Abstract
The use of synthetic materials and the attention towards environmental hazards and toxicity impose the development of green composites with natural origins. Clay is one of the candidates for this approach. Halloysite is a natural clay mineral, a member of the Kaolin group, with characteristic tubular morphology, usually named halloysite nanotubes (HNTs). The different surface chemistry of halloysite allows the selective modification of both the external surface and the inner lumen by supramolecular or covalent interactions. An interesting aspect of HNTs is related to the possibility of introducing different species that can be released more slowly compared to the pristine compound. Due to their unique hollow morphology and large cavity, HNTs can be employed as an optimal natural nanocarrier. This review discusses the structure, properties, and application of HNTs in the biological field, highlighting their high biocompatibility, and analyse the opportunity to use new HNT hybrids as drug carriers and delivery systems.
Keywords: halloysite nanotubes, biocompatibility, drug delivery system, biomedical application
1. Introduction
As emerging materials, nanomaterials have attracted much attention over the years due to their small size, but also due to the countless properties that distinguish them. In fact, the nanometric dimensions of material make it assume particular and different chemical-physical properties compared to conventional materials. These different properties, determined by the chemical composition, structure, surface, and increase in surface reactivity in relation to volume, solubility, and state of aggregation, have raised questions about potential human health and environmental risks [1]. In the past few years, there has been a growing interest in research aimed at the development of new organic or inorganic nanocomposites [2,3,4,5]. The attention of the scientific community has been drawn by nano clays, thanks to their natural origin, worldwide abundance, availability, biocompatibility, and sustainability [6,7,8]. Halloysite, largely known as halloysite nanotubes or HNTs, is a natural mineral clay composed of alternating layers of silica and alumina geologically laminated in mesoporous tubular particles with significant adsorption and loading capabilities [9]. Compared to other tubular nanomaterials, HNTs show some advantages in terms of processability and hydrodynamic properties [10]. In fact, for many years, much attention was focused on carbon nanotubes (CNTs) [11,12,13,14], showing that these nanotubes have a high cost, lower water dispersibility, and higher toxicity than HNTs [15]. The physicochemical properties of HNTs were fully described, disclosing their potential for various applications such as biomedicine and catalysis [16,17,18,19]. HNT-based composites are gaining interest in research aimed at the development of biomaterials for drug delivery vehicles in nanomedicine [20]. In this review, we aim to provide an overview of the properties and biomedical applications of halloysite nanotubes as drug delivery systems. We will discuss their possible application in biotechnology through a focus on HNT composites for biomedical applications. Herein, we aim to provide an overview of the structure, properties, and applicative aspects of halloysite nanotubes in the biomedical field. We start with a brief background about HNTs then provide a brief discussion of their biocompatibility and application.
2. Halloysite Nanotubes
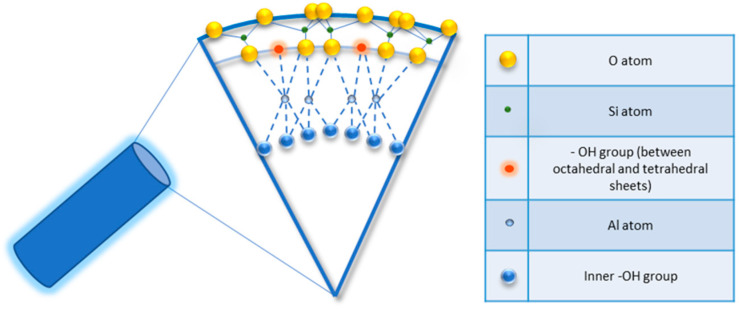
Halloysite is a two-layered aluminosilicate with a chemical composition similar to kaolinite (chemical formula: Al2Si2O5 (OH)4·nH2O) [21,22], and a hollow tubular structure in the sub-micrometre range [23,24,25,26]. Halloysite was first described in 1826 by the French chemist Pierre Berthier and was later given its name in honour of the Belgian geologist Omalius d’Halloy, who found it in the deposits of Angleur, Belgium [27]. Natural tubular halloysite clay has attracted great interest in materials development because it is one of the few inexpensive nanomaterials available in thousands of tons at a low price [28,29]. The nucleation of halloysite crystals occurs in different parts of the world, because of rock erosion due to weathering, pedogenesis, and hydrothermal alteration of ultramafic rocks [30,31]. New Zealand, Belgium, Brazil, France, China, Australia, and Turkey are rich in deposits of halloysite, and it has been shown that with the variation of the deposits it is possible to observe different characteristics that are maintained within the same deposit. Halloysite is defined as a 1:1 phyllosilicate in which a planar layer of tetrahedral silicates alternates with an octahedral geometry layer; these layers are bound together by oxygen bridges [32]. The fundamental unit for the octahedral sheet consists of three octahedrons. In particular, the siloxane groups are bonded via only one oxygen atom to octahedral rings at the outer part and the apical oxygen of tetrahedra becomes the vertices of octahedra [33]. However, under certain geological conditions, halloysite can also take on forms other than classical tubular. It is also possible to distinguish a spheroidal morphology, flat and almost rolled [34,35]. In the “Dragon Mine” deposits of Utah (USA), halloysite is characterized by a good degree of purity and looks like a white stone that can easily be transformed into soft, fine powder. In some deposits, the presence of metals as contaminants induces a colour change that becomes yellowish or brown [30]. Transmission electron microscopy (TEM) structural analysis has shown that halloysite with predominantly tubular morphology and heterogeneous dimensions is present in New Zealand, the United States, and Australian deposits [36]. A very interesting aspect is linked to the different chemical composition between the inner and outer surfaces, in which there are, respectively, aluminolic groups (Al-OH) that give a positive charge and siloxanes (Si-O-Si) that give a negative charge [37,38]. The charges that characterize the internal and external nanotube surfaces are due to the different dielectric and ionization properties of silicon oxides and aluminium. For pH values of 3–10, the positive charges are distributed in the inner lumen and the negative charges on the external surface; present on the edges is a negative/positive charge. In particular, the tubule lumen is positively charged with pH ≤ 8.5, and the outer surface is negatively charged with pH ≥ 1.5 [39]. Generally, because of the O and OH atoms that carry negative charges, the halloysite nanotube is negatively charged. As a result of the tubular shape, on the outer surface only a few hydroxyl groups are present; these are more concentrated in the internal lumen and therefore are more reactive. In fact, for halloysite, it is possible to classify three types of Al-OH, according to their positioning on the surface, at the ends, and between the octahedral and tetrahedral sheets, as shown in Figure 1. All can be reactive and dissociate according to the pH of the solutions, except those placed between the octahedral and tetrahedral sheets, due to steric hindrance [40].
Figure 1.
Detail of schematic illustration of the crystalline structure of halloysite nanotubes.
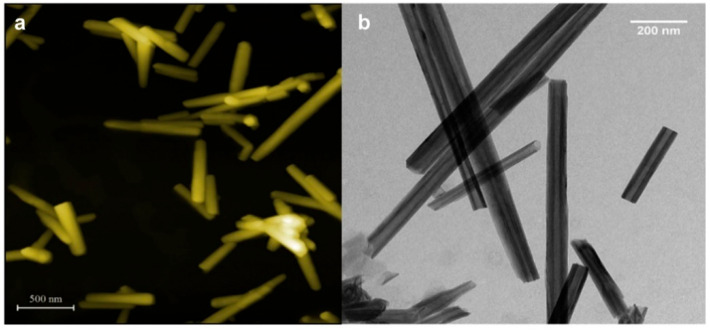
The halloysite nanotubes’ size may vary depending on the extraction site and the purification process they undergo, but they usually have an internal diameter of 10–30 nm, an external diameter of 40–70 nm [41], and a length between 200 and 2000 nm [42] (Figure 2a,b).
Figure 2.
(a) Atomic force microscopy; (b) TEM images of halloysite nanotubes precipitated from aqueous dispersion. Adapted from [43].
Nanotubes with a length between 3 and 5 μm have been found in some deposits, although those with smaller sizes are more interesting from a biological point of view, as they are more suitable for use as drug carriers [44]. Halloysite is chemically like kaolinite, but the halloysite double layers are separated by a monolayer of water molecules. In the hydrated form of halloysite n = 2 in the formula Al2(OH)4Si2O5·nH2O. One layer of water molecules is present between the multilayers and is named “halloysite-(10 Å)”, where “10 Å” indicates the d001-value of the layers. When the n value is 0 (n = 0), the halloysite is named dehydrated or “halloysite-(7 Å)” and may be obtained through the loss of the interlayer water molecules [30,45]. The water present in halloysite-(10 Å) can be classified as “hole water” and “associated water”. In the first case, the water molecules are placed on the surface of the tetrahedral sheet with different orientations and make hydrogen bonds with the basal oxygens. The “associated” water has a greater degree of mobility at room temperature and is located at a different level in the interlayer space, with an ice-like configuration and forming hydrogen bonds with each other and/or with inner-surface hydroxyls [46,47,48,49,50]. Generally, HNT surfaces could be classified into three types. The inner lumen surface has a positive charge and is covered by Al-OH groups. It can undergo a variety of covalent modifications by which certain functional groups can be added. This method allows immobilizing several organic groups on the surface of the lumen stably. The external siloxane surface has a negative charge and can be used to establish covalent bonding with molecules such as organosilanes [51]. Moreover, it can be modified by coating with cationic substances, such as polymers, biopolymers, and surfactants. This type of modification can help to improve the dispersibility and biocompatibility of halloysite. Interstate surfaces, held together by hydrogen bridges, can be modified by direct or indirect intercalation of small organic molecules and some monovalent cationic salts. This, therefore, can lead to a weakening of the hydrogen bonds interstate and an increase in the surface between the various layers that can be understood as additional space for loading or adsorption [52].
