Abstract
This study aimed to investigate the presence/absence of SARS-CoV-2 genome in the air and high-touch surfaces. This cross-sectional study was conducted from late-2020 to mid-2021 in the sections of Intensive Care Unit (ICU), emergency, infectious disease ward, and nursing station of the COVID-19 patient reception center in Kerman, Iran. The presence/absence of SARS-CoV-2 genome in the 60 samples of high-touch surfaces and 23 air samples was analyzed by reverse transcription polymerase chain reaction (RT-PCR). Fisher’s exact test was used to compare the number of positive samples in different sampling sites. The genome of SARS-CoV-2 was found in the eight samples (13.32%) taken from the high-touch surfaces (two samples in COVID-19 ICU, two samples in general ICU, two samples in emergency ward, and two samples in nursing station) and two air samples (8.70%) (one sample in the general ICU and one sample in the emergency ward). Statistical analysis showed that there was no significant difference between the type of sampling site and the positive cases of SARS-CoV-2 in the surface samples (p value = 0.80) and air samples (p value = 0.22). According to the results, the SARS-CoV-2 can find in the high-touch surfaces and indoor air of the COVID-19 patient reception centers. Therefore, suitable safety and health measures should be taken, including regular and accurate disinfection of surfaces and equipment and proper ventilation to protect healthcare workers and prevent disease transmission. More studies are recommended to investigate the SARS-CoV-2 concentration in the high-touch surfaces and air samples in the similar researches, efficacy of different disinfectants used on the high-touch surfaces and compare the effect of type of ventilation (natural or mechanical) on the viral load.
Keywords: SARS-CoV-2, High-touch surfaces, Indoor air, Kerman
Article Highlights
The presence/absence of SARS-CoV-2 genome investigated in the air and high-touch surfaces.
Eight surfaces samples (13.32%) and two air samples (8.70%) were positive for genome.
SARS-CoV-2 genome detected in the general ICU and emergency ward air samples.
There was no significant difference between the sampling site and positive cases.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as the novel coronavirus 2019 is a beta-coronavirus and third known zoonotic coronavirus after SARS and Middle East Respiratory Syndrome (MERS). It spread rapidly across all countries in the world and the WHO declared it as a global pandemic on March 12, 2020. Different ways of SARS-CoV-2 transmission have been introduced (Chia et al. 2020). Generally, it seems that viral respiratory infections are transmitted by direct contact with infected people or surfaces as well as droplets containing viral particles (Morawska 2006).
It is possible for people to be infected with SARS-CoV-2 through contact with contaminated surfaces (Tavakoli et al. 2020). By investigating the studies on SARS-associated coronavirus, it was found that virus can be also transmitted through air in different environments (Morawska et al. 2009; Kenarkoohi et al. 2020). These studies have concluded that airborne transmission is the main way of virus transmission in indoor environments (Morawska and Cao 2020). On July 9, 2020, the WHO officially confirmed that coronavirus-containing droplets could also be transmitted through air and those who are present in crowded and poorly ventilated spaces could be at risk of becoming infected with the virus (Hadei et al. 2020). Measures required to control airborne transmission of the viruses included increased ventilation rate, natural ventilation, social distancing, and reduced number of people in a common environment (Cucinotta and Vanelli 2020).
Different studies have been conducted to investigate the SARS-CoV-2 infection in hospitals. Results of these studies approved airborne transmission of the SARS-CoV-2 (Habibiet al. 2021; Hemati et al. 2021; Mallach et al. 2021). Presence of SARS-CoV-2 was investigated in banks, shopping malls and airports in the study by Hadei et al. The results showed that many samples contained the viral genome (Hadei et al. 2020). Many studies have been also conducted on the assessment of surface infection of the coronavirus. It was reported that the coronavirus genome was found in the surface samples (Dietz et al. 2021; Ge et al. 2021; Kotwa et al. 2021).
Despite the efforts made by international community to identify the SARS-CoV-2 and its transmission ways, many issues still remain unknown. Earlier studies suggested that SARS and other coronaviruses can live on the environmental surfaces and inanimate objects, and on the other hand, coronavirus has been detected on particle size of 1–4 μm and more (Chia et al. 2020). It is necessary to investigate the contamination level of surfaces and indoor air of the hospital wards with this virus to control the transmission of the disease. As a result, this study aimed to investigate the presence/absence of SARS-CoV-2 genome in the air and high-touch surfaces of the COVID-19 patient reception center in Kerman. Compared to other microorganisms, detecting viruses in the air samples poses many challenges. Viruses are present only in very low concentrations in the air, which requires sampling with a larger volume of air as well as keeping the virus alive for reliable estimates of results. In various studies, air sampling methods were used such as impinge (Masoumbeigi et al. 2020; Hemati et al. 2021), MD8 sampler with gelatin filter (Yamagishi et al. 2020; Passos et al. 2021), polytetrafluoroethylene (PTFE) filter (Ong et al. 2020; Bazzazpour et al. 2021). In the current study, for the first time, a PTFE filter with a pore size of 0.22 microns and a diameter of 47 mm was used to collect air samples.
