Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide [1,2]. The efficacy of immune checkpoint inhibitors (ICIs) in several tumor types has prompted a similar development in HCC patients [3,4]. Firstly, two PD-1 inhibitors, nivolumab and pembrolizumab, were approved by the United States Food and Drug Administration (FDA) following the early phase CheckMate 040 and KEYNOTE-224 trials [5,6]. However, the two confirmatory phase III trials—CheckMate 459 and KEYNOTE-240-compared nivolumab versus sorafenib as first-line treatment, and pembrolizumab versus placebo as second-line therapy, respectively—failed to meet their primary endpoints [7,8]. If ICIs alone as a first-line treatment have not achieved the desired effect, clinical trials evaluating combinatorial strategies involving ICIs and other anticancer agents, especially antiangiogenic agents, have produced more compelling results, marking a new era in HCC management [9,10,11,12,13,14].
The practice-changing phase III IMbrave150 trial compared the combination of the PD-L1 inhibitor atezolizumab plus bevacizumab versus sorafenib monotherapy in treatment-naïve patients with advanced HCC [15,16]; of note, the results of IMbrave150 have led to the approval of this immune-based combination given an unprecedented median overall survival (OS) of 19.2 months compared with 13.4 months for sorafenib monotherapy (Hazard Ratio [HR], 0.58; 95% Confidence Interval [CI], 0.42–0.79). Similarly, the study reported a median progression-free survival (PFS) benefit (6.9 months versus 4.3 months, respectively) and higher overall response rate (ORR) in patients treated with the immune-based combination. Based on these results, atezolizumab–bevacizumab is currently considered the new standard of care in front-line HCC and has been approved in several countries worldwide [17,18]. Similarly, other combinations have been tested and are currently being assessed. Among these, the recently published COSMIC-312 phase III trial compared the combination of atezolizumab plus cabozantinib versus sorafenib as first-line treatment for advanced HCC [19]. Although the results indicated a statistically significant benefit in median PFS in patients treated with atezolizumab–cabozantinib, no difference in OS was highlighted. In another phase II/III trial, ORIENT-32, the investigators compared the combination of the PD-1 inhibitor sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib alone, reporting a statistically significant improvement in terms of median PFS and OS in patients treated with the immune-based combination [20].
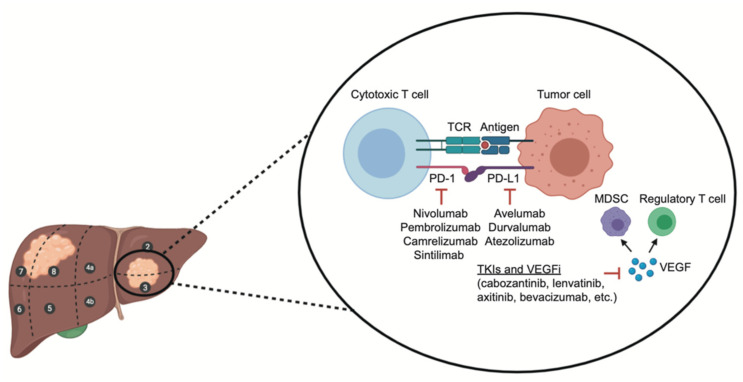
The role of another immune-based combination including two ICIs, the PD-L1 inhibitor durvalumab and the anti-CTLA-4 antibody tremelimumab, has been explored in the HIMALAYA trial (Figure 1) [21,22,23]. In this open-label, multicenter, phase III study, median OS was 16.4 months in patients receiving durvalumab plus tremelimumab versus 13.8 months in the sorafenib monotherapy arm, whereas no significant differences were reported in median PFS. Thus, the results of the HIMALAYA trial support the use of this immune-based combination in this setting. In addition, beyond immunomodulatory antibodies, several other agents and immune-based treatments have been assessed and are currently under evaluation, including adoptive cell transfer (ACT), oncolytic virus therapy, and vaccines [24,25,26,27,28].
Figure 1.
Schematic figure representing the synergistic activity of immune-based combinations (including double checkpoint blockade and immune checkpoint inhibitors plus antiangiogenic agents.
From this point of view, this Special Issue welcomes papers exploring the current state of the art and future perspectives in the immunotherapy of HCC. The Special Issue will aim to assess key open questions in HCC immunotherapy, including preclinical studies, novel immunotherapies and immune-based combinations, biomarkers of response, experimental therapies, real-world experience with immune checkpoint inhibitors, and several other topics.
