Abstract
MicroRNAs (miRNAs) are small non-coding RNAs (ncRNAs) about 22 nucleotides in size, which play an important role in gene regulation and are involved in almost all major cellular physiological processes. In recent years, the abnormal expression of miRNAs has been shown to be associated with human diseases including cancer. In the past ten years, the link between miRNAs and various cancers has been extensively studied, and the abnormal expression of miRNAs has been reported in various malignant tumors, such as lung cancer, gastric cancer, colorectal cancer, liver cancer, breast cancer, and prostate cancer. Due to the high malignancy grade of these cancers, it is more necessary to develop the related diagnostic and prognostic methods. According to the study of miRNAs, many potential cancer biomarkers have been proposed for the diagnosis and prognosis of diseases, especially cancer, thus providing a new theoretical basis and perspective for cancer screening. The use of miRNAs as biomarkers for diagnosis or prognosis of cancer has the advantages of being less invasive to patients, with better accuracy and lower price. In view of the important clinical significance of miRNAs in human cancer research, this article reviewed the research status of miRNAs in the above-mentioned cancers in 2021, especially in terms of diagnosis and prognosis, and provided some new perspectives and theoretical basis for the diagnosis and treatment of cancers.
Keywords: miRNAs, cancer, diagnosis, prognosis, biomarker
1. Introduction
Cancer is a global public health problem and a leading cause of death. As estimated by the WHO in 2019, cancer is the first or second leading cause of death before the age of 70 years in 183 countries around the world [1]. According to the GLOBOCAN 2020 data compiled by the International Agency for Research on Cancer (IARC), there will be 19,292,789 new cancer cases and 9,958,133 cancer deaths worldwide in 2020. Xia et al. conducted statistical analysis on cancer development in China over the years based on data from the GLOBOCAN 2020, the UN world Population Outlook 2019 (Revised Edition), the National Cancer Center of China (NCC) and other websites. According to their results, the incidence and mortality of colorectal cancer (CRC), female breast cancer (BC), and male prostate cancer (PCa) have significantly increased. It is predicted that the top five cancer types in China in 2022 will be lung cancer, CRC, gastric cancer (GC), liver cancer, and BC [2]. The incidence and mortality of cancer show an increasing trend year by year. The early diagnosis of cancer contributes to better patient treatment, markedly improved prognosis, reduced risk of cancer recurrence, and partially decreased cancer patient mortality.
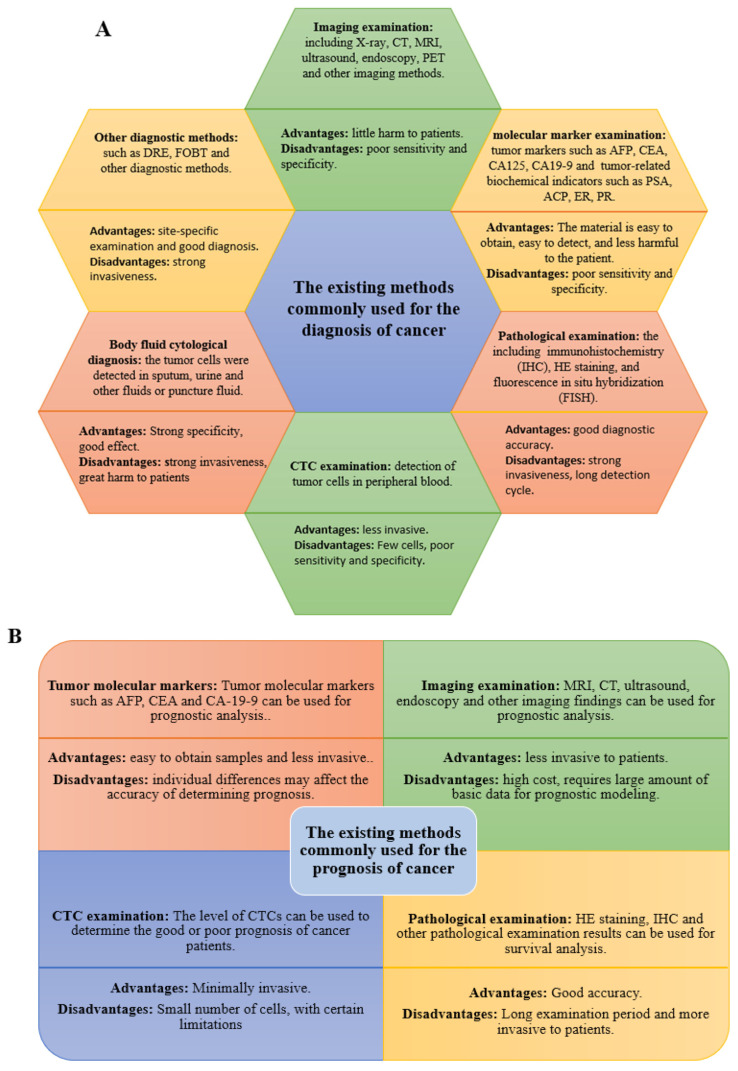
The commonly used methods for the diagnosis and prognosis of cancers are (1) imaging examination: including X-ray, CT, magnetic resonance imaging (MRI), ultrasound, endoscopy, glucose metabolism technology and positron emission tomography (PET), radionuclide imaging examination and other imaging methods [3,4,5]; (2) molecular marker examination: including detection of tumor markers such as carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), and various carbohydrate antigens (such as CA125 and CA19) and some tumor-related biochemical indicators such as acid phosphatase (ACP), estrogen receptor (ER), and progesterone receptor (PR) in serum urine of patients [4,6,7,8,9]; (3) pathological examination where the abnormal tissue samples are collected for pathological examination by techniques such as immunohistochemistry (IHC), HE staining, and fluorescence in situ hybridization (FISH) [10,11]; (4) detection of circulating tumor cells (CTC), such as detection of tumor cells in peripheral blood, which can be used to monitor and predict the prognosis of tumor metastasis [12,13]; (5) body fluid cytological diagnosis, including detection of tumor cells by sputum, urine and other liquids or by means of puncture [14,15]; and (6) other examinations: including digital rectal examination (DRE), fecal occult blood test (FOBT), and other diagnostic methods [16,17].
However, these diagnostic methods are either invasive and expensive, or show poor sensitivity and specificity. For example, colonoscopy for CRC diagnosis and gastroscopy for GC diagnosis will bring extreme discomfort to patients, and making pathological sections will cause greater trauma to patients, the pathological tissue for pathological sections needs to be obtained by biopsy, and the location and size of the samples will affect the test results, which will cause discomfort and even trauma to the patients [10,18]. While the other less invasive detection methods such as ultrasound, MRI, and serum tumor markers CEA, AFP, and other tests exhibit lower sensitivity or specificity [4,18]. Therefore, it is necessary to develop more convenient, rapid, and sensitive diagnostic and prognostic methods. The existing methods commonly used for the diagnosis and prognosis of cancer are summarized in Figure 1.
Figure 1.
(A) The commonly used methods for the diagnosis of cancer. (B) The commonly used methods for the prognosis of cancer.
miRNAs are the non-coding RNAs (ncRNAs) that have been widely investigated in tumors recently. miRNAs are found to play an important role in the diagnosis and prognosis of cancer [19,20,21]. Therefore, the use of miRNAs as biomarkers for cancer diagnosis and prognosis has gradually attracted wide attention from people. In view of the 2021 papers regarding the relationship between miRNAs and different cancers retrieved in NCBI, the GLOBOCAN 2020 data, and the analysis and prediction of cancer development trends in recent years by Xia et al., this article selected six cancer types with high incidence, including GC, CRC, lung cancer, BC, PCa, and liver cancer, and summarized and analyzed the research progress of miRNAs as biomarkers for their diagnosis and prognosis.
2. Biosynthesis and Functions of miRNAs
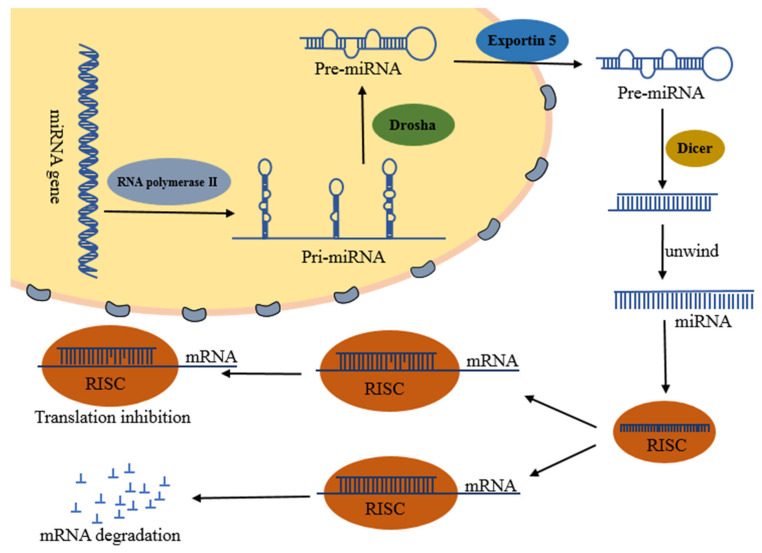
The biosynthesis of miRNA is accomplished by a variety of enzymes. First, primary miRNAs (Pri-miRNAs) are transcribed under the action of RNA polymerase II, and then precursor miRNAs (pre-miRNAs) are formed under the action of RNase III Drosha and its cofactor DGCR8. Then it is cleaved into double-stranded miRNAs by endonuclease Dicer, and finally the mature single-stranded miRNAs are dissociated by helicase [22,23,24]. In addition, there is another miRNA biosynthesis pathway independent of the Drosha/DGCR8 pathway, which is called miRtrons, miRNA is processed by introns of protein-coding genes through mRNA pre-splicing mechanism in this pathway [25].
In general, miRNAs regulate target genes at the post-transcriptional level, and destabilize the target mRNA or inhibit the translation of target mRNA [26]. According to their different modes of action with genes, they can be divided into the following three types [27,28,29,30], namely, ① complementary binding with target gene, which shares extremely similar mode of action and function to siRNA, and finally cleaves mRNA. This is a common mode in plants. ② Incomplete complementary binding with target gene, which prevents translation without affecting the stability of mRNA. It is the most commonly found mode of action and is common in animals. ③ The combination of the above two modes of action. Figure 2 summarizes the miRNA formation and mechanisms of action.
Figure 2.
miRNA formation and mechanism of action.
3. miRNAs and Cancer Diagnosis and Prognosis
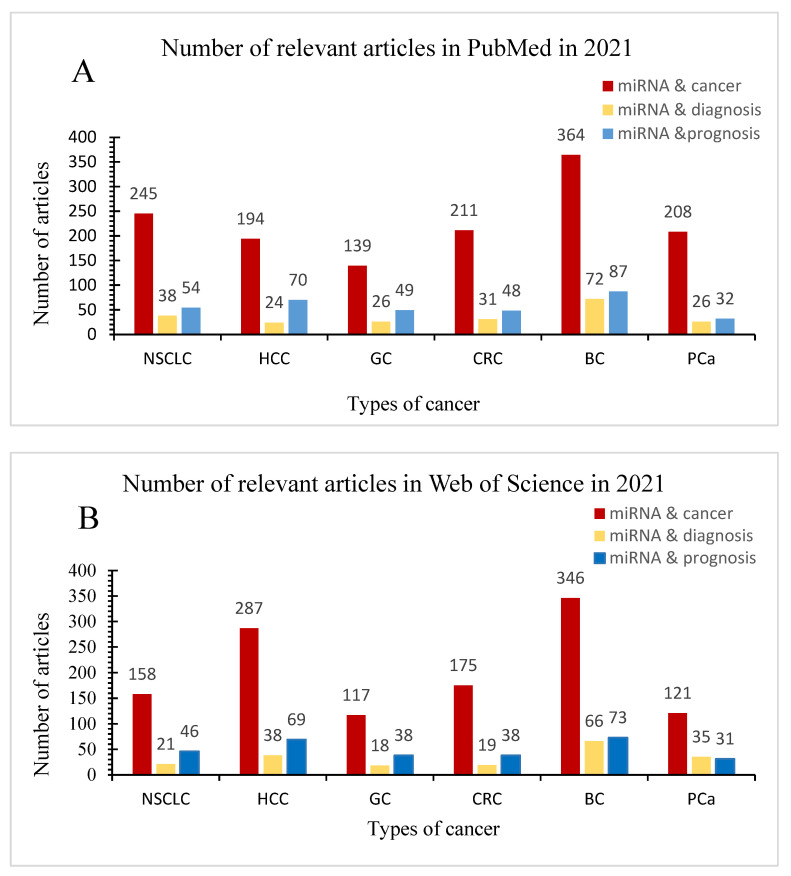
In recent years, miRNAs have attracted the attention of many researchers as biomarkers for cancer diagnosis and prognosis. In order to more systematically understand the research and application of miRNAs in the diagnosis and prognosis of these six cancers in 2021, we searched related papers in PubMed and Web of Science by combining miRNA, diagnosis, and prognosis with lung cancer, colorectal cancer, gastric cancer, liver cancer, breast cancer, and prostate cancer respectively. Articles on the diagnosis and prognosis of miRNAs in six cancers in 2021 are summarized in Figure 3.
Figure 3.
Articles on the diagnosis and prognosis of miRNAs in six cancers in 2021. (A) Number of relevant articles in PubMed in 2021. (B) Number of relevant articles in web of Science in 2021.
Tumor is essentially a multi-gene abnormal disease. Its malignant phenotype is the result of the activation of expression of one or more proto-oncogenes or the loss of mutation of tumor suppressor genes, which enables tumor cells to escape from the normal growth regulation mechanism and to proliferate and invade autonomously. Due to the wide variety of miRNAs, they play different roles in different cancers, suggesting the higher specificity of miRNAs. Therefore, we reviewed the roles of miRNAs according to the characteristics of cancer types.
3.1. Lung Cancer
Lung cancer is a highly malignant cancer, which ranks the second place in terms of its morbidity and mortality in the world, accounting for 11.6% of all new cancer cases and 19.8% of all cancer-related deaths. Typically, non-small cell lung cancer (NSCLC) occupies 80% of lung cancer cases [31]. Recently, great progress has been made in identifying novel diagnostic and prognostic biomarkers for lung cancer, including RBR E3 ubiquitin ligase [32], prostaglandin E synthase 3 (PTGES3) [33], fibroblast activation protein (FAP) [34], checkpoint inhibitor immunotherapy targeting programmed cell death 1 (PD1), and programmed death ligand 1 (PDL1) pathways [35,36]. However, the 5-year overall survival rate of lung cancer remains low among all the cancer types, and its high mortality can be ascribed to the fact that most patients are diagnosed at an advanced stage and the treatment options are thereby limited, with a 5-year survival rate of only 4% [37]. The presence of reliable biomarkers can detect lung cancer at an early stage and can improve prognosis and recurrence risk. Several recent studies have demonstrated that miRNAs can be used as the diagnostic and prognostic biomarkers for lung cancer. The miRNAs associated with lung cancer identified in 2021 are summarized in Table 1.