Materials and Methods
This cross-sectional study was conducted from late-2020 to mid-2021 in Afzalipour Hospital in Kerman as one of the largest and most equipped hospitals in Iran, especially in southeastern Iran (considered as a reception center for COVID-19 patients in Kerman during the pandemic). Research steps included determining sampling sites, high-touch surfaces sampling, air sampling, RNA extraction, and evaluating the presence/absence of the SARS-CoV-2 genome and statistical analysis.
Sampling Sites
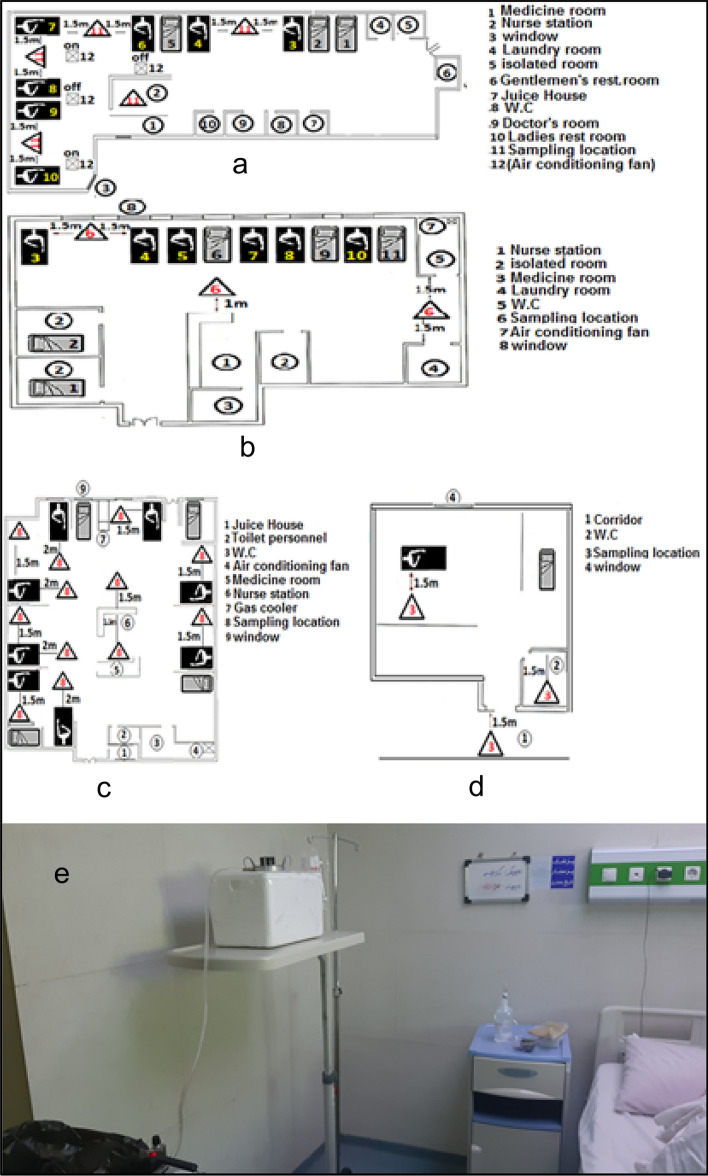
In the current study, samples were taken from high-touch surfaces (objects with high probability of contact) in general Intensive Care Unit (ICU), COVID-19 ICU, emergency ward, infectious disease ward, and nursing station of the COVID-19 ICU. Air samples were taken from COVID-19 ICU, and general ICU, emergency ward, and infectious disease ward (Fig. 1). Temperature and relative humidity in different parts of the study were measured. The average temperature and relative humidity in the wards under study were 24–26 °C and 25–35%, respectively.
Fig. 1.
