Abstract
Visceral leishmaniasis, or kala-azar, a fatal tropical disease, remains problematic, as early diagnosis is difficult and treatment often results in drug resistance and relapse. We have developed a sensitive enzyme-linked immunosorbent assay (ELISA), using leishmanial membrane antigenic extracts (LAg) to detect specific antibody responses in 25 untreated Indian visceral leishmaniasis patients. To investigate the pathogenetic significance of isotype markers in kala-azar, relative levels of specific immunoglobulin G (IgG), IgM, IgA, IgE, and IgG subclasses were analyzed under clinically established diseased conditions. Since LAg showed higher sensitivity for specific IgG than lysate, the immunoglobulin isotype responses were evaluated, with LAg as antigen. Compared to 60 controls, which included patients with malaria, tuberculosis, leprosy, and typhoid and healthy subjects, visceral leishmaniasis patients showed significantly higher IgG (100% sensitivity, 85% specificity), IgM (48% sensitivity, 100% specificity), and IgE (44% sensitivity, 98.3% specificity) responses. Low levels of IgA in visceral leishmaniasis patients contrasted with a 13-fold-higher reactivity in sera from patients with leprosy. Among IgG subclasses, IgG1, -3, and -4 responses were significantly higher in visceral leishmaniasis patients than in the controls. IgG2 response, however, was significantly higher (twofold) in leprosy than even visceral leishmaniasis patients. The rank orders for sensitivity (IgG = IgG1 = IgG3 = IgG4 > IgG2 > IgM > IgE > IgA) and specificity (IgM = IgG3 > IgE > IgG4 > IgG2 > IgG > IgG1 > IgA) for LAg-specific antibody responses suggest the potentiality of IgG3 as a diagnostic marker for visceral leishmaniasis.
Human visceral leishmaniasis, kala-azar, is a tropical disease caused by the protozoan parasites of the Leishmania donovani complex. The parasites multiply in the macrophages of the spleen, liver, bone marrow, and lymph nodes, resulting in a progressive disease which is invariably fatal if untreated. Infection by L. donovani in humans induces T-cell anergy as assessed by the depression of delayed-type hypersensitivity reaction and failure of peripheral blood T cells to proliferate (18, 19) and to produce gamma interferon (IFN-γ) and interleukin (IL)-2 in response to Leishmania antigens (8, 11). Cytokine analysis reveals enhanced induction of IFN-γ, IL-10, and/or IL-4 mRNA in tissues (16, 23), and the enhanced presence of IL-4 in circulation (40) of kala-azar patients. While the presence of these cytokines suggests a coexistence of Th-1- and Th-2-like responses in the clinical stage of the disease, the absence of IL-2 points to the dominance of the Th-2 response. The disease is also characterized by high levels of Leishmania-specific antibodies (3). Since cytokines elaborated by activated T cells are required for the regulation of isotype switch during B-cell development (15, 24, 37), a study of the subclass distribution of the antibodies may shed new light on the processes involved in the polarization of the immune responses during disease. Leishmanial membrane antigens of L. donovani (LAg) have been effectively used to investigate immunological responses during disease progression in murine models of visceral leishmaniasis (2). Herein, we report the subclass distribution and the fine specificity of the antibody response to LAg in the sera of Indian kala-azar patients.
MATERIALS AND METHODS
Study subjects.
The subjects of the present investigation were 25 Indian patients with visceral leishmaniasis admitted to School of Tropical Medicine, Calcutta, India. These patients came from Bihar (eastern India), one of the main areas of endemicity. Diagnosis of these patients was confirmed parasitologically by the demonstration of Leishmania amastigotes in spleen and/or bone marrow aspirates. Blood was obtained after diagnosis, before the initiation of chemotherapy. Sixty individuals included as controls consisted of 15 malaria patients infected with Plasmodium falciparum or Plasmodium vivax or both, 10 typhoid patients, 15 tuberculosis patients, 8 leprosy patients, and 12 healthy controls from the Indian Institute of Chemical Biology (IICB). The endemic diseases were confirmed bacteriologically in the case of typhoid, tuberculosis, and leprosy and parasitologically in the case of malaria, and sera were collected before treatment.