3. Effects of Nanomaterial on Human Health
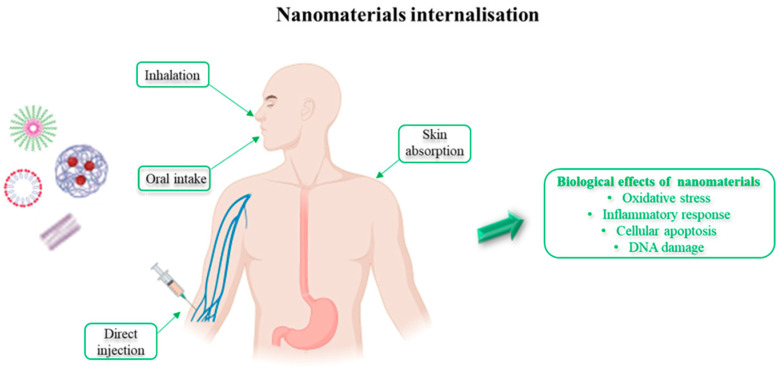
Despite nanomaterials having potential benefits, their interaction with biological systems may cause unpredicted risk to human life. One concern is the variation in dimensions of nanoparticles, which may affect chemical, thermodynamic, optical, biological, spectroscopic, electronic, and electromagnetic processes, resulting in unexpected modifications [53,54,55]. An increasing exposure to different types of nanomaterials makes it essential to determine their possible negative impact on human health and potential toxic effects. For this reason, it is important to evaluate and characterize the chemical-physical properties, dimensions, solubility, chemical composition, surface area, surface energy, etc. [56]. Among these properties, size and surface are the most important for interaction with biological systems as they determine how the materials will respond [57]. It was observed that the mechanisms for cell internalization depend mainly on the material size [58] and that toxicity may also depend on this aspect. The size of nanomaterials depends on the ability to enter the biological systems, to interact with cellular functions by modifying the structure of macromolecules, and thus interfere with biological functions [59,60]. Nanomaterial internalisation can take place by many paths such as inhalation, oral intake, direct injection, or skin absorption (Figure 3), and is followed by their transportation into organs and their succeeding biological effects, including oxidative stress, inflammatory responses, cellular apoptosis, and DNA damage [61,62].
Figure 3.
Nanomaterial internalisation and biological effects on the human body.
Nanostructures, once ingested, administered topically, or inhaled, can be transported by blood and accumulated in various organs [63]. Subsequent to entry into the systemic circulation, the absorption of nanoparticles by blood capillaries allows the distribution in various body districts. Depending on their surface characteristics, they can be recognized and degraded by macrophages [64]. Moreover, nanocarrier size also affects the in vivo fate. In fact, particles larger than 200 nanometres are shown to accumulate in the liver and spleen. Instead, nanoparticles smaller than five nanometres are filtered at the renal level [65]. It has been observed that the optimal size of a nano system for application in the biomedical field is around 100 nanometres [66]. In vivo studies have shown that once nanomaterials enter the bloodstream they can reach the central nervous system [67], induce pulmonary inflammatory reactions [68,69], and cause cardiovascular problems [70]. The toxic effects induced in the cells by contact with nanomaterials can be chemical or physical. Chemical mechanisms may include the production of reactive oxygen species (ROS) [71], disruption of electron and ion transport across the cell membrane [72], and lipid peroxidation with a consequent decrease in the fluidity of the membranes [73]. Concerning physical mechanisms, which depend on the size of the nanomaterial and the surface properties [74], these include rupture of the plasma membrane and interruption of related activities such as transport processes [75,76] and misfolding of proteins resulting in loss of their function [77]. The life cycle of nanomaterials in the human body, their metabolism, and their fate in human organs are dependent on the physical and chemical characteristics of the nanomaterials and on their exposure route [78]. The exposure of humans to nanomaterials requires an improved understanding of their potential toxicity to predict the consequences. Future research efforts should be towards developing easy, cost-effective, and highly biocompatible nanomaterials for their application in the biomedical field.
4. Halloysite Biocompatibility
Thanks to their rheological properties, high interaction, and high binding capacity with biopolymers, the role of mineral clays as a drug carrier has become the subject of extensive research. The physical-chemical properties, including size, morphology, and surface charge density, which depend on the type of mineral clay and the crystalline structure, are the features that make them interesting [79]. The increased use of halloysite nanotubes, their purification, modification, and large-scale preparation lead to an increasing exposure to human society and environments. Therefore, it is important to do a systematic analysis before their use in biomedical applications and to consider the possible consequential effects of their use on human health. The different interactions of HNTs with living cells, encompassing electrostatic, van der Waals, and ion exchange, as well as cellular response, are decisive in determining the behaviour of halloysite nanotubes in biological systems [80]. Due to their abundance and perceived biocompatibility, in recent years halloysite nanotube toxicity has been evaluated in different in vitro and in vivo models. For example, Sawicka et al. investigated both short- (24 or 72 h) and long-term (seven days) cytotoxic effects of HNTs at doses ranging from 10 to 200 μg/mL on human alveolar carcinoma epithelial cells (A549) and human bronchial epithelial cells (BEAS-2B). After 24 h of exposure, the IC50 of HNTs in A549 and BEAS-2B cells was 152 ± 6.4 μg/mL and > 400 μg/mL, respectively. After 72 h of exposure, the IC50 values decreased to 49 ± 3 μg/mL in A549 and 45.1 ± 8 μg/mL, in BEAS-2B cells. Thus, the results showed that cytotoxicity of HNTs depends on cell model, dose, and time of exposure [81]. Table 1 shows some of the toxicity threshold values reported for different concentrations of HNTs regarding cell types.
Table 1.
Cytotoxicity of HNTs towards different cell lines.
| Cell Type | Incubation Time | HNT Concentration | Reference |
|---|---|---|---|
| MCF7, HeLa | 24–72 h | No toxic effect up to 75 μg mL−1 | [25] |
| A549 | 24 h | No toxic effect up to 100 μg mL−1 | [82] |
| Caco-2, HT29-MTX | 6 h | No toxic effect up to 100 μg mL−1 | [83] |
| A549 | 24–48–72 h | No toxic effect up to 100 μg mL−1 | [84] |
| C6 | 24 h | No toxic effect up to 500 μg mL−1 | [85] |
| Colo 320 | 24 h | No toxic effect up to 625 μg mL−1 | [86] |
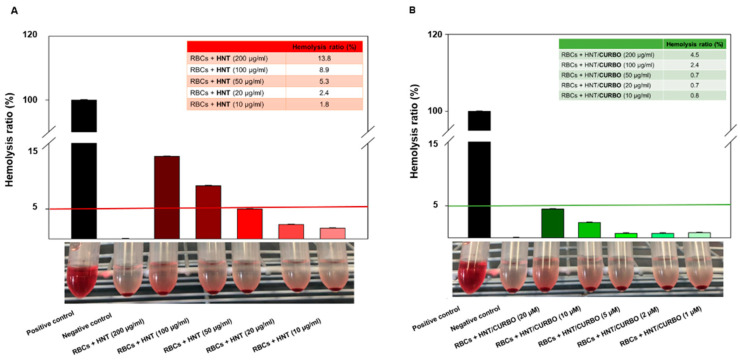
Kamalieva et al., evaluated the internalization of pristine halloysite nanotubes using the A549 cell line. To assess the cytotoxicity, the cells were treated for 24 h with increasing concentrations of pristine HNT (ranging from 33 to 900 μg per 105 cells). Once the treatment was finished, the IC50 of halloysite nanotubes for A549 cells was determined to be 300 μg per 105 cells by MTT assay [87]. Regarding the nanomaterials for applications in the biomedical field, it is also important to verify their haemolytic potency when contacting the blood. To this end, the hemocompatibility of the hybrid system HNT/CURBO was investigated by exposing human red blood cells (HRBCs) both to pristine halloysite and HNT/CURBO for 24 h at 37 °C. After exposure to different concentrations, a non-noticeable haemolytic effect was observed. The haemolysis percentage values induced by the exposition of HRBCs to different concentrations of HNT/CURBO (ranging from 10 µg/mL to 200 µg/mL) were all less than 5% (Figure 4).
Figure 4.
In vitro hemocompatibility assay of (A) raw HNT and (B) HNT/CURBO after incubation at 37 °C for 12 h. The positive control was in ultrapure water (100% lysis), and negative control was in PBS 1× (0% lysis). International Journal of NanoMedicine 2021:16 4755–4768 Originally published by and used with permission from Dove Medical Press Ltd [88].
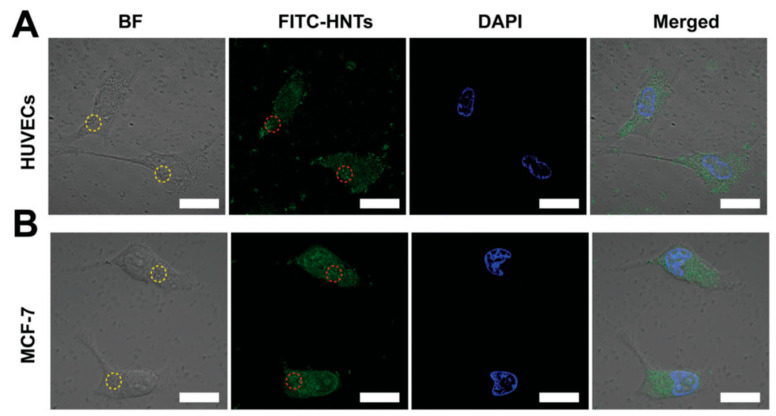
This result suggests that HNTs do not exhibit a significant haemolytic effect [88]. It has also been seen that HUVECs (human umbilical vein endothelial cells) and MCF-7 (human breast cancer) cells show high cell viability after being treated with different concentrations of HNTs for 24 h. For both cell lines, vitality remains above 85% even when the concentration of HNTs reaches up to 200 mg/mL. When incubation times increase (48 h and 72 h), a slight decrease is observed in cell viability. In particular, at 72 h in HUVEC cells viability is 62.1% at the maximum HNT concentration of 200 mg/mL. Furthermore, to investigate the cellular uptake of HNTs in HUVECs and MCF-7, the cells were incubated with FITC-HNTs and monitored using confocal laser scanning microscope (CLSM) as shown in Figure 5 [89].
Figure 5.
CLSM images of (A) HUVECs and (B) MCF-7 cells. Cells incubated with FITC-HNTs (50 mg mL−1); FITC-HNTs and cell nucleus are indicated in green and blue, respectively. The circles in the images represent the FITC-HNT aggregates. Scale bar = 20 mm. Adapted from [89].