Early diagnosis is the key to the successful treatment of lung cancer. As revealed by multiple human studies, miRNAs may serve as the tools for the early diagnosis of lung cancer. For example, Monika Migdalska-Sęk et al. found that miRNA-17 was up-regulated in NSCLC tissue and its surrounding para-cancerous tissues, so miRNA-17 was speculated to have an oncogenic effect. In addition, the expression of miRNA-17 in lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), and the two NSCLC subtypes was significantly different [38]. miRNAs can also be used to diagnose NSCLC metastasis. Leptomeningeal metastasis (LM) is the most serious complication in NSCLC patients. The survival time of patients will be greatly shortened once LM occurs. Xu et al. found that the levels of miRNA-483-5p and miRNA-342-5p in serum exosomes of LM patients were higher than those of NSCLC patients without LM. Therefore, miRNA-483-5p and miRNA-342-5p might replace the routine examination of cerebrospinal fluid (CSF) to diagnose the occurrence of LM in NSCLC patients [39], which greatly reduced the trauma caused by CSF examination to patients. For the screening of miRNAs biomarkers for lung cancer, bioinformatics methods can also be used apart from using samples such as serum and tissues. Nie et al. analyzed the miRNAs expression profiles of lung cancer by bioinformatics analysis and discovered that miRNA-708-5p was significantly up-regulated in NSCLC tissues. To understand its sensitivity and specificity, a receiver operating characteristic (ROC) curve was plotted, and the area under the curve (AUC) was ≥0.9, indicating that miRNA-708-5p exhibited a good diagnostic value for NSCLC [40]. In addition, carcinoembryonic antigen (CEA) as a diagnostic marker for tumor has been widely used in clinical practice, but it has some shortcomings such as poor specificity. Therefore, the combination of CEA and other markers is among the popular cancer detection methods at present. Dong et al. combined CEA with serum miRNA-1247-5P, miRNA-301B-3p, and miRNA-105-5P, respectively, for the early diagnosis of NSCLC. Compared with miRNA alone, the AUC value of CEA combined with serum miRNAs increased from 0.812, 0.788, and 0.793 to 0.900, 0.895, and 0.904, respectively [41]. These results indicated that the combination of multiple markers for the diagnosis of NSCLC outperformed the use of miRNA alone, and the diagnostic effect was greatly improved, enhancing the detection accuracy.
In addition to diagnosis, miRNAs can also serve as the prognostic biomarkers for lung cancer. Similar to other malignancies, tumor stage is the most important determinant of lung cancer prognosis along with other clinical and histological variables [42]. In recent years, miRNAs have been considered as the key factors for predicting cancer prognosis, including lung cancer [43,44,45]. Yang et al. suggested that miRNA-1276, miRNA-767-3p, and miRNA-767-5p were up-regulated in LUAD by bioinformatics approaches, and the up-regulated expression of miRNA-1276 was negatively correlated with patient prognosis, whereas the up-regulated expression of miRNA-767-3p and miRNA-767-5p was negatively correlated with the survival time of patients. At the same time, Yang et al. verified the above bioinformatics results in two lung cancer cell lines A549 and SK-MES-1 [46]. As indicated by Khandelwal et al., the expression of miRNA-320a in the serum of NSCLC patients was significantly down-regulated. Moreover, its expression was correlated with the prognosis of NSCLC patients, and patients with low miRNA-320a expression had significantly decreased survival [47]. In addition, miRNAs can also be used for personalized studies in LUAD patients. Boldrini et al. analyzed miRNAs in 88 LUAD specimens and demonstrated that miR-25 expression was up-regulated in young LUAD patients compared with older LUAD patients, consistent with the results obtained from TCGA database. Furthermore, mechanism research showed that high expression of miR-25 was associated with the low expression of phosphatase and tensin homolog (PTEN) in young patients [48]. This study revealed the differential expression of miRNA-25 in young and elderly LUAD patients, and in particular, the interaction of miRNA-25 and PTEN in young LUAD patients may define a subgroup, highlighting the concept of molecular detection of different age subtypes. It provided a theoretical basis for whether lung adenocarcinoma (LUAD) patients of different ages have different molecular characteristics, and provided a preliminary guarantee for appropriate miRNA individualized treatment of LUAD. It also provided a theoretical basis and direction for the screening of tumor diagnostic and prognostic markers in the future, which should consider whether individual differences of patients (such as age, gender, and the presence of other diseases) will affect the screening results.
Table 1.
MicroRNAs as biomarkers for diagnosis and prognosis of NSCLC.
| miRNA | Sample | Expression | AUC | Biomarker | Reference |
|---|---|---|---|---|---|
| miR-708-5p | Tissue | ↑ | 0.925 | Diagnosis | [40] |
| miR-1247-5p | Serum | ↑ | 0.812 | Diagnosis | [41] |
| miR-301b-3p | Serum | ↑ | 0.788 | Diagnosis | [41] |
| miR-105-5p | Serum | ↑ | 0.793 | Diagnosis | [41] |
| miR-492 | Plasma | ↑ | 3-miR:0.828 | Diagnosis | [49] |
| miR-590-3p | Plasma | ↑ | 3-miR:0.828 | Diagnosis | [49] |
| miR-4507 | EBC | ↑ | 3-miR:0.837 | Diagnosis | [50] |
| miR-451a | EBC | ↑ | 3-miR:0.837 | Diagnosis | [50] |
| miR-483-5p | exosomes | ↑ | / | Diagnosis | [39] |
| miR-342-5p | exosomes | ↑ | / | Diagnosis | [39] |
| miR-520c-3p | exosomes | ↑ | 2-miR:0.857 | Diagnosis | [51] |
| miR-1274b | exosomes | ↑ | 2-miR:0.857 | Diagnosis | [51] |
| miR-96 | exosomes | ↑ | 0.9735 | Diagnosis/Prognosis | [52] |
| miR-1276 | Tissue | ↑ | / | Prognosis | [46] |
| miR-767-3p | Tissue | ↑ | / | Prognosis | [46] |
| miR-767-5p | Tissue | ↑ | / | Prognosis | [46] |
| miR-10b | Tissue | ↑ | / | Prognosis | [53] |
| miR-135b | Tissue | ↑ | / | Prognosis | [54] |
| miR-142-3p | Tissue | ↑ | / | Prognosis | [55] |
| miR-196a | Tissue | ↑ | / | Prognosis | [56] |
| miR-202 | Plasma | ↑ | / | Prognosis | [57] |
| miR-17 | Tissue | ↓ | 0.692 | Diagnosis | [38] |
| miR-125b-5p | Tissue | ↓ | 0.768 | Diagnosis/Prognosis | [44] |
| miR-631 | Plasma | ↓ | 3-miR:0.828 | Diagnosis | [49] |
| miR-6777-5p | EBC | ↓ | 3-miR:0.837 | Diagnosis | [50] |
| miR-23a | exosomes | ↓ | 0.744 | Diagnosis | [58] |
| miR-let7i | exosomes | ↓ | 0.733 | Diagnosis | [58] |
| miR-203 | Tissue | ↓ | / | Prognosis | [53] |
| miR-320a | Serum | ↓ | / | Prognosis | [47] |
EBC: exhaled breath condensate, 3-miR: AUC values of three miRNA combinations appearing in the same paper, 2-miR: AUC values of two miRNA combinations appearing in the same paper, ↑: Up-regulated expression, ↓: Down-regulation expression.
3.2. Liver Cancer
Liver cancer is the fourth leading cause of cancer death worldwide. There are two subtypes of liver cancers, including hepatocellular carcinoma (HCC) and cholangiocarcinoma, of which HCC accounts for 80% [59]. Based on liver cancer data from 204 countries from 1990 to 2019, viral hepatitis B and C are found to be the leading cause of death from liver cancer [60]. Although great progress has been made in the treatment of HCC in recent decades, the 5-year survival rate of HCC patients remains as low as about 20%. This is mainly ascribed to the delayed diagnosis of HCC, chemotherapy resistance, frequent recurrence, and poor patient prognosis [61]. Consequently, the accurate diagnosis of HCC is important, which will improve the therapeutic effect and survival rate of patients. More recent studies have indicated that miRNAs may serve as the diagnostic and prognostic biomarkers for HCC. Notably, circulating miRNAs in serum or exosomes may be more useful biomarkers than those in tissues, since blood samples can be collected repeatedly in a non-invasive manner and show high specificity. miRNAs associated with HCC identified in 2021 are summarized in Table 2.
Early detection of HCC at a surgically resectable stage provides the best chance of survival for patients, and it is needed to find effective biomarkers for detecting HCC and tracking its development. It has been indicated in numerous studies that miRNAs can potentially serve as the tools for the early diagnosis of HCC. For example, Qin et al. performed miRNAs differential expression analysis on TCGA dataset and the miRNAs expression profiling data of 48 HCC patients, and plotted the ROC curve of differentially expressed genes (DEGs) to analyze their diagnostic value. It was found that the AUC values of miRNA-452-5p, miRNA-5589-5p, miRNA-5589-3p, and miRNA-4661-5p in the early diagnosis of HCC were 0.830, 0.879, 0.838, and 0.725, respectively. Moreover, the four miRNAs were differentially expressed in only one or two types of cancer with good specificity, which might be used as the early diagnostic biomarkers for HCC [62]. In addition, Huang et al. found that miRNAs might also serve as the diagnostic biomarkers for lung metastasis in HCC patients, and the combination of multiple miRNAs had better performance than a single miRNA. For example, the AUC values of miRNA-18A, miRNA-20B, and miRNA-221 for the diagnosis of HCC lung metastasis were 0.7722, 0.8661, and 0.5816, respectively, while that of their combination reached 0.904, which greatly improved compared with that of a single miRNA [63]. This phenomenon was also supported by Yu et al., who analyzed and validated five GEO datasets and discovered that miRNA-184, miRNA-532-5p, miRNA-221-3p, miRNA-5589-5p, let-76-3p, and miRNA-26b-3p were significantly differentially expressed in the serum of HCC patients. Moreover, the AUC value of the diagnostic biomarker composed of these six miRNAs reached 0.9559, indicating that they had good diagnostic value [64]. Similarly, the combination of miRNAs and other diagnostic markers is also suitable for HCC. Alpha-fetoprotein (AFP) has been extensively applied in the diagnosis of various cancers, but due to its low accuracy, specificity, and sensitivity, it is not used in the diagnosis of cancer alone, but in combination with other diagnostic methods. Fouda et al. analyzed the combination of AFP with miRNAs as a diagnostic biomarker for HCC, and discovered that the AUC value of the combination of AFP with miRNA-200, miRNA-29, miRNA-21, and miRNA-355 reached 0.92 [65], indicating that combination of miRNAs with AFPs potentially enhanced the diagnostic value of HCC.
A large number of recent studies also support miRNAs as the prognostic biomarkers of HCC. For instance, through differential analysis of HCC miRNAs in TCGA databases and stepwise multivariate Cox regression analysis of the correlation between differentially expressed miRNAs and prognosis, Zhang et al. found that miRNA-139-3p, miRNA-760, and miRNA-7-5p were correlated with the prognosis of HCC patients. Meanwhile, they detected the levels of these three miRNAs in the serum of HCC patients. As a result, compared with normal patients, the levels of miRNA-139-3p in the serum of HCC patients decreased, while those of miRNA-7-5p and miRNA-760 increased. Combined with ROC curve analysis, it was indicated that these three miRNAs were also of great diagnostic value for HCC [66]. Liver transplantation is an effective approach for the treatment of HCC, but there is always a risk of recurrence. miRNAs can be used to predict the recurrence of HCC after liver transplantation. Huang et al. screened miRNA-3200-3p and miRNA-3690 as the relevant prognostic markers for HCC recurrence after liver transplantation by analyzing HCC miRNAs in TCGA and GEO databases [67]. Yokota et al. also demonstrated that miRNA-638 served as a prognostic marker for recurrence after liver transplantation in HCC patients, and the two-year disease-free survival (DFS) rate of HCC patients in the high miRNA-638 expression group was 47.1%, while that in the low expression group was 77.4%, and patients in the high expression group were more likely to develop recurrence after surgery [68]. As discovered by Xie et al., miRNA-1269 and miRNA-421 were significantly over-expressed in tumor tissues through differential analysis of miRNA expression in tumor tissues and surrounding tissues of HCC patients, which was verified by analysis of TCGA database. Subsequent Kaplan–Meier analysis revealed that the high expression of miRNA-638 in HCC tissues was closely related to the shorter patient survival [69].
Table 2.
MicroRNAs as biomarkers for diagnosis and prognosis of HCC.
| miRNA | Sample | Expression | AUC | Biomarker | Reference |
|---|---|---|---|---|---|
| miR-452-5p | Tissue | ↑ | 0.83 | Diagnosis/Prognosis | [62] |
| miR-4661-5p | Tissue | ↑ | 0.725 | Diagnosis/Prognosis | [62] |
| miR-760 | Tissue/Serum | ↑ | 0.707 | Diagnosis/Prognosis | [66] |
| miR-7-5p | Tissue/Serum | ↑ | 0.641 | Diagnosis/Prognosis | [66] |
| miR-27a | exosomes/Tissue | ↑ | 0.8282 | Diagnosis/Prognosis | [63] |
| miR-18a | exosomes/Tissue | ↑ | 3-miR:0.904 | Diagnosis/Prognosis | [63] |
| miR-20b | exosomes/Tissue | ↑ | 3-miR:0.904 | Diagnosis/Prognosis | [63] |
| miR-221 | exosomes/Tissue | ↑ | 3-miR:0.904 | Diagnosis/Prognosis | [63] |
| miR-184 | Serum | ↑ | 6-miR:0.9535 | Diagnosis | [64] |
| miR-532-5p | Serum | ↑ | 6-miR:0.9535 | Diagnosis | [64] |
| miR-221-3p | Serum | ↑ | 6-miR:0.9535 | Diagnosis | [64] |
| miR-200 | Serum | ↑ | 4-miR:0.92 | Diagnosis | [65] |
| miR-21 | Serum | ↑ | 4-miR:0.92 | Diagnosis | [65] |
| miR-355 | Serum | ↑ | 4-miR:0.92 | Diagnosis | [65] |
| miR-1293 | TEP miRNA | ↑ | 0.78 | Diagnosis | [70] |
| miR-3200-3p | Tissue | ↑ | / | Prognosis | [67] |
| miR-3690 | Tissue | ↑ | / | Prognosis | [67] |
| miR-1269a | Tissue | ↑ | / | Prognosis | [69] |
| miR-421 | Tissue | ↑ | / | Prognosis | [69] |
| miR-326 | Tissue | ↑ | / | Prognosis | [71] |
| miR-21 | Tissue | ↑ | / | Prognosis | [71] |
| miR-212-3p | Tissue | ↑ | / | Prognosis | [72] |
| miR-25 | Tissue | ↑ | / | Prognosis | [73] |
| let-7e | Tissue/Cell | ↑ | / | Prognosis | [74] |
| let-7e | exosomes/Tissue | ↑ | / | Prognosis | [63] |
| miR-652 | exosomes/Tissue | ↑ | / | Prognosis | [63] |
| miR-638 | Cell/exosomes | ↑ | / | Prognosis | [68] |
| miR-5589-3p | Tissue | ↓ | 0.838 | Diagnosis/Prognosis | [62] |
| miR-5589-5p | Tissue | ↓ | 0.879 | Diagnosis/Prognosis | [62] |
| miR-139-3p | Tissue/Serum | ↓ | 0.706 | Diagnosis/Prognosis | [66] |
| miR-5589-5p | Serum | ↓ | 6-miR:0.9535 | Diagnosis | [64] |
| miR-26b-3p | Serum | ↓ | 6-miR:0.9535 | Diagnosis | [64] |
| let-7b-3p | Serum | ↓ | 6-miR:0.9535 | Diagnosis | [64] |
| miR-29a | Serum | ↓ | 4-miR:0.92 | Diagnosis | [65] |
| miR-495-3p | TEP miRNA | ↓ | 0.76 | Diagnosis | [70] |
| miR-125b | exosomes | ↓ | / | Diagnosis | [75] |
| miR-144/451a cluster | Tissue | ↓ | / | Prognosis | [76] |
| miR-140 | Tissue | ↓ | / | Prognosis | [77] |
| mir-150-3p | Tissue/exosomes | ↓ | / | Prognosis | [78] |
TEP: tumor-educated platelet, 3-miR: AUC values of three miRNA combinations appearing in the same paper, 4-miR: AUC values of four miRNA combinations appearing in the same paper, 6-miR: AUC values of six miRNA combinations appearing in the same paper, ↑: Up-regulated expression, ↓: Down-regulation expression.