Status of air sampling sites in this study; a general ICU, b acute emergency ward 1, c COVID-19 ICU, d infectious disease ward (patient room), e condition of air sampler
Sampling of High-Touch Surfaces
Distilled water, solutions and Eppendorf tubes were DNase and RNase free in the current study. 60 samples of high-touch surfaces were collected in accordance with the WHO protocols. Sampling was carried out before disinfection. Disinfection was done by the solutions of Sayasept (4%) and Septi Surface for the surface in the form of wipes, sodium hypochlorite (2%) for the surface as the spray and Septidin for equipment.
Sterile swabs were wetted with the viral transport medium (VTM). It is recommended to apply sufficient pressure with the wet swab onto the surface, move them in at least two different directions while rotating the swab stick. According to the WHO protocol, the smallest surface suggested for using swabs was 25 cm2. Two or three swabs were used for larger surfaces.
After sampling, each swab was placed in the Eppendorf tubes containing 2 ml of VTM (200 ml of double distilled water, stabilizing protein, antibiotic and buffer solution). Then, the vials were labeled and placed in a self-sealing bag. The outside of the bag was disinfected with 60–80% ethanol and placed in another similar unused bag. A set of control samples was also included at each sampling round. Sterile swabs were considered as the negative controls. Other control samples were included opening the package and removing the swab from the tube, but without sampling any surfaces that handled in the same way as the environmental samples. Also, other set of control samples remained sealed, but was shipped, stored and tested with the surface samples. They used to exclude contamination later on (WHO 2020).
Air Sampling
A number of 23 air samples was collected by drying method using SKC personal sampling pumps at flow rate of 25 L per minute (L/min) for one hour (1500 L) equipped with hydrophilic polytetrafluoroethylene filter at pore size of 0.22 µm and a diameter of 47 mm in the cassette filter holder (Heneghan et al. 2021). The sampling pump was installed at a height of 1.5 m from the floor and distance of 1.5–2 m from the patient bed. After sampling, the filters were placed in vials included the VTM. Then, the vials were labeled and placed in a sealed bag. The outside of the bag was disinfected with 60–80% ethanol and placed in another similar unused bag. At each sampling round, one sample from each section was considered as a control sample (so that a filter was placed inside the VTM at the sampling site with no specific action, but transport and stored and tested similar to the original samples to exclude secondary contamination). Sterile filters were considered as the negative controls. Date, time, and exact location of sampling were noted for each sample. After sampling, the samples taken from air and surfaces were transported to the virology lab as soon as possible at 4 °C and kept at − 30 °C until analysis.
RNA Extraction and Presence/Absence of Virus
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) (MIC, BMS Company, Australia) was used to determine presence/absence of SARS-CoV-2 genome in samples taken from air and surfaces (Chia et al. 2020). The samples were centrifuged in the lab at 13,000 rpm for 13 min, then the supernatant was discarded and the remaining microtubules were used for RNA extraction. RNA virus was extracted by RNA extraction kit (manufactured by Behgene Company, Shiraz, Iran) in accordance with the manufacturer's instructions. Virus detection kit (manufactured by Pishtaz Company, Iran) included positive and negative controls was used to determine the presence/absence of SARS-CoV-2 genome using RT-PCR in samples. Specific primer and probe targeting RNA-dependent RNA polymerase (RdRp) and S gene was used to determine viral genomes, which simultaneously detected in a multiplex reaction by FAM and ROX fluorochromes, respectively. The internal control gene of RNase p with the JOE reporter dye was used to test the quality of the extracted RNA and to check the accuracy of the technique. According to the manufacturer's instruction, the extracted genome was reacted with other reaction components in the microtube to detect the SARS-CoV-2 genome. Prepared reactions were in 50 °C for 20 min, 95 °C for 3 min, followed by 42 cycles of 95 °C for 3 s, and 55 °C for 20 s. RT-PCR results were interpreted in accordance with the kit’s instruction and the presence/absence of the virus was determined. Experiments of presence/absence of virus are illustrated in Fig. 2.
Fig. 2.

Experiments of presence/absence of virus
Statistical Analysis
Results were quantitatively reported as the number and percentage of positive samples for the presence of SARS-CoV-2. The number of positive samples in different sampling sites included general ICU, COVID-19 ICU, emergency ward, infectious disease ward, and nursing station of the COVID-19 ICU were compared through Fisher’s exact test (Razzini et al. 2020) in R 4.0.3. P value lower than 0.05 was considered as a significant level.
Results and Discussion
In this study, the contamination status of the high-touch surfaces and indoor air of the COVID-19 patient reception center in Kerman was investigated for the presence/absence of SARS-CoV-2 genome.
