Abstract
The mitogen-activated protein kinase (MAPK) pathway is a widely distributed signaling cascade in eukaryotes and is involved in regulating plant growth, development, and stress responses. High temperature, a frequently occurring environmental stressor, causes premature bolting in lettuce with quality decline and yield loss. However, whether MAPKs play roles in thermally induced bolting remains poorly understood. In this study, 17 LsMAPK family members were identified from the lettuce genome. The physical and chemical properties, subcellular localization, chromosome localization, phylogeny, gene structure, family evolution, cis-acting elements, and phosphorylation sites of the LsMAPK gene family were evaluated via in silico analysis. According to phylogenetic relationships, LsMAPKs can be divided into four groups, A, B, C, and D, which is supported by analyses of gene structure and conserved domains. The collinearity analysis showed that there were 5 collinearity pairs among LsMAPKs, 8 with AtMAPKs, and 13 with SlMAPKs. The predicted cis-acting elements and potential phosphorylation sites were closely associated with hormones, stress resistance, growth, and development. Expression analysis showed that most LsMAPKs respond to high temperatures, among which LsMAPK4 is significantly and continuously upregulated upon heat treatments. Under heat stress, the stem length of the LsMAPK4-knockdown lines was significantly shorter than that of the control plants, and the microscope observations demonstrated that the differentiation time of flower buds at the stem apex was delayed accordingly. Therefore, silencing of LsMAPK4 significantly inhibited the high- temperature-accelerated bolting in lettuce, indicating that LsMPAK4 might be a potential regulator of lettuce bolting. This study provides a theoretical basis for a better understanding of the molecular mechanisms underlying the MAPK genes in high-temperature-induced bolting.
Keywords: MAPK, lettuce, genome-wide analysis, bioinformatics analysis, high temperature, expression pattern, bolting, virus-induced gene silencing, LsMAPK4
1. Introduction
Mitogen-activated protein kinase (MAPK) is a serine/threonine protein kinase [1]. Through stepwise phosphorylation, MAPK, MAPKK (mitogen-activated protein kinase kinase), and MAPKKK (mitogen-activated protein kinase kinase kinase) collectively form a highly conserved MAPKKK → MAPKK → MAPK cascade, signal-transduction pathway [2]. When MAPKKK is activated by a sensor/receptor it can phosphorylate serine/threonine residues of S/TXXXXXS/T, in which X represents any amino acid, to activate downstream MAPKK, after which MAPKK continues to phosphorylate the conserved threonine and tyrosine residues in the TXY motif downward to activate MAPK [3,4,5,6,7,8]. MAPK cascades respond to almost all biological or abiotic stresses [9,10,11], such as mechanical damage [12], high salt [13], low temperature [14], drought [15] and pathogen invasion [16] and widely participate in the process of plant growth and development [17,18].
MAPK is located downstream of the entire MAPK signaling cascade. After phosphorylation, MAPK has three targets: cytoplasmic protein kinases or transcription factors (TFs), cytoplasmic cytoskeleton, and nuclear transcription factors [19]. Many plant MAPK genes have been studied, and sequence analysis of the whole genome showed that Arabidopsis thaliana has 20 MAPK genes, which can be separated into four groups, A, B, C, and D, according to their phosphorylation motif. The phosphorylation motif of groups A, B, and C was TEY, and that of group D was TDY [9,20]. The C-terminus of MAPK in groups A, B, and C contains the CD domain, which is speculated to be the binding region of upstream MAPKK, phospholipase, and various downstream substrates. The CD domain of group C is modified. Group D does not contain this region [21,22]. In addition to Arabidopsis, 15 MAPK genes were identified in rice [23], 11 in chrysanthemum [24], 43 in cultivated strawberry [25], and 12 in grape [26]. The functions of many MAPK genes have been identified and are known to participate in almost all activities in the plant life cycle, including plant growth and development, response to stress signals, activation of stress resistance gene expression, and improvement in adaptability to adversity [5]. MAPKs play an important role in biotic and abiotic stress responses. Arabidopsis AtMAPK4 has been reported to negatively regulate plant disease resistance and hypertonic resistance [27,28]. In addition, MAPKs are widely involved in hormone synthesis and signal transduction. AtMAPK6 is involved in regulating the polar transport of auxin and promotes ethylene synthesis together with AtMAPK3 [29,30]. Ethylene/jasmonic acid (JA) and MAPK3/MAPK6 cooperate to regulate plant defense [31,32]. ABA induces the expression of the MKK3–MAPK6–MYC2 cascade and actively regulates ABA biosynthesis and signal gene transcription [33]. Soybean GmMAPK4s negatively regulates salicylic acid accumulation and defense [34]. More importantly, MAPKs are largely involved in plant growth and development. Rice OsMAPK2 is involved in flower development, and AtMAPK4 is required for cell plate formation and cell division of male-specific meiosis in Arabidopsis [35]. The model TES/STUD/AtNACK2-AtANPs-AtMKK6-AtMPK4 was proposed to regulate cell meiosis [36,37]. In addition, MAPK also plays an important role in anther development [38,39], ovule development [40], pollen development [17], and seed development [41].
Lettuce is rich in nutrients and is an important component in modern diet and nutrition [42]. Lettuce is sensitive to high temperatures, which can induce premature bolting, resulting in poor growth and reductions in yield and quality. The symbol of bolting is flower bud differentiation, which is the process of rapid stem elongation after flower bud differentiation at the stem tip, accompanied by the transformation from vegetative growth to reproductive growth [43,44]. Bolting is restricted by its development and environment, such as hormones, temperature, and light, and its genetic characteristics, such as cell division, elongation, differentiation, and aging [45,46]. Moreover, the specific regulatory mechanism of high-temperature bolting in lettuce remains unclear, and MAPK has not been reported to play a role in the high temperature-induced bolting of lettuce. Early in this research, we screened differentially expressed MAPK cascade members involved in lettuce bolting at high temperatures, including LsMAPK3, LsMAPK4, LsMAPK9, and LsMAPKK5 protein, using comparative proteomics [47]. Recognizing the crucial role of MAPK in plant growth, development, and stress response, we conducted a genome-wide screening, identification, and bioinformatics analysis of the MAPK gene family in lettuce, determined the differential expression of the MAPK gene family at high temperature, and conducted a virus-induced gene silencing (VIGS) evaluation on LsMAPK4 with significant differential expression.
2. Results
2.1. Identification, Chromosomal Localization, and Physicochemical Property Analysis of LsMAPK Family Members in Lettuce
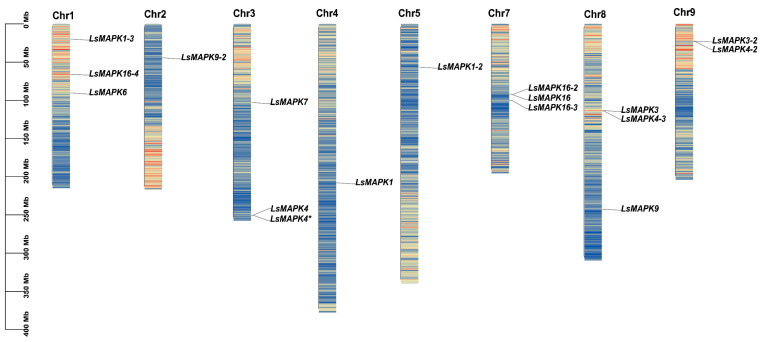
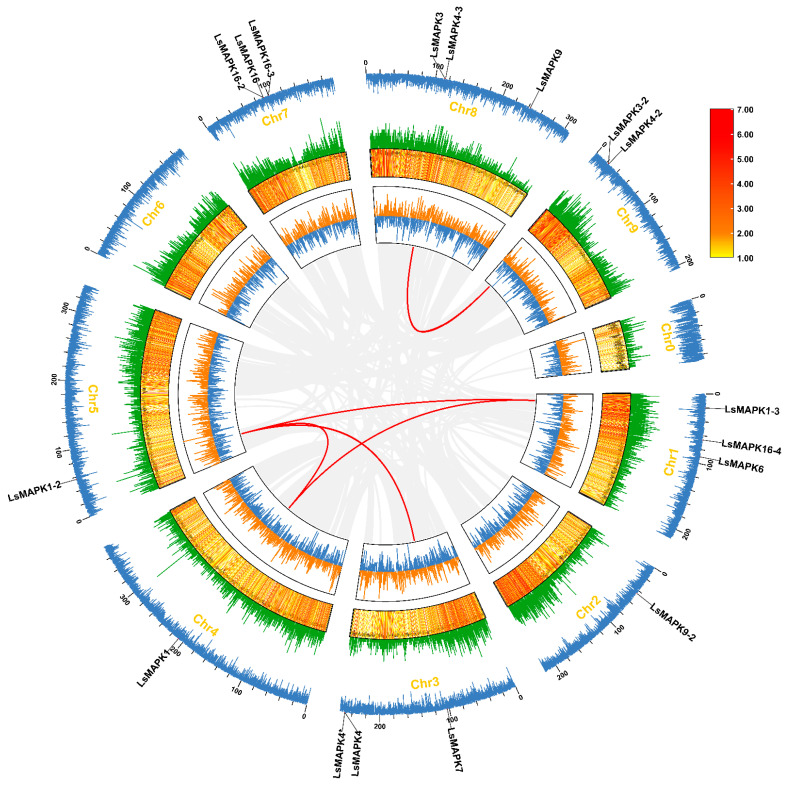
By homology comparison and domain analysis, we identified a total of 17 dependable LsMAPK gene family members in the lettuce genome. According to the unified nomenclature of Arabidopsis AtMAPKs, we comprehensively analyzed the evolutionary relationship between LsMAPKs and AtMAPKs (Figure 1). Based on the analysis, we systematically renamed them according to the phylogenetic relationship (LsMAPKs in Table 1). Among them, LsMAPK4 and LsMAPK4* are identical genes (Figure S1). The corresponding gene name, gene registration number, chromosome position, isomer numbers, protein size, molecular weight, isoelectric point (pI), subcellular localization, and transmembrane domain are summarized in Table 1. The lengths of the LsMAPK proteins ranged from 284 (LsMAPK4-3) to 782 (LsMAPK16-4) amino acids, with a molecular weight ranging from 33.00 to 89.70 kDa. The isomer numbers varied from 1 to 4, and the theoretical pI value scope was from 4.74 (LsMAPK4-3) to 9.08 (LsMAPK16). The subcellular localization of all MAPKs was located in the nucleus without a transmembrane domain. Chromosome mapping results showed that the 17 LsMAPKs were unevenly distributed on 8 lettuce chromosomes, ranging in number from 1 to 3, with none on chromosome 6 (Table 1, Figure 1).