3.3. Gastric Cancer
Gastric cancer (GC) is another major cause of cancer-related death, accounting for 8.2% of all cancer-related deaths. Unfortunately, due to the lack of early diagnostic markers or screening tools, many GC patients are diagnosed as the advanced stage, resulting in the low (3%) 5-year survival rate [79,80]. The sensitivity and specificity of existing biomarkers for GC diagnosis and prognosis are low. Currently, GC is diagnosed based on invasive procedures such as upper gastrointestinal endoscopy [81]. Some studies have shown that the abnormal expression of miRNA in GC may have clinical utility, especially in the diagnosis and prognosis of GC. miRNAs associated with GC identified in 2021 are summarized in Table 3.
Identifying effective biomarkers for the early diagnosis of GC is an effective way to improve the survival rate of GC patients, and ROC curve is an effective indicator for judging the diagnostic effect. Chen et al. analyzed the diagnostic effect of miRNA-130b by drawing ROC curve. According to their results, the AUC value of miRNA-130b was 0.911, which might serve as the potential GC diagnostic marker for further research [79]. The combination of miRNAs can also be used for the early diagnosis of GC, which achieves better diagnostic effect than a single miRNA. For example, Kohrboa et al. identified four significantly up-regulated miRNAs in the serum exosomes of GC patients, namely, miRNA-10a-5p, miRNA-19b-3p, miRNA-215-5p, and miRNA-18a-5p. ROC curve analysis showed that the AUC values were 0.801, 0.721, 0.780, and 0.736, respectively, which were lower than that of 0.813 for the combination of these four miRNAs [82]. Yu et al. selected six differentially expressed miRNAs in tumor tissues and plasma of GC patients, among which, miRNA-3185, miRNA-6083, miRNA-6792-3p, and miRNA-659-3p were up-regulated, whereas miRNA-936 and miRNA-1306-3p were down-regulated. Further analysis of their diagnostic value showed that the AUC values of the four up-regulated miRNAs were 0.825, significantly higher than those of the two down-regulated miRNAs of 0.730 [83]. In addition, in some studies, the combination of miRNAs with tumor markers currently used in the clinical diagnosis of GC also improves the diagnostic effect. For example, So et al. analyzed 578 miRNAs in the serum of 682 GC patients and identified 12 miRNAs for GC diagnosis, with an AUC value of 0.848. However, the AUC value increased to 0.884 if the 12 miRNAs were combined with age, CEA, and Helicobacter pylori serological examination [84].
Similar to diagnosis, the combination of multiple miRNAs as a prognostic marker is definitely better than a single miRNA, so the researchers set out to construct a prognostic model including multiple miRNAs. Xu et al. obtained nine immune-related miRNAs through TCGA database, and verified that these nine miRNAs were closely related to GC prognosis. Among them, six miRNAs were used as the GC-related tumor suppressors, and their high expression was positively correlated with longer patient survival, whereas the other three miRNAs were high-risk miRNAs, and their high expression predicted poor prognosis. Thereafter, the authors constructed the immune related signature (ImmiRSig) based on these nine miRNAs, and divided the patients into high-risk group and low-risk group. According to their results, the expression of high-risk miRNAs in low-risk patients was lower than that in high-risk patients, while that of protective miRNAs showed an opposite trend [85]. Similarly, Liu et al. also constructed the GI-related miRNAs signature (GIMiSig) for predicting the survival rate of GC patients based on eight miRNAs, and compared it with the current clinically commonly used cancer staging methods. As a result, GIMiSig achieved a better prediction effect on the survival rate of GC patients [86]. In addition, Qi et al. built a risk scoring formula based on three miRNAs including miRNA-126-3p, miRNA-143-5p, and miRNA-1275, which was verified and might be used to predict the prognosis of GC patients [87].
Table 3.
MicroRNAs as biomarkers for diagnosis and prognosis of GC.
| miRNA | Sample | Expression | AUC | Biomarker | Reference |
|---|---|---|---|---|---|
| miR-455-3p | Tissue | ↑ | 3-miR:0.91 | Diagnosis | [88] |
| miR-135b-5p | Tissue | ↑ | 3-miR:0.91 | Diagnosis | [88] |
| let-7a-3p | Tissue | ↑ | 3-miR:0.91 | Diagnosis | [88] |
| miR-3185 | Tissue/Plasma | ↑ | 4-miR:0.825 | Diagnosis | [83] |
| miR-6083 | Tissue/Plasma | ↑ | 4-miR:0.825 | Diagnosis | [83] |
| miR-6792-3p | Tissue/Plasma | ↑ | 4-miR:0.825 | Diagnosis | [83] |
| miR-659-3p | Tissue/Plasma | ↑ | 4-miR:0.825 | Diagnosis | [83] |
| miR-140 | Serum | ↑ | 12-miR:0.848 | Diagnosis | [84] |
| miR-183 | Serum | ↑ | 12-miR:0.848 | Diagnosis | [84] |
| miR-30e | Serum | ↑ | 12-miR:0.848 | Diagnosis | [84] |
| miR-103a | Serum | ↑ | 12-miR:0.848 | Diagnosis | [84] |
| miR-93 | Serum | ↑ | 12-miR:0.848 | Diagnosis | [84] |
| miR-142 | Serum | ↑ | 12-miR:0.848 | Diagnosis | [84] |
| miR-21 | Serum | ↑ | 12-miR:0.848 | Diagnosis | [84] |
| miR-29c | Serum | ↑ | 12-miR:0.848 | Diagnosis | [84] |
| miR-424 | Serum | ↑ | 12-miR:0.848 | Diagnosis | [84] |
| miR-340 | Serum | ↑ | 12-miR:0.848 | Diagnosis | [84] |
| miR-1290 | Serum | ↑ | 0.6576 | Diagnosis | [89] |
| miR-320a | Serum | ↑ | ≥0.95 | Diagnosis | [90] |
| miR-1260b | Serum | ↑ | ≥0.95 | Diagnosis | [90] |
| miR-6515-5p | Serum | ↑ | ≥0.95 | Diagnosis | [90] |
| miR-130b | Plasma | ↑ | 0.911 | Diagnosis | [79] |
| miR-10a-5p | exosomes | ↑ | 0.801 | Diagnosis | [82] |
| miR-19b-3p | exosomes | ↑ | 0.721 | Diagnosis | [82] |
| miR-215-5p | exosomes | ↑ | 0.78 | Diagnosis | [82] |
| miR-18a-5p | exosomes | ↑ | 0.736 | Diagnosis | [82] |
| miR-145-3p | Tissue | ↑ | / | Prognosis | [85] |
| miR-328-3p | Tissue | ↑ | / | Prognosis | [85] |
| miR-125b-5p | Tissue | ↑ | / | Prognosis | [85] |
| miR-99a-3p | Tissue | ↑ | / | Prognosis | [85] |
| miR-133a-5p | Tissue | ↑ | / | Prognosis | [85] |
| miR-1292-5p | Tissue | ↑ | / | Prognosis | [85] |
| miR-196b-3p | Tissue | ↑ | / | Prognosis | [86] |
| miR-548v | Tissue | ↑ | / | Prognosis | [86] |
| miR-380-3p | Tissue | ↑ | / | Prognosis | [86] |
| miR-1275 | Tissue | ↑ | / | Prognosis | [86] |
| miR126-3p | Tissue | ↑ | / | Prognosis | [87] |
| miR-143-5p | Tissue | ↑ | / | Prognosis | [87] |
| miR-424-5p | Tissue | ↑ | / | Prognosis | [91] |
| let-7a-5p | Tissue | ↑ | / | Prognosis | [91] |
| miR-27a-3p | Tissue | ↑ | / | Prognosis | [91] |
| miR-126-5p | Tissue | ↑ | / | Prognosis | [91] |
| miR-182 | / | ↑ | / | Prognosis | [92] |
| miR-204-5p | Tissue | ↓ | 4-miR:0.94 | Diagnosis | [88] |
| miR-149-5p | Tissue | ↓ | 4-miR:0.94 | Diagnosis | [88] |
| miR-143-3p | Tissue | ↓ | 4-miR:0.94 | Diagnosis | [88] |
| miR-195-5p | Tissue | ↓ | 4-miR:0.94 | Diagnosis | [88] |
| miR-936 | Tissue/Plasma | ↓ | 2-miR:0.73 | Diagnosis | [83] |
| miR-1306-3p | Tissue/Plasma | ↓ | 2-miR:0.73 | Diagnosis | [83] |
| miR-181a | Serum | ↓ | 12-miR:0.848 | Diagnosis | [84] |
| miR-126 | Serum | ↓ | 12-miR:0.848 | Diagnosis | [84] |
| miR-1343-3p | Serum | ↓ | 1.000 | Diagnosis | [93] |
| miR-590-5p | exosomes | ↓ | 0.81 | Diagnosis/Prognosis | [94] |
| miR-92b-5p | Tissue | ↓ | / | Prognosis | [85] |
| miR-942-3p | Tissue | ↓ | / | Prognosis | [85] |
| miR-675-3p | Tissue | ↓ | / | Prognosis | [85] |
| miR-125b-5p | Tissue | ↓ | / | Prognosis | [86] |
| miR-99a-3p | Tissue | ↓ | / | Prognosis | [86] |
| miR-100-5p | Tissue | ↓ | / | Prognosis | [86] |
| miR-363-3p | Tissue | ↓ | / | Prognosis | [86] |
| miR-1275 | Tissue | ↓ | / | Prognosis | [87] |
| miR-23b | Tissue | ↓ | / | Prognosis | [95] |
| miR-194 | Tissue/Cell | ↓ | / | Prognosis | [96] |
| miR-339 | Tissue/Cell | ↓ | / | Prognosis | [97] |
| miR-148a | Plasma | ↓ | / | Prognosis | [98] |
4-miR: AUC values of four miRNA combinations appearing in the same paper, 2-miR: AUC values of two miRNA combinations appearing in the same paper, 12-miR: AUC values of twelve miRNA combinations appearing in the same paper, 3-miR: AUC values of three miRNA combinations appearing in the same paper, ↑: Up-regulated expression, ↓: Down-regulation expression.
3.4. Colorectal Cancer
Colorectal cancer (CRC) is a common malignant tumor; however, the effective diagnostic and prognostic markers are lacking, and the time of diagnosis will have a significant impact on the patient survival rate. Relevant studies have suggested that the survival rate of stage I CRC is 93%, but it drops sharply to 8% in stage IV CRC [99]. At present, colonoscopy is the gold standard for CRC diagnosis, but it is highly invasive and cumbersome. In addition, fecal occult blood test is also one of the commonly used diagnostic methods for CRC, but it has low sensitivity and specificity [100]. miRNAs are frequently dysregulated in CRC and used as novel and promising biomarkers because they have less invasibility, but good sensitivity and specificity. The miRNAs associated with CRC discovered in 2021 are summarized in Table 4.
Some miRNAs such as miRNA-1290 may have diagnostic value in different tumors, which can be used as the diagnostic markers not only for GC but also for CRC [89]. Silva et al. constructed a signature by combining four miRNAs (let-7e-5p, miRNA-106a-5p, miRNA-28-3p, miRNA-542-5p), which not only distinguished CRC patients from healthy subjects, but also from thickened polyps and adenomas [101]. Meanwhile, miRNAs can also be used to distinguish metastatic from non-metastatic CRC patients. Chen et al. analyzed miRNAs in plasma of CRC patients with liver metastasis, CRC patients without liver metastasis, and healthy subjects. According to their results, the AUC value of miRNA-96 combined with miRNA-99B in CRC patients and healthy subjects was 0.93, and that in patients with liver metastasis was 0.91 [102]. Nassar et al. found that miRNA-210 and miRNA-203 also showed certain diagnostic value in CRC liver metastasis [103]. In addition to its diagnostic role in liver metastasis, miRNAs can also be used in lymph node metastasis of CRC. Dokhanchi et al. discovered a correlation between miRNA-19A-3p, miRNA-203-3p, and miRNA-221-3p expression and lymph node metastasis in serum of CRC patients [104].
Some miRNAs play different roles in CRC, which not only have diagnostic value, but can also be used as prognostic markers for CRC patients. Similarly, miRNA-96 and miRNA-99b have such functions, which are thereby included in the nomogram prognostic model composed of five independent prognostic factors constructed by Chen et al. [102]. In addition, Liu et al. identified and verified that miRNA-216-5p, miRNA-194-3p, and miRNA-3677-3p, which were included in the construction of immune correlation miRNAs prognostic signature (IAMIPS) module, not only predicted the survival of CRC patients well, but also predicted the sensitivity of chemotherapeutic drugs [105]. Lan et al. analyzed autophagy-related miRNAs in tumor tissues of CRC patients and found that low expression of miRNA-449 was associated with autophagy and poor overall survival (OS) [106]. In addition, other miRNAs are studied in CRC-related cell lines. For example, Cho et al. indicated that miRNA-193a and let-7g were significantly differentially expressed between CRC cell lines and peritoneal metastasis (PTM) cell lines. Moreover, they were associated with poorer prognosis (including recurrence, venous invasion, and lymphatic invasion) in plasma exosomes from CRC patients [107]. In most studies, people only focus on miRNAs expression at a certain time point, but ignore the changes of miRNAs in the whole course of disease, as a result, individual differences between patients will affect the results. Fukada et al. prospectively focused on the changes of miRNA-21-5p throughout the disease course of CRC patients, including before surgery, 7 days, 1 month, and 6 months after surgery. According to their results, miRNA-21-5p levels were significantly higher in plasma of CRC patients at recurrence, 1 and 6 months after surgery [108], greatly improving the accuracy of the marker.
Table 4.