Occurrence of SARS-CoV-2 Genome in High-Touch Surfaces
According to the results, the SARS-CoV-2 genome was detected in eight samples (13.32%) out of 60 samples collected from the high-touch surfaces in different hospital wards. The total number and percentage of samples and positive samples collected from the high-touch surfaces in different wards have been shown in Table 1. Statistical analysis showed that there was no significant difference between the type of sampling site and the positive cases of SARS-CoV-2 in samples taken from the surfaces (p value = 0.80).
Table 1.
Analysis results of the presence of SARS-CoV-2 genome in samples taken from the high-touch surfaces from different hospital wards
| Sampling site | Number of samples (%) | Number of positive samples (%) | p value |
|---|---|---|---|
| General ICU | 12 (20) | 2 (3.33) | |
| COVID-19ICU | 16 (26.6) | 2 (3.33) | |
| Acute wars department 1 | 10 (16.7) | 2 (3.33) | 0.80 |
| Infectious disease ward | 10 (16.7) | 0 (0) | |
| Nursing station | 12 (20) | 2 (3.33) | |
| Total | 60 (100) | 8 (13.32) |
Of eight samples taken from the high-touch surfaces, the presence of SARS-CoV-2 genome was confirmed at the sphygmomanometer cuff and vital signs monitor in COVID-19 ICU, stethoscope and ventilator in general ICU, bed rail and manometer in the acute emergency department 1, and the telephone and shift notebook in nursing station of COVID-19 ICU. The virus genome was not detected in other samples taken from the high-touch surfaces.
According to the studies, there is a risk of infection with SARS-CoV-2 by touching contaminated objects or surfaces, so that virus can enter the body after contact with the contaminated surfaces and then touching the mouth, nose, and eyes with the infected hand. Generally, the persistence of coronavirus on surfaces can affect the disease transmission rate. The characteristics of coronavirus-infected surfaces and environmental conditions are important aspects that determine the persistence of the infection and virus transmission rate (Cucinotta and Vanelli 2020; Dargahi et al. 2021).
In the present study, infected surfaces were reported in COVID-19 ICU, general ICU, acute respiratory emergency ward 1, and COVID-19 ICU nursing station. In the study conducted by Hemati et al. on the contamination of the contact surfaces in the ward of patients infected with SARS-CoV-2 in Hajar Hospital in Shahrekord, Iran, 13 samples were collected from different surfaces and the presence of the virus genome was confirmed in 6 samples (46%) (Hemati et al. 2021). Of 50 samples taken from high-touch surfaces in Imam Khomeini Hospital in Ardabil, Iran in the study carried out by Dargahi et al. 2021, SARS-CoV-2 genome was detected in nine samples (18%) (Dargahi et al. 2021). Ryu et al. studied the viral infection level in two hospitals in South Korea where 13 patients infected with COVID-19 were hospitalized, in which 13 samples (45.16%) were positive for presence of SARS-CoV-2 (Ryu et al. 2020).In 2020, a study was conducted by Giordano et al., in the hospitals of Italy. In this study, the presence of SARS-CoV-2 was negative after disinfecting the contact surfaces of waiting rooms and corridors. The study indicated that despite the widespread contamination of high-touch surfaces in patients’ rooms and toilets, the protective measures and disinfection can eliminate the contamination (Giordano et al. 2020). Razzini et al. investigated the contamination of contact surfaces with the SARS-CoV-2 in COVID-19 ward of a hospital in Milan, Italy. A total of 37 samples were collected from infected wards (COVID-19 patients department), semi-infected wards (locker room), and clean areas. The virus genome was found in 24.3% of the samples. The number of positive samples was higher in infected and semi-infected areas than clean areas (Razzini et al. 2020). The results of similar studies on the SARS-CoV-2 genome in the samples of high-touch surfaces are compared in Table 2.
Table 2.