Figure 1.
Chromosomal location of LsMAPKs. The chromosome number is indicated at the top, and the scale on the left represents the chromosome size. Without LsMAPK localization, chromosome 6 is not shown.
Table 1.
Physical and chemical aspects of LsMAPKs.
| Gene Name | Locus ID | Chromosomal Location | Gene Models | Putative Proteins | Subcellular Localization | Transmembrane Domain | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chr | Chr_start | Chr_end | Direction | Length(aa) | MW (kDa) | pI | |||||
| LsMAPK1 | Lsat_1_v5_gn_4_117181 | 4 | 208105012 | 208108237 | − | 2 | 373 | 43.08 | 6.50 | Nucleus | none |
| LsMAPK1-2 | Lsat_1_v5_gn_5_26820 | 5 | 56806062 | 56808833 | − | 2 | 376 | 43.48 | 7.62 | Nucleus | none |
| LsMAPK1-3 | Lsat_1_v5_gn_1_17080 | 1 | 20023975 | 20026544 | − | 1 | 371 | 42.42 | 5.83 | Nucleus | none |
| LsMAPK3 | Lsat_1_v5_gn_8_76860 | 8 | 113432000 | 113434556 | − | 3 | 370 | 42.60 | 5.62 | Nucleus | none |
| LsMAPK3-2 | Lsat_1_v5_gn_9_20480 | 9 | 22563636 | 22565881 | − | 2 | 372 | 42.88 | 5.38 | Nucleus | none |
| LsMAPK4 | Lsat_1_v5_gn_3_137680 | 3 | 250231945 | 250234610 | + | 2 | 378 | 43.46 | 6.32 | Nucleus | none |
| LsMAPK4* | Lsat_1_v5_gn_3_138401 | 3 | 250423836 | 250426191 | + | 1 | 378 | 43.46 | 6.32 | Nucleus | none |
| LsMAPK4-2 | Lsat_1_v5_gn_9_21161 | 9 | 23155272 | 23158265 | + | 2 | 377 | 43.46 | 5.86 | Nucleus | none |
| LsMAPK4-3 | Lsat_1_v5_gn_8_76340 | 8 | 114078543 | 114081325 | − | 2 | 284 | 33.00 | 4.74 | Nucleus | none |
| LsMAPK6 | Lsat_1_v5_gn_1_74260 | 1 | 90387406 | 90392319 | + | 2 | 382 | 43.94 | 5.65 | Nucleus | none |
| LsMAPK7 | Lsat_1_v5_gn_3_77061 | 3 | 103013530 | 103018532 | − | 2 | 410 | 47.51 | 5.89 | Nucleus | none |
| LsMAPK9 | Lsat_1_v5_gn_8_144901 | 8 | 242553674 | 242558284 | − | 2 | 568 | 64.46 | 8.10 | Nucleus | none |
| LsMAPK9-2 | Lsat_1_v5_gn_2_19180 | 2 | 44466873 | 44470986 | − | 3 | 405 | 46.52 | 8.87 | Nucleus | none |
| LsMAPK16 | Lsat_1_v5_gn_7_64441 | 7 | 92076041 | 92080742 | + | 1 | 761 | 85.36 | 9.08 | Nucleus | none |
| LsMAPK16-2 | Lsat_1_v5_gn_7_64461 | 7 | 92052037 | 92059155 | + | 1 | 738 | 84.03 | 7.15 | Nucleus | none |
| LsMAPK16-3 | Lsat_1_v5_gn_7_66400 | 7 | 100166151 | 100169658 | − | 1 | 453 | 51.79 | 6.01 | Nucleus | none |
| LsMAPK16-4 | Lsat_1_v5_gn_1_56481 | 1 | 65962932 | 65970369 | − | 5 | 782 | 89.70 | 8.75 | Nucleus | none |
2.2. Phylogenetic Relationships and Multiple Sequence Alignment Analysis of LsMAPKs
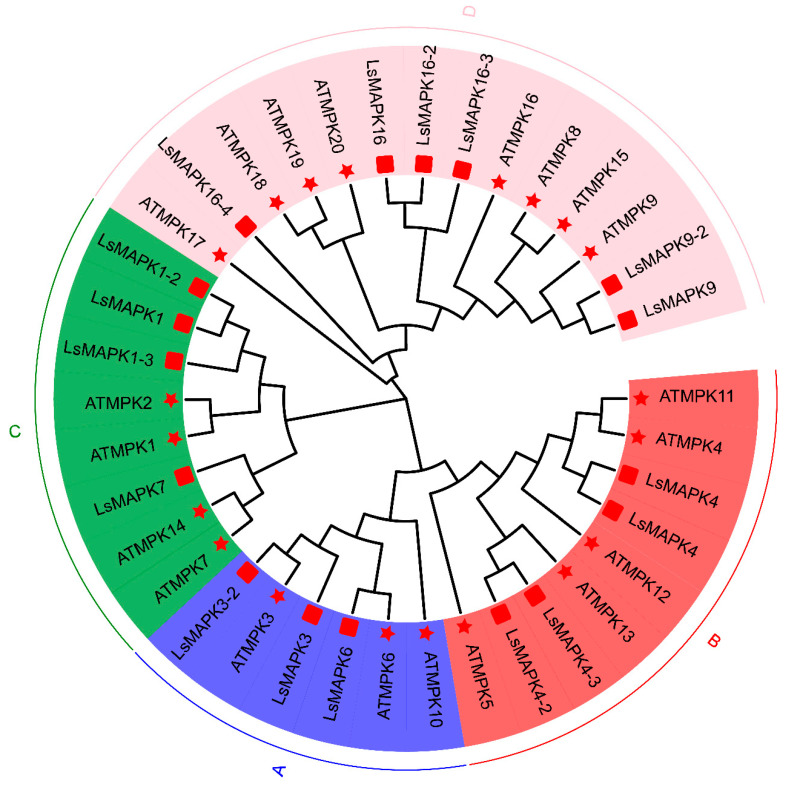
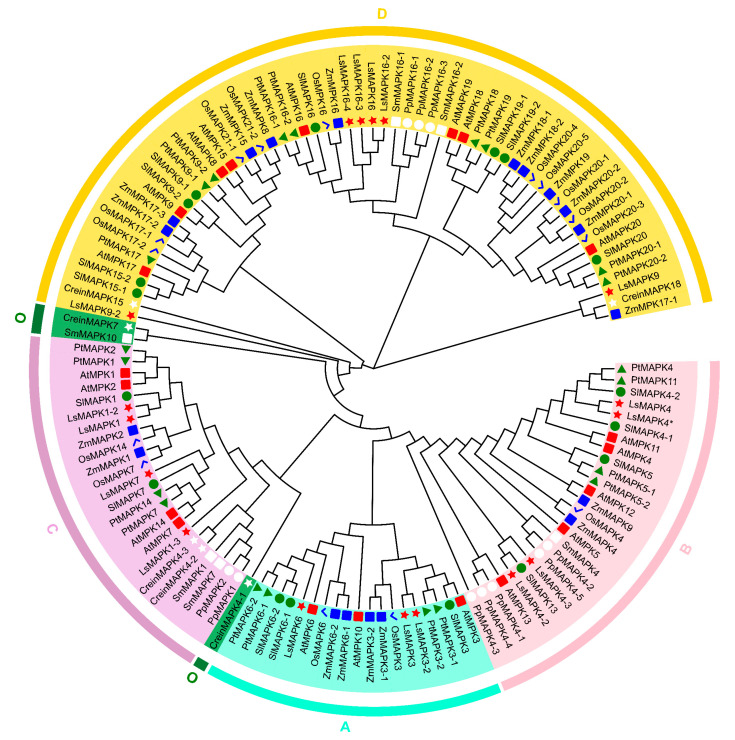
To analyze the phylogenetic relationship and classification of LsMAPKs, a phylogenetic tree was constructed based on AtMAPKs of Arabidopsis thaliana and LsMAPKs of lettuce (Figure 2). LsMAPK members were clustered into four groups: A, B, C, and D, which included 3 (LsMAPK3/3-2/6), 4 (LsMAPK4/4*/4-2/4-3), 4 (LsMAPK1/1-2/1-3/7), and 6 (LsMAPK9/9-2/16/16-2/16-3/16-4) LsMAPKs, respectively. Multisequence alignment analysis showed that all LsMAPKs contained a highly conserved tripeptide motif of TXY (Figure S2). Groups A, B, and C contain TEY, and group D contains TDY. Group A and B members also contain the conserved CD domain in (LH)DXXDE(P)XC, which is considered to be a binding site for MAPKK. In addition, more plant species were used to analyze the differentiation of the MAPK gene family in plants (Figure 3), including Physcomitrella patens, Chlamydomonas reinhardtii, Selaginella moellendorffii, Arabidopsis thaliana, Oryza sativa, Zea mays, Solanum lycopersicum, and Populus trichocarpa. All MAPK genes fell into five groups, and the new group of MAPKs (group O) was mainly composed of lower eukaryotic and fern members, which were CreinMAPK4-1, CreinMAPK7, and SmMAPK10. Compared with higher eukaryotic angiosperms, lower plants encode very few MAPK genes. Among mosses, algae, and ferns, C. reinhardtii lacks the ‘group B’ type of MAPKs. All three species lacked the ‘group A’ type. It can also be seen from the phylogenetic tree that group A had the fewest members and group D had the most members.
