Abstract
Age-induced osteoporosis is a global problem. Essential amino acids (EAAs) work as an energy source and a molecular pathway modulator in bone, but their functions have not been systematically reviewed in aging bone. This study aimed to discuss the contribution of EAAs on aging bone from in vitro, in vivo, and human investigations. In aged people with osteoporosis, serum EAAs were detected changing up and down, without a well-established conclusion. The supply of EAAs in aged people either rescued or did not affect bone mineral density (BMD) and bone volume. In most signaling studies, EAAs were proven to increase bone mass. Lysine, threonine, methionine, tryptophan, and isoleucine can increase osteoblast proliferation, activation, and differentiation, and decrease osteoclast activity. Oxidized L-tryptophan promotes bone marrow stem cells (BMSCs) differentiating into osteoblasts. However, the oxidation product of tryptophan called kynurenine increases osteoclast activity, and enhances the differentiation of adipocytes from BMSCs. Taken together, in terms of bone minerals and volume, more views consider EAAs to have a positive effect on aging bone, but the function of EAAs in bone metabolism has not been fully demonstrated and more studies are needed in this area in the future.
Keywords: essential amino acids, bone mass, bone mineral density, aging
1. Introduction
The aging process is characterized by changes in body composition, such as an increase in body fat, and a reduction in lean mass (sarcopenia) and bone density (osteopenia) [1]. Osteopenia happens as a consequence of imbalanced osteoblast and osteoclast activity. Osteoblasts are differentiated from BMSCs and are responsible for secreting the bone matrix, which will be mineralized in bone development and homeostasis [2]. An osteoclast is the original bone-resorbing cell from monocytes/macrophages [3]. Osteopenia increases the incidence of bone fractures, is one of the largest musculoskeletal diseases, and is a main reason for the death of the elderly. In people older than 65, women have an absolute mortality risk of 12.5% within 1-year post-fracture, while men have a risk of 19.5% [4].
Normally, bone is considered as a supporting structure, but actually, it is also a very active metabolic organ. In addition to mineral metabolism, as the main energy source, glucose, fatty acid, and amino acid metabolism are also studied in bone. Glucose and fatty acids both work as energy sources and are bone structure providers; additionally, they play major roles in inducing internal signaling pathways in bone. For instance, glucose can induce the mammalian target of the rapamycin (mTOR) pathway in osteoblasts [5], which promotes osteoblast differentiation from pre-osteoblasts [6]. Fatty acids function as a critical source of adenosine triphosphate (ATP) in osteoblasts and osteocytes. Intriguingly, disrupting the carnitine palmitoyltransferase 2 (Cpt2)-regulated fatty acid uptake pathway in bone impaired the postnatal bone acquisition in female mice, but not in male mice [7].
Twenty-five years ago, some researchers thought that excess dietary protein would induce more bone resorption by causing extra serum potassium and excreting more acid [8]. However, this view has been challenged by more recent studies. Dietary proteins are recommended to improve bone loss by forming an organic matrix of bone such as collagen, as indicated by a cohort study from 1966 to 2008. This study showed a diet containing 25–30% of total energy from protein every day, and 1.4 g protein/kg/day with three daily servings of dairy had a positive effect on lumbar spine BMD in adult people [9].
Proteins are formed by amino acids. There are eight EAAs: lysine (Lys), tryptophan (Trp), phenylalanine (Phe), methionine (Met), threonine (Thr), isoleucine (Ile), leucine (Leu), and valine (Val). Branched-chain amino acids (BCAAs) are amino acids having an aliphatic side chain with a branch. In EAAs, isoleucine, leucine, and valine are BCAAs [10], which are more intensively studied, mostly because previous studies found that they are effective in losing weight and keeping muscle. People have to absorb EAAs from food. According to the Cleveland Clinic, the best sources of EAAs are animal proteins, as they are the most easily absorbed and used by the human body. Complete proteins, including beef, poultry, fish, eggs, dairy, soy, quinoa, and buckwheat, contain all the EAAs. Incomplete proteins, such as nuts, some grains, and vegetables, mainly contain one or several EAAs [11] (Figure 1). Leucine, isoleucine, valine, serine, and threonine have been reported to correlate with an increase in longevity [12]. Muscle and bone are two interconnected tissues [13]. EAA supplementation has been shown to improve muscle function in the elderly with normal activity [14,15] and during bed rest [16]. However, the effect on age-related osteoporosis was not reported in this study. The functions of EAAs in aging bone have not been systematically reviewed. Since there is more and more knowledge about bone as a metabolic organ, EAAs should be studied more with regard to aging bone in the future. This study aimed to discuss the contribution of EAAs in aging bone as an energy provider and as a molecular signal modulator both in vitro and in vivo.
