Abstract
Deep brain stimulation (DBS) is a promising therapeutic approach for patients with treatment-resistant obsessive compulsive disorder (OCD), a condition linked to abnormalities in corticobasal ganglia networks. Effective targets are placed in one of four subcortical areas with the goal of capturing prefrontal, anterior cingulate, and basal ganglia connections linked to the limbic system. These include the anterior limb of the internal capsule, the ventral striatum, the subthalamic nucleus, and a midbrain target. The goal of this review is to examine these four targets with respect to the similarities and differences of their connections. Following a review of the connections for each target based on anatomic studies in nonhuman primates (NHPs), we examine the accuracy of diffusion magnetic resonance imaging (dMRI) tractography to replicate those connections in the NHP, before evaluating the connections in the human brain based on dMRI tractography. Results demonstrate that the four targets generally involve similar connections, all of which are part of the internal capsule. Nonetheless, some connections are unique to each site. Delineating the similarities and differences across targets is a critical step for evaluating and comparing the effectiveness of each and how circuits contribute to the therapeutic outcome. It also underscores the importance that the terminology used for each target accurately reflects its position and its anatomic connections, so as to enable comparisons across clinical studies and for basic scientists to probe mechanisms underlying DBS.
Keywords: Anterior limb of the internal capsule, medial forebrain bundle, subthalamic nucleus, ventral striatum, anatomic tracing, diffusion magnetic resonance imaging, white matter pathways
Introduction.
Deep brain stimulation (DBS) is a promising therapeutic approach for patients with treatment-resistant obsessive compulsive disorder (OCD(1–4), a condition linked to abnormalities in the cortico-thalamic-basal ganglia network(5–8). Most commonly the targets are centered on the orbitofrontal (OFC) and anterior cingulate cortical (ACC) circuits. There are four main targets: 1. The ventral anterior limb of the internal capsule (ALIC), to modulate OFC/AC-subcortical connections(2, 3); 2. The ventral striatum (VS), to modulate the reward system(4); 3. The medial subthalamic nucleus (mSTN), to modulate the OFC/ACC hyperdirect pathway and/or the STN-ventral pallidal (VP) loop(1, 9); and 4. A midbrain target, to modulate the ascending ventral tegmental area (VTA) fibers(10, 11). Importantly, at the typical parameters used for stimulation, myelinated fibers are preferentially stimulated over nonmyelinated fibers and axons are stimulated more easily than cells although other neuronal elements can also be involved(12). Thus, electrode targets are typically placed to modulate a particular set of myelinated fibers that carry information from (or to) cortical or basal ganglia areas (supplemental Fig. 1). Critical questions regarding these targets are: 1. What connections are likely involved at each? 2. How similar are these connections across the targets? 3. Can we develop a more precise and common terminology for each target based on their connections? The importance of clarifying these issues cannot be underestimated as it centers on the ability to compare which circuits are modulated at each target across clinical sites. These comparisons form the basis for cross evaluation of therapeutic outcomes and for understanding the underlying mechanisms of those outcomes. Diffusion magnetic resonance imaging (dMRI) tractography is increasingly being used to model or position electrodes for DBS (9, 13–19) which raises an additional issue regarding the accuracy of diffusion tractography to correctly label connections.
In this paper we examine the connections involved at the four major DBS target sites for OCD based on animal anatomic studies, and animal and human dMRI studies. Individual groups have also explored other targets, the bed nucleus of the stria terminalis (BNST), the globus pallidus (GP), and the inferior thalamic peduncle (17, 20–22). Given their proximity to the ALIC, VS, and STN, these targets will be addressed within those sections (23). We first review the known anatomic connections passing through each target based on tracing experiments in nonhuman primates (NHPs)(24–35). We then review the accuracy of dMRI to replicate those connections by evaluating the ability of dMRI to identify those connections first in NHPs, including experiments in which animals received both tracer injections and a dMRI scan(25). This step is critical for guiding the evaluation of white matter tracts in human dMRI by testing the accuracy of dMRI to demonstrate the correct connections, based on histology. It sets the stage for using dMRI to follow connections in the human brain where tracing experiments are not possible(25, 36, 37). Combining these stringent criteria, we demonstrate that the four targets generally involve similar connections, all of which are part of the internal capsule (IC) (38). Nonetheless, some connections are unique to each site. Delineating the connectional similarities and differences across the different targets will help define accurate and common terminology that reflects the positions and anatomic connections of each target. This enables researchers to make comparisons across clinical studies and discuss how connections might contribute to the therapeutic outcome. It also lays a common platform to probe mechanisms underlying DBS.
Anatomic connections.
The ALIC. The ALIC is a heavily myelinated fiber bundle that carries the descending and ascending PFC/ACC fibers to the thalamus and brainstem(26). Importantly, many of the PFC/ACC ALIC fibers are embedded within the VS as small myelinated fascicles and, as such, are considered part of the VS rather than the ALIC. Axons from each cortical area remain clustered as they enter and travel through the ALIC. They are organized into two intertwined bundles, those that exit the capsule to the thalamus and those that continue to the brainstem(24, 39). The ALIC is organized topologically and can be segmented into five regions based on positions of PFC/ACC fibers within it (Fig. 1A)(25). These positions generate a ventrodorsal and mediolateral arrangement within the capsule. Fibers originating in ventral cortical regions travel ventral to those that originate in more dorsal regions. Fibers originating in medial regions travel medial to those that originate in more lateral regions. The ventral-most components carry vACC and OFC fibers. Just dorsal to these axons are fibers from the dorsal ACC (dACC) and ventrolateral PFC, positioned medially and laterally respectively. Dorsal to these components are fibers from the dorsomedial and dorsolateral PFC(24, 25). Thus, electrodes placed in the ventral-most part of the ALIC will involve both thalamic and brainstem fibers connecting the vACC and OFC. Those placed more dorsomedially likely involve axons from the dACC, while those placed laterally will involve vlPFC fibers. The most dorsal electrodes will modulate the dorsomedial or dorsolateral PFC (Fig. 1b)(25). The BNST is located adjacent to the caudal ALIC and contains PFC-thalamic fibers existing ALIC. Electrodes placed here will thus, also modulate these fibers, in addition to BNST connections. Indeed, the distinction between the IC and BNST is noted by the authors(21). Electrodes placed in the inferior thalamic peduncle will more specifically modulate OFC fibers (26).
Figure 1.

Connections through the ALIC(A/B) and VS (C/D) sites. A. Organization of PFC/ACC fibers in the ALIC-coronal view. B. electrode position to capture OFC fibers-sagittal view. C. Schematic showing convergence of frontal terminals fields in the striatum. D. Electrode placement positioned to capture OFC fibers. It also illustrates the additional connections of the VS in the area of the electrode. Inset demonstrates the electrode placement with respect to the small fascicles traveling through the VS. Abbreviations: Amy=amygdala, BNST=bed nucleus of the stria terminalis, Cd=caudate nucleus, Pu=putamen, Hipp=hippocampus, hypo=hypothalamus, MD=mediodorsal nucleus of the thalamus, NB=nucleus basalis, PPT=pedunculopontine nucleus, VP/GP=ventral pallidum/globus pallidus, VS=ventral striatum, VTA/SN= ventral tegmental area/substantia nigra. Color code: Fuchsia=vACC/mOFC, red=OFC, dark orange=dACC, light orange=vlPFC, yellow=dPFC, green=premotor cortex, blue=motor cortex.
