Abstract
Preliminary testing has shown in vitro and in vivo that antitumor activity can be obtained with fusion proteins linking tumor-reactive monoclonal antibodies to cytokines, such as granulocyte-macrophage colony-stimulating factor or interleukin 2 (IL-2). Preclinical and clinical testing of these reagents requires their in vitro and in vivo quantitation and pharmacokinetic evaluation. We have focused on the detection of a fusion protein which links one human IL-2 molecule to the carboxy terminus of each heavy chain of the tumor-reactive human-mouse chimeric anti-GD2 antibody, ch14.18. We have developed enzyme-linked immunosorbent assays (ELISAs) to evaluate intact tumor-reactive fusion proteins. By these ELISAs we can reliably measure nanogram quantities of intact ch14.18-IL-2 fusion protein and distinguish the intact protein from its components (ch14.18 and IL-2) in buffer, mouse serum, and human serum with specificity and reproducibility. The measurement of intact ch14.18-IL-2 fusion protein is not confounded by free IL-2 or free ch14.18 when 100 ng or less of total immunoglobulin per ml is used during the assay procedure. Our results indicate that these ELISAs are suitable for preclinical and clinical testing and with slight modifications are applicable to the analysis of a variety of other fusion proteins.
Through molecular engineering, proteins can be altered to enhance their bioactivities. Fusion proteins, designed to combine antibodies with cytokines (2, 3, 5, 6, 13, 16, 17, 19), antibodies with cytokine receptors (1), or cytokines with toxins (8), are currently being evaluated in preclinical and clinical studies (18). The ch14.18-interleukin 2 (IL-2) and hu14.18-IL-2 proteins are two such engineered, antitumor antibody-cytokine fusion proteins (3). Human recombinant IL-2 has been linked to the anti-GD2 human-mouse chimeric or humanized forms of the 14.18 antibody (ch14.18 or hu14.18) at the carboxy terminus of the immunoglobulin heavy chain. The ch14.18-IL-2 fusion protein was shown to enhance in vitro killing of autologous GD2-positive human melanoma cells by a tumor-infiltrating lymphocyte cell line (3). In vivo, ch14.18-IL-2 markedly inhibited the growth of established hepatic metastases in severe combined immunodeficient (SCID) mice, previously reconstituted with human lymphokine-activated killer cells (15), and in immunocompetent mice bearing syngeneic GD2+ tumors (10). Increasing interest in the use of antibody-cytokine fusion proteins such as these in the treatment of malignant diseases warrants a systematic approach for quantifying and assessing their immunopharmacological effects in preclinical and clinical trials.
Many of the standard methods of protein quantitation lack specificity. For example, spectrophotometric assays are confounded by other proteins in the serum (Bradford, Lowry, or bicinchoninic acid protein assay systems), and enzyme-linked immunosorbent assays (ELISAs) quantitating immunoglobulin G (IgG) are unable to distinguish the intact fusion protein from the parent immunoglobulin. The assays described in this report specifically quantitate and distinguish the intact fusion protein from its breakdown or composite products, by utilizing capture reagents directed against one functional group and detection ligands which combine with the other active moiety. The potential use of bioengineered fusion proteins in vivo necessitates the development of assays which accurately determine the quantity of intact fusion protein. The assays presented here should be useful for both in vitro and in vivo evaluations of a wide variety of fusion proteins used in both preclinical and clinical testing.
MATERIALS AND METHODS
Immunologic reagents. (i) Antibody-IL-2 fusion proteins.
Antibody-cytokine fusion proteins used in this study include ch14.18-IL-2 and hu14.18-IL-2 (obtained from Toby Hecht of the National Cancer Institute [NCI], Frederick, Md.), CC49-IL-2 (obtained from Jeff Schlom of the NCI), and KS1/4-IL-2 (Lexigen Pharmaceuticals). The ch14.18-IL-2 fusion protein contains a mouse-human chimeric IgG1 with an anti-GD2 recognition domain and a human IL-2 molecule at the carboxy terminus of each heavy chain (3). The purification of the ch14.18-IL-2 fusion protein used in these studies was performed at the Monoclonal Antibody and Recombinant Protein facilities (NCI), and two independently purified batches (lot numbers 1 and 31403) were used as indicated. Stock concentrations of these two lots, based on ELISAs of their IgG content, were 1.15 and 0.4 mg/ml, respectively. hu14.18-IL-2 was obtained at a concentration of 1.0 mg/ml and stored until use. The CC49-IL-2 fusion protein is a single-chain antibody-cytokine fusion protein. It contains the antigen recognition domain from the murine monoclonal antibody (Mab) CC49, a human IgG1 heavy chain, and human IL-2 (22). The CC49-IL-2 protein was purified from culture supernatants of expressing cells (22, 14) and maintained as a stock solution at a concentration of 200 μg/ml. KS1/4-IL-2 is a humanized antibody-IL-2 fusion protein which is similar in structure to the hu14.18-IL-2 molecule but uses the humanized form of the mouse KS1/4 pan-carcinoma antibody (26). The stock of high pressure liquid chromatography-purified KS1/4-IL-2 determined by spectrophotometric measurement was at a concentration of 1 mg/ml. Concentrations of immunoglobulins were verified by using an ELISA for IgG content. The purity and structural integrity of the proteins were verified by electrophoretic and Western blot analyses. All fusion proteins were stored at −80°C until use.
(ii) Anti-idiotype antibodies.
The anti-idiotype antibodies used include Mab 1A7 (provided by K. Foon and M. Chatterjee of the University of Kentucky, Lexington, and Titan Pharmaceuticals, Inc.), a murine IgG1, which recognizes the hypervariable region of the ch14.18 Mab (20), and A.I. 49-3, a monoclonal anti-idiotype antibody against the mouse CC49 antibody obtained from J. Schlom (NCI). These were stored at −80°C until use.
(iii) Other immunoreagents.
ch14.18 is a mouse-human Mab which recognizes the GD2 antigen found on melanomas and neuroblastomas. The antibody was produced at Repligen Inc. (Boston, Mass.) for the NCI and stored at a stock concentration of 5.34 mg/ml, at −80°C until use. Mab CC49, a gift from Jeff Schlom, is a murine Mab which recognizes the tumor-associated antigen TAG-72 (11, 23). The IL-2 (recombinant human) standard was provided in the IL-2 ELISA kit (Immunotech, Marseille, France). Human recombinant IL-2 (Hoffmann-La Roche, Nutley, N.J.) was reconstituted with phosphate-buffered saline (PBS) at a concentration of 600,000 IU/100 μl and stored at 4°C until use. Purified human IgG1 was obtained from Sigma (St. Louis, Mo.); its concentration was 1 mg/ml based on the reading of optical density at 280 nm (OD280).