MicroRNAs as biomarkers for diagnosis and prognosis of CRC.
| miRNA | Sample | Expression | AUC | Biomarker | Reference |
|---|---|---|---|---|---|
| miR-1290 | Serum | ↑ | 0.7852 | Diagnosis | [89] |
| miR-19a-3p | Serum | ↑ | 0.84 | Diagnosis | [104] |
| miR-203-3p | Serum | ↑ | 0.83 | Diagnosis | [104] |
| miR-221-3p | Serum | ↑ | 0.88 | Diagnosis | [104] |
| let-7f-5p | Serum | ↑ | 0.73 | Diagnosis | [104] |
| miR-21 | Serum/Plasma | ↑ | 0.87 | Diagnosis | [109] |
| let-7e-5p | Plasma/Tissue | ↑ | 4-miR: | Diagnosis | [101] |
| miR-106a-5p | Plasma/Tissue | ↑ | 0.716(Plasma); | Diagnosis | [101] |
| miR-28-3p | Plasma/Tissue | ↑ | 0.998(Tissue) | Diagnosis | [101] |
| miR-542-5p | Plasma/Tissue | ↑ | Diagnosis | [101] | |
| miR-210 | Plasma | ↑ | 2-miR:0.731 | Diagnosis | [103] |
| miR-21 | Plasma | ↑ | 2-miR:0.731 | Diagnosis | [103] |
| miR-203 | Plasma | ↑ | 2-miR:0.833 | Diagnosis | [103] |
| miR-210 | Plasma | ↑ | 2-miR:0.833 | Diagnosis | [103] |
| miR-15b | exosomes | ↑ | 0.82 | Diagnosis | [100] |
| miR-16 | exosomes | ↑ | 0.58 | Diagnosis | [100] |
| miR-21 | exosomes | ↑ | 0.75 | Diagnosis | [100] |
| miR-31 | exosomes | ↑ | 0.75 | Diagnosis | [100] |
| miR-183 | Tissue | ↑ | / | Prognosis | [99] |
| miR-20a | Tissue | ↑ | / | Prognosis | [99] |
| miR-21 | Tissue | ↑ | / | Prognosis | [99] |
| miR-216a-5p | Tissue | ↑ | / | Prognosis | [105] |
| let-7c | Tissue | ↑ | / | Prognosis | [110] |
| let-7e | Tissue | ↑ | / | Prognosis | [110] |
| miR-34c | Tissue | ↑ | / | Prognosis | [110] |
| miR-133b | Tissue | ↑ | / | Prognosis | [110] |
| miR-21-5p | Plasma | ↑ | / | Prognosis | [108] |
| let-7g | exosomes | ↑ | / | Prognosis | [107] |
| miR-96 | Plasma | ↑ | 2-miR:0.93 | Diagnosis/Prognosis | [102] |
| miR-99b | Plasma | ↓ | 2-miR:0.93 | Diagnosis/Prognosis | [102] |
| miR-381-3p | exosomes | ↓ | 2-miR:0.807 | Diagnosis | [111] |
| miR-377-3p | exosomes | ↓ | 2-miR:0.807 | Diagnosis | [111] |
| miR-195 | Tissue | ↓ | / | Prognosis | [99] |
| miRN-139 | Tissue | ↓ | / | Prognosis | [99] |
| miR-145 | Tissue | ↓ | / | Prognosis | [99] |
| miR-194-3p | Tissue | ↓ | / | Prognosis | [105] |
| miR-3677-3p | Tissue | ↓ | / | Prognosis | [105] |
| miR-449a | Tissue | ↓ | / | Prognosis | [106] |
| miR-106a | Tissue | ↓ | / | Prognosis | [110] |
| miR-144 | Tissue | ↓ | / | Prognosis | [110] |
| miR-100 | Tissue | ↓ | / | Prognosis | [112] |
| miR-99a | Tissue | ↓ | / | Prognosis | [112] |
| miR-33b-5p | Tissue | ↓ | / | Prognosis | [113] |
| miR-193a | exosomes | ↓ | / | Prognosis | [107] |
2-miR: AUC values of two miRNA combinations appearing in the same paper, 4-miR: AUC values of four miRNA combinations appearing in the same paper, ↑: Up-regulated expression, ↓: Down-regulation expression.
3.5. Breast Cancer
According to the International Agency for Research on Cancer (IARC) managed by the World Health Organization (WHO), breast cancer (BC) currently accounts for the largest number of cancer cases and deaths in women. Early diagnosis of BC is one of the important strategies to reduce BC-related mortality. Numerous studies have reported that the 5-year survival rate of stage I BC patients can reach 100%, but less than 50% of BC patients are diagnosed in the early stage [114,115]. There is growing evidence that early diagnosis holds the key toward effective treatment outcome. Thus, multiple studies have concentrated on exploring biomarkers for BC detection. BC-related miRNAs discovered in 2021 are summarized in Table 5.
The early and combined diagnosis of miRNAs has an important guiding role in the treatment of BC patients. For example, Anna et al. analyzed miRNAs in plasma of 54 BC patients and found that the combination of miRNA-30b-5p with miRNA-99a-5p showed good diagnostic value for early BC patients, and its AUC value was 0.9273. Such results were validated in the plasma of another 74 BC patients (AUC: 0.7620) [116]. Itani et al. also selected four miRNAs, miRNA-145, miRNA-139-5p, miRNA-130a, and miRNA-425-5p, as the diagnostic markers for early BC patients, and its diagnostic performance was superior to any of the two miRNAs combinations, with an AUC value of 0.97 [117]. Apart from mature miRNAs, pri-miRNA and exosomal miRNAs also served as the important markers for BC diagnosis. Majumder et al. analyzed the initial transcript of miRNAs, according to their results, pri-miRNA-526b was a diagnostic marker for distinguishing stage I BC patients from healthy controls, and the AUC value was 0.7273 [118]. In addition, Kim et al. analyzed the diagnostic power of miRNAs for each subtype of BC in more detail. By analyzing the data from TCGA database and verifying the expression of miRNAs in extracellular vesicles of BC cell line and plasma of BC patient, they obtained four up-regulated miRNAs, namely miRNA-16, miRNA-21, miRNA-9, and miRNA-429. Further studies revealed that these miRNAs not only served as diagnostic markers for BC separately, but could also be used as a combination for the diagnosis of BC with better results [119]. In addition to the combination of multiple miRNAs, some researchers have turned their attention to miRNA clusters. For instance, Lal et al. determined the diagnostic value of miRNA379/656 cluster (the second largest gene cluster in humans, containing 42 miRNA-encoding genes) in BC. As a result, 15 miRNAs were selected as a subset of miRNA379/656, with an AUC value of 0.9843 [120].
Prognosis is an important indicator for the detection of BC recurrence and therapeutic effect, and miRNAs clusters and combinations can also be used as prognostic markers for BC. For example, Lal et al. found that low expression of the miRNA379/656 cluster was associated with poor clinical outcomes in patients [120]. In addition, Tian et al. identified a novel 5-miRNAs (including miRNA-574, miRNA-224, miRNA-210, miRNA-30b, miRNA-130a) prognostic model, which was further used to construct a nomogram by combining the prognostic model with traditional clinical prognostic predictors (like age, tumor size, lymph node metastasis, and subtype) for the better application in clinical settings [121]. Turkistani et al. directly screened three groups of miRNAs combinations, which were respectively used to determine the tumor size (≥5 cm or <5 cm), lymph node metastasis and recurrence of BC patients, so as to better understand the causes of poor prognosis [122] and to improve patient prognosis in a targeted clinical application. In addition to tumor size, lymph node metastasis, and recurrence, resistance to chemotherapeutics was another important cause of the poor prognosis of BC patients. Ai et al. directly focused more on miRNAs related to chemotherapy resistance, by comparing the differences in miRNAs expression profiles in tissue samples between chemo-sensitive and chemo-resistant patients. According to their results, the combination of miRNA-200C-3p, miRNA-214-3p, miRNA-23A-3p, miRNA-451A, and miRNA-638 was applicable for the prediction of chemotherapy resistance in BC patients [123]. Apart from miRNAs, the ncRNAs axis is also an important indicator for future BC prognosis. For instance, Lin et al. found the lncRNA GATA3-AS1/miRNA-495-3p/CENPU axis with high prognostic value in BC tissues by searching and analyzing multiple ncRNAs databases [124], indicating the increasing diversity of BC prognostic biomarkers.
Table 5.
MicroRNAs as biomarkers for diagnosis and prognosis of BC.
| miRNA | Sample | Expression | AUC | Biomarker | Reference |
|---|---|---|---|---|---|
| let-7b-5p | Serum/Tissue/exosomes | ↑ | >0.94 | Diagnosis | [125] |
| miR-106a-5p | Serum/Tissue/exosomes | ↑ | >0.94 | Diagnosis | [125] |
| miR-19a-3p | Serum/Tissue/exosomes | ↑ | >0.94 | Diagnosis | [125] |
| miR-19b-3p | Serum/Tissue/exosomes | ↑ | >0.94 | Diagnosis | [125] |
| miR-20a-5p | Serum/Tissue/exosomes | ↑ | >0.94 | Diagnosis | [125] |
| miR-223-3p | Serum/Tissue/exosomes | ↑ | >0.94 | Diagnosis | [125] |
| miR-25-3p | Serum/Tissue/exosomes | ↑ | >0.94 | Diagnosis | [125] |
| miR-425-5p | Serum/Tissue/exosomes | ↑ | >0.94 | Diagnosis | [125] |
| miR-451a | Serum/Tissue/exosomes | ↑ | >0.94 | Diagnosis | [125] |
| miR-92a-3p | Serum/Tissue/exosomes | ↑ | >0.94 | Diagnosis | [125] |
| miR-93-5p | Serum/Tissue/exosomes | ↑ | >0.94 | Diagnosis | [125] |
| miR-16-5p | Serum/Tissue/exosomes | ↑ | >0.94 | Diagnosis | [125] |
| miR-451a | Serum | ↑ | 6-miR:0.881 | Diagnosis | [126] |
| miR-195-5p | Serum | ↑ | 6-miR:0.881 | Diagnosis | [126] |
| miR-126-5p | Serum | ↑ | 6-miR:0.881 | Diagnosis | [126] |
| miR-30b-5p | Plasma | ↑ | 2-miR:0.9273 | Diagnosis | [116] |
| miR-99a-5p | Plasma | ↑ | 2-miR:0.9273 | Diagnosis | [116] |
| miR-145 | Plasma | ↑ | 4-miR:0.97 | Diagnosis | [117] |
| miR-139-5p | Plasma | ↑ | 4-miR:0.97 | Diagnosis | [117] |
| miR-130a | Plasma | ↑ | 4-miR:0.97 | Diagnosis | [117] |
| miR-425-5p | Plasma | ↑ | 4-miR:0.97 | Diagnosis | [117] |
| pri-miR-526b | Plasma | ↑ | 0.7273 | Diagnosis | [118] |
| miR-19a | Plasma | ↑ | 4-miR:0.802 | Diagnosis | [127] |
| miR-20a | Plasma | ↑ | 4-miR:0.802 | Diagnosis | [127] |
| miR-421 | exosomes | ↑ | 0.835 | Diagnosis | [128] |
| miR-128 | exosomes | ↑ | 0.825 | Diagnosis | [128] |
| miR-16 | EV | ↑ | 4-miR:0.88 | Diagnosis | [119] |
| miR-21 | EV | ↑ | 4-miR:0.88 | Diagnosis | [119] |
| miR-9 | EV | ↑ | 4-miR:0.88 | Diagnosis | [119] |
| miR-429 | EV | ↑ | 4-miR:0.88 | Diagnosis | [119] |
| miR-10b | Tissue | ↑ | / | Prognosis | [53] |
| miR-210 | Tissue | ↑ | / | Prognosis | [121] |
| miR-378c | Tissue | ↑ | / | Prognosis | [122] |
| let-7f-5p | Tissue | ↑ | / | Prognosis | [122] |
| miR-1268b | Tissue | ↑ | / | Prognosis | [122] |
| miR-200c-3p | Tissue | ↑ | / | Prognosis | [122] |
| miR-1271-3p | Tissue | ↑ | / | Prognosis | [122] |
| miR-200c-3p | Tissue | ↑ | / | Prognosis | [123] |
| miR-214-3p | Tissue | ↑ | / | Prognosis | [123] |
| miR-23a-3p | Tissue | ↑ | / | Prognosis | [123] |
| miR-106b-5p | Tissue | ↑ | / | Prognosis | [128] |
| miR-93 | Serum | ↑ | / | Prognosis | [129] |
| miR-210 | Serum | ↑ | / | Prognosis | [129] |
| miR-19a | Serum | ↑ | / | Prognosis | [129] |
| miR-19b | Serum | ↑ | / | Prognosis | [129] |
| miR379/656 cluster | Tissue | ↓ | 0.9843 | Diagnosis/ Prognosis |
[120] |
| miR-423-3p | Serum | ↓ | 6-miR:0.881 | Diagnosis | [126] |
| miR-192-5p | Serum | ↓ | 6-miR:0.881 | Diagnosis | [126] |
| miR-17-5p | Serum | ↓ | 6-miR:0.881 | Diagnosis | [126] |
| miR-126 | Plasma | ↓ | 4-miR:0.802 | Diagnosis | [127] |
| miR-155 | Plasma | ↓ | 4-miR:0.802 | Diagnosis | [127] |
| miR-203 | Tissue | ↓ | / | Prognosis | [53] |
| miR-574 | Tissue | ↓ | / | Prognosis | [121] |
| miR-224 | Tissue | ↓ | / | Prognosis | [121] |
| miR-30b | Tissue | ↓ | / | Prognosis | [121] |
| miR-130a | Tissue | ↓ | / | Prognosis | [121] |
| miR-1200 | Tissue | ↓ | / | Prognosis | [122] |
| miR-1249-3p | Tissue | ↓ | / | Prognosis | [122] |
| miR-1255b-5p | Tissue | ↓ | / | Prognosis | [122] |
| miR-520d | Tissue | ↓ | / | Prognosis | [122] |
| miR-527 | Tissue | ↓ | / | Prognosis | [122] |
| miR-518a-5p | Tissue | ↓ | / | Prognosis | [122] |
| miR-2117 | Tissue | ↓ | / | Prognosis | [122] |
| miR-638 | Tissue | ↓ | / | Prognosis | [123] |
| miR-451a | Tissue | ↓ | / | Prognosis | [123] |
| miR-495-3p | Tissue | ↓ | / | Prognosis | [124] |
| miR-223 | Tissue | ↓ | / | Prognosis | [130] |
| miR-34a | Tissue | ↓ | / | Prognosis | [131] |
| miR-200c | Tissue | ↓ | / | Prognosis | [131] |
| miR-363-5p | Tissue/exosomes | ↓ | / | Prognosis | [132] |
EV: extracellular vesicles, 4-miR: AUC values of four miRNA combinations appearing in the same paper, 2-miR: AUC values of two miRNA combinations appearing in the same paper, 6-miR: AUC values of six miRNA combinations appearing in the same paper, ↑: Up-regulated expression, ↓: Down-regulation expression.
3.6. Prostate Cancer
Prostate cancer (PCa) is the most common and leading cause of death in men. At present, the common methods for clinical diagnosis of PCa include tissue biopsy, digital rectal examination, and serum prostate specific antigen (PSA). Tissue biopsy and digital rectal examination are highly invasive, while the low specificity of PSA has led to missed cases of the prostate-related diseases including PCa and benign prostatic hyperplasia (BPH), thereby limiting its clinical application in PCa [133,134]. These traditional assays do not meet the current needs, so a variety of new methods have been developed with special attention to sensitivity, specificity, and invasiveness. The PCa-related miRNAs identified in 2021 are summarized in Table 6.