Figure 2.
Phylogenetic tree analysis of the MAPK gene family from lettuce and Aradiopsis. Distinct color blocks represent different groups, five-pointed stars represent lettuce, and squares represent Arabidopsis.
Figure 3.
Phylogenetic relationships of the MAPK gene family from Physcomitrella patens (PpMAPKs), Chlamydomonas reinhardtii (CreinMAPKs), Selaginella moellendorffii (SmMAPKs), Arabidopsis thaliana (AtMAPKs), Oryza sativa (OsMAPKs), Zea mays (ZmMAPKs), Solanum lycopersicum (SlMAPKs), Populus trichocarpa (PtMAPKs), and Lactuca sativa (LsMAPKs). Distinct color blocks represent different groups. The different shapes and colors of the symbols indicate different species.
2.3. Gene Structure and Motif Location Analysis of LsMAPKs
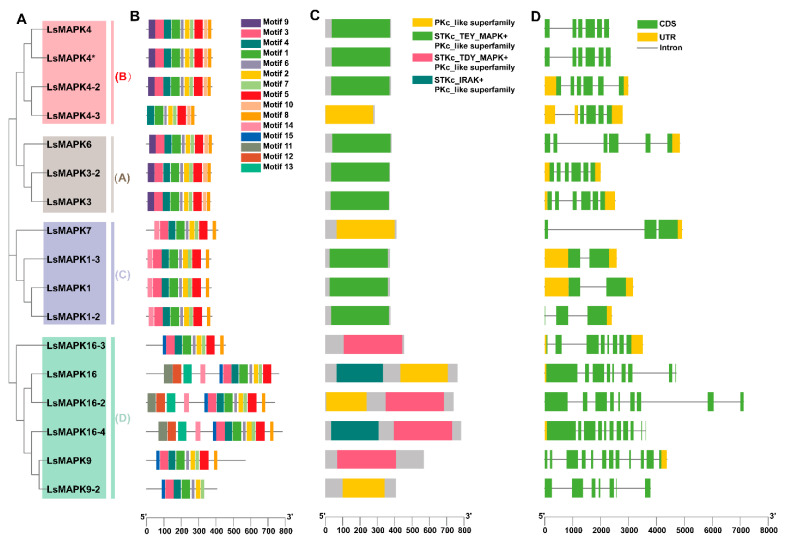
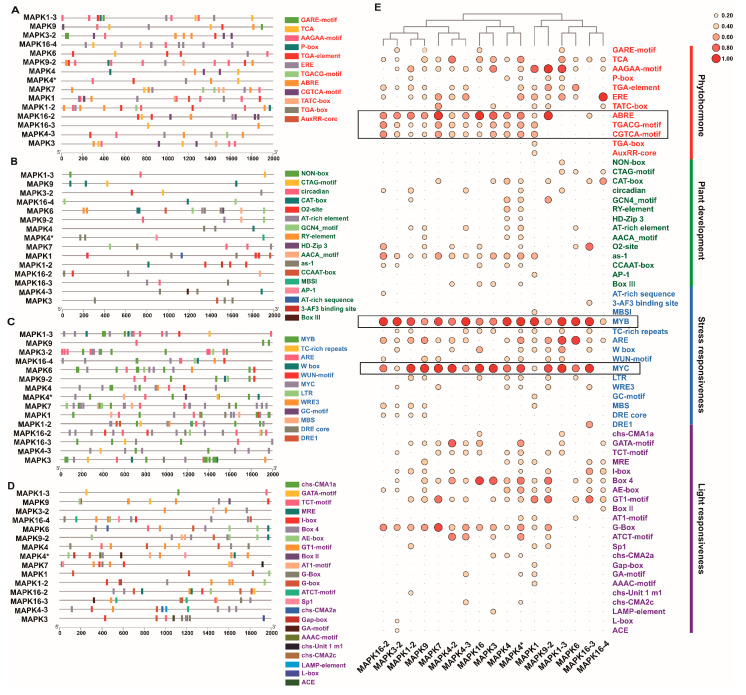
We conducted analyses to characterize the sequences of the 17 LsMAPKs by motif distribution, conserved domain, and exon–intron structure (Figure 4). Fifteen distinct motifs were predicted (Figure 4B), among which motifs 1-8 were highly conserved and appeared in almost the same order and position in all LsMAPKs proteins, although LsMAPK16 lacked motif 8 and LsMAPK9-2 lacked motifs 5 and 8. Motifs 9 and 10 were unique to groups A and B, and motif 15 was specific to group D. Compared with other group D members, LsMAPK16, LsMAPK16-2, and LsMAPK16-4 specifically had motifs 11-14. Conservative domain analysis showed that all LsMAPKs proteins had the same PKc-like superfamily domain (Figure 4C), in which groups A, B, and C generally had the STKc_TEY_MAPK domain, whereas group D generally had the STKc_TDY_MAPK domain. LsMAPK16, 16-2, and 16-4 had one additional domain. The results of exon–intron structure analysis showed that the number of exons and introns in groups A, B, and C was relatively conservative (Figure 4D). In contrast, the number of introns and exons in group D was distinctly different from that in groups A, B, and C, and the numbers in Group D were generally greater than those in groups A, B, and C.
Figure 4.
Gene structure analysis of LsMAPKs. LsMAPK gene family phylogenetic tree (A), motif distribution (B), conservative domain analysis (C), and intron–exon structure (D).
2.4. Synteny Analysis of LsMAPKs
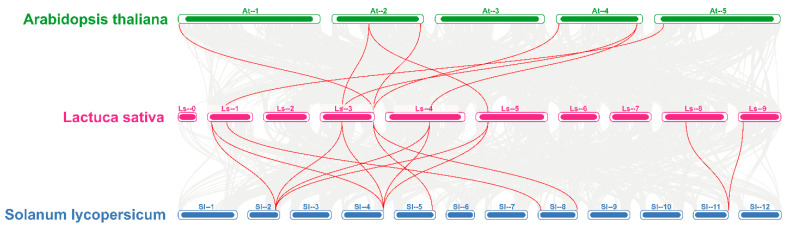
Collinearity analysis can further reveal the potential evolutionary mechanism of species’ gene families. Therefore, the evolutionary relationship and homology between LsMAPK members, lettuce and Arabidopsis, and lettuce and tomato were predicted. Among the 4291 collinear pairs in lettuce, five collinear pairs belong to LsMAPKs, among which LsMAPK1-2 was collinear with LsMAPK1-3, LsMAPK1, and LsMAPK7 at the same time (Figure 5). Furthermore, the comparison of lettuce with Arabidopsis and tomato at the genomic level showed that 12,492 collinear pairs were obtained between lettuce and Arabidopsis, and 15,558 collinear pairs were obtained between lettuce and tomato (Figure 6), among which the numbers belonging to MAPK members were 8 and 13, respectively. The collinearity pairs of LsMAPK1, 4, and 7 accounted for a total of 12 pairs, indicating the substantial role of these genes in evolution.
Figure 5.
Collinearity analysis of the LsMAPK gene family. The gray line in the background represents the homolinear pair in the whole lettuce genome, and the red line represents the homolinear pair of LsMAPKs. The information represented by each circle in the figure is GC skew, genome density heatmap, GC radio, genome density linear map, chromosome name, N radio, chromosome length scale, and LsMAPKs chromosome location annotation from inside to outside.
Figure 6.
Collinearity analysis of lettuce with Arabidopsis and tomato. The gray line in the background represents all collinear pairs of lettuce with Arabidopsis and tomato at the genomic level. The red line represents the collinear pairs belonging to MAPK family members from those three species.
2.5. Cis-Acting Element Analysis of LsMAPKs
After cis-acting element analysis of the LsMAPK promoter region, a total of 64 types of elements were identified and divided into four subgroups: phytohormones, plant development, stress responsiveness, and light responsiveness (Figure 7). The members of each subgroup were evenly distributed on all MAPKs, with the most elements related to stress response and the fewest elements related to development (Figure 7A–D). Among a total of 706 cis-acting elements, MYB elements occurred most frequently at 101 times, followed by MYC elements, which appeared 77 times (Figure 7E). However, MYC elements were absent from LsMAPK16-4. Members with close relatives may not show similar types of cis-acting elements. Here, for example, LsMAPK16 in group D, LsMAPK3 in group A, and LsMAPK4 and 4* in group B were classified into the same group according to the type and number of cis-acting elements, suggesting that they may be closely related in function.
Figure 7.
Promoter analysis of LsMAPKs. Location diagram of cis-acting elements related to phytohormones (A), plant development (B), stress responsiveness (C), and light responsiveness (D). (E) Quantity heatmap of all instantaneously acting elements.
