Abstract
Background:
Ivacaftor is a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator for people with CF and the G551D mutation. We aimed to investigate the biology of CFTR modulation and systemic effects of CFTR restoration by examining changes in circulating measurements of inflammation and growth and novel proteins with ivacaftor treatment.
Methods:
Blood samples from 64 CF subjects with G551D-CFTR were analyzed for inflammatory and growth-related proteins at baseline, 1 and 6 months after ivacaftor initiation. In 30 subjects, plasma was assayed for 1,322 proteins using the SomaScan proteomic platform at baseline and 6 months post-ivacaftor. Correlations with clinical outcomes were assessed.
Measurements and Main Results:
Significant reductions in high mobility group box-1 protein (HMGB-1), calprotectin, serum amyloid A, and granulocyte colony-stimulating factor (G-CSF), and an increase in insulin-like growth factor (IGF-1) occurred 1 month after ivacaftor. This treatment effect was sustained at 6 months for HMGB-1 and calprotectin. Correcting for multiple comparisons in the proteomic analysis, 9 proteins (albumin, afamin, leptin, trypsin, pancreatic stone protein [PSP], pituitary adenylate cyclase-activating polypeptide-38, repulsive guidance molecule A [RGMA], calreticulin, GTPase KRas) changed significantly with ivacaftor. Proteins changing with treatment are involved in lipid digestion and transport and extracellular matrix organization biological processes. Reductions in calprotectin and G-CSF and increases in calreticulin, and RGMA correlated with improved lung function, while increasing IGF-1, leptin and afamin and decreasing PSP correlated with increased weight.
Conclusions:
Ivacaftor led to changes in inflammatory, lipid digestion, and extracellular matrix proteins, lending insights into the extrapulmonary effects of CFTR modulation.
Keywords: CFTR modulation, proteomics, inflammation, extrapulmonary
Introduction
Ivacaftor, a cystic fibrosis transmembrane conductance regulator (CFTR) protein modulator, was found to have profound effects on both CFTR function and clinical outcomes in phase 3 clinical trials(1, 2). The beneficial effects of ivacaftor on lung function, pulmonary exacerbations, and quality of life were the basis for its approval by regulatory agencies. However, its impact on inflammation, a hallmark manifestation of CF lung disease, and its effects on extrapulmonary organs are not fully known.
Studies of ivacaftor and airway inflammation have yielded mixed results. In the G551D Observational (GOAL) trial, there were no significant changes in any sputum markers of inflammation following 6 months of ivacaftor therapy in two distinct cohorts: one with milder lung disease assessed by sputum induction(3), and a second with more impaired lung function who spontaneously expectorated sputum before and on therapy(4). In contrast, a separate study found that ivacaftor markedly reduced sputum markers of inflammation during the first year of treatment in a cohort of 12 adults with the G551D mutation, most of whom were chronically infected with P. aeruginosa(5). Circulating biomarkers of inflammation, which do not always correlate with sputum measurements of inflammation(6), may indicate different biologic and inflammatory pathways that are affected by CFTR modulation.
Studies have also reported beneficial extrapulmonary effects related to the use of ivacaftor (reviewed in (7)) on weight and growth(8, 9), pancreatic function(10, 11), intestinal pH and inflammation and fecal dysbiosis(3, 12), and endocrine manifestations including CF-related diabetes(13, 14) and bone disease(13, 15), reflecting broader benefits of CFTR modulation. Measuring changes in blood-based proteins provides an opportunity to investigate the systemic effects of ivacaftor therapy and further understand the multiorgan biology of CFTR modulation.
The objectives of this study were three-fold: (1) determine changes in pre-determined (targeted) circulating proteins based on biological plausibility and association with CF outcomes: high sensitivity C-reactive protein (hsCRP) and serum amyloid A (SAA) (acute phase reactants and non-specific markers of inflammation), calprotectin and granulocyte colony-stimulating factor (G-CSF) (markers of neutrophilic inflammation), IL-13 (marker of Th2 inflammation), IL-17 (marker of Th17 inflammation), high mobility group box (HMGB)-1, total IgG, and insulin-like growth factor (IGF)-1 pre- and post-ivacaftor treatment; (2) apply a high dimensional, multiplexed aptamer-based proteomic technology(16, 17) to analyze changes in proteome profiles with ivacaftor treatment; and (3) examine relationships between circulating protein measurements and clinical outcome measures (lung function, weight, body mass index [BMI], sweat chloride) pre- (baseline) and post- (changes following) ivacaftor treatment.
Methods
Study Population and Design
Data were derived from participants in the GOAL longitudinal observational cohort study involving 28 centers of the U.S. CF Foundation (CFF) Therapeutics Development Network, designed to capture clinical measures and biospecimens in CF patients 6 years and older with at least 1 copy of the G551D mutation and no prior exposure to ivacaftor(3). Clinical assessments included percent predicted forced expiratory volume in 1 second (ppFEV1), weight, BMI, and sweat chloride analysis performed at baseline and 1, 3, and 6 months after initiation of ivacaftor. Serum and plasma samples were collected at these same time points and stored at the CFF Biorepository for future research.
Plasma was also collected at multiple time points from 12 healthy adult subjects (6 [50%] female, 6 male [50%], mean (SD) age 44 (15) years, range 33–56), separate from the GOAL cohort, to serve as controls for comparing the proteome changes in CF subjects pre- and post-ivacaftor as well as to independently assess the reproducibility of all aptamer-based protein measurements.
Targeted measurements of inflammatory and growth-related proteins
Serum and plasma specimens collected from 64 study participants at baseline, 1 and 6 months after ivacaftor initiation were analyzed in the Clinical Translational Research Center (CTRC) Core Laboratory at Children’s Hospital Colorado and University of Colorado Anschutz Medical Campus (Aurora, CO), which serves as the CFF-funded Center for Biochemical Markers. Serum aliquots were analyzed using validated commercially available assays for the following protein biomarkers: hsCRP (Siemens Nephelometer assay, Siemens Healthcare, Tarrytown, NY), SAA (Milliplex MAP® for Luminex Technology, EMD Millipore, St. Charles, MO), calprotectin (American Laboratory Products Company-ALPCO, Salem, NH), HMGB-1 (HMGB1 ELISA, IBL International, Hamburg, Germany), IL-13 (Human Quantikine IL-13 ELISA, R&D Systems, Minneapolis, MN), total IgG (Siemens Nephelometer assay, Siemens Healthcare, Tarrytown, NY), and IGF-1 (Insulin Like Growth Factor 1, Immunodiagnostic Systems, United Kingdom). EDTA plasma aliquots were analyzed for G-CSF (Milliplex MAP® for Luminex Technology, EMD Millipore, St. Charles, MO) and IL-17 (Milliplex MAP® for Luminex Technology, EMD Millipore, St. Charles, MO). The lower limits of detection for these assays were: hsCRP, 0.007 mg/L; SAA, 500 ng/mL; calprotectin, 0.4 mg/mL; HMGB-1, 0.6 ng/mL; IL-13, 62.5 pg/mL; total IgG, 7 mg/dL; IGF-1, 10 ng/mL; G-CSF, 14 pg/mL; and IL-17, 12 pg/mL. The intra-assay coefficients of variation (CV) were less than 15% for all assays. The rationale for selecting the 9 proteins in the targeted approach is included in the online supplement.