SDS-PAGE.
The purity and structural integrity of the ch14.18-IL-2 fusion protein were evaluated by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing conditions. Samples were solubilized at 95°C for 5 min in a loading buffer containing Tris, SDS, and 2-mercaptoethanol (Bio-Rad, Hercules, Calif.) and then loaded onto polyacrylamide gels. Electrophoresis was performed in a 0.75-mm-thick 12% separating gel topped with a 5% stacking gel, for 2 h under 15 mA of constant current, with a Tris-glycine buffer system (9) in a model SE 260 Mighty Small II vertical electrophoresis unit (Hoefer/Pharmacia, Piscataway, N.J.). After electrophoresis, the gel was removed, fixed for 15 min in isopropanol-acetic acid fixing solution, and then stained with Coomassie brilliant blue R-250 for 2 to 3 h with gentle shaking. The gel was destained in a methanol-acetic acid-water solution, analyzed, and photographed. Titrations done with ch14.18-IL-2 indicated that this method can detect concentrations as low as 250 ng of protein per lane (data not shown).
Western blotting.
For the specific evaluation of the fusion protein components, a semidry blotting system was used. After electrophoresis, as described above, the gel was washed for 5 min in a transfer buffer (Tris-glycine-methanol) (25), placed on an Immobilon-P polyvinylidene difluoride transfer membrane (Millipore, Bedford, Mass.), saturated with methanol, and transferred onto five layers of 3MM filter paper (Whatman, Maidstone, England) saturated with the transfer buffer. The gel was covered with another five layers of filter paper saturated with the transfer buffer, and transfer was carried out for 1.5 h under 200 mA of constant current in the Multiphor II NovaBlot unit (Pharmacia/LKB). After transfer the gel was removed from the transfer membrane, the membrane was washed three times in Tris-buffered saline (TBS) buffer (100 mM Tris-HCl [pH 7.5], 0.9% NaCl), and blocked overnight in 5% nonfat dry milk in TBS at 4°C on a rocking platform. The next day the blotting membrane was washed for 15 min with TBS and then incubated with shaking for 3 h at 25°C with sheep anti-human IgG1 antibody coupled to horseradish peroxidase (The Binding Site, Birmingham, England), diluted to a concentration of 2 μg/ml in TBS–0.05% Tween 20, to immunoprobe the IgG1 component of the fusion protein. After incubation, the blot was washed three times for 5 min with TBS and visualized with Super Signal chemiluminescent substrate (Pierce, Rockford, Ill.) for 5 min at room temperature (RT). Excess substrate was removed, and the blot was wrapped with plastic wrap and placed on New RX GCU X-ray film (Fuji Photo Film Co., Tokyo, Japan) for 30 s. The films were developed in an automatic developer, analyzed for the presence of specific bands, and then photographed. By this method we could visualize as little as 62 ng of specific protein per lane, based on our titration experiments performed with the purified ch14.18-IL-2 fusion protein (data not shown).
ELISA procedures. (i) 1A7-IL-2 ELISA (fusion protein-specific ELISA).
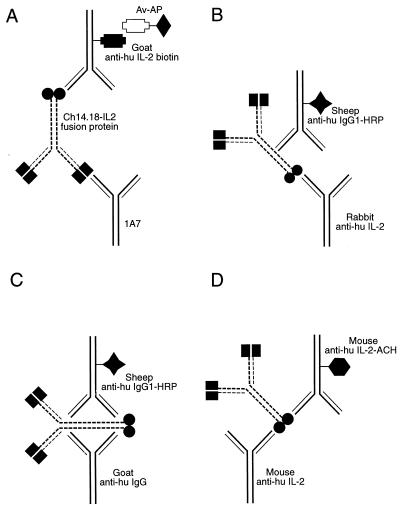
C8 Maxisorp Nunc Immunomodules (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 120 μl of 1A7, a mouse monoclonal anti-idiotype to 14.G2a antibody, per well at a concentration of 2 μg/ml in 15 mM carbonate buffer, pH 9.6 (Fig. 1A). After being washed three times with PBS-Tween, the wells were blocked for 3 h at RT with 200 μl of 5% nonfat dried milk dissolved in PBS per well. After being washed further with PBS-Tween, plates were kept at 4°C in PBS-Tween until use. To analyze samples, PBS-Tween was removed and 100 μl of standards or experimental samples prepared in sample buffer (PBS-Tween plus 2% milk) per well was added, and the plates were incubated in a moist chamber overnight at 4°C. Plates were then washed five times with PBS-Tween, followed by the addition of 100 μl of goat anti-human IL-2 antibody coupled to biotin (R & D Systems, Minneapolis, Minn.), diluted to a concentration of 0.035 μg/ml in 0.1 M Tris-HCl containing 0.05% Tween 20. Plates were then incubated in a humidified chamber for 3 h at RT and washed five times with Tris-Tween, and ExtrAvidin-alkaline phosphatase (Sigma, St. Louis, Mo.) diluted 1:5,000 in Tris-Tween (according to the manufacturer’s specification) was added. After a 1-h incubation at RT, samples were washed five times with Tris-Tween. Staining was performed by adding 100 μl of p-nitrophenyl phosphate (Sigma) as a substrate at a concentration of 1 mg/ml in diethanolamine buffer. The sample was incubated with the substrate at RT in the dark, and the conversion to the colored product was measured at 30, 60, and 75 minutes, at an OD of 405 nm with a 492-nm reference wavelength (OD405/492) on a model EAR 400 AT ELISA reader (SLT, Salzburg, Austria) equipped with SOFT-2000 software. The ODs obtained were used to determine concentrations of the intact fusion protein. A standard curve ranging from 0 to 115 ng/ml was generated by using known concentrations of the ch14.18-IL-2 fusion protein (lot number 1).
FIG. 1.