Comparison of similar studies on the SARS-CoV-2 genome in the samples of high-touch surfaces
| Firs author | Year | Sampling location | Type of sample | Sample size | Sampling method | Results | References |
|---|---|---|---|---|---|---|---|
| Razzini | 2020 | Exposure levels to SARS-CoV-2 virus in the corona ward of a hospital in Milan, Italy | Surfaces | 37 | Sterile swab and transfer to the solution VTM | 9 Positive samples | Razzini et al. (2020) |
| Ryu | 2020 | The environment of two hospitals in South Korea where 13 patients with COVID-19 were hospitalized | Surfaces | 79 | Sterile swab and transfer to the solution VTM |
13 Positive samples |
Ryu et al. (2020) |
| Dargahi | 2021 | Surfaces with common contact of Imam Khomeini Hospital, Ardabil | Surfaces | 50 | Sterile swab and transfer to the solution VTM | 9 Positive samples | Dargahi et al. (2021) |
| Hemati | 2021 | Contact surfaces of hospitalization of patients with SARS-CoV-2 in Hajar Shahrekord Hospital | Surfaces | 13 | Sterile swab and transfer to the solution VTM | 6 Positive samples | Hemati et al. (2021) |
| This study | 2021 | High-touch surfaces (objects with high probability of contact) in general Intensive Care Unit (ICU), COVID-19 ICU, emergency ward, infectious disease ward, and nursing station of the COVID-19 ICU | Surfaces | 60 | Sterile swab and transfer to the solution VTM | 8 Positive samples | – |
Occurrence of SARS-CoV-2 Genome in Indoor Air Samples
Of 23 air samples taken from COVID-19 ICU, general ICU, emergency and infectious diseases wards, the presence of SARS-CoV-2 genome was reported in two samples (8.70%). Analysis results of the presence of the SARS-CoV-2 genome in air samples from different hospital wards and the ventilation status in the studied wards are reported in Table 3. Statistical analysis showed that there was no significant difference between the type of sampling site and the positive cases of SARS-CoV-2 in samples taken from the indoor air (p value = 0.22). The virus genome was detected in one sample of five samples collected from the general ICU (1.5 m distance between patients of beds no.3 and no.4) and one sample of 3 samples collected from acute emergency ward 1 (1.5 m distance between toilet and laundry room).
Table 3.
Analysis results of SARS-CoV-2 genome in air samples taken from different hospital wards
| Sampling site | Type of ventilation | Number of samples (%) | Number of positive cases (%) | p value |
|---|---|---|---|---|
| COVID-19 ICU | Natural and artificial | 12 (52.3) | 0 (00) | |
| General ICU | Natural and artificial | 5 (21.7) | 1 (4.35) | 0.22 |
| Acute emergency ward 1 | Natural and artificial | 3 (13.0) | 1 (4.35) | |
| Infectious disease ward | No ventilation | 3 (13.0) | 0 (00) | |
| Total | 23 (100) | 2 (8.70) | ||
General evidence for the transmission of SARS-CoV-2 through air has been considered as a global challenge. In the present study, indoor air samples were collected from different wards of COVID-19 patient reception center in Kerman to determine the transmission of SARS-CoV-2 through air. Airborne transmission is a major route for infectious agents such as viruses (Noorimotlagh et al. 2020).
In a study conducted by Rahmani et al. on air contamination of the area for the patients with SARS-CoV-2 in Hajar Hospital in Shahrekord, Iran, 15 air samples were collected by impinger, of which four samples (26.66%) were positive for the presence of virus genome (Rahmani et al. 2020). Kenarkoohi et al. investigated the presence of SARS-CoV-2 in the air samples taken from ICU of Shahid Mostafa Khomeini Hospital in Ilam, Iran. The results of the study suggested that transmission of SARS-CoV-2 through air is possible and more accurate methods should be considered for prevention and control of infection in ICUs. In this study, air samples taken from the infectious disease ward of hospitalized patients were free of SARS-CoV-2 (Kenarkoohi et al. 2020). Faridi et al. reported that 10 air samples collected from the ICU wards of Imam Khomeini Hospital in Tehran, Iran were negative for SARS-CoV-2 (Faridi et al. 2020). Ong et al. reported that all air samples collected from infection isolation rooms were negative for SARS-CoV-2 in a hospital in Singapore, which was consistent with the results of this study (Ong et al. 2020).Yuan et al. investigated the distribution RNA of SARS-CoV-2 in samples collected from air and surfaces of different wards of Wuhan Hospital in China. The infection in ICUs was higher than general wards. The presence of the virus was widely detected in the air at a distance of 4 m from the patient bed (Yuan et al. 2020).The results of some studies are in contrast to findings of our study, which can be due to the difference in the ventilation and method and type of disinfection. Chia et al. detected SARS-CoV-2 particles in the air of infection isolation rooms equipped with ventilation in the general department of the hospital. However, they reported that respiratory aerosol is not the main method of transmission of SARS-CoV-2 and more data are required to confirm the potential way of transmission of the virus through the air (Chia et al. 2020). Hemati et al. collected 107 indoor air samples from different wards of Hajar Hospital in Shahrekord, Iran. The presence of SARS-CoV-2 was confirmed in six air samples taken from infectious disease ward 1 and 2, ICU, computed tomography scan (CT), respiratory clinic, and personal protective equipment (PPE) (Hemati et al. 2021). Findings of this research in comparison with the results of studies carried out in relatively similar centers indicated that the probability of finding the virus genome in healthcare centers is higher than other areas (hotels, means of transportation, etc.). It could be attributed to the presence of definite or suspected patients infected with COVID-19.The large number of these patients as well as the closed area of these centers increase the probability of the presence of SARS-CoV-2 in the air, despite the presence of natural and mechanical ventilation. The results of similar studies on the SARS-CoV-2 genome in the air samples are compared in Table 4.