Preparation of antigen.
L. donovani AG83, originally isolated from an Indian kala-azar patient, was cultured in vitro for antigen preparation as described earlier (1). Briefly, stationary-phase promastigotes, harvested after the third or fourth passage, were washed four times in cold phosphate-buffered saline (PBS) (pH 7.2) and resuspended at a concentration of 1.0 g of cell pellet in 50 ml of cold 5 mM Tris-HCl buffer, pH 7.6. The suspension was vortexed and centrifuged at 2,310 × g for 10 min. The crude ghost membrane pellet thus obtained was resuspended in the same Tris buffer and sonicated in an ultrasonicator. The suspension was centrifuged at 4,390 × g for 30 min, and the supernatant containing the LAg was harvested and stored at −70°C until use. The amount of protein obtained from 1.0 g of cell pellet, as assayed by the method of Lowry et al. (26), was 16 mg. The lysate used in this study was prepared from 5 × 107 stationary-phase promastigotes per ml according to the method of Jaffe and Zalis (21). Protein concentration (5 mg/ml) was assessed as described above.
Enzyme-linked immunosorbent assay (ELISA).
For serological studies, microtiter plates (Tarsons) were coated overnight with 2 μg of lysate or LAg per well. For Leishmania-reactive immunoglobulin G (IgG), IgM, IgA, and IgE antibody determination, the antigen-coated plates were incubated with sera diluted 1:1,000-fold, and reacted with peroxidase-conjugated goat anti-human IgG, IgM, IgA, and IgE polyclonal antibodies (Sigma Immunochemicals) at a 1:5,000 dilution and developed with o-phenylenediamine dihydrochloride (1). For IgG subclass determination, human sera were reacted with mouse anti-human IgG1, IgG2, IgG3, and IgG4 monoclonal antibodies (1:3,000 dilution; Sigma Immunochemicals). Bound antibodies were detected with peroxidase-conjugated goat anti-mouse IgG (1:5,000 dilution; Sigma Immunochemicals) (1).
Statistical analysis.
All data comparisons were tested for significance by using Student’s t test; P values of < 0.05 were considered significant. The lower limit of positivity (cutoff) was determined by the mean of healthy controls + 2 standard deviations (13, 14).
RESULTS
Serum IgG specificity for L. donovani lysate and LAg.
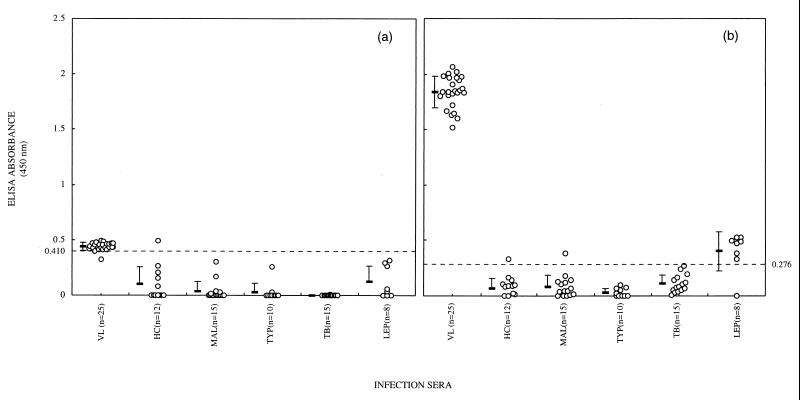
Reactivities of serum IgG antibodies of kala-azar patients to the parasite lysate were compared to those of LAg. At a 1:1,000 dilution of sera, 20 of 25 patients were positive for the lysate, with IgG absorbance values ranging from 0.319 to 0.493 (Fig. 1a). Reactivity with LAg, however, resulted in 100% sensitivity, with significantly higher IgG absorbance values (1.517 to 2.066; Fig. 1b). Serum specimens from patients with diseases such as malaria, typhoid, tuberculosis, and leprosy were negative for the lysate, and only 1 of 12 serum samples from normal control individuals analyzed was found to be positive (Fig. 1a). Conversely, 1 of 12 healthy controls and 1 of 15 malaria patients were positive, while all typhoid and tuberculosis serum specimens were negative for LAg (Fig. 1b). The highest cross-reactivity was observed with sera from leprosy patients (seven of eight samples). However, the mean ± standard deviation of IgG absorbance (0.403 ± 0.176) of these specimens was just above the cutoff value of 0.276 and significantly lower than the mean IgG response observed with kala-azar patient sera. Since antibody reactivities of sera from kala-azar patients with LAg were higher than with lysate and 100% sensitive, Ig subclass distribution analysis was restricted to LAg.