SomaScan Proteomics Assay
Plasma obtained at baseline and 6 months post-ivacaftor from 30 subjects was analyzed for 1,322 proteins using the SomaScan® Platform (SomaLogic, Boulder, CO), a high throughput multiplexing aptamer-based assay described elsewhere(16). The platform consists of a panel of aptamers which bind with high specificity and affinity to target proteins. The presence of proteins are quantified simultaneously on a custom hybridization array (Agilent, Santa Clara, CA) and measured in relative fluorescence units (RFU) (16). Twenty-three of the 30 proteomic cohort subjects were also included in the targeted protein cohort. The remaining subjects in the targeted protein cohort did not have sufficient sample volumes available for proteomic analysis. Proteins were classified into pathways using Reactome(18, 19) (further details are available in the online data supplement).
Statistical Analyses
A linear regression with generalized estimating equations (GEE) and a compound symmetric working correlation matrix were used to assess changes in clinical outcomes over time. Inflammatory protein concentrations were evaluated using a generalized linear model with a log link and GEE. Values below the limit of detection were randomly imputed between 0 and the limit of detection for each assay. Aptamer protein values (RFUs) were log2 transformed prior to analyses. Paired t-tests on the log transformed protein levels were performed to rank aptamer-based protein measurements. P-values <0.05 were considered statistically significant after adjusting for multiple comparisons using False Discovery Rate (20). Pathways were ranked using a functional class scoring approach that uses p-values as the aptamer level statistics and is appropriate for platforms with a priori selected targets (21). Spearman rank-based correlations were calculated between differences in proteins and differences in clinical parameters from baseline. Intraclass correlation coefficients (ICC) and coefficients of variation (CV) values were calculated to evaluate reproducibility of aptamer measurements in repeated samples from the control subjects.
Results
Clinical Characteristics and Outcomes with Ivacaftor Treatment
Demographics and baseline clinical characteristics of the 64 participants undergoing targeted inflammatory and growth-related measurements and the 30 participants undergoing aptamer-based proteomics measurements are shown in Table 1. Participants in the targeted protein cohort ranged in age from 6 to 45 years and had a mean ppFEV1 of 88 while participants in the proteomics cohort ranged in age from 6 to 47 years and had a mean ppFEV1 of 78. The sex distribution was well balanced in both cohorts.
Table 1.
Demographic and baseline clinical characteristics of study participants
| Baseline characteristics mean (SD) [range] | Targeted protein cohort (n = 64) | Proteomics cohort (n = 30) |
|---|---|---|
| Gender F:M | 30:34 | 14:16 |
| Age, years | 18 (10) [6 – 45] | 24 (13) [6 – 47] |
| White | 63 (98%) | 29 (97%) |
| Genotype class of non-G551D allele | ||
| I | 4 (6%) | 1 (3%) |
| II | 48 (75%) | 22 (73%) |
| III | 1 (2%) | 0 |
| IV | 1 (2%) | 0 |
| V | 4 (6%) | 2 (7%) |
| missing | 6 (9%) | 5 (17%) |
| ppFEV1, | 88 (23) [39–137] | 78 (25) [39–120] |
| Weight (kg) | 51 (21) [18 – 121] | 57 (21) [23 – 121] |
| Body mass index | 21 (5) [14–38] | 22 (6) [15–38] |
| Sweat Chloride | 101 (15) [34 – 132] | 100 (15) [34 – 119] |
Improvements in lung function (ppFEV1), weight, BMI, and reductions in sweat chloride occurred in both cohorts (Table 2). These clinical improvements with ivacaftor treatment were similar in magnitude to those observed in the overall GOAL study cohort(3) and appear to be representative of the typical ivacaftor response in the G551D patient population(1).
Table 2.
Changes in clinical outcomes from baseline with ivacaftor treatment
| Mean (95% confidence interval, CI) | Targeted protein cohort (n = 64) | Proteomics cohort (n = 30) | ||||
|---|---|---|---|---|---|---|
| 1 month change from baseline, mean (95% CI) | p-value | 6 month change from baseline, mean (95% CI) | p-value | 6 month change from baseline, mean (95% CI) | p-value | |
| ppFEV1, | 6.1 (3.7, 8.4) | <0.01 | 6.3 (3.9, 8.7) | <0.01 | 7.6 (4.8, 10.3) | <0.01 |
| Weight, kg | 1.11 (0.78, 1.44) | <0.01 | 2.45 (1.54, 3.35) | <0.01 | 3.56 (2.00, 5.61) | <0.01 |
| BMI, kg/m2 | 0.38 (0.25, 0.51) | <0.01 | 0.74 (0.44, 1.04) | <0.01 | 1.23 (0.72, 1.74) | <0.01 |
| Sweat Chloride, mEq/L | −48.1 (−53.8, −42.4) | <0.01 | −53.8 (−59.1, −48.4) | <0.01 | −50.7 (−58.6, −42.8) | <0.01 |
Changes in inflammatory and growth-related proteins
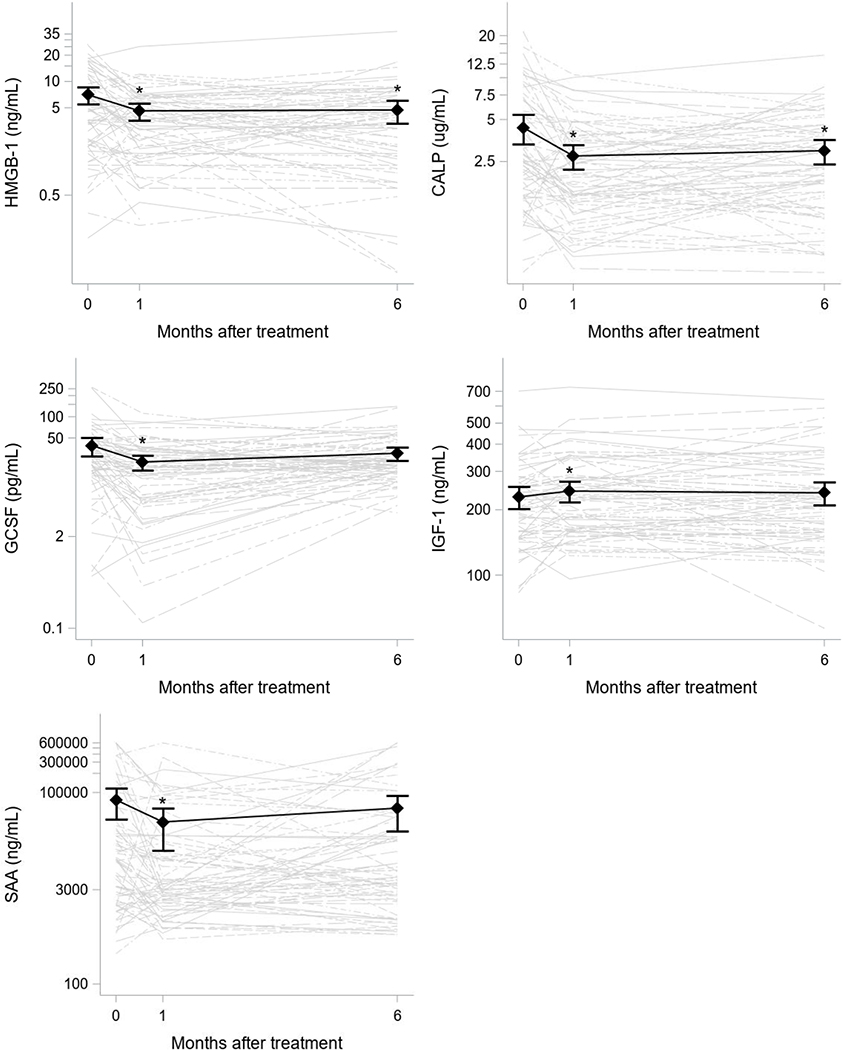
Concentrations of IL-17 were below the assay’s limits of detection in samples from all subjects and time points (thus, no IL-17 data are reported in this manuscript). Concentrations of IL-13, HMGB-1, and G-CSF were below the limits of detection of their assays in 55%, 6%, and 25% of samples, respectively. All other inflammatory markers were detectable and quantifiable in > 95% of samples. The 1- and 6-month changes in circulating protein levels from baseline with ivacaftor treatment are shown in Table E1 (online data supplement). Significant reductions in HMGB-1, calprotectin, SAA and G-CSF, and an increase in IGF-1 occurred 1 month after beginning ivacaftor (Figure 1). This treatment effect was sustained at 6 months for HMGB-1 and calprotectin. No significant changes were detected in hsCRP, IL-13, and IgG concentrations with treatment. Positive correlations were observed between ppFEV1 and 1 and 6 month changes in calprotectin and 6 month changes in HMGB-1, meaning that greater decreases in calprotectin and HMGB-1 were seen in those with lower baseline lung function (Table E2).
