–63. doi: 10.1016/j.metabol.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 55.De Hert M, Detraux J, Vancampfort D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin. Neurosci. 2022;20(1):31–40. doi: 10.31887/DCNS.2018.20.1/mdehert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hawkins ML, et al. Endocrine and metabolic diseases among colorectal cancer survivors in a population-based cohort. JNCI J. Natl. Cancer Inst. 2020;112(1):78–86. doi: 10.1093/jnci/djz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galiero R, et al. Impact of chronic liver disease upon admission on COVID-19 in-hospital mortality: Findings from COVOCA study. Plos One. 2020;15(12):e0243700. doi: 10.1371/journal.pone.0243700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee LY, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020;21(10):1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doolub G, et al. Impact of COVID-19 on inpatient referral of acute heart failure: A single-centre experience from the south-west of the UK. ESC Heart Fail. 2021;8(2):1691–1695. doi: 10.1002/ehf2.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Lusignan S, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal college of general practitioners research and surveillance centre primary care network: A cross-sectional study. Lancet Infect. Dis. 2020;20(9):1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffith GJ, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. commun. 2020;11(1):1–12. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.The Northern Ireland Statistics and Research Agency. Covid-19 related deaths and pre-existing conditions in Northern Ireland: March 2020 to November 2021. nisra.gov.uk/sites/nisra.gov.uk/files/publications/C19-PEC-Nov21_0.pdf (2021).
- 63.Raleigh VS. Ethnic differences in covid-19 death rates. BMJ. 2022 doi: 10.1136/bmj.o427. [DOI] [PubMed] [Google Scholar]
- 64.Bosworth ML, et al. Deaths involving COVID-19 by self-reported disability status during the first two waves of the COVID-19 pandemic in England: A retrospective, population-based cohort study. Lancet Public Health. 2021;6(11):e817–e825. doi: 10.1016/S2468-2667(21)00206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cifuentes MP, Rodriguez-Villamizar LA, Rojas-Botero ML, Alvarez-Moreno CA, Fernández-Niño JA. Socioeconomic inequalities associated with mortality for COVID-19 in Colombia: A cohort nationwide study. J. Epidemiol. Community Health. 2021;75(7):610–615. doi: 10.1136/jech-2020-216275. [DOI] [PubMed] [Google Scholar]
- 66.Paakkari L, Okan O. COVID-19: Health literacy is an underestimated problem. Lancet Public Health. 2020;5(5):e249–e250. doi: 10.1016/S2468-2667(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McKeigue PM, et al. Relation of severe COVID-19 to polypharmacy and prescribing of psychotropic drugs: The REACT-SCOT case-control study. BMC Med. 2021;19(1):1–11. doi: 10.1186/s12916-021-01907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quan H, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 69.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med. Care. 2009;47(6):626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 70.Huang Z. A fast clustering algorithm to cluster very large categorical data sets in data mining. In: Lu H, Motoda H, Luu H, editors. KDD: Techniques and Applications. Singapore: World Scientific; 1997. pp. 21–34. [Google Scholar]
- 71.Shalev-Shwartz S, Ben-David S. Understanding Machine Learning from Theory to Algorithms. Cambridge University Press; 2014. [Google Scholar]
- 72.Vandenbroucke JP, et al. Strobe initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Plos Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified study data are available for access by accredited researchers in accordance with data sharing policies of HSCB Performance Management Service Improvement Directorate.