FIG. 1.
Dot plots showing specific IgG responses (absorbance values) of sera from healthy controls and patients with visceral leishmaniasis, malaria, typhoid, tuberculosis, and leprosy to leishmanial lysate (a) and LAg (b). The horizontal bars represent the means ± standard deviations for the different groups. The dotted line indicates the cutoff value (mean of healthy controls + 2 standard deviations).
LAg-specific serum Ig antibodies.
Antibody reactivities of IgG, IgM, and IgE of sera from patients with visceral leishmaniasis with LAg were significantly higher than those of normal controls and those of patients with other diseases such as malaria, typhoid, tuberculosis, and leprosy (P < 0.05; Table 1). The IgA reactivity with LAg was, however, predominant in sera from patients with leprosy, with titers 13-fold higher than those even of patients with visceral leishmaniasis.
TABLE 1.
Comparison of the serological responses of visceral leishmaniasis to other diseases
| Serogroupb | Antibody
levela
|
No. of patients | |||
|---|---|---|---|---|---|
| IgG | IgM | IgA | IgE | ||
| VL | 1.839 ± 0.143c | 0.878 ± 0.455c | 0.039 ± 0.084 | 0.083 ± 0.043c | 25 |
| HC | 0.094 ± 0.091 | 0.250 ± 0.295 | 0.051 ± 0.93 | 0.022 ± 0.035 | 12 |
| MAL | 0.085 ± 0.100 | 0.140 ± 0.130 | 0.002 ± 0.007 | 0.003 ± 0.004 | 15 |
| TYP | 0.031 ± 0.037 | 0.158 ± 0.205 | 0.091 ± 0.162 | 0 | 10 |
| TB | 0.110 ± 0.078 | 0.315 ± 0.281 | 0.158 ± 0.189 | 0 | 15 |
| LEP | 0.403 ± 0.176c | 0.249 ± 0.246 | 0.518 ± 0.312c | 0.006 ± 0.011 | 8 |
| Total | 85 | ||||
Absorbance values (means ± standard deviations).
VL, visceral leishmaniasis; HC, healthy controls; MAL, malaria; TYP, typhoid; TB, tuberculosis; LEP, leprosy.
Significantly different (P < 0.05) from the value for healthy control group as calculated by Student’s t test.
Antigen-specific distribution of serum IgG subclasses.