2.6. Phosphorylation Site Analysis of LsMAPKs
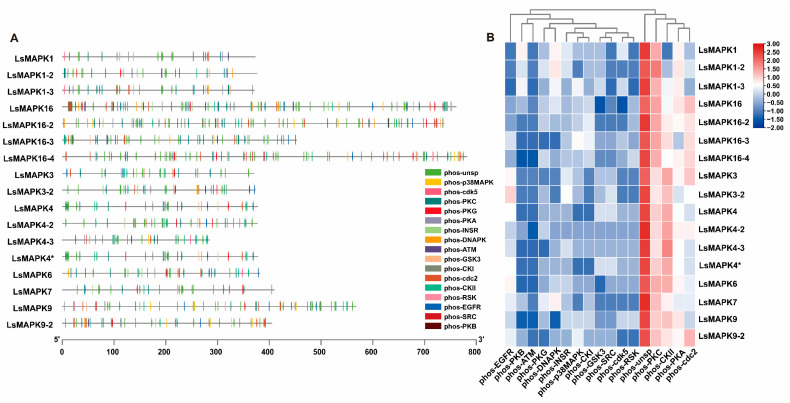
Activation of protein kinases is usually achieved by phosphorylation, so the analysis of phosphorylation sites is vital to understanding the mechanisms of protein action. The predicted phosphorylation sites of LsMAPK gene family members showed that a total of 1131 reliable phosphorylation sites (the same site could be phosphorylated by multiple kinases) were widely distributed in the sequence (Figure 8A). The number of nonspecific sites (phos-unsp) was the largest, with a total of 436, followed by phosphokinase C-specific sites (phos-PKC), with a total of 154, and casein kinase II sites, with a total of 105 (Figure 8B). Only one specific phosphorylation site of protein kinase B (phos-PKB) was predicted in LsMAPK16 and 16-2. LsMAPK16, 16-2, and 16-4 had the most nonspecific and total phosphate sites, which may be attributed to their sequence length. However, the number of phosphorylation sites was not necessarily related to the length of the amino acid sequence. LsMAPK4-3 had the shortest amino acid sequence length, but its phosphorylation sites were still more abundant than those of LsMAPK1, 3, and 7.
Figure 8.
Prediction of phosphorylation sites of LsMAPKs. (A) Distribution of phosphorylation sites of protein kinases in LsMAPKs. (B) Quantitative heatmap of protein kinases at phosphorylatable sites.
2.7. Expression Pattern Analysis of LsMAPKs in Lettuce under High Temperature
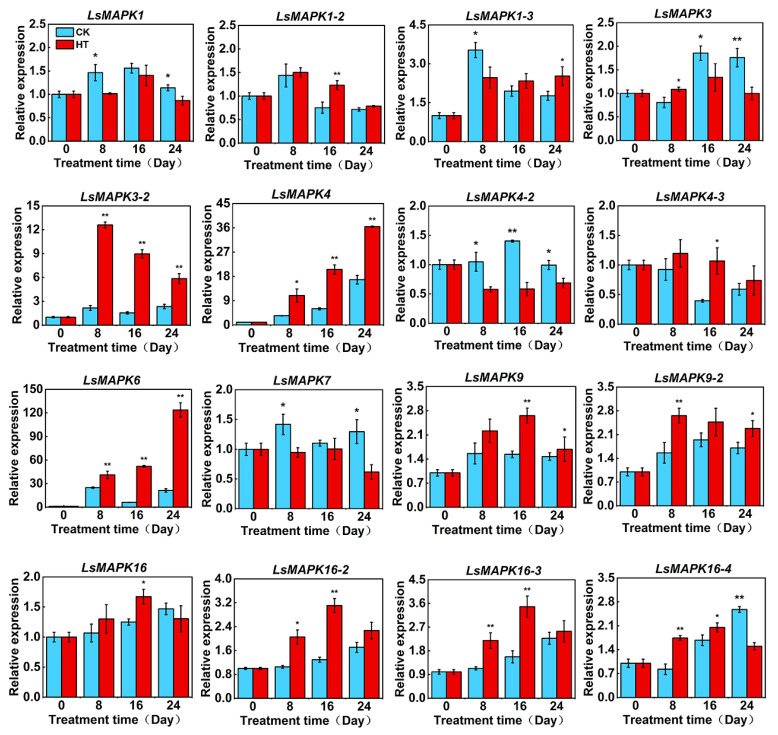
To investigate the potential regulatory mechanism of the MAPK gene in high-temperature bolting, the expression patterns of LsMAPKs were quantitatively assessed in response to high temperature in the stems of lettuce (Figure 9). Our previous research demonstrated that the experimental material GB-30 generally began bolting on the 8th day after high-temperature treatment. The results showed that the 16 LsMAPKs demonstrated a specific expression pattern during high-temperature bolting in lettuce.
Figure 9.
Expression analysis of LsMAPKs in lettuce under high temperature. HT, high temperature. The standard error is indicated, * stands for p < 0.05, and ** stands for p < 0.01.
The expression levels of LsMAPK1, LsMAPK1-3, LsMAPK4-2, and LsMAPK7 on the 8th day were significantly lower than those of the control. Among them, no difference was found on the 16th day in LsMAPK1, LsMAPK1-3, and LsMAPK7. On the 24th day, the expression of LsMAPK1 and LsMAPK7 was lower, but that of LsMAPK1-3 was higher. The expression of LsMAPK4-2 remained lower than that of the control after HT treatment. The transcriptional levels of LsMAPK1-2, LsMAPK4-3, and LsMAPK16 were higher than those of the control only on the 16th day, showing no difference on the 8th and 24th days after HT treatment.
LsMAPK3-2, LsMAPK4, and LsMAPK6 were abundantly expressed after HT treatment. LsMAPK3-2 peaked on the 8th day, and LsMAPK4 and LsMAPK6 peaked on the 24th day. The expression level of LsMAPK9-2 was higher than that of the control on the 8th and 24th days and showed no difference on the 16th day. LsMAPK16-2, LsMAPK16-3, and LsMAPK16-4 presented similar expression patterns. Their expression levels were substantially higher than those of the control on the 8th and 16th days, and except that LsMAPK16-4 was lower, there was no difference on the 24th day. In addition, the expression level of LsMAPK3 was higher than that of the control on the 8th day, then decreased, and was lower than that on the 16th and 24th day. Overall, qRT–PCR analysis suggested that LsMAPKs had a high likelihood of involvement in the bolting of lettuce induced by high temperature.
2.8. VIGS-Induced LsMAPK4 Silencing Delayed Bolting of Lettuce under High Temperature
In the previous comparative proteomics, we found that among LsMAPKs proteins, LsMAPK3/4/9 were differentially expressed in high-temperature bolting of leaf lettuce [47]. The subsequent qRT–PCR indicated that the transcription level of LsMAPK4 among the above three LsMAPKs was markedly and continuously upregulated during high-temperature bolting. To further explore gene function, VIGS-mediated LsMAPK4 gene silencing in lettuce was conducted.
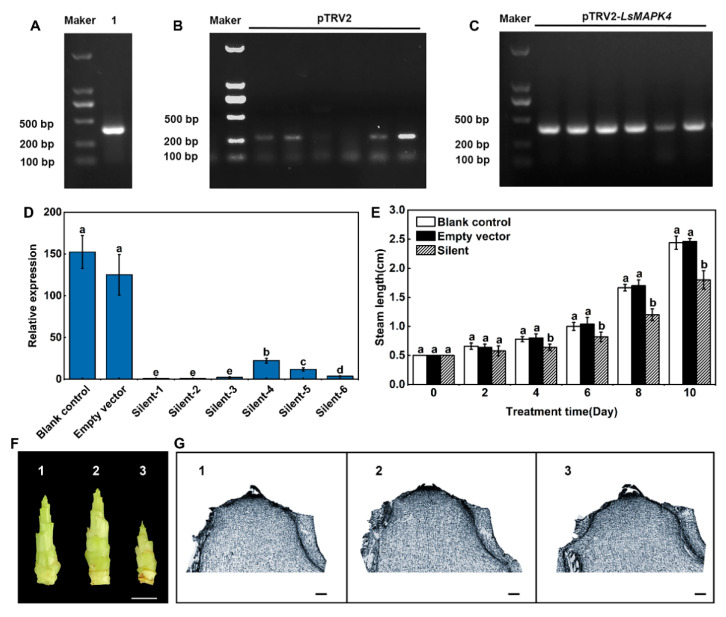
A 355 bp fragment of LsMAPK4 was successfully cloned into the pTRV2 vector (Figure 10A). Three weeks after Agrobacterium infection, RT–PCR amplification results showed that the pTRV2 empty vector and pTRV2-LsMAPK4 vector had been transferred into lettuce and effectively expressed (Figure 10B,C). Then, to finally determine whether LsMAPK4 was silenced, the expression was measured by qRT–PCR (Figure 10D). The results showed that the expression of LsMAPK4 in the silenced group was significantly lower than that in the blank control group and the empty vector group.
Figure 10.
Effect of VIGS-mediated LsMAPK4 gene silencing on high-temperature bolting of lettuce. (A) Construction of the pTRV2-LsMAPK4 vector. (B) Detection of pTRV2 fragments. (C) Detection of pTRV2-LsMAPK4 fragments. (D) Expression of LsMAPK4 in the blank control group, the empty vector group, and the silenced group. (E) Changes in stem length of VIGS-infected plants after HT treatment. (F) Morphology of the stem on the 8th day after HT. Bar = 0.5 cm. (G) Morphology of the shoot tip on the 8th day of HT. Bar = 200 µm. 1. the blank control group; 2. the empty vector group; 3. the silent group. The standard error is indicated. Different letters represent significant differences as determined using one-way ANOVA followed by Duncan’s test. p < 0.05.
