Abstract
Human diseases such as cancer can be caused by aberrant epigenetic regulation. Polyphenols play a major role in mammalian epigenome regulation through mechanisms and proteins that remodel chromatin. In fruits, seeds, and vegetables, as well as food supplements, polyphenols are found. Compounds such as these ones are powerful anticancer agents and antioxidants. Gallic acid, kaempferol, curcumin, quercetin, and resveratrol, among others, have potent anti-tumor effects by helping reverse epigenetic changes associated with oncogene activation and tumor suppressor gene inactivation. The role dietary polyphenols plays in restoring epigenetic alterations in cancer cells with a particular focus on DNA methylation and histone modifications was summarized. We also discussed how these natural compounds modulate gene expression at the epigenetic level and described their molecular targets in cancer. It highlights the potential of polyphenols as an alternative therapeutic approach in cancer since they modulate epigenetic activity.
Keywords: cancer, polyphenol, epigenetics
1. Introduction
Epigenetics is the study of how genes and their products affect an organism’s phenotype [1]. Since the discovery of DNA, epigenetics has not received much attention. In the 1980s, however, studies of chromatin structure made epigenetics respectable after being shrouded in the shadows [2]. In 1987, Robin Holliday redefined epigenetics as nuclear inheritance without DNA sequence differences [3]. It offers possible explanations for cellular differentiation and parental imprinting in mammals, and it enables genetics and developmental embryology to be integrated [4]. Furthermore, epigenetic modifications play a crucial role in developmental patterning, biological processes, and pathology [5,6,7]. In mammalian cells, DNA methylation and histone modifications induce chromatin remodeling, leading to cellular phenotype changes [8,9,10,11]. Diverse epigenetic changes occur in cancer cells in the early stages of tumor development [12]. These epigenetic modifications of chromatin are inherited and reversible, so they could be used to develop drugs targeting the epigenome which could help treat cancer [13,14,15]. The use of new therapeutic drugs and personalized treatment leads to improved patient survival. Dietary supplements have been combined with some of these treatments [16,17,18]. Diets high in vegetables and fruits have been proven to reduce the risk of cancer. This is because they regulate the expression of oncogenes and tumor suppressor genes [19]. Dietary supplements might be an alternative cancer treatment. In this study, we provide an overview of the most common epigenetic alterations seen in cancer, then discuss the most studied dietary patterns and how they might be used to reverse epigenetic changes, and lastly, discuss how they might be used as an alternative therapy for cancer.
2. Oxidative DNA Damage and Polyphenols
Polyphenols have antioxidant properties which as a therapeutic action against cancer. It is found that polyphenol has a dominant antioxidant that mitigates oxidative stress from pathological conditions such as cancer. Polyphenol can scavenge ROS and act on free radicals. This is due to the presence of aromatic rings, the presence of hydroxyl groups in a different region, and has more conjugated system [20]. Polyphenol scavenges Reactive Oxygen Species (ROS) and mitigates the biomolecule oxidative damage [21,22]. Polyphenols with antioxidant capacity suppress the signaling pathways involved in oxidative stress generation at a molecular level. Consumption of a polyphenol diet increases the activity of the antioxidant and inhibits the peroxidation of lipids and cyclooxygenase (COX) pathways [23]. Increased levels of free radical production such as ROS and LPO with oxidative stress cause damage to the tissues inclusive of DNA and increases the possibility of cancer occurrence. Increased ROS level is due to exogenous, antioxidant defense, and endogenous sources. The exogenous sources include X-rays, UV light irradiation, the action of metals, toxins, and γ rays, drugs, and solvents; endogenous sources include peroxisomes, metabolism of cytochrome P450; reactions in mitochondria; and activation of inflammation. This exogenous and endogenous source of ROS is important for oxidative stress-mediated ROS production causes damage to the cell, and alters the signaling pathway, which further causes cancer [24]. Damages to the DNA can cause errors in the replication, arresting transcriptional activities, instability of the DNA damage, and further causes cancer [25,26]. Different studies show a reduction in endogenous DNA damage and protection from ex vivo DNA damage [27]. A diet such as vegetables and fruits has a high content of polyphenols which includes quercetin, ellagic acid, catechins, naringenin, and resveratrol. This polyphenol can decrease the risk of cancer. Polyphenols have a chemopreventive action which includes the involvement of antiestrogenic, arresting cell cycle, proliferation against cancer cells, resistance to the oxidative stress, induction of apoptosis, detoxification enzyme activation, regulation of the host immune system, and cellular signaling improvement in the cancer condition [28]. Polyphenols show protection from cancer when combined with DNA-damaging agents. Polyphenols impair the metabolism of pro-carcinogen by altering the level of enzyme cytochrome P450 which plays an important role in the stimulation of cancer [29]. Polyphenol and quercetin have properties of anti-cancer action by reducing free radical-ROS scavenging activity [30]. The polyphenols present in black tea such as theaflavins, EGCG, and thearubigins have effective properties of anti-cancer [31,32,33]. The catechins present in the tea can prevent cancer by impairing intraepithelial prostate lesions converting them into cancer and decreasing the cancer cell apoptosis, thereby it inhibits carcinogenesis [34]. The flavonoids like catechins, anthocyanins, flavanols, flavanones, flavones, and isoflavones, have a capacity for free radicals neutralization via scavenging ROS and impairs the risk of cancer by cellular growth arrest in cancer cells [35]. There are different types of cancer such as prostate, endometrial, epithelial, breast cancer and colon cancer are mitigated by polyphenols [36]. Resveratrol has an anti-cancer property via an antioxidant defense mechanism which impairs the hydroperoxidase level, matrix metalloproteinase level (MMP-9), Akt signaling pathway, NF-KB pathway, cycloxygenase pathway, protein kinase C, Bcl-2 level and focal adhesion kinase [37].
3. Human Cancer and DNA Methylation
In cancer, epigenetic changes include genome-scale methylation changes, hypermethylation in specific loci, and dysfunction of histone-modifying enzymes. Changes in DNA methylation are good biomarkers since they can be detected and quantified [23,38,39]. Many studies have found DNA methylation patterns specific to liver cancers, including genome-wide studies [40,41,42,43,44,45]. According to DNA methylation and transcriptome mapping in human tumors, a lot of genes are hypomethylated and expressed more, and a lot of genes are hypermethylated and underexpressed. The genes induced by epigenetics were found to be involved in cellular transformation and differentiation, tumor growth, and metastasis. Apoptosis, cell adhesion, and cell cycle progression genes are repressed [46,47,48]. Even though genome-wide DNA methylation studies are a hot topic, there are a few caveats that urge caution about the clinical and biological significance of the data [49]. Most importantly, tumors have a lot of cellular heterogeneity, so observed differences in DNA methylation patterns might just be due to differences in tumor cell numbers, rather than being an epigenetic signature. The DNA methylation profiling needs to be performed on small numbers of histologically verified tumor cells sorted by high-speed cell sorting or laser dissection microscopy. Another caveat is that we shouldn’t assume a simple relationship between DNA methylation and gene expression, even if transcriptome data indicates that. In vivo experiments would need to manipulate DNA methylation site-directedly and demonstrate transcription rate changes (Table 1).
Table 1.
Polyphenols on DNA methylation and histone modification.
| Polyphenols | Molecular Mechanism | Pre Clinical Model | Target Gene |
|---|---|---|---|
| DNA methylation | |||
| Curcumin | DNMT inhibitor | Leukemia, esophageal | NA |
| Epicatechin, epicatechin-gallate, epigalocatechin-3-gallate | DNMT inhibitor | Lung, colon cancer cells, esophageal, oral, breast cancers | RAŘ, MGMT, MLH1, CDKN2A, RECK, TERT, RXⱤ, CDX2, GSTP1, W1F1 |
| Quercetin | DNMT inhibitor | Breast, colon, esophageal cancers | CDKN2A |
| Resveratrol | DNMT inhibitor | Breast, Lungs cancers | NA |
| Histone modifications | |||
| Curcumin | HAT and HDAC inhibitor | anti-inflammatory anticancer, antioxidant, antiproliferative |
GATA4, EOMES, GZMB, PRF1,H3/H4 deacetylation |
| Epicatechin, epicatechin-gallate, epigalocatechin-3-gallate | HAT inhibitor | antioxidant, anticancer, anti-inflammatory |
NF-kB, IL-6, BMI-1, EZH2, SUZ12, H3K27 trimethylation, H3/H4 acetylation |
| Quercetin | SIRTI activator HAT inhibitor | anti-migration, anticancer, antiproliferative, antidiabetic, antioxidant |
Inflammatory diseases |
| Resveratrol | SIRTi activator | anti-migration, anticancer, antiproliferative, antidiabetic, antioxidant |
TNF-˛, IL-8, RBP |
4. Cancer and Histones
DNA is packed into chromatin around an octamer of histones in a chromosome. A nucleosome is a repeating unit of chromatin that is made up of 150 base pairs of DNA and an octamer of histones, H2A, H2B, H3, and H4 [49,50,51,52]. Histone tails are targets for post-translational modifications, including acetylation, methylation, phosphorylation, and ubiquitination [53,54]. DNA modifications can turn the transcription of genes on or off, which affects the accessibility of transcription factors by adjusting how tightly DNA is bound to histones. HATs, which “write” the acetyl mark on histones, are responsible for histone acetylation. By counteracting the positive charge of histones, it loosens the connection between histones and DNA. In contrast, histone deacetylases (HDACs) “erase” those acetyl groups, resulting in tight DNA coiling around the histones, making chromatin transcriptionally inactive. Histone methylation is associated with either transcriptionally active or closed chromatin depending on where the lysine is methylated [55]. The trimethylation of histone 3 lysine 27 (H3K27me3), for example, is associated with transcriptional repression, whereas trimethylation of histone 3 lysine 4 is associated with gene activation [56,57]. Cancer patients with high levels of trimethylated histone H3 lysine 4 (H3K4me3) have a poor prognosis. H3K27me3 levels were linked to poor prognosis and tumor aggressive features including vascular invasion, large tumor sizes, multiple tumors, and poor differentiation in another study [56,58,59]. To fully understand the role of these specific DNA-protein modifications in cancer, further studies using more precise detection methods, such as ChIP-sequencing, will be needed.