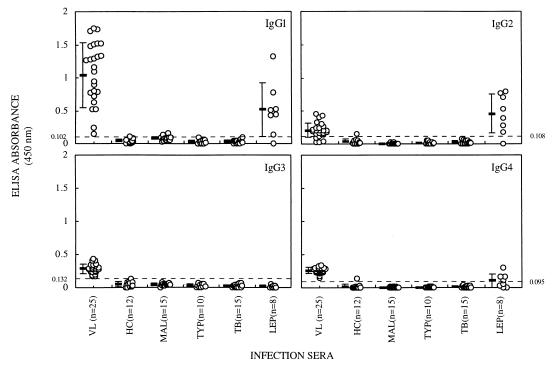
Since the analysis of sera from patients with kala-azar revealed high levels of IgG antibody response to LAg, the IgG subclass specificity was further examined. The results demonstrate that serum samples from all 25 visceral leishmaniasis patients were positive for IgG1, IgG3, and IgG4 antibodies, whereas 21 of 25 had antibodies of the IgG2 subclass (Fig. 2). All these IgG subclasses showed significantly higher reactivity with LAg than normal controls with the dominance of IgG1, in agreement with a previous report (38). While LAg-specific reactivities of all the IgG subclasses were minimal with sera from patients with other diseases, sera from patients with leprosy showed significant levels of antibody responses. Seven of eight samples tested had IgG1 and IgG2 subclasses, and four had IgG4. Moreover, the mean IgG2 response in sera from patients with leprosy was twofold higher even than that of patients with visceral leishmaniasis (Fig. 2). Surprisingly, however, samples of neither leprosy nor any disease other than kala-azar had LAg-specific IgG3 subclass antibodies. Table 2 summarizes the sensitivity and specificity of IgG, IgM, IgE, and IgG subclasses of sera from patients with visceral leishmaniasis.
FIG. 2.
LAg-specific antibody reactivities of IgG subclasses (absorbance values) of individual sera from healthy controls and patients with visceral leishmaniasis, malaria, typhoid, tuberculosis, and leprosy. The horizontal bars represent the means ± standard deviations for the different groups. The dotted line indicates the cutoff value (mean of healthy controls + 2 standard deviations).
TABLE 2.
Percent sensitivity and specificity of IgG, IgM, IgA, IgE, and IgG subclasses in visceral leishmaniasis patients
| Antibody | Sensitivitya (%) | Specificitya (%) |
|---|---|---|
| IgG | 100 | 85.0 |
| IgM | 48 | 100.0 |
| IgA | 4 | 80.0 |
| IgE | 44 | 98.3 |
| IgG1 | 100 | 83.3 |
| IgG2 | 84 | 86.6 |
| IgG3 | 100 | 100.0 |
| IgG4 | 100 | 91.6 |
Calculations were done with respect to the ELISA cutoff values of 0.276, 0.840, 0.237, 0.092, 0.102, 0.108, 0.132, 0.095 optical density for IgG, IgM, IgA, IgE, IgG1, IgG2, IgG3, and IgG4, respectively.
DISCUSSION
We found that while a high proportion of Indian kala-azar patients have elevated levels of anti-LAg IgG, IgM, IgE, and IgG subclass antibodies, IgG, IgG1, IgG3, and IgG4 were present in sera from all the patients, with IgG3 being specifically associated with this disease. Although investigations in murine models of Leishmania major and L. donovani infections clearly demonstrate a Th-2/IL-4/IgG1 relationship with disease progression, and a Th-1/IFN-γ/IgG2a relationship with resistance and protective immunity (1, 2, 4), such a relationship in humans is not fully understood. An association between antibody isotypes, cytokine profiles, and pathogenesis has been made for some diseases such as leprosy (12, 20), AIDS (6, 27), lymphatic filariasis (25), onchocerciasis (32), and malaria (28). In American cutaneous leishmaniasis, strong cell-mediated immunity and the predominance of IgG1, IgG2, and IgG3 isotypes in localized cutaneous and mucocutaneous leishmaniasis have been linked with Th-1 reactivity, whereas IgG4 subclass antibody response in sera of diffuse cutaneous leishmaniasis patients has been correlated with a Th-2 cell response (7, 9, 29, 35). Cytokine analysis of human visceral leishmaniasis suggests that the Th-2 response will be stronger than the Th-1 response during the active phase of the disease (8, 16, 23, 40). Investigations of IgG subclass response during disease show significant stimulation of all the IgG subclasses in Sudanese patients, with higher levels of IgG3 and IgG4 than IgG1 (13). Conversely, Venezuelan patients have a dominant IgG1 response followed by IgG4 (38). Indian kala-azar patients also showed a predominant IgG1 subclass antibody response, but the levels of IgG3, IgG4, and IgG2 were also significant. These subclasses of human IgG are endowed with unique biological and functional properties, including their response to different types of antigens (22). The elicitation of IgG1, IgG3, and IgG4 antibodies in kala-azar sera may be due mostly to the presence of protein antigens, and the elicitation of IgG2 antibodies in kala-azar sera may be due mostly to the