The striatum. The striatum is the main input structure of the basal ganglia. It receives a massive and topographic input from cerebral cortex, thalamus, and midbrain (28, 40–45). The VS refers to the rostral striatal region that includes the nucleus accumbens, ventromedial caudate nucleus, and the ventromedial putamen. The VS contains myelinated connections of the OFC/ACC, the midline thalamic nuclei, the mediodorsal thalamic nucleus, ventral pallidum, and the unmyelinated fibers from the midbrain. The central striatum is linked with the dorso- and ventrolateral PFC, dorsal ACC, mediodorsal nucleus, ventral anterior nuclei of the thalamus, GP, and central substantia nigra (SN). The dorsolateral striatum is connected to cortical, thalamic, pallidal and brainstem motor control areas. Within this general topography, there is extensive convergence of terminals from multiple cortical areas; ACC and OFC fibers converge in the medial caudate, OFC, dACC, and vlPFC converge in central striatal regions, etc. (Fig. 1c)(29, 46, 47). The VS also has unique connections compared to the dorsal striatum. These include connections to the amygdala, nucleus basalis, preoptic area, bed nucleus of the stria terminalis, hippocampus, and pedunculo pontine tegmentum (Fig. 1d) (48–52). Intermingled with these projections and those of the thalamo-striatal, striato-pallidal, brainstem fibers are the small fascicles that carry ALIC axons that do not terminate in the VS (described above, Fig. 1d, inset). Thus, there is a complex mixture of myelinated fibers that run through the VS. Electrodes placed close to, or within, the small fascicles of the ALIC will involve not only descending and ascending OFC/ACC fibers, but also a complex mixture of additional fibers (thalamic, striato-pallidal, amygdala, brainstem, etc.) (Fig. 3D).
Figure 3.
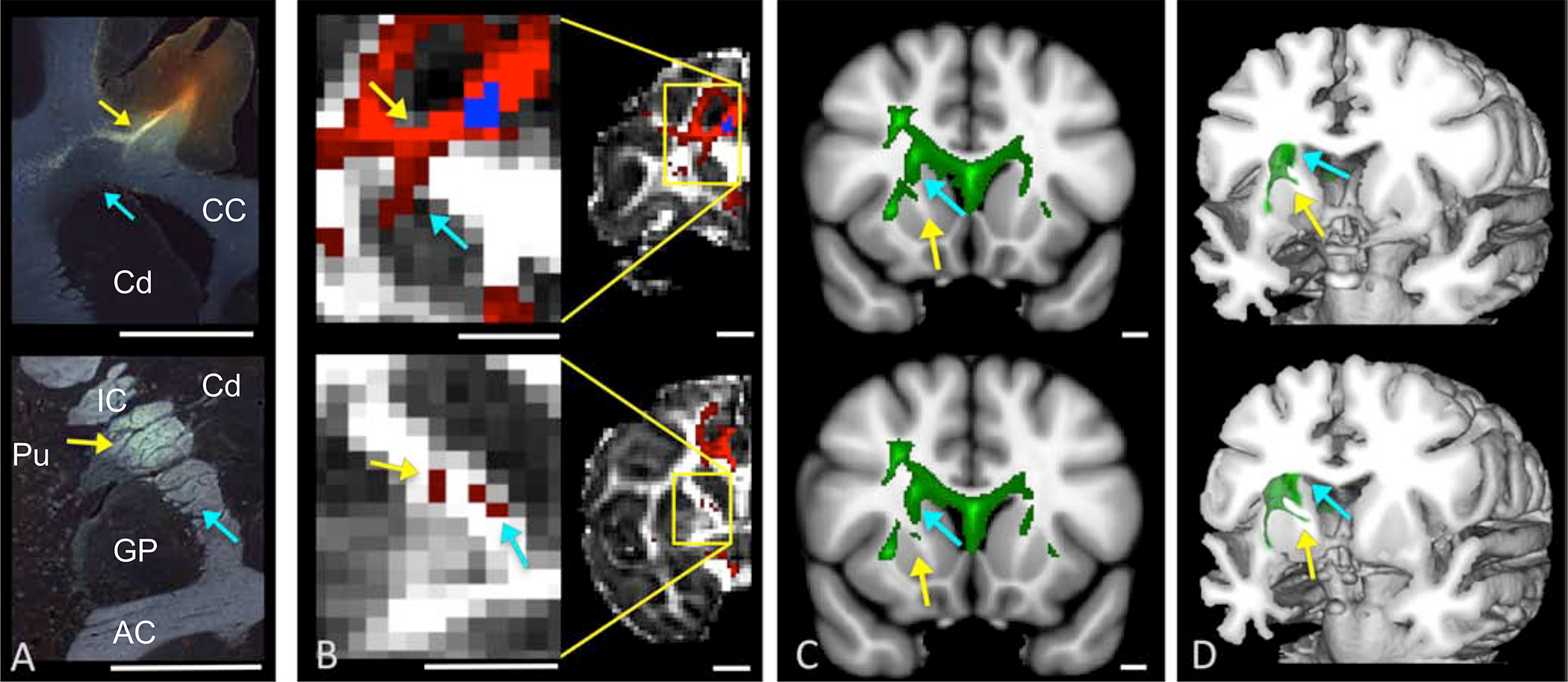
Anatomic tracing and dMRI through the ALIC. (A). Histology showing fiber pathways following an injection site in the dACC. Scale bar=5mm. (B). NHP dMRI streamlines generated from a seed at the injection site location from (A). Scale bar=4.9. The coronal levels match those shown in (A). Correct streamlines are indicated with yellow arrows, incorrect streamlines with blue arrows. (C-D). Human dMRI data illustrating streamlines following placement of a seed in a similar area of dACC. Based on the NHP data, yellow arrows show the likely correct streamlines, and blue arrows show the likely incorrect ones. Scale bar=10mm. Abbreviations: AC=anterior commissure, Cd=caudate, CC=corpus callosum, GP=globus pallidus, IC= internal capsule, Pu=putamen. Republished from Safadi et al.
The medial subthalamic nucleus. The mSTN contains myelinated cortical, pallidal, STN and pedunculopontine nucleus fibers (32–34, 53–55). The two best-known pathways are the indirect pathway (pallido-STN connections) and the hyperdirect pathway (direct cortico-STN connection)(56). The GP connects primarily to the STN via the subthalamic fasciculus, a fiber system that carries both GP-STN axons and STN-GP fibers. The hyperdirect pathway travels through the ALIC, exiting the capsule, lateral to the STN (Fig. 2A)(32). The STN is generally divided into limbic, cognitive, and motor regions(32, 33, 57–59). The OFC/ACC projects to the medial part of the nucleus, extending into the lateral hypothalamus. The dorso- and ventrolateral prefrontal cortical areas project centrally and motor areas terminate in the lateral half of the nucleus (Fig. 2B)(32). However, similar to cortico-striatal connections, there is a high degree of convergence of terminal fields from functionally diverse cortical areas (Fig. 2B, inset)(32). The mSTN(1, 9) targets ACC, OFC and ventral pallidum connections. Its close proximity to the Fields of Forel, zona incerta (ZI), and lateral hypothalamus, and possibly, the ventral inferior thalamic peduncle, suggests the possible involvement of these structures. Importantly, the fields of Forel include, pallidal, cerebellar, tegmental, reticular and corticofugal fibers(60). Taken together, the medial STN target likely modulates the hyperdirect pathway, the ventral pallidal-STN loop, and possibly the fields of Forel and lateral hypothalamus (Fig. 2B). It may also modulate fibers exiting from the IC, including those traveling to (and from) the ZI and several brainstem nuclei.
Figure 2.