5. Inhibitors of DNA Methylation
The epigenome is reprogrammed as soon as embryogenesis begins because DNA methylation decreases. Methylation of DNA requires methylating enzymes, so cellular replication without these enzymes leads to significant demethylation of daughter cells and gene reactivation. This approach has a therapeutic ratio when applied to cancer cells; normal cells usually survive hypomethylation, whereas cancer cells usually die when it occurs, perhaps because they are dependent on gene silencing.
DNA hypomethylation only happens with cytosine analogs with 5’ modifications of the ring. Nucleoside and cytosine analogs do not directly affect DNA methylation. It was determined that the ability of these two main analogs to target DNA methyltransferases (DNMTs) for degradation was attributed to their ability to trap DNA methyltransferases (Table 1). In the absence of these enzymes, DNA synthesis results in hypomethylation in daughter cells, which in turn leads to the reactivation of silenced genes. Some other 5’ modified nucleoside analogs have been described in preclinical or early clinical studies [60]. Inhibiting DNA methylation in cancers works, at least in part, by inhibiting DNA methylation.
6. Inhibitors of Histone Modification
Inhibitors of histone deacetylase (HDACi) decrease HDAC activity, block acetylated histone aggregation, and promote autosomal acetylation. HDACi promotes cancer cell differentiation, induces apoptosis, and inhibits angiogenesis through many mechanisms, including cell cycle arrest, apoptosis, autophagy, and differentiation. HDACi are classified into four classes based on their chemical. In addition to in vitro and in vivo studies, hydroxamates and aliphatic acids have also been tested in clinical trials as a new treatment strategy for hepatobiliary cancer [61,62,63,64]. Panobinostat, trichostatin A, vorinostat, and belinostat are hydroxamates that block HDAC activity by binding to Zn2+ at the HDAC binding site. Besides aliphatic acids, sodium butyrate and valproic acid (VPA) inhibit class I HDACs as well [65,66]. For example, this occurs with VPA, sodium butyrate, and TSA. By downregulating cyclins A and D1 and upregulating P21, VPA could induce cell cycle arrest [67]. Additionally, sodium butyrate upregulated p21 and p27 protein expression [68,69,70]. Furthermore, TSA causes G2/M-phase arrest and G0/G1 arrest in hepatoma cells [71,72]. The fact that apoptosis plays an important role in tumor development makes it an obvious target for cancer therapy. HDACi promotes apoptosis in cancer cells. In addition, HDACi promotes apoptosis by different mechanisms; for example, VPA activates TRAIL-associated cell death and intrinsic apoptosis by upregulating cleaved caspases 3 and 9 [73]. Specifically, TSA upregulates bax and cleaved caspase 3 and downregulates BCL-2 in cancer cells [74,75]. Further, it has been found that HDACi can induce autophagy-mediated cell death, and cancer cell lines showed autophagosome formation, maturation, and aggregation when exposed to panobinostat. Several inhibitors of angiogenesis have been found to interact with HDACi in a synergistic way to inhibit hepatobiliary cancers [76,77]. Some clinical trials have tested HDACi’s anticancer effects, especially when combined with sorafenib.
7. Polyphenol and Cancer
There is usually at least one hydroxyl group attached to an aromatic ring in these compounds. Polyphenolic molecules have been found in thousands of higher plants and hundreds of edible plants [78,79]. There are two types of polyphenols in plants: flavonoids and non-flavonoids [80]. Flavanoids share 15 carbon atoms at their core and are divided into flavanols, flavonols, anthocyanidins, flavones, flavanones, and chalcones [81]. The non-flavonoids contain an aromatic ring with one or more hydroxyl groups. Stilbene, phenolic acids, saponin, and other polyphenols such as curcumin and tannins are not flavonoids. Plants synthesize polyphenols to defend themselves against infection and protect themselves from stress. In recent years, plant polyphenols have received substantial attention for their cancer-preventing effects. Several studies have demonstrated that plant polyphenols are chemopreventive against multiple types of cancer [82,83,84,85,86,87].
7.1. Epigenetics Mechanism of Kaempferol
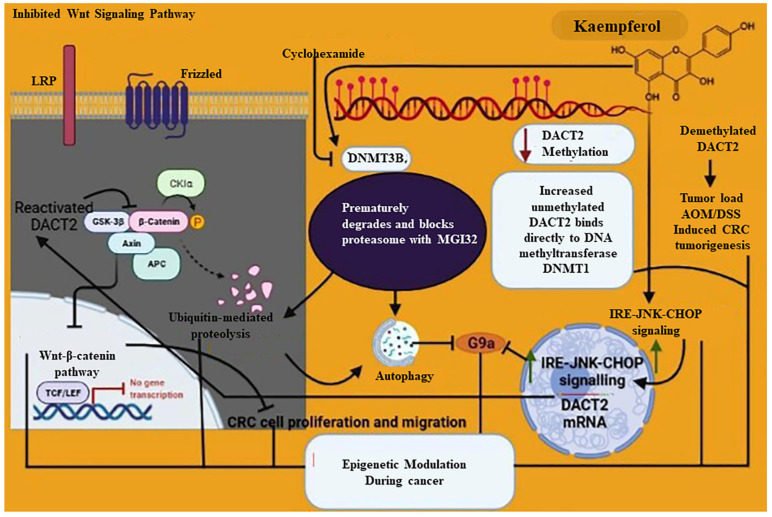
The natural flavonoid kaempferol (KFL) is widely found in fruit and veggies. Many cancers, such as breast cancer, pancreatic cancer, prostate cancer, and lung cancer, have been reported as being susceptible to KFL [88,89,90,91]. A previous study has shown that KFL could affect DNA methylation in nude mice treated with bladder cancer [92]. In their experiments, they found that KFL inhibited the levels of DNMT3B protein without affecting DNMT1 and also found that cycloheximide inhibited protein synthesis of DNMT3B and caused DNMT3B to prematurely degrade and block proteasome with MG132, causing KFL to ubiquitinate DNMT3B. Results suggest that KFL can degrade DNMT3B through the ubiquitin-proteasome pathway [93]. Another study showed treatment with KFL increased DACT2 in three colorectal cancer cells. In addition, KFL reduced DACT2 methylation but increased unmethylated DACT2 by binding directly to DNA methyltransferases DNMT1 [93]. Kaempferol epigenetically reactivates DACT2 transcription to inhibit nuclear β-catenin expression, which in turn inhibits Wnt/β-catenin signaling, which restricts CRC cell proliferation and migration. Additionally, kaempferol-demethylated DACT2 reduced tumor load in AOM/DSS-induced CRC tumorigenesis. Tae et al. found that kaempferol activates autophagy via the inhibition of G9a [94]. According to this study, kaempferol activates IRE1-JNK-CHOP signaling from the cytosol to the nucleus and inhibiting G9a triggers autophagy [95,96,97,98,99,100,101] (Figure 1).
Figure 1.
Molecular mechanism represents the role of kaempferol in epigenetic modulation during cancer and how it protects from cancer. Kaempferol protecting against cancer via a different mechanism such as inhibition of DNMT3B which prematurely degrades and blocks proteasome with MGI32 further causes autophagy via G9a inhibition and ubiquitin-mediated proteolysis; on the other hand, reactivated DACT2 inhibits Wnt-β-catenin signaling pathway and leads to impairment of the CRC cell proliferation and migration further causes epigenetic modulation and protects against cancer; increased localization of the IRE-JNK CHOP from cytosolic to the nucleus further inhibits G9a; decreased DACT2 methylation; increased unmethylated DACT2 binds directly to DNA methyltransferase DNMT1 and is involved in epigenetic modulation during cancer.
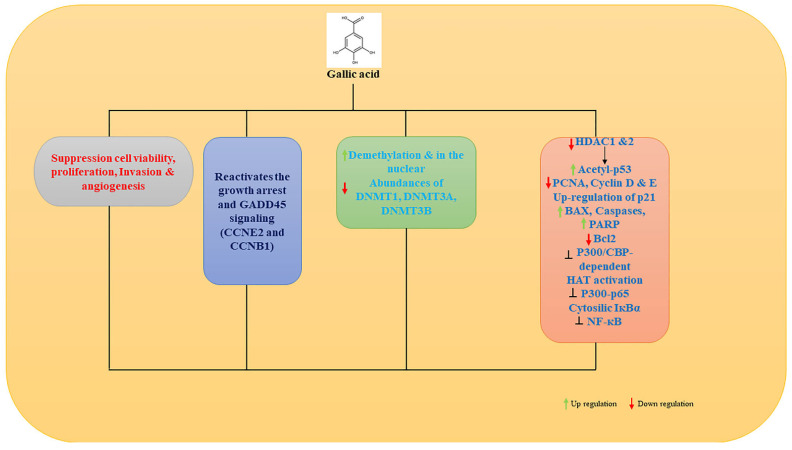
7.2. Epigenetic Mechanism of Gallic Acid
Among the emerging cancer treatments is gallic acid (GA) [102,103,104]. Studies have shown it suppresses hepatic cancer cell viability, proliferation, invasion, and angiogenesis [105,106,107,108]. In addition to being an antioxidant, GA is also a pro-oxidant. In H1299 cells, GA reactivates the growth arrest and DNA damage-inducible 45 (GADD45) signaling pathway through demethylation of CCNE2 and CCNB1 [109]. Through a two-week fermentation process, Aspergillus sojae is able to efficiently increase the GA content in oolong tea, improving the epigenetic anticancer properties of GA. DNMT1, DNMT3A, and DNMT3B in human cancer cell lines were significantly reduced when the fungus dramatically increased GA up to 45 fold [110]. As a result, fermented oolong tea high in GA appears to be an effective dietary intervention strategy for tobacco-associated cancers because of its potent inhibitory effects on DNMTs. As confirmed by histological analysis, oral administration of GA for 8 weeks decreases tumor size, damages tumor structure, and lowers expression of HDAC1 and PCNA in tumor mass [111]. As an HDAC inhibitor and anti-PCa therapy, GA may inhibit PCa progression by inhibiting HDAC1 and two expressions.