presence of carbohydrate antigens, as reported for viral, bacterial, and parasitic infections (6, 12, 20, 25, 32). Induction of IgG1 and IgG2 is IFN-γ dependent, and IgG3 and IgG4 depend on IL-4 and are down regulated by IFN-γ (15, 24). The elevation of IFN-γ in kala-azar patients (23, 40) and the strong reactivity of IgG1 during disease appear to be consistent with the above observations. Their presence, however, fails to control the infection. The absence of IL-2, a Th-1 mediator, suggests a lack of Th-1 response during disease (8, 11). Stimulation of serum IgG3 and IgG4 during infection, together with the expression of cytokines such as IL-10 and IL-4 (16, 40), which are also responsible for the upregulation of these IgG subclasses, provides further evidence in support of a Th-2 cell response in determining the outcome of the disease. One explanation for the presence of IgG1 in kala-azar patients may be due to IFN-γ derived from alternative cell sources such as natural killer cells and γδ T cells (10, 39).
LAg-associated serological responses of patients with diseases other than kala-azar were observed to be maximal for leprosy for all isotypes except IgM, IgE, and IgG3. In contrast to a previous report of low reactions of leprosy sera with soluble extracts of Leishmania promastigote antigen for all isotypes (38), LAg gave strong reactions with IgG, IgA, IgG1, and IgG2 and low reactivity with IgG4. Further, reactions with IgA and IgG2 were 13- and 2-fold higher, respectively, than even kala-azar patient sera. Leprosy sera show reactivity with lipoarabinomannan B (LAM), a carbohydrate component of Mycobacterium leprae, through IgG2 and IgG4 and rarely with IgG3 (12). Phenolic glycolipid (PGL-1), another cell wall carbohydrate of M. leprae, reacts strongly with IgA (31) and IgG1 (12) antibodies in leprosy sera. While it is not understood how antibodies in leprosy sera react with LAg, these observations point to cross-reacting epitopes of LAM and PGL-1 in L. donovani LAg.
Amongst all the Ig isotypes and IgG subclasses studied, only IgG3 showed 100% sensitivity and specificity for LAg in visceral leishmaniasis patients. Hence, IgG3 antibody may be a more specific marker for this disease than IgG, which shows low cross-reactivity with other diseases and significant reactivity with leprosy. Moreover, we have found that although there is a decline in the levels of IgG and its subclasses after successful treatment, the decrease is maximal in IgG3 (data not shown), suggesting that IgG3 may be a useful tool for diagnosis as well as for the prognosis of visceral leishmaniasis. IgG3 elevation during leishmaniasis was reported earlier (13, 29, 34), and high specificity and sensitivity for IgG3 have been found in Sudanese visceral leishmaniasis patients (13). Better sensitivity and specificity for IgG3 observed in our studies may be due largely to the specificities of the antibodies to the antigen studied (35) in addition to ethnic variation and differences in parasite genotypes. The significance of IgG3 specificity in visceral leishmaniasis is not clearly understood. IgG3 in malaria is associated with recovery from the fatal disease (33), and skewing of the response toward the IgG3 subclass is merozoite surface antigen 2 specific (30). In leprosy, disease progression is correlated with selective increases in IgG3, along with IgG1 responses (20). Another example of antibody response which is significantly skewed toward IgG3 is the response to the outer membrane protein of Branhamella catarrhalis (17). While there are reports of involvement of T-cell-derived cytokines in the regulation of switch factors for IgG3 (5, 24), little is known about the factors which may preferentially induce the production of IgG3 in humans. Functionally, IgG3 is considered to be the most effective subclass for activating the complement pathway (22) and is known to mediate cell lysis by monocytes or Fc receptor-bearing lymphocytes (36). However, the protective role of leishmania-specific antibodies in human visceral leishmaniasis is still controversial. In conclusion, our serological data demonstrate the potentiality of LAg as an important antigen in the diagnosis of the outcome of infection with L. donovani, with IgG3 as a marker for the identification of individuals with visceral leishmaniasis.