In the third week after the VIGS infection, the three groups of lettuce were treated with HT. There was no significant difference in stem length among the three groups at 0 and 2 days after HT treatment (Figure 10E). On the 4th day, the stem length of the silent group began and continued to be significantly shorter than that of the blank control group and the empty vector group. On the 8th day, the stems of the blank control and the empty vector group elongated rapidly, and both of them began bolting (Figure 10F). At this time, the flower bud differentiation status of the stem tips of the three groups was detected by paraffin sectioning to judge whether the lettuce was bolting (Figure 10G). The results showed that the growth cone of the stem tips of the blank control and the empty vector group was round, blunt, and hypertrophic, without obvious protuberance, and had entered the early stage of flower bud differentiation. However, the growth cone of the silent group was semicircular with obvious protuberances, indicating that it was still in the vegetative growth stage and had not yet entered the early stage of flower bud differentiation to start bolting. Overall, silencing LsMAPK4 significantly delayed the bolting of lettuce under high temperatures, indicating that LsMAPK4 may play a promoting role in high-temperature bolting in lettuce.
3. Discussion
The MAPK cascade is a functional module that widely exists in eukaryotes [48] and has been well studied in Arabidopsis, rice, and other plants. However, there are few reports regarding its occurrence in lettuce. Our study identified a total of 17 MAPK family members in the lettuce genome, of which LsMAPK4 and LsMAPK4* were found to be identical. The 17 MAPK family members found in lettuce is fewer than the number in Arabidopsis (20 genes) [49], corn (19 genes) [50], and poplar (21 genes) [51] but more than the number in tomato (16 genes) [52], C. reinhardtii (6 genes), P. patens (10 genes), and S. moellendorffii (6 genes) [53] and the same as the number found in rice [51]. This indicates that there is no correlation between the number of MAPKs in plants and a plant species’ genome size. The chromosome distribution pattern of the MAPK family indicates that they are located on chromosomes individually or in clusters, but they do not occur on all chromosomes. None of the LsMAPK family members are located on chromosome 6, just as no MAPK members are distributed on chromosome 5 of Brachypodium distachyon [54] or chromosome 3 of cucumber [55].
Through phylogenetic analysis and multiple sequence alignment, LsMAPK family members can be divided into four groups according to the TXY motif. To study the molecular evolution and phylogenetic relationship of MAPKs in lettuce, we conducted phylogenetic analysis on 132 MAPKs from different plant groups. In lettuce, dicotyledons, and monocotyledons, MAPKs in group A have orthologs and paralogs of MAPK3 and MAPK6 but no orthologs of MAPK10. MAPK3 and MAPK6 are either present together or absent, and the decisive functions of these two MAPKs in growth and development and response to biotic and abiotic stresses have been confirmed in various plants [41,56,57,58], implying that MAPK3 and MAPK6 orthologs are indispensable. Another member of group A, the ortholog of MAPK10, was identified only in mustard plants [53], indicating that the ortholog of MAPK10 is only conserved in the Brassicaceae family of dicotyledons and has been lost from other families during evolution. In group B, MAPK5 and MAPK11 are similar to MAPK10, and MAPK11 has been lost even in the mustard family. In lettuce, all group B LsMAPKs are orthologs or paralogs of MAPK4, and there are two identical LsMAPK4s, indicating that a wide range of repetitive events may occur in its genome. LsMAPKs in group C exist in the form of MAPK1 and MAPK7, similar to the tomato genome. Group D is a type of MAPK unique to plants. None of the studied species lacks group D MAPKs. With the increase in biological complexity, the group D MAPKs expand through gene replication [22], which is indispensable in the green plant lineage. Collinearity analysis further revealed the potential evolutionary mechanism of the species gene family. There were five collinear pairs among LsMAPK members of lettuce and eight pairs among AtMAPKs of Arabidopsis thaliana. There were 13 pairs of SlMAPKs in tomato. This indicated that the genetic relationship between tomato and lettuce was closer than that of Arabidopsis.
Gene structure is closely related to gene expression and function. All LsMAPKs proteins contain motifs 1-7 (except LsMAPK4-3, which lacked motif 3). The C-terminal motif is more conserved than the N-terminal motif. There are unique characteristic motifs among distinct groups, indicating their evolutionary relationship and different functional divisions [59]. Transcript information indicates that LsMAPKs have different exon arrays in their genes. The distribution of introns and exons in the gene structure of LsMAPKs showed regularity among diverse groups. Both group A and group B contain 6 exons, group C contains 2–3 exons, and group D contains more exons, including 8–12 introns. Similar structural patterns were also observed in other plants, with high conservation within groups and an elevated level of variation between groups [49,53]. SmMAPK10 of S. moellendorfii contains up to 15 exons, and some intron-free MAPKs also exist in higher eukaryotic plants, such as PaMAPK2, PaMAPK3, PaMAPK7-1, and PaMAPK20 of Picea abies [53]. The plant MAPK cascade pathway mediates the response to salt, drought, cold, high temperature, and pathogenic bacteria [5] and is cross-linked with the hormone signaling pathway to regulate plant growth and development [60,61]. The promoter of the LsMAPK gene is rich in various cis-acting elements, such as ABA response elements (ABREs), MYB elements, MYC elements, methyl jasmonate reaction motifs (TGACG motifs and CGTCA motifs), root-specific expression elements (as-1 s), and various light response elements, which is strong evidence that LsMAPKs may participate in various life activities. Analysis of phosphorylation sites is very important for understanding the mechanism of action of the LsMAPK protein kinase. In addition to nonspecific sites, phosphokinase C (phos-PKC), casein kinase II (phos-CKII), and cell division cyclin 2 (phos-cdc2) have the largest number of specific phosphorylation sites, which have been reported to participate in plant life activities such as stress resistance, cell apoptosis, flowering regulation, and cell division [62,63,64,65]. It is speculated that LsMAPK is related to growth and development and the regulation of stress resistance.
Plants suffer from various adversity stresses during their growth and development. Among them, high-temperature stress affects almost all aspects of plant growth, development, reproduction, and yield, resulting in shedding, flowering, fruit abortion, and even whole plant death [15,66,67]. Activation of MAPK plays a key role in the perception and transduction of temperature signals [68]. MAPKs in B. distachyon are temperature-sensitive, 60% of which are induced by high temperature [69]. High temperature can induce the activity of AtMAPK6 in Arabidopsis, but it has also been reported that AtMAPK6 negatively regulates the high-temperature response [70,71]. Most cucumber CsMAPKs (except CsMAPK3 and CsMAPK7) were upregulated after high-temperature treatment [55]. In chrysanthemum, except for the increased expression levels of CmMAPK4.1, CmMAPK6, and CmMAPK13 after 1 h of heat shock treatment, the other MAPKs decreased or remained unchanged [24]. The high-temperature treatment times used in the above literature were mostly between 1 and 8 h. In this paper, the changes in LsMAPK expression in lettuce after high-temperature treatment were continuously monitored and detected at 0, 8, 16, and 24 days after high-temperature treatment with a higher sampling frequency and longer observation time. Among them, the most obvious were LsMAPK3-2, LsMAPK4, and LsMAPK6, which were significantly and continuously higher than the control after high temperature. LsMAPK4-2 was significantly and continuously lower than the control, and the rest of the LsMAPKs had a tendency to decrease or to rise first and then decrease compared with the control, but not every stage had a significant difference.
At present, studies on MAPK4 gene function mainly focus on cell division and stress response. Tomato MAPK4 [72], rice MAPK4 [73], and Arabidopsis MAPK4 [74] have been reported to play a role in low-temperature stress. In addition, MAPK4 was more reported to be related to cell division, flower organ formation, and photosynthesis [18,37,75], and little has been reported about the high-temperature stress response. In our study, we screened LsMAPK4 and LsMAPK4* on chromosome 3, which are located in different positions but are very close to each other, with a distance of about 180,000 bp. Their gene sequences and open reading frame sequences are identical. No same gene was found in the front and back of the two chromosome positions, indicating that this is a small and segmental duplication event. Compositae species have experienced the triplication of the whole genome in the evolutionary process, and leaf lettuce also shows the triplication of the genome, which leads to a large number of replication events [76]. The segmental duplication of LsMAPK4 is probably the result of the retention of duplicate copies. We have noticed that the promoter sequences of LsMAPK4 and LsMAPK4* were completely different, and there were differences in cis-acting elements (Figure 7), which may lead to functional differences between the two genes. But it is also very likely that the functions of the two genes will overlap under a specific condition. The gene replication of LsMAPK4 is likely to make its expression more extensive and efficient. Since the expression of LsMAPK4 was significantly upregulated by high temperature, we further conducted VIGS experiments to explore the function of LsMAPK4 in the high-temperature bolting of lettuce. According to the cytological and morphological experiments of flower buds and stems, after 8 days of high-temperature treatment, bolting occurred in the blank control group and the empty vector group but not in the silenced group. Easy-bolting lettuce GB-30 was used as the experimental material. After high-temperature treatment, GB-30 began bolting on the 8th day [46]. This is very consistent with the VIGS phenotype. High temperature significantly promoted the expression of LsMAPK4, which played a positive regulatory role in the bolting process of lettuce at high temperatures.