Figure 1. Changes in HMGB-1, calprotectin, G-CSF, and IGF-1 following 1 and 6 months of ivacaftor.
Protein concentrations for each subject over time are indicated by the grey lines. Means and 95% confidence intervals from the generalized linear regression models are overlaid in black. Stars indicate times at which the average protein concentrations were statistically significant from baseline.
In a small subset of subjects (n=7) who had concomitant sputum samples collected and analyzed at these same study visits (baseline and 6 months following ivacaftor initiation)(4), positive correlations were observed between changes in sputum IL-1β and circulating G-CSF and sputum alpha-1 antitrypsin and circulating hsCRP (Figure E1). A negative correlation was observed between change in sputum IL-8 and circulating IgG (Figure E1).
Changes in aptamer-based protein measurements
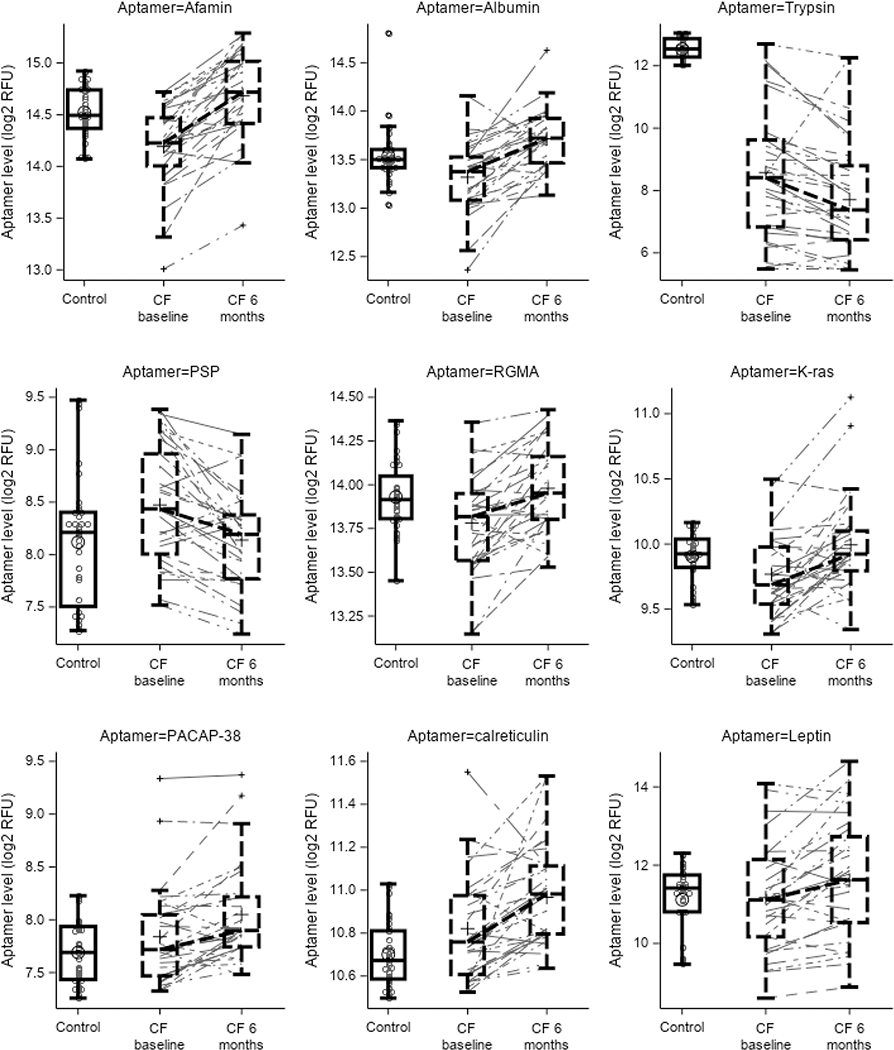
Out of 1,322 proteins assessed by the SOMAscan® platform, 9 proteins (albumin, trypsin, pancreatic stone protein [PSP], afamin, leptin, calreticulin, GTPase KRas [K-ras], repulsive guidance molecule A [RGMA], pituitary adenylate cyclase-activating polypeptide 38 [PACAP-38]) changed significantly with 6 months of ivacaftor treatment after adjusting for multiple comparisons (Table E3, Figure 2). Additional information regarding the biological processes of these proteins is included in Table E2. Changes in concentrations of these 9 proteins in CF subjects pre- and post-ivacaftor treatment were also compared to concentrations in plasma samples from a cohort of healthy adult controls obtained at one time point (Figure 3). All the proteins that changed significantly without adjustment are shown in Supplemental file 1. Among the top ranked pathways that changed with treatment, using Reactome to assign pathways, include: 1) plasma lipoprotein assembly, remodeling, and clearance and 2) extracellular matrix organization (Tables E4 & E5, Supplemental file 2). Changes in multiple aptamers correlated with age and baseline ppFEV1 (Tables E6 & E7). For example, changes in afamin were inversely associated with age with greater increases in afamin being observed in younger subjects (Table E6). Changes in PSP were positive associated with age such that greater decreases in PSP were observed in younger subjects (Table E6).