ACKNOWLEDGMENTS
We gratefully acknowledge the patients of the School of Tropical Medicine, Calcutta, India, who participated in this study. We thank the volunteer blood donors of IICB, Calcutta, India. We thank K. Kabir (Repromed Diagnostic, Calcutta, India) for providing access to blood samples of malaria, typhoid, and tuberculosis.
This work was supported through grants from the CSIR and the DST, Government of India, and the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. K.A. and F.A. are research fellows supported by ICMR and CSIR, respectively. We thank J. Das, director, IICB, Calcutta, for supporting this work.
REFERENCES
- 1.Afrin F, Ali N. Adjuvanticity and protective immunity elicited by Leishmania donovani antigens encapsulated in positively charged liposomes. Infect Immun. 1997;65:2371–2377. doi: 10.1128/iai.65.6.2371-2377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afrin F, Ali N. Isotype profiles of Leishmania donovani infected BALB/c mice: preferential stimulation of IgG2a/b by liposome associated promastigote antigens. J Parasitol. 1998;84:743–748. [PubMed] [Google Scholar]
- 3.Bray R S. Immunodiagnosis of leishmaniasis. In: Cohen S, Sadun E H, editors. Immunology of parasitic infections. Oxford, England: Blackwell Scientific Publications; 1976. pp. 65–76. [Google Scholar]
- 4.Bretscher P A, Wei G, Menon J N, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes ‘susceptible’ mice resistant to Leishmania major. Science. 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 5.Briere F, Servet-Delprat C, Bridon J-M, Saint-Remy J-M, Bancherau J. Human interleukin 10 induces naïve surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J Exp Med. 1994;179:757–762. doi: 10.1084/jem.179.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broliden P A, Morfeldt-Mansson L, Rosen J, Jondal M, Wahren B. Fine specificity of IgG subclass response to group antigens in HIV-1-infected patients. Clin Exp Immunol. 1989;76:216–221. [PMC free article] [PubMed] [Google Scholar]
- 7.Caceres-Dittmar G, Tapia F J, Sanchez M A, Yamamura M, Uyemura K, Modlin R L, Bloom B R, Convit J. Determination of the cytokine profile in American cutaneous leishmaniasis using the polymerase chain reaction. Clin Exp Immunol. 1993;91:500–505. doi: 10.1111/j.1365-2249.1993.tb05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho E M, Badaro R, Reed S G, Jones T C, Johnson W D. Absence of gamma-interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Investig. 1985;76:2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castes M, Cabrera M, Trujillo D, Convit J. T-cell subpopulations, expression of interleukin-2 receptor, and production of interleukin-2 and gamma interferon in human American cutaneous leishmaniasis. J Clin Microbiol. 1988;26:1207–1213. doi: 10.1128/jcm.26.6.1207-1213.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christmas S E, Meager A. Production of interferon-gamma and tumor necrosis factor-alpha by human T-cell clones expressing different forms of the gamma delta receptor. Immunology. 1990;71:486–492. [PMC free article] [PubMed] [Google Scholar]
- 11.Cillari E, Liew F Y, Campo P L, Milano S, Mansueto S, Salerno A. Suppression of IL-2 production by cryopreserved peripheral blood mononuclear cells from patients with active visceral leishmaniasis in Sicily. J Immunol. 1988;140:2721–2726. [PubMed] [Google Scholar]