Gallic acid from Rosa rugosa inhibited the majority of histone acetyltransferases (HATs) in another study [112], Including sirtuin, histone deacetylase, and histone methyltransferase. Gallic acid inhibits p300/CBP-dependent HAT activities uncompetitively. Both in vitro and in vivo, GA inhibits p300-induced p65 acetylation. It prevents the nuclear translocation of p65 caused by lipopolysaccharide (LPS). It suppresses LPS-induced nuclear factor-κB activation in A549 lung cancer cells [113] (Figure 2) [110,111,112].
Figure 2.
Gallic acid protects from cancer. Gallic acid suppresses cell viability, proliferation, invasion, and angiogenesis; reactivates growth arrest and GADD45; increases demethylation and increases the DNMT1, DNMT3A, and DNMT3B found to be more in the nucleus; decreases HDAC1 and 2; increases acetyl-p53; decreases PCNA; cyclin D and E; up-regulation of p21, decreases BAX, Cas3, and PARP, and increases Bcl2; inhibits P300/CBP-dependent HAT activation; P300-p65 inhibition; cytosolic IKBα; inhibition of NF-kB; and further protects from cancer.
7.3. Epigenetic Modulation of Curcumin in Cancer
Curcumin modulates HDAC or HAT activity in a variety of in vitro cancer cell models [114,115,116]. Curcumin inhibits Notch-1 and the pro-inflammatory nuclear transcription factor-kappa B by inhibiting p300-mediated acetylation of its RelA isoform [117]. The researchers showed that curcumin reduces HAT activity in human acute monocytic leukemia THP-1 cells by hypoacetylation p65 at Lys310 and preventing nuclear transcription factor kappaB activity [118]. It has been shown that curcumin induces histone hypoacetylation in brain glioma cells, which results in subsequent apoptotic cell death through PARP- and caspase-3-mediated mechanisms [106]. Recent studies have shown curcumin acts as an epigenetic modulator of TREM-1 gene expression by inhibiting p300 activity in the TREM-1 promoter region, causing hypoacetylation of histones 3 and 4 [118].
During prostate cancer progression in TRAMP mice, Nrf2, the master regulator of cellular antioxidant defenses is epigenetically silenced. Furthermore, curcumin induces hypomethylation of Fanconi anemia (FANCF) promoter that leads to an increase in FANCF protein and gene expression in SiHa cells and a subsequent reduction in ovarian tumor cell proliferation [119]. In MV4-11 leukemia cells, curcumin induces global hypomethylation [120]. Based on molecular docking, curcumin covalently binds to the catalytic thiolate of DNMT1 with an IC50 of 30 nM treatment, causing DNA methylation to be inhibited. More recently, in vitro methylation assays showed that M.SssI, a DNMT1 analog, was inhibited by 50 µM of curcumin in SiHa ovarian cancer cells [108]. This epigenetic event may be related to NF-кB/Sp1 disrupting DNMT1’s promoter. Another study showed that at 5 µM concentration, curcumin also reversed CpG methylation in Neurog1, a cancer methylation marker known to be highly methylated and whose expression is disrupted in human prostate cancer cells [121]. Therefore, curcumin could be a DNMT inhibitor. Using a 2.5 µM dose of curcumin can also restore Nrf2 expression via promoter CpGs demethylation in TRAMP C1 prostate cancer cells [122].
The p300/CREB-binding protein-specific inhibitor curcumin inhibited acetyltransferase activity in HeLa cancer cells when applied at different concentrations [18]. Inhibiting p53 acetylation by histones and p300 leads to apoptosis. In LNCaP, prostate cancer cells treated with curcumin upregulates both phosphorylations at serine and acetylation of p53. The same natural compound induces acetylation of histone H3 and H4 at the same concentration applied for 4 h [123], suggesting that curcumin regulates apoptosis through global regulation of genes related to cell survival and/or apoptosis.
In vivo and ex vivo models of acute myeloid leukemia (AML), curcumin downregulated the expression of DNMT1. DNMT1, p65, and Sp1 become less active for binding to the promoter region of DNMT1 with curcumin. Furthermore, curcumin restored p15INK4b expression by hypomethylating its promoter, resulting in cell cycle arrest in the G1 phase and apoptosis. Curcumin suppressed tumor growth in mice implanted with the MV4-11 cell line of AML [124]. In a TRAMP mouse model of prostate cancer, curcumin inhibited tumor growth by reverting methylation of the Nrf2 promoter. In addition, curcumin inhibited JNK signaling and repressed the H3K4me3 epigenetic mark in LNCaP cells [99]. Curcumin and JQ-1 together suppress prostate cancer development. HT29 colon cancer cells were treated with curcumin and the colony formation and methylation of the DLEC1 promoter were inhibited. Curcumin induced specific changes in DNA methylation of a subset of genes involved in cell viability and proliferation in colorectal cancer cells as opposed to the global hypomethylation induced by 5-aza-CdR [125].
7.4. Epigenetic Modulation of Resveratrol in Cancer
Researchers are studying resveratrol (RVT) as a possible treatment for cancer, diabetes, cardiovascular diseases, neurodegenerative diseases, and metabolic diseases [126,127,128,129]. With a focus on epigenetic mechanisms, RVT has biological activities. SIRT1 is downregulated and NF-кB is upregulated in colon cancer (CC), but RVT reverses it [130]. Colon cell lines overexpressing SIRT1 showed antiproliferative effects [131]. By modulating cell cycle-regulating genes, enhancing apoptosis via p53 upregulation, and inhibiting anti-apoptotic genes, RVT inhibited colorectal cancer (CRC) cell proliferation, invasion, and metastasis [132]. In CRC cell lines, NF-кB was down-regulated after RVT. RVT inhibits nuclear translocation of NF-кB when SIRT1 mRNA levels are suppressed, suggesting RVT is SIRT1-dependent. RVT also induced premature senescence in human dermal fibroblasts, which is associated with DNA damage [133]. In vitro and in vivo, RVT has been shown to inhibit tumor growth by affecting the HDAC pathway [134,135,136]. RVT also downregulates MTA1 by acetylating p53 in PCa cells [114]. PTEN, a tumor suppressor, is deleted on chromosome 10 in prostate cancer or inactivated by MTA1/HDAC inhibitors. By abrogating MTA1/HDACs’ negative epigenetic effect, RVT may stimulate PTEN reactivation [137]. Downregulation of MTA1 by RVT triggers apoptosis in prostate cancer cells (PCa) by activating pro-apoptotic genes Bax and p21.
RVT induced apoptosis in Hodgkin lymphoma cells by inhibiting SIRT1 and hyperacetylating p53/FOXO3a [138]. Acyl-p53 and acetyl-FOXO3a levels were increased after RVT treatment, indicating that deacetylase inhibition is a critical step in the apoptosis of lymphoma cells [139]. Hepatoma cell lines treated with RVT showed dose-dependent antiproliferative effects [140]. Another study found the effects of RVT on growth and apoptosis in osteoblastoma cells. Compared with normal osteoblasts, osteosarcoma cells overexpressed the SIRT1 protein after RVT [31].
7.5. Epigenetic Modulation of EGCG in Cancer
Worldwide, green tea is widely consumed and believed to prevent cancer. Green tea contains catechins, a type of polyphenol. Most abundant and most biologically active is epigallocatechin-gallate (EGCG). The anticancer properties of EGCG have been extensively studied [141,142,143]. DNA methylation is inhibited by GTPs, which leads to hypomethylation and activation of epigenetically silenced genes. To evaluate the effect of GTPs on DNA methylation, several in vitro experiments have been carried out. A review article summarizes the effect of EGCG on DNA methylation in cell culture. There could be several reasons for the discrepancy between their findings and previous studies, including different methods of analysis, possible gene specificity or cell line specificity of EGCG, or an ineffective treatment method. According to Strassmann et al. [144], in some in vitro cell culture conditions, cellular effects caused by EGCG might be attributed to the oxidative stress caused by it. In an alkaline environment, EGCG undergoes autooxidation, forming homo- and heterodimers of EGCG and EGC, resulting in the formation of H2O2. There is no doubt that auto-oxidation occurs in cell culture and during digestion, but how much it happens depends on the medium [144]. H2O2 formation, however, was mostly ignored in cell culture experiments. As a result of GTP treatment, silenced genes are re-expressed in in vitro cell culture. DNA methylation will be changed by EGCG concentrations of 20–50 mol/L for 3–6 days. The level is far higher than what mice or humans can physiologically handle [145]. Therefore, GT interventions might not be suitable for therapeutic purposes, but they might affect DNA methylation long term.
Sulforaphane has been shown to enhance EGCG’s DNMT1-inhibitory effect, and at the same time, enhance hTERT inhibition [146]. GT treatment has anticarcinogenic properties in vitro, but EGCG’s limited chemical stability at alkaline pH under normal physiological conditions makes it difficult to translate to the clinic. EGCG and trichostatin A boost ER-α expression in ER-α-negative breast cancer cells [147]. Synthetic analogs have been developed to increase the chemical stability of EGCG, showing stronger anticancer activity and more stability and efficacy [148,149,150]. EGCG inhibits hTERT expression by inducing DNA hypomethylation and promoter deacetylation via DNMTs and histone acetylases, respectively [151]. EGCG and pEGCG inhibited the proliferation of human ER-positive in MCF-7 and ER-negative in MDA-MB-231 breast cancer cells, but not normal cells [152]. In addition, EGCG treatment inhibited the gene and protein expression of DNMT1, DNMT3a, and DNMT3b [153]. Treatment with EGCG led to a significant boost in RECK mRNA expression in oral carcinoma cells (SCC9 and HSC3) [154].
