Abstract
Cellular senescence is characterized by irreversible cell cycle arrest in response to different triggers and an inflammatory secretome. Although originally described in fibroblasts and cell types of solid organs, cellular senescence affects most tissues with advancing age, including the lymphoid tissue, causing chronic inflammation and dysregulation of both innate and adaptive immune functions. Besides its normal occurrence, persistent microbial challenge or pathogenic microorganisms might also accelerate the activation of cellular aging, inducing the premature senescence of immune cells. Therapeutic strategies counteracting the detrimental effects of cellular senescence are being developed. Their application to target immune cells might have the potential to improve immune dysfunctions during aging and reduce the age-dependent susceptibility to infections. In this review, we discuss how immune senescence influences the host’s ability to resolve more common infections in the elderly and detail the different markers proposed to identify such senescent cells; the mechanisms by which infectious agents increase the extent of immune senescence are also reviewed. Finally, available senescence therapeutics are discussed in the context of their effects on immunity and against infections.
Keywords: aging, senescence, exhaustion, immunity, infections, COVID-19
1. Introduction
Cellular senescence is an irreversible state of cell cycle arrest and is triggered, in normal cells, by exposure to different stresses (oxidative stress, chemotherapeutic drugs, inflammation, oncogene activation, etc.) or irreparable damage. Originally described in fibroblasts, cellular senescence affects most tissues during aging, including lymphoid tissues, causing chronic inflammation and the deregulation of innate and adaptive immune functions. Senescent cells (SnCs) display key features, such as an enlarged nucleus, flattened morphology, a granular phenotype, increased production of lipofuscin, the activity of B-galactosidase (B-gal), the activation of cyclin kinase inhibitor proteins (e.g., p15INK4b, p16INK4a and p21Cip1) and resistance to apoptosis [1].
In addition, SnCs secrete chemokines, cytokines, metalloproteases, reactive oxygen species (ROS), metabolites, miRNAs and extracellular vesicles in a complex defined as the senescent secretory phenotype (SASP) [2,3]. With advanced age, SnCs accumulate in most tissues, likely due to their inefficient removal by the immune system [4,5]. An increased number of SnCs and the chronic secretome are the drivers of “inflammaging”, defined as persistent low-grade inflammation contributing to the enhancement of the frequency and severity of elderly pathologies [6,7]. Of note, the excessive generation of the SASP can also contribute to hyperinflammation (cytokine storm) associated pathogenic responses to stimuli [8].
Because of its numerous consequences on tissue homeostasis, cellular senescence is currently the focus of considerable interest as a pathway that could be targeted to ameliorate aging across multiple tissues [9]. In this review, we discuss the current understanding of age-related changes affecting immune cells and how they influence protective immunity to the most common pathogen infections in the elderly. Moreover, we summarize the strategies that are currently being explored for targeting senescent immune cells to improve immunity against infections.
2. Aging and Immunosenescence
The natural aging process differentially impacts all organ systems and results in the loss of organismal homeostasis, leading to increased mortality and morbidity in the elderly population. It is therefore the greatest risk factor for most chronic diseases [10]. The gradual decline in the immune system during aging is regarded as immunosenescence [11] and arises from two complementary processes: the direct effect of cellular senescence in immune cells and the indirect consequence of tissue cellular senescence. The latter is characterized by weakened organism barriers and fosters the release of various signaling molecules to which immune cells respond [12,13]. In this regard, Chambers et al. described how the recruitment of inflammatory monocytes induced by senescent fibroblasts inhibited tissue-resident memory T-cell activation and proliferation, impairing antigen-specific immunity [14]. This phenomenon of age-related immune remodeling leads to immune response dysregulation and deterioration, causing high susceptibility to infections, reduced vaccination response, deregulated inflammation and cancer [15,16].
Notably, the aged immune system can also induce senescence in peripheral tissues and cause tissue damage. A recent paper defines the contribution of immune system aging to organismal aging. The selective deletion of Ercc1, encoding a crucial DNA repair protein in mouse hematopoietic cells, resulted in the accumulation of spontaneous DNA damage and the premature onset of immunosenescence in mice, which was associated with increased levels of cellular senescence and damage in non-lymphoid organs [17]. Another study described T cells with dysfunctional mitochondria working as accelerators of senescence after selectively deleting the Tfam gene in CD4 and CD8 T cells [18]. T cells from mutated mice showed mitochondrial dysfunction, like those present in aged mice, and higher secretion of proinflammatory cytokines, resulting in premature inflammaging. Of note, these high levels of cytokines act as systemic inducers of senescence, contributing to several age-related impairments, including reduced responses to infections and cardiovascular and cognitive alterations. This suggests that senescent, aged immune cells have a causal role in driving systemic aging and therefore may represent a key therapeutic target to promote healthy aging.
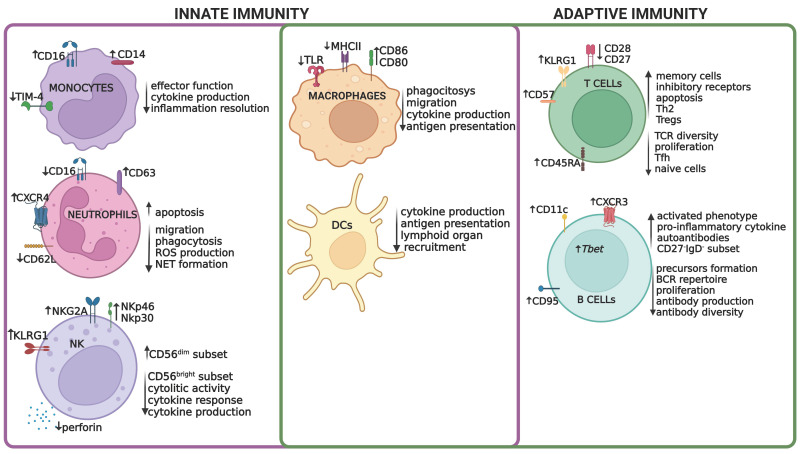
Although immunosenescence is mostly related to failures in adaptive immunity, aging also impacts various functions of the innate immune system, which in turn have been associated with failures in long-term humoral and cellular immunity. The characteristics of immunosenescence have normally been assessed through changes in surface marker expression and in the functional properties of different immune cell populations (Figure 1). Here, we summarize these phenotypic and functional alterations and report how they impact the overall response to infections.
Figure 1.
Simplified picture of phenotypical and functional modifications occurring in innate and adaptive immune cell populations during aging.
2.1. Immunosenescence in the Adaptive Immune System
During aging, progressive thymic involution, linked to the reduced export of naïve T cells, is paralleled by an increase in antigen-experienced and memory effector T cells so that the absolute number of T cells is maintained [19,20].
However, while CD4+ T-cell blood counts are not affected, the CD8+ subset shows a strong decrease in the elderly [21]. Interestingly, the CD4/CD8 ratio value has been proposed as an integrative marker of biological age in older people, correlating with thymic output, phenotypic and functional alterations and general health status [21].
In humans, most T cells express the CD28 antigen. The proportion of CD28+ cells consistently decreases with age and is therefore considered a prognostic indicator of immunosenescence in the older population [22,23]. In addition, CD4+ and CD8+ T cells have been shown to express several inhibitory receptor molecules, including programmed cell death protein 1 (PD-1), lymphocyte activation gene 3 (LAG-3), cytotoxic T-lymphocyte antigen-4 (CTLA-4) and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), also associated with T-cell exhaustion [24]. Highly differentiated CD27-CD28-CD8+ populations display senescence features and can be further identified by the expression of Killer Cell Lectin-Like Receptor G1 (KLRG1) and CD57 markers and intracellular (MAP kinase p38 and γH2AX) molecules [25,26,27]. They also upregulate receptors associated with NK cells, such as inhibitory (NKG2A) and activator proteins (NKG2C and NKG2D) [28]. Recently, it was shown that Sestrins, stress-sensing proteins known to inhibit T-cell proliferation [29], are also responsible for the acquisition of this innate-like killing phenotype [30]. Human senescent T cells can also be characterized by the re-expression of the CD45RA antigen in highly differentiated T cells [31]. Moreover, senescent CD4 T cells expressing PD-1 and CD153 have been shown to accumulate in the spleens of aged mice [32].
T-cell receptor (TCR) diversity contracts with the increased risk of the generation of oligoclonal cell populations. In murine lymphocytes, studies have shown that TCR functionality diminishes with age, with reduced Signal Transducer and Activator of Transcription (STAT) phosphorylation, cfos expression and extracellular signal-regulated kinase (ERK) activation [33]. Impaired responses to stimuli in elderly T cells are associated with their pre-activation state, mirrored by the increased expression of CD25 and HLADR markers [34]. This also resulted in lower proliferative ability (reduced IL7 production) [35] and increased apoptosis (pro-apoptotic shift) [36].
In addition to shortened telomeres, DNA damage responses, constitutive MAP kinase activity and reduced proliferative activity, senescent T cells also exhibit a SASP profile, consisting of proteases and inflammatory cytokines (IL1β and TNFα) [37]. In humans, high SA-β-gal activity and p16 expression in T cells are considered reliable markers of senescence and are correlated with age and disease [38,39]. Similarly, in aged mice, Quinn et al. [40] reported a population of virtual memory CD8 cells displaying high p21 and gH2AX expression and reduced TCR-driven proliferation.
In the context of cytokine production, T-helper type 1 (Th1) cells, which secrete IL-2, IFN-γ and lymphotoxin, have a major role in cellular immunity to intracellular pathogens, virus infections and cancers, whereas Th2 cells, which secrete IL-4, IL-5 and IL-10, play a predominant role in humoral immunity against extracellular parasites [41]. A bias toward a Th2-type cytokine response (IL-4 and IL-10) has been shown to reflect the age-related decline in Th1 activation and differentiation [42]. Reduced differentiation toward T follicular helper (Tfh) cells has been reported as well and correlated with the reduced age-dependent expression of the transcription factor TCF1 [35,43,44]. Moreover, Tfh cells displayed reduced expression of the costimulatory molecule CD40L, which is important for a proper germinal center response [45]. Th17 cells were significantly increased in older individuals, whereas regulatory T cells (Tregs) were reduced. However, the overall Th17/Treg ratio decreased with age, which was associated with elevated Foxp3 mRNA and IL-10 protein expression [46]. These alterations might contribute to imbalanced proinflammatory and anti-inflammatory immune responses, which sustain a higher susceptibility to age-related inflammatory diseases [46]. Specifically in the elderly, augmented Treg function may favor tumor progression, chronic infections or tissue degeneration, whereas impaired Treg function may cause autoimmunity and/or chronic inflammation [47].
Several studies in mice indicated that pre-B, pro-B and immature B cells develop in lower numbers during aging [48], reflecting reduced levels of IL-7 in the bone marrow [48]. Interestingly, the impaired generation of pro-B cells has also been linked to TNF-α, secreted in high amounts by proinflammatory “age-associated B cells” (ABCs), which populate the bone marrow of old mice [49]. Two groups described ABCs in the spleens of aged mice [50,51]: these cells displace FO B cells with advancing age, are refractory to BCR but TLR7/TLR9-sensitive and have a high propensity to proinflammatory and regulatory cytokine production (IFN-γ and TNF-α) [50,51]. Accordingly, they are able to skew T-cell polarization toward an inflammatory subset, suggesting a role for ABCs as players in basal inflammatory states associated with aging [52]. In humans, B-cell percentages and numbers significantly decrease with age [53]. Regulatory B cells (Bregs) are potent suppressors of immune responses, both through the secretion of IL-10 and through other contact-dependent mechanisms [54]. It has been shown that these cells decrease with age and maintain unaltered TNF-α production, while the production of IL-10 declines significantly, correlating with the appearance of autoantibodies [55,56]. Aged B cells produce fewer antibodies with lower affinity since affinity maturation by hypermutation is disturbed as well. This results in the reduced quality and quantity of antibody production in response to infection [57]. There is a shift in the proportions of the different B cell subsets: aging induces a significant decrease in the percentage of switched memory B cells and a significant increase in the percentage of naive and CD27−IgD− double-negative (DN) late memory (LM) B cells, whereas no changes were observed in IgM memory [58,59]. In other reports, IgM memory B cells were found to be reduced in the elderly, resulting in a predisposition to pneumococcal infection [60,61]. In older people, DN LM B cells share similar characteristics with the above-mentioned murine ABCs. They are the most proinflammatory B-cell subset, which has also been described in the blood of patients with infectious diseases [62,63,64]. In the elderly, the CXCR3 and CD11c markers are expressed at high levels, indicating their ability to migrate to inflamed tissues and to contribute to local inflammation [58]. DN LM B cells affect the microenvironment by secreting proinflammatory mediators that, in turn, amplify the inflammatory response [59]. Moreover, they display senescence features, such as a poor ability to proliferate, reduced telomerase activity, high levels of SASP factors and elevated p16 expression [65,66].
2.2. Immunosenescence in the Innate Immune System
Aging results in altered numbers and functions of different innate immune cell subsets. Neutrophils are the first cells to reach the sites of infection. The number of circulating neutrophils does not change in the elderly compared to young people, but their function is significantly compromised [67]. Neutrophil adhesion to the endothelium appears to be unaltered in the aging population [68,69], suggesting that the extravasation of neutrophils is not affected. Activated neutrophils, in both aged humans and mice, are more prone to apoptosis and less effective in phagocytosis, chemotactic activity, and ROS production [70,71,72]. Interestingly, impaired neutrophil migration has been linked to increased constitutive PI3K signaling, and PI3K-blocking strategies were able to restore neutrophil chemotaxis. Targeting PI3K signaling may therefore be beneficial in improving neutrophil functions during infections and reducing inappropriate inflammation in older patients [73]. Aged neutrophils have been characterized by the increased expression of CXCR4 and the low expression of CD62L markers [74]. Increased degranulation is supported by the higher surface expression of CD63 and increased neutrophil elastase degradation products [73]. There is a reduction in the phagocytic ability for opsonized bacteria [69,71], especially for Staphylococcus Aureus [75], to which the elderly are particularly susceptible. The CD16 marker is reduced with age, impacting Fc-receptor-mediated phagocytosis and the oxidative burst [71]. In contrast, the phagocytic deficit for unopsonized bacteria does not seem to be compromised [76]. Moreover, aged mice have been demonstrated to have impaired neutrophil extracellular trap (NET) formation, leading to the increased dissemination of S. Aureus [77]. Finally, NET release in response to several physiological and pathological stimuli, such as lipopolysaccharide (LPS) and interleukin-8 (IL-8), was reduced in cells isolated from old subjects [78].
Natural killer (NK) cells have the key ability to produce cytokines after cell recognition, thus having an important part in innate immunity during aging. Several studies have reported changes in the absolute number of circulating NK cells as well as their subset distribution during aging [79]. In contrast, NK progenitor numbers in the peripheral blood or in the bone marrow are not affected [80]. Nonetheless, most studies reported an increase in the mature NK cell number, associated with a decline in the CD56bright subset and a concomitant increase in the CD56dim subset, reflecting reduced cytolytic activity and a reduced ability to produce cytokines [81,82,83,84]. The expression of NK cell receptors NKp46, NKp30, KLRG1 and NKG2A has been shown to decline with age, whereas NKG2D and CD16 expression remains unaltered [83]. NK cell cytotoxicity (NKCC) mediated by perforin exocytosis is reduced with age [81,82]. Indeed, defects in the polarization of perforin have been described at the immunological synapse between NK and cancer cells [81]. In contrast, NK-cell-mediated antibody-dependent cell cytotoxicity (ADCC) is preserved with age [85]. Persistent NK cell signaling via CD158d-HLA-G interaction induced a DNA damage response pathway, leading to senescent NK cells expressing p21 and secreting proinflammatory and proangiogenic mediators [86].
Macrophages create a heterogeneous population with different phenotypes and behaviors, depending on their anatomical location [87]. Studies in aged mice showed augmented myeloid progenitor cells in the bone marrow [88] and an increased number of macrophages in the spleen and bone marrow [89]. Conversely, mono/macrophage cells are reduced in the blood and in the bone marrow of older adults [90]. Aged macrophages from both humans and mice showed the reduced expression of MHCII molecules, which could limit T-cell responses in the elderly [91].