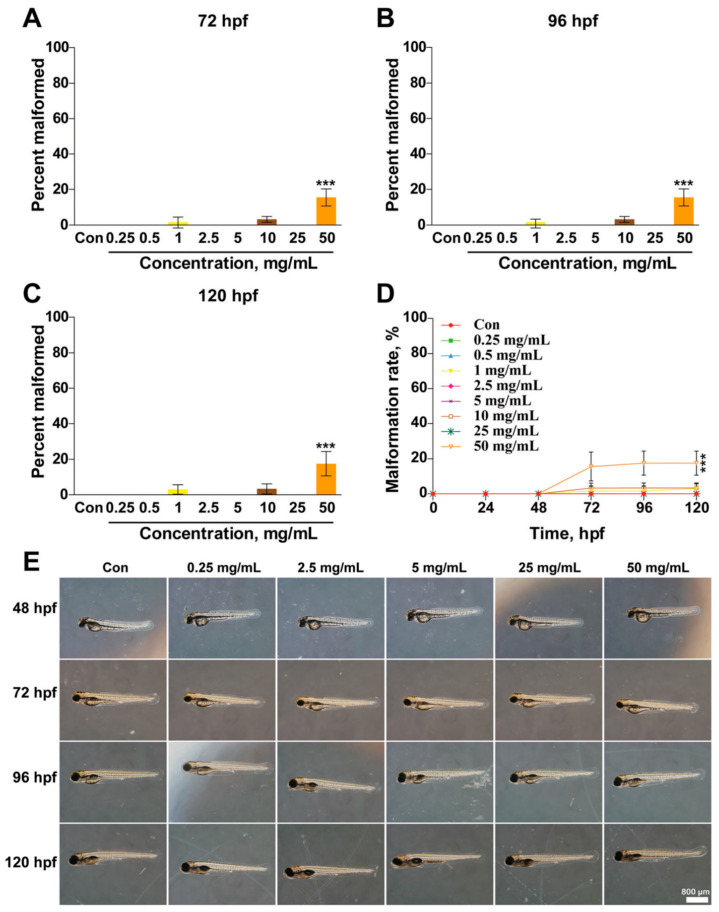
Halloysite nanotube toxicity was further evaluated against human peripheral lymphocytes by means of mitotic index assay. Different concentrations of HNTs (10, 100, 500, and 1000 µg/mL) were incubated with the peripheral lymphocyte culture. The mitotic index assay revealed inhibition of the proliferation of lymphocytes only at the highest concentration (1000 µg/mL) [90]. Despite many in vitro studies indicating that HNTs exhibit a high level of biocompatibility, the in vivo toxicity of HNTs remains unclear. HNT toxicity has been evaluated in in vivo models and in all cases, results show that this nanomaterial is non-toxic or scarcely toxic depending on the tested condition. Several concentrations of HNTs (ranging from 0.25 to 50 mg/mL) were tested towards zebrafish embryos and larvae from 24 h post-fertilization (hpf) to 120 hpf and the results showed that the percent survival of zebrafish embryos and larvae have no significant changes at different developmental stages (24, 48, 72, 96, and 120 hpf) except at the highest concentrations (25 and 50 mg/mL) (Figure 6).
Figure 6.
Effects of HNTs on the morphology of developing zebrafish. Embryos were treated with different concentrations of HNTs (0, 0.25, 2.5, 5, 25, and 50 mg mL−1) starting from 6 hpf. (A–D) Percent of malformed zebrafish was analysed at 72, 96, and 120 hpf. (E) Morphology of zebrafish larvae treated with HNTs was photographed using a microscope at 48, 72, 96, and 120 hpf. The values are represented as mean ± SD (n = 24). Each bar represents the collated data of three separate experiments. The data were analysed using Graph Prim 6 for one-way ANOVA and a Tukey’s post hoc test. *** p < 0.001 versus control group. Adapted from [89].
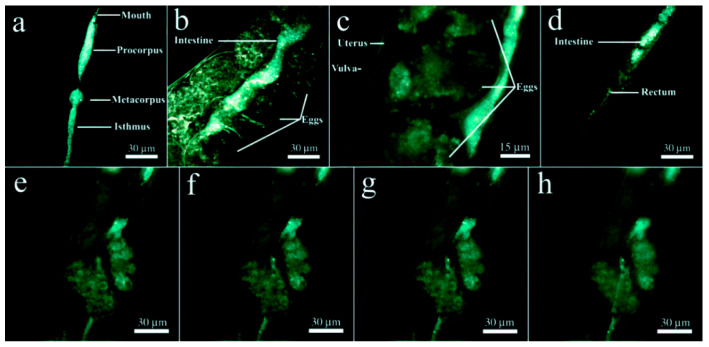
Following the treatment at relatively low HNT concentrations, the percent survival for all the hpf data sets increases slightly above 100%, then the HNTs increase the survival of the zebrafish at low concentrations and have little effect on the survival, proving to be weakly toxic [89]. Fakhrullin et al. evaluated the in vivo toxicity of HNTs by using as a model organism the nematode Caenorhabditis elegans. The results showed that HNTs within the investigated concentrations, ranging from 0.05 mg/mL to 1 mg/mL, were not toxic to the nematode. They only induce mechanical stress in the digestive system, which is restored once the treatment is finished. In extended depth of field (EDF) microscopy images, halloysite nanotubes were found exclusively in the alimentary system of the worms (Figure 7) [91].
Figure 7.
HNT localization in the nematode’s intestines by EDF microscopy: (a) inside the foregut; (b,c) in the midgut; (d) inside the hindgut; (e–h) EDF images of the intestine near the uterus taken at different focal planes demonstrating the localisation of HNTs exclusively inside the intestine. Adapted from [91].
In addition, a phytotoxic study carried out on Raphanus sativus has shown that halloysite nanotubes do not affect the germination process, xylem differentiation, and development, thus demonstrating a high biocompatibility also with respect to plant species [92]. Another study evaluates the biodistribution and pulmonary toxicity of the purified HNTs in mice, following an intragastric administration for 30 days. The results showed that HNT oral administration caused considerable aluminium accumulation mainly in the lungs. Oral administration of HNTs stimulated the growth of the mice at low dose (5 mg/kg BW) with no pulmonary toxicity. When concentrations increased ten times (50 mg/kg BW), the mouse growth was inhibited and resulted in lung inflammation and oxidative stress [93]. An additional study instead showed normal mice weight gain at an oral dose of 5 mg kg−1 and a reversible inflammation of the small intestine [94]. It is possible to state that HNTs have been shown to be almost non toxic under the tested conditions, and no cell toxicity makes HNTs suitable as safe materials for biomedical applications.
5. Halloysite Nanotube Application in Drug Delivery
Clay minerals are commonly used in the pharmaceutical industry either as excipients or activated ingredients. Indeed, it is known that when drugs and clay mineral are administered simultaneously the absorption capacity reduces by interacting with the drug. These kinds of interactions can represent advantages, for example in drug release [95]. Research has recently focused on the development of new drug delivery systems based on the use of nanomaterials, with the aim of improving the efficacy from the therapeutic point of view and reducing the side effects, especially, in cancer treatment. In several studies HNTs have been used as nanocontainers or nanocarriers for drug delivery. Since unmodified halloysite nanotubes have been shown to establish weak interaction with the drugs, several methods of modification have been developed, for example, tubular entrapment, adsorption, or intercalation [96,97,98]. Commonly, the methods used for the HNT loading consists of three steps. The first step is mixing the clay dry powder with the saturated solution of the guest molecule; the second step is the sonication and stirring of the HNT/guest molecule dispersion; and the last step is the vacuum pumping in/out operation, in which the dispersion is transferred from atmospheric pressure to a vacuum jar. The third step was introduced to optimize the quantity of active molecules loaded inside the nanotubes by keeping the system under vacuum and then cycling it back to atmospheric pressure [99]. Thanks to hydroxyl groups present both on the outer and inner surface, HNTs can be functionalized and used as delivery vehicles of drugs [18,100]. In fact, thanks to the structural properties, high surface area (up to 184.9 m2/g), and the large pore volume (up to 0.353 cm3/g), over the last few years, halloysite has attracted increasing attention in several areas such as carriers for drug delivery, adsorbents, photocatalysts, etc. [101]. The functional groups of external (Si-OH) and internal (Al-OH) surfaces affect the loading of molecules of interest into HNTs. The negative charge of HNTs at pH above three, is due to silanol group deprotonation. The anionic nature of the external HNT surface enables it to interact with cationic compounds, while aluminol groups located on the internal lumen surface carry a positive charge and support the loading of anionic molecules. Moreover, interactions between HNTs and molecules can take place by van der Waals, hydrogen binding, or other specific interactions [102]. Due to the unique properties mentioned in the previous sections, HNTs have attracted research interest for application in drug delivery. In fact, drug molecules could be encapsulated inside the tube lumen or might be adsorbed on the outer surface [103,104,105]. Furthermore, the tubular morphology allows an increase in tensile and bending strength [106]. Taking advantage of the hollow tubular shape and the large cavity volume, HNTs can be used as desirable natural nanocarriers for biologically active agents. Drug therapy is the main approach in cancer treatment. Due to the poor loading capacity of hydrophobic molecules commonly used in cancer therapy, as for example curcumin, doxorubicin, and paclitaxel, halloysite nanotubes are not considered as an efficient carrier system for cancer drug therapy [107,108,109]. Since the surface of halloysite nanotubes is negatively charged, polycations such as chitosan can be coated onto HNTs. Thanks to this type of approach, drugs released from the HNT lumen may be sustained over a long period of time. Following this reasoning, curcumin was entrapped into the lumen of halloysite with the aid of vacuum suction and release obtaining drug-loaded halloysite nanotubes (DLHNTs). Then, the DLHNTs were coated with chitosan (DLHNTs-CH). The viability assay performed on MCF7 cells showed that polycationic coated HNTs have the potential to serve as a drug carrier [110]. Following surface modification of distearoyl phosphoethanolamine (DSPE), paclitaxel (PTX) is successfully loaded onto HNT surfaces with different inner lumen diameters giving rise to the system DSPE-HNTs-PTX, designed to deliver this drug to cancer cells. The antitumoral effects of the DSPE-HNTs-PTX system were evaluated on MDA-MB-231-bearing mice. The results showed that the system can inhibits tumour growth, suggesting a good anticancer effect [111]. Another study was conducted to evaluate the anticancer effect of chitosan-modified HNT loaded with curcumin-gold hybrid nanoparticles (HNT@CUR-Au/CS). This HNT hybrid system consisted of AuNP which have near-infrared (NIR) responsive property and pH-responsive curcumin release. The anticancer efficacy of HNT@CUR-Au/CS was tested on MCF-7 breast cancer cells showing more effective anticancer activity at pH 5.5 (intracellular tumour environment) than at a pH value of 7.4 (extracellular conditions) [112]. Taheri-Ledari et al. exploiting the properties of HNTs and gold nanoparticles (AuNPs), proposed a new system for controlled release of docetaxel (DTX), a cytotoxic anticancer agent. The DTX@HNT/Au-SORT system is composed of HNTs conjugated with monoclonal antibody as a biologically active agent for targeted drug delivery and small plasmonically active AuNPs included in the HNT pores. In vitro cytotoxicity assay performed on 3T3 (human normal fibroblast) and caov-4 (human ovarian cancer) cell lines showed high selectivity of DTX@HNT/Au-SORT in cell adhesion and internalization. At a concentration of DTX@HNT/Au-SORT equal to 50 μg mL−1 the cytotoxicity was approximately 90% for caov-4 cells and 16% for the 3T3 cell line demonstrating that it could be a promising system to use in the treatment of ovarian cancer [113]. In recent years, various methods have been developed to functionalize HNTs and allow them to be used as delivery systems for anticancer drugs. In Table 2 we summarize literature reports with respect to some anticancer drugs entrapped in HNTs.
Table 2.