Figure 1.
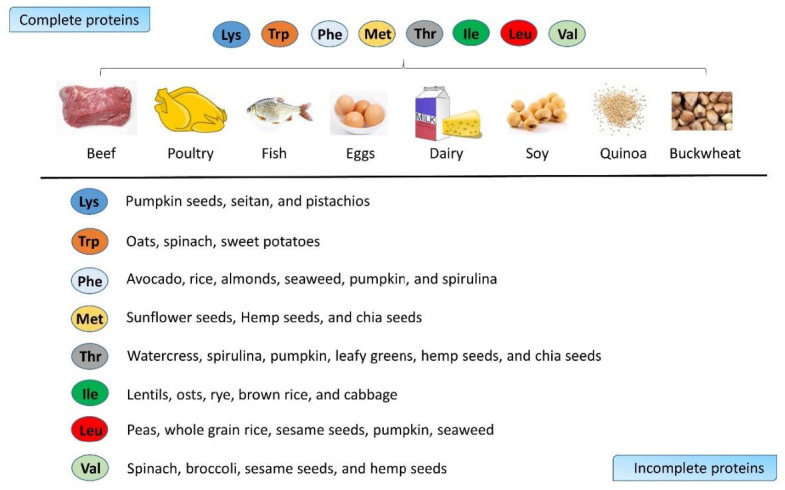
Sources of EAAs from complete proteins and incomplete proteins.
2. Amino Acid as Energy Source and Tissue Builder in Bones
Citrate is an intermediate product in the tricarboxylic acid cycle (TCA cycle, Krebs cycle), which means it can be generated by the metabolism of glucose, and fatty and amino acids. Dickens et al. proved that approximately 90% of citrate was in mineralized tissue in mammals [17]. In the recent 10 years, with solid-state nuclear magnetic resonance spectroscopy (NMR) and other higher technologies, citrate has been found incorporated in the calcium–phosphate structure, to form crystals in mineralized bone, which is the foundation of hard tissue [18,19,20]. The citrate content levels of bone and plasma are both reduced in osteoporotic and aged animals [21]. Therefore, the source of mineral citrate has been an interesting topic in the recent few years. In 2018, X Fu et al. proved that part of the citrate is directly generated from glucose from BMSCs upon differentiation into osteoblasts [22].
The mass spectrometry technique is used to detect metabolic tracking from stable isotope tracers [23]. 13C is the predominant isotope used because of its universal distribution in almost every cellular organic molecule [24]. In metabolic substrate analysis, M + 0 is an unlabeled molecule, while M + 1, M + 2, M + 3, etc., are different numbers of carbon labeled with 13C [25]. Using 13C-tracing technology, Y Yu et al. demonstrated that in differentiated mineralized skeletal stem cells (SSCs), glutamine’s contribution to citrate (M + 4 and M + 5) was reduced compared with undifferentiated SSCs, which means glutamine contributes more during the differentiation procedure, but is not the major energetic substrate in osteoblasts [26]. Although there is no direct evidence of citrate produced by EAAs, from Yu’s study, we can hypothesize that EAAs also might not be the major energy source in osteoblasts, while whether they are the source of citrate during differentiation from SSCs to osteoblasts still needs to be investigated.
3. Amino Acid Sensing and Bone Metabolism
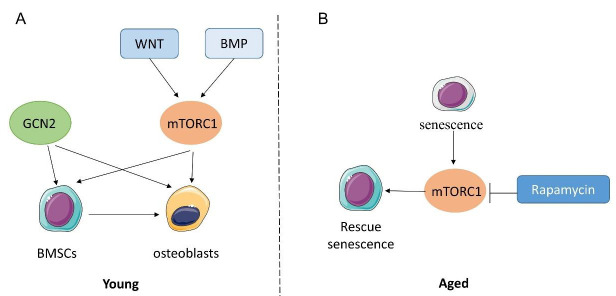
The ability to sense and react to amino acids is important in bone as well as other tissues. General control nonderepressible 2 (GCN2) is an important amino acid sensor triggered by amino acid deprivation. In drosophila, GCN2 is associated with diet restriction-induced life span extension [27]. The Eukaryotic Translation Initiation Factor 2 Alpha Kinase 4 (Eif2ak4)/GCN2/Activating Transcription Factor 4 (ATF4) pathway is required for the uptake of amino acids and proliferation of cells in primary BMSCs [28]. In osteoblasts, the GCN2/ATF4 pathway is also needed for osteoblast differentiation and bone formation in response to wingless and INT (WNT) signaling, but Eif2ak4 is not required in osteoblasts differentiation [29] (Figure 2A).