- 12.Dhandayuthapani S, Izumi S, Anandan D, Bhatia V N. Specificity of IgG subclass antibodies in different clinical manifestations of leprosy. Clin Exp Immunol. 1992;88:253–257. doi: 10.1111/j.1365-2249.1992.tb03069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elassad A M S, Younis S A, Siddig M, Grayson J, Peterson E, Ghalib H W. The significance of blood levels of IgM, IgA, IgG and IgG subclasses in Sudanese visceral leishmaniasis patients. Clin Exp Immunol. 1994;95:294–299. doi: 10.1111/j.1365-2249.1994.tb06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fargeas C, Hommel M, Maingon R, Dourado C, Monsigny M, Mayer R. Synthetic peptide-based enzyme-linked immunosorbent assay for serodiagnosis of visceral leishmaniasis. J Clin Microbiol. 1996;34:241–248. doi: 10.1128/jcm.34.2.241-248.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gascan H, Gauchat J-F, Roncarolo M-G, Yssel H, Spits H, de Vries J E. Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by interleukin 4 and a signal provided by activated CD4+ T cell clones. J Exp Med. 1991;173:747–750. doi: 10.1084/jem.173.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghalib H W, Piuvezam M R, Skeiky Y A W, Siddiq M, Hashim F A, El-Hassan A M, Russo D M, Reed S G. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Investig. 1993;92:324–329. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldblatt D, Turner M W, Levinsky R J. Branhamella catarrhalis: antigenic determinants and the development of the IgG subclass response in childhood. J Infect Dis. 1990;162:1128–1135. doi: 10.1093/infdis/162.5.1128. [DOI] [PubMed] [Google Scholar]
- 18.Haldar J P, Ghose S, Saha K C, Ghose A C. Cell-mediated immune response in Indian kala azar and post-kala azar dermal leishmaniasis. Infect Immun. 1983;42:702–707. doi: 10.1128/iai.42.2.702-707.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho M, Koech D K, Iha D W, Bryceson A D M. Immunosuppression in Kenyan visceral leishmaniasis. Clin Exp Immunol. 1983;51:207–214. [PMC free article] [PubMed] [Google Scholar]
- 20.Hussain R, Kifayet A, Chiang T J. Immunoglobulin G1 (IgG1) and IgG3 antibodies are markers of progressive disease in leprosy. Infect Immun. 1995;63:410–415. doi: 10.1128/iai.63.2.410-415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaffe C L, Zalis M. Purification of two Leishmania donovani membrane proteins recognized by sera from patients with visceral leishmaniasis. Mol Biochem Parasitol. 1988;27:53–62. doi: 10.1016/0166-6851(88)90024-2. [DOI] [PubMed] [Google Scholar]
- 22.Jefferis R. Structure/function relationships of IgG subclasses. In: Shakib F, editor. The human IgG subclasses: molecular analysis of structure, function and regulation. London, England: Pergamon Press; 1990. pp. 93–108. [Google Scholar]
- 23.Karp C L, El-Safi S H, Wynn T A, Satti M M H, Kordofani A M, Hashim F A, Hag-Ali M, Neva F A, Nutman T B, Sacks D L. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin 10 and interferon gamma. J Clin Investig. 1993;91:1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitani A, Strober W. Regulation of Cγ subclass germ-line transcripts in human peripheral blood B cells. J Immunol. 1993;151:3478–3488. [PubMed] [Google Scholar]
- 25.Kurniawan A, Yazdanbakhsh M, van Ree R, Aalberse R, Selkirk M E, Partono F, Maizels R M. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J Immunol. 1993;150:3941–3950. [PubMed] [Google Scholar]
- 26.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Ouaaz F, Ruscetti F W, Dugas B, Mikovits J, Agut H, Debre P, Mossalayi M D. Role of IgE immune complexes in the regulation of HIV-1 replication and increased cell death of infected U1 monocytes: involvement of CD23/FcɛRII-mediated nitric oxide and cyclic AMP pathways. Mol Med. 1996;2:38–49. [PMC free article] [PubMed] [Google Scholar]