Accurate diagnosis provides a basic guarantee for the treatment of PCa, and serum PSA is an important indicator for the determination of PCa. When the PSA concentration is 4–10 ng/mL, it is difficult to distinguish PCa from BPH, so other biomarkers are necessary to achieve the accurate diagnosis of PCa. MiRNAs are the most commonly used auxiliary markers, especially miRNAs in serum and urine, which are characterized by their invasiveness and less damage to patients. For example, Mello-Grand et al. analyzed 102 plasma samples with the PSA concentration of 4–16 ng/mL (53 PCa-negative and 49 PCa-positive). According to their results, the up-regulated miRNA-5100 helped to distinguish PCa from BPH [135]. In addition, Giglio et al. analyzed miRNAs in the plasma of 290 PCa patients, and the screened combination of miRNA-26b-5p and miRNA-98-5p performed well in distinguishing PCa from BPH patients (AUC of 0.944). Meanwhile, another combination of miRNA-26b-5p and miRNA-4732-3p also exhibited favorable effect (AUC of 0.80) on diagnosing advanced and early PCa [136]. Urine miRNAs can also be used to differentiate PCa from BPH. Compared with serum and tissues, urine samples can be obtained without any harm to patients. Markert et al. obtained six miRNAs (miRNA-15a-5p, miRNA-3126-3p, miRNA-324-5p, miRNA-150-5p, miRNA-425-3p, miRNA-6078) by analyzing the miRNAs in the urine of PCa and BPH patients. As a result, these miRNAs could be used to distinguish PCa from BPH, and their AUC values were all ≥0.70 [137]. Metastasis is another key factor affecting the survival of patients with PCa, and bone metastasis plays a dominant role. Wang et al. found that exosomes miRNA-181A-5p served as a biomarker for the early diagnosis of PCa bone metastasis in the serum of PCa patients [138]. At present, one of the treatments for metastatic PCa is androgen-deprivation therapy (ADT), but many patients develop resistance to ADT and progressed into ADT-resistant prostate cancer (CRPC). By comparing the differentially expressed miRNAs in tumor tissues between CRPC and primary PCa patients, Ronnau et al. obtained the combination of three miRNAs (miRNA-205, miRNA-3195 and miRNA-4417), which could be used as a potential biomarker for differentiating CRPC from primary PCa [139]. The abnormal expression of miRNAs in the tissues of PCa patients can be used to determine patient prognosis as well, and the prognosis is directly related to the clinical therapeutic effect and metastasis of PCa. Stoen et al. discovered that the high expression of miRNA-17-5p in tumor epithelium was associated with biochemical disorder (BF) and clinical failure (CF) in PCa patients, which was one of the factors contributing to the poor prognosis of PCa patients [140]. Additionally, Liu et al. indicated that the expression of miRNA-199b-3p in PCa was significantly lower than that in BPH by analyzing the expression of miRNAs in tumor tissues from 60 PCa and BPH patients. The low expression of miRNA-199b-3p was positively correlated with poor progression-free survival (PFS) in PCa patients [141]. Combinations of multiple miRNAs can be used to predict the prognosis of PCa patients. Bian et al. obtained the combination of 15 miRNAs by TCGA databases, which was adopted to predict the relapse-free survival (RFS) rate of PCa patients [142]. Similar to diagnosis, urinary miRNAs served as the prognostic markers for PCa. Kim et al. discovered that exosomal miRNA-532-5p was significantly up-regulated in urine of biochemical recurrence (BRC) patients compared with non-BRC patients, which was used as a marker to predict BRC in PCa patients after radical prostatectomy (RP) by KM analysis [143].
Table 6.
MicroRNAs as biomarkers for diagnosis and prognosis of PCa.
| miRNA | Sample | Expression | AUC | Biomarker | Reference |
|---|---|---|---|---|---|
| miR-3195 | Tissue | ↑ | 3-miR:0.994 | Diagnosis | [139] |
| miR-4417 | Tissue | ↑ | 3-miR:0.994 | Diagnosis | [139] |
| miR-301a | Serum/Tissue | ↑ | / | Diagnosis | [144] |
| miR-4732-3p | Plasma | ↑ | 2-miR:0.8 | Diagnosis | [136] |
| miR-5100 | Plasma | ↑ | / | Diagnosis | [135] |
| miR-1255-5p | Plasma | ↑ | 0.885 | Diagnosis | [145] |
| miR-181a-5p | exosomes | ↑ | 0.738 | Diagnosis | [138] |
| miR-451a | Urine exosomes | ↑ | 0.757 | Diagnosis | [146] |
| miR-486-3p | Urine exosomes | ↑ | 0.704 | Diagnosis | [146] |
| miR-486-5p | Urine exosomes | ↑ | 0.796 | Diagnosis | [146] |
| miR-6078 | Urine | ↑ | 0.7 | Diagnosis | [137] |
| miR-1913 | Urine | ↑ | 2-miR:0.821 | Diagnosis | [147] |
| miR-3659 | Urine | ↑ | 2-miR:0.821 | Diagnosis | [147] |
| miR-451a | SEVs | ↑ | 0.65 | Diagnosis | [148] |
| miR-141 | SEVs | ↑ | 0.64 | Diagnosis | [148] |
| miR-145 | SEVs | ↑ | 0.76 | Diagnosis | [148] |
| miR-221 | SEVs | ↑ | 0.7 | Diagnosis | [148] |
| miR-17-5p | Tissue | ↑ | / | Prognosis | [140] |
| miR-222-3p | Tissue | ↑ | / | Prognosis | [142] |
| miR-582-5p | Tissue | ↑ | / | Prognosis | [142] |
| miR-582-3p | Tissue | ↑ | / | Prognosis | [142] |
| miR-505-3p | Tissue | ↑ | / | Prognosis | [142] |
| miR-326 | Tissue | ↑ | / | Prognosis | [142] |
| miR-212-3p | Tissue | ↑ | / | Prognosis | [142] |
| miR-296-5p | Tissue | ↑ | / | Prognosis | [142] |
| miR-144-3p | Tissue | ↑ | / | Prognosis | [142] |
| miR-532-5p | Urine exosomes | ↑ | / | Prognosis | [143] |
| miR-425-5p | Cell exosomes | ↑ | / | Prognosis | [149] |
| miR-205 | Tissue | ↓ | 3-miR:0.994 | Diagnosis | [139] |
| miR-26b-5p | Plasma | ↓ | 2-miR:0.944 | Diagnosis | [136] |
| miR-98-5p | Plasma | ↓ | 2-miR:0.944 | Diagnosis | [136] |
| miR-26b-5p | Plasma | ↓ | 2-miR:0.8 | Diagnosis | [136] |
| miR-15a-5p | Urine | ↓ | 0.71 | Diagnosis | [137] |
| miR-3126-3p | Urine | ↓ | 0.76 | Diagnosis | [137] |
| miR-324-5p | Urine | ↓ | 0.74 | Diagnosis | [137] |
| miR-150-5p | Urine | ↓ | 0.76 | Diagnosis | [137] |
| miR-425-3p | Urine | ↓ | 0.71 | Diagnosis | [137] |
| miR-375 | Urine exosomes | ↓ | 0.788 | Diagnosis | [146] |
| miR-199b-3p | Tissue | ↓ | / | Prognosis | [141] |
| miR-21-5p | Tissue | ↓ | / | Prognosis | [142] |
| miR-192-5p | Tissue | ↓ | / | Prognosis | [142] |
| miR-15b-5p | Tissue | ↓ | / | Prognosis | [142] |
| miR-106b-5p | Tissue | ↓ | / | Prognosis | [142] |
| miR181a-5p | Tissue | ↓ | / | Prognosis | [142] |
| miR-18a-5p | Tissue | ↓ | / | Prognosis | [142] |
| miR-301a-3p | Tissue | ↓ | / | Prognosis | [142] |
SEVs: small extracellular vesicles, 2-miR: AUC values of two miRNA combinations appearing in the same paper, 3-miR: AUC values of three miRNA combinations appearing in the same paper, ↑: Up-regulated expression, ↓: Down-regulation expression.
4. Conclusions and Prospects
At present, there are numerous studies about miRNAs and cancer diagnosis and prognosis, but few results have been actually applied in the clinic. However, there are some miRNAs products used to detect tumor patient samples, such as miRNAs detection kits, test strips, and sensors. For instance, Zhou et al. developed a dual colorimetric miRNAs detection kit based on constant-temperature PCR technology, which rapidly and qualitatively detected miRNA-223 and miRNA-200b at room temperature, and was used to distinguish lung cancer patients from healthy people [150]. Yu et al. developed a novel surface-enhanced Raman scattering (SERS)-lateral flow assay (LFA) strip that simultaneously measured miRNA-21 and miRNA-196a-5p on a single test line in urine samples of NSCLC patients, which improved the detection efficiency and was adopted for the diagnosis of NSCLC [151]. Additionally, Zhuang et al. developed electrochemical biosensors for the detection of miRNA-100 in serum of GC patients [152], and Li et al. developed a sensor for the detection of miRNA-21 in serum exosomes of BC patients [153]. In China, some miRNAs detection kits have completed product registration and can thereby be used in clinical practice, including 7 miRNA detection kits (Jusbio, China), miR-92a detection kits (GeneBiohealth, China), and miRNA-25 detection kit (MicroMedMark, China), which can be utilized for the diagnosis and efficacy monitoring of liver cancer, colon cancer and pancreatic cancer, respectively.
Ideally, miRNAs may be more effective than the methods that have been applied in clinical diagnosis and prognosis, but there are still some problems in practical research. For instance, (1) small sample size: in the literature retrieved in this work, the maximum sample size is 682 serum samples from GC patients [84], whereas the smaller sample size may be only a few cases, and some are completely based on databases such as TCGA and GEO. (2) The sampling method was highly invasive to patients: many studies used tissue biopsy samples instead of blood, urine, and other samples that were less harmful to patients. (3) A single miRNA was used as a marker for the diagnosis and prognosis of some diseases: It was obvious that a combination of multiple miRNAs or a combination of miRNAs with clinically applied markers would be better than a single miRNA. (4) Biomarker detection at a single time point of the disease: in many studies, researchers only focused on miRNAs expression at a certain time point of the disease, but ignored the changes of miRNAs during the whole course of disease, resulting in the deviation of results due to individual differences of patients. (5) Tumor heterogeneity of miRNAs: the expression and functions of some miRNAs were different in different cancers, if the miRNA screened from one cancer directly applied to the clinic, it may interfere with the detection results of other diseases, and their clinical application will be limited. Therefore, miRNAs as biomarkers for disease diagnosis and prognosis can be appropriately improved in subsequent studies. For example, the relevant medical staff should be trained regularly, so that they can understand the detailed information of a miRNA as a marker of disease diagnosis and prognosis as comprehensively as possible, so that the accuracy of detection can be greatly improved. First, as many patient samples as possible should be collected. Second, less invasive sample types such as blood and urine should be used. Third, many combinations of miRNAs, miRNAs, and other ncRNAs, and miRNAs and biochemical markers should be used to improve disease diagnosis and prognosis. Fourth, changes in miRNAs expression during the entire course of disease should be explored as far as possible to eliminate the influence of individual differences on screening results. Fifth, the heterogeneity of miRNAs in diseases should be fully understood and verified as far as possible to reduce the impact on the detection results of other diseases.
Certainly, miRNAs also have as many advantages as tumor biomarkers. For example, miRNAs are stable and can be easily detected in different tissues, blood, urine, stool, saliva, and ascites. There are various sample types, especially blood and urine samples, which are less invasive to patients and easy to be accepted by patients. Furthermore, their accuracy as diagnostic or prognostic markers is higher than AFP, CEA, PSA, and other biochemical markers that have been applied in clinical practice. Therefore, in the near future, miRNA will be studied more widely. With the further improvement of biomarker research system, as well as the development of bioinformatics and diagnostic technology, more miRNAs will be gradually applied in the clinical diagnosis and prognosis of cancer.
Abbreviations
| miRNAs(miR) | MicroRNAs |
| ncRNAs | non-coding RNAs |
| IARC | International Agency for Research on Cancer |
| NCC | National Cancer Center of China |
| CT | Computed Tomography |
| MRI | magnetic resonance |
| PET | Positron emission computed tomography |
| CEA | carcinoembryonic antigen |
| AFP | alpha-fetoprotein |
| PSA | Prostate cancer specific antigen |
| CTC | circulating tumor cells |
| NSCLC | non-small cell lung cancer |
| RISC | RNA induced silencing complex |
| pri-miRNA | primary miRNA |
| pre-miRNA | precursor miRNA |
| UTR | untranslated region |
| PTGES3 | prostaglandin E synthase 3 |
| FAP | ibroblast activation protein |
| PD1 | programmed cell death 1 |
| PDL1 | programmed death ligand 1 |
| LUAD | lung adenocarcinoma |
| LUSC | lung squamous cell carcinoma |
| CSF | cerebrospinal fluid |
| ROC | receiver operating characteristic |
| AUC | area under the curve |
| PTEN | phosphatase and tensin homolog |
| HCC | hepatocellular carcinoma |
| DFS | disease-free survival |
| GC | Gastric cancer |
| ImmiRSig | immune-related nine-miRNA signature |
| GIMiSig | GI-related miRNA signature |
| CRC | Colorectal cancer |
| IAMIPS | immune-associated miRNA prognostic signature |
| PTM | peritoneal metastasis |
| LM | Leptomeningeal metastasis |
| BC | breast cancer |
| TCGA | The Cancer Genome Atlas |
| GEO | Gene Expression Omnibus |
| PCa | Prostatic cancer |
| BPH | benign prostatic hyperplasia |
| PSA | prostate specific antigen |
| ADT | androgen-deprivation therapy |
| CRPC | castration-resistant prostate cancer |
| BF | biochemical disorder |
| CF | clinical failure |
| PFS | progression-free survival |
| RFS | relapse free survival |
| BRC | biochemical relapse |
| RP | radical prostatectomy |
| SERS-LFA | surface-enhanced Raman scattering-lateral flow assay |
| TEP | tumor-educated platelet |
| SEVs | small extracellular vesicles |
| EV | extracellular vesicles |
| EBC | exhaled breath condensate |
| ACP | acid phosphatase |
| ER | estrogen receptor |
| PR | progesterone receptor |
| DRE | digital rectal examination |
| FOBT | fecal occult blood test |
Author Contributions
C.S. and Y.Z. searched and sorted literatures. Q.W. and J.G. refined the drafted manuscript. C.Z. and G.Y. conceived and coordinated the project. C.S. and B.Y. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors have announced that there is no conflict of interest in our research.