7.6. Epigenetic Modulation of Quercetin in Cancer
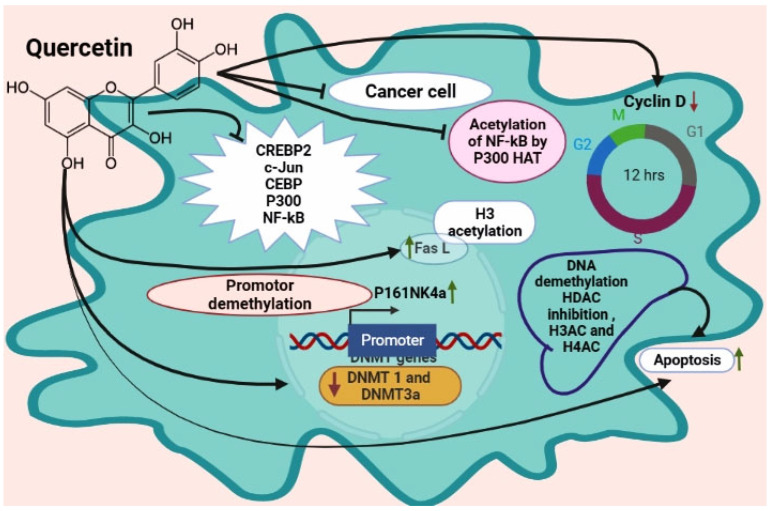
Flavonoids such as quercetin are found in apples, onions, red wine, and green tea [155,156]. Quercetin causes pro-apoptotic effects in tumor cells without affecting normal ones [157]. DNMT, HDAC, and HMT are all reduced by quercetin in a dose-dependent manner in human cervical cancer cells [158]. Quercetin seems to have an anticancer effect through epigenetic pathways [159,160]. A study used human acute myeloid leukemia cells and HL60 and U937 cell lines. Quercetin treatment eliminated DNMT1 and DNMT3a effects [161]. Quercetin treatment increases apoptosis by DNA demethylation, HDAC inhibition, and H3Ac and H4Ac enrichment in promoter regions of apoptotic genes.
Researchers found quercetin and sodium butyrate downregulated DNMT1, HDAC1, and cyclin D1 [162]. HDAC-NF-кB signaling is inhibited by this combination. Quercetin and curcumin combined treatment resulted in the sensitization of resistant prostate cancer cells to anti-androgen therapy [163]. However, quercetin inhibits HDAC1 and DNMT1 in hamster buccal pouch carcinoma [164], which has a pivotal role in cell survival, and invasiveness, angiogenesis, and cell proliferation. In addition, another researcher found quercetin blocks bound transactivators CREB2, C-Jun, C/EBP*, and NF-кB and the recruitment of the coactivator p300 (Figure 3) [142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160]. Quercetin inhibited the acetylation of NF-кB by p300 HAT. Another study found that quercetin inhibited colorectal cancer growth by activating p16INK4a induced by promoter demethylation [161]. Quercetin promotes cell death in leukemic HL-60 cells by activating FasL and H3 acetylation. Curcumin and quercetin together restored AR protein levels in androgen-receptor negative prostate cancer cells. DNMT decreased, resulting in global hypomethylation and mitochondrial depolarization, which induced apoptosis.
Figure 3.
Quercetin protects from cancer via an epigenetic mechanism. Quercetin inhibits NF-kB acetylation by p300 HAT; decreases cyclin D; increases Fas L via H3 acetylation; increases P161NK4a via promoter demethylation; decreases DNMT1 and DNMT3a; DNA methylation HDAC inhibition, H3AC, and H4AC, and increases apoptosis; inhibits the transcriptional CREBP2, c-Jun, CEBP; P300 and NF-kB.
8. Conclusions
Many kinds of cancer are triggered by epigenetic changes. Thus, epigenetic therapy is a valid strategy to stop cancer from spreading. There are a significant number of active molecules in natural compounds, including some that modulate gene expression epigenetically, which deserves more study. DNA aberrations that cause neoplastic transformation can be reversed by many dietary polyphenols. Oncogenes and tumor suppressor genes are epigenetic targets of polyphenol action, which are indirectly controlled by epigenetic enzymes such as DNMTs, HATs, and HDACs. Several polyphenols, including kaempferol, gallic acid, curcumin, resveratrol, EGCG, and quercetin, have been found to downregulate HDAC expression, mostly HDAC 1 and HDAC 3. Polyphenols regulate epigenetic machineries, which may prevent normal cells from turning into tumors. In this review, the majority of studies on polyphenols and epigenetic modifications used cancer cell culture models. The epigenetic modifying effect of these polyphenols on cancer needs further study in vivo.
Acknowledgments
Deanship of Scientific Research at King Faisal University, Saudi Arabia.
Author Contributions
Conceptualization, P.R. and E.A.A.; writing—original draft preparation, P.R., K.R., R.B.A., and V.V.; writing—review and editing, P.R., R.B.A., and S.A.A.; supervision, P.R. and E.A.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The author acknowledged the Deanship of Scientific Research, Vice Presidency at King Faisal University Saudi Arabia for financial support under the annual funding track (Grant 1028).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vandegehuchte M.B., Janssen C.R. Epigenetics and its implications for ecotoxicology. Ecotoxicology. 2011;20:607–624. doi: 10.1007/s10646-011-0634-0. [DOI] [PubMed] [Google Scholar]
- 2.Foolchand A. Ph.D. Dissertation. University of Kwazulu-Natal; Berea, South Africa: 2019. Methyl Picolinic Acid Plays a Role in Epigenetic Modifications in Human HepG2 Liver Cells. [Google Scholar]
- 3.Jablonka E., Lamb M.J. The expanded evolutionary synthesis—A response to Godfrey-Smith, Haig, and West-Eberhard. Biol. Philos. 2007;22:453–472. doi: 10.1007/s10539-007-9064-z. [DOI] [Google Scholar]
- 4.Deans C., Maggert K.A. What do you mean, “epigenetic”? Genetics. 2015;199:887–896. doi: 10.1534/genetics.114.173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maccani M.A., Marsit C.J. Epigenetics in the placenta. Am. J. Reprod. Immunol. 2009;62:78–89. doi: 10.1111/j.1600-0897.2009.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiesel-Motiuk N., Assaraf Y.G. The key roles of the lysine acetyltransferases KAT6A and KAT6B in physiology and pathology. Drug Resist. Updat. 2020;53:100729. doi: 10.1016/j.drup.2020.100729. [DOI] [PubMed] [Google Scholar]
- 7.Tost J. DNA methylation: An introduction to the biology and the disease-associated changes of a promising biomarker. Mol. Biotechnol. 2010;44:71–81. doi: 10.1007/s12033-009-9216-2. [DOI] [PubMed] [Google Scholar]
- 8.Kim S., Kaang B.-K. Epigenetic regulation and chromatin remodeling in learning and memory. Exp. Mol. Med. 2017;49:e281. doi: 10.1038/emm.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastar I., Marjanovic J., Stone R.C., Chen V., Burgess J.L., Mervis J.S., Tomic-Canic M. Epigenetic regulation of cellular functions in wound healing. Exp. Dermatol. 2021;30:1073–1089. doi: 10.1111/exd.14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar H., Chaudhary A., Singh A., Sukhija N., Panwar A., Saravanan K., Bhaladhare A., Kaisa K., Panigrahi M. A review on epigenetics: Manifestations, modifications, methods & challenges. J. Entomol. Zool. Stud. 2020;8:1–6. [Google Scholar]
- 11.Andersen G.B., Tost J. A summary of the biological processes, disease-associated changes, and clinical applications of DNA methylation. DNA Methylation Protoc. 2018;1708:3–30. doi: 10.1007/978-1-4939-7481-8_1. [DOI] [PubMed] [Google Scholar]
- 12.Castilho R.M., Squarize C.H., Almeida L.O. Epigenetic modifications and head and neck cancer: Implications for tumor progression and resistance to therapy. Int. J. Mol. Sci. 2017;18:1506. doi: 10.3390/ijms18071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biswas S., Rao C.M. Epigenetics in cancer: Fundamentals and beyond. Pharmacol. Ther. 2017;173:118–134. doi: 10.1016/j.pharmthera.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Johnson C., Warmoes M.O., Shen X., Locasale J.W. Epigenetics and cancer metabolism. Cancer Lett. 2015;356:309–314. doi: 10.1016/j.canlet.2013.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinnaird A., Zhao S., Wellen K.E., Michelakis E.D. Metabolic control of epigenetics in cancer. Nat. Rev. Cancer. 2016;16:694–707. doi: 10.1038/nrc.2016.82. [DOI] [PubMed] [Google Scholar]
- 16.Shankar E., Kanwal R., Candamo M., Gupta S. Proceedings of the Seminars in Cancer Biology. Elsevier; Amsterdam, The Netherlands: 2016. Dietary phytochemicals as epigenetic modifiers in cancer: Promise and challenges; pp. 82–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sapienza C., Issa J.-P. Diet, nutrition, and cancer epigenetics. Annu. Rev. Nutr. 2016;36:665–681. doi: 10.1146/annurev-nutr-121415-112634. [DOI] [PubMed] [Google Scholar]
- 18.Carlos-Reyes Á., López-González J.S., Meneses-Flores M., Gallardo-Rincón D., Ruíz-García E., Marchat L.A., Astudillo-De La Vega H., Hernández de la Cruz O.N., López-Camarillo C. Dietary compounds as epigenetic modulating agents in cancer. Front. Genet. 2019;10:79. doi: 10.3389/fgene.2019.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lévesque S., Pol J.G., Ferrere G., Galluzzi L., Zitvogel L., Kroemer G. Trial watch: Dietary interventions for cancer therapy. Oncoimmunology. 2019;8:e1591878. doi: 10.1080/2162402X.2019.1591878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salisbury D., Bronas U. Reactive Oxygen and Nitrogen Species: Impact on Endothelial Dysfunction. Nurs. Res. 2015;64:53–66. doi: 10.1097/NNR.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H., Tsao R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-inflammatory Effects. Curr. Opin. Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- 22.Reis J.F., Monteiro V.V.S., De Souza Gomes R., Do Carmo M.M., Da Costa G.V., Ribera P.C., Monteiro M.C. Action Mechanism and Cardiovascular Effect of Anthocyanins: A Systematic Review of Animal and Human Studies. J. Transl. Med. 2016;14:315. doi: 10.1186/s12967-016-1076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudrapal M., Khairnar S.J., Khan J., Dukhyil A.B., Ansari M.A., Alomary M.N., Alshabrmi F.M., Palai S., Deb P.K., Devi R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism (s) of Action. Front. Pharmacol. 2022;13:806470. doi: 10.3389/fphar.2022.806470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Federico A., Morgillo F., Tuccillo C., Ciardiello F., Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 25.Khansari N., Shakiba Y., Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 26.Azqueta A., Collins A. Polyphenols and DNA Damage: A Mixed Blessing. Nutrients. 2016;8:785. doi: 10.3390/nu8120785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papiez M.A. The influence of curcumin and (−)-epicatechin on the genotoxicity and myelosuppression induced by etoposide in bone marrow cells of male rats. Drug Chem. Toxicol. 2013;36:93–101. doi: 10.3109/01480545.2012.726626. [DOI] [PubMed] [Google Scholar]