During aging, macrophages may present reduced phagocytic activity [92], reduced TLR expression [93] and altered cytokine responses to LPS [94]. During their activation, macrophages can polarize into M1 macrophages, which are capable of proinflammatory responses producing cytokines such as IL-6, IL-12 and TNF-α, or into M2 macrophages, which exert anti-inflammatory responses and are involved in damaged tissue repair [95]. There are reports suggesting that macrophage polarization is affected during aging: the nature of skewing is still controversial, but perturbations in the normal M1-M2 balance might dysregulate the host immune response, contributing to increased susceptibility to inflammatory and infectious diseases [96,97,98]. In line with this, the observed reduction in TLR expression in the elderly might be responsible, at least in part, for the higher susceptibility to bacterial, mycotic and viral infections [89]. On the contrary, Qian et al. reported a significant increase in the TLR5-induced production of IL-8 in monocytes from older individuals, accompanied by the increased expression of TLR5 and the phosphorylation of downstream MAPK, suggesting that targeting TLR5 may be a notable mechanism to enhance immune responsiveness in elderly [99]. In vitro studies have shown that human macrophages subjected to external stressors develop p21- and p53-mediated cell cycle arrest, elongated morphology and increased B-gal activity [100]. Some studies have reported a characteristic SASP in macrophages [101,102]. Interestingly, macrophages, which are naturally fusogenic, are primarily involved in senescent giant cell formation, which is a hallmark of chronic inflammation. Senescent multinucleated giant cells with their secretomes are a source of inflammation in aging arteries and gonads. These cells can revert their senescence state, leading to cancer progression and metastasis [103].
In the context of antigen-presenting cells, monocyte subsets did not reveal significant age-related alterations under physiological conditions; however, upon stimulation, elderly monocytes showed reduced production of IFN-α, IFN-γ, IL1-β, CCL20 and CCL8 and increased CX3CR1 [104,105]. In particular, nonclassical proinflammatory CD14+CD16+ monocytes [106] accumulate with age and show senescence features such as telomere shortening, reduced proliferation and SASP secretion [107]. In addition, a recent study revealed that aging alters critical pathways important in the production of type I interferon in CD14dimCD16+ monocytes, resulting in impaired innate immune responses to pathogen recognition receptor (PRR) agonists [108]. The frequency of circulating myeloid-derived suppressor cells (MDSCs) increases with age in both humans and mice [109]. Interestingly, proinflammatory factors produced by senescent cells promote both the proliferation and activation of MDSCs, which, in turn, secrete the anti-inflammatory cytokine TGF-β, a potent inducer of cellular senescence in inflamed tissues [110].
Dendritic cells (DCs), as macrophages, represent a bridge between innate and adaptive immunity. The numbers of circulating plasmacytoid (pDCs) and conventional (cDCs) dendritic cells are reduced in healthy and frail elderly people [111], while their distribution in different tissues seems to be unchanged [112]. Specifically, the number of skin Langherans DCs has been shown to decrease with age [113]. Several reports have documented that pDCs from elderly donors produced lower levels of IFN-γ in response to TLR ligands [111,114]. Despite the limited data available, DCs seem to be compromised with aging, helping to explain several phenotypes of immunosenescence (increased susceptibility to infection, loss of tolerance and lower vaccination response) [115].
3. Immune Response to Infection in Aging
Older adults exhibit increased susceptibility to infections and their complications. For this reason, vaccinations are strongly recommended in the elderly, but because of age-related changes in immune functions, vaccine efficacy and effectiveness also decline with age [116]. Influenza virus is one of the most studied viruses in relation to changes that occur with aging. Murine models of aging and influenza infection revealed dysregulated innate immune responses to viruses. Neutrophils accumulated in the lungs of aged mice as early as day 1 post-infection, and this increase was maintained throughout the course of infection as compared with young mice and correlated with higher pulmonary CXCL1 and CXCL2 levels [117]. Likewise, during influenza infection, aged rhesus macaques exhibited increased pulmonary levels of IL-8, inducing neutrophil chemotaxis, compared with younger macaques [118]. Interestingly, whereas neutrophil depletion at the time of infection increased mortality, depletion occurring during later stages improved survival in aged mice and reduced the levels of proinflammatory cytokines [117], suggesting that excessive neutrophil retention in the lung during infection is detrimental. Consistently, high levels of neutrophil-related transcripts in whole blood were correlated with severe outcomes and morbidity in individuals with influenza infection [119,120]. Alveolar macrophages (AMs) play a crucial role in viral clearance and in inflammation resolution during influenza infection, and aging severely impacts these functions. The adoptive transfer of AMs from young mice into the airways of aged mice has been shown to reduce lung tissue injury upon influenza infection [121]. A diminished ability to clear apoptotic cells from the lung environment could further contribute to inflammation and immune system dysfunction. Similar findings have been reported in humans [122,123]. Adaptive immune responses to influenza infection are also impaired during aging. Reduced numbers of both CD4+ and CD8+ influenza-specific pulmonary T cells were reported during influenza infection in aged rhesus macaques and mice [118,124]. Additionally, virus-infected aged mice generated a restricted repertoire of influenza-specific T cells, mainly in the CD8+ compartment [125,126]. Likewise, alterations in the TCR repertoire have also been documented in older people [127], with important implications for vaccination. In mice, aging delayed T-cell infiltration into the lungs following influenza infection [128,129], and adoptive transfer experiments indicated that this defect might be partially due to the aged microenvironment, though the exact mechanisms are not well understood [130,131]. On the other hand, the reduced cDC priming of T cells might play a role [132]. Following influenza infection, cDCs similarly displayed delayed infiltration kinetics into the lungs of aged mice [129] and impaired migration to the lung dLN [133]. The age-associated defect in DC migration to lymph nodes could be ascribed to both environmental and cell-intrinsic factors [132,134,135]. In the context of B-cell responses, the production of influenza-neutralizing Abs was found to be compromised in aged mice, rhesus macaques and humans [118,129,136]. Reductions in Abs may reflect intrinsic defects or reduced CD4+ T-cell help [131,136,137]. Diminished Ab titers during influenza infection could also explain the reduced efficacy of vaccines in the elderly [138].
Respiratory syncytial virus (RSV) leads to rates of infection and disease severity comparable to influenza in older people. In mice, aging impaired the RSV-specific CD8+ T-cell response [139]. The viral load was higher in aged mice even if they displayed higher pulmonary levels of antiviral type I IFN signaling [139,140]. Aged humans have reduced numbers of RSV-specific CD4+ and CD8+ T cells and reduced IFN-γ secretion by RSV-specific T cells [141]. In addition to influenza and RSV, other respiratory viruses, such as rhinovirus, metapneumovirus and parainfluenza, cause severe burdens in older people [142,143]. The impact of aging on the immune response to these respiratory viruses is understudied and thus not well understood. However, the findings in RSV or influenza models may inform on these other respiratory viruses, since they activate common immune pathways, such as type I IFN and Th1 responses.
The bacterium Streptococcus pneumoniae is also a common cause of severe pneumonia in the elderly, often leading to sepsis [144]. In response to pneumococcal pneumonia, aged mice displayed increased neutrophil recruitment in the lung, even though this did not affect protective immunity [145]. Likewise, a higher percentage of neutrophilic granulocytes was immunohistochemically quantified in lung tissue specimens from the elderly compared to young patients with microbiologically proven S. pneumoniae pneumonia [146]. Conversely, young patients versus elderly patients had more alveolar macrophages [146]. The activation of c-Jun N-terminal kinase/activator protein-1 (JNK/AP-1), one of the MAPK signaling pathways, was significantly upregulated in response to S. pneumoniae infection in PMNs from old mice compared to young controls, and its pharmacological inhibition reversed the defect in pneumococcal killing by PMNs, indicating that this pathway can potentially be targeted to reverse the age-related dysregulation of PMN responses [147]. Lower IL-10 production was associated with higher levels of chemokine ligands CXCL9, CXCL12, CCL3, CCL4, CCL5, CCL11 and CCL17 in infected old mice, suggesting a defect in anti-inflammatory cytokine production by immune cells in the lung [145]. The neutralization of IL-10 in infected young mice was associated with increased neutrophil recruitment but no decrease in bacterial outgrowth, similar to what was observed in aged mice [145]. A decrease in proinflammatory cytokine (TNF-α, IL-1β and IL-6) production by macrophages from aged mice has been observed after pulmonary infection with S. pneumoniae, suggesting that the reduction in TLR signaling, especially TLR2, occurs in vivo during an active infection [148,149]. Other functional impairments observed in aged macrophages during S. pneumoniae infection include the decreased expression and function of the NLRP3 inflammasome [150] and LC3-associated phagocytosis [151]. Alterations in marginal zone macrophages and marginal zone B cells were also reported in old mice [151,152], which may affect the ability to clear blood-borne antigens and mount proper T-independent immune responses. This is consistent with the evidence of reduced serum levels of anti-pneumococcal IgM in elderly persons compared with those in adults after pneumococcal vaccination [61]. Furthermore, declines in sTNFR-I and in the TNF-α/IL-10 ratio during pneumococcal infections were correlated with age in humans [153].
4. Immune Senescence Driven by Pathogen Exposure
In addition to natural aging, chronic infections induce a phenotype in immune cells that shares features with immunosenescence [154]. After the primary infection, many viruses can evade the immune system and persist in the host. In some circumstances, these infections become chronic because, due to the lack of immune containment, the virus continuously replicates, generating persistent antigenic stimulation for several months or even decades. Other chronic infections become latent, where very little viral replication occurs and almost no systemic viral antigens are present or hide from the immune system. Many latent infections, however, can become reactivated (i.e., caused by stress), leading to productive viral replication and the increased systemic presentation of viral particles [155]. The antigenic overload imposed by persistent infection and latent viruses exposes the immune system to permanent stress that leads to host T-cell exhaustion [156]. T-cell exhaustion was first described in mice during chronic lymphocytic choriomeningitis virus (LCMV) infection [156], but exhausted T cells have also been observed during infection with simian immunodeficiency virus (SIV) [157] and in individuals infected with a multitude of chronic human viruses. Unlike normal memory cells, the proliferation of exhausted T cells is only sustained by the presence of the viral antigen, partly due to losses of interleukin-2 receptor-b (CD122) and interleukin-7 receptor (CD127) [158,159]. Because the viral antigen is constantly or intermittently supplied, viral-specific T cells never cease proliferating. These dysfunctional memory T cells lack telomerase, and thus, the progressive erosion of chromosome telomeres, induced by repeated rounds of cell division, triggers mechanisms for replicative senescence, leading to premature cellular proliferative arrest [160]. Paradoxically, telomere erosion is coupled with increased resistance to apoptosis, allowing these senescent T cells to accumulate in the blood and tissues at the expense of functional naïve T cells, leading to biological aging and impaired immunity [161]. During chronic viral infections, CD8+/CD28− T cells have reduced expression of effector molecules (granzyme B and perforin) and reduced cytotoxic T-lymphocyte (CTL) activity [162]. Regulatory T cells (Tregs) also have altered functions during chronic viral infections, particularly enhanced proliferation and immune-suppressive actions, as highlighted by the upregulation of PD-1 and CTLA-4 markers on their cell surfaces [163,164].
Critical telomere shortening has been shown to contribute to persistent DNA damage and the consequent induction of the DNA damage response (DDR); if DNA damage is not repaired, either programmed cell death (apoptosis) or the irreversible loss of division potential (replicative senescence) occurs. In the absence of apoptosis, cells harboring dysfunctional telomeres are at risk of developing genomic defects. Although, in some cases, apoptotic markers have been found in T cells progressing to senescence, replicative senescence is permanent. In vitro studies have shown that repeated antigenic stimulation led to activation-induced cell death resistance [165]. These cells increased PI3K/AKT signaling through Phosphatase and Tensin Homolog (PTEN) loss, leading to impaired CD95 activation. Long-term antigen exposure also increased the intracellular expression of Bcl-2-Like Protein-1 (Bcl-xl) and reduced the expression of the pro-apoptotic proteins Bad and Bax. The choice of apoptosis versus replicative senescence may be dependent on the state of cell differentiation, with less differentiated states (CD27+) more prone to apoptosis and late differentiated cells (CD27−) more prone to replicative senescence.
4.1. Immune Senescence during Chronic Persistent Viral Infections
Although antiretroviral therapy for human immunodeficiency virus (HIV) infection reduces AIDS morbidity and mortality, it does not fully restore health. Long-term-treated patients retain a higher risk of a number of complications, partially related to HIV-associated immunologic dysfunction [166]. Indeed, it is recognized that, with antiretroviral therapy (ART), HIV-specific T cells are exposed to viral antigens for a longer period, potentially causing increased T-cell exhaustion and immune senescence. Consistent with this evidence, aggressive forms of the disease present more cells with markers of T-cell exhaustion [167]. In HIV-infected individuals, the enhanced expression of inhibitory markers (PD-1, CTLA-4 and LAG-3), low CD127, reduced proliferative and self-renewal potential, impaired production of effector cytokines and CD4+ T-cell help have been widely documented [168]. Remarkably, CD8+ T cells also bear an exhausted phenotype in HIV patients and, in contrast to CD4+ T cells, which retain their replicative potential despite undetectable telomerase activity, become immunosenescent [169]. Telomerase addition resulted in increased viral targeting and reduced p16 and p21 levels [170].
Like HIV, Hepatitis B and C (HBV and HCV) viruses are persistent chronic viruses, leading to T-cell exhaustion from telomere shortening. Exhausted HCV-specific T cells have been found in the liver, spleen, and blood. In HCV-specific T cells expressing low CD127 levels and high levels of inhibitory markers (PD-1, TIM-3 and CD57), impaired proliferation and CTL responses were reported [171]. During chronic HCV infection, exhausted T cells undergo senescence and show all hallmarks of DNA damage, including γ-H2AX and p53 serine phosphorylation. Immune impairment was seen in CD8+ γ-H2AX+ T cells, which failed to signal through the JAK/STAT pathway [172].
The presence of exhausted T cells is also reported in patients suffering from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Along with this, inhibitory receptors (TIM-3 and PD-1) are also detected in a subpopulation of T cells isolated from hosts infected with SARS-CoV-2 [173].
4.2. Immune Senescence during Chronic Latent Viral Infections
Hallmarks of T-cell exhaustion have been found in two latent viruses, VZV and herpes simplex-1/2 virus (HSV-1/2). VZV-specific T cells declined with age, lost T-cell functions and expressed T-cell exhaustion markers [174]. Exhausted CD8+ T cells have been documented in in vivo models [175]. However, telomere lengths in VZV- and HSV-specific T cells were not significantly shortened or senescent, probably due to the viruses’ ability to remain hidden from immune surveillance due to their location in neurons [176].
On the contrary, human T-cell leukemia virus type-1 (HTLV-I) predominantly infects immune cells and CD4+ T cells. However, there is evidence that replicative senescence occurs in HTLV-I-specific CD8+ T cells. HTLV-I is responsible for two different diseases: a fatal T-cell cancer developing after a very long latency period, Adult T-cell Leukemia/lymphoma (ATLL), and a neurodegenerative disease, Tropical Spastic Paraparesis/HTLV-I-associated myelopathy (TSP/HAM) [177]. T cells from both ATL and TSP patients display exhaustion markers, including PD-1, TIGIT, TIM-2 and 2B4 [178,179]. T cells from TSP patients were also deficient in CD28 and CD27 expression [180]. Senescent CD8+ T cells showed impaired proliferation and increased levels of proinflammatory and pro-senescence cytokines, such as IL-6 and TNFα. Total PBMCs from ATL patients have defects in telomerase expression and harbor shortened telomeres. These are recognized as DNA double-strand breaks and activate the DDR pathway, elevating ATM and p53 activities and culminating in replicative senescence [181].
Epstein–Barr virus (EBV) is one of the most common human viruses in the world and is responsible for a wide spectrum of human diseases, including those associated with defective B cells, such as acute infectious mononucleosis (AIM), and a variety of non-B-cell diseases. Moreover, HTLV-I is oncogenic. Studies have reported high PD-1 and TIM-3 expression on EBV CD8+ T cells from healthy individuals, which were increased in EBV/HIV-co-infected individuals [182]. EBV-specific exhausted T cells have also been found in EBV-associated diseases, such as chronic fatigue syndrome (CFS), multiple sclerosis (MS) and systemic lupus erythematosus (SLE). Signs of T-cell exhaustion encompassed elevated PD-1 and decreased cytotoxic T-cell functions in EBV CD8+ T cells from SLE patients, diminished T- and B-cell memory responses in CFS and reduced EBV CD8+ T-cell functionality and exhaustion in MS [183,184]. Uncontrolled chronic viral exposure leads to replicative senescence in EBV CD8+ T cells, as also reported in HIV-infected immunocompromised individuals and in X-linked lymphoproliferative syndrome (XLP) [185,186,187].
Human cytomegalovirus (CMV) is a member of Herpesviridae, infecting ~50% of the developed world’s population. The immune response elicited to control the primary infection involves natural killer (NK) cells, inflammatory cytokines, B cells and T cells, but CMV often persists, establishing a latent infection with periodic reactivation. During infection, CMV-specific CD8+ T cells undergo massive clonal proliferation and expand into as much as the totality of memory CD8+ T cells. These cells display an altered phenotype and are dysfunctional, likely representing a stage in the transition to replicative senescence [188]. Expansion also occurs in CMV-specific CD4+ T-cell populations, but to a much lesser extent [189]. The CMV-specific memory T cells are CD28−CD27−CD8+ and do not rely on costimulatory signals for their activation [190]. Increased CD57 expression and the loss of CD122 and CD127 have also been documented [191]. The occurrence of telomere shortening presumably limits the proliferative potential of CD28-CD57+ antigen-specific T cells. Antigen-exposed CMV-specific CD8+ T cells can simultaneously produce IFN-γ and TNF-α more quickly and at higher levels than other virus-specific CD8+ T cells [192], evocative of the secretory features (SASP) typical of replicative T-cell senescence.