Figure 2. SomaScan® plasma proteomic changes with ivacaftor treatment.
Volcano plot displaying the significance versus log2 fold change in protein levels following treatment. The y-axis represents the significance of the change (−log p value) and the x-axis the magnitude of the change (log-relative fluorescence unit (RFU) difference between time points). The horizontal line indicates the p value cutoff < 0.05 after adjustment for multiple testing. Red dots represent proteins that increased and blue dots proteins that decreased with ivacaftor treatment. FDR = false discovery rate, PSP = pancreatic stone protein, RGMA = repulsive guidance molecule A, PACAP = pituitary adenylate cyclase-activating polypeptide.
Figure 3. Protein concentrations in log-relative fluorescence units (RFU) for CF subjects at baseline and after 6 months of ivacaftor treatment compared to control subjects.
Data are plotted on a log2 scale. Boxes highlight the interquartile range with the group median; bars display the total distribution range (minimum to maximum). PSP = pancreatic stone protein, RGMA = repulsive guidance molecule A, PACAP = pituitary adenylate cyclase-activating polypeptide.
Relationships between circulating proteins and clinical outcome measures
Correlations between the targeted inflammatory and growth-related measurements and the aptamer-based proteomic measurements and clinical outcome measures (ppFEV1, weight, BMI, and sweat chloride) at baseline and changes following 1 and 6 months of ivacaftor treatment are shown in Tables E8 and E9, respectively. Changes in calprotectin and G-CSF negatively correlated with changes in ppFEV1 at 6 months after treatment initiation while changes in trypsin, calreticulin, and RGMA correlated positively with changes in ppFEV1 (Table E9). Increases in IGF-1, leptin and afamin and decreases in PSP correlated with increased weight at 6 months while changes in IGF-1 and leptin correlated positively with changes in BMI at 6 months. Increases in afamin were associated with reductions in sweat chloride (Figures E2 and E3).
Reproducibility of aptamer-based protein measurements
As a quality control check on the proteomic data, we assessed the reproducibility of proteins detected by the SomaScan assay by examining data generated from 35 plasma samples from 12 healthy adult controls (3 subjects with two samples, 7 subjects with three samples, 2 subjects with 4 samples). The 99th percentile of all the CV values was 5.1% (Figure E4). There were five CV values greater than 20% corresponding to three proteins (none of which significantly changed with ivacaftor or are mentioned in this manuscript). The majority of proteins (77%) had ICC values greater than 0.90 (Figure E4), further suggesting a high degree of reproducibility.
Discussion
In this study of individuals with CF and a G551D CFTR mutation treated with ivacaftor, significant and sustained reductions in circulating HMGB-1 and calprotectin were observed through 6 months of therapy, while a transient decrease in SAA and G-CSF and an increase in IGF-1 occurred 1 month after treatment initiation. Using a high-dimensional approach to biomarker discovery with a highly multiplexed proteomic platform, we identified 9 additional proteins (albumin, trypsin, PSP, afamin, leptin, calreticulin, K-ras, RGMA, PACAP-38) that changed significantly following 6 months of ivacaftor treatment after adjusting for multiple comparisons. Pathways representing lipid digestion, mobilization and transport and extracellular matrix organization were highly enriched with proteins that changed in response to ivacaftor. Reductions in calprotectin and G-CSF and increases in trypsin, calreticulin, and RGMA correlated with improvements in lung function, while increasing IGF-1, leptin and afamin and decreasing PSP correlated with increased weight, providing indirect evidence that these changes were associated with clinically meaningful outcomes.
The reductions in HMGB-1, calprotectin, SAA, and G-CSF indicate that ivacaftor modulates systemic inflammation. HMGB-1, a protein with cytokine activity that plays an important role in immune and inflammatory responses, correlates with lung function and appears to be a predictive biomarker of pulmonary exacerbations in CF, at least when measured in the airway(22, 23). While circulating HMGB-1 was associated with lung function at baseline, declines in HMGB-1 concentrations with ivacaftor treatment were not associated with changes in any clinical outcomes in our study participants. We cannot address whether changes in sputum HMGB-1 are associated with clinical changes following ivacaftor initiation since HMGB-1 was not measured in previously analyzed sputum specimens (4). Calprotectin and G-CSF, markers of neutrophilic inflammation, are proving to be useful markers of disease severity (6, 24) and treatment response(25, 26) in CF. These proteins primarily reflect systemic neutrophilic inflammation and are modestly related to measures of airway inflammation(6). The correlations between changes in calprotectin and ppFEV1 suggest that reducing systemic neutrophilic inflammation may be associated with lung function benefits. This may be due to improved mucociliary clearance and a subsequent decrease in infection, as evidenced by a reduction in exacerbations, leading to reduced systemic neutrophilic inflammation. Calprotectin has been shown to predict pulmonary exacerbations and lung function decline(27). We could not determine whether reductions in calprotectin were also associated with fewer exacerbations due to too few subjects experiencing an exacerbation event. Only 8% of GOAL participants (approximately 12 subjects) experienced an exacerbation in the first 6 months following ivacaftor initiation, not all of whom were included in our study cohort(3).
Ivacaftor was associated with a transient reduction in the acute phase reactant protein, SAA, following 1 month of treatment. Decreasing concentrations of hsCRP and SAA, non-specific markers of systemic inflammation, were previously observed in response to azithromycin therapy(26) and intravenous antibiotics for treatment of pulmonary exacerbations(28). Ivacaftor treatment was not associated with appreciable changes in either IL-13, a representative marker of Th2 mediated inflammation(29), or total IgG, which historically was associated with lung function decline in CF(30, 31).
Low circulating IGF-1 concentrations have been associated with impaired linear growth in CF(32) and the loss of CFTR function may have a direct effect on growth hormone release and reduced growth potential in CF(33). It is intriguing to speculate that improved growth outcomes associated with ivacaftor(9) may be due in part to increased IGF-1 levels through partial restoration of CFTR activity. Here, we demonstrate transient increases in circulating IGF-1 in a study cohort with an average age of 18 years. Certainly, the growth potential of ivacaftor therapy is more likely to be realized when started in infancy and early childhood, and the sensitivity to detect a signal in IGF-1 would be improved in a pre-pubescent population.