- 28.García-Lafuente A., Guillamón E., Villares A., Rostagno M.A., Martínez J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 29.Hazel T.G., Nathans D., Lau L.F. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proc. Natl. Acad. Sci. USA. 1988;85:8444–8448. doi: 10.1073/pnas.85.22.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamaraj S., Vinodhkumar R., Anandakumar P., Jagan S., Ramakrishnan G., Devaki T. The effects of quercetin on antioxidant status and tumor markers in the lung and serum of mice treated with benzo (a) pyrene. Biol. Pharm. Bull. 2007;30:2268–2273. doi: 10.1248/bpb.30.2268. [DOI] [PubMed] [Google Scholar]
- 31.Shankar S., Ganapathy S., Hingorani S.R., Srivastava R.K. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front.Biosci. J. Virtual Libr. 2008;13:440–452. doi: 10.2741/2691. [DOI] [PubMed] [Google Scholar]
- 32.Sharma V., Rao L.J.M. A thought on the biological activities of black tea. Crit. Rev. Food Sci. Nutr. 2009;49:379–404. doi: 10.1080/10408390802068066. [DOI] [PubMed] [Google Scholar]
- 33.Kim S.Y., Park C., Jang H.-J., Kim B.-O., Bae H.-W., Chung I.-Y., Kim E.S., Cho Y.-H. Antibacterial strategies inspired by the oxidative stress and response networks. J. Microbiol. 2019;57:203–212. doi: 10.1007/s12275-019-8711-9. [DOI] [PubMed] [Google Scholar]
- 34.Wang S., Moustaid-Moussa N., Chen L., Mo H., Shastri A., Su R., Bapat P., Kwun I., Shen C.-L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014;25:1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holásková I., Elliott M., Hanson M.L., Schafer R., Barnett J.B. Prenatal cadmium exposure produces persistent changes to thymus and spleen cell phenotypic repertoire as well as the acquired immune response. Toxicol. Appl. Pharmacol. 2012;265:181–189. doi: 10.1016/j.taap.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewinska A., Wnuk M., Grabowska W., Zabek T., Semik E., Sikora E., Bielak-Zmijewska A. Curcumin induces oxidation-dependent cell cycle arrest mediated by sirt7 inhibition of rdna transcription in human aortic smooth muscle cells. Toxicol. Lett. 2015;233:227–238. doi: 10.1016/j.toxlet.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Majidinia M., Bishayee A., Yousefi B. Polyphenols: Major regulators of key components of DNA damage response in cancer. DNA Repair. 2019;82:102679. doi: 10.1016/j.dnarep.2019.102679. [DOI] [PubMed] [Google Scholar]
- 39.Rajendran P., Alzahrani A.M., Rengarajan T., Kaushik R., Arulselvan P., Umamaheswari A. Frontiers in Anti-Cancer Drug Discovery. Volume 10. Bentham Science Publishers; Singapore: 2019. Polyphenols and Cancer; p. 62. [Google Scholar]
- 40.Locke W.J., Guanzon D., Ma C., Liew Y.J., Duesing K.R., Fung K.Y., Ross J.P. DNA methylation cancer biomarkers: Translation to the clinic. Front. Genet. 2019;10:1150. doi: 10.3389/fgene.2019.01150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch A., Joosten S.C., Feng Z., de Ruijter T.C., Draht M.X., Melotte V., Smits K.M., Veeck J., Herman J.G., Van Neste L. Analysis of DNA methylation in cancer: Location revisited. Nat. Rev. Clin. Oncol. 2018;15:459–466. doi: 10.1038/s41571-018-0004-4. [DOI] [PubMed] [Google Scholar]
- 42.Paska A.V., Hudler P. Aberrant methylation patterns in cancer: A clinical view. Biochem. Med. 2015;25:161–176. doi: 10.11613/BM.2015.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Udali S., Guarini P., Ruzzenente A., Ferrarini A., Guglielmi A., Lotto V., Tononi P., Pattini P., Moruzzi S., Campagnaro T. DNA methylation and gene expression profiles show novel regulatory pathways in hepatocellular carcinoma. Clin. Epigenetics. 2015;7:1–13. doi: 10.1186/s13148-015-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu R.-h., Wei W., Krawczyk M., Wang W., Luo H., Flagg K., Yi S., Shi W., Quan Q., Li K. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat. Mater. 2017;16:1155–1161. doi: 10.1038/nmat4997. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Petropoulos S., Liu J., Cheishvili D., Zhou R., Dymov S., Li K., Li N., Szyf M. The signature of liver cancer in immune cells DNA methylation. Clin. Epigenetics. 2018;10:1–17. doi: 10.1186/s13148-017-0436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hao X., Luo H., Krawczyk M., Wei W., Wang W., Wang J., Flagg K., Hou J., Zhang H., Yi S. DNA methylation markers for diagnosis and prognosis of common cancers. Proc. Natl. Acad. Sci. USA. 2017;114:7414–7419. doi: 10.1073/pnas.1703577114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villanueva A., Portela A., Sayols S., Battiston C., Hoshida Y., Méndez-González J., Imbeaud S., Letouzé E., Hernandez-Gea V., Cornella H. DNA methylation-based prognosis and epidrivers in hepatocellular carcinoma. Hepatology. 2015;61:1945–1956. doi: 10.1002/hep.27732. [DOI] [PubMed] [Google Scholar]
- 48.Li L., Li W. Epithelial–mesenchymal transition in human cancer: Comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacol. Ther. 2015;150:33–46. doi: 10.1016/j.pharmthera.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Feinberg A.P., Koldobskiy M.A., Göndör A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat. Rev. Genet. 2016;17:284–299. doi: 10.1038/nrg.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qian Z., Shen Q., Yang X., Qiu Y., Zhang W. The role of extracellular vesicles: An epigenetic view of the cancer microenvironment. BioMed Res. Int. 2015;2015:649161. doi: 10.1155/2015/649161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ilyas M. Next-generation sequencing in diagnostic pathology. Pathobiology. 2017;84:292–305. doi: 10.1159/000480089. [DOI] [PubMed] [Google Scholar]
- 52.Kurumizaka H., Kujirai T., Takizawa Y. Contributions of histone variants in nucleosome structure and function. J. Mol. Biol. 2021;433:166678. doi: 10.1016/j.jmb.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 53.Bilokapic S., Strauss M., Halic M. Histone octamer rearranges to adapt to DNA unwrapping. Nat. Struct. Mol. Biol. 2018;25:101–108. doi: 10.1038/s41594-017-0005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou T., Hashiya F., Wei Y., Yu Z., Pandian G.N., Sugiyama H. Direct observation of H3–H4 octasome by high-speed AFM. Chem. A Eur. J. 2018;24:15998–16002. doi: 10.1002/chem.201804010. [DOI] [PubMed] [Google Scholar]
- 55.Ramazi S., Allahverdi A., Zahiri J. Evaluation of post-translational modifications in histone proteins: A review on histone modification defects in developmental and neurological disorders. J. Biosci. 2020;45:1–29. doi: 10.1007/s12038-020-00099-2. [DOI] [PubMed] [Google Scholar]
- 56.Millán-Zambrano G., Burton A., Bannister A.J., Schneider R. Histone post-translational modifications—Cause and consequence of genome function. Nat. Rev. Genet. 2022;23:1–18. doi: 10.1038/s41576-022-00468-7. [DOI] [PubMed] [Google Scholar]
- 57.van Wijnen A.J., Westendorf J.J. Epigenetics as a new frontier in orthopedic regenerative medicine and oncology. J. Orthop. Res. 2019;37:1465–1474. doi: 10.1002/jor.24305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borkiewicz L. Histone 3 Lysine 27 Trimethylation Signature in Breast Cancer. Int. J. Mol. Sci. 2021;22:12853. doi: 10.3390/ijms222312853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimizu J., Kawano F. Exercise-induced histone H3 trimethylation at lysine 27 facilitates the adaptation of skeletal muscle to exercise in mice. J. Physiol. 2022;600:3331–3353. doi: 10.1113/JP282917. [DOI] [PubMed] [Google Scholar]
- 60.Lu K., Tao H., Si X., Chen Q. The histone H3 lysine 4 presenter WDR5 as an oncogenic protein and novel epigenetic target in cancer. Front. Oncol. 2018;8:502. doi: 10.3389/fonc.2018.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai C.-C., Chien M.-N., Chang Y.-C., Lee J.-J., Dai S.-H., Cheng S.-P. Overexpression of histone H3 lysine 27 trimethylation is associated with aggressiveness and dedifferentiation of thyroid cancer. Endocr. Pathol. 2019;30:305–311. doi: 10.1007/s12022-019-09586-1. [DOI] [PubMed] [Google Scholar]