Funding Statement
Our research has been funded by National Key Research and Development Program (2019YFE19500), Special Program for COVID-19 Prevention and Control of High-end Foreign Experts Introduction Program (GX20200226002), the National Fostering Science Fund Project of Henan Normal University (20A180014), Doctoral Research Foundation of Henan Normal University (5101049170192), Scientific and technological breakthroughs Project of Henan (222102310291, 222102310589) as well as Higher Education Research Key Project of Henan (22A180018, 21A180009).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Xia C., Dong X., Li H., Cao M., Sun D., He S., Yang F., Yan X., Zhang S., Li N., et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022;135:584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bi W.L., Hosny A., Schabath M.B., Giger M.L., Birkbak N.J., Mehrtash A., Allison T., Arnaout O., Abbosh C., Dunn I.F., et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019;69:127–157. doi: 10.3322/caac.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jafari S.H., Saadatpour Z., Salmaninejad A., Momeni F., Mokhtari M., Nahand J.S., Rahmati M., Mirzaei H., Kianmehr M. Breast cancer diagnosis: Imaging techniques and biochemical markers. J. Cell. Physiol. 2018;233:5200–5213. doi: 10.1002/jcp.26379. [DOI] [PubMed] [Google Scholar]
- 5.Wong V.K., Ganeshan D., Jensen C.T., Devine C.E. Imaging and Management of Bladder Cancer. Cancers. 2021;13:1396. doi: 10.3390/cancers13061396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C., Zhang D., Pang X., Pu H., Lei M., Fan B., Lv J., You D., Li Z., Zhang T. Trajectories of Perioperative Serum Tumor Markers and Colorectal Cancer Outcomes: A Retrospective, Multicenter Longitudinal Cohort Study. EBioMedicine. 2021;74:103706. doi: 10.1016/j.ebiom.2021.103706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarwar S., Adil M.A., Nyamath P., Ishaq M. Biomarkers of Prostatic Cancer: An Attempt to Categorize Patients into Prostatic Carcinoma, Benign Prostatic Hyperplasia, or Prostatitis Based on Serum Prostate Specific Antigen, Prostatic Acid Phosphatase, Calcium, and Phosphorus. Prost. Cancer. 2017;2017:5687212. doi: 10.1155/2017/5687212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W., Xu X., Tian B., Wang Y., Du L., Sun T., Shi Y., Zhao X., Jing J. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin. Chim. Acta Int. J. Clin. Chem. 2017;470:51–55. doi: 10.1016/j.cca.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Xia Y., Hu X., Di K., Liu C., Tan T., Lin Y., Xu H., Xie H., Wang S., Yang Z., et al. Combined Detection of Exosome Concentration and Tumor Markers in Gastric Cancer. J. Biomed. Nanotechnol. 2020;16:252–258. doi: 10.1166/jbn.2020.2887. [DOI] [PubMed] [Google Scholar]
- 10.Broggi G., Salvatorelli L. Bio-Pathological Markers in the Diagnosis and Therapy of Cancer. Cancers. 2020;12:3113. doi: 10.3390/cancers12113113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Q.Z., Qi C.B., Xu M.L., Yue J.Q., Guo F. Easily misdiagnosed pulmonary benign metastasizing leiomyoma with rich-mucus degeneration in intraoperative pathological section: Report of a case. Zhonghua Bing Li Xue Za Zhi Chin. J. Pathol. 2021;50:1177–1179. doi: 10.3760/cma.j.cn112151-20210223-00160. [DOI] [PubMed] [Google Scholar]
- 12.Ning D., Cui K., Liu M., Ou Y., Wang Z., Zou B., Shen Y., Lu X., Li S., Li P. Comparison of CellSearch and Circulating Tumor Cells (CTC)-Biopsy Systems in Detecting Peripheral Blood Circulating Tumor Cells in Patients with Gastric Cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021;27:e926565. doi: 10.12659/MSM.926565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolan J., Nedosekin D.A., Galanzha E.I., Zharov V.P. Detection of Apoptotic Circulating Tumor Cells Using in vivo Fluorescence Flow Cytometry. Cytom. Part A J. Int. Soc. Anal. Cytol. 2019;95:664–671. doi: 10.1002/cyto.a.23642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bizzarro T., Bernardi L., Buda R., Rossi G. Cytological diagnosis of a rare synchronous non-small cell lung cancer metastatic to the thyroid gland. Cytopathol. Off. J. Br. Soc. Clin. Cytol. 2020;31:329–332. doi: 10.1111/cyt.12832. [DOI] [PubMed] [Google Scholar]
- 15.Ma H., Wang P., Shang D., Liu Y. Spatial-domain low-coherence quantitative phase microscopy to improve the cytological diagnosis of pancreatic cancer. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2020;68:60–67. doi: 10.1136/jim-2019-000997. [DOI] [PubMed] [Google Scholar]
- 16.Halpern J.A., Shoag J.E., Mittal S., Oromendia C., Ballman K.V., Hershman D.L., Wright J.D., Shih Y.T., Nguyen P.L., Hu J.C. Prognostic Significance of Digital Rectal Examination and Prostate Specific Antigen in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Arm. J. Urol. 2017;197:363–368. doi: 10.1016/j.juro.2016.08.092. [DOI] [PubMed] [Google Scholar]
- 17.Ladabaum U., Dominitz J.A., Kahi C., Schoen R.E. Strategies for Colorectal Cancer Screening. Gastroenterology. 2020;158:418–432. doi: 10.1053/j.gastro.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 18.He J., Wu F., Han Z., Hu M., Lin W., Li Y., Cao M. Biomarkers (mRNAs and Non-Coding RNAs) for the Diagnosis and Prognosis of Colorectal Cancer—From the Body Fluid to Tissue Level. Front. Oncol. 2021;11:632834. doi: 10.3389/fonc.2021.632834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mousavi S.M., Amin Mahdian S.M., Ebrahimi M.S., Taghizadieh M., Vosough M., Sadri Nahand J., Hosseindoost S., Vousooghi N., Javar H.A., Larijani B., et al. Microfluidics for detection of exosomes and microRNAs in cancer: State of the art. Mol. Ther. Nucleic Acids. 2022;28:758–791. doi: 10.1016/j.omtn.2022.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raza A., Khan A.Q., Inchakalody V.P., Mestiri S., Yoosuf Z., Bedhiafi T., El-Ella D.M.A., Taib N., Hydrose S., Akbar S., et al. Dynamic liquid biopsy components as predictive and prognostic biomarkers in colorectal cancer. J. Exp. Clin. Cancer Res. 2022;41:99. doi: 10.1186/s13046-022-02318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong C., Xie Z., Zeng L.H., Yuan C., Duan S. MIR4435-2HG Is a Potential Pan-Cancer Biomarker for Diagnosis and Prognosis. Front. Immunol. 2022;13:855078. doi: 10.3389/fimmu.2022.855078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 23.Jet T., Gines G., Rondelez Y., Taly V. Advances in multiplexed techniques for the detection and quantification of microRNAs. Chem. Soc. Rev. 2021;50:4141–4161. doi: 10.1039/D0CS00609B. [DOI] [PubMed] [Google Scholar]
- 24.Tang L. Recapitulating miRNA biogenesis in cells. Nat. Methods. 2022;19:35. doi: 10.1038/s41592-021-01385-z. [DOI] [PubMed] [Google Scholar]
- 25.Ho P.T.B., Clark I.M., Le L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022;23:7167. doi: 10.3390/ijms23137167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xuan J., Liu Y., Zeng X., Wang H. Sequence Requirements for miR-424-5p Regulating and Function in Cancers. Int. J. Mol. Sci. 2022;23:4037. doi: 10.3390/ijms23074037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bazzini A.A., Lee M.T., Giraldez A.J. Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science. 2012;336:233–237. doi: 10.1126/science.1215704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huntzinger E., Izaurralde E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 29.Park J.H., Shin C. MicroRNA-directed cleavage of targets: Mechanism and experimental approaches. BMB Rep. 2014;47:417–423. doi: 10.5483/BMBRep.2014.47.8.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subtelny A.O., Eichhorn S.W., Chen G.R., Sive H., Bartel D.P. Poly(A)-tail profiling reveals an embryonic switch in translational control. Nature. 2014;508:66–71. doi: 10.1038/nature13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzmaurice C., Allen C., Barber R.M., Barregard L., Bhutta Z.A., Brenner H., Dicker D.J., Chimed-Orchir O., Dandona R., Dandona L., et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding H., Wang Y., Cui Y., Chen Z., Li Y., Yang J., Yang Y., Chen T., Xia D., Li C., et al. Comprehensive analysis of the expression and prognosis for RBR E3 ubiquitin ligases in lung adenocarcinoma. Thorac. Cancer. 2022;13:2459–2472. doi: 10.1111/1759-7714.14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao P., Zou K., Xiao L., Zhou H., Xu X., Zeng Z., Zhang W. High expression of PTGES3 is an independent predictive poor prognostic biomarker and correlates with immune infiltrates in lung adenocarcinoma. Int. Immunopharmacol. 2022;110:108954. doi: 10.1016/j.intimp.2022.108954. [DOI] [PubMed] [Google Scholar]
- 34.Yanagawa N., Sugai M., Shikanai S., Sugimoto R., Osakabe M., Uesugi N., Saito H., Maemondo M., Sugai T. High expression of fibroblast-activating protein is a prognostic marker in non-small cell lung carcinoma. Thorac. Cancer. 2022;13:2377–2384. doi: 10.1111/1759-7714.14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y., He H. Prognostic value of soluble programmed cell death ligand-1 in patients with non-small-cell lung cancer: A meta-analysis. Immunotherapy. 2022;14:945–956. doi: 10.2217/imt-2021-0238. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi O., Kaira K., Naruse I., Umeda Y., Honda T., Watanabe S., Ichikawa K., Tateishi K., Kasahara N., Higuchi T., et al. Prospective assessment using (18)F-FDG PET/CT as a novel predictor for early response to PD-1 blockade in non-small-cell lung cancer. Sci. Rep. 2022;12:11832. doi: 10.1038/s41598-022-15964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 38.Migdalska-Sęk M., Modrzewska B., Kordiak J., Pastuszak-Lewandoska D., Kiszałkiewicz J.M., Bielec F., Antczak A., Brzeziańska-Lasota E. Diagnostic value of PPARδ and miRNA-17 expression levels in patients with non-small cell lung cancer. Sci. Rep. 2021;11:24136. doi: 10.1038/s41598-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Q., Ye L., Huang L., Zhou L., Chen X., Ye M., Wu G., Zhan P., Lv T., Song Y. Serum Exosomal miRNA Might Be a Novel Liquid Biopsy to Identify Leptomeningeal Metastasis in Non-Small Cell Lung Cancer. OncoTargets Ther. 2021;14:2327–2335. doi: 10.2147/OTT.S291611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nie R., Niu W., Tang T., Zhang J., Zhang X. Integrating microRNA expression, miRNA-mRNA regulation network and signal pathway: A novel strategy for lung cancer biomarker discovery. PeerJ. 2021;9:e12369. doi: 10.7717/peerj.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong X., Chang M., Song X., Ding S., Xie L., Song X. Plasma miR-1247-5p, miR-301b-3p and miR-105-5p as potential biomarkers for early diagnosis of non-small cell lung cancer. Thorac. Cancer. 2021;12:539–548. doi: 10.1111/1759-7714.13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klotz L.V., Courty Y., Lindner M., Petit-Courty A., Stowasser A., Koch I., Eichhorn M.E., Lilis I., Morresi-Hauf A., Arendt K.A.M., et al. Comprehensive clinical profiling of the Gauting locoregional lung adenocarcinoma donors. Cancer Med. 2019;8:1486–1499. doi: 10.1002/cam4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang X., Yuan Y., Tang L., Wang J., Zhang D., Duan L. Systematic Analysis and Validation of the Prognosis, Immunological Role and Biology Function of the Ferroptosis-Related lncRNA GSEC/miRNA-101-3p/CISD1 Axis in Lung Adenocarcinoma. Front. Mol. Biosci. 2021;8:793732. doi: 10.3389/fmolb.2021.793732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang L., Yuan Y., Zhai H., Wang J., Zhang D., Liang H., Shi Y., Duan L., Jiang X. MicroRNA-125b-5p Correlates with Prognosis and Lung Adenocarcinoma Progression. Front. Mol. Biosci. 2021;8:788690. doi: 10.3389/fmolb.2021.788690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan Y., Jiang X., Tang L., Wang J., Zhang D., Cho W.C., Duan L. FOXM1/lncRNA TYMSOS/miR-214-3p-Mediated High Expression of NCAPG Correlates with Poor Prognosis and Cell Proliferation in Non-Small Cell Lung Carcinoma. Front. Mol. Biosci. 2021;8:785767. doi: 10.3389/fmolb.2021.785767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J., Hao R., Zhang Y., Deng H., Teng W., Wang Z. Construction of circRNA-miRNA-mRNA network and identification of novel potential biomarkers for non-small cell lung cancer. Cancer Cell Int. 2021;21:611. doi: 10.1186/s12935-021-02278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khandelwal A., Sharma U., Barwal T.S., Seam R.K., Gupta M., Rana M.K., Vasquez K.M., Jain A. Circulating miR-320a Acts as a Tumor Suppressor and Prognostic Factor in Non-small Cell Lung Cancer. Front. Oncol. 2021;11:645475. doi: 10.3389/fonc.2021.645475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boldrini L., Giordano M., Melfi F., Lucchi M., Fontanini G. Expression of miRNA-25 in young and old lung adenocarcinoma. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2021;26:132. doi: 10.4103/jrms.JRMS_830_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duan X., Qiao S., Li D., Li S., Zheng Z., Wang Q., Zhu X. Circulating miRNAs in Serum as Biomarkers for Early Diagnosis of Non-small Cell Lung Cancer. Front. Genet. 2021;12:673926. doi: 10.3389/fgene.2021.673926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Sánchez C., Barbarroja N., Pantaleão L.C., López-Sánchez L.M., Ozanne S.E., Jurado-Gámez B., Aranda E., Lopez-Pedrera C., Rodríguez-Ariza A. Clinical Utility of microRNAs in Exhaled Breath Condensate as Biomarkers for Lung Cancer. J. Pers. Med. 2021;11:111. doi: 10.3390/jpm11020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong Y., Ding X., Bian Y., Wang J., Zhou W., Wang X., Li P., Shen Y., Wang J.J., Li J., et al. Discovery and validation of extracellular vesicle-associated miRNAs as noninvasive detection biomarkers for early-stage non-small-cell lung cancer. Mol. Oncol. 2021;15:2439–2452. doi: 10.1002/1878-0261.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng Q., Ding H., Wang L., Yan Y., Wan Y., Yi Y., Tao L., Zhu C. Circulating Exosomal miR-96 as a Novel Biomarker for Radioresistant Non-Small-Cell Lung Cancer. J. Oncol. 2021;2021:5893981. doi: 10.1155/2021/5893981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu S.B., Fan R.H., Qin X., Han R.M. microRNA Prognostic Signature for Postoperative Success of Metastatic Orthopedic Cancers: Implications for Precision Microsurgery. Front. Cell Dev. Biol. 2021;9:704505. doi: 10.3389/fcell.2021.704505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao J., Wang X., Mi Z., Jiang X., Sun L., Zheng B., Wang J., Meng M., Zhang L., Wang Z., et al. STAT3/miR-135b/NF-κB axis confers aggressiveness and unfavorable prognosis in non-small-cell lung cancer. Cell Death Dis. 2021;12:493. doi: 10.1038/s41419-021-03773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye Q., Putila J., Raese R., Dong C., Qian Y., Dowlati A., Guo N.L. Identification of Prognostic and Chemopredictive microRNAs for Non-Small-Cell Lung Cancer by Integrating SEER-Medicare Data. Int. J. Mol. Sci. 2021;22:7658. doi: 10.3390/ijms22147658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S., Hong J.H., Kim J.S., Yoon J.S., Chun S.H., Hong S.A., Kim E.J., Kang K., Lee Kang J., Ko Y.H., et al. Cancer-associated fibroblasts activated by miR-196a promote the migration and invasion of lung cancer cells. Cancer Lett. 2021;508:92–103. doi: 10.1016/j.canlet.2021.03.021. [DOI] [PubMed] [Google Scholar]