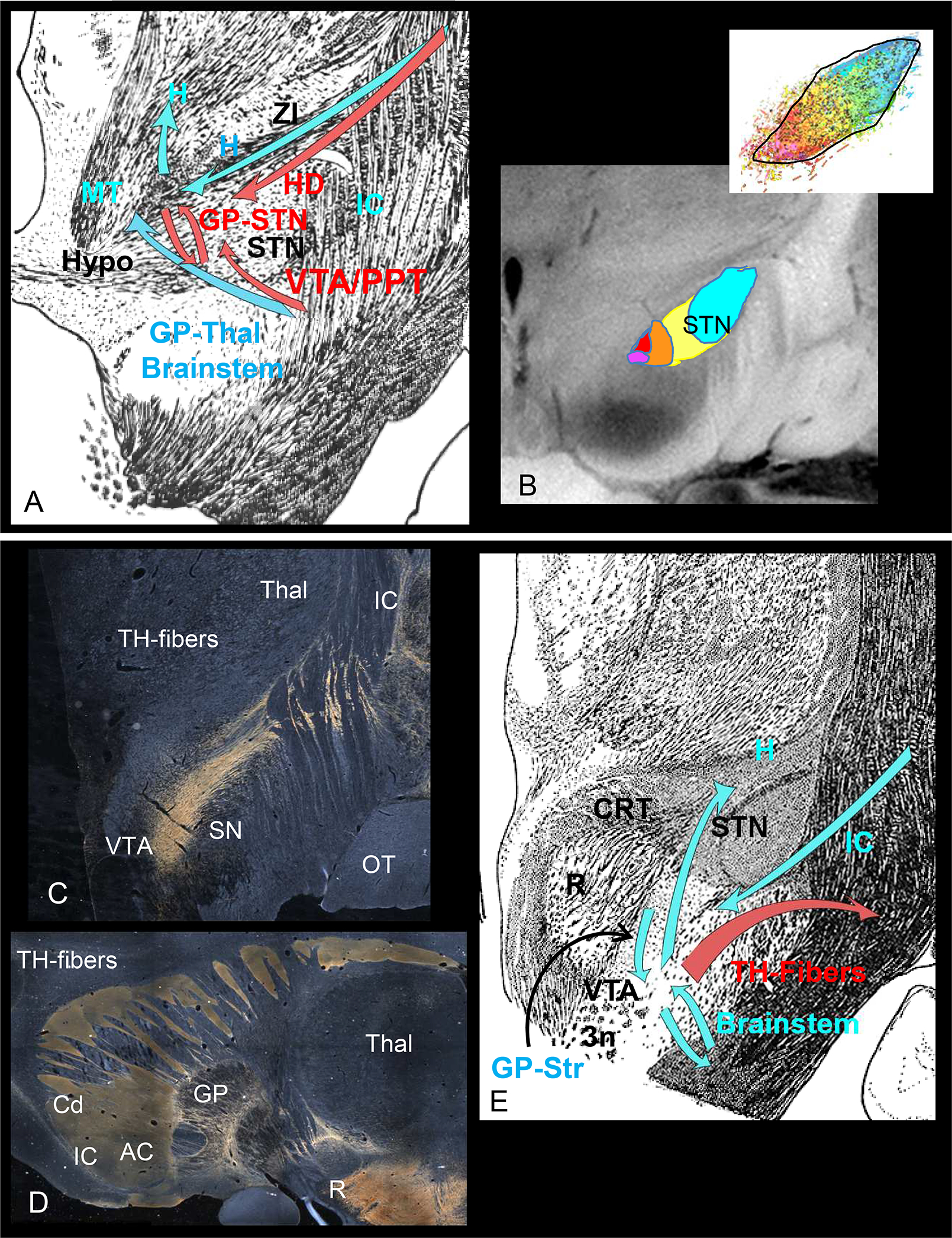
Connections through the STN(A/B) and midbrain (C/D) sites. A. Fiber bundles and connections through the STN. Red arrows=targeted connections, blue arrows=other pathways through the area. B. Organization of cortical terminals in the STN. Color code: Fuchsia=vACC/mOFC, red=OFC, orange=dACC, yellow=dPFC/vlPFC, blue= premotor/motor cortex. C. Dark field illumination demonstrating TH-positive fiber-positive staining (appears gold) as they travel from the VTA laterally, cross the IC to terminate in the globus pallidus and striatum. D. Sagittal section demonstrating the trajectory of TH-positive fibers to frontal cortex. Note, the TH-positive fibers are only found in the striatum, but not in the IC. E. Fibers bundles and connections through the midbrain site. Red arrows=targeted connections, blue arrows=other pathways through the area. Abbreviations: AC=anterior commissure, ALIC=anterior limb of the internal capsule, Cd=caudate, GP=globus pallidus, H=H fields of Forel, HD=hyperdirect pathway, Hypo=hypothalamus, IC= internal capsule, MT=mammillothalamic tract. PPT=pedunculopontine nucleus, OT=optic tract, R=red nucleus, SN=substantia nigra, STN=subthalamic nucleus, TH=tyrosine hydroxylase, Thal=thalamus, VTA=ventral tegmental area, ZI=ZI, 3n=third nerve. Scale bar=5mm.
The midbrain target. The midbrain DBS location was chosen to target the VTA ascending fibers (10, 61). In contrast to the other DBS targets, the VTA contains thinly or unmyelinated fibers (30). Based on dMRI tractography, this target was hypothesized to involve ascending VTA fibers traveling through a newly named pathway, the superior limb of the medial forebrain bundle (slMFB), which runs within the IC(11). However, the ascending VTA dopaminergic axons do not travel in the IC(62). Rather, they exit the VTA, arch laterally over the SN, and cross the IC to enter the striatum (Fig.2C). To reach the frontal cortex, VTA fibers travel within the classic MFB. The MFB courses rostrally through the ventral forebrain, terminating in the lateral hypothalamus, preoptic area, septum, the bed nucleus of the stria terminalis, amygdala, olfactory tubercle before arching dorsally around the corpus callosum, to enter the frontal cortex(30, 31, 62–65). Tyrosine hydroxylase (TH)-positive-staining is a standard marker for dopamine fibers across species. The classical MFB(66) contains TH-positive staining but, in both NHPs and humans, the IC does not (Fig. 2D) (63, 67, 68). Importantly, frontal cortical fibers connecting the thalamus, the STN, and most brainstem regions travel in the IC, not in the MFB (24–26, 32). The dMRI tractography streamlines labeled as the slMFB (10, 61) represent fibers of the IC, not the MFB. Nonetheless, this target appears to have clinical efficacy, which raises the question, what pathways are involved? This complex midbrain area contains tightly packed intermixed myelinated bundles. As such, it likely modulates descending and ascending STN, ZI, and VTA/substantia nigra fibers entering and exiting the IC. The area also contains striato-brainstem, pallido-midbrain, cortico-brainstem, and hypothalamo-brainstem fibers (Fig. 2E).
Anatomy and dMRI.
While much of the work on understanding brain connectivity originated in the tract-tracing anatomic literature, human dMRI studies have now taken the lead in this endeavor. This noninvasive approach has the advantage of allowing the study of connections in vivo and is now playing a role in determining DBS electrode placement (9, 13–18, 69). However, tractography does not reconstruct axons but demonstrates so called ‘streamlines’, i.e. paths of least hindrance to diffusion(70). It is an indirect and relatively low-resolution method that has limitations(37, 71–73). The method simply summarizes local orientation information within a relatively low-resolution voxel to estimate the dominant axonal orientations. Axonal trajectories that give rise to those orientations can be ambiguous, as multiple configurations of axon populations can give rise to similar diffusion profiles. Thus, more dominant directions are emphasized, and smaller bundles with different orientations may be deemphasized or lost. These issues lead to errors in tractography, i.e., false positive and false negative connections(25, 37, 71, 72, 74) or biases in the distributions of cortical terminations(73). Therefore, while tractography is helpful for visualizing known pathways in vivo, it cannot be used on its own to reliably identify ‘new’ pathways in vivo that have not previously been demonstrated using invasive, experimental methods (lesions, animal tracing experiments)(25, 36, 71). Tracing experiments in NHPs are considered the ‘gold standard’ for guiding the interpretation of dMRI connections, by providing the specific origin of axons, their organization within the major fiber bundles, and their terminal fields.
Cross-species, cross-modal information (NHP tracing, NHP dMRI in the same animal, and dMRI in humans) is a powerful approach for determining true connectivity patterns in the human brain. Below we review how well the combination of tracer experiments and dMRI tractography in NHP replicates the anatomy for each DBS site. This allows comparing the position of the axons and the position of streamlines produced by dMRI seeds placed at the same site as the tracer injections, demonstrating the correct and incorrect connections identified using dMRI tractography alone.
Anterior limb of the internal capsule. Tractography seeds placed in the same PFC/ACC positions as tracer injections, produce a streamline pattern in the ALIC that is correctly positioned and other streamlines that are not (Fig. 3)(25, 36). Notably, the incorrect streamlines cover a larger region, are more robust, and travel dorsal to the correct ones (Fig. 3A–B). This spurious streamline pattern is the result of navigating through the complexity of the corona radiata(25). Importantly, a similar pattern is shown with dMRI tractography in the human brain, with one streamline pattern in the position that is expected by the NHP tracing and dMRI and a second, larger one, in a similar position as the false positive in the NHP dMRI (Fig. 3C–D). Without the histological data as a guide, the more robust, but incorrect streamline pattern, would be assumed to be the correct one in both the NHP and human data(25, 36). Using this cross-species, cross-modal information as a guide, the human ALIC can also be segmented based on the positions of axons from different cortical regions within the capsule, showing a similar topology as the NHP (supplemental figure 2). While these studies focus on descending cortical pathways, ascending connections are likely to be organized similarly. In contrast to the fiber positions in the ALIC, those passing through the fascicles of the striatum cannot be followed using dMRI, due to their small size relative to the resolution of the dMRI data, demonstrating a false negative(36).