Several other biologically plausible and novel proteins were identified by the SomaScan assay as changing significantly in response to ivacaftor therapy, indicating extrapulmonary effects of CFTR modulation. Increasing circulating albumin and afamin, which changed most significantly from a statistical perspective, give confidence in our proteomic findings as they are consistent with the nutritional improvements observed in patients treated with ivacaftor. It is likely that ivacaftor decreases intestinal protein malabsorption, just as it does fat malabsorption(8). We cannot determine whether increases in albumin and afamin are a consequence of improvements in nutritional status, or whether they are due to a direct mechanistic effect of ivacaftor. There have been limited reports of afamin, a member of the albumin gene family and a vitamin E-binding glycoprotein(34), in CF. In one publication, afamin was increased in CF compared to controls(35), whereas we observed afamin being decreased in CF subjects pre-ivacaftor compared with controls and increasing with ivacaftor treatment. In another study, afamin increased following intravenous antibiotic treatment, with the authors suggesting that afamin may represent a negative acute phase reactant(36). While increasing afamin was modestly associated with weight gain in our study cohort, afamin was strongly associated with weight gain in transgenic mice overexpressing afamin and with metabolic syndrome in humans(37). As an increasing percentage of people with CF treated with long-term ivacaftor therapy shift into overweight and obese weight categories(38), it is possible that increasing afamin is associated with weight changes over time. We also found a significant increase in leptin, a hormone that regulates energy balance by inhibiting hunger and food intake, and associations between change in leptin, weight, and BMI in our cohort. Data on leptin in CF have been conflicting in terms of concentration differences with matched controls and relationships with weight and BMI(39–42). Despite inconclusive results with regard to leptin levels, mouse models suggest that CFTR function interacts directly or indirectly with leptin signaling(43), implying that partial restoration of CFTR function could affect leptin signaling and have additional implications for weight gain in people with CF.
Two pancreas-derived proteins, trypsin and PSP, significantly decreased with ivacaftor therapy and decreasing PSP correlated with increased BMI. Reductions of immunoreactive trypsinogen (IRT), a pancreatic enzyme precursor to trypsin, have been observed in clinical trials of ivacaftor in infants and young children(11, 44). While it is encouraging that restoration of CFTR activity is associated with improvements in markers of exocrine pancreatic function including trypsin and IRT in young children, it is fascinating that we see significant declines in trypsin in our older population, considering that exocrine pancreatic organ damage has historically been thought to be an irreversible early childhood event(45). Although, it is worth noting that trypsin concentrations in our CF subjects at both time points are significantly lower than those in the control subjects (p<0.01 at both time points). Identifying PSP as another protein highly responsive to ivacaftor is intriguing. This protein, also termed “Reg” for regenerating, was first identified in the human pancreas and was thought to be a secretory protein. A study of pancreatobiliary secretions of CF patients suggested this protein could precipitate and plug pancreatic ducts, contributing to pancreatic disease in CF(46). Another study reported higher circulating concentrations of PSP in CF patients with and without pancreatic insufficiency compared with controls and speculated that rather than being a secretory exocrine protein like other digestive enzymes, PSP may be a hormone-like secretory protein with an endocrine or paracrine function(47).
Two proteins that are either expressed in or have activity in the central nervous system (CNS), PACAP-38 and RGMA, increased with ivacaftor therapy. PACAP, a neuropeptide belonging to the secretin/glucagon/vasoactive intestinal polypeptide family, exists in two biologically active forms, PACAP-38 and PACAP-27. It has diverse biological functions including control of anterior pituitary hormone secretion, vasodilation, adrenaline secretion, insulin secretion, and immunosuppression(48). PACAP-27 stimulates CFTR-dependent chloride secretion in human bronchial epithelial cells(49). Interestingly, PACAP has been implicated in anxiety and depression(50, 51). As there have been emerging case reports of worsening depression and anxiety temporally related to the initiation of CFTR modulator therapy(52, 53), increasing PACAP-38 may be involved. PACAP may exert a modulating role on stress-related centers in the brain, as PACAP-deficient mice have reduced anxiety and increased depressive symptoms as well as changes in gene expression in certain brain areas, leading the authors to propose that PACAP might contribute to stress-related mood disorders(54). PACAP-38 concentrations are also associated with post-traumatic stress disorder symptoms in women, but not in men(55). The authors speculate that PACAP-PAC1 receptor expression and signaling may be involved in stress responses(55), and could suggest a biologically plausible mechanism for increasing PACAP modulating anxiety and depression in people being treated with ivacaftor. As for RGMA, there are no reports linking this protein with CF. RGMA was originally identified for its role during CNS development. However, relevant to CF, RGMA is expressed by epithelium and leukocytes, inhibits leukocyte migration, and suppresses inflammatory responses(56, 57). It will be worth exploring whether its association with improvements in lung function are due to anti-inflammatory effects.
The reason and significance for the increased concentrations of calreticulin, an endoplasmic reticulum chaperone protein, are unclear. Related to CF, this protein was found to negatively regulate cell surface CFTR expression and activity(58). While the primary roles of calreticulin are in protein-folding and calcium homeostasis, it is involved in a wide array of cellular responses important in physiological and pathological processes, including wound healing, immunologic responses, angiogenesis, and cancer(59). Another unique protein that significantly increased with ivacaftor was K-ras, a GTPase protein involved in signaling pathways that govern cell proliferation and differentiation. This protein is implicated in various malignancies, with RAS genes comprising the most frequently mutated oncogene family in human cancers(60, 61). In fact, mutations of RAS are frequently identified in three of the top four cancer killers in the U.S. (lung, colorectal, pancreatic)(60, 61). Further studies will be required to elucidate the significance of increasing K-ras concentrations in CF and whether there are any implications for cancer development, particularly gastrointestinal malignancies, in people with CF(62).
This study has important limitations worth noting. First, the sample size is relatively small with samples obtained from subjects with variable age and lung disease severity. However, these samples afforded the opportunity to analyze data from a heterogenous cohort of children and adults across multiple CF centers. Also, the control plasma samples analyzed using the SomaScan assay were collected from a small cohort of healthy adult subjects who were not age-matched to the CF subjects. The lack of a control population limits the conclusions that can be made regarding the changes observed in circulating proteins pre- and post-ivacaftor within this cohort. Additionally, by measuring circulating proteins we were investigating systemic changes with ivacaftor therapy, which do not necessarily reflect organ-specific biomarkers such as those measured in sputum or bronchoalveolar lavage fluid to indicate CF lung disease. These results will need to be validated in additional cohorts, including those being treated with the highly efficacious elexacaftor/tezacaftor/ivacaftor CFTR modulator combination therapy and in those with less robust CFTR modulation.
In conclusion, ivacaftor treatment is associated with decreased circulating concentrations of a proinflammatory mediator (HMGB-1) and neutrophilic inflammatory markers (calprotectin and G-CSF), which may be indirectly related to improved pulmonary health, or possibly a direct effect on systemic inflammation. Ivacaftor also transiently increases circulating IGF-1 in an older study cohort and may have a measurable impact on growth potential when started in infancy and early childhood. The unique SomaScan proteomic platform identified changes in several proteins involved in lipid digestion, mobilization and transport and extracellular matrix organization. These proteins lend insights into the systemic, extrapulmonary effects of CFTR modulation on organs such as the pancreas and the CNS. With the emergence of even more efficacious triple combination CFTR modulator therapies, and the initiation of these therapies at earlier ages, it will be imperative to continue to investigate their extrapulmonary effects and their relationships with disease outcomes.
Supplementary Material
HIGHLIGHTS – EFFECTS OF IVACAFTOR ON SYSTEMIC INFLAMMATION AND THE PLASMA PROTEOME IN PEOPLE WITH CF AND G551D.