ELISAs used to evaluate structures of immunoglobulin-cytokine fusion proteins. In each panel the ch14.18-IL-2 fusion protein is represented by the immunoglobulin structure with its paired idiotype (black boxes) at the antibody binding domains and a human IL-2 (black circles) attached to the carboxy terminus of the human immunoglobulin heavy chains. (A) 1A7-IL-2 assay uses a mouse monoclonal anti-idiotype antibody, 1A7 (which recognizes the idiotype component of the ch14.18 Mab), to coat the plate. After the addition of standard dilutions (or unknown quantities) of the ch14.18-IL-2 fusion protein and washing of the plate, the reporter antibody, biotinylated goat anti-human IL-2, is added. This assay can specifically detect the intact fusion protein in human or mouse serum. Neither purified ch14.18 nor purified IL-2 is detectable in this assay. Av-AP, avidin coupled to alkaline phosphatase. (B) IL-2-IgG1 ELISA uses plates coated with rabbit antibody to human IL-2. The reporter antibody is a sheep anti-human IgG1 conjugated with horseradish peroxidase (HRP). (C) IgG1 ELISA uses a goat anti-human IgG to coat the plate. The reporter antibody is a sheep anti-human IgG1 conjugated with horseradish peroxidase (HRP). This ELISA quantitates the total IgG1 present, including free IgG1 as well as the IgG1 immunoglobulin component of the fusion protein. (D) IL-2 ELISA uses a mouse monoclonal anti-human IL-2 capture antibody and a mouse monoclonal anti-human IL-2 antibody conjugated with acetylcholinesterase (ACH) as the reporter antibody. Both free IL-2 and the IL-2 component of the ch14.18-IL-2 fusion protein can be detected with this assay.
(ii) IL-2-IgG1 ELISA (fusion protein-specific ELISA).
C8 Maxisorp Nunc Immunomodules (Nunc) were coated overnight at 4°C with 120 μl of rabbit anti-human IL-2 antibody (Endogen, Woburn, Mass.) per well at a concentration of 2 μg/ml in 15 mM carbonate buffer, pH 9.6 (Fig. 1B). After being washed three times with PBS-Tween, the wells were blocked for 3 h at RT with 200 μl of 5% nonfat dried milk dissolved in PBS per well. The plates were washed with PBS-Tween and kept filled with buffer at 4°C until use. For the assay, 100 μl of standards (dilutions of ch14.18-IL-2 lot number 1) or samples was added to each well and incubated overnight at 4°C in a humidified chamber. Following incubation, plates were washed five times with 0.1 M Tris-Tween, and 100 μl of sheep anti-human IgG1 antibody coupled to horseradish peroxidase (0.4 μg/ml) per well (The Binding Site) was added. Incubation was done for 3 h at RT, plates were then washed five times, and tetramethyl benzidine substrate (DAKO, Carpinteria, Calif.) was added (100 μl/well, as provided by the company). After incubation with shaking for 30 min at RT, the reaction was stopped with 50 μl of 2 N H2SO4 per well, and plates were read at 450 nm with a 570-nm reference wavelength with a model EAR 400 AT ELISA reader (SLT), equipped with SOFT-2000 software for analysis.
(iii) IgG1 ELISA.
C8 Maxisorp Nunc Immunomodule microtiter wells (Nunc) were coated overnight at 4°C with 150 μl of goat anti-human IgG antibody (Southern Biotech Associates, Birmingham, Ala.) per well at a concentration of 2 μg/ml in 15 mM carbonate buffer, pH 9.6 (Fig. 1C). After being washed three times with PBS containing 0.05% Tween 20, the wells were blocked for at least 1 h at RT with 5% nonfat dried milk dissolved in PBS. The plates were washed with PBS-Tween and kept at 4°C in PBS-Tween until use. To analyze samples, PBS-Tween was removed, 100 μl of standards or samples was added to the wells, and the samples were incubated overnight (18 h) at 4°C in a humidified chamber. The wells were then washed five times with 0.1 M Tris-Tween. Sheep anti-human IgG1 antibody coupled to horseradish peroxidase (100 μl/well) (The Binding Site) was added at a concentration of 0.4 μg/ml, and the plates were incubated for 3 h at 25°C. Then the plates were washed five times before the addition (100 μl/well) of tetramethyl benzidine substrate (DAKO). After incubation with shaking for 30 min at RT, 50 μl of a stop solution (2 N H2SO4) per well was added, and the plates were read at 450 nm with a 570-nm reference wavelength in a model EAR 400 AT ELISA reader (SLT), equipped with SOFT-2000 software for analysis. Chimeric antibody ch14.18 from a stock at a concentration of 5.34 mg/ml was used as a standard in this assay.
(iv) IL-2 ELISA.
The commercially available human IL-2 ELISA kit (Immunotech) was used. The assay was performed according to the specifications in the manufacturer’s manual. This kit was calibrated against the World Health Organization international standard for IL-2 and contained microtiter plates coated with a mouse monoclonal anti-human IL-2 antibody. Monoclonal anti-human IL-2 linked to acetylcholinesterase was used as the reporter antibody in this assay (Fig. 1D). Bound enzymatic activity was measured after the addition of a chromogenic substrate provided in the kit.
(v) A.I. 49-3-IL-2 ELISA.
The A.I. 49-3-IL-2 assay was prepared as described above for the 1A7-IL-2 ELISA (Fig. 1A), except that A.I. 49-3, a Mab against the idiotype of the CC49 Mab, was used to coat the wells instead of the 1A7 antibody (schema not shown). The remaining steps of the assay protocol were exactly the same as those followed for the 1A7-IL-2 ELISA (Fig. 1A).
Mouse and human sera.
Pooled mouse serum was obtained from Sigma and stored at 4°C. After informed consent had been obtained, human serum was obtained from nine healthy volunteer donors, pooled, and stored at 4°C.
RESULTS
Purity of the ch14.18-IL-2 fusion protein. (i) SDS-PAGE.