Striatal and subthalamic targets.
The striatal and STN targets are centered in grey matter, which, with the exception of the thalamus, tends to be isotropic in dMRI. The VS target, as indicated above likely involves the small white matter fascicles that pass through the striatum. However, as the resolution of dMRI tractography cannot accurately follow streamlines from the small fascicles that are embedded within the striatum, we must rely on its anatomic organization to predict which fibers are likely to be involved and the conservation of these connections across species. A similar argument applies to the STN. However, dMRI tractography can demonstrate the STN functional subdivisions (14).
Midbrain target.
Placing a tractography seed in the NHP dMRI in the same position as a tracer injection into the MFB(75), lateral to the VTA, also produces correct and incorrect streamlines in the NHP (Fig. 4). Streamlines cross the IC to enter the striatum, as in the histological material. However, dMRI also produce erroneous streamlines travelling through the IC (Fig. 4A). At more rostral levels, the dorsal streamlines continue through the ALIC to frontal cortex (Fig. 4B–C). This clear false positive likely results from the seed’s location within the heavily compacted and myelinated fibers that flow to and from the IC. Importantly, the same false positive streamlines can be detected following placement of a tractography seed in the human VTA (Fig.4 A–C). Taken together, the lack of TH-positive staining in both the monkey and human IC, coupled with the anatomic tracing and false positive streamlines in the IC in both monkeys and humans, indicate that VTA dopaminergic fibers do not take this route to reach the frontal cortex(62, 63). The streamlines derived following a tractography seed placed in this midbrain position are, in fact, cortical connections through the IC that connect other brainstem regions with cortex and subcortical areas.
4.
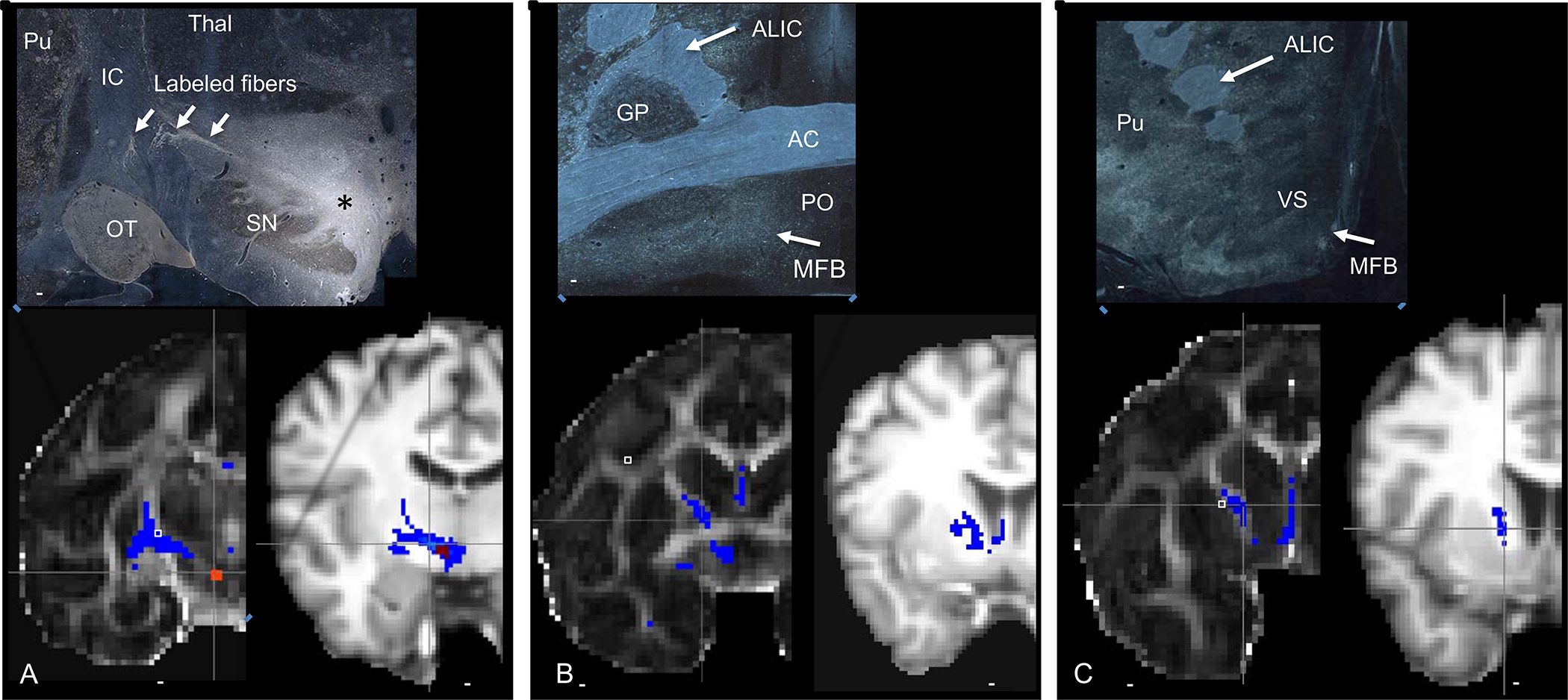
Projections from the VTA. Top panels= NHP histology following a tracer injection into the VTA, bottom panels, left=NHP dMRI tractography in NHP, right= human dMRI tractography. Top panel. A. asterisk=tracer injection site, Red=seed placement at the same site as the injection in monkey and human dMRI. Similar to the anatomic tracing, streamlines cross the IC, to the striatum. However, unlike the anatomic tracing experiment, streamlines also enter the IC and continue to travel rostrally, through the ALIC. B-C. Histology demonstrates fibers in the MFB, ventral and medial to the anterior commissure. There are no fibers in the ALIC. In contrast, the dMRI tractography in both the monkey and human show streamlines in the ALIC. Abbreviations: AC=anterior commissure, ALIC= anterior limb of the internal capsule, GP=globus pallidus, IC=internal capsule, MFB=medial forebrain bundle, OT=optic tract, PO=preoptic area, SN=substantia nigra, VS=ventral striatum. Scale bars: top panel-5mm; bottom panel, NHP-4.9mm; human-10mm.
Discussion
The cortico-thalamo-basal ganglia network is comprised of a complex set of recurrent excitatory and inhibitory loop systems (Fig. 5). Thus, modulation at several points within the system may have similar overall effects (38) (9). The ALIC, VS, STN, and midbrain target placements are chosen to affect this network, with a main focus on the connections associated with reward, avoidance, and cognitive control, primarily the OFC/ACC connections. The electrode position within the ALIC has changed over the years to optimize outcomes (76–78), but is now positioned to primarily modulate OFC/ACC fibers. Although the precise position of these fibers varies across individuals, their topology is consistent, allowing predictions about the relative positions of fibers from different cortical regions(25, 79). This organization can help guide the positioning of electrodes to optimize capturing the desired OFC/ACC fibers or change the stimulated contacts based on outcome (Fig. 5B). Moreover, the topology can guide electrode adjustments made to optimize the modulation of the best combinations of cortical fiber. For example, recent evidence suggests that the ventro- and dorsolateral prefrontal cortex may play a role in behaviors associated with OCD (80–85) and may thus be a future target. The VS target is also typically positioned to modulate a similar set of OFC/ACC connections as the ALIC target. Additionally, the VS target also includes the thalamo-striatal, striato-pallidal and brainstem connections. Depending on its position, it may also include projections unique to the VS (Fig. 5C). The VS is also topologically organized. This includes not only the cortical projections, but also thalamic and midbrain connections (28, 43, 86, 87). Finally, an electrode placed in the VS will likely modulate the fascicles passing through the VS to join the ALIC.
5.
