Abstract
We developed a murine model of kidney abscess by direct renal injection of either Escherichia coli (1 × 106 to 7 × 106 organisms) or sterile medium. Bacterial infection produced renal abscesses, bacteremia, and late-onset leukocytosis in all animals. Controls were unaffected. This model may be useful for the study of various sequelae of kidney infection.
Kidney infection is associated with a variety of medical sequelae, including hypertension and uremia (5). In pregnancy, renal infection is linked to decreased maternal renal function, low birthweight, preterm birth, and preeclampsia (4, 7, 9, 11, 14).
Mice are innately resistant to urinary infection (1). However, both intravenous (2) and transurethral (3, 6, 8, 13) bacterial inoculations have been utilized to induce nephritis in mice. Injection of bacteria directly into the kidney affords certain advantages over the above-mentioned two techniques for the generation of urinary tract infections. These advantages include the facts that both male and female mice can be utilized, as bladder catheterization is not required, and that this method establishes a unilateral infection in 100% of subjects, with the contralateral kidney available as an internal control.
Virginal female CD-1 mice (Charles River Laboratories, Wilmington, Mass.), aged 8 weeks or older and housed in a modified barrier facility, were used in this experiment. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Columbia University College of Physicians and Surgeons.
Mice were anesthetized with 0.015 ml of Avertin (2.5% tribromoethyl alcohol–2.5% tert-amyl alcohol in phosphate-buffered saline) per g of body weight. A left-flank incision was made, and 0.02 ml of either an Escherichia coli suspension (1 × 106 to 7 × 106 organisms of a log-phase-growth culture) or sterile Luria-Bertani (LB) medium (15) was injected into the lower pole of the left kidney. This range of inocula was used because in a pilot study abscesses formed reliably after inoculations of 106 bacteria and above. The muscle and skin were closed with interrupted, synthetic, absorbable, 4:0 glycolide-lactide copolymer sutures (Polysorb; United States Surgical Corporation, Norwalk, Conn.) and 9-mm stainless steel wound clips (Clay Adams; Becton Dickinson, Franklin Lakes, N.J.), respectively.
Mice were monitored in the postoperative period for nonspecific signs of illness, such as decreased mobility and piloerection, and were sacrificed by carbon dioxide inhalation at 24 h, 48 h, or 7 days after inoculation. Blood samples obtained by cardiac puncture were either cultured in LB broth or anticoagulated with EDTA (Microtainer tubes; Becton Dickinson, Franklin Lakes, N.J.) prior to complete blood cell count determination with an automated analyzer (Baker 9110+; Biochem Immunosystems, Allentown, Pa.). Differential leukocyte (WBC) count determinations were performed manually. Blood cultures were shaken at 37°C and then spread onto LB plates. Broth cultures which were negative at 24 h were reincubated and replated at 72 h. Randomly selected positive cultures were serotyped (MicroScan; Dade, West Sacramento, Calif.) to verify the recovery of the original E. coli strain. Histologic examinations of right- and left-kidney sections were performed in a blinded fashion by a veterinary pathologist (S.R.B.). Categorical and continuous data were analyzed by Fisher’s exact and Student’s t tests.
Twenty-one animals were inoculated with E. coli, and eight animals received sterile medium. All animals were asymptomatic through a 7-day course. Histology and bacteriology results are summarized in Table 1. Control mice were not bacteremic, but bacterial injections caused all challenged mice to test positive for bacteremia at 24 h (n = 7) and 48 h (n = 7) after inoculation (P = 0.0083). Bacteremia was still present in two of seven mice at 7 days (P = 0.58).
TABLE 1.
Histology and blood culture results following renal inoculation with either E. coli or sterile medium
| Time and no. tested | Inoculum | Fraction (%) of mice:
|
|
|---|---|---|---|
| Positive for histologic abscess | With blood culture positive for bacteremia | ||
| 24 h (n = 10) | E. coli | 6/7 (86)a | 7/7 (100)a |
| Controlb | 0/3 (0) | 0/3 (0) | |
| 48 h (n = 10) | E. coli | 7/7 (100)a | 7/7 (100)a |
| Control | 0/3 (0) | 0/3 (0) | |
| 7 days (n = 9) | E. coli | 7/7 (100)a | 2/7 (29) |
| Control | 0/2 (0) | 0/2 (0) | |
Significantly different from control group (P < 0.05) by Fisher’s exact test.
Sterile LB medium.