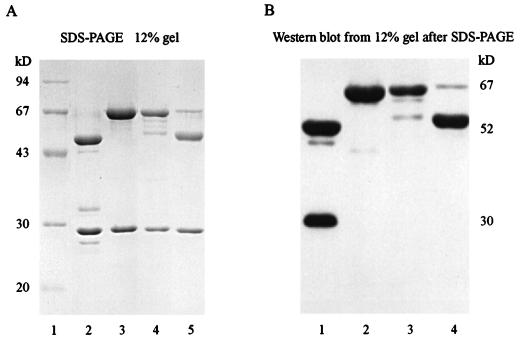
To define the standards used in the 1A7-IL-2 and IL-2-IgG1 ELISA systems, the purities of the different ch14.18-IL-2 preparations were assessed by SDS-PAGE. Three separate batches of ch14.18-IL-2 (lots 1 and 31403 and an aliquot from a sample which was maintained in the presence of fetal calf serum) were evaluated by electrophoresis under reducing conditions with purified ch14.18 and molecular size markers as standards (Fig. 2A). The protein bands located below 30 kDa correspond to the antibody light chains (Fig. 2A, lanes 2 to 5); bands visible at 67 kDa represent the heavy chains of the ch14.18 antibody attached to IL-2 (lanes 3 and 4 and traces in lane 5). The 50-kDa band corresponds to the heavy chain of the chimeric antibody (Fig. 2A, lanes 2 and 5). The lot 1 preparation of ch14.18-IL-2 (Fig. 2A, lane 3) had greater purity of intact ch14.18 heavy chains linked to IL-2 than that of lot 31403 (lane 4). The extra band noted in the lot 31403 sample (50 kDa) is consistent with a fraction of the heavy chains in this preparation lacking a complete IL-2 molecule. The immunoglobulin light chain (25 kDa) appears similar in each of the different lots of ch14.18-IL-2 fusion protein and in the ch14.18 antibody.
FIG. 2.
(A) Evaluation of the purities of different ch14.18-IL-2 fusion protein preparations by SDS-PAGE. Lane 1, molecular size markers (94, 67, 43, 30, and 20 kDa); lane 2, purified ch14.18; lane 3, ch14.18-IL-2 (lot 1); lane 4, ch14.18-IL-2 (lot 31403); lane 5, lower-molecular-weight derivative of the ch14.18-IL-2 fusion protein (an aliquot from a partially purified batch of ch14.18-IL-2 that had been obtained from a cell line which was maintained in the presence of fetal calf serum). (B) Western blot analysis with anti-human IgG1 antibody conjugated with horseradish peroxidase. Lane 1, purified ch14.18; lane 2, ch14.18-IL-2 (lot 1); lane 3, ch14.18-IL-2 (lot 31403); lane 4, lower-molecular-weight derivative of the ch14.18-IL-2 fusion protein.
(ii) Western blot analysis.
The components of the fusion protein detected by SDS-PAGE were further defined by Western blot analysis (Fig. 2B). Recognition of the heavy chains by anti-human IgG1-horseradish peroxidase indicates that the 67-kDa bands noted in the ch14.18-IL-2 samples contain human immunoglobulin (Fig. 2B, lanes 2 and 3 and a faint band in lane 4). These correspond to the IgG heavy chain linked to IL-2. Bands visible in the 50-kDa area correspond to the heavy chain of the chimeric antibody (Fig. 2B, lanes 1 and 4). The band visible in the 30-kDa area (Fig. 2B, lane 1) represents reactivity against a smaller-molecular-size derivative of the heavy chain seen only in the ch14.18 stock preparation and not in the ch14.18-IL-2 or KS1/4-IL-2 fusion protein preparations or in the human IgG1 or mouse IgG1 controls, and it is distinct from the immunoglobulin light chain (data not shown).
Standardization and performance characteristics of the 1A7-IL-2 ELISA which quantitates intact fusion protein. (i) Standard curves.
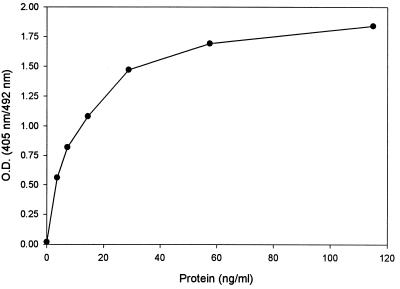
Figure 3 shows the typical standard curve obtained by using the lot 1 preparation of ch14.18-IL-2 as a standard for the 1A7-IL-2 ELISA. The minimal detection limit in this assay was calculated based on the mean ODs plus 2 standard deviations for readings done on samples containing the buffer and 0 ng of fusion protein per ml. This OD was then compared to the ODs obtained from the mean of nine separate replicate curves (Fig. 3). The background level of absorbance remained low in the buffer (PBS-Tween-milk) (OD405/492 = 0.02). The calculated minimal detection limit was thus found to be 0.26 ng/ml.
FIG. 3.
Standard curve from the 1A7-IL-2 ELISA. Serial dilutions of ch14.18-IL-2 (lot 1) in PBS-Tween buffer were evaluated in several replicate quantitations with this ELISA system. OD readings were determined at 405/492 nm. The graph demonstrates a representative curve from a typical assay.
(ii) Intra-assay precision (precision within an assay).
Three samples of fusion protein at concentrations in the low, intermediate, and high ranges of the standard curve, dissolved in sample buffer, were assayed 20 times on one plate to assess intra-assay precision (Table 1).
TABLE 1.
Intra-assay precision of the 1A7-IL-2 ELISAa
| Measurement | Result for sample
|
||
|---|---|---|---|
| 1 | 2 | 3 | |
| No. of determinations | 20 | 20 | 20 |
| Mean concn (ng/ml) | 14.4 | 62.9 | 91.8 |
| CV (%) | 5.6 | 6.3 | 6.2 |
Three samples of known concentrations of the fusion protein were assayed 20 times on one plate. The obtained mean concentration was calculated as well as the coefficient of variation (defined as the standard deviation/mean).
(iii) Interassay precision (precision between assays).
Seven different samples of fusion protein were assayed in duplicate in nine separate runs to assess interassay precision (Table 2). The concentrations of ch14.18-IL-2 (lot 1), used to generate the standard curve for these ELISAs, were in the range of 0 to 115.0 ng/ml, based on an OD280 reading indicating the total protein concentration. As determined by a densitometer evaluation of the gel after SDS-PAGE (Fig. 1), the lot 1 protein is >95% pure. The mean concentrations of ch14.18-IL-2 measured in nine replicate experiments with the 1A7-IL-2 ELISA are listed along with the calculated coefficients of variation (CV) (standard deviation/mean). These assays demonstrate the reliability of the 1A7-IL-2 ELISA in quantifying the ch14.18-IL-2 fusion protein at concentrations ranging from 0.26 to 115 ng/ml.
TABLE 2.