Figure 2.
GCN2 and mTORC1 signaling in BMSC-osteoblast lineage cells in vitro at young (A) and aged (B) stage.
Another key amino acid sensor is mTOR, which is decreased in calorie restriction-induced longevity [30]. Both rapamycin treatment [31] and the genetic deletion of mTOR downstream p70 ribosomal protein S6 kinase 1 (S6K1) [32] can extend lifespan. There are two protein complexes of mTOR, mTORC1 and mTORC2. mTORC1 can be stimulated by amino acids while mTORC2 is not sensitive to amino acid sensing [33]. The inhibition of mTORC1 with rapamycin impairs BMSC proliferation and differentiation, inducing trabecular bone loss [34]. WNT promotes mTORC1 signaling to mediate glutamine catabolism, resulting in increased osteoblast anabolism [29]. Moreover, bone morphogenetic proteins (BMP) also induce osteoblast differentiation via mTORC1 signaling in vivo [35]. These studies elucidate that in young mice, mTORC1 works as an anabolic signal for bone formation, mainly through increasing BMSC and osteoblast proliferation and differentiation (Figure 2A). In 2021, L Stukenforg et al. found that in BMSCs isolated from older individuals characterized by cell senescence, the inhibition of the mTOR pathway could prevent senescence, extend BMSC proliferation, and maintain stemness of the cells [36]. In an accelerated aging disorder mouse model with Hutchinson–Gilford progeria syndrome (HGPS), H Liu et al. showed that mTORC1 signaling was increased in stress-induced MSC senescence, and also in a D-galactose-induced osteoporosis model in rats. The inhibition of the mTORC1 pathway rescued the senescence phenotype in vitro [37] (Figure 2B). Taken together, the GCN2 signal has not been investigated in aging bone yet, while the role of mTOR in aged-induced osteoporosis models remains controversial, especially in vivo. There is a lot to be done to study the role of mTOR and GCN2 signaling in an aging bone model.
4. The Effect of EAAs on Bone Health in Animals and Cells
Studies with mouse models and cells have mostly focused on the mechanism underlying how EAAs regulate bone health. In addition to population-based surveys, there are also studies regarding EAAs in primary cells isolated from human bone. Residing in the bone marrow niche, BMSCs are multipotent stem cells able to differentiate into osteoblasts, adipocytes, and chondroblasts [38]. In human primary osteoblasts derived from both healthy bone and osteopenic bone, the supplementation of lysine has a positive effect on osteoblast proliferation, activation, and differentiation by inducing nitric oxide (NO), insulin-like growth factor-I (IGF1), osteocalcin, and alkaline phosphatase (ALP) activities [39]. In cultured human osteoblasts, the administration of lysine, but not arginine, reduced levels of transforming growth factor-β1 (TGF-β1) [40], which is produced by osteoblast-like cells and mediates normal bone remodeling. In addition to TGF-β1, lysine exposure also inhibited IL-6 levels, suggesting an anti-resorptive effect [39,40].
The effect of BCAA-enriched short peptides in human BMSCs has also been observed [41]. M Huttunen et al. discovered that using 50 μM isoleucine-proline-proline (IPP) could increase BMSC proliferation. However, in contrast, 50 μM leucine-proline-proline (LPP) or 50 μM valine-proline-proline (VPP) showed a very modest influence on osteoblastic gene expression. In the follow-up experiment, IPP was shown to up-regulate Parathyroid Hormone-Related Peptide (PTHrP), BMP-5, and cAMP response element binding protein-5 (CREB-5), and down-regulate Vitamin D receptor (VDR) and caspase-8, which reflects increasing osteoblast proliferation and differentiation.
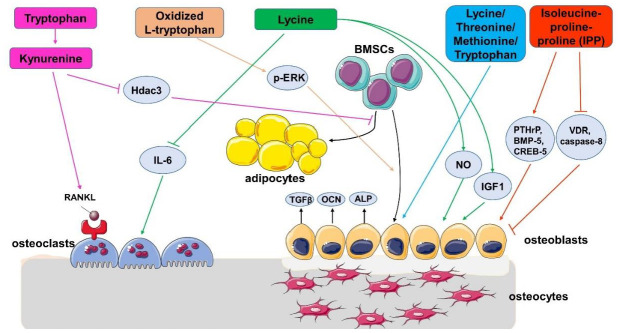
In 1957, Wasserman et al. observed the effect of lysine on calcium absorption in rat and chicken [42]. In primary osteoblasts, obtained from newborn SD rats, adding lysine, threonine, methionine, or tryptophan can enhance cell growth and ALP activity, as well as collagen synthesis at lower degrees, causing an increase in bone formation [43]. Age-induced bone loss has been associated with the increased generation of reactive oxygen species (ROS), which oxidize the surrounding proteins and enzymes to reduce their normal function. In mouse BMSCs cultured in osteogenic media, oxidized L-tryptophan enhanced cell proliferation and differentiation by enhancing the runt-related transcription factor 2 (Runx2)-osteocalcin and ALP expression. Oxidized L-tryptophan also increased extracellular-signal-regulated kinase (ERK) phosphorylation, which increased cell survival and proliferation [44].