- 62.Blecua P., Martinez-Verbo L., Esteller M. The DNA methylation landscape of hematological malignancies: An update. Mol. Oncol. 2020;14:1616–1639. doi: 10.1002/1878-0261.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou M., Yuan M., Zhang M., Lei C., Aras O., Zhang X., An F. Combining histone deacetylase inhibitors (HDACis) with other therapies for cancer therapy. Eur. J. Med. Chem. 2021;226:113825. doi: 10.1016/j.ejmech.2021.113825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banik D., Moufarrij S., Villagra A. Immunoepigenetics combination therapies: An overview of the role of HDACs in cancer immunotherapy. Int. J. Mol. Sci. 2019;20:2241. doi: 10.3390/ijms20092241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roca M.S., Di Gennaro E., Budillon A. Implication for cancer stem cells in solid cancer chemo-resistance: Promising therapeutic strategies based on the use of HDAC inhibitors. J. Clin. Med. 2019;8:912. doi: 10.3390/jcm8070912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Movafagh S., Munson A. Epigenetics of Cancer Prevention. Elsevier; Amsterdam, The Netherlands: 2019. Histone deacetylase inhibitors in cancer prevention and therapy; pp. 75–105. [Google Scholar]
- 67.Rojas-Espinosa O., Moreno-García S., Arce-Paredes P., Becerril-Villanueva E., Juárez-Ortega M. Effect of dialyzable leukocyte extract, sodium butyrate, and valproic acid in the development of anergy in murine leprosy. Int. J. Mycobacteriology. 2020;9:268. doi: 10.4103/ijmy.ijmy_31_20. [DOI] [PubMed] [Google Scholar]
- 68.Perona M., Thomasz L., Rossich L., Rodriguez C., Pisarev M.A., Rosemblit C., Cremaschi G.A., Dagrosa M.A., Juvenal G.J. Radiosensitivity enhancement of human thyroid carcinoma cells by the inhibitors of histone deacetylase sodium butyrate and valproic acid. Mol. Cell. Endocrinol. 2018;478:141–150. doi: 10.1016/j.mce.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 69.Sanaei M., Kavoosi F. Effect of Valproic Acid on the Class I Histone Deacetylase 1, 2 and 3, Tumor Suppressor Genes p21WAF1/CIP1 and p53, and Intrinsic Mitochondrial Apoptotic Pathway, Pro-(Bax, Bak, and Bim) and anti-(Bcl-2, Bcl-xL, and Mcl-1) Apoptotic Genes Expression, Cell Viability, and Apoptosis Induction in Hepatocellular Carcinoma HepG2 Cell Line. Asian Pac. J. Cancer Prev. 2021;22:89–95. doi: 10.31557/APJCP.2021.22.S1.89. [DOI] [PubMed] [Google Scholar]
- 70.Elnozahi N.A., Abd ELAziz E.A., Helmy M.W., Bistawroos A.E. Modulatory Effect of Sodium Butyrate on Anticancer Activity of Abemaciclib in MDA-MB-231 Human Breast Cancer Cells. Research Square; Durham, NC, USA: 2022. [DOI] [Google Scholar]
- 71.Sanaei M., Kavoosi F., Moezzi M.A. Effect of 5′-fluoro-2′-deoxycytidine and sodium butyrate on the genes of the intrinsic apoptotic pathway, p21, p53, cell viability, and apoptosis in human hepatocellular carcinoma cell lines. Iran. J. Pediatr. Hematol. Oncol. 2021;11:216–230. doi: 10.18502/ijpho.v11i4.7163. [DOI] [Google Scholar]
- 72.Psilopatis I., Pergaris A., Giaginis C., Theocharis S. Histone Deacetylase Inhibitors: A Promising Therapeutic Alternative for Endometrial Carcinoma. Dis. Markers. 2021;2021:1–9. doi: 10.1155/2021/7850688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garmpis N., Damaskos C., Garmpi A., Georgakopoulou V.E., Sarantis P., Antoniou E.A., Karamouzis M.V., Nonni A., Schizas D., Diamantis E. Histone deacetylase inhibitors in the treatment of hepatocellular carcinoma: Current evidence and future opportunities. J. Pers. Med. 2021;11:223. doi: 10.3390/jpm11030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsilimigras D.I., Ntanasis-Stathopoulos I., Moris D., Spartalis E., Pawlik T.M. Histone deacetylase inhibitors in hepatocellular carcinoma: A therapeutic perspective. Surg. Oncol. 2018;27:611–618. doi: 10.1016/j.suronc.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 75.Zhang H., Zhao X., Liu H., Jin H., Ji Y. Trichostatin A inhibits proliferation of PC3 prostate cancer cells by disrupting the EGFR pathway. Oncol. Lett. 2019;18:687–693. doi: 10.3892/ol.2019.10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jang Y.G., Hwang K.A., Choi K.C. Rosmarinic Acid, a Component of Rosemary Tea, Induced the Cell Cycle Arrest and Apoptosis through Modulation of HDAC2 Expression in Prostate Cancer Cell Lines. Nutrients. 2018;10:1784. doi: 10.3390/nu10111784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hontecillas-Prieto L., Flores-Campos R., Silver A., de Álava E., Hajji N., García-Domínguez D.J. Synergistic Enhancement of Cancer Therapy Using HDAC Inhibitors: Opportunity for Clinical Trials. Front. Genet. 2020;11:578011. doi: 10.3389/fgene.2020.578011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaidya G.N., Rana P., Venkatesh A., Chatterjee D.R., Contractor D., Satpute D.P., Nagpure M., Jain A., Kumar D. Paradigm shift of “classical” HDAC inhibitors to “hybrid” HDAC inhibitors in therapeutic interventions. Eur. J. Med. Chem. 2021;209:112844. doi: 10.1016/j.ejmech.2020.112844. [DOI] [PubMed] [Google Scholar]
- 79.Hazafa A., Rehman K.U., Jahan N., Jabeen Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer. 2020;72:386–397. doi: 10.1080/01635581.2019.1637006. [DOI] [PubMed] [Google Scholar]
- 80.Davatgaran-Taghipour Y., Masoomzadeh S., Farzaei M.H., Bahramsoltani R., Karimi-Soureh Z., Rahimi R., Abdollahi M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017;12:2689–2702. doi: 10.2147/IJN.S131973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williamson G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017;42:226–235. doi: 10.1111/nbu.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rana A., Samtiya M., Dhewa T., Mishra V., Aluko R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022;13:e14264. doi: 10.1111/jfbc.14264. [DOI] [PubMed] [Google Scholar]
- 84.Del Rio D., Costa L.G., Lean M.E., Crozier A. Polyphenols and health: What compounds are involved? Nutr. Metab. Cardiovasc. Dis. 2010;20:1–6. doi: 10.1016/j.numecd.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 85.Rajendran P., Rengarajan T., Nandakumar N., Divya H., Nishigaki I. Mangiferin in cancer chemoprevention and treatment: Pharmacokinetics and molecular targets. J. Recept. Signal Transduct. Res. 2015;35:76–84. doi: 10.3109/10799893.2014.931431. [DOI] [PubMed] [Google Scholar]
- 86.Rajendran P., Rengarajan T., Nishigaki I., Ekambaram G., Sakthisekaran D. Potent chemopreventive effect of mangiferin on lung carcinogenesis in experimental Swiss albino mice. J. Cancer Res. Ther. 2014;10:1033–1039. doi: 10.4103/0973-1482.137966. [DOI] [PubMed] [Google Scholar]
- 87.Drețcanu G., Iuhas C.I., Diaconeasa Z. The Involvement of Natural Polyphenols in the Chemoprevention of Cervical Cancer. Int. J. Mol. Sci. 2021;22:8812. doi: 10.3390/ijms22168812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shah D., Gandhi M., Kumar A., Cruz-Martins N., Sharma R., Nair S. Current insights into epigenetics, noncoding RNA interactome and clinical pharmacokinetics of dietary polyphenols in cancer chemoprevention. Crit. Rev. Food Sci. Nutr. 2021:1–37. doi: 10.1080/10408398.2021.1968786. [DOI] [PubMed] [Google Scholar]
- 89.Rajendran P., Rengarajan T., Nandakumar N., Palaniswami R., Nishigaki Y., Nishigaki I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur. J. Med. Chem. 2014;86:103–112. doi: 10.1016/j.ejmech.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 90.Amjad E., Sokouti B., Asnaashari S. A systematic review of anti-cancer roles and mechanisms of kaempferol as a natural compound. Cancer Cell Int. 2022;22:260. doi: 10.1186/s12935-022-02673-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mohan Shankar G., Swetha M., Keerthana C.K., Rayginia T.P., Anto R.J. Cancer Chemoprevention: A Strategic Approach Using Phytochemicals. Front. Pharmacol. 2021;12:809308. doi: 10.3389/fphar.2021.809308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma N., Biswas S., Al-Dayan N., Alhegaili A.S., Sarwat M. Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma. Antioxidants. 2021;10:1419. doi: 10.3390/antiox10091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qiu W., Lin J., Zhu Y., Zhang J., Zeng L., Su M., Tian Y. Kaempferol Modulates DNA Methylation and Downregulates DNMT3B in Bladder Cancer. Cell. Physiol. Biochem. 2017;41:1325–1335. doi: 10.1159/000464435. [DOI] [PubMed] [Google Scholar]
- 94.Lu L., Wang Y., Ou R., Feng Q., Ji L., Zheng H., Guo Y., Qi X., Kong A.N., Liu Z. DACT2 Epigenetic Stimulator Exerts Dual Efficacy for Colorectal Cancer Prevention and Treatment. Pharmacol. Res. 2018;129:318–328. doi: 10.1016/j.phrs.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 95.Kim T.H., Ku S.-K., Lee I.-C., Bae J.-S. Anti-inflammatory effects of kaempferol-3-O-sophoroside in human endothelial cells. Inflamm. Res. 2012;3:217–224. doi: 10.1007/s00011-011-0403-9. [DOI] [PubMed] [Google Scholar]
- 96.Kim T.W., Lee S.Y., Kim M., Cheon C., Ko S.G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018;9:875. doi: 10.1038/s41419-018-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Imran M., Salehi B., Sharifi-Rad J., Aslam Gondal T., Saeed F., Imran A., Shahbaz M., Tsouh Fokou P.V., Umair Arshad M., Khan H., et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules. 2019;24:2277. doi: 10.3390/molecules24122277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhosale P.B., Ha S.E., Vetrivel P., Kim H.H., Kim S.M., Kim G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020;9:7619–7631. doi: 10.21037/tcr-20-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang Y., Pei J., Zheng Y., Miao Y.J., Duan B.Z., Huang L.F. Gallic Acid: A Potential Anti-Cancer Agent. Chin. J. Integr. Med. 2022;28:661–671. doi: 10.1007/s11655-021-3345-2. [DOI] [PubMed] [Google Scholar]