HNT nanocomposites for controlled and sustained anticancer drug delivery.
| Anticancer Drug | Cell Type | HNT Modifications | Reference |
|---|---|---|---|
| Anthocyanins | MCF-7, HT-29 | HNT-Anth | [114] |
| Atorvastatin | Caco-2, HT-29 | HNT-ATV@HF-CEL | [115] |
| Camptothecin | HeLa | f-HNT/CPT and Fmoc-F/f-HNT/CPT | [116] |
| Camptothecin | Caco-2 | CPT@COS/MHNTs and CPT@FA-COS/MHNTs |
[117] |
| Curcumin | Caco-2 | HNT-APT-PMVEMA@MF | [118] |
| Curcumin | HepG2, MCF-7, SV-HUC-1, EJ, CaSki, HeLa | HNT-COOH/Chitosan | [119] |
| Curcumin | SUM 149, MDA-MB-231, HL60, HL60R | f-Hal-1, 2, 4, 5, 6, and 7 | [120] |
| Curcumin | MCF-7 | PCL/PEO-Cur/HNT, PCL/PEO-Cur/HNT-GPTMS, and PCL/PEO-Cur/HNT-APTES |
[121] |
| Doxorubicin | MCF-7 | DOX@HNTs-g-COS | [122] |
| Doxorubicin | A549 | DNA-wrapped HNTs | [123] |
| Doxorubicin | SKOV3, 293T | DOX@HNTs-S-S-β-CD-Ad-PEG-FA | [124] |
| Doxorubicin | MCF-7 | DOX@HNTs-PEG-FA | [125] |
| Doxorubicin | HeLa | DOX loaded Fe3O4@HNT | [126] |
| Doxorubicin | MCF-7 | Au-HNT-DOX@BSA-FA | [127] |
| Doxorubicin | MCF-7, COLO 205 | HNT-liposome-coated surfaces | [128] |
| Doxorubicin | HeLa, MCF-7 | HNTs-DOX conjugated with anti-EpCAM antibody |
[129] |
Changes in the HNTs surface are important to improve their hydrophilicity and compatibility. One of the advantages of the use of clay nanomaterials is the ability to protect drugs against degradation by chemicals and enzymes while extending the drug release rate [130]. In the inner cavity of HNTs is possible to load not only small drug molecules but also proteins, DNA, or antibacterial agents [131,132,133,134]. Various modifications of the HNTs’ lumen can increase the affinity of the drug towards the HNTs, thereby controlling the release rate [135]. Molecules and drugs released from HNTs’ lumen can take place by diffusion from the lumen or by desorption from the external surface. Release from the lumen can be controlled by the tube diameter or by addition of tube-end stoppers [136,137]. The functionalization of HNTs with stimuli-responsive materials, for example with thermosensitive polymers, allows the release profile to be adjusted [138]. Kartogenin is a small molecule that promotes the selective differentiation of multipotent mesenchymal stem cells into chondrocytes, stimulating the repair of damaged cartilage. Unfortunately, like most organic molecules with biological properties, it possesses short-term stability in an aqueous medium. As an efficient treatment for osteoarthritis, halloysite nanotubes have been proposed as a carrier system for potential intra-articular delivery of KGN by means of laponite hydrogel (HNT/KGN/Lap). The cytotoxicity of the hybrid hydrogels was evaluated in human liver HepG2 cells. The efficacy of HNT/Lap hydrogel as a carrier for KGN was proved by in vitro release experiments performed at pH 7.4 and in ex vivo synovial fluid at 37 °C and it was observed that KGN has a slower release in synovial fluid than that of phosphate buffer at pH 7.4 [139]. Alternative approaches can be employed for HNT surface modifications before the loading of drugs. One of these is the modification through (3-Aminopropyl) triethoxysilane (APTES), known for the functionalization of surfaces due to their ease of use and low toxicity. Its role is to introduce a silanol group on the surfaces of halloysite nanotubes to establish a bond with hydroxyl groups [95]. Pristine HNTs and APTES-modified HNTs were tested as drug carriers for loading and release of ibuprofen (IBU) that was encapsulated into the lumen and partially loaded onto the external surface. When unmodified halloysite nanotubes are used as a carrier for ibuprofen, a low loading rate and rapid release is achieved because the only established interactions between HNTs and ibuprofen are weak bonds (Van der Waals). HNT surface modification with APTES increases the loading of IBU by creating an electrostatic attraction between the introduced aminopropyl groups of the grafted APTES and the carboxyl group of ibuprofen and induces a delay in the release from the lumen of nanotubes [140]. In addition to exploiting the HNT lumen for drug encapsulation, it is possible to adsorb or add by covalent bonding molecules on the outer surface of nanotubes. By combining both, it is possible to obtain a system that can release the drug from the external surface initially and subsequently, in a slow and controlled way, that of the lumen. This type of system can be obtained through the functionalization of the external surface, for example, with some linkers. It has been observed that the functionalisation of the outer surface of nanotubes by the addition of triazolium salts is useful for the transport of curcumin. This system, in addition to demonstrating high efficiency for curcumin encapsulation and for controlled and prolonged release capacity, shows cytotoxic effects against different tumour cell lines [141]. The bioavailability of ciprofloxacin (CIP), due to its complexation with iron present in the body, decreases subsequent to administration. Entrapping the drug in a carrier could be a solution. To this end, CIP was loaded onto APTES modified halloysite nanotubes. The result was a high adsorption capacity in modified HNTs for CIP (70% ± 1.7%), compared to that of pristine halloysite nanotubes [142]. A multi-layered polylactic acid (PLA)/HNT porous membrane encapsulated with gentamicin was prepared for use in bone regeneration as antibacterial membrane. The membrane was shown to have good antibacterial efficacy against both Gram-negative and Gram-positive bacteria, suggesting that it could be used in the prevention of infection in bone regeneration applications [143]. Over the years, systems for pulmonary drug delivery systems have been developed to treat lung disease. Jermi et al. designed a clay-based system with release capability of dexamethasone (Dex), to be used in coronavirus disease (COVID-19) treatment. They designed the system ZnFe2O4/Hal/DEX/PEG (Dex 5% wt/wt) sensitive to pH 5.6, able to release Dex at pulmonary infectious pH conditions [144]. Bordini et al. have developed an injectable GelMA-based nanotube modified hydrogel for controlled release of dexamethasone, illustrating the potential of this system for mineralized tissue regeneration. The DEX-loaded nanotube modified GelMA hydrogel (GelMA + 5.0%HNT-DEX10%) showed relevant properties from a mechanical point of view, but also biodegradability and cytocompatibility with mesenchymal stem cells from human exfoliated deciduous teeth (SHEDs). Moreover, the system had in vivo biocompatibility, and it also supported bone regeneration in vivo [145]. Phototherapy is described as a safe and secure way to destroy cancer cells when light waves of a particular wavelength are used with appropriate activating agents. Li et al. developed halloysite nanotubes decorated with poly(sodium-p-styrenesulfonate) (PSS) to enhance the biocompatibility, and further functionalized by lumen loading the type-II photosensitizer indocyanine green (ICG) to obtain a biomimetic nanocarrier platform for target-specific delivery of phototherapeutic agents. The obtained system, HNT-PSS-ICG, showed an excellent in vivo phototherapeutic effect against breast cancer in model mice [146]. Tan et al. developed a system of HNT-based multifunctional nanoparticles designed for tumour targeting and phototherapy in breast cancer treatment. Fluorescein isothiocyanate (FITC) was adsorbed on the HNT surfaces, and indocyanine green (ICG) was loaded as the photothermal agent into the lumen. To enhance the biocompatibility, the system was wrapped with red blood cell membrane (RBCM). Finally, anti-EpCAM was conjugated with HNTs-FITC-ICG-RBCM with the assistance of streptavidin to improve the specific uptake of breast cancer cells. The cell viability assay results for MCF-7 cells indicated that the HNTs-FITC-ICG-RBCM cytotoxicity was irradiation time and concentration dependent and that this could be potentially used in breast cancer treatment [147]. As evidenced by Lisuzzo et al., HNTs are excellent candidates as interfacially active inorganic particles for the formation of Pickering emulsions thanks to their advantages, as for example low cost, biocompatibility, mechanical strength, tubular morphology, etc. [148]. The functionalization of HNT surfaces allows the synthesis of infinite nano-architectures which have different properties and can be modulated according to the desired performance and the field of application. It is, therefore, clear why it has led to a deepening of the research for their possible applications.
6. Conclusions
Halloysite nanotubes are naturally occurring and cost-effective nanomaterials that are finding applications in several areas, some discussed here. Several types of functionalisation of HNT have been discussed, including the advantageous tubular structure that has given it numerous roles as drug delivery and gene delivery agents or nanocarriers. Recent studies demonstrate the potential of halloysite clay nanotubes for life science applications since results suggest that HNTs are a safe nanomaterial which can be used in biomaterials without serious side effects. However, more studies are needed to clarify the in vivo outcomes of long and chronic oral exposure to HNTs, which seems to depend on the administered concentration. Overall, the results obtained to date open wide prospects of investigation to better understand the use of these systems for a potential application as drug carrier and delivery systems. The combination of the innumerable properties of halloysite nanotubes, together with their biocompatibility and the possibility of functionalising the surfaces, makes them ideal candidates for the development of an innovative therapeutic approach.