Author Contributions
Conceptualization, all authors; methodology, all authors; software, all authors; validation, all authors; formal analysis, all authors; investigation, all authors; resources, all authors; data curation, A.R.; writing—original draft preparation, A.R.; writing—review and editing, all authors; visualization, all authors; supervision, all authors; project administration, all authors; funding acquisition, all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rizzo A., Nannini M., Novelli M., Dalia Ricci A., Scioscio V.D., Pantaleo M.A. Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020;12:1758835920936932. doi: 10.1177/1758835920936932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llovet J.M., Castet F., Heikenwalder M., Maini M.K., Mazzaferro V., Pinato D.J., Pikarsky E., Zhu A.X., Finn R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2021;19:151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo A., Ricci A.D., Gadaleta-Caldarola G., Brandi G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: Current management and future challenges. Expert Rev. Gastroenterol. Hepatol. 2021;15:1245–1251. doi: 10.1080/17474124.2021.1973431. [DOI] [PubMed] [Google Scholar]
- 4.Sangro B., Sarobe P., Hervás-Stubbs S., Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021;18:525–543. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Khoueiry A.B., Sangro B., Yau T., Crocenzi T.S., Kudo M., Hsu C., Kim T.-Y., Choo S.-P., Trojan J., Welling T.H., 3rd, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu A.X., Finn R.S., Edeline J., Cattan S., Ogasawara S., Palmer D., Verslype C., Zagonel V., Fartoux L., Vogel A., et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 7.Yau T., Park J.-W., Finn R.S., Cheng A.-L., Mathurin P., Edeline J., Kudo M., Harding J.J., Merle P., Rosmorduc O., et al. Nivolumab versus sorafenib in advanced hepato-cellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 8.Finn R.S., Ryoo B.-Y., Merle P., Kudo M., Bouattour M., Lim H.Y., Breder V., Edeline J., Chao Y., Ogasawara S., et al. Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang T., Kan X., Zheng C. Immune Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma: Monotherapies and Combined Therapies. Front. Oncol. 2022;12:898964. doi: 10.3389/fonc.2022.898964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzo A., Cusmai A., Gadaleta-Caldarola G., Palmiotti G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expert Rev. Gastroenterol. Hepatol. 2022;16:333–339. doi: 10.1080/17474124.2022.2064273. [DOI] [PubMed] [Google Scholar]
- 11.Wen W., Zhang Y., Zhang H., Chen Y. Clinical outcomes of PD-1/PD-L1 inhibitors in patients with advanced hepatocellular carcinoma: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2022. pp. 1–10. in press . [DOI] [PubMed]
- 12.Rizzo A., Ricci A.D., Di Federico A., Frega G., Palloni A., Tavolari S., Brandi G. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy in Hepatocellular Carcinoma: Where Do We Stand? Front. Oncol. 2021;11:803133. doi: 10.3389/fonc.2021.803133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., Lencioni R., Koike K., Zucman-Rossi J., Finn R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 14.Rizzo A. Locoregional treatments plus immunotherapy in hepatocellular carcinoma: Where do we stand? Future Oncol. 2022;18:1665–1668. doi: 10.2217/fon-2021-1623. [DOI] [PubMed] [Google Scholar]
- 15.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.-Y., Kudo M., Breder V., Merle P., Kaseb A.O., et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 16.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.-Y., Lim H.Y., Kudo M., Breder V.V., Merle P., et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC) J. Clin. Oncol. 2021;39((Suppl. 3)):267. doi: 10.1200/JCO.2021.39.3_suppl.267. [DOI] [Google Scholar]
- 17.Rizzo A., Ricci A.D., Brandi G. Atezolizumab in advanced hepatocellular carcinoma: Good things come to those who wait. Immunotherapy. 2021;13:637–644. doi: 10.2217/imt-2021-0026. [DOI] [PubMed] [Google Scholar]
- 18.Benson A.B., D’Angelica M.I., Abbott D.E., Anaya D.A., Anders R., Are C., Bachini M., Borad M., Brown D., Burgoyne A., et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021;19:541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 19.Kelley R.K., Rimassa L., Cheng A.L., Kaseb A., Qin S., Zhu A.X., Chan S.L., Melkadze T., Sukeepaisarnjaroen W., Breder V., et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:995–1008. doi: 10.1016/S1470-2045(22)00326-6. [DOI] [PubMed] [Google Scholar]
- 20.Ren Z., Xu J., Bai Y., Xu A., Cang S., Du C., Li Q., Lu Y., Chen Y., Guo Y., et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22:977–990. doi: 10.1016/S1470-2045(21)00252-7. Erratum in Lancet Oncol. 2021, 22, e347. [DOI] [PubMed] [Google Scholar]
- 21.Abou-Alfa G.K., Chan S.L., Kudo M., Lau G., Kelley R.K., Furuse J., Sukeepaisarnjaroen W., Kang Y.-K., Dao T.V., De Toni E.N., et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepato-cellular carcinoma (uHCC): HIMALAYA. JCO. J. Clin. Oncol. 2022;40((Suppl. S4)):379. doi: 10.1200/JCO.2022.40.4_suppl.379. [DOI] [Google Scholar]
- 22.Wong K.M., King G.G., Harris W.P. The Treatment Landscape of Advanced Hepatocellular Carcinoma. Curr. Oncol. Rep. 2022;24:917–927. doi: 10.1007/s11912-022-01247-7. [DOI] [PubMed] [Google Scholar]
- 23.Feng M.Y., Chan L.L., Chan S.L. Drug Treatment for Advanced Hepatocellular Carcinoma: First-Line and Beyond. Curr. Oncol. 2022;29:5489–5507. doi: 10.3390/curroncol29080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foerster F., Gairing S.J., Ilyas S.I., Galle P.R. Emerging immunotherapy for HCC: A guide for hepatologists. Hepatology. 2022;75:1604–1626. doi: 10.1002/hep.32447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzo A., Brandi G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat. Res. Commun. 2021;27:100328. doi: 10.1016/j.ctarc.2021.100328. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z., Liu X., Liang J., Liu Y., Hou X., Zhang M., Li Y., Jiang X. Immunotherapy for Hepatocellular Carcinoma: Current Status and Future Prospects. Front. Immunol. 2021;12:765101. doi: 10.3389/fimmu.2021.765101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oura K., Morishita A., Tani J., Masaki T. Tumor Immune Microenvironment and Immunosuppressive Therapy in Hepatocellular Carcinoma: A Review. Int. J. Mol. Sci. 2021;22:5801. doi: 10.3390/ijms22115801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzo A., Ricci A.D., Brandi G. Immune-based combinations for advanced hepatocellular carcinoma: Shaping the direction of first-line therapy. Future Oncol. 2021;17:755–757. doi: 10.2217/fon-2020-0986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.