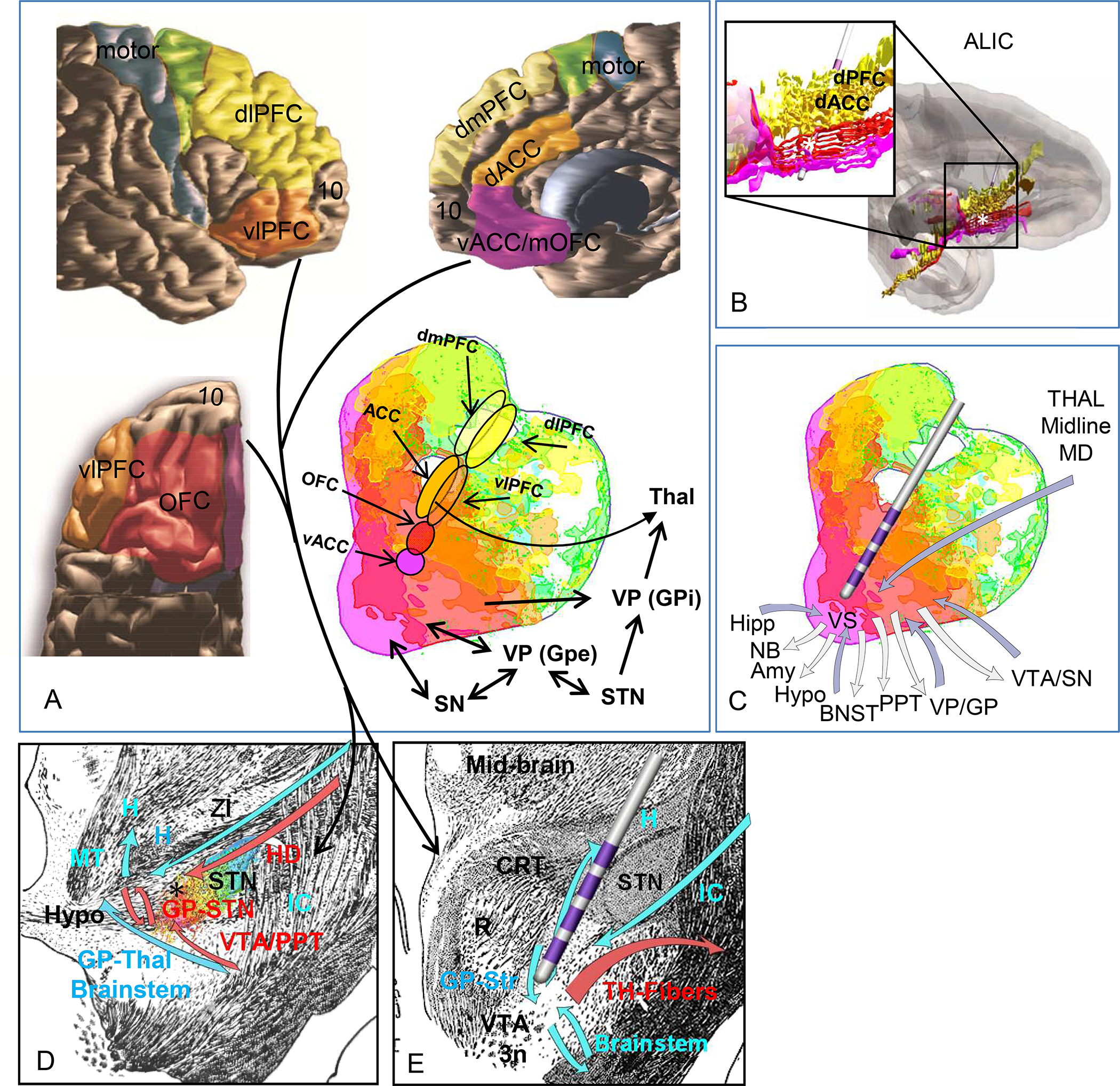
Summary of connections through the four DBS target: A. summary of the PFC-subcortical circuits. Color coding represents the topography of cortico-striatal connections. Arrows indication the projections through each structures. Color code: Fuchsia=vACC/mOFC, red=OC, dark orange=dACC, light orange=vlPFC, yellow=dPFC, green=premotor cortex, blue=motor cortex.
Abbreviations: dACC= dorsal anterior cingulate cortex, dPFC=dorsal prefrontal cortex, GPe=globus pallidus, external segment, GPi=globus pallidus, internal segment, mOFC=medial orbitofrontal cortex, OFC=orbitofrontal cortex, STN=subthalamic nucleus, Thal=thalamus, vACC=ventral anterior cingulate cortex, vlPFC=ventrolateral prefrontal cortex, SN=substantia nigra. B. Electrode placement demonstrates cortical pathways likely modulated through the ALIC site. C. Electrode placement demonstrates cortical and subcortical pathways likely modulated through the VS site. D. Black asterisk indicates cortical and basal ganglia pathways likely modulated through the STN site. E. Electrode placement demonstrates cortical pathways likely modulated through the midbrain site. Asterisks=electrode contacts. Color code: Fuchsia=vACC/mOFC, red=OC, dark orange=dACC, light orange=vlPFC, yellow=dPFC, green=premotor cortex, blue=motor cortex. Abbreviations: Amy=amygdala, BNST=bed nucleus of the stria terminalis, Cd=caudate nucleus, dACC= dorsal anterior cingulate cortex, dPFC=dorsal prefrontal cortex, GPe=globus pallidus, external segment, GPi=globus pallidus, internal segment, Hipp=hippocampus, hypo=hypothalamus, MD=mediodorsal nucleus of the thalamus, mOFC=medial orbitofrontal cortex, PPT=pedunculopontine nucleus, Pu=putamen, OFC=orbitofrontal cortex, NB=nucleus basalis, STN=subthalamic nucleus, Thal=thalamus, vACC=ventral anterior cingulate cortex, vlPFC=ventrolateral prefrontal cortex, SN=substantia nigra, VP/GP=ventral pallidum/globus pallidus, VS=ventral striatum, VTA/SN= ventral tegmental area/substantia nigra.
The mSTN involves several connections, including the hyperdirect pathway, primarily from the OFC/ACC, reciprocal VP-STN and STN-VTA connections. These connections have an immediate secondary effect on the VS and medial thalamus. It also may involve fibers passing through the region, including those entering the ZI (Fig. 5D). The midbrain target is positioned in a region that contains several different myelinated bundles, including those exiting and entering the IC. Thus, streamlines extending into the capsule are captured by placing a seed in this midbrain region. Importantly, these are not part of the MFB. In addition to the general modulation of IC fibers, this target will also involve specific cortical projections to the STN, VTA/SN, and ZI, pallido-STN connections, and several ascending and descending brainstem pathways (Fig. 5E). While stimulation may modulate VTA fibers, it’s main effects will be on myelinated fibers entering and exiting the capsule and those coursing through the region.
Important considerations:
To understand the actual fibers involved at each structure, several additional factors should be kept in mind. First, the area within each target that is actually stimulated depends on the electric field. The size of the field depends on a combination of factors specific to each site, including the electrodes used, axon orientation, and diameter, stimulation parameters, etc. This makes it difficult to estimate the actual area stimulated across targets and across studies. Therefore, we focused here only on the electrode placement and its adjacent region. However, when the precise location of the contacts are identified in individual patients and field modeling is applied, care must be taken to consider the entire area that might be included within the electric field. Thus, a primary consideration for what might be necessary and sufficient for a positive outcome should include not only the target itself, but its surrounding structures.
Second, there is individual variation in the position of specific PFC/ACC fibers at each site (16, 25, 88). Moreover, symptoms vary across OCD individuals (16). Thus, the desired targeted structures may also vary and may include, for example, ventro-or dorsolateral cortical circuits. Taken together, understanding the anatomic organization of the brain region both serves as an initial guide for electrode placement and can play a critical role for choosing the contacts to activate based on symptoms and outcomes in individual patients. Third, stimulation is non-directional, and consideration must also be given to possible antidromic stimulation. Thus, the electrodes in the ALIC effect both afferent and efferent cortico-thalamic, and brain stem fibers. Those in the VS involves bidirectional ventro-pallido-VS and brainstem-VS fibers.