Acknowledgments
We thank Daniele Francofonte for his excellent assistance and technical support for images and table realization.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boverhof D.R., Bramante C.M., Butala J.H., Clancy S.F., Lafranconi M., West J., Gordon S.C. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. 2015;73:137–150. doi: 10.1016/j.yrtph.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad H., Fan M., Hui D. Graphene oxide incorporated functional materials: A review. Compos. Part B Eng. 2018;145:270–280. doi: 10.1016/j.compositesb.2018.02.006. [DOI] [Google Scholar]
- 3.Guo S., Fu D., Utupova A., Sun D., Zhou M., Jin Z., Zhao K. Applications of polymer-based nanoparticles in vaccine field. Nanotechnol. Rev. 2019;8:143–155. doi: 10.1515/ntrev-2019-0014. [DOI] [Google Scholar]
- 4.Yang Z., Yang J., Liu A., Fu J. Nonlinear in-plane instability of functionally graded multilayer graphene reinforced composite shallow arches. Compos. Struct. 2018;204:301–312. doi: 10.1016/j.compstruct.2018.07.072. [DOI] [Google Scholar]
- 5.Tam M., Yang Z., Zhao S., Yang J. Vibration and Buckling Characteristics of Functionally Graded Graphene Nanoplatelets Reinforced Composite Beams with Open Edge Cracks. Materials. 2019;12:1412. doi: 10.3390/ma12091412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou C.H., Keeling J. Fundamental and applied research on clay minerals: From climate and environment to nanotechnology. Appl. Clay Sci. 2013;74:3–9. doi: 10.1016/j.clay.2013.02.013. [DOI] [Google Scholar]
- 7.Moraes J.D.D., Bertolino S.R.A., Cuffini S.L., Ducart D.F., Bretzke P.E., Leonardi G.R. Clay minerals: Properties and applications to dermocosmetic products and perspectives of natural raw materials for therapeutic purposes—A review. Int. J. Pharm. 2017;534:213–219. doi: 10.1016/j.ijpharm.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Aguzzi C., Cerezo P., Viseras C., Caramella C. Use of clays as drug delivery systems: Possibilities and limitations. Appl. Clay Sci. 2007;36:22–36. doi: 10.1016/j.clay.2006.06.015. [DOI] [Google Scholar]
- 9.Joussein E., Petit S., Fialips C.I., Vieillard P., Righi D. Differences in the Dehydration-Rehydration Behavior of Halloysites: New Evidence and Interpretations. Clays Clay Miner. 2006;54:473–484. doi: 10.1346/CCMN.2006.0540408. [DOI] [Google Scholar]
- 10.Ventrapragada L.K., Creager S.E., Rao A.M., Podila R. Carbon nanotubes coated paper as current collectors for secondary Li-ion batteries. Nanotechnol. Rev. 2019;8:18–23. doi: 10.1515/ntrev-2019-0002. [DOI] [Google Scholar]
- 11.Moniruzzaman M., Winey K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules. 2006;39:5194–5205. doi: 10.1021/ma060733p. [DOI] [Google Scholar]
- 12.Zakaria M.R., Akil H.M., Kudus M.H.A., Ullah F., Javed F., Nosbi N. Hybrid carbon fiber-carbon nanotubes reinforced polymer composites: A review. Compos. Part B Eng. 2019;176:107313. doi: 10.1016/j.compositesb.2019.107313. [DOI] [Google Scholar]
- 13.Munir K.S., Wen C., Li Y. Carbon nanotubes and graphene as nanoreinforcements in metallic biomaterials: A review. Adv. Biosyst. 2019;3:1800212. doi: 10.1002/adbi.201800212. [DOI] [PubMed] [Google Scholar]
- 14.Gohari G., Safai F., Panahirad S., Akbari A., Rasouli F., Dadpour M.R., Fotopoulos V. Modified multiwall carbon nanotubes display either phytotoxic or growth promoting and stress protecting activity in Ocimum basilicum L. in a concentration-dependent manner. Chemosphere. 2020;249:126171. doi: 10.1016/j.chemosphere.2020.126171. [DOI] [PubMed] [Google Scholar]
- 15.Lampropoulou P., Papoulis D. Halloysite in Different Ceramic Products: A Review. Materials. 2021;14:5501. doi: 10.3390/ma14195501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahmani S., Maroufkhani M., Mohammadzadeh-Komuleh S., Khoubi-Arani Z. Polymer nanocomposites for biomedical applications. In: Barhoum A., Jeevanandam J., Danquah M.K., editors. Micro and Nano Technologies, Fundamentals of Bionanomaterials. Elsevier; Amsterdam, The Netherlands: 2022. pp. 175–215. [Google Scholar]
- 17.Liu M., Fakhrullin R., Novikov A., Panchal A., Lvov Y. Tubule Nanoclay-Organic Heterostructures for Biomedical Applications. Macromol. Biosci. 2019;19:e1800419. doi: 10.1002/mabi.201800419. [DOI] [PubMed] [Google Scholar]
- 18.Satish S., Tharmavaram M., Rawtani D. Halloysite nanotubes as a nature’s boon for biomedical applications. Nanobiomedicine. 2019;6:1849543519863625. doi: 10.1177/1849543519863625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X., Zhou C., Liu M. Self-assembled structures of halloysite nanotubes: Towards the development of high-performance biomedical materials. J. Mater. Chem. B. 2020;8:838–851. doi: 10.1039/C9TB02460C. [DOI] [PubMed] [Google Scholar]
- 20.Ghalei S., Hopkins S., Douglass M., Garren M., Mondal A., Handa H. Nitric oxide releasing halloysite nanotubes for biomedical applications. J. Colloid Interface Sci. 2021;590:277–289. doi: 10.1016/j.jcis.2021.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prishchenko D.A., Zenkov E.V., Mazurenko V.V., Fakhrullin R.W., Lvov Y.M., Mazurenko V.G. Molecular dynamics of the halloysite nanotubes. Phys. Chem. Chem. Phys. 2018;20:5841–5849. doi: 10.1039/C7CP06575B. [DOI] [PubMed] [Google Scholar]
- 22.Santos A.C., Ferreira C., Veiga F., Ribeiro A.J., Panchal A., Lvov Y., Agarwal A. Halloysite clay nanotubes for life sciences applications: From drug encapsulation to bioscaffold. Adv. Colloid Interface Sci. 2018;257:58–70. doi: 10.1016/j.cis.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Hasani M., Abdouss M., Shojaei S. Nanocontainers for drug delivery systems: A review of Halloysite nanotubes and their properties. Int. J. Artif. Organs. 2020;44:426–433. doi: 10.1177/0391398820968836. [DOI] [PubMed] [Google Scholar]
- 24.Kotova O., Sun S., Kotova E., Ponariaydov A., Brodskaya R. Aluminosilicates: Interphase boundary interactions and nature engineering of nanostructures. J. Phys. Conf. Ser. 2022;2315:012003. doi: 10.1088/1742-6596/2315/1/012003. [DOI] [Google Scholar]
- 25.Vergaro V., Abdullayev E., Lvov Y.M., Zeitoun A., Cingolani R., Rinaldi R., Leporatti S. Cytocompatibility and Uptake of Halloysite Clay Nanotubes. Biomacromolecules. 2010;11:820–826. doi: 10.1021/bm9014446. [DOI] [PubMed] [Google Scholar]
- 26.Massaro M., Cavallaro G., Colletti C.G., Lazzara G., Milioto S., Noto R., Riela S. Chemical modification of halloysite nanotubes for controlled loading and release. J. Mater. Chem. B. 2018;6:3415–3433. doi: 10.1039/C8TB00543E. [DOI] [PubMed] [Google Scholar]
- 27.Churchman G., Carr R.M. The Definition and Nomenclature of Halloysites. Clays Clay Miner. 1975;23:382–388. doi: 10.1346/CCMN.1975.0230510. [DOI] [Google Scholar]
- 28.Massaro M., Lazzara G., Milioto S., Noto R., Riela S. Covalently modified halloysite clay nanotubes: Synthesis, properties, biological and medical applications. J. Mater. Chem. B. 2017;5:4246. doi: 10.1039/C7TB90071F. [DOI] [PubMed] [Google Scholar]
- 29.Lvov Y., Panchal A., Fu Y., Fakhrullin R., Kryuchkova M., Batasheva S., Stavitskaya A., Glotov A., Vinokurov V. Interfacial Self-Assembly in Halloysite Nanotube Composites. Langmuir. 2019;35:8646–8657. doi: 10.1021/acs.langmuir.8b04313. [DOI] [PubMed] [Google Scholar]
- 30.Chow W.S., Tham W.L., Seow P.C. Effects of maleated-PLA compatibilizer on the properties of poly(lactic acid)/halloysite clay composites. J. Thermoplast. Compos. Mater. 2013;26:1349–1363. doi: 10.1177/0892705712439569. [DOI] [Google Scholar]
- 31.Wilson I., Keeling J. Global occurrence, geology and characteristics of tubular halloysite deposits. Clay Miner. 2016;51:309–324. doi: 10.1180/claymin.2016.051.3.12. [DOI] [Google Scholar]
- 32.Teo Z.X., Chow W.S. Impact, thermal, and morphological properties of poly(lactic acid)/poly(methyl methacrylate)/halloysite nanotube nanocomposites. Polym.-Plast. Technol. Eng. 2016;55:1474–1480. doi: 10.1080/03602559.2015.1132464. [DOI] [Google Scholar]
- 33.Duarte H.A., Lourenço M.P., Heine T., Guimarães L. Clay Mineral Nanotubes: Stability, Structure and Properties. In: Innocenti A., Kamarulzaman N., editors. Stoichiometry and Materials Science—When Numbers Matter. 1st ed. Intech Open; London, UK: 2012. [DOI] [Google Scholar]
- 34.Du M., Guo B., Jia D. Newly emerging applications of halloysite nanotubes: A review. Polym. Int. 2010;59:574–582. doi: 10.1002/pi.2754. [DOI] [Google Scholar]
- 35.Pasbakhsh P., Churchman G.J., Keeling J.L. Characterisation of properties of various halloysites relevant to their use as nanotubes and microfibre fillers. Appl. Clay Sci. 2013;74:47–57. doi: 10.1016/j.clay.2012.06.014. [DOI] [Google Scholar]
- 36.Daraie M., Bagheri D., Malmir M., Heravi M.M. Investigation of halloysite nanotubes and Schiff base combination with deposited copper iodide nanoparticles as a novel heterogeneous catalytic system. Sci. Rep. 2021;11:23658. doi: 10.1038/s41598-021-02991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guimaraes L., Enyashin A.N., Seifert G., Duarte H.A. Structural, electronic, and mechanical properties of single-walled halloysite nanotube models. J. Chem. Phys. 2010;114:11358–11363. doi: 10.1021/jp100902e. [DOI] [Google Scholar]
- 38.Joo Y., Sim J.H., Jeon Y., Lee S.U., Sohn D. Opening and blocking the inner-pores of Halloysite. Chem. Commun. 2013;49:4519–4521. doi: 10.1039/c3cc40465j. [DOI] [PubMed] [Google Scholar]