Table 4.
Comparison of similar studies on the SARS-CoV-2 genome in the air samples
| Firs author | Year | Sampling location | Type of sample | Sample size | Sampling method | Results | References |
|---|---|---|---|---|---|---|---|
| Kenarkoohi | 2020 |
ICU, Laboratory ward, CT scan, Radiology, Men and Woman internal ward, Emergency ward, ICU and Hospital entrance hall in Iran |
Air | 14 | Impinger | 2 Positive samples | Kenarkoohi et al. (2020) |
| Razzini | 2020 | Corridor for patients, ICU, dressing and undressing room | Air | 5 |
MD8 Airport Portable Air Sampler with gelatine Membrane Filters |
5 Positive samples | Razzini et al. (2020) |
| Lednicky | 2020 | Ward rooms | Air | 22 |
BioSpot-VIVAS Sampler |
12 Positive samples | Lednicky et al. (2020) |
| Ong | 2020 | Infection isolation rooms | Air | 3 | 37 mm filter cassettes and 0.3 μm PTFE filters | 2 Positive samples | Ong et al. (2020) |
| Hemati | 2021 |
Infectious wards, ICU, Pediatric ward, CT scan, Emergency ward, Laundry, Respiratory patients clinic |
Air | 45 | Impinger | 6 Positive samples | Hemati et al. (2021) |
| Passos | 2021 | different hospital facilities (indoor environments) and public spaces (outdoor environments) | Air | 62 | Samplers with filters) and passive method (petri dishes) | 5 Positive samples | Passos et al. (2021) |
| Jin | 2021 | ICU | Air | 7 | WA 400 Portable viral aerosol sampler |
1 Positive samples |
Jin et al. (2021) |
| Mallach | 2021 | hospital ward and ICU rooms, rooms in long-term care homes experiencing outbreaks, and a correctional facility | Air | 138 | Gelatin filters by Ultrasonic Personal Air Samplers (UPAS) |
15 Positive samples |
Mallach et al. (2021) |
| This study | 2021 |
dental clinics COVID-19 ICU, General ICU, Acute emergency ward 1, Infectious disease ward |
Air | 23 |
47 mm filter cassettes and 0.22 μm PTFE filters |
2 Positive samples |
– |
Management of Virus Dissemination
To manage virus dissemination, proper safety and health measures should be adopted including suitable disinfectants (Kotwa et al. 2021), regular and accurate disinfection of surfaces and equipment (Ge et al. 2021), proper ventilation of different departments (Passos et al. 2021), and also ensuring the appropriate function of hospital ventilation systems (Faridi et al. 2020) and its improvement to protect the healthcare personnel and prevent the disease transmission.
Conclusion
The presence/absence of SARS-CoV-2 in the samples from indoor air and high-touch surfaces was investigated in COVID-19 patient reception center in Kerman. The results of this study demonstrated that the SARS-CoV-2 genome was found in samples taken from air (13.32%) and surfaces (8.70%). Significant difference was not observed between the type of sampling site, Intensive Care Unit (ICU), emergency, infectious disease ward, and nursing station, and the positive cases of SARS-CoV-2. Generally, based on the results of the present research, it can be concluded that the transmission of SARS-CoV-2 is possible through surfaces and indoor air. There were some restrictions during the present study: (1) Specific filters of this virus at pore size of 0.3 μm and diameter of 37 mm is very efficient for sampling the SARS-CoV-2, where this filter could not be prepared in this study. (2) In this study, merely the presence/absence of SARS-CoV-2 genome in the samples was examined and it was not possible to detect the virus concentration. Therefore, more studies are recommended to investigate the SARS-CoV-2 concentration in the high-touch surfaces and air samples via filters 0.3 μm and diameter of 37 mm, efficacy of different disinfectants used on the high-touch surfaces and compare the effect of type of ventilation (natural or mechanical) on the viral load.