M Refaey et al. discovered that a 50 μM phenylalanine-tryptophan combination could decrease the early and late markers of osteoclasts in cell culture, including the RNA levels of the vitronectin receptor, cathepsin K, indicating that this may inhibit osteoclast differentiation [45]. However, in the in vitro resorption experiment, both phenylalanine and tryptophan increased the resorption pits, which is contradictory to the RNA result. The author guessed that maybe phenylalanine and tryptophan mainly regulate the attachment of osteoclasts. Later, M Refaey continued the research of tryptophan in bone metabolism. In 2017, M Refaey found that kynurenine, which is an oxidation product of tryptophan, accumulated with aging [46]. Subsequent research has demonstrated that kynurenine can induce osteoclast activity by inducing the receptor activator of the NF-κB ligand (RANKL) pathway, and increase the differentiation of adipocytes from BMSCs by inhibiting Hdac3 signaling (Figure 3).
Figure 3.
Potential mechanisms of action of essential amino acids in bone metabolism.
5. Relationship between Body EAAs Levels and Bone Quality in Human Research
The role of body EAA content on bone health is inconsistent across various studies. Using Mendelian randomization analysis, Z Cui et al. revealed that isoleucine and valine levels were positively associated with total BMD in European adults [47]. In middle-aged men (31–66 years) with idiopathic osteoporosis, most serum EAAs were not different from that of age-matched controls, except for a significant increase in the threonine level. Erythrocyte amino acids have also been tested and revealed a significant decrease in lysine, phenylalanine, and tryptophan levels in idiopathic osteoporosis patients [48].
A study in China found that in older community-dwelling adults of 1424 men and 1573 women with a mean age of 72 years, serum phenylalanine, tryptophan, methionine, valine, leucine, and isoleucine were significantly lower in osteoporosis subjects [49] (Table 1). These studies suggest that in old people with osteoporosis, the serum EAA levels are usually down-regulated. Most of the studies revealed that EAA levels are positively related with bone conditions, with only Pernow’s study in Sweden showing threonine negatively changing with bone quality [48]. This might be because a very small sample number was used in this study, as the 20 participants, with a large age range from 31 to 66 years old, resulted in a big age variation; thus, this conclusion is not as strong as others.
Table 1.
EAA content in aging people with osteoporosis.
| Participants Number | Participants Background | Age | Tissue | EAAs | Relationship between EAA Changes and Bone Condition | Publication Year | Reference |
|---|---|---|---|---|---|---|---|
| 502,639 | European | 37–70 | Serum | Isoleucine, valine | Positively associated with total BMD | 2021 | [47] |
| 20–22 | Sweden | 31–66 years | Serum | Threonine | Significant increase in people with osteoporosis | 2010 | [48] |
| 20–22 | Sweden | 31–66 years | Erythrocyte | Lysine, phenylalanine, and tryptophan | Significant decrease in people with osteoporosis | 2010 | [48] |
| 1424 men and 1573 women | Chinese | mean age 72 | Serum | Phenylalanine, tryptophan, methionine, valine leucine, and isoleucine | Significantly lower in osteoporosis subjects | 2019 | [49] |
6. Supplementation of EAAs on Bone Quality in Aging People
In 1120 postmenopausal females aged over 50 years, the food source of amino acids was investigated and it was found that leucine and lysine from vegetables are related to a significantly less prevalence of osteoporosis ratios [50]. In addition, monozygotic twins with an intake of leucine and lysine were associated with higher BMD at the spine and forearm, notably, leucine has the strongest association [50]. As a BCAA, there are a lot of studies that have investigated the effect of leucine on bone health, based on the results of this monozygotic twin study.