Overall, outcome measures across targets demonstrate that approximately 50% of patients show improvement (2, 16, 38, 89). Functional changes in cortex and striatum linked to DBS targets have been reported, some of which are consistent with the expected anatomic connections, others are not (14, 90–94). The effects on relatively large areas (i.e. medial or lateral prefrontal cortex) and/or regions within the stimulated area (i.e. striatum). Before we can link changes to specific connections and assess precisely which connections are necessary and sufficient, we need: 1. To be more precise about electrode locations to assess all fiber, both targets and those passing through that elicit these responses; 2. To be consistent in how we subdivide cortical and subcortical regions. 3. To take into account individual variations.
Summary
All four OCD DBS sites likely involve OFC/ACC connections passing through, entering, or leaving the IC, albeit at different brain locations (Fig.8) which might explain similar outcomes(9, 38, 89). The specific cortical region or regions stimulated depend on the specific electrode location. For example, a more ventral ALIC site may involve OFC connections, but a more dorsal and medial ALIC site will involve dACC connections. All sites involve a variety of subcortical pathways including, but not limited to basal ganglia, thalamic and brainstem connections. While all four sites were chosen to involve OFC/ACC connections, each preferentially targets different components of the circuit. The ALIC targets all ascending and descending connections, and connections in the adjacent VS. The VS target involve VS connections (see Fig.1D), but also other ascending and descending connections, including, but not limited to, ALIC and amygdala fibers. Both the STN and the midbrain also target connections of the OFC/ACC along with their basal ganglia components. Importantly, these sites likely involve a wider range of diencephalic and brainstem connections through passing fibers. The midbrain site targets a combination of the striato-midbrain, pallido-midbrain, cortico-midbrain, cortico-STN, cortico ZI, and a variety of brainstem targets.
Diffusion MRI tractography is error prone and must be evaluated in the context of known connections from anatomic studies(25, 36, 37, 71, 72). One argument used when dMRI tractography in humans differs from NHP anatomic experiments centers on evolution and the expansion of the cortex, suggesting that the full extent of a pathway can only be discovered in the human by dMRI(10). However, the four DBS subcortical targets are highly conserved across mammalian species, particularly primates(26, 62, 66, 95). While these pathways may increase or decrease in size through evolution, they are unlikely to take different routes. Effective use of tractography for surgical planning and interpreting outcomes should be informed by anatomic experiments, particularly for pathways conserved across species. The false-positive labeling of an MFB component in the IC has led to a misleading concept: that the IC is divided into a medial anterior thalamic radiation (ATR) and a lateral slMFB, component (15, 61). The ALIC is topographically organized and fibers from the medial prefrontal regions, including the ACC and medial dorsal PFC, travel medially to those from lateral prefrontal regions (25). Based on this topography, the streamlines identified as slMFB should represent fibers of the lateral PFC, and those of the ATR should represent fibers of medial cortex. However, the streamlines identified as the slMFB appear to target midline cortical areas.
Translational neuroscience depends on developing a common terminology for brain regions across species. DBS is not only an effective clinical treatment, it also provides an important opportunity for researchers to understand the mechanism underlying disease and the effectiveness of treatment. As such, a standard nomenclature is essential for facilitating, not only comparisons across clinical studies, but also mechanistically probe circuits in animals. The ALIC, striatum, STN, and the MFB, are well studied, definable, different, and separable structures and it is important to use the correct nomenclature regarding each. Specifically, the definitions of the MFB and IC are consistent across species and should not be conflated based solely on dMRI for the reasons outlined above. Clear cross-species definitions are critical to move the field forward, not only for developing the best DBS targets and parameters for stimulation for each disease, but also for probing the circuits experimentally so as to better understand the underlying mechanisms, and to eventually develop individualized targeting. Taken together, finding the stimulation ‘sweet spot’ for DBS treatment for psychiatric disorders, including but not limited to OCD, requires a solid understanding of the connections that pass through each target combined with imaging, network analyses and physiological data.
Supplementary Material
Acknowledgments
We thank Drs. Andreas Horn, Helen Mayberg, and Steven Rasmussen for their helpful comments and suggestions. This work was supported by the National Institute of Health (Grants Nos. MH106435 and MH045573 to Dr. Haber).
Footnotes
Financial Disclosures.
Drs. Haber, Yendiki, and Jbabdi reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, et al. (2008): Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 359:2121–2134. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg BD, Rauch SL, Haber SN (2010): Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology. 35:317–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman WK, Foote KD, Greenberg BD, Ricciuti N, Bauer R, Ward H, et al. (2010): Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. 67:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, et al. (2010): Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 67:1061–1068. [DOI] [PubMed] [Google Scholar]
- 5.Admon R, Bleich-Cohen M, Weizmant R, Poyurovsky M, Faragian S, Hendler T (2012): Functional and structural neural indices of risk aversion in obsessive-compulsive disorder (OCD). Psychiatry Res. 203:207–213. [DOI] [PubMed] [Google Scholar]
- 6.Versace A, Graur S, Greenberg T, Lima Santos JP, Chase HW, Bonar L, et al. (2019): Reduced focal fiber collinearity in the cingulum bundle in adults with obsessive-compulsive disorder. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Posner J, Marsh R, Maia TV, Peterson BS, Gruber A, Simpson HB (2014): Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 35:2852–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlop K, Woodside B, Olmsted M, Colton P, Giacobbe P, Downar J (2016): Reductions in Cortico-Striatal Hyperconnectivity Accompany Successful Treatment of Obsessive-Compulsive Disorder with Dorsomedial Prefrontal rTMS. Neuropsychopharmacology. 41:1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyagi H, Apergis-Schoute AM, Akram H, Foltynie T, Limousin P, Drummond LM, et al. (2019): A Randomized Trial Directly Comparing Ventral Capsule and Anteromedial Subthalamic Nucleus Stimulation in Obsessive-Compulsive Disorder: Clinical and Imaging Evidence for Dissociable Effects. Biol Psychiatry. 85:726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coenen VA, Schumacher LV, Kaller C, Schlaepfer TE, Reinacher PC, Egger K, et al. (2018): The anatomy of the human medial forebrain bundle: Ventral tegmental area connections to reward-associated subcortical and frontal lobe regions. Neuroimage Clin. 18:770–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coenen VA, Honey CR, Hurwitz T, Rahman AA, McMaster J, Burgel U, et al. (2009): Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson’s disease. Neurosurgery. 64:1106–1114; discussion 1114–1105. [DOI] [PubMed] [Google Scholar]
- 12.McIntyre CC, Grill WM, Sherman DL, Thakor NV (2004): Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 91:1457–1469. [DOI] [PubMed] [Google Scholar]
- 13.Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, et al. (2018): A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. 23:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann CJ, Chaturvedi A, Lujan JL (2015): Quantitative analysis of axonal fiber activation evoked by deep brain stimulation via activation density heat maps. Front Neurosci. 9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liebrand LC, Caan MWA, Schuurman PR, van den Munckhof P, Figee M, Denys D, et al. (2019): Individual white matter bundle trajectories are associated with deep brain stimulation response in obsessive-compulsive disorder. Brain Stimul. 12:353–360. [DOI] [PubMed] [Google Scholar]