Acknowledgements
This work was Master of Science thesis that supported by the Vice-Chancellor for Research and Technology of Kerman University of Medical Sciences. The authors would like to acknowledge the Environmental Health Engineering Research Center of Kerman University of Medical Sciences for financial supports.
Author Contributions
IH collected samples, MH developed the theory and performed the computations, GA analyzed samples, DKN wrote the manuscript, AH supervised the findings of this work, TD contributed to the revision of the manuscript and MF conceived of the presented idea.
Funding
This work was Master of Science thesis that supported by the Vice-Chancellor for Research and Technology of Kerman University of Medical Sciences under grant number 99000629.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
This work was supported by the Vice-Chancellor for Research and Technology of Kerman University of Medical Sciences under code of research ethics certificate IR.KMU.REC.1399.590.
Consent to Participate
Not applicable.
Consent for Publication
Not Applicable.
References
- Bazzazpour S, Rahmatinia M, Mohebbi SR, Hadei M, Shahsavani A, Hopke PK, Houshmand B, Raeisi A, Jafari AJ, Yarahmadi M. The detection of SARS-CoV-2 RNA in indoor air of dental clinics during the COVID-19 pandemic. Environ Sci Pollut Res. 2021 doi: 10.1007/s11356-021-15607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia PY, Coleman KK, Tan YK, Ong SWX, Gum M, Lau SK, Lim XF, Lim AS, Sutjipto S, Lee PH. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun. 2020;11(1):1–7. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio Medica Atenei Parmensis. 2020;91(1):157. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargahi A, Jeddi F, Vosoughi M, Karami C, Hadisi A, Mokhtari SA, Ghobadi H, Alighadri M, Haghighi SB, Sadeghi H. Investigation of SARS CoV-2 virus in environmental surface. Environ Res. 2021;195:110765. doi: 10.1016/j.envres.2021.110765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz L, Constant DA, Fretz M, Horve PF, Martinez-Olsen A, Stenson J, Wilkes A, Martindale RG, Messer WB, Van Den Wymelenberg KG (2021) Exploring Integrated Environmental Viral Surveillance of Indoor Environments: a comparison of surface and bioaerosol environmental sampling in hospital rooms with COVID-19 patients. medRxiv
- Faridi S, Niazi S, Sadeghi K, Naddafi K, Yavarian J, Shamsipour M, Jandaghi NZS, Sadeghniiat K, Nabizadeh R, Yunesian M. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci Total Environ. 2020;725:138401. doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge T, Lu Y, Zheng S, Zhuo L, Yu L, Ni Z, Zhou Y, Ni L, Qu T, Zhong Z. Evaluation of disinfection procedures in a designated hospital for COVID-19. Am J Infect Control. 2021;49(4):447–451. doi: 10.1016/j.ajic.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G, Blanchini F, Bruno R, Colaneri P, Di Filippo A, Di Matteo A, Colaneri M. Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy. Nat Med. 2020;26(6):855–860. doi: 10.1038/s41591-020-0883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi N, Uddin S, Al-Salameen F, Al-Amad S, Kumar V, Al-Otaibi M, Razzack NA, Shajan A, Shirshikar F. SARS-CoV-2, other respiratory viruses and bacteria in aerosols: report from Kuwait's hospitals. Indoor Air. 2021 doi: 10.1111/ina.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadei M, Hopke PK, Jonidi A, Shahsavani A. A letter about the airborne transmission of SARS-CoV-2 based on the current evidence. Aerosol Air Qual Res. 2020;20(5):911–914. doi: 10.4209/aaqr.2020.04.0158. [DOI] [Google Scholar]