Ivacaftor led to sustained reductions in serum HMGB-1 and calprotectin
SAA and G-CSF decreased and IGF-1 increased 1 month after treatment initiation
Proteomic analyses identified 9 proteins that changed significantly with ivacaftor
Ivacaftor altered lipid digestion/transport and extracellular matrix organization proteins
These changes lend insight into the extrapulmonary effects of CFTR modulation
ACKNOWLEDGMENTS
We thank all site investigators and research coordinators who were involved in the GOAL study and all the people living with CF and their families who consented to participate in this study and provided blood samples that were used in this study. We also want to thank Linh Do who oversees the CF Foundation Biorepository and assisted with sample acquisition.
Sources of Support: This work was supported by the Cystic Fibrosis Foundation (#HOPPE16A0, GOAL11K1, GOAL13K1, SAGEL15K0) and the National Institutes of Health: NIH/NCATS Grant Numbers UL1 TR002535 and UL1TR003096; NIH/NIDDK P30 DK089507, P30DK072482.
Footnotes
CONFLICT OF INTEREST STATEMENT
JEH reports grants from Cystic Fibrosis Foundation, outside the submitted work, SMR reports grants, personal fees, non-financial support and other from Vertex Pharmaceuticals Inc., during the conduct of the study; grants and personal fees from Novartis, grants and personal fees from Bayer, grants from Translate Bio, non-financial support from Proteostasis, grants, personal fees and non-financial support from Galapagos/Abbvie, grants, personal fees and other from Synedgen/Synspira, grants from Eloxx, grants and personal fees from Celtaxsys, grants, personal fees, non-financial support and other from Vertex Pharmaceuticals Inc, personal fees from Renovion, grants and personal fees from Arrowhead, grants and other from Ionis, grants from Astra Zenica, personal fees from Cystetic Medicines, personal fees from Arcturus, outside the submitted work; EMD reports other from EvoEndoscopy (formerly Triple Endoscopy), outside the submitted work and is a consultant for Boehringer Ingelheim, not related to this work. SDS reports grants from the Cystic Fibrosis Foundation that funds the work under consideration, and grants from the Cystic Fibrosis Foundation and National Institutes of Health funding activities outside the submitted work. BDW, JKH and SLH have nothing to disclose.
Credit Author Statement
Conception and design of the study: S.M.R, S.L.H and S.D.S; Data acquisition: S.M.R and S.D.S; Analysis and Interpretation of Data: J.E.H, B.D.W, J.K.H, S.M.R, S.L.H, E.M.D, and S.D.S; J.E.H drafted the initial manuscript and B.D.W, J.K.H, S.M.R, S.L.H, E.M.D, and S.D.S critically reviewed it for intellectual content. All authors approve this final version.
Subject descriptor number of manuscript: 9.17 Cystic Fibrosis: Translational & Clinical Studies
This article has an online data supplement, which is accessible from this issue’s table of content online at www.atsjournals.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordonez C, Elborn JS, Group VXS. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011; 365: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, Mainz JG, Rodriguez S, Li H, Yen K, Ordonez CL, Ahrens R, Group VXS. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med 2013; 187: 1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, Sagel SD, Khan U, Mayer-Hamblett N, Van Dalfsen JM, Joseloff E, Ramsey BW. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. American journal of respiratory and critical care medicine 2014; 190: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris JK, Wagner BD, Zemanick ET, Robertson CE, Stevens MJ, Heltshe SL, Rowe SM, Sagel SD. Changes in Airway Microbiome and Inflammation with Ivacaftor Treatment in Patients with Cystic Fibrosis and the G551D Mutation. Ann Am Thorac Soc 2020; 17: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hisert KB, Heltshe SL, Pope C, Jorth P, Wu X, Edwards RM, Radey M, Accurso FJ, Wolter DJ, Cooke G, Adam RJ, Carter S, Grogan B, Launspach JL, Donnelly SC, Gallagher CG, Bruce JE, Stoltz DA, Welsh MJ, Hoffman LR, McKone EF, Singh PK. Restoring Cystic Fibrosis Transmembrane Conductance Regulator Function Reduces Airway Bacteria and Inflammation in People with Cystic Fibrosis and Chronic Lung Infections. American journal of respiratory and critical care medicine 2017; 195: 1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain R, Baines A, Khan U, Wagner BD, Sagel SD. Evaluation of airway and circulating inflammatory biomarkers for cystic fibrosis drug development. J Cyst Fibros 2021; 20: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sergeev V, Chou FY, Lam GY, Hamilton CM, Wilcox PG, Quon BS. The Extrapulmonary Effects of Cystic Fibrosis Transmembrane Conductance Regulator Modulators in Cystic Fibrosis. Annals of the American Thoracic Society 2020; 17: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stallings VA, Sainath N, Oberle M, Bertolaso C, Schall JI. Energy Balance and Mechanisms of Weight Gain with Ivacaftor Treatment of Cystic Fibrosis Gating Mutations. The Journal of pediatrics 2018; 201: 229–237.e224. [DOI] [PubMed] [Google Scholar]
- 9.Stalvey MS, Pace J, Niknian M, Higgins MN, Tarn V, Davis J, Heltshe SL, Rowe SM. Growth in Prepubertal Children With Cystic Fibrosis Treated With Ivacaftor. Pediatrics 2017; 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies JC, Cunningham S, Harris WT, Lapey A, Regelmann WE, Sawicki GS, Southern KW, Robertson S, Green Y, Cooke J, Rosenfeld M. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2–5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. The Lancet Respiratory medicine 2016; 4: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenfeld M, Wainwright CE, Higgins M, Wang LT, McKee C, Campbell D, Tian S, Schneider J, Cunningham S, Davies JC. Ivacaftor treatment of cystic fibrosis in children aged 12 to <24 months and with a CFTR gating mutation (ARRIVAL): a phase 3 single-arm study. The Lancet Respiratory medicine 2018; 6: 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ooi CY, Syed SA, Rossi L, Garg M, Needham B, Avolio J, Young K, Surette MG, Gonska T. Impact of CFTR modulation with Ivacaftor on Gut Microbiota and Intestinal Inflammation. Scientific reports 2018; 8: 17834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bessonova L, Volkova N, Higgins M, Bengtsson L, Tian S, Simard C, Konstan MW, Sawicki GS, Sewall A, Nyangoma S, Elbert A, Marshall BC, Bilton D. Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax 2018; 73: 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly A, De Leon DD, Sheikh S, Camburn D, Kubrak C, Peleckis AJ, Stefanovski D, Hadjiliadis D, Rickels MR, Rubenstein RC. Islet Hormone and Incretin Secretion in Cystic Fibrosis after Four Months of Ivacaftor Therapy. American journal of respiratory and critical care medicine 2019; 199: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sermet-Gaudelus I, Delion M, Durieu I, Jacquot J, Hubert D. Bone demineralization is improved by ivacaftor in patients with cystic fibrosis carrying the p.Gly551Asp mutation. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society 2016; 15: e67–e69. [DOI] [PubMed] [Google Scholar]