- 28.Perlmann P, Perlmann H, Flyg B W, Hagstedt M, Elghazali G, Worku S, Fernandez V, Rutta A S M, Troye-Blomberg M. Immunoglobulin E, a pathogenetic factor in Plasmodium falciparum malaria. Infect Immun. 1997;65:116–121. doi: 10.1128/iai.65.1.116-121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez V, Centeno M, Ulrich M. The IgG isotypes of specific antibodies in patients with American cutaneous leishmaniasis: relationship to the cell-mediated immune response. Parasite Immunol. 1996;18:341–345. doi: 10.1046/j.1365-3024.1996.d01-113.x. [DOI] [PubMed] [Google Scholar]
- 30.Rzepczyk C M, Hale K, Woodroffe N, Bobogare A, Csurhes P, Ishii A, Ferrante A. Humoral immune responses of Solomon Islanders to the merozoite surface antigen 2 of Plasmodium falciparum show pronounced skewing towards antibodies of the immunoglobulin G3 subclass. Infect Immun. 1997;65:1098–1100. doi: 10.1128/iai.65.3.1098-1100.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saad M H, Gormus B J, Cho S N, Bernheimer H, Schwerer B. Detection of IgA anti-PGL-1 specific antigen to Mycobacterium leprae in mangabey monkeys inoculated with M. leprae. Leprosy Rev. 1995;66:296–306. [PubMed] [Google Scholar]
- 32.Salinas G, Sinha K, Cooper J P, Whitworth J A G, Taylor D W. Human isotype antibody responses to an Onchocerca volvulus glutathione S-transferase. Parasite Immunol. 1996;18:377–386. doi: 10.1046/j.1365-3024.1996.d01-124.x. [DOI] [PubMed] [Google Scholar]
- 33.Sarthou J-L, Angel G, Aribot G, Rogier C, Dieye A, Balde A T, Diatta B, Seignot P, Roussilhon C. Prognostic value of anti-Plasmodium falciparum-specific immunoglobulin G3, cytokines, and their soluble receptors in West African patients with severe malaria. Infect Immun. 1997;65:3271–3276. doi: 10.1128/iai.65.8.3271-3276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiddo S A, Huldt G, Nilsson L-A, Ouchterlony O, Thorstensson R. Visceral leishmaniasis in Somalia. Significance of IgG subclasses and of IgE response. Immunol Lett. 1996;50:87–93. doi: 10.1016/0165-2478(96)02529-1. [DOI] [PubMed] [Google Scholar]
- 35.Skeiky Y A W, Benson D R, Costa J L M, Badaro R, Reed S G. Association of Leishmania heat shock protein 83 antigen and immunoglobulin G4 antibody titers in Brazilian patients with diffuse cutaneous leishmaniasis. Infect Immun. 1997;65:5368–5370. doi: 10.1128/iai.65.12.5368-5370.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speigelberg H L. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol. 1974;19:259–294. doi: 10.1016/s0065-2776(08)60254-0. [DOI] [PubMed] [Google Scholar]
- 37.Stavnezer J. Immunoglobulin class switching. Curr Opin Immunol. 1996;8:199–205. doi: 10.1016/s0952-7915(96)80058-6. [DOI] [PubMed] [Google Scholar]
- 38.Ulrich M, Rodriguez V, Centeno M, Convit J. Differing antibody IgG isotypes in the polar forms of leprosy and cutaneous leishmaniasis characterized by antigen-specific T cell anergy. Clin Exp Immunol. 1995;100:54–58. doi: 10.1111/j.1365-2249.1995.tb03603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wherry J C, Schreiber R D, Unanue E R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991;59:1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zwingenberger K, Harms G, Pedrosa C, Omena S, Sandkamp B, Neifer S K. Determinants of the immune response in visceral leishmaniasis: evidence for predominance of endogenous interleukin 4 over interferon-γ production. Clin Immunol Immunopathol. 1990;57:242–249. doi: 10.1016/0090-1229(90)90038-r. [DOI] [PubMed] [Google Scholar]