- 39.Bugatti V., Sorrentino A., Gorrasi G. Encapsulation of Lysozyme into halloysite nanotubes and dispersion in PLA: Structural and physical properties and controlled release analysis. Eur. Polym. J. 2017;93:495–506. doi: 10.1016/j.eurpolymj.2017.06.024. [DOI] [Google Scholar]
- 40.Albdiry M.T., Yousif B.F. Role of silanized halloysite nanotubes on structural, mechanical properties and fracture toughness of thermoset nanocomposites. Mater. Des. 2014;57:279–288. doi: 10.1016/j.matdes.2013.12.017. [DOI] [Google Scholar]
- 41.Abdullayev E., Lvov Y. Halloysite clay nanotubes as a ceramic “skeleton” for functional biopolymer composites with sustained drug release. J. Mater. Chem. B. 2013;1:2894–2903. doi: 10.1039/c3tb20059k. [DOI] [PubMed] [Google Scholar]
- 42.Pereira I., Saleh M., Nunes C., Reis S., Veiga F., Paiva-Santos A.C. Preclinical developments of natural-occurring halloysite clay nanotubes in cancer therapeutics. Adv. Colloid Interface Sci. 2021;291:102406. doi: 10.1016/j.cis.2021.102406. [DOI] [PubMed] [Google Scholar]
- 43.Yendluri R., Lvov Y., De Villiers M.M., Vinokurov V., Naumenko E., Tarasova E., Fakhrullin R. Paclitaxel Encapsulated in Halloysite Clay Nanotubes for Intestinal and Intracellular Delivery. J. Pharm. Sci. 2017;10:3131–3139. doi: 10.1016/j.xphs.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 44.Massaro M., Lazzara G., Noto R., Riela S. Halloysite nanotubes: A green resource for materials and life sciences. Rend. Lincei. Sci. Fis. Nat. 2020;31:213–221. doi: 10.1007/s12210-020-00886-x. [DOI] [Google Scholar]
- 45.Joussein E., Petit S., Delvaux B. Behavior of halloysite clay under formamide treatment. Appl. Clay Sci. 2007;35:17–24. doi: 10.1016/j.clay.2006.07.002. [DOI] [Google Scholar]
- 46.Hillier S., Ryan P.C. Identification of halloysite (7 Å) by ethylene glycol solvation: The ‘MacEwan effect’. Clay Miner. 2002;37:487–496. doi: 10.1180/0009855023730047. [DOI] [Google Scholar]
- 47.Fisher G.B., Ryan P.C. The smectite-to-disordered kaolinite transition in a tropical soil chrono sequence, Pacific coast, Costa Rica. Clays Clay Miner. 2006;54:571–586. doi: 10.1346/CCMN.2006.0540504. [DOI] [Google Scholar]
- 48.Hendricks S.B., Jefferson M.E. Structures of kaolin and talc-pyrophyllite hydrates and their bearing on water sorption of the clays. Am. Mineral. 1938;23:863–875. [Google Scholar]
- 49.Lipsicas M., Straley C., Costanzo P.M., Giese R.F., Jr. Static and dynamic structure of water in hydrated kaolinites. II. The dynamic structure. J. Colloid Interface Sci. 1985;107:221–230. doi: 10.1016/0021-9797(85)90165-1. [DOI] [Google Scholar]
- 50.Smirnov K.S., Bougeard D. A molecular dynamics study of structure and short-time dynamics of water in kaolinite. J. Phys. Chem. B. 1999;103:5266–5273. doi: 10.1021/jp9900281. [DOI] [Google Scholar]
- 51.Yuan P., Southon P.D., Liu Z., Green M.E.R., Hook J.M., Antill S.J., Kepert C.J. Functionalization of Halloysite Clay Nanotubes by grafting with γ-Aminopropyltriethoxysilane. J. Phys. Chem. C. 2008;112:15742–15751. doi: 10.1021/jp805657t. [DOI] [Google Scholar]
- 52.Tan D., Yuan P., Liu D., Du P. Developments in Clay Science. Volume 7. Elsevier; Amsterdam, The Netherlands: 2016. Surface Modifications of Halloysite in Nanosized Tubular Clay Minerals; pp. 167–201. Chapter 8. [Google Scholar]
- 53.Peer D., Karp J., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 54.Xia Y., Xiong Y., Lim B., Skrabalak S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. 2009;48:60–103. doi: 10.1002/anie.200802248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zielińska A., Costa B., Ferreira M.V., Miguéis D., Louros J.M.S., Durazzo A., Lucarini M., Eder P., Chaud M.V., Morsink M., et al. Nanotoxicology and Nanosafety: Safety-By-Design and Testing at a Glance. Int. J. Environ. Res. Public Health. 2020;13:4657. doi: 10.3390/ijerph17134657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gatoo M.A., Naseem S., Arfat M.Y., Dar A.M., Qasim K., Zubair S. Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations. BioMed. Res. Int. 2014;2014:498420. doi: 10.1155/2014/498420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Powers K.W., Palazuelos M., Moudgil B.M., Roberts S.M. Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies. Nanotoxicology. 2007;1:42–51. doi: 10.1080/17435390701314902. [DOI] [Google Scholar]
- 58.Foroozandeh P., Aziz A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018;13:339. doi: 10.1186/s11671-018-2728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang X.Q., Xu X., Bertrand N., Pridgen E., Swami A., Farokhzad O.C. Interactions of nanomaterials and biological systems: Implications to personalized nanomedicine. Adv. Drug Deliv. Rev. 2012;64:1363–1384. doi: 10.1016/j.addr.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gehr P. Interaction of nanoparticles with biological systems. Colloids Surf. B Biointerfaces. 2018;172:395–399. doi: 10.1016/j.colsurfb.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 61.Yah C.S., Iyuke S.E., Simate G.S. A review of nanoparticles toxicity and their routes of exposures. Iran. J. Pharm. Sci. 2012;8:299–314. [PubMed] [Google Scholar]
- 62.Teleanu D.M., Negut I., Grumezescu V., Grumezescu A.M., Teleanu R.I. Nanomaterials for Drug Delivery to the Central Nervous System. Nanomaterials. 2019;9:371. doi: 10.3390/nano9030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Jong W.H., Hagens W.I., Krystek P., Burger M.C., Sips A.J., Geertsma R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 64.Rizvi S.A.A., Saleh A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018;26:64–70. doi: 10.1016/j.jsps.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi H.S., Liu W., Misra P., Tanaka E., Zimmer J.P., Itty Ipe B., Bawendi M.G., Frangioni J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang S., Li Y., Fan J., Wang Z., Zeng X., Sun Y., Song P., Ju D. The role of autophagy in the neurotoxicity of cationic PAMAM dendrimers. Biomaterials. 2014;35:7588–7597. doi: 10.1016/j.biomaterials.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 68.Cullen R.T., Searl A., Miller B.G., Davis J.M., Jones A.D. Pulmonary and intraperitoneal inflammation induced by cellulose fibres. J. Appl. Toxicol. 2000;20:49–60. doi: 10.1002/(SICI)1099-1263(200001/02)20:1<49::AID-JAT627>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 69.Huang Y.C., Vieira A., Huang K.L., Yeh M.K., Chiang C.H. Pulmonary inflammation caused by chitosan microparticles. J. Biomed. Mater. Res. 2005;75:283–287. doi: 10.1002/jbm.a.30421. [DOI] [PubMed] [Google Scholar]
- 70.Kim J.Y., Kim H.H., Cho K.H. Acute cardiovascular toxicity of sterilizers, PHMG, and PGH: Severe inflammation in human cells and heart failure in zebrafish. Cardiovasc. Toxicol. 2013;13:148–160. doi: 10.1007/s12012-012-9193-8. [DOI] [PubMed] [Google Scholar]
- 71.Yu Z., Li Q., Wang J., Yu Y., Wang Y., Zhou Q., Li P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020;15:115. doi: 10.1186/s11671-020-03344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Auffan M., Achouak W., Rose J., Roncato M.A., Chanéac C., Waite D.T., Masion A., Woicik J.C., Wiesner M.R., Bottero J.Y. Relation between the redox state of iron-based nanoparticles and their cytotoxicity toward Escherichia coli. Environ. Sci. Technol. 2008;42:6730–6735. doi: 10.1021/es800086f. [DOI] [PubMed] [Google Scholar]
- 73.Kamat J.P., Devasagayam T.P., Priyadarsini K.I., Mohan H. Reactive oxygen species mediated membrane damage induced by fullerene derivatives and its possible biological implications. Toxicology. 2000;155:55–61. doi: 10.1016/S0300-483X(00)00277-8. [DOI] [PubMed] [Google Scholar]
- 74.Walczyk D., Bombelli F.B., Monopoli M.P., Lynch I., Dawson K.A. What the Cell ‘Sees’ in Bionanoscience. J. Am. Chem. Soc. 2010;132:5761–5768. doi: 10.1021/ja910675v. [DOI] [PubMed] [Google Scholar]
- 75.Hussain S.M., Hess K.L., Gearhart J.M., Geiss K.T., Schlager J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In Vitro. 2005;19:975–983. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 76.Leroueil P.R., Berry S.A., Duthie K., Han G., Rotello V.M., McNerny D.Q., Baker J.R., Jr., Orr B.G., Holl M.M. Wide varieties of cationic nanoparticles induce defects in supported lipid bilayers. Nano Lett. 2008;8:420–424. doi: 10.1021/nl0722929. [DOI] [PubMed] [Google Scholar]
- 77.Hauck T.S., Ghazani A.A., Chan W.C.W. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small. 2008;4:153–159. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]
- 78.Gupta R., Xie H. Nanoparticles in daily life: Applications, toxicity and regulations. J. Environ. Pathol. Toxicol. Oncol. 2018;37:209–230. doi: 10.1615/JEnvironPatholToxicolOncol.2018026009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mousa M., Evans N.D., Oreffo R.O.C., Dawson J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials. 2018;159:204–214. doi: 10.1016/j.biomaterials.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 80.Setter O.F., Segal E. Halloysite nanotubes—the nano-bio interface. Nanoscale. 2020;12:23444–23460. doi: 10.1039/D0NR06820A. [DOI] [PubMed] [Google Scholar]