- 16.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, Carter J, Dalby AB, Eaton BE, Fitzwater T, Flather D, Forbes A, Foreman T, Fowler C, Gawande B, Goss M, Gunn M, Gupta S, Halladay D, Heil J, Heilig J, Hicke B, Husar G, Janjic N, Jarvis T, Jennings S, Katilius E, Keeney TR, Kim N, Koch TH, Kraemer S, Kroiss L, Le N, Levine D, Lindsey W, Lollo B, Mayfield W, Mehan M, Mehler R, Nelson SK, Nelson M, Nieuwlandt D, Nikrad M, Ochsner U, Ostroff RM, Otis M, Parker T, Pietrasiewicz S, Resnicow DI, Rohloff J, Sanders G, Sattin S, Schneider D, Singer B, Stanton M, Sterkel A, Stewart A, Stratford S, Vaught JD, Vrkljan M, Walker JJ, Watrobka M, Waugh S, Weiss A, Wilcox SK, Wolfson A, Wolk SK, Zhang C, Zichi D. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One 2010; 5: e15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeBoer EM, Kroehl ME, Wagner BD, Accurso FJ, Harris JK, Lynch DA, Sagel SD, Deterding RR. Proteomic profiling identifies novel circulating markers associated with bronchiectasis in cystic fibrosis. Proteomics Clin Appl 2017; 11. [DOI] [PubMed] [Google Scholar]
- 18.Fabregat A, Sidiropoulos K, Garapati P, Gillespie M, Hausmann K, Haw R, Jassal B, Jupe S, Korninger F, McKay S, Matthews L, May B, Milacic M, Rothfels K, Shamovsky V, Webber M, Weiser J, Williams M, Wu G, Stein L, Hermjakob H, D’Eustachio P. The Reactome pathway Knowledgebase. Nucleic Acids Res 2016; 44: D481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milacic M, Haw R, Rothfels K, Wu G, Croft D, Hermjakob H, D’Eustachio P, Stein L. Annotating cancer variants and anti-cancer therapeutics in reactome. Cancers (Basel) 2012; 4: 1180–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001; 125: 279–284. [DOI] [PubMed] [Google Scholar]
- 21.Khatri P, Sirota M, Butte AJ. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Comput Biol 2012; 8: e1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liou TG, Adler FR, Keogh RH, Li Y, Jensen JL, Walsh W, Packer K, Clark T, Carveth H, Chen J, Rogers SL, Lane C, Moore J, Sturrock A, Paine R 3rd, Cox DR, Hoidal JR. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PloS one 2012; 7: e42748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chirico V, Lacquaniti A, Leonardi S, Grasso L, Rotolo N, Romano C, Di Dio G, Lionetti E, David A, Arrigo T, Salpietro C, La Rosa M. Acute pulmonary exacerbation and lung function decline in patients with cystic fibrosis: high-mobility group box 1 (HMGB1) between inflammation and infection. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2015; 21: 368 e361–369. [DOI] [PubMed] [Google Scholar]
- 24.Sagel SD, Wagner BD, Ziady A, Kelley T, Clancy JP, Narvaez-Rivas M, Pilewski J, Joseloff E, Sha W, Zelnick L, Setchell KDR, Heltshe SL, Muhlebach MS. Utilizing centralized biorepository samples for biomarkers of cystic fibrosis lung disease severity. J Cyst Fibros 2020; 19: 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray RD, Imrie M, Boyd AC, Porteous D, Innes JA, Greening AP. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society 2010; 9: 193–198. [DOI] [PubMed] [Google Scholar]
- 26.Ratjen F, Saiman L, Mayer-Hamblett N, Lands LC, Kloster M, Thompson V, Emmett P, Marshall B, Accurso F, Sagel S, Anstead M. Effect of azithromycin on systemic markers of inflammation in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa. Chest 2012; 142: 1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid PA, McAllister DA, Boyd AC, Innes JA, Porteous D, Greening AP, Gray RD. Measurement of serum calprotectin in stable patients predicts exacerbation and lung function decline in cystic fibrosis. Am J Respir Crit Care Med 2015; 191: 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagel SD, Thompson V, Chmiel JF, Montgomery GS, Nasr SZ, Perkett E, Saavedra MT, Slovis B, Anthony MM, Emmett P, Heltshe SL. Effect of treatment of cystic fibrosis pulmonary exacerbations on systemic inflammation. Ann Am Thorac Soc 2015; 12: 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiringer K, Treis A, Fucik P, Gona M, Gruber S, Renner S, Dehlink E, Nachbaur E, Horak F, Jaksch P, Döring G, Crameri R, Jung A, Rochat MK, Hörmann M, Spittler A, Klepetko W, Akdis CA, Szépfalusi Z, Frischer T, Eiwegger T. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. American journal of respiratory and critical care medicine 2013; 187: 621–629. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler WB, Williams M, Matthews WJ Jr., Colten HR. Progression of cystic fibrosis lung disease as a function of serum immunoglobulin G levels: a 5-year longitudinal study. The Journal of pediatrics 1984; 104: 695–699. [DOI] [PubMed] [Google Scholar]
- 31.Proesmans M, Els C, Vermeulen F, De Boeck K. Change in IgG and evolution of lung function in children with cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society 2011; 10: 128–131. [DOI] [PubMed] [Google Scholar]
- 32.Laursen EM, Juul A, Lanng S, Høiby N, Koch C, Müller J, Skakkebaek NE. Diminished concentrations of insulin-like growth factor I in cystic fibrosis. Archives of disease in childhood 1995; 72: 494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogan MP, Reznikov LR, Pezzulo AA, Gansemer ND, Samuel M, Prather RS, Zabner J, Fredericks DC, McCray PB Jr., Welsh MJ, Stoltz DA. Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proceedings of the National Academy of Sciences of the United States of America 2010; 107: 20571–20575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dieplinger H, Dieplinger B. Afamin--A pleiotropic glycoprotein involved in various disease states. Clinica chimica acta; international journal of clinical chemistry 2015; 446: 105–110. [DOI] [PubMed] [Google Scholar]
- 35.Benabdelkamel H, Alamri H, Okla M, Masood A, Abdel Jabar M, Alanazi IO, Alfadda AA, Nizami I, Dasouki M, Abdel Rahman AM. Serum-Based Proteomics Profiling in Adult Patients with Cystic Fibrosis. Int J Mol Sci 2020; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts JM, Dai DLY, Hollander Z, Ng RT, Tebbutt SJ, Wilcox PG, Sin DD, Quon BS. Multiple reaction monitoring mass spectrometry to identify novel plasma protein biomarkers of treatment response in cystic fibrosis pulmonary exacerbations. J Cyst Fibros 2018; 17: 333–340. [DOI] [PubMed] [Google Scholar]