T-cell exhaustion leading to immune senescence has been less characterized during some other chronic viral infections, such as human herpesvirus-8 (HHV-8) and human papilloma virus (HPV) infections. Senescence markers, such as increased CD57+/CD28− on CD4+ and CD8+ T cells and PD-1 on NK cells, have been found in HHV-8-infected individuals with HIV-associated or classic Kaposi’s sarcoma [193,194]. T-cell exhaustion may not occur in HPV to the same extent as in other infections due to its low antigenic loads. Otherwise, it is difficult to detect the possible localization of HPV-specific T cells to the mucosal tissue. In high-grade HPV infection, T cells and dendritic cells from cervical tissue showed positivity for the PD-1 marker. In addition, reductions in IFN-γ and IL-12 from helper T cells were observed [195]. Finally, other helper cells, including DCs, become dysfunctional in the context of chronic infections. DCs isolated from patients with HIV, HBV and HCV infections exhibited a diminished ability to produce cytokines and to induce T-cell activation. Such reductions impaired the CD8 T-cell antiviral response and drove exhaustion [196].
4.3. Immune Senescence during Bacterial Infections
Like viruses, many bacteria have the ability to circumvent immune surveillance and establish chronic infections, which, in some instances, may also evolve in host cell transformation and cancers. Evidence suggests that, to establish infections, pathogenic bacteria elicit DNA damage in host cells, but the effect on host immune cells remains poorly investigated. Mathiasen et al. recently reported that genotoxins, cytolethal distending toxins (CDTs) produced by a wide range of Gram-negative bacteria, including Helicobacter hepaticus, Escherichia coli and Shigella dysenteriae, induce DNA damage, DDR activation and premature senescence in activated CD4 T cells both in vitro and in vivo. The CDT-induced SASP phenotype in senescent CD4 T cells was partially orchestrated by ATM-p38 signaling [197]. The study, however, did not address whether these senescent T cells are long-lived; further studies are needed to assess whether they eventually die from the cytotoxic effect of the toxin or are cleared by immune-mediated destruction. Salmonella pathogens rely on typhoid toxin (TT) for dissemination and pathogen colonization. Another recent work revealed that TT induces a non-canonical DDR in macrophages that drives a senescence-like phenotype characterized by the accumulation of γH2AX and SASP [198]. Importantly, the SASP caused transmissible senescence in bystander cells, which became, in turn, more susceptible to Salmonella infection [198]. These findings demonstrate that pathogenic bacteria exploit senescence to dysregulate the host’s immune response and to promote a favorable niche for successful infection. Many questions regarding bacteria-induced senescence remain to be addressed, including the detailed analysis of the markers of immune senescence and of the SASP and whether they would be different from those induced in the context of viral infections. The current knowledge is limited and mostly related to infections with Mycobacterium tuberculosis (Mtb). Murine studies have demonstrated that chronic Mtb infection is associated with the differentiation of CD4 T cells toward an exhausted memory phenotype, with high expression levels of KLRG-1 and IFN-γ-monofunctional capacity and poor ability to contribute to antigen clearance [199]. Human Mtb-specific CD4+ and CD8+ T cells ex vivo display reduced production of INF-γ, TNF-α and IL-2. In addition, increased expression levels of PD-1 and its ligands are found on T cells, neutrophils, monocytes, macrophages and B cells from patients with active pulmonary tuberculosis [200]. The blockade of PD-1/PD-L1 signaling could enhance the specific degranulation of CTLs and restore specific IFN-γ activity [201,202]. This is in accordance with the fact that PD-1 and PD-L1 knockout (KO) mice display increased susceptibility to infection [203]. LAG-3 and TIM-3 are increasingly expressed on CD8+ T cells during the chronic phase of infection if not supported by the CD4+ compartment [203].
Studies in patients experiencing HIV-Mtb co-infection confirm that Mtb plays a major role in accelerating HIV disease progression by directly or indirectly facilitating factors associated with immune senescence. In fact, Mtb enhanced the expression of CD38, CD57, CD69, human leukocyte antigen-DR and the downregulated CD28, CD27, CD40L and CD127 markers on HIV-specific T cells [204]. Interestingly, T cells with a senescent phenotype (high expression of CD57, KLRG-1 and γH2AX and short telomeres) accumulate during cutaneous infections with the protozoa Leishmaniasis braziliensis, contributing to the inflammatory response in this disease [205]. This suggests that the cell fate of immune senescence might be a common endpoint in infections elicited by different pathogen species other than viruses. Future efforts providing mechanistic insights into the immunomodulatory properties of these pathogens and the link to immune senescence will be important in developing therapeutic strategies to eradicate them.
5. Immunometabolism and Cellular Senescence
Age-related alterations in metabolic homeostasis and nutrition have been linked to changes in different physiological functions, including immune functions. In this regard, the recent literature suggests that age-associated dysfunctional metabolism, driven by the accumulation of key host metabolites (saturated fatty acids, cholesterol, ceramides and lactate) and the loss of others (glutamine, tryptophan and short-chain fatty acids), might play a role in driving immune senescence and inflammaging and ultimately lead to diseases in the elderly [206]. Furthermore, the reduced availability of any one of these metabolites could negatively impact the magnitude of the antiviral response. Despite their proliferative arrest, immune cells with a senescent phenotype show high metabolic activity to cope with the high energetic demand of the senescence program, including SASP-coupled proteotoxic stress [207,208]. Generally, senescent immune cells choose glycolysis instead of OXPHOS, leading to an unbalanced bioenergetic condition caused by the accumulation of dysfunctional mitochondria. The consequent Ca++ buffering deficits found in aged mitochondria have the effect of reducing Ca++-mediated signaling in the target cells. Senescent T cells produce less ATP, with the consequences of the defective induction of several metabolites and reduced T cell activation. Specifically, the loss of the CD28 antigen has been associated with the loss of metabolic fitness. The preferential use of glycolysis in senescent T cells is supported by reduced pyruvate dehydrogenase kinase activity and the downregulation of Sirtuin1 [209]. In senescent Tregs, glucose consumption is accelerated, supporting their function. TLR8 signaling restored metabolic control and Treg function, improving antitumor immunity in vitro and in vivo [210]. Fewer studies have addressed senescence-associated changes in B-cell metabolism. Kurupati et al. reported that circulating switched memory B cells from aged people present high mitochondrial mass and ROS and lower FOXO1, a transcription factor controlling metabolic homeostasis in response to oxidative stress [211]. As a consequence, aged B cells exhibit a strong reduction in oxidative phosphorylation after activation. Moreover, it has been reported that DN B cells from the elderly utilize higher amounts of glucose and aerobic glycolysis to support survival and function [212]. Finally, reduced respiratory capacity due to insufficient NAD levels and decreased mitochondrial function (reduced ATP and increased ROS) have been described in monocytes and macrophages from elderly individuals [213].
Similarly, chronic infections can induce metabolic alterations in infected cells both indirectly, by altering helper cell function as described above, or directly, causing T-cell exhaustion. For instance, Kaposi’s sarcoma-associated herpesvirus (KHSV) has been shown to alter infected host cell glucose metabolism in infected cells by upregulating HIF1α, reducing mitochondrial capacity and releasing micro-RNAs, which are able to guide metabolic reprogramming, into the microenvironment [214]. HIV increased glycolysis and Glut1 expression in T cells. In Glut1hi CD4+ T cells, viral transcription is linked to cholesterol metabolism via SREBP2 and to enhanced fatty acid synthesis [215]. Similarly, increased glucose metabolism and surface Glut1 and Glut 3 expression have been reported in monocytes from HIV subjects [216]. In infected B cells, EBV induced the activation of the PI3K/Akt pathway and enhanced HIF-1α expression, increasing glucose uptake and glycolytic flux [217]. HBV impacts the infected host cell’s metabolism by upregulating the expression of glucose transporters (GLUT) through mTOR signaling and promoting glycolysis [218]. Interestingly, it has been reported that Mtb also perturbs metabolic circuits and alters effector functions in lung CD8+ T cells. As Mtb infection become chronic, mitochondrial metabolism deteriorates in CD8+ T cells, resulting in an increased dependency on glycolysis that, in turn, potentiates inflammatory cytokine production [219]. Overall, the evidence that senescent immune cells rely on metabolic reprogramming for survival and activity suggests that their state could be reversed by manipulating cell metabolism, offering therapeutic strategies for the treatment of infections.
6. Targeting of Senescent Immune Cells
In line with the increased understanding of the immune system during aging, in the last years, different potential therapeutic approaches to counteract immune dysfunctions in the elderly have been proposed [6]. In this review, we focus on approaches targeting cellular senescence, whereas details and more extensive information on other immune-targeting approaches can be retrieved elsewhere [57].
Senotherapeutics are a new class of drugs that consists of two members, senolytics and senomorphics, with the goal of removing senescent cells and/or blocking the SASP [220]. Senolytics classically relied on the use of apoptosis-inducing drugs, yet the harnessing of innate and humoral immunity, targeting specific receptors, has been proposed more recently as an attractive strategy to stimulate senescent cell clearance. Senomorphics act as SASP inhibitors without cell killing and without compromising cell cycle arrest. These drugs primarily target pathways related to SASP expression and transcription factors. In this context, metabolic or epigenetic targeting has also been proposed as a means of reverting the state of cellular senescence. A second approach is based on the neutralization of the activity and function of specific SASP factors, such as cytokines, with specific antibodies [220].
Most of these approaches have shown efficacy in modulating the senescence of cells from multiple tissues, with a bystander benefit for the aged immune system and for the treatment of a variety of diseases of old age [221,222]; fewer studies have described their direct effectiveness in senescent immune cells. Here, we discuss this evidence, considering their application shown in the context of aging or immunity against infection (Table 1).
Table 1.
Main potential therapeutic strategies to target senescent immune cells with implication for ageing and immunity against infections.
| Approach | Target (molecular/cellular) |
Experimental outcome | Benefits | Limitations | Ref. |
|---|---|---|---|---|---|
| Senolytics | |||||
|
CD153 peptide (vaccination) |
CD4+ CD44high CD62Llow PD-1+ CD153+ cells in VAT tissue | Elimination of infiltrating senescent T cells in adipose tissue in obese mice | Improved glucose tolerance and insulin resistance | Contraindicated in case of mycobacterial infections |
[226,227] |
| Prodrug SSK1 (oral administration) | SA- β -gal positive cells | Clearance of senescent macrophages | Dampened inflammation and restored physical function in aged mice | Off-target effects | [224] |
| Genetic deletion | p16(INK4a) in lymphocytes | Improved T cell immune function | Ameliorated several aging phenotypes | B lineage-specific ablation was associated with a markedly increased incidence of systemic, high-grade B-cell neoplasms | [229] |
| Senomorphics | |||||
|
Leniolisib (oral administration) |
PI3Kd | Reduction in PD-1+CD4+ and senescent CD57+CD4− T cells | Improved immune dysregulation and decreased fatigue in ongoing clinical trials | Potential genomic instability by augmenting off-target activity of activation-induced cytidine deaminase | [231,232] |
| Genetic inhibition | Sestrins in T cells | Restored T cell proliferation and cytokine production via p38 MAPK inhibition in senescent-like CD27−CD28−CD8+ T cells. | Enhanced vaccine responsiveness in old mice; enhanced immune function in primary human T cells |
Prolonged inhibition of sestrins may result in malignancy | [29,30] |
|
Losmapimod (oral administration) |
p38 MAPK | Reduced systemic symptoms of inflamm-ageingr; escued TIM-4 expression; cleared apoptotic bodies and restored efferocytosis in macrophages | Improved skin inflammation resolution in aged people | Tested on a small cohort of individuals | [122] |
| BEZ235 (oral administration) | PI3K/mTOR | Augment the type I IFN response; reversed infection-induced changes in metabolism | Increased lifespan and health and reduced the incidence of respiratory tract infections in mice | Controversial results obtained in humans | [239,240,241,242] |
| Metformin (oral administration) | AMPK | Enhanced T cell autophagy, normalized mitochondrial function, and alleviated senes-cence-associated inflammation | Extended health span and lifespan in multiple animal models | Focus on CD4 T cells as sources of inflammageing; studies in humans not conclusive |
[246,247,248] |
| Resveratrol (oral administration) | SIRT1 | Reduced ROS, inhibited COX, and activated anti-inflammatory pathways (Sirt1) | Anti-ageing in human trials; Restored T-cell function and NK cell activities | Possible risks related to nephrotoxicity | [249] |
| Spermidine (oral administration) | eIF5A | Reduced B cells senescence; improved autophagy in T cells | Restored response to vaccination and infection of CD8+ T cells in old mice | Toxic at high dose | [251] |
| Antibodies against cytokines | |||||
| Anti-IL10 | IL-10 | Enhanced T-cell functions | Inhibited viral persistence during LCMC infection in mice | Potential side-effects due to its role in immune tolerance | [278] |
| Anti-IFN-I | IFN receptor 2 | Decreased T cell exhaustion marker expression, restored viral-specific CD8 T cell function | Decreased viral replication in conjunction with ART treatment in HIV-infected humanized mice | Potential side-effect due to different role of IFN-I signaling during acute and chronic infection | [282] |
| Inhibitory receptors blockade | |||||
| Anti-PD-1 | PD-1 |
Reduced exhaustion, expanded and increased functionality of virus-specific CD8+ T cells, | Significantly reduced plasma SIV RNA and prolonged survival in SIV-infected macaques | Potential side-effect related to break of tolerance | [157,263] |
| Reversed the exhausted phenotype, Increased IFN-g effector functions | Clearance of HBV virus in an in vivo model of HBV | [266] | |||
| BMS-936558 | No substantial changes in immune phenotype | Reduced viral load in a subset of HCV patients enrolled in the trial | Immune-related adverse events of mild-to-moderate intensity | [267] | |
| Anti-PD-L1 | PD-L1 | Restored viral-specific T cell functions | Reduced HIV-1 replication in HIV-infected humanized mice | No effect on HIV viral load in humans, likely due to low dosage used. | [265] |
| Anti-CTLA-4 | CTLA-4 | Enhanced viral specific responses | Better control of EBV and HIV infections in combination with anti-PD-1 | No effect in viral infection when used as monotherapy | [270,271] |
6.1. Therapeutic Opportunities for Aging
The main hurdle in eliminating senescent immune cells is the fact that exclusive criteria for discriminating immunosenescent cells from highly differentiated but non-senescent immune cells are still largely missing. Although not completely validated, immunological markers for senescent immune cells have been proposed and might provide additional therapeutic options in addition to traditional markers. The classical senescent marker, an increase in SA-β-gal activity, was observed in many aged subsets of peripheral blood mononuclear cells, including T and B lymphocytes, plasmacytoid dendritic cells (pDCs), natural killer (NK) cells and monocytes [38], whereas it might not be reliable enough to completely differentiate senescent from activated macrophages [223]. The use of a prodrug processed into a cytotoxic compound by β-gal has been demonstrated to be extremely effective in dampening inflammation and restoring physical function in aged mice, with this effect being partly mediated by the depletion of SA-β-gal-positive macrophages [224]. Thus, although this strategy could suffer from off-target effects, lysosomal β-gal can be effectively leveraged to selectively eliminate immunosenescent cells [225]. A study recently reported the development of a CD153 peptide vaccine able to induce the production of anti-CD153 antibodies and to decrease the number of senescent CD153+ T cells infiltrating the adipose tissue of obese mice [225]. Notably, vaccinated animals showed improved glucose tolerance and insulin resistance [226]. On the other hand, this approach may be contraindicated in the case of mycobacterial infection [227]. In addition, the clinical translation of vaccination strategies targeting senescence epitopes may require careful consideration, especially considering their lasting effects, which might be difficult to reverse. The p16INK4a tumor suppressor inhibits the cell cycle, promotes cellular senescence and has been linked to aging in mammalian systems. In particular, p16INK4a-expressing T cells accumulate in human peripheral blood with aging, correlating with plasma interleukin-6 concentration, a marker of human frailty [228], and p16+ T cells seem to have a reduced immune capacity, as the specific deletion of p16 in these cells enhances immune function in aged mice [229].