In a double-blind study of 380 participants older than 65 years, a leucine-enriched whey protein supplement (with a daily supplement of 6 g leucine) for 13 weeks improved the BMD in a small but significant range [51]. C Lin et al. obtained a similar conclusion in another open-label, parallel-group study. Twenty-eight people received a vitamin D- and leucine-enriched whey protein supplement for 12 weeks, and achieved a significant improvement in walking speed in the age 65–74 group [52]. However, other studies have shown neither a benefit nor a harmful effect of leucine supplementation on bone health. In a Japanese study, the aging people were divided into two groups, one taking 0.8 g citrulline and 1.6 g leucine twice daily for 20 weeks, and another group taking a placebo. Both groups were accompanied by exercise, and the results showed no difference in BMD and bone area in the two groups [53]. Another study took place in Northwest England, giving leucine-enriched whey protein drinks to aged 60–90 people for 16 weeks, which also did not show any difference in bone mass between groups [54] (Table 2). The difference in the leucine supplementation effects in Table 2 might be caused by the differences in the sample number, leucine content in the supplement, and the measured index of bone. A small group number (13–30) is not as reliable as hundreds or thousands of sample quantities, and BMD, bone mass, and walking speed do not always change together. In a study in Taipei, leucine was supplied together with vitamin D, which may better improve the absorption of calcium to enhance walking ability [52].
Table 2.
Impact of EAA supplementation in aging bone.
| Participants Number in Each Group | Participant Background | Age | EAAs Supplement | EAA Treatment Time | Changes after EAA Supplementation | Publication Year | Reference |
|---|---|---|---|---|---|---|---|
| 184–196 | 6 European countries: Belgium, Germany, Ireland, Italy, Sweden, and the UK | Older than 65 years | 6 g of leucine with 40 g of whey protein daily | 13 weeks | BMD increasing in a small but significant range | 2019 | [51] |
| 28 | Taipei | Older than 65 | 1.2 g leucine enriched whey protein supplement | 12 weeks | Significant improvement in walking speed in age 65–74 group | 2021 | [52] |
| 13 | Japanese | 65–80 years | 1.6 g leucine twice daily | 20 weeks | No effect on BMD and bone area | 2021 | [53] |
| 22–31 | Northwest England | 60–90 years, mean age 68.73 ± 5.80 years | 1.50 g/kg BW/day whey protein, plus 0.03 g/kg BW leucine | 16 weeks | No effect on bone mass | 2021 | [54] |
7. Conclusions
EAAs are important in aging as a nutritional source and as a signaling regulator. In aging people with bone loss, serum EAA levels are not always consistent in different studies; thus, there is not a well-recognized conclusion about the correlation between serum EAAs and bone volume. In people supplied with extra EAAs, most of the studies showed that EAAs are beneficial for BMD; although, some also showed no effect. The inconsistency of these results is mainly because of the small number of people in the testing groups, and the different nutrients supplied with EAAs at the same time. Therefore, the studies with larger sample numbers are more reliable, which illustrated that EAAs improved BMD. EAAs regulated bone health by increasing the osteogenic differentiation of BMSCs/osteoblasts and by inducing the RANKL-regulated osteoclast pathway (Figure 3).
The function of EAAs in aging bone will be more extensively studied in the future, to better understand it as a metabolic organ. Currently, there are many shortfalls in the human studies, such as the small group of samples, the variations in age, no detection of the absorption rate after amino acid supplementation, etc. In animal and cell studies, some conclusions concerning the functions of EAA-related pathways are conflicted with others. With more studies likely in the future, the correlation between specific EAAs and aging bone metabolism will become clearer.
Acknowledgments
We thank Kai Fu (Xiangya Hospital, Central South University) for the comments and the editing of our manuscript. Part of Figure 2 and Figure 3 was modified from Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License: http://smart.servier.com/.