Interassay variation of the 1A7-IL-2 ELISAa
| Concn of ch14.18-IL-2 (ng/ml) | ELISA result for ch14.18-IL-2
|
||
|---|---|---|---|
| No. of samples | Mean concn (ng/ml) | CV (%) | |
| 115.0 | 9 | 113.9 | 5.4 |
| 57.5 | 9 | 60.2 | 7.3 |
| 28.8 | 9 | 27.9 | 6.5 |
| 14.4 | 9 | 14.6 | 3.5 |
| 7.2 | 9 | 7.3 | 4.2 |
| 3.6 | 9 | 3.6 | 9.9 |
| 0 | 9 | 0 | 0 |
The ch14.18-IL-2 fusion protein (lot 1) was serially diluted in a sample buffer at concentrations from 3.6 to 115.0 mg/ml, shown in the left column. The concentration of ch14.18-IL-2 was then quantitated by using the ELISA described in Fig. 1A.
(iv) Effects of serum.
In order to examine the usefulness of this method for measuring levels of intact fusion protein in serum, aliquots of the ch14.18-IL-2 (lot 1) placed in either mouse serum, human serum, or PBS-Tween-milk buffer were evaluated in the 1A7-IL-2 ELISA. Table 3 lists the background OD readings obtained with mouse serum or buffer, as well as the OD readings resulting from the presence of intact fusion protein in undiluted serum or buffer. These OD readings indicated that equivalent concentrations of fusion protein were detectable in buffer and mouse serum, with no effect of mouse serum on background OD readings. Undiluted human serum (Table 3) increased the background OD reading. Dilutions of the human serum of 1:2 or greater eliminated this nonspecific color development, enhancing the specificity of the OD reading. These data indicate that the 1A7-IL-2 system is useful for the quantitation of the ch14.18-IL-2 fusion protein in buffer, mouse serum, and human serum.
TABLE 3.
1A7-IL-2 ELISA of ch14.18-IL-2 in serum relative to backgrounda
| Buffer or serum | Mean OD405 for
1A7-IL-2 ELISA
|
|
|---|---|---|
| Without protein | With protein | |
| PBS-Tween-milk | 0.002 | 1.674 |
| Mouse serum (100%) | −0.005 | 1.372 |
| Human serum (%) | ||
| 100 | 0.242 | 0.997 |
| 50 | −0.012 | 1.495 |
| 10 | −0.009 | 1.673 |
| 2 | −0.006 | 1.732 |
| 0.5 | −0.002 | 1.759 |
| 0.1 | 0.001 | 1.587 |
ch14.18-IL-2 fusion protein (lot 1) was added to PBS-Tween-milk, undiluted pooled mouse serum, or various dilutions of human serum and compared to buffer or serum alone as evaluated in the 1A7-IL-2 ELISA. Values listed indicate the relative OD405/492 readings obtained. Data presented are the means of duplicate samples.
(v) Recovery (dilution test).
Levels of fusion protein were spiked in three different matrices (sample buffer, 50% human serum, and 50% mouse serum), and the protein was serially diluted to create three sets of four samples throughout the range of the assay for the evaluation of recovery in the 1A7-IL-2 ELISA (Table 4).
TABLE 4.
Dilution test and recovery calculations for the 1A7-IL-2 ELISAa
| Sample | Dilution factor | Observed FP concn (ng/ml) | Expected FP concn (ng/ml) | Recovery (%) |
|---|---|---|---|---|
| A | Undiluted | 100.0 | ||
| 1/2 | 55.4 | 50.0 | 110.8 | |
| 1/4 | 31.9 | 25.0 | 127.6 | |
| 1/8 | 11.5 | 12.5 | 92.0 | |
| B | Undiluted | 83.2 | ||
| 1/2 | 47.6 | 41.6 | 114.4 | |
| 1/4 | 24.1 | 20.8 | 115.9 | |
| 1/8 | 11.9 | 10.4 | 114.4 | |
| C | Undiluted | 73.3 | ||
| 1/2 | 41.9 | 36.7 | 114.2 | |
| 1/4 | 21.9 | 18.4 | 119.0 | |
| 1/8 | 11.2 | 9.2 | 121.7 |
Fusion protein (FP) levels spiked in sample buffer (A), 50% human serum (B), and 50% mouse serum (C) and its serial dilutions were assayed. Recovery was calculated as the ratio of values obtained by ELISA measurements to concentrations expected from the dilutions of primary samples. Every sample was assayed in duplicate.
Validity of comparative quantitation.
A known concentration of ch14.18-IL-2 fusion protein (lot 31403) was tested in the 1A7-IL-2 (Fig. 1A), IgG (Fig. 1C), and IL-2 (Fig. 1D) ELISAs, to determine if quantitative comparisons between these three assays are valid (Table 5). The expected concentration, based on the known amount and molecular weight of the fusion protein and the proportion of its IgG1 and IL-2 components, was calculated for each ELISA. The observed mean was determined in each assay. These data indicate that a quantitative comparison of the concentrations of the ch14.18-IL-2 fusion protein is possible with these three assays. The sensitivity of the IL-2 ELISA to detect the intact fusion protein was lower than that of the other two assays, with the IL-2 ELISA detecting only 76% of the expected product.
TABLE 5.
Detection of ch14.18-IL-2 and its components within the intact molecule by various ELISAsa
| ELISA for: | Concn (ng/ml)
|
% Expected | |
|---|---|---|---|
| Expected | Observed | ||
| 1A7-IL-2 | 7,500 | 6,882 | 92 |
| IgG | 6,250 | 5,673 | 91 |
| IL-2 | 1,250 | 949 | 76 |
Three separate experiments were performed with the ch14.18-IL-2 fusion protein (lot 31403) diluted in a sample buffer and tested with all three ELISA methods. The stock fusion protein concentration was standardized by using the IgG1 ELISA. This was diluted with PBS-Tween-milk so that 7,500 ng of fusion protein per ml was tested in the assay. The expected concentrations of IL-2 and ch14.18 components of the fusion protein were based on the calculation that the IL-2 component is one-sixth and the antibody component is five-sixths of the mass of the fusion protein. Observed data are presented as the means of three experiments. Standard deviations are 508 ng/ml for the 1A7-IL-2 ELISA, 32 ng/ml for the IgG ELISA, and 90 ng/ml for the IL-2 ELISA. Percent expected was calculated as the observed concentration/expected concentration × 100.
Specificity of the intact fusion protein ELISAs.