Many of the strategies to modulate SASP target key activation pathways, such as mitogen-activated protein kinase (MAPK) signaling, phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) signaling, which lead to NF-kB activation [220]. The p110 Delta subunit of phosphoinositide 3-kinase (PI(3)K), an upstream activator of mTOR, is selectively expressed in leukocytes and is critical for lymphocyte biology. Patients with activated PI3K Delta syndrome present a dominant mutation in the PI3K catalytic subunit p110d, resulting in T-cell senescence and immunodeficiency [230]. Notably, the administration of a selective PI3Kd inhibitor, Leniolisib (CDZ173), reduced senescent T cells and decreased inflammatory markers in ongoing clinical trials [231,232]. The inhibition of PI3-kinase also corrected the aberrant migration seen in old neutrophils [73]. The switch to NK-like activity has been described in senescent CD8+ T cells during aging. The increased expression of the NK receptor and the concomitant decrease in TCR levels is mediated by sestrins, a family of stress-sensing proteins, and their blockade increased telomere activity, restored T cell function and enhanced the immune response to vaccination in aged mice [29,30]. Importantly, the silencing of sestrin expression in primary T-cell populations from old humans similarly enhanced antigen-specific proliferation and cytokine production in vitro via p38 MAPK inhibition [29]. Moreover, p38 MAPK blockade reversed CD8+ T-cell senescence via an mTOR-independent pathway, increasing autophagy [31]. Altered p38 activity is also associated with a reduction in TIM-4 receptor expression on aged senescent macrophages, leading to impaired efferocytosis [122]. Administering a p38 inhibitor rescued TIM-4 expression and restored a macrophage resolution phenotype both in vitro and in vivo in humans [122].
Senescent cells are “hypermetabolic”, and this metabolic reprogramming may potentially be therapeutically targetable [233], though it remains unclear how metabolism-targeted drugs can achieve sufficient specificity for senescent over non-senescent cells to allow clinical translation. Dietary interventions have the potential to limit the accumulation of senescent cells with age. Calorie restriction (CR) was one of the first interventions identified and able to extend lifespan [234]. CR impinges on several pathways, including mTOR inhibition, sirtuin activation and enhanced AMP kinase function, which in turn induces autophagy [235,236]. CR lowered markers of senescence in T cells, whereas the effect on NK cells remains controversial [237,238]. Several metabolic regulators and CR mimickers have been identified and tested for their anti-aging effects mediated by mTOR/AMPK pathway inhibition. The inhibition of this pathway could increase lifespan and health during aging in pre-clinical models and also reduce the incidence of respiratory tract infections in mice and augment the type I IFN response [239]. However, in aged people with increased senescent PD-1+ T cells, the mTORC1 inhibitors rapamycin and its analogs showed controversial results in enhancing immune function and reducing respiratory illness [240,241,242]. Further work is needed to evaluate rapamycin efficacy in reducing the clinical effect of aging and to investigate whether mTOR inhibition could be useful in preventing respiratory tract infections in specific subpopulations or whether inhibition is more effective against specific types of viruses. The results could help in the evaluation of drug repurposing, which has been proposed for patients affected by severe SARS-CoV-2 infection [243,244,245]. Another CR agent, Metformin, was able to enhance T-cell autophagy, normalize mitochondrial function and alleviate senescence-associated inflammation [246]. Metformin also extended the health span and lifespan in multiple animal models, both alone and in combination with rapamycin [247,248]. Resveratrol, a natural polyphenol compound, is drawing interest for its anti-aging activities mediated by the reduction in reactive oxygen species (ROS), the inhibition of cyclooxygenase (COX) and the activation of many anti-inflammatory pathways, including the one controlled by Sirtuin-1 (Sirt1) [249]. Resveratrol functions as an immunomodulator. It has been shown to restore T-cell function by targeting the immune-checkpoint signaling molecules PD-1/PD-L1 and to activate NK cells through AKT- and mTORC2-mediated c-Myb upregulation [249]. Remarkably, resveratrol also induced apoptosis and cellular senescence in primary and cancer cells [250]. Finally, spermidine, an endogenous metabolite, had a potent effect in reducing B-cell senescence and was able to restore the age-related decline in autophagy and responses to vaccination and infection of CD8+ T cells in old mice [251]. Considering how the pathways regulating metabolism are linked to immune cell function and senescence, they could hold a high therapeutic potential for treating clinical conditions associated with aging.
The hallmarks of aging include genomic instability, telomere attrition and epigenetic alterations [10]. In particular, epigenetic control is essential to the healthy development of effector cells of the immune system [252,253,254,255]. It is therefore not surprising that epigenetic pathways are dysregulated in aged immune cells and are responsible for their altered transcriptional profiles [256]. In the last several years, various epigenetic drugs have been developed to target epigenetic changes in different diseases and contexts [257], and investigating their effects on immunosenescence may provide novel ways to restore functional protective immunity during aging. In this regard, recent work showed that therapeutic efforts to reverse T-cell exhaustion during infection may require new approaches that increase the epigenetic plasticity of exhausted T cells [258,259].
Interestingly, metabolism and epigenetics are closely linked, together affecting the aging of the immune system. For example, CR modulates the sirtuin protein family, one of the first known epigenetic enzymes and a key regulator of aging [260]. Moreover, Metformin regulates the activation of AMPK, which directly governs the activities of several epigenetic enzymes, such as HATs, HDACs and DNMTs [261]. Studies using metabolism-targeted drugs specifically evaluating these chromatin-modifying pathways are still warranted.
6.2. Therapeutic Opportunities for Persistent Infections
Blocking early senescence in exhausted T cells in patients with chronic viral infection may enhance their efficacy [262]. In this regard, antibody blockade of inhibitory receptors has shown promise in re-activating T-cells. Importantly, these strategies enable the reversal of metabolic dysfunction by restoring TCR signaling, which stimulates glycolysis-enhancing pathways. In vitro studies showed that blocking PD-1 pathways restored effector functions in patients’ HIV-specific CD8+ T cells, improving pathogen control [167]. Moreover, the in vivo administration of anti-PD-1 antibody expanded and increased the functionality of virus-specific CD8+ T cells, significantly reduced plasma SIV RNA, prolonged survival and reduced markers of immune activation in SIV-infected macaques [157,263]. Anti-PD-L1 antibody treatment reduced HIV-1 replication and also increased CD4+ T and CD8+ T cells in HIV-infected humanized mice [264].
More recently, single-dose anti-PD-L1 therapy was evaluated in healthy HIV-1-infected persons on suppressive ART therapy. In this study, Gay et al. reported enhanced HIV-specific CD8+ T-cell responses in the blood of a subset of patients, but without any effects on HIV viral load. This result can likely be explained by the low dosage of anti-PD-L1 antibodies used [265]. PD-1 blockade was also shown to be effective in increasing IFN-γ effector functions and the clearance of the HBV virus in an in vivo model of HBV [266]. In chronic HCV infection, anti-PD-1 therapy led to only a moderate response [267]. The direct in vivo blockade of CTLA-4 during chronic viral infections such as LCMV, SIV and HIV failed to decrease the viral load or increase T-cell functionalities [268,269]. Overall, this evidence suggests that targeting multiple inhibitory receptors may be more efficacious. In HCV infections, the concurrent blockade of CTLA-4 and PD-1 reinvigorated HCV-specific CD8+ T cells in a CD4+ T-cell-independent manner [270]. A combination of anti-PD-1 and anti-CTLA4 therapies elicited better control of EBV infection, increasing EBV effector responses and decreasing EBV-infected B cells [271]. An additional promising target is P-Selectin Glycoprotein Ligand 1, PSGL-1. Its knockdown led to the downregulation of several inhibitory receptors, including PD-1, CD160, TIM-3, BTLA and LAG-3, improving antiviral responses [272]. LAG-3 expression has been associated with reduced LCMV-specific CD8 T-cell responses, and its blockade, in combination with anti-PD1/PD-L1 therapy, restored immune activity [273,274]. Moreover, LAG3 inhibition was shown to be effective in chronic HBV infection [275]. Unlike anti-PD-1 treatment, TIM-3 blockade has been reported to restore T cells from exhaustion and reduce Mtb replication within macrophages, thus improving outcomes in tuberculosis infection. Further, double blocking PD-1 and TIM-3 receptors increased the secretion of IL-1β and promoted Mtb clearance [276].
In addition to inhibitory receptors, cytokines have been identified as potential targets to reinvigorate exhausted T cells. IL-10 was positively associated with immune evasion and the persistence of viral infections such as HCV, HIV and EBV [277]. IL-10 blockade was effective in inhibiting viral persistence and enhancing T-cell functions during LCMC infection [278]. Consistently, IL-10Rα blockade resulted in the markedly increased secretion of IFN-γ by HIV-specific CD4+ T cells. However, combining IL-10 and PD-1 blockade could only partially restore T-cell-driven cytokine production [279]. On the contrary, in a mouse model of LCMV infection, combined anti-IL-10 and anti-PD-1 therapy synergistically enhanced the antiviral response of T cells [280]. Despite its benefits, there are several concerns regarding the use of anti-IL-10 therapy related to its role in maintaining oral tolerance and the potential emergence of undesirable side effects. Grint et al. demonstrated that IL-2 treatment successfully decreased the virus RNA levels in HCV in HCV/HIV-co-infected patients [281]. Targeting the type I IFN response in combination with ART was effective in reducing viral loads and restoring CD8+ T-cell functions in humanized mice with chronic HIV infection [282].
Several reports have suggested that metabolic deregulation contributes to T-cell exhaustion [158]. Bengsch et al. demonstrated that during chronic LCMV infection, PD-1 signals inhibited glycolytic and mitochondrial metabolism through peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) in early effector CD8+ T cells. Interestingly, the overexpression of PGC1α corrected some metabolic alterations in exhausted T cells and improved their effector function [283]. Altered mitochondrial metabolism was also described in exhausted HBV-specific T cells. Interleukin (IL)-12 recovered the HBV-specific T-cell effector function, increased their mitochondrial potential and reduced their dependence on glycolysis [284]. Finally, Metformin rejuvenates mitochondrial metabolism in T cells from Mtb-infected mice [219].
7. Conclusions and Future Perspectives
The immune system involves the interplay of many different cell types, collectively providing protection against infectious pathogens. Aging affects immune cell functions, resulting in increased severity of infections, enhanced predisposition to their complications and increased mortality in older people. The induction of senescence in immune cells can also occur in response to chronic viral infections, though information regarding a link between bacterial infections and immune senescence is emerging [197]. Despite the increased interest in developing therapeutic and preventive strategies targeting senescence in immunity, many of the underlying molecular processes, both intrinsic and extrinsic, are still not completely understood. In fact, whether the observed decline in immune function is a cause or a consequence of the cross-talk among various systems within organismal aging is still not well understood.
Mouse models have been particularly informative in aging studies; however, it remains to be established to what extent these models faithfully recapitulate the mechanisms underlying senescence processes in humans and their effects. Novel tools to track small populations of peripheral blood immune cells from clinical samples have enabled studies of cell exhaustion leading to senescence directly in humans, especially during chronic infections. Nonetheless, these studies did not consider potential changes in senescent cells residing in tissues. A combination of multiple scientific approaches (e.g., multiparametric analyses, high-resolution omics-technologies, system biology and big data analyses) would allow a better understanding of immune senescence during human aging and its outcomes for infections.
Targeting senescent cells has become an attractive alternative therapy for treating aging-related immune dysfunction, thus preventing or reducing the impact of different diseases in the elderly. Senotherapeutic strategies to eliminate senescent cells or disrupt their impact have achieved promising results in a variety of contexts. SASP modulators have been actively exploited to reduce mortality in hospitalized COVID-19 patients based on their potential to counteract the detrimental cytokine storm [285]. Future studies are needed to determine whether SASP modulators can also be beneficial for people suffering from chronic viral infections, such as HIV. However, some of them may have negligible direct effects on immune cells [286]. Senolytics typically target senescent cell anti-apoptotic pathways, survival pathways utilized by senescent cells to maintain their viability. Interestingly, ABT-263 has been shown to sensitize macrophages infected with a variety of virus types (influenza, herpes simplex and measles) to undergo cell death [287]. However, it is not known whether the drug was effective on infected senescent cells. The development of CAR-T cells has shown efficacy in cancer therapy [288] and autoimmune diseases [289,290]. In addition, recent evidence suggests their potential application as senolytic agents during aging for the treatment of age-associated diseases [291]. The use of CAR-T cells directed against senescence-specific surface antigens would open new possibilities to modify the immune system to restore active immune surveillance. Furthermore, CAR-T cells might be modified to be more resistant to their own exhaustion [292]. Strategies based on immune-mediated clearance should consider immune evasion mechanisms that senescent cells have developed to prevent their removal [293,294].
Studies in animal models in which senotherapeutics were tested have revealed various beneficial effects, but they also experienced adverse side effects [295]. Consistently, strategies proposed for SASP inhibition might result in off-target side effects affecting the immune system, such as neutrophilia, nephrotoxicity and changes in the T-cell phenotype, due to the close interconnection among the various pathways involved in inflammatory signaling [296]. Specifically, NF-kB pathway inhibition, either by genetic deletion or small molecules, has been shown to inhibit the development of mature lymphocytes and lymphoid progenitors [296,297]; to induce apoptosis of thymocytes, macrophages and B cells [298,299]; to impair lymphocyte growth and cytokine production [300]; and to potentially increase susceptibility to various infections [301]. Interestingly, the combination of Dasatinib, a kinase inhibitor, and quercetin, a flavonoid targeting the PI3K/AKT pathways, has been proven effective in pilot clinical trials in reducing senescent cells and alleviating physical dysfunction in patients with pulmonary fibrosis [302,303]. Despite this important proof of concept demonstrating the feasibility of clinical translation, larger controlled trials are needed to fully assess the efficacy of the therapy by identifying biomarkers of senescence, the potential off-target effects of the drugs and their mechanisms of action in humans before moving to age-related diseases.
The identification of specific intracellular and extracellular markers of cellular senescence will be instrumental in developing next-generation senotherapeutics characterized by increased selectivity and reduced off-target effects. In this regard, recent studies corroborated the use of nanoparticles for the specific delivery of drugs into senescent cells in vivo [225]. This approach may help to overcome the problem of specificity in conditions requiring chronic administration.
Given the complexity of the immune system and aging, targeting multiple components of the immune system at once could be required. This would need an extremely balanced approach to avoid undesired side effects. Indeed, while the strong stimulation of the immune system to recover its complete functionality could lead to acute inflammation or autoimmune diseases, exaggerating immune suppression to reduce inflammaging could result in increased susceptibility to infections. Future challenges will be, therefore, to identify the right combination of the various therapeutic approaches and to select the group of patients that would benefit the most. Finally, the large differences between individuals, particularly in the heterogeneity of immune system changes with age, must be considered. Thanks to multiomics analyses that provide personalized genetic and epigenomic information, precision medicine is now the gold standard approach in oncology, where treatments are decided based on the mutational profile of the patient [304]. In aging, distinct individuals respond differently to Metformin, with some developing side effects [305]. This evidence suggests that specific genetic or epigenetic characteristics might influence treatment efficacy. Stratification of the aged population based on biomarkers identified from multiomics data could help in recognizing subgroups of patients who would benefit most from specific treatments, thus allowing more tailored approaches.