- 100.Shi C.J., Zheng Y.B., Pan F.F., Zhang F.W., Zhuang P., Fu W.M. Gallic Acid Suppressed Tumorigenesis by an LncRNA MALAT1-Wnt/β-Catenin Axis in Hepatocellular Carcinoma. Front. Pharmacol. 2021;12:708967. doi: 10.3389/fphar.2021.708967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ko E.B., Jang Y.G., Kim C.W., Go R.E., Lee H.K., Choi K.C. Gallic Acid Hindered Lung Cancer Progression by Inducing Cell Cycle Arrest and Apoptosis in A549 Lung Cancer Cells via PI3K/Akt Pathway. Biomol. Ther. 2022;30:151–161. doi: 10.4062/biomolther.2021.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aglan H.A., Ahmed H.H., El-Toumy S.A., Mahmoud N.S. Gallic acid against hepatocellular carcinoma: An integrated scheme of the potential mechanisms of action from in vivo study. Tumour Biol. 2017;39:1010428317699127. doi: 10.1177/1010428317699127. [DOI] [PubMed] [Google Scholar]
- 103.Lima K.G., Krause G.C., Schuster A.D., Catarina A.V., Basso B.S., De Mesquita F.C., Pedrazza L., Marczak E.S., Martha B.A., Nunes F.B., et al. Gallic acid reduces cell growth by induction of apoptosis and reduction of IL-8 in HepG2 cells. Biomed. Pharmacother. 2016;84:1282–1290. doi: 10.1016/j.biopha.2016.10.048. [DOI] [PubMed] [Google Scholar]
- 104.Dorniani D., Saifullah B., Barahuie F., Arulselvan P., Hussein M.Z., Fakurazi S., Twyman L.J. Graphene Oxide-Gallic Acid Nanodelivery System for Cancer Therapy. Nanoscale Res. Lett. 2016;11:491. doi: 10.1186/s11671-016-1712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Silva I.C., Polaquini C.R., Regasini L.O., Ferreira H., Pavan F.R. Evaluation of cytotoxic, apoptotic, mutagenic, and chemopreventive activities of semi-synthetic esters of gallic acid. Food Chem. Toxicol. 2017;105:300–307. doi: 10.1016/j.fct.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 106.Weng Y.P., Hung P.F., Ku W.Y., Chang C.Y., Wu B.H., Wu M.H., Yao J.Y., Yang J.R., Lee C.H. The inhibitory activity of gallic acid against DNA methylation: Application of gallic acid on epigenetic therapy of human cancers. Oncotarget. 2018;9:361–374. doi: 10.18632/oncotarget.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bhattacharya R., Chatterjee R., Mandal A.K.A., Mukhopadhyay A., Basu S., Giri A.K., Chatterji U., Bhattacharjee P. Theaflavin-Containing Black Tea Extract: A Potential DNA Methyltransferase Inhibitor in Human Colon Cancer Cells and Ehrlich Ascites Carcinoma-Induced Solid Tumors in Mice. Nutr. Cancer. 2021;73:2447–2459. doi: 10.1080/01635581.2020.1828943. [DOI] [PubMed] [Google Scholar]
- 108.Jang Y.G., Ko E.B., Choi K.C. Gallic acid, a phenolic acid, hinders the progression of prostate cancer by inhibition of histone deacetylase 1 and 2 expression. J. Nutr. Biochem. 2020;84:108444. doi: 10.1016/j.jnutbio.2020.108444. [DOI] [PubMed] [Google Scholar]
- 109.Lee W., Lee S.Y., Son Y.J., Yun J.M. Gallic Acid Decreases Inflammatory Cytokine Secretion Through Histone Acetyltransferase/Histone Deacetylase Regulation in High Glucose-Induced Human Monocytes. J. Med. Food. 2015;18:793–801. doi: 10.1089/jmf.2014.3342. [DOI] [PubMed] [Google Scholar]
- 110.Soflaei S.S., Momtazi-Borojeni A.A., Majeed M., Derosa G., Maffioli P., Sahebkar A. Curcumin: A Natural Pan-HDAC Inhibitor in Cancer. Curr. Pharm. Des. 2018;24:123–129. doi: 10.2174/1381612823666171114165051. [DOI] [PubMed] [Google Scholar]
- 111.Lee S.J., Krauthauser C., Maduskuie V., Fawcett P.T., Olson J.M., Rajasekaran S.A. Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC Cancer. 2011;11:144. doi: 10.1186/1471-2407-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu J., Peng Y., Wu L.C., Xie Z., Deng Y., Hughes T., He S., Mo X., Chiu M., Wang Q.E., et al. Curcumin down-regulates DNA methyltransferase 1 and plays an anti-leukemic role in acute myeloid leukemia. PLoS ONE. 2013;8:e55934. doi: 10.1371/journal.pone.0055934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Teiten M.H., Dicato M., Diederich M. Curcumin as a regulator of epigenetic events. Mol. Nutr. Food Res. 2013;57:1619–1629. doi: 10.1002/mnfr.201300201. [DOI] [PubMed] [Google Scholar]
- 114.Soltani B., Ghaemi N., Sadeghizadeh M., Najafi F. Curcumin confers protection to irradiated THP-1 cells while its nanoformulation sensitizes these cells via apoptosis induction. Cell Biol. Toxicol. 2016;32:543–561. doi: 10.1007/s10565-016-9354-9. [DOI] [PubMed] [Google Scholar]
- 115.Kang S.K., Cha S.H., Jeon H.G. Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 2006;15:165–174. doi: 10.1089/scd.2006.15.165. [DOI] [PubMed] [Google Scholar]
- 116.Yuan Z., Syed M.A., Panchal D., Rogers D., Joo M., Sadikot R.T. Curcumin mediated epigenetic modulation inhibits TREM-1 expression in response to lipopolysaccharide. Int. J. Biochem. Cell Biol. 2012;44:2032–2043. doi: 10.1016/j.biocel.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 117.Parashar G., Parashar N.C., Capalash N. Curcumin causes promoter hypomethylation and increased expression of FANCF gene in SiHa cell line. Mol. Cell. Biochem. 2012;365:29–35. doi: 10.1007/s11010-012-1240-z. [DOI] [PubMed] [Google Scholar]
- 118.Shu L., Khor T.O., Lee J.H., Boyanapalli S.S., Huang Y., Wu T.Y., Saw C.L., Cheung K.L., Kong A.N. Epigenetic CpG demethylation of the promoter and reactivation of the expression of Neurog1 by curcumin in prostate LNCaP cells. AAPS J. 2011;13:606–614. doi: 10.1208/s12248-011-9300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang C., Su Z.Y., Khor T.O., Shu L., Kong A.N. Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP C1 cells through epigenetic regulation. Biochem. Pharmacol. 2013;85:1398–1404. doi: 10.1016/j.bcp.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kutluay S.B., Doroghazi J., Roemer M.E., Triezenberg S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology. 2008;373:239–247. doi: 10.1016/j.virol.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cui L., Miao J., Cui L. Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: Inhibition of histone acetylation and generation of reactive oxygen species. Antimicrob. Agents Chemother. 2007;51:488–494. doi: 10.1128/AAC.01238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao W., Zhou X., Qi G., Guo Y. Curcumin suppressed the prostate cancer by inhibiting JNK pathways via epigenetic regulation. J. Biochem. Mol. Toxicol. 2018;32:e22049. doi: 10.1002/jbt.22049. [DOI] [PubMed] [Google Scholar]
- 123.Fernandes G.F.S., Silva G.D.B., Pavan A.R., Chiba D.E., Chin C.M., Dos Santos J.L. Epigenetic Regulatory Mechanisms Induced by Resveratrol. Nutrients. 2017;9:1201. doi: 10.3390/nu9111201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Imperador C.H.L., Scarim C.B., Bosquesi P.L., Lopes J.R., Cardinalli Neto A., Giarolla J., Ferreira E.I., Dos Santos J.L., Chin C.M. Resveratrol and Curcumin for Chagas Disease Treatment-A Systematic Review. Pharmaceuticals. 2022;15:609. doi: 10.3390/ph15050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Albani D., Polito L., Signorini A., Forloni G. Neuroprotective properties of resveratrol in different neurodegenerative disorders. BioFactors. 2010;36:370–376. doi: 10.1002/biof.118. [DOI] [PubMed] [Google Scholar]
- 126.Wahab A., Gao K., Jia C., Zhang F., Tian G., Murtaza G., Chen J. Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules. 2017;22:1329. doi: 10.3390/molecules22081329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carrizzo A., Forte M., Damato A., Trimarco V., Salzano F., Bartolo M., Maciag A., Puca A.A., Vecchione C. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem. Toxicol. 2013;61:215–226. doi: 10.1016/j.fct.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 128.Gong W.H., Zhao N., Zhang Z.M., Zhang Y.X., Yan L., Li J.B. The inhibitory effect of resveratrol on COX-2 expression in human colorectal cancer: A promising therapeutic strategy. Eur. Rev. Med. Pharmacol. Sci. 2017;21:1136–1143. [PubMed] [Google Scholar]
- 129.Afrin S., Giampieri F., Gasparrini M., Forbes-Hernández T.Y., Cianciosi D., Reboredo-Rodriguez P., Zhang J., Manna P.P., Daglia M., Atanasov A.G., et al. Dietary phytochemicals in colorectal cancer prevention and treatment: A focus on the molecular mechanisms involved. Biotechnol. Adv. 2020;38:107322. doi: 10.1016/j.biotechadv.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 130.Vernousfaderani E.K., Akhtari N., Rezaei S., Rezaee Y., Shiranirad S., Mashhadi M., Hashemi A., Khankandi H.P., Behzad S. Resveratrol and Colorectal Cancer: A Molecular Approach to Clinical Researches. Curr. Top. Med. Chem. 2021;21:2634–2646. doi: 10.2174/1568026621666211105093658. [DOI] [PubMed] [Google Scholar]