- Hemati S, Mobini GR, Heidari M, Rahmani F, Babadi AS, Farhadkhani M, Nourmoradi H, Raeisi A, Ahmadi A, Khodabakhshi A. Simultaneous monitoring of SARS-CoV-2, bacteria, and fungi in indoor air of hospital: a study on Hajar Hospital in Shahrekord, Iran. Environ Sci Pollut Res. 2021 doi: 10.1007/s11356-021-13628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneghan C, Spencer EA, Brassey J, Plüddemann A, Onakpoya IJ, Evans D, Conly JM, Jefferson T. SARS-CoV-2 and the role of airborne transmission: a systematic review. F1000Research. 2021;10(232):232. doi: 10.12688/f1000research.52091.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Li J, Yang J, Li J, Hong F, Long H, Deng Q, Qin Y, Jiang J, Zhou X. SARS-CoV-2 presented in the air of an intensive care unit (ICU) Sustain Cities Soc. 2021;65:102446. doi: 10.1016/j.scs.2020.102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenarkoohi A, Noorimotlagh Z, Falahi S, Amarloei A, Mirzaee SA, Pakzad I, Bastani E. Hospital indoor air quality monitoring for the detection of SARS-CoV-2 (COVID-19) virus. Sci Total Environ. 2020;748:141324. doi: 10.1016/j.scitotenv.2020.141324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwa JD, Jamal AJ, Mbareche H, Yip L, Aftanas P, Barati S, Bell NG, Bryce E, Coomes ED, Crowl G (2021) Surface and air contamination with SARS-CoV-2 from hospitalized COVID-19 patients in Toronto, Canada. medRxiv [DOI] [PMC free article] [PubMed]
- Lednicky JA, Lauzard M, Fan ZH, Jutla A, Tilly TB, Gangwar M, Usmani M, Shankar SN, Mohamed K, Eiguren-Fernandez A. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallach G, Kasloff SB, Kovesi T, Kumar A, Kulka R, Krishnan J, Robert B, McGuinty M, den Otter-Moore S, Yazji B (2021) Aerosol SARS-CoV-2 in hospitals and long-term care homes during the COVID-19 pandemic. medRxiv [DOI] [PMC free article] [PubMed]
- Masoumbeigi H, Ghanizadeh G, Arfaei RY, Heydari S, Goodarzi H, Sari RD, Tat M. Investigation of hospital indoor air quality for the presence of SARS-Cov-2. J Environ Health Sci Eng. 2020;18(2):1259–1263. doi: 10.1007/s40201-020-00543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air. 2006;16(5):335–347. doi: 10.1111/j.1600-0668.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- Morawska L, Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L, Johnson G, Ristovski Z, Hargreaves M, Mengersen K, Corbett S, Chao CYH, Li Y, Katoshevski D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2009;40(3):256–269. doi: 10.1016/j.jaerosci.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorimotlagh Z, Jaafarzadeh N, Martínez SS, Mirzaee SA. A systematic review of possible airborne transmission of the COVID-19 virus (SARS-CoV-2) in the indoor air environment. Environ Res. 2020 doi: 10.1016/j.envres.2020.110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos RG, Silveira MB, Abrahão JS. Exploratory assessment of the occurrence of SARS-CoV-2 in aerosols in hospital facilities and public spaces of a metropolitan center in Brazil. Environ Res. 2021;195:110808. doi: 10.1016/j.envres.2021.110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani AR, Leili M, Azarian G, Poormohammadi A. Sampling and detection of corona viruses in air: a mini review. Sci Total Environ. 2020;740:140207. doi: 10.1016/j.scitotenv.2020.140207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzini K, Castrica M, Menchetti L, Maggi L, Negroni L, Orfeo NV, Pizzoccheri A, Stocco M, Muttini S, Balzaretti CM. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Sci Total Environ. 2020;742:140540. doi: 10.1016/j.scitotenv.2020.140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu B-H, Cho Y, Cho O-H, Hong SI, Kim S, Lee S. Environmental contamination of SARS-CoV-2 during the COVID-19 outbreak in South Korea. Am J Infect Control. 2020;48(8):875–879. doi: 10.1016/j.ajic.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli A, Vahdat K, Keshavarz M. Novel coronavirus disease 2019 (COVID-19): an emerging infectious disease in the 21st century. ISMJ. 2020;22(6):432–450. [Google Scholar]
- WHO . Laboratory testing for coronavirus disease ( COVID-19) in suspected human cases: interim guidance, 19 March 2020. World Health Organization; 2020. [Google Scholar]
- Yamagishi T, Ohnishi M, Matsunaga N, Kakimoto K, Kamiya H, Okamoto K, Suzuki M, Gu Y, Sakaguchi M, Tajima T. Environmental sampling for severe acute respiratory syndrome coronavirus 2 during a COVID-19 outbreak on the Diamond Princess cruise ship. J Infect Dis. 2020;222(7):1098–1102. doi: 10.1093/infdis/jiaa437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Zhi N, Yu C, Ming G, Yingle L, Kumar GN, Li S, Yusen D, Jing C, Dane W. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak. BioRxiv. 2020 doi: 10.1101/2020.03.08.982637. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.