The specificities of the IL-2-IgG1 ELISA (Fig. 1B) and 1A7-IL-2 ELISA (Fig. 1A) were tested. The IgG1 ELISA was used to verify the concentrations of Mabs ch14.18 and CC49 and those of their respective fusion proteins, ch14.18-IL-2 and CC49-IL-2. The IL-2 ELISA with recombinant human IL-2 as a standard was used to detect IL-2, the IL-2 portion of the ch14.18-IL-2 fusion protein, and the IL-2 portion of the CC49-IL-2 fusion protein (Table 6). Samples were diluted in PBS-Tween-milk to fall into the effective range of the standard curves.
TABLE 6.
Specificities of fusion protein detection for various ELISAsa
| ELISA detection system | Result for:
|
||||||
|---|---|---|---|---|---|---|---|
| ch14.18 | ch14.18-IL-2 | CC49 | CC49-IL-2 | IL-2 | KS1/4-IL-2 | hu14.18-IL-2 | |
| 1A7-IL-2 | − | + | − | − | − | − | + |
| IL-2-IgG1 | − | + | − | + | − | + | + |
| IgG1 | + | + | + | + | − | + | + |
| IL-2 | − | + | − | + | + | + | + |
| A.I. 49-3-IL-2 | − | − | − | + | − | − | − |
Purified immunoglobulin (ch14.18 or CC49), purified IL-2, or immunoglobulin-cytokine fusion proteins (ch14.18-IL-2, CC49-IL-2, KS1/4-IL-2, or hu14.18-IL-2) were compared. +, protein was detected, at the expected level, in that assay system; −, samples were below detection limits. Each result represents two to three replicate experiments.
As shown in Table 6, the IL-2-IgG1 ELISA, the 1A7-IL-2 ELISA, and the A.I. 49-3-IL-2 ELISA each measured only intact fusion protein; free IL-2, free ch14.18, and free CC49 were not detectable in these ELISAs. The anti-GD2 fusion proteins, ch14.18-IL-2 and hu14.18-IL-2, were both recognized in the 1A7-IL-2 ELISA, while all the fusion proteins tested were detectable in the IL-2-IgG1 ELISA. As expected, only the anti-TAG 72 fusion protein (CC49-IL-2) was detected in the A.I. 49-3-IL-2 ELISA. The chimeric IgG1 antibodies, ch14.18 and CC49, were also detectable, as expected, in the IgG1 ELISA. Thus, the IgG1 ELISA is one of the least selective ELISAs evaluated in this study for detecting the intact fusion protein, as any protein with a human IgG1 component can be specifically detected. This IgG ELISA could potentially overestimate the concentration of intact fusion protein if a sample believed to contain the intact fusion protein also contained the free immunoglobulin component of the fusion protein lacking the cytokine. Intact fusion proteins can be quantitated without the confounding influence of free immunoglobulin or free IL-2 by using the IL-2-IgG1 ELISA system, thus providing a level of selectivity. As shown in Table 6, the use of anti-idiotype antibodies in these assays (i.e., 1A7-IL-2 and A.I. 49-3-IL-2 systems) can further improve the specificity in quantitating these antibody-IL-2 fusion proteins.
Competition.
While the above data demonstrate the ability of these ELISAs to discriminate between the different proteins when they are assayed separately, the quantitation of fusion protein may be necessary when fusion proteins are mixed with other immunoglobulins (as in human serum) or with components of the fusion protein. Furthermore, potential therapies with these fusion proteins might involve the coadministration of fusion protein with soluble IL-2 or with the Mab itself. Thus, it is desirable to be able to quantitate the fusion protein even in the presence of free IL-2 or Mab. Competition experiments were performed to determine the range in which the intact fusion protein could accurately be assessed, based on the total amount of protein added per well and the proportion of intact ch14.18-IL-2 fusion protein in each well. When a concentration of 100 ng of protein per ml was used, decreasing concentrations of the fusion protein could be accurately detected in the presence of increasing concentrations of ch14.18 antibody (Table 7). Mixing 100 ng of ch14.18-IL-2 per ml with ch14.18 to increase the total protein concentration to either 140, 180, or 420 ng/ml results in an increase in the ch14.18/ch14.18-IL-2 ratio (Table 7). This causes a confounding influence on the accuracy of the detection of the ch14.18-IL-2 fusion protein. This likely reflects the partial saturation of the bound 1A7 molecules on the plate when more than 100 ng of ch14.18 or ch14.18-IL-2 per ml is added. This indicates the importance of limiting the immunoglobulin concentration to less than 100 ng/ml, to avoid exceeding the capacity of the system. Similar experiments were performed to test the influence of free IL-2 on the quantitation of ch14.18-IL-2 in the 1A7-IL-2 ELISA. Increasing concentrations of IL-2 (7.5, 15.0, and 60.0 ng/ml) were mixed with 80 ng of ch14.18-IL-2 per ml which contained 13.3 ng of IL-2 per ml as part of the fusion protein (lot 31403) and evaluated in the 1A7-IL-2 ELISA. In this case, there was no influence of free IL-2 on the assay (data not shown).
TABLE 7.
Competition of ch14.18 and ch14.18-IL-2 fusion protein in the 1A7-IL-2 ELISAa
| Total concn (ng/ml) | Concn
(ng/ml) of:
|
Concn of ch14.18-IL-2 detected by ELISA (ng/ml) | |
|---|---|---|---|
| ch14.18-IL-2 | ch14.18 | ||
| 100 | 100 | 0 | 98 |
| 100 | 90 | 10 | 92 |
| 100 | 80 | 20 | 81 |
| 100 | 50 | 50 | 47 |
| 100 | 20 | 80 | 22 |
| 100 | 10 | 90 | 9 |
| 100 | 5 | 95 | 5 |
| 100 | 0 | 100 | 0 |
| 140 | 100 | 40 | 77 |
| 180 | 100 | 80 | 46 |
| 420 | 100 | 320 | 28 |
ch14.18-IL-2 (lot 1) (100 ng/ml) was serially diluted in a buffer. ch14.18 was added to achieve a total concentration of 100 ng of protein per ml in each sample. Data are presented in nanograms of total fusion protein per milliliter in each sample. Then ch14.18-IL-2 (lot 1) (100 ng/ml) was mixed with increasing concentrations of ch14.18, so that the total amount of protein per well was increased. The mixture was run on the 1A7-IL-2 ELISA. Data are shown as concentrations of intact fusion protein and represent the means of two separate experiments.