- 37.Kronenberg F, Kollerits B, Kiechl S, Lamina C, Kedenko L, Meisinger C, Willeit J, Huth C, Wietzorrek G, Altmann ME, Thorand B, Melmer A, Dähnhardt D, Santer P, Rathmann W, Paulweber B, Koenig W, Peters A, Adham IM, Dieplinger H. Plasma concentrations of afamin are associated with the prevalence and development of metabolic syndrome. Circulation Cardiovascular genetics 2014; 7: 822–829. [DOI] [PubMed] [Google Scholar]
- 38.Guimbellot JS, Baines A, Paynter A, Heltshe SL, VanDalfsen J, Jain M, Rowe SM, Sagel SD Long term clinical effectiveness of ivacaftor in people with the G551D CFTR mutation. Journal of Cystic Fibrosis 2020: S1569–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stylianou C, Galli-Tsinopoulou A, Koliakos G, Fotoulaki M, Nousia-Arvanitakis S. Ghrelin and leptin levels in young adults with cystic fibrosis: relationship with body fat. J Cyst Fibros 2007; 6: 293–296. [DOI] [PubMed] [Google Scholar]
- 40.Ahme ML, Ong KK, Thomson AH, Dunger DB. Reduced gains in fat and fat-free mass, and elevated leptin levels in children and adolescents with cystic fibrosis. Acta Paediatr 2004; 93: 1185–1191. [PubMed] [Google Scholar]
- 41.Schmitt-Grohe S, Hippe V, Igel M, von Bergmann K, Posselt HG, Krahl A, Smaczny C, Wagner TO, Nikolaizik W, Lentze MJ, Zielen S. Serum leptin and cytokines in whole blood in relation to clinical and nutritional status in cystic fibrosis. J Pediatr Gastroenterol Nutr 2006; 43: 228–233. [DOI] [PubMed] [Google Scholar]
- 42.Cohen RI, Tsang D, Koenig S, Wilson D, McCloskey T, Chandra S. Plasma ghrelin and leptin in adult cystic fibrosis patients. J Cyst Fibros 2008; 7: 398–402. [DOI] [PubMed] [Google Scholar]
- 43.Bederman IR, Pora G, O’Reilly M, Poleman J, Spoonhower K, Puchowicz M, Perez A, Erokwu BO, Rodriguez-Palacios A, Flask CA, Drumm ML. Absence of leptin signaling allows fat accretion in cystic fibrosis mice. American journal of physiology Gastrointestinal and liver physiology 2018; 315: G685–g698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies JC, Wainwright CE, Sawicki GS, Higgins MN, Campbell D, Harris C, Panorchan P, Haseltine E, Tian S, Rosenfeld M. Ivacaftor in Infants Aged 4 to <12 Months With Cystic Fibrosis and a Gating Mutation: Results of a 2-Part Phase 3 Clinical Trial. American journal of respiratory and critical care medicine 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nichols AL, Davies JC, Jones D, Carr SB. Restoration of exocrine pancreatic function in older children with cystic fibrosis on ivacaftor. Paediatric respiratory reviews 2020; 35: 99–102. [DOI] [PubMed] [Google Scholar]
- 46.Forstner GG, Vesely SM, Durie PR. Selective precipitation of 14 kDa stone/thread proteins by concentration of pancreaticobiliary secretions: relevance to pancreatic ductal obstruction, pancreatic failure, and CF. Journal of pediatric gastroenterology and nutrition 1989; 8: 313–320. [DOI] [PubMed] [Google Scholar]
- 47.Carrère J, Guy-Crotte O, Gaia E, Figarella C. Immunoreactive pancreatic Reg protein in sera from cystic fibrosis patients with and without pancreatic insufficiency. Gut 1999; 44: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacological reviews 2009; 61: 283–357. [DOI] [PubMed] [Google Scholar]
- 49.Dérand R, Montoni A, Bulteau-Pignoux L, Janet T, Moreau B, Muller JM, Becq F. Activation of VPAC1 receptors by VIP and PACAP-27 in human bronchial epithelial cells induces CFTR-dependent chloride secretion. British journal of pharmacology 2004; 141: 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashimoto R, Hashimoto H, Shintani N, Ohi K, Hori H, Saitoh O, Kosuga A, Tatsumi M, Iwata N, Ozaki N, Kamijima K, Baba A, Takeda M, Kunugi H. Possible association between the pituitary adenylate cyclase-activating polypeptide (PACAP) gene and major depressive disorder. Neuroscience letters 2010; 468: 300–302. [DOI] [PubMed] [Google Scholar]
- 51.Lutfy K, Shankar G. Emerging evidence for the role of pituitary adenylate cyclase-activating peptide in neuropsychiatric disorders. Prog Mol Biol Transl Sci 2019; 167: 143–157. [DOI] [PubMed] [Google Scholar]
- 52.Talwalkar JS, Koff JL, Lee HB, Britto CJ, Mulenos AM, Georgiopoulos AM. Cystic Fibrosis Transmembrane Regulator Modulators: Implications for the Management of Depression and Anxiety in Cystic Fibrosis. Psychosomatics 2017; 58: 343–354. [DOI] [PubMed] [Google Scholar]
- 53.McKinzie CJ, Goralski JL, Noah TL, Retsch-Bogart GZ, Prieur MB. Worsening anxiety and depression after initiation of lumacaftor/ivacaftor combination therapy in adolescent females with cystic fibrosis. J Cyst Fibros 2017; 16: 525–527. [DOI] [PubMed] [Google Scholar]
- 54.Gaszner B, Kormos V, Kozicz T, Hashimoto H, Reglodi D, Helyes Z. The behavioral phenotype of pituitary adenylate-cyclase activating polypeptide-deficient mice in anxiety and depression tests is accompanied by blunted c-Fos expression in the bed nucleus of the stria terminalis, central projecting Edinger-Westphal nucleus, ventral lateral septum, and dorsal raphe nucleus. Neuroscience 2012; 202: 283–299. [DOI] [PubMed] [Google Scholar]
- 55.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 2011; 470: 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mirakaj V, Brown S, Laucher S, Steinl C, Klein G, Köhler D, Skutella T, Meisel C, Brommer B, Rosenberger P, Schwab JM. Repulsive guidance molecule-A (RGM-A) inhibits leukocyte migration and mitigates inflammation. Proceedings of the National Academy of Sciences of the United States of America 2011; 108: 6555–6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mirakaj V, Rosenberger P. Immunomodulatory Functions of Neuronal Guidance Proteins. Trends in immunology 2017; 38: 444–456. [DOI] [PubMed] [Google Scholar]
- 58.Harada K, Okiyoneda T, Hashimoto Y, Ueno K, Nakamura K, Yamahira K, Sugahara T, Shuto T, Wada I, Suico MA, Kai H. Calreticulin negatively regulates the cell surface expression of cystic fibrosis transmembrane conductance regulator. J Biol Chem 2006; 281: 12841–12848. [DOI] [PubMed] [Google Scholar]
- 59.Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, Michalak M, Murphy-Ullrich JE. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2010; 24: 665–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: Mission possible? Nature reviews Drug discovery 2014; 13: 828–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papke B, Der CJ. Drugging RAS: Know the enemy. Science (New York, NY) 2017; 355: 1158–1163. [DOI] [PubMed] [Google Scholar]
- 62.Maisonneuve P, Marshall BC, Knapp EA, Lowenfels AB. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. Journal of the National Cancer Institute 2013; 105: 122–129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.