- 81.Sawicka D., Zapor L., Chojnacka-Puchta L., Miranowicz-Dzierzawska K. The in vitro toxicity evaluation of halloysite nanotubes (HNTs) in human lung cells. Toxicol. Res. 2020;37:301–310. doi: 10.1007/s43188-020-00062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verma N.K., Moore E., Blau W., Volkov Y., Babu R.P. Cytotoxicity evaluation of nanoclays in human epithelial cell line A549 using high content screening and real-time impedance analysis. J. Nanopart. Res. 2012;14:1137. doi: 10.1007/s11051-012-1137-5. [DOI] [Google Scholar]
- 83.Lai X., Agarwal M., Lvov Y.M., Pachpande C., Varahramyan K., Witzmann F.A. Proteomic profiling of halloysite clay nanotube exposure in intestinal cell co-culture. J. Appl. Toxicol. 2013;11:1316–1329. doi: 10.1002/jat.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu H.Y., Du L., Zhao Y.T., Tian W.Q. In Vitro Hemocompatibility and Cytotoxicity Evaluation of Halloysite Nanotubes for Biomedical Application. J. Nanomater. 2015;16:685323. doi: 10.1155/2015/685323. [DOI] [Google Scholar]
- 85.Sánchez-Fernández A., Peña-Parás L., Vidaltamayo R., Cué-Sampedro R., Mendoza-Martínez A., Zomosa-Signoret V.C., Rivas-Estilla A.M., Riojas P. Synthesization, Characterization, and in Vitro Evaluation of Cytotoxicity of Biomaterials Based on Halloysite Nanotubes. Materials. 2014;7:7770–7780. doi: 10.3390/ma7127770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khodzhaeva V., Makeeva A., Ulyanova V., Zelenikhin P., Evtugyn V., Hardt M., Rozhina E., Lvov Y., Fakhrullin R., Ilinskaya O. Binase Immobilized on Halloysite Nanotubes Exerts Enhanced Cytotoxicity toward Human Colon Adenocarcinoma Cells. Front. Pharmacol. 2017;8:631. doi: 10.3389/fphar.2017.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamalieva R.F., Ishmukhametov I.R., Batasheva S.N., Rozhina E.V., Fakhrullin R.F. Uptake of halloysite clay nanotubes by human cells: Colourimetric viability tests and microscopy study. Nano-Struct. Nano-Objects. 2018;15:54–60. doi: 10.1016/j.nanoso.2018.03.009. [DOI] [Google Scholar]
- 88.Biddeci G., Spinelli G., Massaro M., Riela S., Bonaccorsi P., Barattucci A., Di Blasi F. Study of Uptake Mechanisms of Halloysite Nanotubes in Different Cell Lines. Int. J. Nanomed. 2021;16:4755–4768. doi: 10.2147/IJN.S303816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Long Z., Wu Y.P., Gao H.Y., Zhang J., Ou X., He R.R., Liu M. In vitro and in vivo toxicity evaluation of halloysite nanotubes. J. Mater. Chem. B. 2018;6:7204–7216. doi: 10.1039/C8TB01382A. [DOI] [PubMed] [Google Scholar]
- 90.Ahmed F.R., Shoaib M.H., Azhar M., Um S.H., Yousuf R.I., Hashmi S., Dar A. In-vitro assessment of cytotoxicity of halloysite nanotubes against HepG2, HCT116 and human peripheral blood lymphocytes. Colloids Surf. B Biointerfaces. 2015;135:50–55. doi: 10.1016/j.colsurfb.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 91.Fakhrullin G.I., Akhatova F.S., Lvov Y.M., Fakhrullin R.F. Toxicity of halloysite clay nanotubes in vivo: A Caenorhabditis elegans study. Environ. Sci. Nano. 2015;2:54–59. doi: 10.1039/C4EN00135D. [DOI] [Google Scholar]
- 92.Bellani L., Giorgetti L., Riela S., Lazzara G., Scialabba A., Massaro M. Ecotoxicity of halloysite nanotube-supported palladium nanoparticles in Raphanus sativus L. Environ. Toxicol. 2016;35:2503–2510. doi: 10.1002/etc.3412. [DOI] [PubMed] [Google Scholar]
- 93.Wang X., Gong J., Rong R., Gui Z., Hu T., Xu X. Halloysite Nanotubes-Induced Al Accumulation and Fibrotic Response in Lung of Mice after 30-Day Repeated Oral Administration. J. Agric. Food Chem. 2018;66:2925–2933. doi: 10.1021/acs.jafc.7b04615. [DOI] [PubMed] [Google Scholar]
- 94.Hu T., Wang X., Tan W., Nie K., Xu X. Nitric oxide synthase-mediated sub-chronic injury and recovery in the small intestine of mice after oral administration with halloysite nanotubes. Environ. Sci. Pollut. Res. Int. 2020;15:17730–17737. doi: 10.1007/s11356-020-08314-1. [DOI] [PubMed] [Google Scholar]
- 95.Khatoon N., Chub M.Q., Zhou C.H. Nanoclay-based drug delivery systems and their therapeutic potentials. J. Mater. Chem. B. 2020;8:7335–7351. doi: 10.1039/D0TB01031F. [DOI] [PubMed] [Google Scholar]
- 96.Veerabadran N.G., Price R.R., Lvov Y.M. Clay nanotubes for encapsulation and sustained release of drugs. Nano. 2007;2:115–120. doi: 10.1142/S1793292007000441. [DOI] [Google Scholar]
- 97.Hanif M., Jabbar F., Sharif S., Abbas G., Farooq A., Aziz M. Halloysite nanotubes as a new drug-delivery system: A review. Clay Miner. 2016;51:469–477. doi: 10.1180/claymin.2016.051.3.03. [DOI] [Google Scholar]
- 98.Price R.R., Gaber B.P., Lvov Y. In-vitro release characteristics of tetracycline HCl, khellin and nicotinamide adenine dineculeotide from halloysite; a cylindrical mineral. J. Microencapsul. 2001;18:713–722. doi: 10.1080/02652040010019532. [DOI] [PubMed] [Google Scholar]
- 99.Lisuzzo L., Cavallaro G., Pasbakhsh P., Milioto S., Lazzara G. Why does vacuum drive to the loading of halloysite nanotubes? The key role of water confinement. J. Colloid Interface Sci. 2019;547:361–369. doi: 10.1016/j.jcis.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 100.Yang Y., Chen Y., Leng F., Huang L., Wang Z., Tian W. Recent Advances on Surface Modification of Halloysite Nanotubes for Multifunctional Applications. Appl. Sci. 2017;7:1215. doi: 10.3390/app7121215. [DOI] [Google Scholar]
- 101.Danyliuk N., Tomaszewska J., Tatarchuk T. Halloysite nanotubes and halloysite-based composites for environmental and biomedical applications. J. Mol. Liq. 2020;309:113077. doi: 10.1016/j.molliq.2020.113077. [DOI] [Google Scholar]
- 102.Lvov Y.M., DeVilliers M.M., Fakhrullin R.F. The application of halloysite tubule nanoclay in drug delivery. Expert Opin. Drug Deliv. 2016;13:977–986. doi: 10.1517/17425247.2016.1169271. [DOI] [PubMed] [Google Scholar]
- 103.Lvov Y., Abdullayev E. Functional polymer–clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013;38:1690–1719. doi: 10.1016/j.progpolymsci.2013.05.009. [DOI] [Google Scholar]
- 104.Massaro M., Amorati R., Cavallaro G., Guernelli S., Lazzara G., Milioto S., Noto R., Poma P., Riela S. Direct chemical grafted curcumin on halloysite nanotubes as dual-responsive prodrug for pharmacological applications. Colloids Surf. B Biointerfaces. 2016;140:505–513. doi: 10.1016/j.colsurfb.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 105.Massaro M., Noto R., Riela S. Past, Present and Future Perspectives on Halloysite Clay Minerals. Molecules. 2020;25:4863. doi: 10.3390/molecules25204863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lu D., Chen H., Wu J., Chan C.M. Direct measurements of the Young’s modulus of a single halloysite nanotube using a transmission electron microscope with a bending stage. J. Nanosci. Nanotechnol. 2011;11:7789–7793. doi: 10.1166/jnn.2011.4720. [DOI] [PubMed] [Google Scholar]
- 107.Fakhrullina G., Khakimova E., Akhatova F., Lazzara G., Parisi F., Fakhrullin R. Selective Antimicrobial Effects of Curcumin@Halloysite Nanoformulation: A Caenorhabditis elegans Study. ACS Appl. Mater. Interfaces. 2019;26:23050–23064. doi: 10.1021/acsami.9b07499. [DOI] [PubMed] [Google Scholar]
- 108.Luo X., Zhang J., Wu Y.P., Yang X., Kuang X.P., Li W.X., Li Y.F., He R.R., Liu M. Multifunctional HNT@Fe3O4@PPy@DOX Nanoplatform for Effective Chemo-Photothermal Combination Therapy of Breast Cancer with MR Imaging. ACS Biomater. Sci. Eng. 2020;6:3361–3374. doi: 10.1021/acsbiomaterials.9b01709. [DOI] [PubMed] [Google Scholar]
- 109.Massaro M., Poma P., Cavallaro G., García-Villén F., Lazzara G., Notarbartolo M., Muratore N., Sánchez-Espejo R., Viseras Iborra C., Riela S. Prodrug based on halloysite delivery systems to improve the antitumor ability of methotrexate in leukemia cell lines. Colloids Surf. B Biointerfaces. 2022;213:112385. doi: 10.1016/j.colsurfb.2022.112385. [DOI] [PubMed] [Google Scholar]
- 110.Nyankson E., Aboagye S.O., Efavi J.K., Agyei-Tuffour B., Paemka L., Asimeng B.O., Balapangu S., Arthur P.K., Tiburu E.K. Chitosan-Coated Halloysite Nanotubes As Vehicle for Controlled Drug Delivery to MCF-7 Cancer Cells In Vitro. Materials. 2021;14:2837. doi: 10.3390/ma14112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liao J., Wang D., Tang A., Fu L., Ouyang J., Yang H. Surface modified halloysite nanotubes with different lumen diameters as drug carriers for cancer therapy. Chem. Commun. 2021;57:9470–9473. doi: 10.1039/D1CC01879E. [DOI] [PubMed] [Google Scholar]
- 112.Rao K.M., Kumar A., Suneetha M., Han S.S. pH and near-infrared active; chitosan-coated halloysite nanotubes loaded with curcumin-Au hybrid nanoparticles for cancer drug delivery. Int. J. Biol. Macromol. 2018;112:119–125. doi: 10.1016/j.ijbiomac.2018.01.163. [DOI] [PubMed] [Google Scholar]
- 113.Taheri-Ledari R., Zhang W., Radmanesh M., Cathcart N., Maleki A., Kitaev V. Plasmonic photothermal release of docetaxel by gold nanoparticles incorporated onto halloysite nanotubes with conjugated 2D8-E3 antibodies for selective cancer therapy. J Nanobiotechnol. 2021;19:239. doi: 10.1186/s12951-021-00982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hamedi S., Koosha M. Designing a pH-responsive drug delivery system for the release of black-carrot anthocyanins loaded in halloysite nanotubes for cancer treatment. Appl. Clay Sci. 2020;197:105770. doi: 10.1016/j.clay.2020.105770. [DOI] [Google Scholar]