DISCUSSION
As other immunoglobulin-derived fusion proteins are entering preclinical and clinical testing, assays are needed to study the pharmacokinetics of these proteins. Our study demonstrates the sensitivities, specificities, and reliabilities of ELISAs that require the corecognition of each component of the fusion protein (ch14.18-IL-2 and CC49-IL-2). The concentration of intact fusion protein can be assessed with greater accuracy and specificity by using the IL-2-IgG1 ELISA than by using the other usual methods of protein quantitation. Our analyses of some partially purified preparations (Fig. 2) and evaluation of plasmin-induced cleavage products (3) indicate that some of these fusion proteins may be subject to degradation that could influence fusion protein function. In order to assess the possibility of in vivo degradation in preclinical (murine or primate) and clinical testing, accurate assays to detect the intact fusion protein in serum are essential. Also, since the adequate dilution of test samples (Table 7) can prevent free ch14.18 and free IL-2 from affecting the ability of the 1A7-IL-2 ELISA to quantitate the intact fusion protein, these ELISAs may also provide simple, reliable methods for evaluating the concentrations of intact antibody-cytokine fusion proteins, even in the presence of some of the degradative products potentially expected to occur in vivo.
The use of ELISAs to measure either antibodies or cytokines from patients receiving biologic therapies has been well documented (7, 24). The unique structure of fusion proteins, such as the ch14.18-IL-2 fusion protein, allows us to use antibodies directed against each component of the fusion protein in a sandwich ELISA to detect only the intact fusion protein. The incorporation of the anti-idiotype antibody in the sandwich ELISA allows us to specifically detect the ch14.18-IL-2 fusion protein which still retains its idiotypic determinant. Several advantages of this method of analysis include (i) the specificity with which one can measure the intact protein in both buffer and serum, (ii) the simplicity with which one can reliably test samples of relatively small volumes, and (iii) the extrapolation of this system to similar genetically engineered proteins. Both the IL-2-IgG1 ELISA and the 1A7-IL-2 ELISA are useful in quantitating the intact fusion protein; however, the 1A7-IL-2 ELISA is particularly useful in testing for proteins in human serum because of its enhanced specificity and low background, thus providing an assay for future clinical trial use. The separate assays allowing the detection of total IL-2 (IL-2 ELISA) and total ch14.18 antibody (IgG ELISA) are also very useful. By subtracting the values obtained in the 1A7-IL-2 ELISA from the data obtained in these two assays, it is possible to calculate the levels of free IL-2 and free ch14.18 antibody (data not shown). A comparison of the concentration of the intact fusion protein with that of its components can provide a reliable means of evaluating the clearance and degradation of the intact fusion protein, even in the presence of some degraded products potentially expected to occur in vivo. Other similarly constructed fusion proteins can also be evaluated by modifications of these methods. When an anti-idiotype antibody is not available, antibody-cytokine fusion proteins can be quantitated with the IL-2-IgG ELISA.
One of the potentially confounding variables in these assays is the effect of the amount of total ch14.18 protein per well on the sensitivity of the 1A7-IL-2 assay. Our data suggest that the saturation of 1A7 (capture antibody) occurs in this system at a concentration of ≥100 ng of ch14.18 antibody per ml. Thus, when the total concentration of ch14.18 and ch14.18-IL-2 exceeds 100 ng/ml, the ch14.18 molecules can interfere with the accurate detection of ch14.18-IL-2. Samples containing a mixture of ch14.18 antibody and ch14.18-IL-2 fusion protein need to be diluted to a concentration of <100 ng of total ch14.18 per ml to prevent an underestimation of the ch14.18-IL-2 fusion protein concentration.
The assays developed here provide an accurate means for the specific measurement of intact fusion proteins by simultaneously detecting both components of the fusion protein molecule. These studies have focused on the detection of antibody-cytokine fusion proteins, particularly the ch14.18-IL-2 immunocytokine, the humanized form of which has just entered clinical testing (5a). Slight modifications of the ELISAs presented here should allow the specific detection of any intact immunocytokine, by requiring the simultaneous corecognition of the fusion protein’s antibody idiotype and its cytokine. As new genetically engineered fusion proteins are developed for clinical testing, their in vitro and in vivo evaluations should be facilitated by adaptations of these methods, allowing the accurate quantitation of the intact molecules and distinguishing them from their components.
ACKNOWLEDGMENTS
We acknowledge the kind provision of reagents by Toby Hecht and Jeff Schlom, NCI; Ken Foon and Malaya Chatterjee, University of Kentucky; and Titan Pharmaceuticals, Inc. We also thank Ralph Reisfeld (of The Scripps Research Institute), Mark Albertini and Charles Nicolet (University of Wisconsin—Madison), and Richard Hong (University of Vermont—Burlington) for helpful discussions.
This research was supported by grants RPG-82-001-16 of the American Cancer Society and grants CA-332685-15, CA-14520-25, CA-68334-02, CA-0961-08, and CA-0961-09 of the National Cancer Institute.
J. Gan and K. Kendra contributed equally to this work.
REFERENCES
- 1.Baker D, Butler D, Scallon B J, O’Neill J K, Turk J L, Feldmann M. Control of established experimental allergic encephalomyelitis by inhibition of tumor necrosis factor (TNF) activity within the central nervous system using monoclonal antibodies and TNF receptor-immunoglobulin fusion proteins. Eur J Immunol. 1994;24:2040–2048. doi: 10.1002/eji.1830240916. [DOI] [PubMed] [Google Scholar]
- 2.Bogers W M, Lang F, Parker K E, LeMauff B, Anegon I, Jacques Y, Soulillou J P. Rat interleukin-2 immunoglobulin M fusion proteins are cytotoxic in vitro for cells expressing the IL-2 receptor and can abolish cell-mediated immunity in vivo. Transplantation. 1994;58:932–939. doi: 10.1097/00007890-199410270-00013. [DOI] [PubMed] [Google Scholar]
- 3.Gillies S D, Reilly E B, Lo K M, Reisfeld R A. Antibody-targeted interleukin 2 stimulates T-cell killing of autologous tumor cells. Proc Natl Acad Sci USA. 1992;89:1428–1432. doi: 10.1073/pnas.89.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillies S D, Young D, Lo K, Roberts S. Biological activity and in vivo clearance of antitumor antibody/cytokine fusion proteins. Bioconjug Chem. 1993;4:230–235. doi: 10.1021/bc00021a008. [DOI] [PubMed] [Google Scholar]
- 5.Hand P H, Kashmiri S V S, Schlom J. Potential for recombinant immunoglobulin constructs in the management of carcinoma. Cancer. 1994;73(Suppl.):1005–1113. doi: 10.1002/1097-0142(19940201)73:3+<1105::aid-cncr2820731351>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5a.Handgretinger, R. (Tübingen, Germany). Personal communication.