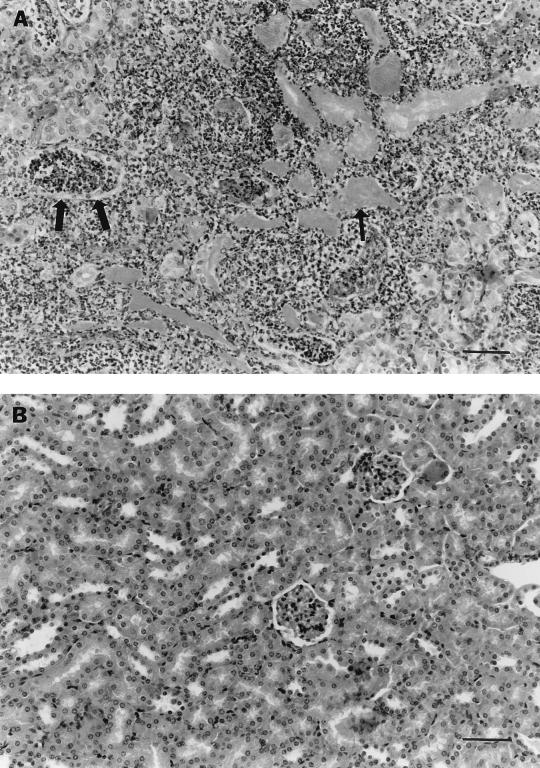
Renal abscesses were induced in 100% of infected animals by 48 h (Fig. 1) and were still present at 7 days. Abscesses were restricted to the site of injection at the lower kidney pole, while the upper pole and contralateral kidney remained histologically normal. No abscesses formed in LB medium-injected kidneys (P < 0.001).
FIG. 1.
Histologic findings following renal injection. (A) E. coli-injected kidney demonstrating massive neutrophil infiltration, bacterial aggregates (thin arrow), and tubular necrosis involving both the cortex and medulla (thick arrows); (B) control kidney demonstrating normal histology. Specimens were obtained 48 h after inoculation. Bar = 100 μm.
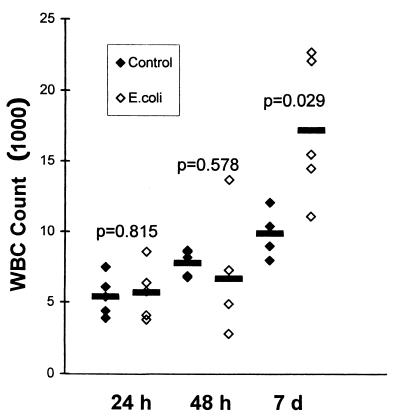
WBC counts were obtained for 28 of 29 mice and were found to be significantly elevated 7 days after the animals were infected, but not after 24 or 48 h (Fig. 2). Granulocytes and lymphocytes increased in equal proportions (data not shown).
FIG. 2.
WBC counts of infected and control animals at 24 h, 48 h, and 7 days (d) after inoculation. Horizontal lines indicate means. P values result from the comparison of infected with control animals at each time point (Student’s t test).
The findings that 100% of mice showed persistent abscess and that 29% of mice displayed bacteremia for 7 days suggest that direct renal inoculation may serve as a model for chronic infection and are consistent with prior observations that experimental kidney abscesses may persist for several weeks (10, 12, 16). Although it is possible that such a persistent infection could result in bacterial seeding of other organs, we did not find gross or microscopic evidence of secondary infection of either the opposite pole of the injected kidney or the contralateral kidney.
In clinical practice, patients may present to clinical practitioners with flank pain before pyrexia or systemic leukocytosis develops. It may be of interest that we observed delayed rather than acute leukocytosis after kidney infection. The kidney, perhaps owing to its inherent drainage, may allow advanced local infection before a systemic response can be detected.
Miller and Robinson have previously described a method of inducing pyelonephritis in rats by direct injection (12). This method has not been previously validated for mice. The mouse provides several advantages over the rat in studies of experimental kidney infection. Mice possess globoseries glycolipid receptors for P pili similar to those found in human uroepithelium (1). Rat uroepithelial cells lack these receptors. Additionally, a wealth of information regarding murine genetics and immunology exists, and current techniques allow the manipulation of the murine genome. Our mouse model extends the observations of Miller and Robinson (12) by including a control group, demonstrating delayed leukocytosis, and developing abscesses within the renal parenchyma.