- 115.Li W., Liu D., Zhang H., Correia A., Mäkilä E., Salonen J., Hirvonen J., Santos H.A. Microfluidic assembly of a nano-in-micro dual drug delivery platform composed of halloysite nanotubes and a pH-responsive polymer for colon cancer therapy. Acta Biomater. 2017;48:238–246. doi: 10.1016/j.actbio.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 116.Rizzo C., Arrigo R., D’Anna F., Di Blasi F., Dintcheva N.T., Lazzara G., Parisi F., Riela S., Spinelli G., Massaro M.J. Hybrid supramolecular gels of Fmoc-F/halloysite nanotubes: Systems for sustained release of camptothecin. J. Mater. Chem. B. 2017;5:3217–3229. doi: 10.1039/C7TB00297A. [DOI] [PubMed] [Google Scholar]
- 117.Dramou P., Fizir M., Taleb A., Itatahine A., Dahiru N.S., Mehdi Y.A., Wei L., Zhang J., He H. Folic acid-conjugated chitosan oligosaccharide-magnetic halloysite nanotubes as a delivery system for camptothecin. Carbohydr. Polym. 2018;197:117–127. doi: 10.1016/j.carbpol.2018.05.071. [DOI] [PubMed] [Google Scholar]
- 118.Kerdsakundee N., Li W., Martins J.P., Liu Z., Zhang F., Kemell M., Correia A., Ding Y., Airavaara M., Hirvonen J., et al. Multifunctional Nanotube-Mucoadhesive Poly (methyl vinyl ether-co-maleic acid) @ Hydroxypropyl Methylcellulose Acetate Succinate Composite for Site-Specific Oral Drug Delivery. Adv. Healthc. Mater. 2017;20:1700629. doi: 10.1002/adhm.201700629. [DOI] [PubMed] [Google Scholar]
- 119.Liu M., Chang Y., Yang J., You Y., He R., Chen T., Zhou C. Functionalized halloysite nanotube by chitosan grafting for drug delivery of curcumin to achieve enhanced anticancer efficacy. J. Mater. Chem. B. 2016;4:2253–2263. doi: 10.1039/C5TB02725J. [DOI] [PubMed] [Google Scholar]
- 120.Massaro M., Poma P., Colletti C.G., Barattucci A., Bonaccorsi P.M., Lazzara G., Nicotra G., Parisi F., Salerno T.M.G., Spinella C., et al. Chemical and biological evaluation of cross-linked halloysite-curcumin derivatives. Appl. Clay Sci. 2020;184:105400. doi: 10.1016/j.clay.2019.105400. [DOI] [Google Scholar]
- 121.Bulbul Y.E., Okur M., Demirtas-korkmaz F., Dilsiz N. Development of PCL/PEO electro spun fibrous membranes blended with silane-modified halloysite nanotube as a curcumin release system. Appl. Clay Sci. 2020;186:105430. doi: 10.1016/j.clay.2019.105430. [DOI] [Google Scholar]
- 122.Yang J., Wu Y., Shen Y., Zhou C., Li Y.-F., He R.-R., Liu M. Enhanced therapeutic efficacy of doxorubicin for breast cancer using chitosan oligosaccharide-modified halloysite nanotubes. ACS Appl. Mater. Interfaces. 2016;8:26578–26590. doi: 10.1021/acsami.6b09074. [DOI] [PubMed] [Google Scholar]
- 123.Lee Y., Jung G.E., Cho S.J., Geckeler K.E., Fuchs H. Cellular interactions of doxorubicin-loaded DNA-modified halloysite nanotubes. Nanoscale. 2013;5:8577–8585. doi: 10.1039/c3nr02665e. [DOI] [PubMed] [Google Scholar]
- 124.Hu Y., Chen J., Li X., Sun Y., Huang S., Li Y., Zhong S. Multifunctional halloysite nanotubes for targeted delivery and controlled release of doxorubicin in-vitro and in-vivo studies. Nanotechnology. 2017;28:375101. doi: 10.1088/1361-6528/aa8393. [DOI] [PubMed] [Google Scholar]
- 125.Wu Y.P., Yang J., Gao H.Y., Shen Y., Jiang L., Zhou C., Li Y.F., He R.R., Liu M. Folate-Conjugated Halloysite Nanotubes, an Efficient Drug Carrier, Deliver Doxorubicin for Targeted Therapy of Breast Cancer. ACS Appl. Nano Mater. 2018;1:595–608. doi: 10.1021/acsanm.7b00087. [DOI] [Google Scholar]
- 126.Guo M., Wang A., Muhammad F., Qi W., Ren H., Guo Y., Zhu G. Halloysite nanotubes, a multifunctional nanovehicle for anticancer drug delivery. Chin. J. Chem. 2012;30:2115–2120. doi: 10.1002/cjoc.201200657. [DOI] [Google Scholar]
- 127.Zhang J., Luo X., Wu Y.P., Wu F., Li Y.F., He R.R., Liu M. Rod in Tube: A Novel Nanoplatform for Highly Effective Chemo-Photothermal Combination Therapy toward Breast Cancer. ACS Appl. Mater. Interfaces. 2019;11:3690–3703. doi: 10.1021/acsami.8b17533. [DOI] [PubMed] [Google Scholar]
- 128.Mitchell M.J., Castellanos C.A., King M.R. Nanostructured surfaces to target and kill circulating tumor cells while repelling leukocytes. J. Nanomater. 2012;2012:831263. doi: 10.1155/2012/831263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.He R., Liu M., Shen Y., Liang R., Liu W., Zhou C. Simple fabrication of rough halloysite nanotubes coatings by thermal spraying for high performance tumor cells capture. Mater. Sci. Eng. C. 2018;85:170–181. doi: 10.1016/j.msec.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 130.Lazzara G., Riela S., Fakhrullin R.F. Clay-based drug delivery systems: What does the future hold? Ther. Deliv. 2017;8:633–646. doi: 10.4155/tde-2017-0041. [DOI] [PubMed] [Google Scholar]
- 131.Saleh M.Y., Prajapati N., DeCoster M.A., Lvov Y. Tagged Halloysite Nanotubes as a Carrier for Intercellular Delivery in Brain Microvascular Endothelium. Front. Bioeng. Biotechnol. 2020;8:451. doi: 10.3389/fbioe.2020.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fizir M., Dramou P., Dahiru N.S., Ruya W., Huang T., He H. Halloysite nanotubes in analytical sciences and in drug delivery: A review. Microchim. Acta. 2018;185:389. doi: 10.1007/s00604-018-2908-1. [DOI] [PubMed] [Google Scholar]
- 133.Lvov Y., Wang W., Zhang L., Fakhrullin R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater. 2016;28:1227–1250. doi: 10.1002/adma.201502341. [DOI] [PubMed] [Google Scholar]
- 134.Patel S., Jammalamadaka U., Sun L., Tappa K., Mills D.K. Sustained release of antibacterial agents from doped Halloysite nanotubes. Bioengineering. 2015;3:1. doi: 10.3390/bioengineering3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang H. Selective modification of inner surface of halloysite nanotubes: A review. Nanotechnol. Rev. 2017;6:573–581. doi: 10.1515/ntrev-2017-0163. [DOI] [Google Scholar]
- 136.Joshi A., Abdullayev E., Vasiliev A., Volkova O., Lvov Y. Interfacial modification of clay nanotubes for the sustained release of corrosion inhibitors. Langmuir. 2013;29:7439–7448. doi: 10.1021/la3044973. [DOI] [PubMed] [Google Scholar]
- 137.Dzamukova M.R., Naumenko E.A., Lvov Y.M., Fakhrullin R.F. Enzyme-activated intracellular drug delivery with tubule clay nanoformulation. Sci. Rep. 2015;5:10560. doi: 10.1038/srep10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cavallaro G., Lazzara G., Massaro M., Milioto S., Noto R., Parisi F., Riela S. Biocompatible Poly(N-isopropylacrylamide)-halloysite Nanotubes for Thermoresponsive Curcumin Release. J. Phys. Chem. C. 2015;119:8944–8951. doi: 10.1021/acs.jpcc.5b00991. [DOI] [Google Scholar]
- 139.Massaro M., Buscemi G., Arista L., Biddeci G., Cavallaro G., D’Anna F., Di Blasi F., Ferrante A., Lazzara G., Rizzo C., et al. Multifunctional Carrier Based on Halloysite/Laponite Hybrid Hydrogel for Kartogenin Delivery. ACS Med. Chem. Lett. 2018;10:419–424. doi: 10.1021/acsmedchemlett.8b00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tan D., Yuan P., Annabi-Bergaya F., Liu D., Wang L., Liu H., He H. Loading and in vitro release of ibuprofen in tubular halloysite. Appl. Clay Sci. 2014;96:50–55. doi: 10.1016/j.clay.2014.01.018. [DOI] [Google Scholar]
- 141.Riela S., Massaro M., Colletti C.G., Bommarito A., Giordano C., Milioto S., Noto R., Poma P., Lazzara G. Development and characterization of co-loaded curcumin/triazole-halloysite systems and evaluation of their potential anticancer activity. Int. J. Pharm. 2014;475:613–623. doi: 10.1016/j.ijpharm.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 142.Rawtani D., Pandey G., Tharmavaram M., Pathak P., Akkireddy S., Agrawal Y.K. Development of a novel ‘nanocarrier’ system based on Halloysite Nanotubes to overcome the complexation of ciprofloxacin with iron: An in vitro approach. Appl. Clay Sci. 2017;150:293–302. doi: 10.1016/j.clay.2017.10.002. [DOI] [Google Scholar]
- 143.Pierchala M.K., Makaremi M., Tan H.L., Pushpamalar J., Muniyandy S., Solouk A., Lee S.M., Pasbakhsh P. Nanotubes in nanofibers: Antibacterial multilayered polylactic acid/halloysite/gentamicin membranes for bone regeneration application. Appl. Clay Sci. 2018;160:95–105. doi: 10.1016/j.clay.2017.12.016. [DOI] [Google Scholar]
- 144.Jermy B.R., Ravinayagam V., Almohazey D., Alamoudi W.A., Dafalla H., Akhtar S., Tanimu G. PEGylated green halloysite/spinel ferrite nanocomposites for pH sensitive delivery of dexamethasone: A potential pulmonary drug delivery treatment option for COVID-19. Appl. Clay Sci. 2022;216:106333. doi: 10.1016/j.clay.2021.106333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bordini E.A.F., Ferreira J.A., Dubey N., Ribeiro J.S., De Souza Costa C.A., Soares D.G., Bottino M.C. Injectable Multifunctional Drug Delivery System for Hard Tissue Regeneration under Inflammatory Microenvironments. ACS Appl. BioMater. 2021;4:6993–7006. doi: 10.1021/acsabm.1c00620. [DOI] [PubMed] [Google Scholar]
- 146.Li L.Y., Zhou Y.M., Gao R.Y., Liu X.C., Du H.H., Zhang J.L., Ai X.C., Zhang J.P., Fu L.M., Skibsted L.H. Naturally occurring nanotube with surface modification as biocompatible, target-specific nanocarrier for cancer phototherapy. Biomaterials. 2019;190–191:86–96. doi: 10.1016/j.biomaterials.2018.10.046. [DOI] [PubMed] [Google Scholar]
- 147.Tan C., Zheng J., Feng Y., Liu M. Cell Membrane-Coated Halloysite Nanotubes for Target-Specific Nanocarrier for Cancer Phototherapy. Molecules. 2021;26:4483. doi: 10.3390/molecules26154483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lisuzzo L., Cavallaro G., Milioto S., Lazzara G. Pickering Emulsions Stabilized by Halloysite Nanotubes: From General Aspects to Technological Applications. Adv. Mater. Interfaces. 2022;9:2102346. doi: 10.1002/admi.202102346. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.