- 57.Monastirioti A., Papadaki C., Rounis K., Kalapanida D., Mavroudis D., Agelaki S. A Prognostic Role for Circulating microRNAs Involved in Macrophage Polarization in Advanced Non-Small Cell Lung Cancer. Cells. 2021;10:1988. doi: 10.3390/cells10081988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kryczka J., Migdalska-Sęk M., Kordiak J., Kiszałkiewicz J.M., Pastuszak-Lewandoska D., Antczak A., Brzeziańska-Lasota E. Serum Extracellular Vesicle-Derived miRNAs in Patients with Non-Small Cell Lung Cancer-Search for Non-Invasive Diagnostic Biomarkers. Diagnostics. 2021;11:425. doi: 10.3390/diagnostics11030425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 60.Yang J., Pan G., Guan L., Liu Z., Wu Y., Liu Z., Lu W., Li S., Xu H., Ouyang G. The burden of primary liver cancer caused by specific etiologies from 1990 to 2019 at the global, regional, and national levels. Cancer Med. 2022;11:1357–1370. doi: 10.1002/cam4.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oura K., Morishita A., Masaki T. Molecular and Functional Roles of MicroRNAs in the Progression of Hepatocellular Carcinoma-A Review. Int. J. Mol. Sci. 2020;21:8362. doi: 10.3390/ijms21218362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qin S., Xu J., Yi Y., Jiang S., Jin P., Xia X., Ma F. Transcription Factors and Methylation Drive Prognostic miRNA Dysregulation in Hepatocellular Carcinoma. Front. Oncol. 2021;11:691115. doi: 10.3389/fonc.2021.691115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang C., Tang S., Shen D., Li X., Liang L., Ding Y., Xu B. Circulating plasma exosomal miRNA profiles serve as potential metastasis-related biomarkers for hepatocellular carcinoma. Oncol. Lett. 2021;21:168. doi: 10.3892/ol.2021.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu B., Zhou S., Liang H., Ye Q., Wang Y. Development and Validation of a Novel Circulating miRNA-Based Diagnostic Score for Early Detection of Hepatocellular Carcinoma. Dig. Dis. Sci. 2022;67:2283–2292. doi: 10.1007/s10620-021-07031-0. [DOI] [PubMed] [Google Scholar]
- 65.Fouda M.S., Omran M.M., Tarek G., Hady A.A.W.A. Development of a novel panel based on micro-RNAs (21, 29a, 200 and 335) and alpha-fetoprotein as diagnostic biomarkers for hepatocellular carcinoma associated with hepatitis C infection. Arab J. Gastroenterol. Off. Publ. Pan-Arab Assoc. Gastroenterol. 2021;22:28–33. doi: 10.1016/j.ajg.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X., Ma L., Zhai L., Chen D., Li Y., Shang Z., Zhang Z., Gao Y., Yang W., Li Y., et al. Construction and validation of a three-microRNA signature as prognostic biomarker in patients with hepatocellular carcinoma. Int. J. Med. Sci. 2021;18:984–999. doi: 10.7150/ijms.49126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang X.C., Pang F.X., Ou S.S., Wei X.J., Xu Y.J., Lai Y.H. Risk Score Based on Two microRNAs as a Prognostic Marker of Hepatocellular Carcinoma and the Corresponding Competitive Endogenous RNA Network. Int. J. Gen. Med. 2021;14:3377–3385. doi: 10.2147/IJGM.S318516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yokota Y., Noda T., Okumura Y., Kobayashi S., Iwagami Y., Yamada D., Tomimaru Y., Akita H., Gotoh K., Takeda Y., et al. Serum exosomal miR-638 is a prognostic marker of HCC via downregulation of VE-cadherin and ZO-1 of endothelial cells. Cancer Sci. 2021;112:1275–1288. doi: 10.1111/cas.14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie Y., Wang Y., Xue W., Zou H., Li K., Liu K., Zhao W., Zhu C., Cao J. Profiling and Integrated Analysis of Differentially Expressed MicroRNAs as Novel Biomarkers of Hepatocellular Carcinoma. Front. Oncol. 2021;11:770918. doi: 10.3389/fonc.2021.770918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu B., Gu S., Wu X., He W., Zhou H. Bioinformatics analysis of tumor-educated platelet microRNAs in patients with hepatocellular carcinoma. Biosci. Rep. 2021;41:BSR20211420. doi: 10.1042/BSR20211420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu B., Ma X., Fu P., Sun Q., Tang W., Sun H., Yang Z., Yu M., Zhou J., Fan J., et al. miRNA-mRNA Regulatory Network and Factors Associated with Prediction of Prognosis in Hepatocellular Carcinoma. Genom. Proteom. Bioinform. 2021;19:913. doi: 10.1016/j.gpb.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang D.D., Wang W.E., Ma Y.S., Shi Y., Yin J., Liu J.B., Yang X.L., Xin R., Fu D., Zhang W.J. A miR-212-3p/SLC6A1 Regulatory Sub-Network for the Prognosis of Hepatocellular Carcinoma. Cancer Manag. Res. 2021;13:5063–5075. doi: 10.2147/CMAR.S308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.El-Mezayen H., Yamamura K., Yusa T., Nakao Y., Uemura N., Kitamura F., Itoyama R., Yamao T., Higashi T., Hayashi H., et al. MicroRNA-25 Exerts an Oncogenic Function by Regulating the Ubiquitin Ligase Fbxw7 in Hepatocellular Carcinoma. Ann. Surg. Oncol. 2021;28:7973–7982. doi: 10.1245/s10434-021-09778-2. [DOI] [PubMed] [Google Scholar]
- 74.Liang H., Xu M., Xiong Z., Hu K., Yang J., Cao M., Zhong Z., Yao Z., Deng M., Liu B. Identification of miRNAs as diagnostic and prognostic markers in hepatocellular carcinoma. Aging. 2021;13:6115–6133. doi: 10.18632/aging.202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim H.S., Kim J.S., Park N.R., Nam H., Sung P.S., Bae S.H., Choi J.Y., Yoon S.K., Hur W., Jang J.W. Exosomal miR-125b Exerts Anti-Metastatic Properties and Predicts Early Metastasis of Hepatocellular Carcinoma. Front. Oncol. 2021;11:637247. doi: 10.3389/fonc.2021.637247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao J., Li H., Zhao S., Wang E., Zhu J., Feng D., Zhu Y., Dou W., Fan Q., Hu J., et al. Epigenetic silencing of miR-144/451a cluster contributes to HCC progression via paracrine HGF/MIF-mediated TAM remodeling. Mol. Cancer. 2021;20:46. doi: 10.1186/s12943-021-01343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kong C.Q., Chen X.C., Qiu G.H., Liang J.C., Wang D., Liu X.Y., Liu J.J., Han Y.Q., Fan X.H. Effects of miRNA-140 on the Growth and Clinical Prognosis of SMMC-7721 Hepatocellular Carcinoma Cell Line. BioMed Res. Int. 2021;2021:6638915. doi: 10.1155/2021/6638915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yugawa K., Yoshizumi T., Mano Y., Itoh S., Harada N., Ikegami T., Kohashi K., Oda Y., Mori M. Cancer-associated fibroblasts promote hepatocellular carcinoma progression through downregulation of exosomal miR-150-3p. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2021;47:384–393. doi: 10.1016/j.ejso.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 79.Chen J., Liu Z., Gao G., Mo Y., Zhou H., Huang W., Wu L., He X., Ding J., Luo C., et al. Efficacy of circulating microRNA-130b and blood routine parameters in the early diagnosis of gastric cancer. Oncol. Lett. 2021;22:725. doi: 10.3892/ol.2021.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsuoka T., Yashiro M. Biomarkers of gastric cancer: Current topics and future perspective. World J. Gastroenterol. 2018;24:2818–2832. doi: 10.3748/wjg.v24.i26.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Necula L., Matei L., Dragu D., Neagu A.I., Mambet C., Nedeianu S., Bleotu C., Diaconu C.C., Chivu-Economescu M. Recent advances in gastric cancer early diagnosis. World J. Gastroenterol. 2019;25:2029–2044. doi: 10.3748/wjg.v25.i17.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kahroba H., Samadi N., Mostafazadeh M., Hejazi M.S., Sadeghi M.R., Hashemzadeh S., Eftekhar Sadat A.T., Karimi A. Evaluating the presence of deregulated tumoral onco-microRNAs in serum-derived exosomes of gastric cancer patients as noninvasive diagnostic biomarkers. BioImpacts. 2022;12:127–138. doi: 10.34172/bi.2021.22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu Z., Rong Z., Sheng J., Luo Z., Zhang J., Li T., Zhu Z., Fu Z., Qiu Z., Huang C. Aberrant Non-Coding RNA Expressed in Gastric Cancer and Its Diagnostic Value. Front. Oncol. 2021;11:606764. doi: 10.3389/fonc.2021.606764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.So J.B.Y., Kapoor R., Zhu F., Koh C., Zhou L., Zou R., Tang Y.C., Goo P.C.K., Rha S.Y., Chung H.C., et al. Development and validation of a serum microRNA biomarker panel for detecting gastric cancer in a high-risk population. Gut. 2021;70:829–837. doi: 10.1136/gutjnl-2020-322065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu J., Wen J., Li S., Shen X., You T., Huang Y., Xu C., Zhao Y. Immune-Related Nine-MicroRNA Signature for Predicting the Prognosis of Gastric Cancer. Front. Genet. 2021;12:690598. doi: 10.3389/fgene.2021.690598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y., Cheng L., Huang W., Cheng X., Peng W., Shi D. Genome Instability-Related miRNAs Predict Survival, Immune Landscape, and Immunotherapy Responses in Gastric Cancer. J. Immunol. Res. 2021;2021:2048833. doi: 10.1155/2021/2048833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qi W., Zhang Q. Development and clinical validation of a 3-miRNA signature to predict prognosis of gastric cancer. PeerJ. 2021;9:e10462. doi: 10.7717/peerj.10462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu X., Pu K., Wang Y., Chen Y., Zhou Y. Gastric cancer-associated microRNA expression signatures: Integrated bioinformatics analysis, validation, and clinical significance. Ann. Transl. Med. 2021;9:797. doi: 10.21037/atm-21-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu L., Cai Y., Chen X., Zhu Y., Cai J. Circulating MiR-1290 as a potential diagnostic and disease monitoring biomarker of human gastrointestinal tumors. BMC Cancer. 2021;21:989. doi: 10.1186/s12885-021-08729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yao Y., Ding Y., Bai Y., Zhou Q., Lee H., Li X., Teng L. Identification of Serum Circulating MicroRNAs as Novel Diagnostic Biomarkers of Gastric Cancer. Front. Genet. 2020;11:591515. doi: 10.3389/fgene.2020.591515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang Y., Zhao Y., Li L., Wei H., Huang T., Zhang H., Chen X., Yun H., Sun W., Wang Y. MicroRNA profiles in five pairs of early gastric cancer tissues and adjacent non-cancerous tissues. Oncol. Lett. 2021;22:595. doi: 10.3892/ol.2021.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao D., Xie J., Wu S. MicroRNA-182 is a potential biomarker for prognosis of gastric cancer: A protocol for meta-analysis and bioinformatics analysis. Medicine. 2021;100:e25830. doi: 10.1097/MD.0000000000025830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gilani N., Arabi Belaghi R., Aftabi Y., Faramarzi E., Edgünlü T., Somi M.H. Identifying Potential miRNA Biomarkers for Gastric Cancer Diagnosis Using Machine Learning Variable Selection Approach. Front. Genet. 2021;12:779455. doi: 10.3389/fgene.2021.779455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zheng G.D., Xu Z.Y., Hu C., Lv H., Xie H.X., Huang T., Zhang Y.Q., Chen G.P., Fu Y.F., Cheng X.D. Exosomal miR-590-5p in Serum as a Biomarker for the Diagnosis and Prognosis of Gastric Cancer. Front. Mol. Biosci. 2021;8:636566. doi: 10.3389/fmolb.2021.636566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mei D., Qi Y., Xia Y., Ma J., Hu H., Ai J., Chen L., Wu N., Liao D. Microarray profile analysis identifies ETS1 as potential biomarker regulated by miR-23b and modulates TCF4 in gastric cancer. World J. Surg. Oncol. 2021;19:311. doi: 10.1186/s12957-021-02417-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang J., Zhang M., Hu X., She J., Sun R., Qin S., Li D. miRNA-194 predicts favorable prognosis in gastric cancer and inhibits gastric cancer cell growth by targeting CCND1. FEBS Open Bio. 2021;11:1814–1826. doi: 10.1002/2211-5463.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang H., Liu Y., Hu K., Xia Y., Liang L., Zhu X., Cheng X. MiRNA-339 targets and regulates ZNF689 to inhibit the proliferation and invasion of gastric cancer cells. Transl. Cancer Res. 2021;10:3516–3526. doi: 10.21037/tcr-21-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Komatsu S., Imamura T., Kiuchi J., Takashima Y., Kamiya H., Ohashi T., Konishi H., Shiozaki A., Kubota T., Okamoto K., et al. Depletion of tumor suppressor miRNA-148a in plasma relates to tumor progression and poor outcomes in gastric cancer. Am. J. Cancer Res. 2021;11:6133–6146. [PMC free article] [PubMed] [Google Scholar]
- 99.Feng J., Wei Q., Yang M., Wang X., Liu B., Li J. Development and validation of a novel miRNA classifier as a prognostic signature for stage II/III colorectal cancer. Ann. Transl. Med. 2021;9:747. doi: 10.21037/atm-20-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han L., Shi W.J., Xie Y.B., Zhang Z.G. Diagnostic value of four serum exosome microRNAs panel for the detection of colorectal cancer. World J. Gastrointest. Oncol. 2021;13:970–979. doi: 10.4251/wjgo.v13.i8.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Silva C.M.S., Barros-Filho M.C., Wong D.V.T., Mello J.B.H., Nobre L.M.S., Wanderley C.W.S., Lucetti L.T., Muniz H.A., Paiva I.K.D., Kuasne H., et al. Circulating let-7e-5p, miR-106a-5p, miR-28-3p, and miR-542-5p as a Promising microRNA Signature for the Detection of Colorectal Cancer. Cancers. 2021;13:1493. doi: 10.3390/cancers13071493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Y., Liu H., Ning S., Wei C., Li J., Wei W., Zhang L. The High Ratio of the Plasma miR-96/miR-99b Correlated with Poor Prognosis in Patients with Metastatic Colorectal Cancer. Front. Mol. Biosci. 2021;8:799060. doi: 10.3389/fmolb.2021.799060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nassar F.J., Msheik Z.S., Itani M.M., Helou R.E., Hadla R., Kreidieh F., Bejjany R., Mukherji D., Shamseddine A., Nasr R.R., et al. Circulating miRNA as Biomarkers for Colorectal Cancer Diagnosis and Liver Metastasis. Diagnostics. 2021;11:341. doi: 10.3390/diagnostics11020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dokhanchi M., Pakravan K., Zareian S., Hussen B.M., Farid M., Razmara E., Mossahebi-Mohammadi M., Cho W.C., Babashah S. Colorectal cancer cell-derived extracellular vesicles transfer miR-221-3p to promote endothelial cell angiogenesis via targeting suppressor of cytokine signaling 3. Life Sci. 2021;285:119937. doi: 10.1016/j.lfs.2021.119937. [DOI] [PubMed] [Google Scholar]