Abbreviations
| AKT | Protein kinase B |
| ATM | Ataxia Telangiectasia Mutated |
| BCL-xl | Bcl-2-Like Protein-1 |
| BCR | B-cell receptor |
| B-gal | B-galactosidase |
| BTLA | B- and T-Lymphocyte Attenuator |
| CAR-T | Chimeric Antigen Receptor-T |
| CD122 | Interleukin-2 receptor-b |
| CD127 | Interleukin-7 receptor |
| CDKN | Cyclin-Dependent Kinase inhibitor |
| CMV | Human cytomegalovirus |
| CR | Calorie restriction |
| CTLA-4 | Cytotoxic T-lymphocyte antigen-4 |
| CXCR | C-X-C Motif Chemokine Receptor |
| DDR | DNA damage response |
| DNMTs | DNA methyltransferases |
| EBV | Epstein–Barr virus |
| ERK | Extracellular signal-regulated kinase |
| FOXP3 | Forkhead box P3 |
| gH2AX | Gamma H2A histone family member X |
| HAT | Histone Acetyltransferases |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HDAC | Histone Deacetylases |
| HIV | Human immunodeficiency virus |
| HHV-8 | Human herpesvirus-8 |
| HLA-DR | Human Leukocyte Antigen DR |
| HSV-1/2 | Herpes simplex-1/2 virus |
| HTLV-I | Human T-cell leukemia virus type-1 (HTLV-I) |
| JNK/AP-1 | c-Jun N-terminal kinase/activator protein-1 |
| KLRG1 | Killer Cell Lectin-Like Receptor G1 |
| LAG-3 | Lymphocyte activation gene 3 |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 |
| LCMV | Lymphocytic choriomeningitis virus |
| LPS | Lipopolysaccharide |
| MAP | Mitogen-activated protein |
| Mtb | Mycobacterium tuberculosis |
| mTOR | Mammalian target of rapamycin |
| NKCC | Natural Killer Cellular Cytotoxicity |
| NET | Neutrophil extracellular trap |
| NLRP3 | NLR family pyrin domain containing 3 |
| PD-1 | Programmed cell death protein 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| PTEN | Phosphatase and Tensin Homolog |
| ROS | Reactive oxygen species |
| RSV | Respiratory syncytial virus |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SASP | Senescence-Associated Secretory Phenotype |
| SIV | Simian immunodeficiency virus |
| STAT | Signal Transducer and Activator of Transcription |
| TCF1 | T Cell Factor 1 |
| TCR | T-cell receptor |
| TFH | T follicular helper |
| TIGIT | T-cell immunoglobulin and ITIM domain |
| TH | T helper |
| TIM-3 | T-cell immunoglobulin and mucin-domain containing-3 |
| TLR | Toll-Like Receptor |
| TNF | Tumor Necrosis Factor |
| VZV | Varicella-zoster virus |
Author Contributions
Conceptualization, B.C.; writing—original draft preparation B.C., V.M. and A.F.; writing—review and editing B.C., V.M. and A.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The APC have been funded by IRCCS Humanitas Research Hospital, 20089 Milan, Italy.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gorgoulis V., Adams P.D., Alimonti A., Bennett D.C., Bischof O., Bishop C., Campisi J., Collado M., Evangelou K., Ferbeyre G., et al. Cellular Senescence: Defining a Path Forward. Cell. 2019;179:813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Coppé J.P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J., Nelson P.S., Desprez P.Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodier F., Coppé J.P., Patil C.K., Hoeijmakers W.A., Muñoz D.P., Raza S.R., Freund A., Campeau E., Davalos A.R., Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coppé J.P., Desprez P.Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prata L.G.P.L., Ovsyannikova I.G., Tchkonia T., Kirkland J.L. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin. Immunol. 2018;40:101275. doi: 10.1016/j.smim.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 7.Frasca D., Blomberg B.B. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016;17:7–19. doi: 10.1007/s10522-015-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camell C.D., Yousefzadeh M.J., Zhu Y., Prata L.G.P.L., Huggins M.A., Pierson M., Zhang L., O’Kelly R.D., Pirtskhalava T., Xun P., et al. Senolytics reduce coronavirus-related mortality in old mice. Science. 2021;373:eabe4832. doi: 10.1126/science.abe4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Micco R., Krizhanovsky V., Baker D., d’Adda di Fagagna F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021;22:75–95. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulop T., Larbi A., Dupuis G., Le Page A., Frost E.H., Cohen A.A., Witkowski J.M., Franceschi C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawelec G. Hallmarks of human “immunosenescence”: Adaptation or dysregulation? Immun. Ageing. 2012;9:15. doi: 10.1186/1742-4933-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vicente R., Mausset-Bonnefont A.L., Jorgensen C., Louis-Plence P., Brondello J.M. Cellular senescence impact on immune cell fate and function. Aging Cell. 2016;15:400–406. doi: 10.1111/acel.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers E.S., Vukmanovic-Stejic M., Shih B.B., Trahair H., Subramanian P., Devine O.P., Glanville J., Gilroy D., Rustin M.H.A., Freeman T.C., et al. Recruitment of inflammatory monocytes by senescent fibroblasts inhibits antigen-specific tissue immunity during human aging. Nat. Aging. 2021;1:101–113. doi: 10.1038/s43587-020-00010-6. [DOI] [PubMed] [Google Scholar]
- 15.Aw D., Silva A.B., Palmer D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McElhaney J.E., Zhou X., Talbot H.K., Soethout E., Bleackley R.C., Granville D.J., Pawelec G. The unmet need in the elderly: How immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012;30:2060–2067. doi: 10.1016/j.vaccine.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yousefzadeh M.J., Flores R.R., Zhu Y., Schmiechen Z.C., Brooks R.W., Trussoni C.E., Cui Y., Angelini L., Lee K.A., McGowan S.J., et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594:100–105. doi: 10.1038/s41586-021-03547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desdín-Micó G., Soto-Heredero G., Aranda J.F., Oller J., Carrasco E., Gabandé-Rodríguez E., Blanco E.M., Alfranca A., Cussó L., Desco M., et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science. 2020;368:1371–1376. doi: 10.1126/science.aax0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goronzy J.J., Fang F., Cavanagh M.M., Qi Q., Weyand C.M. Naive T cell maintenance and function in human aging. J. Immunol. 2015;194:4073–4080. doi: 10.4049/jimmunol.1500046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stulnig T., Maczek C., Böck G., Majdic O., Wick G. Reference intervals for human peripheral blood lymphocyte subpopulations from ‘healthy’ young and aged subjects. Int. Arch. Allergy Immunol. 1995;108:205–210. doi: 10.1159/000237155. [DOI] [PubMed] [Google Scholar]
- 21.Garrido-Rodríguez V., Herrero-Fernández I., Castro M.J., Castillo A., Rosado-Sánchez I., Galvá M.I., Ramos R., Olivas-Martínez I., Bulnes-Ramos Á., Cañizares J., et al. Immunological features beyond CD4/CD8 ratio values in older individuals. Aging. 2021;13:13443–13459. doi: 10.18632/aging.203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanni F., Vescovini R., Biasini C., Fagnoni F., Zanlari L., Telera A., Di Pede P., Passeri G., Pedrazzoni M., Passeri M., et al. Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: A contribution to understand the relationship between inflammation and immunosenescence. Exp. Gerontol. 2003;38:981–987. doi: 10.1016/S0531-5565(03)00160-8. [DOI] [PubMed] [Google Scholar]
- 23.Weng N.P., Akbar A.N., Goronzy J. CD28− T cells: Their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wherry E.J. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 25.Henson S.M., Akbar A.N. KLRG1—More than a marker for T cell senescence. Age. 2009;31:285–291. doi: 10.1007/s11357-009-9100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenchley J.M., Karandikar N.J., Betts M.R., Ambrozak D.R., Hill B.J., Crotty L.E., Casazza J.P., Kuruppu J., Migueles S.A., Connors M., et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 27.Xu W., Larbi A. Markers of T Cell Senescence in Humans. Int. J. Mol. Sci. 2017;18:1742. doi: 10.3390/ijms18081742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarazona R., DelaRosa O., Alonso C., Ostos B., Espejo J., Peña J., Solana R. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech. Ageing Dev. 2000;121:77–88. doi: 10.1016/S0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 29.Lanna A., Gomes D.C., Muller-Durovic B., McDonnell T., Escors D., Gilroy D.W., Lee J.H., Karin M., Akbar A.N. A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat. Immunol. 2017;18:354–363. doi: 10.1038/ni.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira B.I., De Maeyer R.P.H., Covre L.P., Nehar-Belaid D., Lanna A., Ward S., Marches R., Chambers E.S., Gomes D.C.O., Riddell N.E., et al. Sestrins induce natural killer function in senescent-like CD8. Nat. Immunol. 2020;21:684–694. doi: 10.1038/s41590-020-0643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henson S.M., Lanna A., Riddell N.E., Franzese O., Macaulay R., Griffiths S.J., Puleston D.J., Watson A.S., Simon A.K., Tooze S.A., et al. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8⁺ T cells. J. Clin. Investig. 2014;124:4004–4016. doi: 10.1172/JCI75051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirakawa K., Yan X., Shinmura K., Endo J., Kataoka M., Katsumata Y., Yamamoto T., Anzai A., Isobe S., Yoshida N., et al. Obesity accelerates T cell senescence in murine visceral adipose tissue. J. Clin. Investig. 2016;126:4626–4639. doi: 10.1172/JCI88606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li G., Yu M., Lee W.W., Tsang M., Krishnan E., Weyand C.M., Goronzy J.J. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat. Med. 2012;18:1518–1524. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sansoni P., Cossarizza A., Brianti V., Fagnoni F., Snelli G., Monti D., Marcato A., Passeri G., Ortolani C., Forti E. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82:2767–2773. doi: 10.1182/blood.V82.9.2767.2767. [DOI] [PubMed] [Google Scholar]
- 35.Pangrazzi L., Meryk A., Naismith E., Koziel R., Lair J., Krismer M., Trieb K., Grubeck-Loebenstein B. “Inflamm-aging” influences immune cell survival factors in human bone marrow. Eur. J. Immunol. 2017;47:481–492. doi: 10.1002/eji.201646570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLeod J.D. Apoptotic capability in ageing T cells. Mech. Ageing Dev. 2000;121:151–159. doi: 10.1016/S0047-6374(00)00206-2. [DOI] [PubMed] [Google Scholar]
- 37.Callender L.A., Carroll E.C., Beal R.W.J., Chambers E.S., Nourshargh S., Akbar A.N., Henson S.M. Human CD8. Aging Cell. 2018;17:e12675. doi: 10.1111/acel.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martínez-Zamudio R.I., Dewald H.K., Vasilopoulos T., Gittens-Williams L., Fitzgerald-Bocarsly P., Herbig U. Senescence-associated β-galactosidase reveals the abundance of senescent CD8+ T cells in aging humans. Aging Cell. 2021;20:e13344. doi: 10.1111/acel.13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pustavoitau A., Barodka V., Sharpless N.E., Torrice C., Nyhan D., Berkowitz D.E., Shah A.S., Bandeen Roche K.J., Walston J.D. Role of senescence marker p16 INK4a measured in peripheral blood T-lymphocytes in predicting length of hospital stay after coronary artery bypass surgery in older adults. Exp. Gerontol. 2016;74:29–36. doi: 10.1016/j.exger.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinn K.M., Fox A., Harland K.L., Russ B.E., Li J., Nguyen T.H.O., Loh L., Olshanksy M., Naeem H., Tsyganov K., et al. Age-related decline in primary CD8+ T cell responses is associated with the development of senescence in virtual memory CD8+ T cells. Cell Rep. 2018;23:3512–3524. doi: 10.1016/j.celrep.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 41.Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 42.Rink L., Cakman I., Kirchner H. Altered cytokine production in the elderly. Mech. Ageing Dev. 1998;102:199–209. doi: 10.1016/S0047-6374(97)00153-X. [DOI] [PubMed] [Google Scholar]
- 43.Goronzy J.J., Weyand C.M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 2019;19:573–583. doi: 10.1038/s41577-019-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Utzschneider D.T., Delpoux A., Wieland D., Huang X., Lai C.Y., Hofmann M., Thimme R., Hedrick S.M. Active Maintenance of T Cell Memory in Acute and Chronic Viral Infection Depends on Continuous Expression of FOXO1. Cell Rep. 2018;22:3454–3467. doi: 10.1016/j.celrep.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linterman M.A. How T follicular helper cells and the germinal centre response change with age. Immunol. Cell Biol. 2014;92:72–79. doi: 10.1038/icb.2013.77. [DOI] [PubMed] [Google Scholar]
- 46.Schmitt V., Rink L., Uciechowski P. The Th17/Treg balance is disturbed during aging. Exp. Gerontol. 2013;48:1379–1386. doi: 10.1016/j.exger.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Rocamora-Reverte L., Melzer F.L., Würzner R., Weinberger B. The Complex Role of Regulatory T Cells in Immunity and Aging. Front. Immunol. 2020;11:616949. doi: 10.3389/fimmu.2020.616949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frasca D., Diaz A., Romero M., Garcia D., Blomberg B.B. B Cell Immunosenescence. Annu. Rev. Cell Dev. Biol. 2020;36:551–574. doi: 10.1146/annurev-cellbio-011620-034148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ratliff M., Alter S., Frasca D., Blomberg B.B., Riley R.L. In senescence, age-associated B cells secrete TNFα and inhibit survival of B-cell precursors. Aging Cell. 2013;12:303–311. doi: 10.1111/acel.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao Y., O’Neill P., Naradikian M.S., Scholz J.L., Cancro M.P. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubtsov A.V., Rubtsova K., Fischer A., Meehan R.T., Gillis J.Z., Kappler J.W., Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c⁺ B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cancro M.P. Age-Associated B Cells. Annu. Rev. Immunol. 2020;38:315–340. doi: 10.1146/annurev-immunol-092419-031130. [DOI] [PubMed] [Google Scholar]
- 53.Ademokun A., Wu Y.C., Dunn-Walters D. The ageing B cell population: Composition and function. Biogerontology. 2010;11:125–137. doi: 10.1007/s10522-009-9256-9. [DOI] [PubMed] [Google Scholar]
- 54.Mauri C., Menon M. The expanding family of regulatory B cells. Int Immunol. 2015;27:479–486. doi: 10.1093/intimm/dxv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duggal N.A., Upton J., Phillips A.C., Sapey E., Lord J.M. An age-related numerical and functional deficit in CD19+ CD24hi CD38hi B cells is associated with an increase in systemic autoimmunity. Aging Cell. 2013;12:873–881. doi: 10.1111/acel.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Geest K.S., Lorencetti P.G., Abdulahad W.H., Horst G., Huitema M., Roozendaal C., Kroesen B.J., Brouwer E., Boots A.M. Aging-dependent decline of IL-10 producing B cells coincides with production of antinuclear antibodies but not rheumatoid factors. Exp. Gerontol. 2016;75:24–29. doi: 10.1016/j.exger.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Borgoni S., Kudryashova K.S., Burka K., de Magalhães J.P. Targeting immune dysfunction in aging. Ageing Res. Rev. 2021;70:101410. doi: 10.1016/j.arr.2021.101410. [DOI] [PubMed] [Google Scholar]
- 58.Bulati M., Buffa S., Candore G., Caruso C., Dunn-Walters D.K., Pellicanò M., Wu Y.C., Colonna Romano G. B cells and immunosenescence: A focus on IgG+IgD−CD27− (DN) B cells in aged humans. Ageing Res. Rev. 2011;10:274–284. doi: 10.1016/j.arr.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Frasca D., Diaz A., Romero M., Blomberg B.B. Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp. Gerontol. 2017;87:113–120. doi: 10.1016/j.exger.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Buffa S., Bulati M., Pellicanò M., Dunn-Walters D.K., Wu Y.C., Candore G., Vitello S., Caruso C., Colonna-Romano G. B cell immunosenescence: Different features of naive and memory B cells in elderly. Biogerontology. 2011;12:473–483. doi: 10.1007/s10522-011-9353-4. [DOI] [PubMed] [Google Scholar]
- 61.Shi Y., Yamazaki T., Okubo Y., Uehara Y., Sugane K., Agematsu K. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J. Immunol. 2005;175:3262–3267. doi: 10.4049/jimmunol.175.5.3262. [DOI] [PubMed] [Google Scholar]
- 62.Chang L.Y., Li Y., Kaplan D.E. Hepatitis C viraemia reversibly maintains subset of antigen-specific T-bet+ tissue-like memory B cells. J. Viral Hepat. 2017;24:389–396. doi: 10.1111/jvh.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Illingworth J., Butler N.S., Roetynck S., Mwacharo J., Pierce S.K., Bejon P., Crompton P.D., Marsh K., Ndungu F.M. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J. Immunol. 2013;190:1038–1047. doi: 10.4049/jimmunol.1202438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moir S., Ho J., Malaspina A., Wang W., DiPoto A.C., O’Shea M.A., Roby G., Kottilil S., Arthos J., Proschan M.A., et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colonna-Romano G., Bulati M., Aquino A., Pellicanò M., Vitello S., Lio D., Candore G., Caruso C. A double-negative (IgD−CD27−) B cell population is increased in the peripheral blood of elderly people. Mech. Ageing Dev. 2009;130:681–690. doi: 10.1016/j.mad.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Martorana A., Balistreri C.R., Bulati M., Buffa S., Azzarello D.M., Camarda C., Monastero R., Caruso C., Colonna-Romano G. Double negative (CD19+IgG+IgD−CD27−) B lymphocytes: A new insight from telomerase in healthy elderly, in centenarian offspring and in Alzheimer’s disease patients. Immunol. Lett. 2014;162:303–309. doi: 10.1016/j.imlet.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Simell B., Vuorela A., Ekström N., Palmu A., Reunanen A., Meri S., Käyhty H., Väkeväinen M. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine. 2011;29:1929–1934. doi: 10.1016/j.vaccine.2010.12.121. [DOI] [PubMed] [Google Scholar]