Author Contributions
Conceptualization, Q.Z.; writing—original draft preparation: Z.L. and W.S.; writing—review and editing: W.S. and Q.Z.; supervision: Q.Z. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Chinese Universities Scientific Fund (2022RC040).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vianna D., Resende G.F., Torres-Leal F.L., Pantaleao L.C., Donato J., Jr., Tirapegui J. Long-term leucine supplementation reduces fat mass gain without changing body protein status of aging rats. Nutrition. 2012;28:182–189. doi: 10.1016/j.nut.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Chen X., Wang Z., Duan N., Zhu G., Schwarz E.M., Xie C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018;59:99–107. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teitelbaum S.L. Osteoclasts: What do they do and how do they do it? Am. J. Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown J.P., Adachi J.D., Schemitsch E., Tarride J.E., Brown V., Bell A., Reiner M., Oliveira T., Motsepe-Ditshego P., Burke N., et al. Mortality in older adults following a fragility fracture: Real-world retrospective matched-cohort study in Ontario. BMC Musculoskelet. Disord. 2021;22:105. doi: 10.1186/s12891-021-03960-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tangseefa P., Martin S.K., Chin P.Y., Breen J., Mah C.Y., Baldock P.A., Wittert G.A., Page A.J., Proud C.G., Fitter S., et al. The mTORC1 complex in pre-osteoblasts regulates whole-body energy metabolism independently of osteocalcin. Bone Res. 2021;9:10. doi: 10.1038/s41413-020-00123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J., Long F. mTORC1 Signaling Promotes Osteoblast Differentiation from Preosteoblasts. PLoS ONE. 2015;10:e0130627. doi: 10.1371/journal.pone.0130627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S.P., Li Z., Zoch M.L., Frey J.L., Bowman C.E., Kushwaha P., Ryan K.A., Goh B.C., Scafidi S., Pickett J.E., et al. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner. JCI Insight. 2017;2:e92704. doi: 10.1172/jci.insight.92704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barzel U.S., Massey L.K. Excess dietary protein can adversely affect bone. J. Nutr. 1998;128:1051–1053. doi: 10.1093/jn/128.6.1051. [DOI] [PubMed] [Google Scholar]
- 9.Darling A.L., Millward D.J., Torgerson D.J., Hewitt C.E., Lanham-New S.A. Dietary protein and bone health: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2009;90:1674–1692. doi: 10.3945/ajcn.2009.27799. [DOI] [PubMed] [Google Scholar]
- 10.Atherton P.J., Smith K., Etheridge T., Rankin D., Rennie M.J. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38:1533–1539. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 11.Wu G. Amino acids: Metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 12.Mirzaei H., Suarez J.A., Longo V.D. Protein and amino acid restriction, aging and disease: From yeast to humans. Trends Endocrinol. Metab. 2014;25:558–566. doi: 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tagliaferri C., Wittrant Y., Davicco M.J., Walrand S., Coxam V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015;21:55–70. doi: 10.1016/j.arr.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Katsanos C.S., Kobayashi H., Sheffield-Moore M., Aarsland A., Wolfe R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiology. Endocrinol. Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 15.Jihyun Im H.P.a.K.P. Dietary Essential Amino Acid Intake Is Associated with High Muscle Strength in Korean Older Adults. Nutritents. 2022;14:3104. doi: 10.3390/nu14153104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrando A.A., Paddon-Jones D., Hays N.P., Kortebein P., Ronsen O., Williams R.H., McComb A., Symons T.B., Wolfe R.R., Evans W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin. Nutr. 2010;29:18–23. doi: 10.1016/j.clnu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Dickens F. The citric acid content of animal tissues, with reference to its occurrence in bone and tumour. Biochem. J. 1941;35:1011–1023. doi: 10.1042/bj0351011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lotsari A., Rajasekharan A.K., Halvarsson M., Andersson M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018;9:4170. doi: 10.1038/s41467-018-06570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies E., Muller K.H., Wong W.C., Pickard C.J., Reid D.G., Skepper J.N., Duer M.J. Citrate bridges between mineral platelets in bone. Proc. Natl. Acad. Sci. USA. 2014;111:E1354–E1363. doi: 10.1073/pnas.1315080111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delgado-Lopez J.M., Bertolotti F., Lyngso J., Pedersen J.S., Cervellino A., Masciocchi N., Guagliardi A. The synergic role of collagen and citrate in stabilizing amorphous calcium phosphate precursors with platy morphology. Acta. Biomater. 