- 105.Liu Z., Lu T., Wang Y., Jiao D., Li Z., Wang L., Liu L., Guo C., Zhao Y., Han X. Establishment and experimental validation of an immune miRNA signature for assessing prognosis and immune landscape of patients with colorectal cancer. J. Cell. Mol. Med. 2021;25:6874–6886. doi: 10.1111/jcmm.16696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lan S.H., Lin S.C., Wang W.C., Yang Y.C., Lee J.C., Lin P.W., Chu M.L., Lan K.Y., Zuchini R., Liu H.S., et al. Autophagy Upregulates miR-449a Expression to Suppress Progression of Colorectal Cancer. Front. Oncol. 2021;11:738144. doi: 10.3389/fonc.2021.738144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cho W.C., Kim M., Park J.W., Jeong S.Y., Ku J.L. Exosomal miR-193a and let-7g accelerate cancer progression on primary colorectal cancer and paired peritoneal metastatic cancer. Transl. Oncol. 2021;14:101000. doi: 10.1016/j.tranon.2020.101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fukada M., Matsuhashi N., Takahashi T., Sugito N., Heishima K., Yoshida K., Akao Y. Postoperative changes in plasma miR21-5p as a novel biomarker for colorectal cancer recurrence: A prospective study. Cancer Sci. 2021;112:4270–4280. doi: 10.1111/cas.15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu T., Liu D., Guan S., Dong M. Diagnostic role of circulating MiR-21 in colorectal cancer: A update meta-analysis. Ann. Med. 2021;53:87–102. doi: 10.1080/07853890.2020.1828617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Song H., Ruan C., Xu Y., Xu T., Fan R., Jiang T., Cao M., Song J. Survival stratification for colorectal cancer via multi-omics integration using an autoencoder-based model. Exp. Biol. Med. 2022;247:898–909. doi: 10.1177/15353702211065010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang L., Song X., Yu M., Niu L., Zhao Y., Tang Y., Zheng B., Song X., Xie L. Serum exosomal miR-377-3p and miR-381-3p as diagnostic biomarkers in colorectal cancer. Future Oncol. 2022;18:793–805. doi: 10.2217/fon-2021-1130. [DOI] [PubMed] [Google Scholar]
- 112.Wang X., Gao G., Chen Z., Chen Z., Han M., Xie X., Jin Q., Du H., Cao Z., Zhang H. Identification of the miRNA signature and key genes in colorectal cancer lymph node metastasis. Cancer Cell Int. 2021;21:358. doi: 10.1186/s12935-021-02058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cai R., Lu Q., Wang D. Construction and prognostic analysis of miRNA-mRNA regulatory network in liver metastasis from colorectal cancer. World J. Surg. Oncol. 2021;19:7. doi: 10.1186/s12957-020-02107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ginsburg O., Yip C.H., Brooks A., Cabanes A., Caleffi M., Dunstan Yataco J.A., Gyawali B., McCormack V., McLaughlin de Anderson M., Mehrotra R., et al. Breast cancer early detection: A phased approach to implementation. Cancer. 2020;126((Suppl. 10)):2379–2393. doi: 10.1002/cncr.32887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller K.D., Nogueira L., Mariotto A.B., Rowland J.H., Yabroff K.R., Alfano C.M., Jemal A., Kramer J.L., Siegel R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 116.Adam-Artigues A., Garrido-Cano I., Carbonell-Asins J.A., Lameirinhas A., Simón S., Ortega-Morillo B., Martínez M.T., Hernando C., Constâncio V., Burgues O., et al. Identification of a Two-MicroRNA Signature in Plasma as a Novel Biomarker for Very Early Diagnosis of Breast Cancer. Cancers. 2021;13:2848. doi: 10.3390/cancers13112848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Itani M.M., Nassar F.J., Tfayli A.H., Talhouk R.S., Chamandi G.K., Itani A.R.S., Makoukji J., Boustany R.N., Hou L., Zgheib N.K., et al. A Signature of Four Circulating microRNAs as Potential Biomarkers for Diagnosing Early-Stage Breast Cancer. Int. J. Mol. Sci. 2021;22:6121. doi: 10.3390/ijms22116121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Majumder M., Ugwuagbo K.C., Maiti S., Lala P.K., Brackstone M. Pri-miR526b and Pri-miR655 Are Potential Blood Biomarkers for Breast Cancer. Cancers. 2021;13:3838. doi: 10.3390/cancers13153838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim M.W., Park S., Lee H., Gwak H., Hyun K.A., Kim J.Y., Jung H.I., Il Kim S. Multi-miRNA panel of tumor-derived extracellular vesicles as promising diagnostic biomarkers of early-stage breast cancer. Cancer Sci. 2021;112:5078–5087. doi: 10.1111/cas.15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lal M., Ansari A.H., Agrawal A., Mukhopadhyay A. Diagnostic and Prognostic Potential of MiR-379/656 MicroRNA Cluster in Molecular Subtypes of Breast Cancer. J. Clin. Med. 2021;10:4071. doi: 10.3390/jcm10184071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tian B., Hou M., Zhou K., Qiu X., Du Y., Gu Y., Yin X., Wang J. A Novel TCGA-Validated, MiRNA-Based Signature for Prediction of Breast Cancer Prognosis and Survival. Front. Cell Dev. Biol. 2021;9:717462. doi: 10.3389/fcell.2021.717462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Turkistani S., Sugita B.M., Fadda P., Marchi R., Afsari A., Naab T., Apprey V., Copeland R.L., Jr., Campbell M.C., Cavalli L.R., et al. A panel of miRNAs as prognostic markers for African-American patients with triple negative breast cancer. BMC Cancer. 2021;21:861. doi: 10.1186/s12885-021-08573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xing A.Y., Wang B., Li Y.H., Chen X., Wang Y.W., Liu H.T., Gao P. Identification of miRNA Signature in Breast Cancer to Predict Neoadjuvant Chemotherapy Response. Pathol. Oncol. Res. POR. 2021;27:1609753. doi: 10.3389/pore.2021.1609753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin S., Zhao M., Lv Y., Mao G., Ding S., Peng F. The lncRNA GATA3-AS1/miR-495-3p/CENPU axis predicts poor prognosis of breast cancer via the PLK1 signaling pathway. Aging. 2021;13:13663–13679. doi: 10.18632/aging.202909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zou X., Xia T., Li M., Wang T., Liu P., Zhou X., Huang Z., Zhu W. MicroRNA profiling in serum: Potential signatures for breast cancer diagnosis. Cancer Biomark. Sect. A Dis. Markers. 2021;30:41–53. doi: 10.3233/CBM-201547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zou R., Loke S.Y., Tan V.K., Quek S.T., Jagmohan P., Tang Y.C., Madhukumar P., Tan B.K., Yong W.S., Sim Y., et al. Development of a microRNA Panel for Classification of Abnormal Mammograms for Breast Cancer. Cancers. 2021;13:2130. doi: 10.3390/cancers13092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thomopoulou K., Papadaki C., Monastirioti A., Koronakis G., Mala A., Kalapanida D., Mavroudis D., Agelaki S. MicroRNAs Regulating Tumor Immune Response in the Prediction of the Outcome in Patients with Breast Cancer. Front. Mol. Biosci. 2021;8:668534. doi: 10.3389/fmolb.2021.668534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bao S., Hu T., Liu J., Su J., Sun J., Ming Y., Li J., Wu N., Chen H., Zhou M. Genomic instability-derived plasma extracellular vesicle-microRNA signature as a minimally invasive predictor of risk and unfavorable prognosis in breast cancer. J. Nanobiotechnol. 2021;19:22. doi: 10.1186/s12951-020-00767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qattan A., Al-Tweigeri T., Alkhayal W., Suleman K., Tulbah A., Amer S. Clinical Identification of Dysregulated Circulating microRNAs and Their Implication in Drug Response in Triple Negative Breast Cancer (TNBC) by Target Gene Network and Meta-Analysis. Genes. 2021;12:549. doi: 10.3390/genes12040549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen L., Zhu X., Han B., Ji L., Yao L., Wang Z. High Expression of microRNA-223 Indicates a Good Prognosis in Triple-Negative Breast Cancer. Front. Oncol. 2021;11:630432. doi: 10.3389/fonc.2021.630432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mansoori B., Silvestris N., Mohammadi A., Khaze V., Baghbani E., Mokhtarzadeh A., Shanehbandi D., Derakhshani A., Duijf P.H.G., Baradaran B. miR-34a and miR-200c Have an Additive Tumor-Suppressive Effect on Breast Cancer Cells and Patient Prognosis. Genes. 2021;12:267. doi: 10.3390/genes12020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang X., Qian T., Bao S., Zhao H., Chen H., Xing Z., Li Y., Zhang M., Meng X., Wang C., et al. Circulating exosomal miR-363-5p inhibits lymph node metastasis by downregulating PDGFB and serves as a potential noninvasive biomarker for breast cancer. Mol. Oncol. 2021;15:2466–2479. doi: 10.1002/1878-0261.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Srivastava S., Koay E.J., Borowsky A.D., De Marzo A.M., Ghosh S., Wagner P.D., Kramer B.S. Cancer overdiagnosis: A biological challenge and clinical dilemma. Nat. Rev. Cancer. 2019;19:349–358. doi: 10.1038/s41568-019-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Welch H.G., Albertsen P.C. Reconsidering Prostate Cancer Mortality—The Future of PSA Screening. N. Engl. J. Med. 2020;382:1557–1563. doi: 10.1056/NEJMms1914228. [DOI] [PubMed] [Google Scholar]
- 135.Mello-Grand M., Bruno A., Sacchetto L., Cristoni S., Gregnanin I., Dematteis A., Zitella A., Gontero P., Peraldo-Neia C., Ricotta R., et al. Two Novel Ceramide-Like Molecules and miR-5100 Levels as Biomarkers Improve Prediction of Prostate Cancer in Gray-Zone PSA. Front. Oncol. 2021;11:769158. doi: 10.3389/fonc.2021.769158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Giglio S., De Nunzio C., Cirombella R., Stoppacciaro A., Faruq O., Volinia S., Baldassarre G., Tubaro A., Ishii H., Croce C.M., et al. A preliminary study of micro-RNAs as minimally invasive biomarkers for the diagnosis of prostate cancer patients. J. Exp. Clin. Cancer Res. 2021;40:79. doi: 10.1186/s13046-021-01875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Markert L., Holdmann J., Klinger C., Kaufmann M., Schork K., Turewicz M., Eisenacher M., Savelsbergh A. Small RNAs as biomarkers to differentiate benign and malign prostate diseases: An alternative for transrectal punch biopsy of the prostate? PLoS ONE. 2021;16:e0247930. doi: 10.1371/journal.pone.0247930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y., Fang Y.X., Dong B., Du X., Wang J., Wang X., Gao W.Q., Xue W. Discovery of extracellular vesicles derived miR-181a-5p in patient’s serum as an indicator for bone-metastatic prostate cancer. Theranostics. 2021;11:878–892. doi: 10.7150/thno.49186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rönnau C.G.H., Fussek S., Smit F.P., Aalders T.W., van Hooij O., Pinto P.M.C., Burchardt M., Schalken J.A., Verhaegh G.W. Upregulation of miR-3195, miR-3687 and miR-4417 is associated with castration-resistant prostate cancer. World J. Urol. 2021;39:3789–3797. doi: 10.1007/s00345-021-03723-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stoen M.J., Andersen S., Rakaee M., Pedersen M.I., Ingebriktsen L.M., Bremnes R.M., Donnem T., Lombardi A.P.G., Kilvaer T.K., Busund L.T., et al. High expression of miR-17-5p in tumor epithelium is a predictor for poor prognosis for prostate cancer patients. Sci. Rep. 2021;11:13864. doi: 10.1038/s41598-021-93208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu J., Quan Z., Gao Y., Wu X., Zheng Y. MicroRNA-199b-3p suppresses malignant proliferation by targeting Phospholipase Cε and correlated with poor prognosis in prostate cancer. Biochem. Biophys. Res. Commun. 2021;576:73–79. doi: 10.1016/j.bbrc.2021.08.078. [DOI] [PubMed] [Google Scholar]
- 142.Bian Z., Huang X., Chen Y., Meng J., Feng X., Zhang M., Zhang L., Zhou J., Liang C. Fifteen-MiRNA-Based Signature Is a Reliable Prognosis-Predicting Tool for Prostate Cancer Patients. Int. J. Med. Sci. 2021;18:284–294. doi: 10.7150/ijms.49412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim M.Y., Shin H., Moon H.W., Park Y.H., Park J., Lee J.Y. Urinary exosomal microRNA profiling in intermediate-risk prostate cancer. Sci. Rep. 2021;11:7355. doi: 10.1038/s41598-021-86785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saran U., Chandrasekaran B., Kolluru V., Tyagi A., Nguyen K.D., Valadon C.L., Shaheen S.P., Kong M., Poddar T., Ankem M.K., et al. Diagnostic molecular markers predicting aggressive potential in low-grade prostate cancer. Transl. Res. J. Lab. Clin. Med. 2021;231:92–101. doi: 10.1016/j.trsl.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 145.Zhao Y., Tang X., Zhao Y., Yu Y., Liu S. Diagnostic significance of microRNA-1255b-5p in prostate cancer patients and its effect on cancer cell function. Bioengineered. 2021;12:11451–11460. doi: 10.1080/21655979.2021.2009413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li Z., Li L.X., Diao Y.J., Wang J., Ye Y., Hao X.K. Identification of Urinary Exosomal miRNAs for the Non-Invasive Diagnosis of Prostate Cancer. Cancer Manag. Res. 2021;13:25–35. doi: 10.2147/CMAR.S272140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Byun Y.J., Piao X.M., Jeong P., Kang H.W., Seo S.P., Moon S.K., Lee J.Y., Choi Y.H., Lee H.Y., Kim W.T., et al. Urinary microRNA-1913 to microRNA-3659 expression ratio as a non-invasive diagnostic biomarker for prostate cancer. Investig. Clin. Urol. 2021;62:340–348. doi: 10.4111/icu.20200488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zabegina L., Nazarova I., Nikiforova N., Slyusarenko M., Sidina E., Knyazeva M., Tsyrlina E., Novikov S., Reva S., Malek A. A New Approach for Prostate Cancer Diagnosis by miRNA Profiling of Prostate-Derived Plasma Small Extracellular Vesicles. Cells. 2021;10:2372. doi: 10.3390/cells10092372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rode M.P., Silva A.H., Cisilotto J., Rosolen D., Creczynski-Pasa T.B. miR-425-5p as an exosomal biomarker for metastatic prostate cancer. Cell. Signal. 2021;87:110113. doi: 10.1016/j.cellsig.2021.110113. [DOI] [PubMed] [Google Scholar]
- 150.Zhou P., Gong S., Liu B., Shi M., Lu F., Li N., Tang B. A hybridization-based dual-colorimetric kit for circulating cancer miRNA detection. Chem. Commun. 2021;57:6058–6061. doi: 10.1039/D1CC01607E. [DOI] [PubMed] [Google Scholar]
- 151.Mao Y., Sun Y., Xue J., Lu W., Cao X. Ultra-sensitive and high efficiency detection of multiple non-small cell lung cancer-related miRNAs on a single test line in catalytic hairpin assembly-based SERS-LFA strip. Anal. Chim. Acta. 2021;1178:338800. doi: 10.1016/j.aca.2021.338800. [DOI] [PubMed] [Google Scholar]
- 152.Zhuang J., Wan H., Zhang X. Electrochemical detection of miRNA-100 in the sera of gastric cancer patients based on DSN-assisted amplification. Talanta. 2021;225:121981. doi: 10.1016/j.talanta.2020.121981. [DOI] [PubMed] [Google Scholar]
- 153.Li T., Liang Y., Li J., Yu Y., Xiao M.M., Ni W., Zhang Z., Zhang G.J. Carbon Nanotube Field-Effect Transistor Biosensor for Ultrasensitive and Label-Free Detection of Breast Cancer Exosomal miRNA21. Anal. Chem. 2021;93:15501–15507. doi: 10.1021/acs.analchem.1c03573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.