- 131.Moreira H., Szyjka A., Grzesik J., Pelc K., Żuk M., Kulma A., Emhemmed F., Muller C.D., Gąsiorowski K., Barg E. Celastrol and Resveratrol Modulate SIRT Genes Expression and Exert Anticancer Activity in Colon Cancer Cells and Cancer Stem-like Cells. Cancers. 2022;14:1372. doi: 10.3390/cancers14061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Venturelli S., Berger A., Böcker A., Busch C., Weiland T., Noor S., Leischner C., Schleicher S., Mayer M., Weiss T.S., et al. Resveratrol as a pan-HDAC inhibitor alters the acetylation status of histone [corrected] proteins in human-derived hepatoblastoma cells. PLoS ONE. 2013;8:e73097. doi: 10.1371/annotation/5b9a8614-1009-40ca-b90b-db817fe445c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dhar S., Kumar A., Li K., Tzivion G., Levenson A.S. Resveratrol regulates PTEN/Akt pathway through inhibition of MTA1/HDAC unit of the NuRD complex in prostate cancer. Biochim. Biophys. Acta. 2015;1853:265–275. doi: 10.1016/j.bbamcr.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 134.Bankole O., Scambi I., Parrella E., Muccilli M., Bonafede R., Turano E., Pizzi M., Mariotti R. Beneficial and Sexually Dimorphic Response to Combined HDAC Inhibitor Valproate and AMPK/SIRT1 Pathway Activator Resveratrol in the Treatment of ALS Mice. Int. J. Mol. Sci. 2022;23:1047. doi: 10.3390/ijms23031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Han G., Xia J., Gao J., Inagaki Y., Tang W., Kokudo N. Anti-tumor effects and cellular mechanisms of resveratrol. Drug Discov. Ther. 2015;9:1–12. doi: 10.5582/ddt.2015.01007. [DOI] [PubMed] [Google Scholar]
- 136.Kai L., Samuel S.K., Levenson A.S. Resveratrol enhances p53 acetylation and apoptosis in prostate cancer by inhibiting MTA1/NuRD complex. Int. J. Cancer. 2010;126:1538–1548. doi: 10.1002/ijc.24928. [DOI] [PubMed] [Google Scholar]
- 137.Frazzi R., Valli R., Tamagnini I., Casali B., Latruffe N., Merli F. Resveratrol-mediated apoptosis of hodgkin lymphoma cells involves SIRT1 inhibition and FOXO3a hyperacetylation. Int. J. Cancer. 2013;132:1013–1021. doi: 10.1002/ijc.27748. [DOI] [PubMed] [Google Scholar]
- 138.Adibi P., Faghihzadeh F., Hekmatdoost A. Resveratrol and liver: A systematic review. J. Res. Med. Sci. 2015;20:797–810. doi: 10.4103/1735-1995.168405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Y., Bäckesjö C.M., Haldosén L.A., Lindgren U. Resveratrol inhibits proliferation and promotes apoptosis of osteosarcoma cells. Eur. J. Pharmacol. 2009;609:13–18. doi: 10.1016/j.ejphar.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 140.Negri A., Naponelli V., Rizzi F., Bettuzzi S. Molecular Targets of Epigallocatechin-Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients. 2018;10:1936. doi: 10.3390/nu10121936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saffari Y., Sadrzadeh S.M. Green tea metabolite EGCG protects membranes against oxidative damage in vitro. Life Sci. 2004;74:1513–1518. doi: 10.1016/j.lfs.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 142.Wei Y., Chen P., Ling T., Wang Y., Dong R., Zhang C., Zhang L., Han M., Wang D., Wan X., et al. Certain (-)-epigallocatechin-3-gallate (EGCG) auto-oxidation products (EAOPs) retain the cytotoxic activities of EGCG. Food Chem. 2016;204:218–226. doi: 10.1016/j.foodchem.2016.02.134. [DOI] [PubMed] [Google Scholar]
- 143.Henning S.M., Wang P., Carpenter C.L., Heber D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics. 2013;5:729–741. doi: 10.2217/epi.13.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li Y., Yuan Y.Y., Meeran S.M., Tollefsbol T.O. Synergistic epigenetic reactivation of estrogen receptor-α (ERα) by combined green tea polyphenol and histone deacetylase inhibitor in ERα-negative breast cancer cells. Mol. Cancer. 2010;9:274. doi: 10.1186/1476-4598-9-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen D., Wan S.B., Yang H., Yuan J., Chan T.H., Dou Q.P. EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Adv. Clin. Chem. 2011;53:155–177. doi: 10.1016/b978-0-12-385855-9.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ferrari E., Bettuzzi S., Naponelli V. The Potential of Epigallocatechin Gallate (EGCG) in Targeting Autophagy for Cancer Treatment: A Narrative Review. Int. J. Mol. Sci. 2022;23:6075. doi: 10.3390/ijms23116075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bansal S., Vyas S., Bhattacharya S., Sharma M. Catechin prodrugs and analogs: A new array of chemical entities with improved pharmacological and pharmacokinetic properties. Nat. Prod. Rep. 2013;30:1438–1454. doi: 10.1039/c3np70038k. [DOI] [PubMed] [Google Scholar]
- 148.Meeran S.M., Patel S.N., Chan T.H., Tollefsbol T.O. A novel prodrug of epigallocatechin-3-gallate: Differential epigenetic hTERT repression in human breast cancer cells. Cancer Prev. Res. 2011;4:1243–1254. doi: 10.1158/1940-6207.CAPR-11-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zeng L., Holly J.M., Perks C.M. Effects of physiological levels of the green tea extract epigallocatechin-3-gallate on breast cancer cells. Front. Endocrinol. 2014;5:61. doi: 10.3389/fendo.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sheng J., Shi W., Guo H., Long W., Wang Y., Qi J., Liu J., Xu Y. The Inhibitory Effect of (-)-Epigallocatechin-3-Gallate on Breast Cancer Progression via Reducing SCUBE2 Methylation and DNMT Activity. Molecules. 2019;24:2899. doi: 10.3390/molecules24162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kato K., Long N.K., Makita H., Toida M., Yamashita T., Hatakeyama D., Hara A., Mori H., Shibata T. Effects of green tea polyphenol on methylation status of RECK gene and cancer cell invasion in oral squamous cell carcinoma cells. Br. J. Cancer. 2008;99:647–654. doi: 10.1038/sj.bjc.6604521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rodríguez-García C., Sánchez-Quesada C., Gaforio J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants. 2019;8:137. doi: 10.3390/antiox8050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dabeek W.M., Marra M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients. 2019;11:2288. doi: 10.3390/nu11102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vafadar A., Shabaninejad Z., Movahedpour A., Fallahi F., Taghavipour M., Ghasemi Y., Akbari M., Shafiee A., Hajighadimi S., Moradizarmehri S., et al. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020;10:32. doi: 10.1186/s13578-020-00397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kedhari Sundaram M., Hussain A., Haque S., Raina R., Afroze N. Quercetin modifies 5′CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J. Cell. Biochem. 2019;120:18357–18369. doi: 10.1002/jcb.29147. [DOI] [PubMed] [Google Scholar]
- 156.Izzo S., Naponelli V., Bettuzzi S. Flavonoids as Epigenetic Modulators for Prostate Cancer Prevention. Nutrients. 2020;12:1010. doi: 10.3390/nu12041010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Septembre-Malaterre A., Boumendjel A., Seteyen A.S., Boina C., Gasque P., Guiraud P., Sélambarom J. Focus on the high therapeutic potentials of quercetin and its derivatives. Phytomedicine Plus. 2022;2:100220. doi: 10.1016/j.phyplu.2022.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alvarez M.C., Maso V., Torello C.O., Ferro K.P., Saad S.T.O. The polyphenol quercetin induces cell death in leukemia by targeting epigenetic regulators of pro-apoptotic genes. Clin. Epigenetics. 2018;10:139. doi: 10.1186/s13148-018-0563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bouyahya A., El Hachlafi N., Aanniz T., Bourais I., Mechchate H., Benali T., Shariati M.A., Burkov P., Lorenzo J.M., Wilairatana P., et al. Natural Bioactive Compounds Targeting Histone Deacetylases in Human Cancers: Recent Updates. Molecules. 2022;27:2568. doi: 10.3390/molecules27082568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sharma V., Kumar L., Mohanty S.K., Maikhuri J.P., Rajender S., Gupta G. Sensitization of androgen refractory prostate cancer cells to anti-androgens through re-expression of epigenetically repressed androgen receptor—Synergistic action of quercetin and curcumin. Mol. Cell. Endocrinol. 2016;431:12–23. doi: 10.1016/j.mce.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 161.Priyadarsini R.V., Vinothini G., Murugan R.S., Manikandan P., Nagini S. The flavonoid quercetin modulates the hallmark capabilities of hamster buccal pouch tumors. Nutr. Cancer. 2011;63:218–226. doi: 10.1080/01635581.2011.523503. [DOI] [PubMed] [Google Scholar]
- 162.Xiao X., Shi D., Liu L., Wang J., Xie X., Kang T., Deng W. Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PLoS ONE. 2011;6:e22934. doi: 10.1371/journal.pone.0022934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gibellini L., Pinti M., Nasi M., Montagna J.P., De Biasi S., Roat E., Bertoncelli L., Cooper E.L., Cossarizza A. Quercetin and cancer chemoprevention. J. Evid. Based Integr. Med. 2011;2011:591356. doi: 10.1093/ecam/neq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bidian C., Mitrea D.R., Vasile O.G., Filip A., Cătoi A.F., Moldovan R., Decea N., Albu A. Quercetin and curcumin effects in experimental pleural inflammation. Med. Pharm. Rep. 2020;93:260–266. doi: 10.15386/mpr-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.