- 6.Hu P, Hornick J L, Glasky M S, Yun A, Milkie M N, Khawli L A, Anderson P M, Epstein A L. A chimeric Lym-1/interleukin 2 fusion protein for increasing tumor vascular permeability and enhancing antibody uptake. Cancer Res. 1996;56:4998–5004. [PubMed] [Google Scholar]
- 7.Ida N, Sakurai S, Hosoi K, Kunitomo T. A highly sensitive enzyme-linked immunosorbent assay for the measurement of interleukin-8 in biological fluids. J Immunol Methods. 1992;156:27–38. doi: 10.1016/0022-1759(92)90007-g. [DOI] [PubMed] [Google Scholar]
- 8.Jabara H H, Vercelli D, Schneider L C, Williams D P, Genbauffe F S, Poisson L R, Waters C A, Geha R S. Interleukin-4 receptor expression by human B cells: functional analysis with a human interleukin-4 toxin, DAB389IL-4. J Allergy Clin Immunol. 1995;95:893–900. doi: 10.1016/s0091-6749(95)70134-6. [DOI] [PubMed] [Google Scholar]
- 9.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 10.Lode H N, Xiang R, Varki N M, Dolman C S, Gillies S D, Reisfeld R A. Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J Natl Cancer Inst. 1997;89:1586–1594. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
- 11.Milenic D E, Yokota T, Filpula D R, Finkelman M A J, Dodd S W, Wood J F, Whitlow M, Snoy P, Schlom J. Construction, binding properties, metabolism and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Res. 1991;51:6363–6371. [PubMed] [Google Scholar]
- 12.Morrison S L, Wims L A, Oi V T. Genetically engineered antibody molecules: new tools for cancer therapy. Cancer Investig. 1988;6:185–192. doi: 10.3109/07357908809077046. [DOI] [PubMed] [Google Scholar]
- 13.Naramura M, Gillies S D, Mendelsohn J, Reisfeld R A, Mueller B M. Mechanisms of cellular cytotoxicity mediated by a recombinant antibody-IL2 fusion protein against human melanoma cells. Immunol Lett. 1994;39:91–99. doi: 10.1016/0165-2478(93)90169-3. [DOI] [PubMed] [Google Scholar]
- 14.Nicolet C M, Burkholder J K, Gan J, Culp J, Kashmiri S V S, Schlom J, Yang N-S, Sondel P M. Expression of a tumor-reactive antibody-interleukin 2 fusion protein after in vivo particle-mediated gene delivery. Cancer Gene Ther. 1995;2:161–170. [PubMed] [Google Scholar]
- 15.Pancook J D, Becker J C, Gillies S D, Reisfeld R A. Eradication of established hepatic human neuroblastoma metastases in mice with severe combined immunodeficiency by antibody-targeted interleukin-2. Cancer Immunol Immunother. 1996;42:88–92. doi: 10.1007/s002620050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reisfeld R A, Gillies S D. Antibody-interleukin 2 fusion proteins: a new approach to cancer therapy. J Clin Lab Anal. 1996;10:160–166. doi: 10.1002/(SICI)1098-2825(1996)10:3<160::AID-JCLA9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 17.Sabzevari H, Gillies S D, Mueller B M, Pancook J D, Reisfeld R A. A recombinant antibody-interleukin 2 fusion protein suppresses growth of hepatic human neuroblastoma metastases in severe combined immunodeficiency mice. Proc Natl Acad Sci USA. 1994;91:9626–9630. doi: 10.1073/pnas.91.20.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saleh M N, Khazaeli M B, Wheeler R H, Bucy R P, Liu T, Everson M P, Munn D H, Schlom J, LoBuglio A F. Phase II trial of murine monoclonal antibody D612 combined with recombinant human monocyte colony-stimulating factor (rhM-CSF) in patients with metastatic gastrointestinal cancer. Cancer Res. 1995;55:4339–4346. [PubMed] [Google Scholar]
- 19.Savage P, So A, Spooner R A, Epentos A A. A recombinant single chain antibody interleukin-2 fusion protein. Br J Cancer. 1993;67:304–310. doi: 10.1038/bjc.1993.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sen G, Chakraborty M, Foon K A, Reisfeld R A, Bhattacharya-Chatterjee M. Preclinical evaluation in nonhuman primates of murine monoclonal anti-idiotype antibody that mimics the disialoganglioside GD2. Clin Cancer Res. 1997;3:1969–1976. [PubMed] [Google Scholar]
- 21.Shin S U, Morrison S L. Production and properties of chimeric antibody molecules. Methods Enzymol. 1989;178:459–478. doi: 10.1016/0076-6879(89)78034-4. [DOI] [PubMed] [Google Scholar]
- 22.Shu L, Qi C-F, Schlom J, Kashmiri S V S. Secretion of a single-gene-encoded immunoglobulin from myeloma cells. Proc Natl Acad Sci USA. 1993;90:7995–7999. doi: 10.1073/pnas.90.17.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slavin-Chiorini D C, Horan Hand P H, Kashmiri S V S, Calvo B, Zaremba S, Schlom J. Biologic properties of CH2 domain-deleted recombinant immunoglobulins. Int J Cancer. 1993;53:97–103. doi: 10.1002/ijc.2910530119. [DOI] [PubMed] [Google Scholar]
- 24.Thavasu P W, Longhurst S, Joel S P, Slevin M L, Balkwill F R. Measuring cytokine levels in blood: importance of anticoagulants, processing, and storage conditions. J Immunol Methods. 1992;153:115–134. doi: 10.1016/0022-1759(92)90313-i. [DOI] [PubMed] [Google Scholar]
- 25.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang R, Lode H N, Dolman C S, Dreier T, Varki N M, Qian X, Lo K-M, Lan Y, Super M, Gillies S D, Reisfeld R A. Elimination of established murine colon carcinoma metastases by antibody-interleukin 2 fusion protein therapy. Cancer Res. 1997;57:4948–4955. [PubMed] [Google Scholar]