4. Materials and Methods
4.1. Genome-Wide Identification of LsMAPK Family Members in Lettuce
Whole-genome data for lettuce (https://phytozome.jgi.doe.gov/pz/portal.html; accessed on 6 August 2021) were downloaded and constructed into a local protein database. The Arabidopsis thaliana MAPK protein sequences from the Arabidopsis thaliana genome database (http://www.arabidopsis.org/; accessed on 6 August 2021) were used as the search seed for a BLASTP homology search of lettuce whole-genome data with an e-value of 1 × 10−5 and a minimum identity of 50% as a threshold. The HMMER3.0 program (http://hmmer.org/; accessed on 10 September 2021) was used to remove redundant sequences by applying the serine/threonine protein kinase-like domain (PF00069) as a query for hidden Markov model (HMM) searches with a threshold of 1 × 10−5.
4.2. Bioinformatics Analysis of LsMAPKs
By querying the annotation file of the lettuce genome, the chromosome location, direction, and isomer number of MAPK proteins were obtained, and the chromosome location was visualized by TBtools. The amino acid number, isoelectric point, and molecular weight of the MAPK protein were predicted by using the ProParam tool (https://web.expasy.org/protparam/; accessed on 5 July 2022). Cell-PLoc 2.0 was used to predict subcellular localization (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc-2/; accessed on 5 July 2022). TMHMM was used to predict transmembrane domains (https://dtu.biolib.com/DeepTMHMM; accessed on 5 July 2022). The MAPK members of each species were obtained from the corresponding plant database, and the species database was downloaded from Phytozome V13 (https://phytozome-next.jgi.doe.gov/; accessed on 11 July 2022). The construction of the phylogenetic tree was completed by MEGA 7.0 software and enhanced using Evolview (http://www.evolgenius.info/evolview/#/login; accessed on 13 July 2022). The conserved domains of proteins were analyzed by NCBI-CD search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi; accessed on 12 July 2022). MEME Suite analyzed conserved motifs of proteins (http://meme-suite.org/tools/meme; accessed on 12 July 2022). The cis-acting elements were analyzed by Plant CARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/; accessed on 6 July 2022). Analysis of phosphorylation sites was completed by using netphos (https://services.healthtech.dtu.dk/service.php?NetPhos-3.1; accessed on 24 July 2022). Intron–exon structure, whole genome collinearity analysis of lettuce, and collinearity analysis of lettuce with Arabidopsis and tomato were analyzed by TBtools. The visualization of all the above analyses was completed by TBtools and further enhanced using Adobe Illustrator.
4.3. Plant Materials and Treatments
Easily bolting lettuce variety ‘GB30’ was used as the experimental material, which was from seeding plant protection Co., LTD in Inner Mongolia Bameng Fidelity, conserved in our laboratory. It was planted in a matrix of sand, soil, and peat (1:1:1 by volume) and cultured in the computer greenhouse of Beijing Agricultural College under the following conditions: temperature of 20 ± 2 °C (day)/13 ± 2 °C (night), photoperiod of 14 h (day)/10 h (night), relative humidity of 60 ± 5%, and light intensity of 12,000 lux. The plants were transplanted at the trefoil stage, and high-temperature treatment was carried out when lettuce had six true leaves [46]. The control group maintained the original culture conditions, whereas the high-temperature group increased the temperature to 33 ± 2 °C (day)/25 ± 2 °C (night), with the other conditions unchanged. Samples were taken at 0, 8, 16, and 24 days of treatment, and the sampling site was the stem. Every five plants were used as a replicate, and the sampling was repeated three times independently.
4.4. RNA Extraction and Quantitative Real-Time PCR Analysis (qRT–PCR)
Total RNA was extracted using a Quick RNA Isolation kit (Huayueyang Biotech, Beijing, China). The first-stand cDNA of lettuce was synthesized by TransScript® Uni All-in-One First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China). Primer3 Plus software was used to design qRT–PCR primers (Table S1), and 18S rRNA (HM047292.1) was used as an internal reference gene. qRT–PCR was performed using TB Green® Premix Ex Taq™ II (Takara Bio, Beijing, China) and a CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). The amplification program was as follows: pre-denaturation at 95 °C for 3 min, denaturation at 95 °C for 20 s, and annealing at 55 °C for 30 s for 40 cycles. The 2−ΔΔCT method was used to analyze the data.
4.5. Virus-Induced Gene Silencing Analysis
A 355 bp sequence in the CDS region of LsMAPK4 was amplified by VIGS-LsMAPK4-F/R primers (Table S1). The pTRV2 vector was linearized by EcoRI and XhoI. A ClonExpress Ⅱ One Step Cloning Kit (Vazyme Biotech, Beijing, China) was used to complicate the homologous recombination of the amplified band and linearized vector. After the recombinant plasmid was transferred into Trans1-T1 Phage Resistant Chemically Competent Cells and sequenced correctly, it was transferred into GV3101 Chemically Competent Cells (Weidi Biotechnology, Shanghai, China).
Before VIGS injection, the same amount of pTRV11 and pTRV2 bacterial solution was mixed. There were 30 lettuces in each group, and there were three treatment groups in total: the blank control group (no injection), empty vector group (injection with equal pTRV2 and pTRV1), and silent group (injection with equal pTRV2-LsMAPK4 and pTRV1). Three weeks after injection, a high-temperature treatment was carried out. Before treatment, RNA from new leaves was taken to detect whether the vector was transferred and expressed, and the expression level of LsMAPK4 was measured. The vector detection primers were pTRV2-F/R for the empty vector group and VIGS-LsMAPK4-F/R for the silencing group (Table S1). The stem length of each group was measured at 0, 2, 4, 6, 8, and 10 days after high temperature, and the stem tips of the plants were paraffin-sectioned on the 8th day.
4.6. Statistical Analysis
All tests were performed in triplicate. One-way ANOVA was performed on the data using statistical analysis software SPSS 12.5 (International Business Machine, Chicago, IL, USA), and graphs were drawn by Origin 9 (Origin Lab, Northampton, MA, USA). The standard error is indicated. The * stands for p < 0.05 and the ** stands for p < 0.01, followed by Student’s t-test. Different letters represent significant differences as determined using one-way ANOVA followed by Duncan’s test. p < 0.05.
5. Conclusions
In this study, we identified a total of 17 MAPK gene family members from the whole genome of lettuce. The physicochemical properties, chromosomal localization, phylogeny, gene structure, family evolution, cis-acting elements, and phosphorylation sites were comprehensively analyzed. To explore the role of LsMAPKs in high-temperature bolting of lettuce, the expression pattern of LsMAPKs was evaluated within 24 days after high-temperature treatment, and the positive regulatory role of LsMAPK4 in high-temperature bolting was verified by VIGS. This provides a theoretical basis for understanding high-temperature bolting mechanisms in lettuce and identifies potential paths for improving the annual production of lettuce.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/1422-0067/23/19/11129/s1.
Author Contributions
Conceptualization, J.H. and S.F.; Data curation, Y.H., C.L. and S.F.; Formal analysis, T.W., Y.W. and Y.T.; Funding acquisition, M.L., J.H. and S.F.; Investigation, T.W., M.L., Y.W. and Y.T.; Methodology, Y.H., J.H. and S.F.; Project administration, C.L.; Resources, J.H. and SF; Supervision, Y.H., C.L., J.H. and S.F.; Validation, M.L.; Visualization, T.W. and M.L.; Writing—original draft, T.W.; Writing—review & editing, T.W. and M.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 31972400), the Support Plan for Spark Action on Scientific and Technological Innovation of Beijing University of Agriculture (BUA-HHXD2022003), and the Beijing Joint Research Program for Germplasm Innovation and New Variety Breeding (G20220628003). The funding institution was not involved in the design of the study, collection, analysis, and interpretation of data, or in writing the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jagodzik P., Tajdel-Zielinska M., Ciesla A., Marczak M., Ludwikow A. Mitogen-Activated Protein Kinase Cascades in Plant Hormone Signaling. Front. Plant Sci. 2018;9:1387. doi: 10.3389/fpls.2018.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rayapuram N., Bigeard J., Alhoraibi H., Bonhomme L., Hesse A., Vinh J., Hirt H., Pflieger D. Quantitative Phosphoproteomic Analysis Reveals Shared and Specific Targets of Arabidopsis Mitogen-Activated Protein Kinases (MAPKs) MPK3, MPK4, and MPK6. Mol. Cell. Proteom. 2018;17:61–80. doi: 10.1074/mcp.RA117.000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kazuya I.E.A.M., Ichimura K., Shinozaki K., Tena G., Sheen J., Henry Y., Champion A., Kreis M., Zhang S., Hirt H. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002;7:301–308. doi: 10.1016/s1360-1385(02)02302-6. [DOI] [PubMed] [Google Scholar]