- 16.Barcia JA, Avecillas-Chasin JM, Nombela C, Arza R, Garcia-Albea J, Pineda-Pardo JA, et al. (2019): Personalized striatal targets for deep brain stimulation in obsessive-compulsive disorder. Brain Stimul. 12:724–734. [DOI] [PubMed] [Google Scholar]
- 17.Azriel A, Farrand S, Di Biase M, Zalesky A, Lui E, Desmond P, et al. (2019): Tractography-Guided Deep Brain Stimulation of the Anteromedial Globus Pallidus Internus for Refractory Obsessive-Compulsive Disorder: Case Report. Neurosurgery. [DOI] [PubMed] [Google Scholar]
- 18.Coenen VA, Sajonz B, Reisert M, Bostroem J, Bewernick B, Urbach H, et al. (2018): Tractography-assisted deep brain stimulation of the superolateral branch of the medial forebrain bundle (slMFB DBS) in major depression. Neuroimage Clin. 20:580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramasubbu R, Clark DL, Golding S, Dobson KS, Mackie A, Haffenden A, et al. (2020): Long versus short pulse width subcallosal cingulate stimulation for treatment-resistant depression: a randomised, double-blind, crossover trial. Lancet Psychiatry. 7:29–40. [DOI] [PubMed] [Google Scholar]
- 20.Raymaekers S, Vansteelandt K, Luyten L, Bervoets C, Demyttenaere K, Gabriels L, et al. (2017): Long-term electrical stimulation of bed nucleus of stria terminalis for obsessive-compulsive disorder. Mol Psychiatry. 22:931–934. [DOI] [PubMed] [Google Scholar]
- 21.Luyten L, Hendrickx S, Raymaekers S, Gabriels L, Nuttin B (2016): Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry. 21:1272–1280. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez F, Velasco F, Salin-Pascual R, Velasco M, Nicolini H, Velasco AL, et al. (2007): Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 97:393–398. [DOI] [PubMed] [Google Scholar]
- 23.Neudorfer C, Maarouf M (2018): Neuroanatomical background and functional considerations for stereotactic interventions in the H fields of Forel. Brain Struct Funct. 223:17–30. [DOI] [PubMed] [Google Scholar]
- 24.Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, Haber SN (2011): Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J Neurosci. 31:10392–10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safadi Z, Grisot G, Jbabdi S, Behrens TE, Heilbronner SR, McLaughlin NCR, et al. (2018): Functional Segmentation of the Anterior Limb of the Internal Capsule: Linking White Matter Abnormalities to Specific Connections. J Neurosci. 38:2106–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmahmann J, Pandya D (2006): Fiber Pathways of the Brain. New York: Oxford University Press, Inc. [Google Scholar]
- 27.Dejerine J (1895): Anatomie des centres nerveux. Paris,: Rueff. [Google Scholar]
- 28.Selemon LD, Goldman-Rakic PS (1985): Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 5:776–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haber SN, Kim KS, Mailly P, Calzavara R (2006): Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 26:8368–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieuwenhuys R, Geeraedts LM, Veening JG (1982): The medial forebrain bundle of the rat. I. General introduction. J Comp Neurol. 206:49–81. [DOI] [PubMed] [Google Scholar]
- 31.Nieuwenhuys R (1996): The greater limbic system, the emotional motor system and the brain. Prog Brain Res. 107:551–580. [DOI] [PubMed] [Google Scholar]
- 32.Haynes WI, Haber SN (2013): The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J Neurosci. 33:4804–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haber SN, Lynd-Balta E, Mitchell SJ (1993): The organization of the descending ventral pallidal projections in the monkey. J Comp Neurol. 329:111–128. [DOI] [PubMed] [Google Scholar]
- 34.Parent A, Smith Y (1987): Organization of efferent projections of the subthalamic nucleus in the squirrel monkey as revealed by retrograde labeling methods. Brain Research. 436:296–310. [DOI] [PubMed] [Google Scholar]
- 35.Parent A, Smith Y, Filion M, Dumas J (1989): Distinct afferents to internal and external pallidal segments in the squirrel monkey. Neuroscience Letters. 96:140–144. [DOI] [PubMed] [Google Scholar]
- 36.Jbabdi S, Lehman JF, Haber SN, Behrens TE (2013): Human and monkey ventral prefrontal fibers use the same organizational principles to reach their targets: tracing versus tractography. J Neurosci. 33:3190–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jbabdi S, Sotiropoulos SN, Haber SN, Van Essen DC, Behrens TE (2015): Measuring macroscopic brain connections in vivo. Nat Neurosci. 18:1546–1555. [DOI] [PubMed] [Google Scholar]
- 38.Li N, Baldermann JC, Kibleur A, Treu S, Akram H, Elias GJB, et al. (2020): Toward a unified connectomic target for deep brain stimulation in obsessive-compulsive disorder. Nature Communications (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Axer H, Keyserlingk DG (2000): Mapping of fiber orientation in human internal capsule by means of polarized light and confocal scanning laser microscopy. J Neurosci Methods. 94:165–175. [DOI] [PubMed] [Google Scholar]
- 40.Haber SN, Gdowski MJ (2004): The Basal Ganglia. In: Paxinos G, Mai JK, editors. The Human Nervous System, Second Edition ed: Elsevier Press, pp 677–738. [Google Scholar]
- 41.Lynd-Balta E, Haber SN (1994): The organization of midbrain projections to the striatum in the primate: sensorimotor-related striatum versus ventral striatum. Neuroscience. 59:625–640. [DOI] [PubMed] [Google Scholar]
- 42.McFarland NR, Haber SN (2001): Organization of thalamostriatal terminals from the ventral motor nuclei in the macaque. J Comp Neurol. 429:321–336. [DOI] [PubMed] [Google Scholar]
- 43.Gimenez-Amaya JM, McFarland NR, de las Heras S, Haber SN (1995): Organization of thalamic projections to the ventral striatum in the primate. J Comp Neurol. 354:127–149. [DOI] [PubMed] [Google Scholar]
- 44.Smith Y, Raju DV, Pare JF, Sidibe M (2004): The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 27:520–527. [DOI] [PubMed] [Google Scholar]
- 45.Smith Y, Galvan A, Raju D, Wichmann T (2010): Anatomical and Functional Organization of the Thalamostriatal Systems. In: Steiner H, Tseng K-Y, editors. Handbook of basal ganglia structure and function. London: Academic, pp 381–396. [Google Scholar]
- 46.Calzavara R, Mailly P, Haber SN (2007): Relationship between the corticostriatal terminals from areas 9 and 46, and those from area 8A, dorsal and rostral premotor cortex and area 24c: an anatomical substrate for cognition to action. Eur J Neurosci. 26:2005–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Averbeck BB, Lehman J, Jacobson M, Haber SN (2014): Estimates of projection overlap and zones of convergence within frontal-striatal circuits. J Neurosci. 34:9497–9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russchen FT, Bakst I, Amaral DG, Price JL (1985): The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res. 329:241–257. [DOI] [PubMed] [Google Scholar]
- 49.Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN (2002): Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 110:257–275. [DOI] [PubMed] [Google Scholar]
- 50.Haber SN, Lynd E, Klein C, Groenewegen HJ (1990): Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study. J Comp Neurol. 293:282–298. [DOI] [PubMed] [Google Scholar]
- 51.Zaborszky L, Cullinan WE (1992): Projections from the nucleus accumbens to cholinergic neurons of the ventral pallidum: a correlated light and electron microscopic double-immunolabeling study in rat. Brain Res. 570:92–101. [DOI] [PubMed] [Google Scholar]
- 52.Haber S (1987): Anatomical relationship between the basal ganglia and the basal nucleus of Meynert in human and monkey forebrain. Proc Natl Acad Sci U S A. 84:1408–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parent M, Parent A (2007): The microcircuitry of primate subthalamic nucleus. ParkinsonismRelat Disord 13 Suppl 3:S292–S295. [DOI] [PubMed] [Google Scholar]
- 54.Mena-Segovia J, Ross HM, Magill PJ, Bolam JP (2005): The pedunculopontine nucleus: towards a functional integration with the basal ganglia. New York: Springer Science and Business Media. [Google Scholar]
- 55.Lavoie B, Parent A (1994): Pedunculopontine nucleus in the squirrel monkey: Projections to the basal ganglia as revealed by anterograde tract-tracing methods. J Comp Neurol. 344:210–231. [DOI] [PubMed] [Google Scholar]
- 56.Haber SN, Adler A, Bergman H (2012): The Basal Ganglia. In: Mai JK, Paxinos G, editors. The Human Nervous System, 3 ed. San Diego, CA: Academic Press, pp 680–740. [Google Scholar]