2017;49:555–562. doi: 10.1016/j.actbio.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 21.Chen H., Wang Y., Dai H., Tian X., Cui Z.K., Chen Z., Hu L., Song Q., Liu A., Zhang Z., et al. Bone and plasma citrate is reduced in osteoporosis. Bone. 2018;114:189–197. doi: 10.1016/j.bone.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Fu X., Li Y., Huang T., Yu Z., Ma K., Yang M., Liu Q., Pan H., Wang H., Wang J., et al. Runx2/Osterix and Zinc Uptake Synergize to Orchestrate Osteogenic Differentiation and Citrate Containing Bone Apatite Formation. Adv. Sci. 2018;5:1700755. doi: 10.1002/advs.201700755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tea I., De Luca A., Schiphorst A.M., Grand M., Barille-Nion S., Mirallie E., Drui D., Krempf M., Hankard R., Tcherkez G. Stable Isotope Abundance and Fractionation in Human Diseases. Metabolites. 2021;11:370. doi: 10.3390/metabo11060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Falco F.G.B., Carteni F., Mazzoleni S., Kim D.-H. Metabolic flux analysis: A comprehensive review on sample preparation, analytical techniques, data analysis, computational modelling, and main application areas. RSC Adv. 2022;12 doi: 10.1039/d2ra03326g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long C.P., Antoniewicz M.R. High-resolution (13)C metabolic flux analysis. Nat. Protoc. 2019;14:2856–2877. doi: 10.1038/s41596-019-0204-0. [DOI] [PubMed] [Google Scholar]
- 26.Yu Y., Newman H., Shen L., Sharma D., Hu G., Mirando A.J., Zhang H., Knudsen E., Zhang G.F., Hilton M.J., et al. Glutamine Metabolism Regulates Proliferation and Lineage Allocation in Skeletal Stem Cells. Cell Metab. 2019;29:966–978.e964. doi: 10.1016/j.cmet.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava A., Lu J., Gadalla D.S., Hendrich O., Gronke S., Partridge L. The Role of GCN2 Kinase in Mediating the Effects of Amino Acids on Longevity and Feeding Behaviour in Drosophila. Front. Aging. 2022;3:944466. doi: 10.3389/fragi.2022.944466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu G., Yu Y., Tang Y.J., Wu C., Long F., Karner C.M. The Amino Acid Sensor Eif2ak4/GCN2 Is Required for Proliferation of Osteoblast Progenitors in Mice. J. Bone Miner. Res. 2020;35:2004–2014. doi: 10.1002/jbmr.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karner C.M., Esen E., Okunade A.L., Patterson B.W., Long F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J. Clin. Investig. 2015;125:551–562. doi: 10.1172/JCI78470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg E.L., Romero-Aleshire M.J., Renkema K.R., Ventevogel M.S., Chew W.M., Uhrlaub J.L., Smithey M.J., Limesand K.H., Sempowski G.D., Brooks H.L., et al. Lifespan-extending caloric restriction or mTOR inhibition impair adaptive immunity of old mice by distinct mechanisms. Aging Cell. 2015;14:130–138. doi: 10.1111/acel.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., Nadon N.L., Wilkinson J.E., Frenkel K., Carter C.S., et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selman C., Tullet J.M., Wieser D., Irvine E., Lingard S.J., Choudhury A.I., Claret M., Al-Qassab H., Carmignac D., Ramadani F., et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki T., Inoki K. Spatial regulation of the mTORC1 system in amino acids sensing pathway. Acta Biochim. Et. Biophys. Sin. 2011;43:671–679. doi: 10.1093/abbs/gmr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singha U.K., Jiang Y., Yu S., Luo M., Lu Y., Zhang J., Xiao G. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J. Cell. Biochem. 2008;103:434–446. doi: 10.1002/jcb.21411. [DOI] [PubMed] [Google Scholar]
- 35.Karner C.M., Lee S.Y., Long F. Bmp Induces Osteoblast Differentiation through both Smad4 and mTORC1 Signaling. Mol. Cell. Biol. 2017;37 doi: 10.1128/MCB.00253-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stukenborg L., Sell C., Potnis M. MTOR Inhibition Alters miRNA Profile in Cultured Bone Marrow Mesenchymal Stem Cells. Innov. Aging. 2021;5:667. doi: 10.1093/geroni/igab046.2516. [DOI] [Google Scholar]
- 37.Liu H., Huang B., Xue S., U K.P., Tsang L.L., Zhang X., Li G., Jiang X. Functional crosstalk between mTORC1/p70S6K pathway and heterochromatin organization in stress-induced senescence of MSCs. Stem Cell Res. Ther. 2020;11:279. doi: 10.1186/s13287-020-01798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiozawa Y., Havens A.M., Pienta K.J., Taichman R.S. The bone marrow niche: Habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008;22:941–950. doi: 10.1038/leu.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torricelli P., Fini M., Giavaresi G., Giardino R. Human osteopenic bone-derived osteoblasts: Essential amino acids treatment effects. Artif. Cells Blood Substit. Immobil. Biotechnol. 2003;31:35–46. doi: 10.1081/BIO-120018002. [DOI] [PubMed] [Google Scholar]