- 68.MacGregor R.R., Shalit M. Neutrophil function in healthy elderly subjects. J. Gerontol. 1990;45:M55-60. doi: 10.1093/geronj/45.2.M55. [DOI] [PubMed] [Google Scholar]
- 69.Butcher S., Chahel H., Lord J.M. Review article: Ageing and the neutrophil: No appetite for killing? Immunology. 2000;100:411–416. doi: 10.1046/j.1365-2567.2000.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brubaker A.L., Rendon J.L., Ramirez L., Choudhry M.A., Kovacs E.J. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J. Immunol. 2013;190:1746–1757. doi: 10.4049/jimmunol.1201213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Butcher S.K., Chahal H., Nayak L., Sinclair A., Henriquez N.V., Sapey E., O’Mahony D., Lord J.M. Senescence in innate immune responses: Reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J. Leukoc. Biol. 2001;70:881–886. doi: 10.1189/jlb.70.6.881. [DOI] [PubMed] [Google Scholar]
- 72.Fulop T., Larbi A., Douziech N., Fortin C., Guérard K.P., Lesur O., Khalil A., Dupuis G. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3:217–226. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 73.Sapey E., Greenwood H., Walton G., Mann E., Love A., Aaronson N., Insall R.H., Stockley R.A., Lord J.M. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: Toward targeted treatments for immunosenescence. Blood. 2014;123:239–248. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kolaczkowska E. The older the faster: Aged neutrophils in inflammation. Blood. 2016;128:2280–2282. doi: 10.1182/blood-2016-09-739680. [DOI] [PubMed] [Google Scholar]
- 75.Wenisch C., Patruta S., Daxböck F., Krause R., Hörl W. Effect of age on human neutrophil function. J. Leukoc. Biol. 2000;67:40–45. doi: 10.1002/jlb.67.1.40. [DOI] [PubMed] [Google Scholar]
- 76.Emanuelli G., Lanzio M., Anfossi T., Romano S., Anfossi G., Calcamuggi G. Influence of age on polymorphonuclear leukocytes in vitro: Phagocytic activity in healthy human subjects. Gerontology. 1986;32:308–316. doi: 10.1159/000212809. [DOI] [PubMed] [Google Scholar]
- 77.Tseng C.W., Kyme P.A., Arruda A., Ramanujan V.K., Tawackoli W., Liu G.Y. Innate immune dysfunctions in aged mice facilitate the systemic dissemination of methicillin-resistant S. aureus. PLoS ONE. 2012;7:e41454. doi: 10.1371/journal.pone.0041454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hazeldine J., Harris P., Chapple I.L., Grant M., Greenwood H., Livesey A., Sapey E., Lord J.M. Impaired neutrophil extracellular trap formation: A novel defect in the innate immune system of aged individuals. Aging Cell. 2014;13:690–698. doi: 10.1111/acel.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gayoso I., Sanchez-Correa B., Campos C., Alonso C., Pera A., Casado J.G., Morgado S., Tarazona R., Solana R. Immunosenescence of human natural killer cells. J. Innate Immun. 2011;3:337–343. doi: 10.1159/000328005. [DOI] [PubMed] [Google Scholar]
- 80.Muzzioli M., Stecconi R., Moresi R., Provinciali M. Zinc improves the development of human CD34+ cell progenitors towards NK cells and increases the expression of GATA-3 transcription factor in young and old ages. Biogerontology. 2009;10:593–604. doi: 10.1007/s10522-008-9201-3. [DOI] [PubMed] [Google Scholar]
- 81.Hazeldine J., Hampson P., Lord J.M. Reduced release and binding of perforin at the immunological synapse underlies the age-related decline in natural killer cell cytotoxicity. Aging Cell. 2012;11:751–759. doi: 10.1111/j.1474-9726.2012.00839.x. [DOI] [PubMed] [Google Scholar]
- 82.Almeida-Oliveira A., Smith-Carvalho M., Porto L.C., Cardoso-Oliveira J., Ribeiro A.o.S., Falcão R.R., Abdelhay E., Bouzas L.F., Thuler L.C., Ornellas M.H., et al. Age-related changes in natural killer cell receptors from childhood through old age. Hum. Immunol. 2011;72:319–329. doi: 10.1016/j.humimm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 83.Solana R., Campos C., Pera A., Tarazona R. Shaping of NK cell subsets by aging. Curr. Opin. Immunol. 2014;29:56–61. doi: 10.1016/j.coi.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 84.Ogata K., An E., Shioi Y., Nakamura K., Luo S., Yokose N., Minami S., Dan K. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin. Exp. Immunol. 2001;124:392–397. doi: 10.1046/j.1365-2249.2001.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lutz C.T., Moore M.B., Bradley S., Shelton B.J., Lutgendorf S.K. Reciprocal age related change in natural killer cell receptors for MHC class I. Mech. Ageing Dev. 2005;126:722–731. doi: 10.1016/j.mad.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rajagopalan S., Lee E.C., DuPrie M.L., Long E.O. TNFR-associated factor 6 and TGF-β-activated kinase 1 control signals for a senescence response by an endosomal NK cell receptor. J. Immunol. 2014;192:714–721. doi: 10.4049/jimmunol.1302384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kohut M.L., Senchina D.S., Madden K.S., Martin A.E., Felten D.L., Moynihan J.A. Age effects on macrophage function vary by tissue site, nature of stimulant, and exercise behavior. Exp. Gerontol. 2004;39:1347–1360. doi: 10.1016/j.exger.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 88.Geiger H., Van Zant G. The aging of lympho-hematopoietic stem cells. Nat. Immunol. 2002;3:329–333. doi: 10.1038/ni0402-329. [DOI] [PubMed] [Google Scholar]
- 89.De Maeyer R.P.H., Chambers E.S. The impact of ageing on monocytes and macrophages. Immunol. Lett. 2021;230:1–10. doi: 10.1016/j.imlet.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Ogawa T., Kitagawa M., Hirokawa K. Age-related changes of human bone marrow: A histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech. Ageing Dev. 2000;117:57–68. doi: 10.1016/S0047-6374(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 91.Herrero C., Marqués L., Lloberas J., Celada A. IFN-gamma-dependent transcription of MHC class II IA is impaired in macrophages from aged mice. J. Clin. Investig. 2001;107:485–493. doi: 10.1172/JCI11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong C.O., Gregory S., Hu H., Chao Y., Sepúlveda V.E., He Y., Li-Kroeger D., Goldman W.E., Bellen H.J., Venkatachalam K. Lysosomal Degradation Is Required for Sustained Phagocytosis of Bacteria by Macrophages. Cell Host Microbe. 2017;21:719–730. doi: 10.1016/j.chom.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Renshaw M., Rockwell J., Engleman C., Gewirtz A., Katz J., Sambhara S. Cutting edge: Impaired Toll-like receptor expression and function in aging. J. Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 94.Chelvarajan R.L., Liu Y., Popa D., Getchell M.L., Getchell T.V., Stromberg A.J., Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J. Leukoc. Biol. 2006;79:1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- 95.Sica A., Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Beek A.A., Van den Bossche J., Mastroberardino P.G., de Winther M.P.J., Leenen P.J.M. Metabolic Alterations in Aging Macrophages: Ingredients for Inflammaging? Trends Immunol. 2019;40:113–127. doi: 10.1016/j.it.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 97.Mahbub S., Deburghgraeve C.R., Kovacs E.J. Advanced age impairs macrophage polarization. J. Interfer. Cytokine Res. 2012;32:18–26. doi: 10.1089/jir.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qian F., Wang X., Zhang L., Chen S., Piecychna M., Allore H., Bockenstedt L., Malawista S., Bucala R., Shaw A.C., et al. Age-associated elevation in TLR5 leads to increased inflammatory responses in the elderly. Aging Cell. 2012;11:104–110. doi: 10.1111/j.1474-9726.2011.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nyunoya T., Powers L.S., Yarovinsky T.O., Butler N.S., Monick M.M., Hunninghake G.W. Hyperoxia induces macrophage cell cycle arrest by adhesion-dependent induction of p21Cip1 and activation of the retinoblastoma protein. J. Biol. Chem. 2003;278:36099–36106. doi: 10.1074/jbc.M304370200. [DOI] [PubMed] [Google Scholar]
- 101.Wang H., Fu H., Zhu R., Wu X., Ji X., Li X., Jiang H., Lin Z., Tang X., Sun S., et al. BRD4 contributes to LPS-induced macrophage senescence and promotes progression of atherosclerosis-associated lipid uptake. Aging. 2020;12:9240–9259. doi: 10.18632/aging.103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prattichizzo F., De Nigris V., Mancuso E., Spiga R., Giuliani A., Matacchione G., Lazzarini R., Marcheselli F., Recchioni R., Testa R., et al. Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biol. 2018;15:170–181. doi: 10.1016/j.redox.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kloc M., Uosef A., Subuddhi A., Kubiak J.Z., Piprek R.P., Ghobrial R.M. Giant Multinucleated Cells in Aging and Senescence-An Abridgement. Biology. 2022;11:1121. doi: 10.3390/biology11081121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Metcalf T.U., Wilkinson P.A., Cameron M.J., Ghneim K., Chiang C., Wertheimer A.M., Hiscott J.B., Nikolich-Zugich J., Haddad E.K. Human Monocyte Subsets Are Transcriptionally and Functionally Altered in Aging in Response to Pattern Recognition Receptor Agonists. J. Immunol. 2017;199:1405–1417. doi: 10.4049/jimmunol.1700148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Molony R.D., Nguyen J.T., Kong Y., Montgomery R.R., Shaw A.C., Iwasaki A. Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes. Sci. Signal. 2017;10:eaan2392. doi: 10.1126/scisignal.aan2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wong K.L., Yeap W.H., Tai J.J., Ong S.M., Dang T.M., Wong S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 107.Ong S.M., Hadadi E., Dang T.M., Yeap W.H., Tan C.T., Ng T.P., Larbi A., Wong S.C. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis. 2018;9:266. doi: 10.1038/s41419-018-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Connors J., Taramangalam B., Cusimano G., Bell M.R., Matt S.M., Runner K., Gaskill P.J., DeFilippis V., Nikolich-Žugich J., Kutzler M.A., et al. Aging alters antiviral signaling pathways resulting in functional impairment in innate immunity in response to pattern recognition receptor agonists. Geroscience. 2022:1–18. doi: 10.1007/s11357-022-00612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bleve A., Motta F., Durante B., Pandolfo C., Selmi C., Sica A. Immunosenescence, Inflammaging, and Frailty: Role of Myeloid Cells in Age-Related Diseases. Clin. Rev. Allergy Immunol. 2022:1–22. doi: 10.1007/s12016-021-08909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Consonni F.M., Porta C., Marino A., Pandolfo C., Mola S., Bleve A., Sica A. Myeloid-Derived Suppressor Cells: Ductile Targets in Disease. Front. Immunol. 2019;10:949. doi: 10.3389/fimmu.2019.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jing Y., Shaheen E., Drake R.R., Chen N., Gravenstein S., Deng Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 2009;70:777–784. doi: 10.1016/j.humimm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Granot T., Senda T., Carpenter D.J., Matsuoka N., Weiner J., Gordon C.L., Miron M., Kumar B.V., Griesemer A., Ho S.H., et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity. 2017;46:504–515. doi: 10.1016/j.immuni.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chambers E.S., Vukmanovic-Stejic M. Skin barrier immunity and ageing. Immunology. 2020;160:116–125. doi: 10.1111/imm.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Panda A., Qian F., Mohanty S., van Duin D., Newman F.K., Zhang L., Chen S., Towle V., Belshe R.B., Fikrig E., et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Agrawal A., Gupta S. Impact of aging on dendritic cell functions in humans. Ageing Res. Rev. 2011;10:336–345. doi: 10.1016/j.arr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gustafson C.E., Kim C., Weyand C.M., Goronzy J.J. Influence of immune aging on vaccine responses. J. Allergy Clin. Immunol. 2020;145:1309–1321. doi: 10.1016/j.jaci.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kulkarni D.H., Gustafsson J.K., Knoop K.A., McDonald K.G., Bidani S.S., Davis J.E., Floyd A.N., Hogan S.P., Hsieh C.S., Newberry R.D. Goblet cell associated antigen passages support the induction and maintenance of oral tolerance. Mucosal Immunol. 2020;13:271–282. doi: 10.1038/s41385-019-0240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Josset L., Engelmann F., Haberthur K., Kelly S., Park B., Kawoaka Y., García-Sastre A., Katze M.G., Messaoudi I. Increased viral loads and exacerbated innate host responses in aged macaques infected with the 2009 pandemic H1N1 influenza A virus. J. Virol. 2012;86:11115–11127. doi: 10.1128/JVI.01571-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang B.M., Shojaei M., Teoh S., Meyers A., Ho J., Ball T.B., Keynan Y., Pisipati A., Kumar A., Eisen D.P., et al. Neutrophils-related host factors associated with severe disease and fatality in patients with influenza infection. Nat. Commun. 2019;10:3422. doi: 10.1038/s41467-019-11249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dunning J., Blankley S., Hoang L.T., Cox M., Graham C.M., James P.L., Bloom C.I., Chaussabel D., Banchereau J., Brett S.J., et al. Author Correction: Progression of whole-blood transcriptional signatures from interferon-induced to neutrophil-associated patterns in severe influenza. Nat. Immunol. 2019;20:373. doi: 10.1038/s41590-019-0328-y. [DOI] [PubMed] [Google Scholar]
- 121.Wong C.K., Smith C.A., Sakamoto K., Kaminski N., Koff J.L., Goldstein D.R. Aging Impairs Alveolar Macrophage Phagocytosis and Increases Influenza-Induced Mortality in Mice. J. Immunol. 2017;199:1060–1068. doi: 10.4049/jimmunol.1700397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Maeyer R.P.H., van de Merwe R.C., Louie R., Bracken O.V., Devine O.P., Goldstein D.R., Uddin M., Akbar A.N., Gilroy D.W. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat. Immunol. 2020;21:615–625. doi: 10.1038/s41590-020-0646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aprahamian T., Takemura Y., Goukassian D., Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin. Exp. Immunol. 2008;152:448–455. doi: 10.1111/j.1365-2249.2008.03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Decman V., Laidlaw B.J., Dimenna L.J., Abdulla S., Mozdzanowska K., Erikson J., Ertl H.C., Wherry E.J. Cell-intrinsic defects in the proliferative response of antiviral memory CD8 T cells in aged mice upon secondary infection. J. Immunol. 2010;184:5151–5159. doi: 10.4049/jimmunol.0902063. [DOI] [PubMed] [Google Scholar]
- 125.Yager E.J., Ahmed M., Lanzer K., Randall T.D., Woodland D.L., Blackman M.A. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lanzer K.G., Johnson L.L., Woodland D.L., Blackman M.A. Impact of ageing on the response and repertoire of influenza virus-specific CD4 T cells. Immun. Ageing. 2014;11:9. doi: 10.1186/1742-4933-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lanfermeijer J., Borghans J.A.M., van Baarle D. How age and infection history shape the antigen-specific CD8. Aging Cell. 2020;19:e13262. doi: 10.1111/acel.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Po J.L., Gardner E.M., Anaraki F., Katsikis P.D., Murasko D.M. Age-associated decrease in virus-specific CD8+ T lymphocytes during primary influenza infection. Mech. Ageing Dev. 2002;123:1167–1181. doi: 10.1016/S0047-6374(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 129.Toapanta F.R., Ross T.M. Impaired immune responses in the lungs of aged mice following influenza infection. Respir. Res. 2009;10:112. doi: 10.1186/1465-9921-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiang J., Bennett A.J., Fisher E., Williams-Bey Y., Shen H., Murasko D.M. Limited expansion of virus-specific CD8 T cells in the aged environment. Mech. Ageing Dev. 2009;130:713–721. doi: 10.1016/j.mad.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lefebvre J.S., Masters A.R., Hopkins J.W., Haynes L. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Sci. Rep. 2016;6:25051. doi: 10.1038/srep25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zacca E.R., Crespo M.I., Acland R.P., Roselli E., Núñez N.G., Maccioni M., Maletto B.A., Pistoresi-Palencia M.C., Morón G. Aging Impairs the Ability of Conventional Dendritic Cells to Cross-Prime CD8+ T Cells upon Stimulation with a TLR7 Ligand. PLoS ONE. 2015;10:e0140672. doi: 10.1371/journal.pone.0140672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao J., Legge K., Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J. Clin. Investig. 2011;121:4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Agrawal A., Agrawal S., Cao J.N., Su H., Osann K., Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: A role of phosphoinositide 3-kinase-signaling pathway. J. Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 135.Aksoy E., Saveanu L., Manoury B. The Isoform Selective Roles of PI3Ks in Dendritic Cell Biology and Function. Front. Immunol. 2018;9:2574. doi: 10.3389/fimmu.2018.02574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Frasca D., Diaz A., Romero M., Blomberg B.B. The generation of memory B cells is maintained, but the antibody response is not, in the elderly after repeated influenza immunizations. Vaccine. 2016;34:2834–2840. doi: 10.1016/j.vaccine.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nipper A.J., Smithey M.J., Shah R.C., Canaday D.H., Landay A.L. Diminished antibody response to influenza vaccination is characterized by expansion of an age-associated B-cell population with low PAX5. Clin Immunol. 2018;193:80–87. doi: 10.1016/j.clim.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goodwin K., Viboud C., Simonsen L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Y., Wang Y., Gilmore X., Xu K., Wyde P.R., Mbawuike I.N. An aged mouse model for RSV infection and diminished CD8+ CTL responses. Exp. Biol. Med. 2002;227:133–140. doi: 10.1177/153537020222700208. [DOI] [PubMed] [Google Scholar]