- 57.Nambu A, Takada M, Inase M, Tokuno H (1996): Dual somatotopical representations in the primate subthalamic nucleus: Evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplimentary motor area. J Neurosci. 16:2671–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karachi C, Yelnik J, Tande D, Tremblay L, Hirsch EC, Francois C (2005): The pallidosubthalamic projection: an anatomical substrate for nonmotor functions of the subthalamic nucleus in primates. Mov Disord. 20:172–180. [DOI] [PubMed] [Google Scholar]
- 59.Shink E, Bevan MD, Bolam JP, Smith Y (1996): The subthalamic nucleus and the external pallidum: two tightly interconnected structures that control the output of the basal ganglia in the monkey. Neuroscience. 73:335–357. [DOI] [PubMed] [Google Scholar]
- 60.Neubert FX, Mars RB, Sallet J, Rushworth MF (2015): Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc Natl Acad Sci U S A. 112:E2695–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coenen VA, Panksepp J, Hurwitz TA, Urbach H, Madler B (2012): Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J Neuropsychiatry Clin Neurosci. 24:223–236. [DOI] [PubMed] [Google Scholar]
- 62.Oades RD, Halliday GM (1987): Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Research. 434:117–165. [DOI] [PubMed] [Google Scholar]
- 63.Levitt P, Rakic P, Goldman-Rakic P (1984): Region-specific distribution of catecholamine afferents in primate cerebral cortex: A fluorescence histochemical analysis. J Comp Neurol. 227:23–36. [DOI] [PubMed] [Google Scholar]
- 64.Veazey RB, Amaral DG, Cowan WM (1982): The morphology and connections of the posterior hypothalamus in the cynomolgus monkey (Macaca fascicularis). I. Cytoarchitectonic organization. J Comp Neurol. 207:114–134. [DOI] [PubMed] [Google Scholar]
- 65.Nauta WJ (1982): Limbic innervation of the striatum. Advances in Neurology. 35:41–47. [PubMed] [Google Scholar]
- 66.Mai J, Paxinos G, Voss T (2008): Atlas of the Human Brain. Elsevier. [Google Scholar]
- 67.Sutoo D, Akiyama K, Yabe Y, Kohno K (1994): Quantitative analysis of immunohistochemical distributions of cholinergic and catecholaminergic systems in the human brain. Neuroscience. 58:227–234. [DOI] [PubMed] [Google Scholar]
- 68.Holt DJ, Graybiel AM, Saper CB (1997): Neurochemical architecture of the human striatum. J Comp Neurol. 384:1–25. [DOI] [PubMed] [Google Scholar]
- 69.Coenen VA, Prescher A, Schmidt T, Picozzi P, Gielen FL (2008): What is dorso-lateral in the subthalamic Nucleus (STN)?--a topographic and anatomical consideration on the ambiguous description of today’s primary target for deep brain stimulation (DBS) surgery. Acta Neurochir (Wien). 150:1163–1165; discussion 1165. [DOI] [PubMed] [Google Scholar]
- 70.Jbabdi S, Behrens TE, Smith SM (2010): Crossing fibres in tract-based spatial statistics. Neuroimage. 49:249–256. [DOI] [PubMed] [Google Scholar]
- 71.Thomas C, Ye FQ, Irfanoglu MO, Modi P, Saleem KS, Leopold DA, et al. (2014): Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci U S A. 111:16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maier-Hein KH, Neher PF, Houde JC, Cote MA, Garyfallidis E, Zhong J, et al. (2017): The challenge of mapping the human connectome based on diffusion tractography. Nat Commun. 8:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reveley C, Seth AK, Pierpaoli C, Silva AC, Yu D, Saunders RC, et al. (2015): Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography. Proc Natl Acad Sci U S A. 112:E2820–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jbabdi S, Johansen-Berg H (2011): Tractography: where do we go from here? Brain Connect. 1:169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haber SN, Fudge JL, McFarland NR (2000): Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 20:2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. (2006): Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 31:2384–2393. [DOI] [PubMed] [Google Scholar]
- 77.Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B (1999): Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 354:1526. [DOI] [PubMed] [Google Scholar]
- 78.Nuttin BJ, Gabriels LA, Cosyns PR, Meyerson BA, Andreewitch S, Sunaert SG, et al. (2003): Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 52:1263–1272; discussion 1272–1264. [DOI] [PubMed] [Google Scholar]
- 79.Petersen MV, Mlakar J, Haber SN, Parent M, Smith Y, Strick PL, et al. (2019): Holographic Reconstruction of Axonal Pathways in the Human Brain. Neuron. 104:1056–1064 e1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lawrence NS, An SK, Mataix-Cols D, Ruths F, Speckens A, Phillips ML (2007): Neural responses to facial expressions of disgust but not fear are modulated by washing symptoms in OCD. Biol Psychiatry. 61:1072–1080. [DOI] [PubMed] [Google Scholar]
- 81.Vaghi MM, Vertes PE, Kitzbichler MG, Apergis-Schoute AM, van der Flier FE, Fineberg NA, et al. (2017): Specific Frontostriatal Circuits for Impaired Cognitive Flexibility and Goal-Directed Planning in Obsessive-Compulsive Disorder: Evidence From Resting-State Functional Connectivity. Biol Psychiatry. 81:708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sha Z, Versace A, Edmiston EK, Fournier J, Graur S, Greenberg T, et al. (2020): Functional disruption in prefrontal-striatal network in obsessive-compulsive disorder. Psychiatry Research: Neuroimaging. 300:111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saxena S, Brody AL, Ho ML, Alborzian S, Maidment KM, Zohrabi N, et al. (2002): Differential cerebral metabolic changes with paroxetine treatment of obsessive-compulsive disorder vs major depression. Arch Gen Psychiatry. 59:250–261. [DOI] [PubMed] [Google Scholar]
- 84.van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, et al. (2005): Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry. 62:301–309. [DOI] [PubMed] [Google Scholar]
- 85.Bleich-Cohen M, Hendler T, Weizman R, Faragian S, Weizman A, Poyurovsky M (2014): Working memory dysfunction in schizophrenia patients with obsessive-compulsive symptoms: an fMRI study. Eur Psychiatry. 29:160–166. [DOI] [PubMed] [Google Scholar]
- 86.Haber SN (2016): Corticostriatal circuitry. Dialogues Clin Neurosci. 18:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lynd-Balta E, Haber SN (1994): Primate striatonigral projections: a comparison of the sensorimotor-related striatum and the ventral striatum. J Comp Neurol. 345:562–578. [DOI] [PubMed] [Google Scholar]
- 88.Makris N, Rathi Y, Mouradian P, Bonmassar G, Papadimitriou G, Ing WI, et al. (2016): Variability and anatomical specificity of the orbitofrontothalamic fibers of passage in the ventral capsule/ventral striatum (VC/VS): precision care for patient-specific tractography-guided targeting of deep brain stimulation (DBS) in obsessive compulsive disorder (OCD). Brain imaging and behavior. 10:1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Koning PP, Figee M, van den Munckhof P, Schuurman PR, Denys D (2011): Current status of deep brain stimulation for obsessive-compulsive disorder: a clinical review of different targets. Curr Psychiatry Rep. 13:274–282. [DOI] [PubMed] [Google Scholar]
- 90.Dougherty DD, Chou T, Corse AK, Arulpragasam AR, Widge AS, Cusin C, et al. (2016): Acute deep brain stimulation changes in regional cerebral blood flow in obsessive-compulsive disorder. J Neurosurg.1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Figee M, Luigjes J, Smolders R, Valencia-Alfonso CE, van Wingen G, de Kwaasteniet B, et al. (2013): Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 16:386–387. [DOI] [PubMed] [Google Scholar]
- 92.Widge AS, Zorowitz S, Basu I, Paulk AC, Cash SS, Eskandar EN, et al. (2019): Deep brain stimulation of the internal capsule enhances human cognitive control and prefrontal cortex function. Nat Commun. 10:1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Figee M, Vink M, de Geus F, Vulink N, Veltman DJ, Westenberg H, et al. (2011): Dysfunctional reward circuitry in obsessive-compulsive disorder. Biol Psychiatry. 69:867–874. [DOI] [PubMed] [Google Scholar]
- 94.Van Laere K, Nuttin B, Gabriels L, Dupont P, Rasmussen S, Greenberg BD, et al. (2006): Metabolic imaging of anterior capsular stimulation in refractory obsessive-compulsive disorder: a key role for the subgenual anterior cingulate and ventral striatum. J Nucl Med. 47:740–747. [PubMed] [Google Scholar]
- 95.Krieg W (1973): Architectonics of the human cerebral fiber systems. Evanston, IL: Brain Books. [Google Scholar]
- 96.Denys D, Mantione M (2009): Deep brain stimulation in obsessive-compulsive disorder. Prog Brain Res. 175:419–427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