- 40.Torricelli P., Fini M., Giavaresi G., Giardino R., Gnudi S., Nicolini A., Carpi A. L-arginine and L-lysine stimulation on cultured human osteoblasts. Biomed. Pharmacother. Biomed. Pharmacother. 2002;56:492–497. doi: 10.1016/S0753-3322(02)00287-1. [DOI] [PubMed] [Google Scholar]
- 41.Huttunen M.M., Pekkinen M., Ahlstrom M.E., Lamberg-Allardt C.J. Effects of bioactive peptides isoleucine-proline-proline (IPP), valine-proline-proline (VPP) and leucine-lysine-proline (LKP) on gene expression of osteoblasts differentiated from human mesenchymal stem cells. Br. J. Nutr. 2007;98:780–788. doi: 10.1017/S0007114507744434. [DOI] [PubMed] [Google Scholar]
- 42.Wasserman R.H., Comar C.L., Schooley J.C., Lengemann F.W. Interrelated effects of L-lysine and other dietary factors on the gastrointestinal absorption of calcium 45 in the rat and chick. J. Nutr. 1957;62:367–376. doi: 10.1093/jn/62.3.367. [DOI] [PubMed] [Google Scholar]
- 43.Conconi M.T., Tommasini M., Muratori E., Parnigotto P.P. Essential amino acids increase the growth and alkaline phosphatase activity in osteoblasts cultured in vitro. Farmaco. 2001;56:755–761. doi: 10.1016/S0014-827X(01)01126-0. [DOI] [PubMed] [Google Scholar]
- 44.El Refaey M., Watkins C.P., Kennedy E.J., Chang A., Zhong Q., Ding K.H., Shi X.M., Xu J., Bollag W.B., Hill W.D., et al. Oxidation of the aromatic amino acids tryptophan and tyrosine disrupts their anabolic effects on bone marrow mesenchymal stem cells. Mol. Cell. Endocrinol. 2015;410:87–96. doi: 10.1016/j.mce.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Refaey M.E., Zhong Q., Ding K.H., Shi X.M., Xu J., Bollag W.B., Hill W.D., Chutkan N., Robbins R., Nadeau H., et al. Impact of dietary aromatic amino acids on osteoclastic activity. Calcif. Tissue Int. 2014;95:174–182. doi: 10.1007/s00223-014-9878-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Refaey M.E., McGee-Lawrence M.E., Fulzele S., Kennedy E.J., Bollag W.B., Elsalanty M., Zhong Q., Ding K.H., Bendzunas N.G., Shi X.M., et al. Kynurenine, a Tryptophan Metabolite That Accumulates With Age, Induces Bone Loss. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017;32:2182–2193. doi: 10.1002/jbmr.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui Z., Feng H., He B., He J., Tian Y. Relationship Between Serum Amino Acid Levels and Bone Mineral Density: A Mendelian Randomization Study. Front. Endocrinol. 2021;12:763538. doi: 10.3389/fendo.2021.763538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pernow Y., Thoren M., Saaf M., Fernholm R., Anderstam B., Hauge E.M., Hall K. Associations between amino acids and bone mineral density in men with idiopathic osteoporosis. Bone. 2010;47:959–965. doi: 10.1016/j.bone.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Su Y., Elshorbagy A., Turner C., Refsum H., Chan R., Kwok T. Circulating amino acids are associated with bone mineral density decline and ten-year major osteoporotic fracture risk in older community-dwelling adults. Bone. 2019;129:115082. doi: 10.1016/j.bone.2019.115082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jennings A., MacGregor A., Spector T., Cassidy A. Amino Acid Intakes Are Associated With Bone Mineral Density and Prevalence of Low Bone Mass in Women: Evidence From Discordant Monozygotic Twins. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016;31:326–335. doi: 10.1002/jbmr.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hill T.R., Verlaan S., Biesheuvel E., Eastell R., Bauer J.M., Bautmans I., Brandt K., Donini L.M., Maggio M., Mets T., et al. A Vitamin D, Calcium and Leucine-Enriched Whey Protein Nutritional Supplement Improves Measures of Bone Health in Sarcopenic Non-Malnourished Older Adults: The PROVIDE Study. Calcif. Tissue Int. 2019;105:383–391. doi: 10.1007/s00223-019-00581-6. [DOI] [PubMed] [Google Scholar]
- 52.Lin C.C., Shih M.H., Chen C.D., Yeh S.L. Effects of adequate dietary protein with whey protein, leucine, and vitamin D supplementation on sarcopenia in older adults: An open-label, parallel-group study. Clin. Nutr. 2021;40:1323–1329. doi: 10.1016/j.clnu.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 53.Kim M., Isoda H., Okura T. Effect of Citrulline and Leucine Intake with Exercises on Body Composition, Physical Activity, and Amino Acid Concentration in Older Women: A Randomized Double-Blind Placebo-Controlled Study. Foods. 2021;10:3117. doi: 10.3390/foods10123117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirk B., Mooney K., Vogrin S., Jackson M., Duque G., Khaiyat O., Amirabdollahian F. Leucine-enriched whey protein supplementation, resistance-based exercise, and cardiometabolic health in older adults: A randomized controlled trial. J. Cachexia Sarcopenia Muscle. 2021;12:2022–2033. doi: 10.1002/jcsm.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]