- 140.Pennings J.L.A., Mariman R., Hodemaekers H.M., Reemers S.S.N., Janssen R., Guichelaar T. Transcriptomics in lung tissue upon respiratory syncytial virus infection reveals aging as important modulator of immune activation and matrix maintenance. Sci. Rep. 2018;8:16653. doi: 10.1038/s41598-018-35180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cherukuri A., Patton K., Gasser R.A., Zuo F., Woo J., Esser M.T., Tang R.S. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin. Vaccine Immunol. 2013;20:239–247. doi: 10.1128/CVI.00580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Johnstone J., Parsons R., Botelho F., Millar J., McNeil S., Fulop T., McElhaney J., Andrew M.K., Walter S.D., Devereaux P.J., et al. Immune biomarkers predictive of respiratory viral infection in elderly nursing home residents. PLoS ONE. 2014;9:e108481. doi: 10.1371/journal.pone.0108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Falsey A.R., Dallal G.E., Formica M.A., Andolina G.G., Hamer D.H., Leka L.L., Meydani S.N. Long-term care facilities: A cornucopia of viral pathogens. J. Am. Geriatr. Soc. 2008;56:1281–1285. doi: 10.1111/j.1532-5415.2008.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Krone C.L., van de Groep K., Trzciński K., Sanders E.A., Bogaert D. Immunosenescence and pneumococcal disease: An imbalance in host-pathogen interactions. Lancet Respir. Med. 2014;2:141–153. doi: 10.1016/S2213-2600(13)70165-6. [DOI] [PubMed] [Google Scholar]
- 145.Williams A.E., José R.J., Brown J.S., Chambers R.C. Enhanced inflammation in aged mice following infection with Streptococcus pneumoniae is associated with decreased IL-10 and augmented chemokine production. Am. J. Physiol. Cell. Mol. Physiol. 2015;308:L539–L549. doi: 10.1152/ajplung.00141.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Menter T., Giefing-Kroell C., Grubeck-Loebenstein B., Tzankov A. Characterization of the inflammatory infiltrate in Streptococcus pneumoniae pneumonia in young and elderly patients. Pathobiology. 2014;81:160–167. doi: 10.1159/000360165. [DOI] [PubMed] [Google Scholar]
- 147.Bhalla M., Heinzinger L.R., Morenikeji O.B., Marzullo B., Thomas B.N., Bou Ghanem E.N. Transcriptome Profiling Reveals CD73 and Age-Driven Changes in Neutrophil Responses against Streptococcus pneumoniae. Infect. Immun. 2021;89:e00258-21. doi: 10.1128/IAI.00258-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hinojosa E., Boyd A.R., Orihuela C.J. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J. Infect. Dis. 2009;200:546–554. doi: 10.1086/600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boyd A.R., Shivshankar P., Jiang S., Berton M.T., Orihuela C.J. Age-related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Exp. Gerontol. 2012;47:507–518. doi: 10.1016/j.exger.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cho S.J., Rooney K., Choi A.M.K., Stout-Delgado H.W. NLRP3 inflammasome activation in aged macrophages is diminished during Streptococcus pneumoniae infection. Am. J. Physiol. Cell. Mol. Physiol. 2018;314:L372–L387. doi: 10.1152/ajplung.00393.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Inomata M., Xu S., Chandra P., Meydani S.N., Takemura G., Philips J.A., Leong J.M. Macrophage LC3-associated phagocytosis is an immune defense against. Proc. Natl. Acad. Sci. USA. 2020;117:33561–33569. doi: 10.1073/pnas.2015368117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Birjandi S.Z., Ippolito J.A., Ramadorai A.K., Witte P.L. Alterations in marginal zone macrophages and marginal zone B cells in old mice. J. Immunol. 2011;186:3441–3451. doi: 10.4049/jimmunol.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bruunsgaard H., Skinhøj P., Qvist J., Pedersen B.K. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. J. Infect. Dis. 1999;180:551–554. doi: 10.1086/314873. [DOI] [PubMed] [Google Scholar]
- 154.Aiello A., Farzaneh F., Candore G., Caruso C., Davinelli S., Gambino C.M., Ligotti M.E., Zareian N., Accardi G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019;10:2247. doi: 10.3389/fimmu.2019.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Virgin H.W., Wherry E.J., Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 156.Kahan S.M., Wherry E.J., Zajac A.J. T cell exhaustion during persistent viral infections. Virology. 2015;479–480:180–193. doi: 10.1016/j.virol.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Velu V., Titanji K., Zhu B., Husain S., Pladevega A., Lai L., Vanderford T.H., Chennareddi L., Silvestri G., Freeman G.J., et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wherry E.J., Ha S.J., Kaech S.M., Haining W.N., Sarkar S., Kalia V., Subramaniam S., Blattman J.N., Barber D.L., Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 159.Shin H., Blackburn S.D., Blattman J.N., Wherry E.J. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moro-García M.A., Alonso-Arias R., López-Larrea C. Molecular mechanisms involved in the aging of the T-cell immune response. Curr. Genom. 2012;13:589–602. doi: 10.2174/138920212803759749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Voehringer D., Blaser C., Brawand P., Raulet D.H., Hanke T., Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J. Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 162.Gillespie G.M., Wills M.R., Appay V., O’Callaghan C., Murphy M., Smith N., Sissons P., Rowland-Jones S., Bell J.I., Moss P.A. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8+ T lymphocytes in healthy seropositive donors. J. Virol. 2000;74:8140–8150. doi: 10.1128/JVI.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Park H.J., Park J.S., Jeong Y.H., Son J., Ban Y.H., Lee B.H., Chen L., Chang J., Chung D.H., Choi I., et al. Correction: PD-1 Upregulated on Regulatory T Cells during Chronic Virus Infection Enhances the Suppression of CD8+ T Cell Immune Response via the Interaction with PD-L1 Expressed on CD8+ T Cells. J. Immunol. 2015;195:5841–5842. doi: 10.4049/jimmunol.1502256. [DOI] [PubMed] [Google Scholar]
- 164.Catakovic K., Klieser E., Neureiter D., Geisberger R. T cell exhaustion: From pathophysiological basics to tumor immunotherapy. Cell Commun. Signal. 2017;15:1. doi: 10.1186/s12964-016-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Strauss G., Knape I., Melzner I., Debatin K.M. Constitutive caspase activation and impaired death-inducing signaling complex formation in CD95-resistant, long-term activated, antigen-specific T cells. J. Immunol. 2003;171:1172–1182. doi: 10.4049/jimmunol.171.3.1172. [DOI] [PubMed] [Google Scholar]
- 166.Collaboration A.T.C. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Trautmann L., Janbazian L., Chomont N., Said E.A., Gimmig S., Bessette B., Boulassel M.R., Delwart E., Sepulveda H., Balderas R.S., et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 168.Cao W., Mehraj V., Kaufmann D.E., Li T., Routy J.P. Elevation and persistence of CD8 T-cells in HIV infection: The Achilles heel in the ART era. J. Int. AIDS Soc. 2016;19:20697. doi: 10.7448/IAS.19.1.20697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Palmer L.D., Weng N., Levine B.L., June C.H., Lane H.C., Hodes R.J. Telomere length, telomerase activity, and replicative potential in HIV infection: Analysis of CD4+ and CD8+ T cells from HIV-discordant monozygotic twins. J. Exp. Med. 1997;185:1381–1386. doi: 10.1084/jem.185.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dagarag M., Evazyan T., Rao N., Effros R.B. Genetic manipulation of telomerase in HIV-specific CD8+ T cells: Enhanced antiviral functions accompany the increased proliferative potential and telomere length stabilization. J. Immunol. 2004;173:6303–6311. doi: 10.4049/jimmunol.173.10.6303. [DOI] [PubMed] [Google Scholar]
- 171.Golden-Mason L., Palmer B.E., Kassam N., Townshend-Bulson L., Livingston S., McMahon B.J., Castelblanco N., Kuchroo V., Gretch D.R., Rosen H.R. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J. Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hoare M., Shankar A., Shah M., Rushbrook S., Gelson W., Davies S., Akbar A., Alexander G.J. γ-H2AX + CD8+ T lymphocytes cannot respond to IFN-α, IL-2 or IL-6 in chronic hepatitis C virus infection. J. Hepatol. 2013;58:868–874. doi: 10.1016/j.jhep.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rha M.S., Shin E.C. Activation or exhaustion of CD8. Cell. Mol. Immunol. 2021;18:2325–2333. doi: 10.1038/s41423-021-00750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.James S.F., Traina-Dorge V., Deharo E., Wellish M., Palmer B.E., Gilden D., Mahalingam R. T cells increase before zoster and PD-1 expression increases at the time of zoster in immunosuppressed nonhuman primates latently infected with simian varicella virus. J. Neurovirol. 2014;20:309–313. doi: 10.1007/s13365-014-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Allen S.J., Mott K.R., Zandian M., Ghiasi H. Immunization with different viral antigens alters the pattern of T cell exhaustion and latency in herpes simplex virus type 1-infected mice. J. Virol. 2010;84:12315–12324. doi: 10.1128/JVI.01600-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.O’Bryan J.M., Woda M., Co M., Mathew A., Rothman A.L. Telomere length dynamics in human memory T cells specific for viruses causing acute or latent infections. Immun. Ageing. 2013;10:37. doi: 10.1186/1742-4933-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gonçalves D.U., Proietti F.A., Ribas J.G., Araújo M.G., Pinheiro S.R., Guedes A.C., Carneiro-Proietti A.B. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin. Microbiol. Rev. 2010;23:577–589. doi: 10.1128/CMR.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kozako T., Yoshimitsu M., Akimoto M., White Y., Matsushita K., Soeda S., Shimeno H., Kubota R., Izumo S., Arima N. Programmed death-1 (PD-1)/PD-1 ligand pathway-mediated immune responses against human T-lymphotropic virus type 1 (HTLV-1) in HTLV-1-associated myelopathy/tropical spastic paraparesis and carriers with autoimmune disorders. Hum. Immunol. 2011;72:1001–1006. doi: 10.1016/j.humimm.2011.07.308. [DOI] [PubMed] [Google Scholar]
- 179.Ezinne C.C., Yoshimitsu M., White Y., Arima N. HTLV-1 specific CD8+ T cell function augmented by blockade of 2B4/CD48 interaction in HTLV-1 infection. PLoS ONE. 2014;9:e87631. doi: 10.1371/journal.pone.0087631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sabouri A.H., Usuku K., Hayashi D., Izumo S., Ohara Y., Osame M., Saito M. Impaired function of human T-lymphotropic virus type 1 (HTLV-1)-specific CD8+ T cells in HTLV-1-associated neurologic disease. Blood. 2008;112:2411–2420. doi: 10.1182/blood-2008-02-140335. [DOI] [PubMed] [Google Scholar]
- 181.Datta A., Nicot C. Telomere attrition induces a DNA double-strand break damage signal that reactivates p53 transcription in HTLV-I leukemic cells. Oncogene. 2008;27:1135–1141. doi: 10.1038/sj.onc.1210718. [DOI] [PubMed] [Google Scholar]
- 182.Jones R.B., Ndhlovu L.C., Barbour J.D., Sheth P.M., Jha A.R., Long B.R., Wong J.C., Satkunarajah M., Schweneker M., Chapman J.M., et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Larsen M., Sauce D., Deback C., Arnaud L., Mathian A., Miyara M., Boutolleau D., Parizot C., Dorgham K., Papagno L., et al. Exhausted cytotoxic control of Epstein-Barr virus in human lupus. PLoS Pathog. 2011;7:e1002328. doi: 10.1371/journal.ppat.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pender M.P., Csurhes P.A., Burrows J.M., Burrows S.R. Defective T-cell control of Epstein-Barr virus infection in multiple sclerosis. Clin. Transl. Immunol. 2017;6:e126. doi: 10.1038/cti.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Annels N.E., Callan M.F., Tan L., Rickinson A.B. Changing patterns of dominant TCR usage with maturation of an EBV-specific cytotoxic T cell response. J. Immunol. 2000;165:4831–4841. doi: 10.4049/jimmunol.165.9.4831. [DOI] [PubMed] [Google Scholar]
- 186.van Baarle D., Hovenkamp E., Callan M.F., Wolthers K.C., Kostense S., Tan L.C., Niesters H.G., Osterhaus A.D., McMichael A.J., van Oers M.H., et al. Dysfunctional Epstein-Barr virus (EBV)-specific CD8+ T lymphocytes and increased EBV load in HIV-1 infected individuals progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2001;98:146–155. doi: 10.1182/blood.V98.1.146. [DOI] [PubMed] [Google Scholar]
- 187.Plunkett F.J., Franzese O., Belaramani L.L., Fletcher J.M., Gilmour K.C., Sharifi R., Khan N., Hislop A.D., Cara A., Salmon M., et al. The impact of telomere erosion on memory CD8+ T cells in patients with X-linked lymphoproliferative syndrome. Mech. Ageing Dev. 2005;126:855–865. doi: 10.1016/j.mad.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 188.van de Berg P.J., van Stijn A., Ten Berge I.J., van Lier R.A. A fingerprint left by cytomegalovirus infection in the human T cell compartment. J. Clin. Virol. 2008;41:213–217. doi: 10.1016/j.jcv.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 189.Pourgheysari B., Khan N., Best D., Bruton R., Nayak L., Moss P.A. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J. Virol. 2007;81:7759–7765. doi: 10.1128/JVI.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.van Leeuwen E.M., van Buul J.D., Remmerswaal E.B., Hordijk P.L., ten Berge I.J., van Lier R.A. Functional re-expression of CCR7 on CMV-specific CD8+ T cells upon antigenic stimulation. Int. Immunol. 2005;17:713–719. doi: 10.1093/intimm/dxh251. [DOI] [PubMed] [Google Scholar]
- 191.Cicin-Sain L., Brien J.D., Uhrlaub J.L., Drabig A., Marandu T.F., Nikolich-Zugich J. Cytomegalovirus infection impairs immune responses and accentuates T-cell pool changes observed in mice with aging. PLoS Pathog. 2012;8:e1002849. doi: 10.1371/journal.ppat.1002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chiu Y.L., Lin C.H., Sung B.Y., Chuang Y.F., Schneck J.P., Kern F., Pawelec G., Wang G.C. Cytotoxic polyfunctionality maturation of cytomegalovirus-pp65-specific CD4 + and CD8 + T-cell responses in older adults positively correlates with response size. Sci. Rep. 2016;6:19227. doi: 10.1038/srep19227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Unemori P., Leslie K.S., Hunt P.W., Sinclair E., Epling L., Mitsuyasu R., Effros R.B., Dock J., Dollard S.G., Deeks S.G., et al. Immunosenescence is associated with presence of Kaposi’s sarcoma in antiretroviral treated HIV infection. AIDS. 2013;27:1735–1742. doi: 10.1097/QAD.0b013e3283601144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Beldi-Ferchiou A., Lambert M., Dogniaux S., Vély F., Vivier E., Olive D., Dupuy S., Levasseur F., Zucman D., Lebbé C., et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget. 2016;7:72961–72977. doi: 10.18632/oncotarget.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang W., Song Y., Lu Y.L., Sun J.Z., Wang H.W. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology. 2013;139:513–522. doi: 10.1111/imm.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu B., Woltman A.M., Janssen H.L., Boonstra A. Modulation of dendritic cell function by persistent viruses. J. Leukoc. Biol. 2009;85:205–214. doi: 10.1189/jlb.0408241. [DOI] [PubMed] [Google Scholar]
- 197.Mathiasen S.L., Gall-Mas L., Pateras I.S., Theodorou S.D.P., Namini M.R.J., Hansen M.B., Martin O.C.B., Vadivel C.K., Ntostoglou K., Butter D., et al. Bacterial genotoxins induce T cell senescence. Cell Rep. 2021;35:109220. doi: 10.1016/j.celrep.2021.109220. [DOI] [PubMed] [Google Scholar]