This simple, reproducible model may be particularly useful in studying responses to infectious insults, as there is rapid induction of kidney abscess and bacteremia without extrarenal organ failure or host death. We have, for example, observed the induction of preterm delivery for 60% of pregnant mice following bacterial inoculation of the kidney (12a). This model may additionally be suitable for studying antibiotic efficacy as well as hypertension and preeclampsia, both of which are sequelae of kidney infection.
Acknowledgments
We thank Sheetal P. Mehta for her technical assistance.
REFERENCES
- 1.Fernandes P B, Shipkowitz N L, Bower R R. Murine models for studying the pathogenesis and treatment of pyelonephritis. Adv Exp Med Biol. 1987;224:35–51. doi: 10.1007/978-1-4684-8932-3_4. [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto T, Sato M, Katami K, Osada Y, Tachizawa H, Une T. Chemotherapeutic efficacy of ofloxacin on renal and subcutaneous infection models with Staphylococcus aureus in mice. Chemotherapy (Basel) 1986;32:291–298. doi: 10.1159/000238426. [DOI] [PubMed] [Google Scholar]
- 3.Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris R E, Thomas V L, Shelokov A. Asymptomatic bacteriuria in pregnancy: antibody-coated bacteria, renal function, and intrauterine growth retardation. Am J Obstet Gynecol. 1976;126:20–25. doi: 10.1016/0002-9378(76)90458-0. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson S H, Eklof O, Eriksson C G, Lins L-E, Tidgren B, Winberg J. Development of hypertension and uraemia after pyelonephritis in childhood: 27 year follow up. BMJ. 1989;299:703–706. doi: 10.1136/bmj.299.6701.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson D E, Russell R G, Lockatell C V, Zulty J C, Warren J W. Urethral obstruction of 6 hours or less causes bacteriuria, bacteremia, and pyelonephritis in mice challenged with “nonuropathogenic” Escherichia coli. Infect Immun. 1993;61:3422–3428. doi: 10.1128/iai.61.8.3422-3428.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaitz A. Urinary concentrating ability in pregnant women with asymptomatic bacteriuria. J Clin Investig. 1961;40:1331–1338. doi: 10.1172/JCI104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalil A, Brauner A, Bakhiet M, Burman L G, Jaremko G, Wretlind B, Tullus K. Cytokine gene expression during experimental Escherichia coli pyelonephritis in mice. J Urol. 1997;158:1576–1580. [PubMed] [Google Scholar]
- 9.Kincaid-Smith P, Bullen M. Bacteriuria in pregnancy. Lancet. 1965;i:395–399. doi: 10.1016/s0140-6736(65)90001-2. [DOI] [PubMed] [Google Scholar]
- 10.Lee J C, Betley M J, Hopkins C A, Perez N E, Pier G B. Virulence studies, in mice, of transposon-induced mutants of Staphylococcus aureus differing in capsule size. J Infect Dis. 1987;156:741–750. doi: 10.1093/infdis/156.5.741. [DOI] [PubMed] [Google Scholar]
- 11.McGrady G A, Daling J R, Peterson D R. Maternal urinary tract infection and adverse fetal outcomes. Am J Epidemiol. 1985;121:377–381. doi: 10.1093/oxfordjournals.aje.a114009. [DOI] [PubMed] [Google Scholar]
- 12.Miller T E, Robinson K B. Experimental pyelonephritis: a new method for inducing pyelonephritis in the rat. J Infect Dis. 1973;127:307–310. doi: 10.1093/infdis/127.3.307. [DOI] [PubMed] [Google Scholar]
- 12a.Mussalli, G. M., and E. Hirsch. Unpublished data.
- 13.Rugo H S, O’Hanley P, Bishop A G, Pearce M K, Abrams J S, Howard M, O’Garra A. Local cytokine production in a murine model of Escherichia coli pyelonephritis. J Clin Investig. 1992;89:1032–1039. doi: 10.1172/JCI115644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks S H, Jones K V, Roberts R, Asscher A W, Ledingham J G G. Effect of symptomless bacteriuria in childhood on subsequent pregnancy. Lancet. 1987;ii:991–994. doi: 10.1016/s0140-6736(87)92558-x. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. pp. 1117–1123. [Google Scholar]
- 16.Vivaldi E, Zangwill D P, Cotran R, Kass E H. Experimental pyelonephritis consequent to induction of bacteriuria. In: Quinn E L, Kass E H, editors. Biology of pyelonephritis. Boston, Mass: Little, Brown and Company; 1960. pp. 27–37. [Google Scholar]