- 4.Wang W., Feng B., Zhou J.M., Tang D. Plant immune signaling: Advancing on two frontiers. J. Integr. Plant Biol. 2020;62:2–24. doi: 10.1111/jipb.12898. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M., Su J., Zhang Y., Xu J., Zhang S. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr. Opin. Plant Biol. 2018;45:1–10. doi: 10.1016/j.pbi.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Thulasi D.K., Li X., Zhang Y. MAP kinase signaling: Interplays between plant PAMP- and effector-triggered immunity. Cell. Mol. Life Sci. 2018;75:2981–2989. doi: 10.1007/s00018-018-2839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gustin M.C., Albertyn J., Alexander M., Davenport K. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62:1264–1300. doi: 10.1128/MMBR.62.4.1264-1300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang L., Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 9.Andreasson E., Ellis B. Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci. 2010;15:106–113. doi: 10.1016/j.tplants.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M., Zhang S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022;64:301–341. doi: 10.1111/jipb.13215. [DOI] [PubMed] [Google Scholar]
- 11.Chen X., Ding Y., Yang Y., Song C., Wang B., Yang S., Guo Y., Gong Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021;63:53–78. doi: 10.1111/jipb.13061. [DOI] [PubMed] [Google Scholar]
- 12.Bögre L., Meskiene I., Heberle-Bors E., Hirt H. Stressing the role of MAP kinases in mitogenic stimulation. Plant Mol. Biol. 2000;43:705–718. doi: 10.1023/A:1006301614690. [DOI] [PubMed] [Google Scholar]
- 13.Verma D., Jalmi S.K., Bhagat P.K., Verma N., Sinha A.K. A bHLH transcription factor, MYC2, imparts salt intolerance by regulating proline biosynthesis in Arabidopsis. FEBS J. 2020;287:2560–2576. doi: 10.1111/febs.15157. [DOI] [PubMed] [Google Scholar]
- 14.Li H., Ding Y., Shi Y., Zhang X., Zhang S., Gong Z., Yang S. MPK3 and MPK6-Mediated ICE1 Phosphorylation Negatively Regulates ICE1 Stability and Freezing Tolerance in Arabidopsis. Dev. Cell. 2017;43:630–642. doi: 10.1016/j.devcel.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 15.de Zelicourt A., Colcombet J., Hirt H. The Role of MAPK Modules and ABA during Abiotic Stress Signaling. Trends Plant Sci. 2016;21:677–685. doi: 10.1016/j.tplants.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Ayatollahi Z., Kazanaviciute V., Shubchynskyy V., Kvederaviciute K., Schwanninger M., Rozhon W., Stumpe M., Mauch F., Bartels S., Ulm R., et al. Dual control of MAPK activities by AP2C1 and MKP1 MAPK phosphatases regulates defence responses in Arabidopsis. J. Exp. Bot. 2022;73:2369–2384. doi: 10.1093/jxb/erac018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan Y., Meng X., Khanna R., LaMontagne E., Liu Y., Zhang S. Phosphorylation of a WRKY transcription factor by MAPKs is required for pollen development and function in Arabidopsis. PLoS Genet. 2014;10:e1004384. doi: 10.1371/journal.pgen.1004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng Q., Chen J.G., Ellis B.E. AtMPK4 is required for male-specific meiotic cytokinesis in Arabidopsis. Plant J. 2011;67:895–906. doi: 10.1111/j.1365-313X.2011.04642.x. [DOI] [PubMed] [Google Scholar]
- 19.Xu J., Zhang S. Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 2015;20:56–64. doi: 10.1016/j.tplants.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y., Li Z., Schwarz E.M., Lin A., Guan K., Ulevitch R.J., Han J. Structure-function studies of p38 mitogen-activated protein kinase. Loop 12 influences substrate specificity and autophosphorylation, but not upstream kinase selection. J. Biol. Chem. 1997;272:11096–11102. doi: 10.1074/jbc.272.17.11096. [DOI] [PubMed] [Google Scholar]
- 21.Tanoue T., Adachi M., Moriguchi T., Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell Biol. 2000;2:110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- 22.Doczi R., Okresz L., Romero A.E., Paccanaro A., Bogre L. Exploring the evolutionary path of plant MAPK networks. Trends Plant Sci. 2012;17:518–525. doi: 10.1016/j.tplants.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Reyna N.S., Yang Y. Molecular analysis of the rice MAP kinase gene family in relation to Magnaporthe grisea infection. Mol. Plant Microbe Interact. 2006;19:530–540. doi: 10.1094/MPMI-19-0530. [DOI] [PubMed] [Google Scholar]
- 24.Song A., Hu Y., Ding L., Zhang X., Li P., Liu Y., Chen F. Comprehensive analysis of mitogen-activated protein kinase cascades in chrysanthemum. PeerJ. 2018;6:e5037. doi: 10.7717/peerj.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M., Li B., Yang M., Wang L., Hou G., Lin Y., Zhang Y., Zhang Y., Chen Q., Wang Y., et al. Genome-Wide Identification and Expression of MAPK Gene Family in Cultivated Strawberry and Their Involvement in Fruit Developing and Ripening. Int. J. Mol. Sci. 2022;23:5201. doi: 10.3390/ijms23095201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G., Lovato A., Polverari A., Wang M., Liang Y.H., Ma Y.C., Cheng Z.M. Genome-wide identification and analysis of mitogen activated protein kinase kinase kinase gene family in grapevine (Vitis vinifera) BMC Plant Biol. 2014;14:219. doi: 10.1186/s12870-014-0219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andreasson E., Jenkins T., Brodersen P., Thorgrimsen S., Petersen N.H., Zhu S., Qiu J.L., Micheelsen P., Rocher A., Petersen M., et al. The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J. 2005;24:2579–2589. doi: 10.1038/sj.emboj.7600737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J.Z., Horstman H.D., Braun E., Graham M.A., Zhang C., Navarre D., Qiu W.L., Lee Y., Nettleton D., Hill J.H., et al. Soybean homologs of MPK4 negatively regulate defense responses and positively regulate growth and development. Plant Physiol. 2011;157:1363–1378. doi: 10.1104/pp.111.185686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li G., Meng X., Wang R., Mao G., Han L., Liu Y., Zhang S. Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genet. 2012;8:e1002767. doi: 10.1371/journal.pgen.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia W., Li B., Li S., Liang Y., Wu X., Ma M., Wang J., Gao J., Cai Y., Zhang Y., et al. Mitogen-Activated Protein Kinase Cascade MKK7-MPK6 Plays Important Roles in Plant Development and Regulates Shoot Branching by Phosphorylating PIN1 in Arabidopsis. PLoS Biol. 2016;14:e1002550. doi: 10.1371/journal.pbio.1002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi F., Yoshida R., Ichimura K., Mizoguchi T., Seo S., Yonezawa M., Maruyama K., Yamaguchi-Shinozaki K., Shinozaki K. The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell. 2007;19:805–818. doi: 10.1105/tpc.106.046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoo S.D., Cho Y.H., Tena G., Xiong Y., Sheen J. Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signalling. Nature. 2008;451:789–795. doi: 10.1038/nature06543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma D., Bhagat P.K., Sinha A.K. MKK3-MPK6-MYC2 module positively regulates ABA biosynthesis and signalling in Arabidopsis. J. Plant Biochem. Biot. 2020;29:785–795. [Google Scholar]
- 34.Zhai Q., Zhang X., Wu F., Feng H., Deng L., Xu L., Zhang M., Wang Q., Li C. Transcriptional Mechanism of Jasmonate Receptor COI1-Mediated Delay of Flowering Time in Arabidopsis. Plant Cell. 2015;27:2814–2828. doi: 10.1105/tpc.15.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang H.J., Fu S.F., Tai Y.H., Chou W.C., Huang D.D. Expression of Oryza sativa MAP kinase gene is developmentally regulated and stress-responsive. Physiol Plant. 2002;114:572–580. doi: 10.1034/j.1399-3054.2002.1140410.x. [DOI] [PubMed] [Google Scholar]
- 36.Krysan P.J., Jester P.J., Gottwald J.R., Sussman M.R. An Arabidopsis mitogen-activated protein kinase kinase kinase gene family encodes essential positive regulators of cytokinesis. Plant Cell. 2002;14:1109–1120. doi: 10.1105/tpc.001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosetsu K., Matsunaga S., Nakagami H., Colcombet J., Sasabe M., Soyano T., Takahashi Y., Hirt H., Machida Y. The MAP kinase MPK4 is required for cytokinesis in Arabidopsis thaliana. Plant Cell. 2011;22:3778–3790. doi: 10.1105/tpc.110.077164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bush S.M., Krysan P.J. Mutational evidence that the Arabidopsis MAP kinase MPK6 is involved in anther, inflorescence, and embryo development. J. Exp. Bot. 2007;58:2181–2191. doi: 10.1093/jxb/erm092. [DOI] [PubMed] [Google Scholar]
- 39.Hord C.L., Sun Y.J., Pillitteri L.J., Torii K.U., Wang H., Zhang S., Ma H. Regulation of Arabidopsis early anther development by the mitogen-activated protein kinases, MPK3 and MPK6, and the ERECTA and related receptor-like kinases. Mol. Plant. 2008;1:645–658. doi: 10.1093/mp/ssn029. [DOI] [PubMed] [Google Scholar]
- 40.Wang H., Liu Y., Bruffett K., Lee J., Hause G., Walker J.C., Zhang S. Haplo-insufficiency of MPK3 in MPK6 mutant background uncovers a novel function of these two MAPKs in Arabidopsis ovule development. Plant Cell. 2008;20:602–613. doi: 10.1105/tpc.108.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.López-Bucio J.S., Dubrovsky J.G., Raya-González J., Ugartechea-Chirino Y., López-Bucio J., de Luna-Valdez L.A., Ramos-Vega M., León P., Guevara-García A.A. Arabidopsis thaliana mitogen-activated protein kinase 6 is involved in seed formation and modulation of primary and lateral root development. J. Exp. Bot. 2014;65:169–183. doi: 10.1093/jxb/ert368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Assefa A.D., Choi S., Lee J.E., Sung J.S., Hur O.S., Ro N.Y., Lee H.S., Jang S.W., Rhee J.H. Identification and quantification of selected metabolites in differently pigmented leaves of lettuce (Lactuca sativa L.) cultivars harvested at mature and bolting stages. BMC Chem. 2019;13:56. doi: 10.1186/s13065-019-0570-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komeda Y. Genetic regulation of time to flower in Arabidopsis Thaliana. Annu. Rev. Plant Biol. 2004;55:521–535. doi: 10.1146/annurev.arplant.55.031903.141644. [DOI] [PubMed] [Google Scholar]
- 44.Miki N., Koji T., Masaki H., Yoshifumi F., Yasunobu O., Yasuhisa K., Kyuya H. Mapping of QTLs for Bolting Time in Brassica rapa (syn. campestris) under Different Environmental Conditions. Breed. Sci. 2005;55:127–133. [Google Scholar]
- 45.Song Y., Dou L.D., Zhang H.J. Molecular and Genetic Mechanisms of Control of Floral Induction in Higher Plants. Plant Physiol. J. 2014;50:1459–1468. [Google Scholar]
- 46.Hao J.-H., Zhang L.-L., Li P.-P., Sun Y.-C., Li J.-K., Qin X.-X., Wang L., Qi Z.-Y., Xiao S., Han Y.-Y., et al. Quantitative Proteomics Analysis of Lettuce (Lactuca sativa L.) Reveals Molecular Basis-Associated Auxin and Photosynthesis with Bolting Induced by High Temperature. Int. J. Mol. Sci. 2018;19:2967. doi: 10.3390/ijms19102967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao J.H., Su H.N., Zhang L.L., Liu C.J., Han Y.Y., Qin X.X., Fan S.X. Quantitative proteomic analyses reveal that energy metabolism and protein biosynthesis reinitiation are responsible for the initiation of bolting induced by high temperature in lettuce (Lactuca sativa L.) BMC Genom. 2021;22:427. doi: 10.1186/s12864-021-07664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chardin C., Schenk S.T., Hirt H., Colcombet J., Krapp A. Review: Mitogen-Activated Protein Kinases in nutritional signaling in Arabidopsis. Plant Sci. 2017;260:101–108. doi: 10.1016/j.plantsci.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z., Wan Y., Meng X., Zhang X., Liang Y. Genome-Wide Identification and Analysis of MKK and MAPK Gene Families in Brassica Species and Response to Stress in Brassica napus. Int. J. Mol. Sci. 2021;22:544. doi: 10.3390/ijms22020544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y., Zhang D., Wang L., Li D. Genome-Wide Analysis of Mitogen-Activated Protein Kinase Gene Family in Maize. Plant Mol. Biol. Rep. 2013;31:1446–1460. doi: 10.1007/s11105-013-0623-y. [DOI] [Google Scholar]
- 51.Hamel L., Nicole M., Sritubtim S., Morency M., Ellis M., Ehlting J., Beaudoin N., Barbazuk B., Klessig D., Lee J., et al. Ancient signals: Comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci. 2006;11:192–198. doi: 10.1016/j.tplants.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Wu J., Wang J., Pan C., Guan X., Wang Y., Liu S., He Y., Chen J., Chen L., Lu G., et al. Genome-wide identification of MAPKK and MAPKKK gene families in tomato and transcriptional profiling analysis during development and stress response. PLoS ONE. 2014;9:e103032. doi: 10.1371/journal.pone.0103032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohanta T.K., Arora P.K., Mohanta N., Parida P., Bae H. Identification of new members of the MAPK gene family in plants shows diverse conserved domains and novel activation loop variants. BMC Genom. 2015;16:1–20. doi: 10.1186/s12864-015-1244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen L., Hu W., Tan S., Wang M., Ma Z., Zhou S., Deng X., Zhang Y., Huang C., Yang G., et al. Genome-wide identification and analysis of MAPK and MAPKK gene families in Brachypodium distachyon. PLoS ONE. 2012;7:e46744. doi: 10.1371/journal.pone.0046744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J., Pan C., Wang Y., Ye L., Wu J., Chen L., Zou T., Lu G. Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families and transcriptional profiling analysis during development and stress response in cucumber. BMC Genom. 2015;16:1–22. doi: 10.1186/s12864-015-1621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinha A.K., Jaggi M., Raghuram B., Tuteja N. Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal. Behav. 2011;6:196–203. doi: 10.4161/psb.6.2.14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez M.C., Petersen M., Mundy J. Mitogen-Activated Protein Kinase Signaling in Plants. Annu. Rev. Plant Biol. 2010;61:621–649. doi: 10.1146/annurev-arplant-042809-112252. [DOI] [PubMed] [Google Scholar]
- 58.Raina S.K., Wankhede D.P., Jaggi M., Singh P., Jalmi S.K., Raghuram B., Sheikh A.H., Sinha A.K. CrMPK3, A mitogen activated protein kinase from Catharanthus roseus and its possible role in stress induced biosynthesis of monoterpenoid indole alkaloids. BMC Plant Biol. 2012;12:134. doi: 10.1186/1471-2229-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meng X., Zhang S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013;51:245–266. doi: 10.1146/annurev-phyto-082712-102314. [DOI] [PubMed] [Google Scholar]
- 60.Huang R., Zheng R., He J., Zhou Z., Wang J., Xiong Y., Xu T. Noncanonical auxin signaling regulates cell division pattern during lateral root development. Proc. Natl. Acad. Sci. USA. 2019;116:21285–21290. doi: 10.1073/pnas.1910916116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J.B., Wang X.P., Wang Y.C., Chen Y.H., Luo J.W., Li D.D., Li X.B. Genome-wide identification and functional characterization of cotton (Gossypium hirsutum) MAPKKK gene family in response to drought stress. BMC Plant Biol. 2020;20:217. doi: 10.1186/s12870-020-02431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe E., Mano S., Nishimura M., Yamada K. AtUBL5 regulates growth and development through pre-mRNA splicing in Arabidopsis thaliana. PLoS ONE. 2019;14:e224795. doi: 10.1371/journal.pone.0224795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhuang S., Demirs J.T., Kochevar I.E. Protein kinase C inhibits singlet oxygen-induced apoptosis by decreasing caspase-8 activation. Oncogene. 2001;20:6764–6776. doi: 10.1038/sj.onc.1204867. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y., Cao Y., Zheng H., Feng W., Qu J., Fu F., Li W., Yu H. Ectopic expression of antifreeze protein gene from Ammopiptanthus nanus confers chilling tolerance in maize. Crop J. 2021;9:924–933. doi: 10.1016/j.cj.2020.08.011. [DOI] [Google Scholar]
- 65.Feng Y.Z., Yu Y., Zhou Y.F., Yang Y.W., Lei M.Q., Lian J.P., He H., Zhang Y.C., Huang W., Chen Y.Q. A Natural Variant of miR397 Mediates a Feedback Loop in Circadian Rhythm. Plant Physiol. 2020;182:204–214. doi: 10.1104/pp.19.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mittler R., Blumwald E. Genetic engineering for modern agriculture: Challenges and perspectives. Annu. Rev. Plant Biol. 2010;61:443–462. doi: 10.1146/annurev-arplant-042809-112116. [DOI] [PubMed] [Google Scholar]
- 67.Moreno A.A., Orellana A. The physiological role of the unfolded protein response in plants. Biol. Res. 2011;44:75–80. doi: 10.4067/S0716-97602011000100010. [DOI] [PubMed] [Google Scholar]
- 68.Sangwan V., Dhindsa R.S. In vivo and in vitro activation of temperature-responsive plant map kinases. FEBS Lett. 2002;531:561–564. doi: 10.1016/S0014-5793(02)03626-8. [DOI] [PubMed] [Google Scholar]
- 69.Jiang M., Wen F., Cao J., Li P., She J., Chu Z. Genome-wide exploration of the molecular evolution and regulatory network of mitogen-activated protein kinase cascades upon multiple stresses in Brachypodium distachyon. BMC Genom. 2015;16:228. doi: 10.1186/s12864-015-1452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suri S.S., Dhindsa R.S. A heat-activated MAP kinase (HAMK) as a mediator of heat shock response in tobacco cells. Plant Cell Environ. 2008;31:218–226. doi: 10.1111/j.1365-3040.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 71.Evrard A., Kumar M., Lecourieux D., Lucks J., von Koskull-Döring P. Regulation of the heat stress response in Arabidopsis by MPK6-targeted phosphorylation of the heat stress factor HsfA2. PeerJ. 2013;1:672–676. doi: 10.7717/peerj.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao R., Xie H., Lv S., Zheng Y., Yu M., Shen L., Sheng J. LeMAPK4 participated in cold-induced ethylene production in tomato fruit. J. Sci. Food Agric. 2013;93:1003–1009. doi: 10.1002/jsfa.5790. [DOI] [PubMed] [Google Scholar]
- 73.Fu S.F., Chou W.C., Huang D.D., Huang H.J. Transcriptional regulation of a rice mitogen-activated protein kinase gene, OsMAPK4, in response to environmental stresses. Plant Cell Physiol. 2002;43:958–963. doi: 10.1093/pcp/pcf111. [DOI] [PubMed] [Google Scholar]
- 74.Du X., Jin Z., Liu D., Yang G., Pei Y. Hydrogen sulfide alleviates the cold stress through MPK4 in Arabidopsis thaliana. Plant Physiol. Biochem. 2017;120:112–119. doi: 10.1016/j.plaphy.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 75.Witoń D., Gawroński P., Czarnocka W., Lesak I., Karpiński S. Mitogen Activated Protein Kinase 4 (MPK4) Influences Growth in Populus tremula L. x tremuloides. Environ. Exp. Bot. 2016;130:189–205. [Google Scholar]
- 76.Reyes-Chin-Wo S., Wang Z., Yang X., Kozik A., Arikit S., Song C., Xia L., Froenicke L., Lavelle D.O., Truco M.J., et al. Genome assembly with in vitro proximity ligation data and whole-genome triplication in lettuce. Nat. Commun. 2017;8:14953. doi: 10.1038/ncomms14953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.