- 198.Ibler A.E.M., ElGhazaly M., Naylor K.L., Bulgakova N.A., F El-Khamisy S., Humphreys D. Typhoid toxin exhausts the RPA response to DNA replication stress driving senescence and Salmonella infection. Nat. Commun. 2019;10:4040. doi: 10.1038/s41467-019-12064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sakai S., Mayer-Barber K.D., Barber D.L. Defining features of protective CD4 T cell responses to Mycobacterium tuberculosis. Curr. Opin. Immunol. 2014;29:137–142. doi: 10.1016/j.coi.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.McNab F.W., Berry M.P., Graham C.M., Bloch S.A., Oni T., Wilkinson K.A., Wilkinson R.J., Kon O.M., Banchereau J., Chaussabel D., et al. Programmed death ligand 1 is over-expressed by neutrophils in the blood of patients with active tuberculosis. Eur. J. Immunol. 2011;41:1941–1947. doi: 10.1002/eji.201141421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jurado J.O., Alvarez I.B., Pasquinelli V., Martínez G.J., Quiroga M.F., Abbate E., Musella R.M., Chuluyan H.E., García V.E. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J. Immunol. 2008;181:116–125. doi: 10.4049/jimmunol.181.1.116. [DOI] [PubMed] [Google Scholar]
- 202.Singh A., Mohan A., Dey A.B., Mitra D.K. Inhibiting the programmed death 1 pathway rescues Mycobacterium tuberculosis-specific interferon γ-producing T cells from apoptosis in patients with pulmonary tuberculosis. J. Infect. Dis. 2013;208:603–615. doi: 10.1093/infdis/jit206. [DOI] [PubMed] [Google Scholar]
- 203.Barber D.L., Mayer-Barber K.D., Feng C.G., Sharpe A.H., Sher A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J. Immunol. 2011;186:1598–1607. doi: 10.4049/jimmunol.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shankar E.M., Velu V., Kamarulzaman A., Larsson M. Mechanistic insights on immunosenescence and chronic immune activation in HIV-tuberculosis co-infection. World J. Virol. 2015;4:17–24. doi: 10.5501/wjv.v4.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Covre L.P., Martins R.F., Devine O.P., Chambers E.S., Vukmanovic-Stejic M., Silva J.A., Dietze R., Rodrigues R.R., de Matos Guedes H.L., Falqueto A., et al. Circulating Senescent T Cells Are Linked to Systemic Inflammation and Lesion Size During Human Cutaneous Leishmaniasis. Front. Immunol. 2018;9:3001. doi: 10.3389/fimmu.2018.03001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Conway J., Certo M., Lord J.M., Mauro C., Duggal N.A. Understanding the role of host metabolites in the induction of immune senescence: Future strategies for keeping the ageing population healthy. Br. J. Pharmacol. 2022;179:1808–1824. doi: 10.1111/bph.15671. [DOI] [PubMed] [Google Scholar]
- 207.Lee K.A., Robbins P.D., Camell C.D. Intersection of immunometabolism and immunosenescence during aging. Curr. Opin. Pharmacol. 2021;57:107–116. doi: 10.1016/j.coph.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wiley C.D., Campisi J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021;3:1290–1301. doi: 10.1038/s42255-021-00483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jeng M.Y., Hull P.A., Fei M., Kwon H.S., Tsou C.L., Kasler H., Ng C.P., Gordon D.E., Johnson J., Krogan N., et al. Metabolic reprogramming of human CD8. J. Exp. Med. 2018;215:51–62. doi: 10.1084/jem.20161066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li L., Liu X., Sanders K.L., Edwards J.L., Ye J., Si F., Gao A., Huang L., Hsueh E.C., Ford D.A., et al. TLR8-Mediated Metabolic Control of Human Treg Function: A Mechanistic Target for Cancer Immunotherapy. Cell Metab. 2019;29:103–123. doi: 10.1016/j.cmet.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kurupati R.K., Haut L.H., Schmader K.E., Ertl H.C. Age-related changes in B cell metabolism. Aging. 2019;11:4367–4381. doi: 10.18632/aging.102058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Frasca D., Diaz A., Romero M., Thaller S., Blomberg B.B. Metabolic requirements of human pro-inflammatory B cells in aging and obesity. PLoS ONE. 2019;14:e0219545. doi: 10.1371/journal.pone.0219545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chougnet C.A., Thacker R.I., Shehata H.M., Hennies C.M., Lehn M.A., Lages C.S., Janssen E.M. Loss of Phagocytic and Antigen Cross-Presenting Capacity in Aging Dendritic Cells Is Associated with Mitochondrial Dysfunction. J. Immunol. 2015;195:2624–2632. doi: 10.4049/jimmunol.1501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ma T., Patel H., Babapoor-Farrokhran S., Franklin R., Semenza G.L., Sodhi A., Montaner S. KSHV induces aerobic glycolysis and angiogenesis through HIF-1-dependent upregulation of pyruvate kinase 2 in Kaposi’s sarcoma. Angiogenesis. 2015;18:477–488. doi: 10.1007/s10456-015-9475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Palmer C.S., Duette G.A., Wagner M.C.E., Henstridge D.C., Saleh S., Pereira C., Zhou J., Simar D., Lewin S.R., Ostrowski M., et al. Metabolically active CD4+ T cells expressing Glut1 and OX40 preferentially harbor HIV during in vitro infection. FEBS Lett. 2017;591:3319–3332. doi: 10.1002/1873-3468.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Palmer C.S., Anzinger J.J., Zhou J., Gouillou M., Landay A., Jaworowski A., McCune J.M., Crowe S.M. Glucose transporter 1-expressing proinflammatory monocytes are elevated in combination antiretroviral therapy-treated and untreated HIV+ subjects. J. Immunol. 2014;193:5595–5603. doi: 10.4049/jimmunol.1303092. [DOI] [PubMed] [Google Scholar]
- 217.Wakisaka N., Kondo S., Yoshizaki T., Murono S., Furukawa M., Pagano J.S. Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1 alpha. Mol. Cell. Biol. 2004;24:5223–5234. doi: 10.1128/MCB.24.12.5223-5234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Teng C.F., Hsieh W.C., Wu H.C., Lin Y.J., Tsai H.W., Huang W., Su I.J. Hepatitis B Virus Pre-S2 Mutant Induces Aerobic Glycolysis through Mammalian Target of Rapamycin Signal Cascade. PLoS ONE. 2015;10:e0122373. doi: 10.1371/journal.pone.0122373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Russell S.L., Lamprecht D.A., Mandizvo T., Jones T.T., Naidoo V., Addicott K.W., Moodley C., Ngcobo B., Crossman D.K., Wells G., et al. Compromised Metabolic Reprogramming Is an Early Indicator of CD8. Cell Rep. 2019;29:3564–3579. doi: 10.1016/j.celrep.2019.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lagoumtzi S.M., Chondrogianni N. Senolytics and senomorphics: Natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic. Biol. Med. 2021;171:169–190. doi: 10.1016/j.freeradbiomed.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 221.Tchkonia T., Zhu Y., van Deursen J., Campisi J., Kirkland J.L. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Investig. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ovadya Y., Krizhanovsky V. Strategies targeting cellular senescence. J. Clin. Investig. 2018;128:1247–1254. doi: 10.1172/JCI95149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hall B.M., Balan V., Gleiberman A.S., Strom E., Krasnov P., Virtuoso L.P., Rydkina E., Vujcic S., Balan K., Gitlin I., et al. Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging. 2016;8:1294–1315. doi: 10.18632/aging.100991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cai Y., Zhou H., Zhu Y., Sun Q., Ji Y., Xue A., Wang Y., Chen W., Yu X., Wang L., et al. Elimination of senescent cells by β-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 2020;30:574–589. doi: 10.1038/s41422-020-0314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Muñoz-Espín D., Rovira M., Galiana I., Giménez C., Lozano-Torres B., Paez-Ribes M., Llanos S., Chaib S., Muñoz-Martín M., Ucero A.C., et al. A versatile drug delivery system targeting senescent cells. EMBO Mol. Med. 2018;10:e9355. doi: 10.15252/emmm.201809355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yoshida S., Nakagami H., Hayashi H., Ikeda Y., Sun J., Tenma A., Tomioka H., Kawano T., Shimamura M., Morishita R., et al. The CD153 vaccine is a senotherapeutic option for preventing the accumulation of senescent T cells in mice. Nat. Commun. 2020;11:2482. doi: 10.1038/s41467-020-16347-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sallin M.A., Kauffman K.D., Riou C., Du Bruyn E., Foreman T.W., Sakai S., Hoft S.G., Myers T.G., Gardina P.J., Sher A., et al. Host resistance to pulmonary Mycobacterium tuberculosis infection requires CD153 expression. Nat. Microbiol. 2018;3:1198–1205. doi: 10.1038/s41564-018-0231-6. [DOI] [PubMed] [Google Scholar]
- 228.Liu Y., Sanoff H.K., Cho H., Burd C.E., Torrice C., Ibrahim J.G., Thomas N.E., Sharpless N.E. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8:439–448. doi: 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Liu Y., Johnson S.M., Fedoriw Y., Rogers A.B., Yuan H., Krishnamurthy J., Sharpless N.E. Expression of p16(INK4a) prevents cancer and promotes aging in lymphocytes. Blood. 2011;117:3257–3267. doi: 10.1182/blood-2010-09-304402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lucas C.L., Kuehn H.S., Zhao F., Niemela J.E., Deenick E.K., Palendira U., Avery D.T., Moens L., Cannons J.L., Biancalana M., et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat. Immunol. 2014;15:88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Paez-Ribes M., González-Gualda E., Doherty G.J., Muñoz-Espín D. Targeting senescent cells in translational medicine. EMBO Mol. Med. 2019;11:e10234. doi: 10.15252/emmm.201810234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rao V.K., Webster S., Dalm V.A.S.H., Šedivá A., van Hagen P.M., Holland S., Rosenzweig S.D., Christ A.D., Sloth B., Cabanski M., et al. Effective “activated PI3Kδ syndrome”-targeted therapy with the PI3Kδ inhibitor leniolisib. Blood. 2017;130:2307–2316. doi: 10.1182/blood-2017-08-801191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Dörr J.R., Yu Y., Milanovic M., Beuster G., Zasada C., Däbritz J.H., Lisec J., Lenze D., Gerhardt A., Schleicher K., et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501:421–425. doi: 10.1038/nature12437. [DOI] [PubMed] [Google Scholar]
- 234.Heilbronn L.K., Ravussin E. Calorie restriction and aging: Review of the literature and implications for studies in humans. Am. J. Clin. Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 235.Cantó C., Auwerx J. Calorie restriction: Is AMPK a key sensor and effector? Physiology. 2011;26:214–224. doi: 10.1152/physiol.00010.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Barbosa M.C., Grosso R.A., Fader C.M. Hallmarks of Aging: An Autophagic Perspective. Front. Endocrinol. 2018;9:790. doi: 10.3389/fendo.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Messaoudi I., Warner J., Fischer M., Park B., Hill B., Mattison J., Lane M.A., Roth G.S., Ingram D.K., Picker L.J., et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc. Natl. Acad. Sci. USA. 2006;103:19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.White M.J., Beaver C.M., Goodier M.R., Bottomley C., Nielsen C.M., Wolf A.F., Boldrin L., Whitmore C., Morgan J., Pearce D.J., et al. Calorie Restriction Attenuates Terminal Differentiation of Immune Cells. Front. Immunol. 2016;7:667. doi: 10.3389/fimmu.2016.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Smallwood H.S., Duan S., Morfouace M., Rezinciuc S., Shulkin B.L., Shelat A., Zink E.E., Milasta S., Bajracharya R., Oluwaseum A.J., et al. Targeting Metabolic Reprogramming by Influenza Infection for Therapeutic Intervention. Cell Rep. 2017;19:1640–1653. doi: 10.1016/j.celrep.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mannick J.B., Del Giudice G., Lattanzi M., Valiante N.M., Praestgaard J., Huang B., Lonetto M.A., Maecker H.T., Kovarik J., Carson S., et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- 241.Mannick J.B., Morris M., Hockey H.P., Roma G., Beibel M., Kulmatycki K., Watkins M., Shavlakadze T., Zhou W., Quinn D., et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aaq1564. [DOI] [PubMed] [Google Scholar]
- 242.Mannick J.B., Teo G., Bernardo P., Quinn D., Russell K., Klickstein L., Marshall W., Shergill S. Targeting the biology of ageing with mTOR inhibitors to improve immune function in older adults: Phase 2b and phase 3 randomised trials. Lancet Healthy Longev. 2021;2:e250–e262. doi: 10.1016/S2666-7568(21)00062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Patocka J., Kuca K., Oleksak P., Nepovimova E., Valis M., Novotny M., Klimova B. Rapamycin: Drug Repurposing in SARS-CoV-2 Infection. Pharmaceuticals. 2021;14:217. doi: 10.3390/ph14030217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bischof E., Siow R.C., Zhavoronkov A., Kaeberlein M. The potential of rapalogs to enhance resilience against SARS-CoV-2 infection and reduce the severity of COVID-19. Lancet Healthy Longev. 2021;2:e105–e111. doi: 10.1016/S2666-7568(20)30068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Omarjee L., Janin A., Perrot F., Laviolle B., Meilhac O., Mahe G. Targeting T-cell senescence and cytokine storm with rapamycin to prevent severe progression in COVID-19. Clin. Immunol. 2020;216:108464. doi: 10.1016/j.clim.2020.108464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bharath L.P., Agrawal M., McCambridge G., Nicholas D.A., Hasturk H., Liu J., Jiang K., Liu R., Guo Z., Deeney J., et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020;32:44–55. doi: 10.1016/j.cmet.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Martin-Montalvo A., Mercken E.M., Mitchell S.J., Palacios H.H., Mote P.L., Scheibye-Knudsen M., Gomes A.P., Ward T.M., Minor R.K., Blouin M.J., et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Strong R., Miller R.A., Antebi A., Astle C.M., Bogue M., Denzel M.S., Fernandez E., Flurkey K., Hamilton K.L., Lamming D.W., et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15:872–884. doi: 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sorrenti V., Benedetti F., Buriani A., Fortinguerra S., Caudullo G., Davinelli S., Zella D., Scapagnini G. Immunomodulatory and Antiaging Mechanisms of Resveratrol, Rapamycin, and Metformin: Focus on mTOR and AMPK Signaling Networks. Pharmaceuticals. 2022;15:912. doi: 10.3390/ph15080912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Borriello A., Bencivenga D., Caldarelli I., Tramontano A., Borgia A., Zappia V., Della Ragione F. Resveratrol: From basic studies to bedside. Adv. Nutr. Cancer. 2014;159:167–184. doi: 10.1007/978-3-642-38007-5_10. [DOI] [PubMed] [Google Scholar]
- 251.Zhang H., Alsaleh G., Feltham J., Sun Y., Napolitano G., Riffelmacher T., Charles P., Frau L., Hublitz P., Yu Z., et al. Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol. Cell. 2019;76:110–125. doi: 10.1016/j.molcel.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Allan R.S., Zueva E., Cammas F., Schreiber H.A., Masson V., Belz G.T., Roche D., Maison C., Quivy J.P., Almouzni G., et al. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature. 2012;487:249–253. doi: 10.1038/nature11173. [DOI] [PubMed] [Google Scholar]
- 253.Pace L., Goudot C., Zueva E., Gueguen P., Burgdorf N., Waterfall J.J., Quivy J.P., Almouzni G., Amigorena S. The epigenetic control of stemness in CD8. Science. 2018;359:177–186. doi: 10.1126/science.aah6499. [DOI] [PubMed] [Google Scholar]
- 254.Schönheit J., Leutz A., Rosenbauer F. Chromatin dynamics during differentiation of myeloid cells. J. Mol. Biol. 2015;427:670–687. doi: 10.1016/j.jmb.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 255.Sciumè G., Shih H.Y., Mikami Y., O’Shea J.J. Epigenomic Views of Innate Lymphoid Cells. Front. Immunol. 2017;8:1579. doi: 10.3389/fimmu.2017.01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Keenan C.R., Allan R.S. Epigenomic drivers of immune dysfunction in aging. Aging Cell. 2019;18:e12878. doi: 10.1111/acel.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ganesan A., Arimondo P.B., Rots M.G., Jeronimo C., Berdasco M. The timeline of epigenetic drug discovery: From reality to dreams. Clin. Epigenet. 2019;11:174. doi: 10.1186/s13148-019-0776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yates K.B., Tonnerre P., Martin G.E., Gerdemann U., Al Abosy R., Comstock D.E., Weiss S.A., Wolski D., Tully D.C., Chung R.T., et al. Epigenetic scars of CD8. Nat. Immunol. 2021;22:1020–1029. doi: 10.1038/s41590-021-00979-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Tonnerre P., Wolski D., Subudhi S., Aljabban J., Hoogeveen R.C., Damasio M., Drescher H.K., Bartsch L.M., Tully D.C., Sen D.R., et al. Differentiation of exhausted CD8. Nat. Immunol. 2021;22:1030–1041. doi: 10.1038/s41590-021-00982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kanfi Y., Naiman S., Amir G., Peshti V., Zinman G., Nahum L., Bar-Joseph Z., Cohen H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 261.Serrano M., Barzilai N. Targeting senescence. Nat. Med. 2018;24:1092–1094. doi: 10.1038/s41591-018-0141-4. [DOI] [PubMed] [Google Scholar]
- 262.Bellon M., Nicot C. Telomere Dynamics in Immune Senescence and Exhaustion Triggered by Chronic Viral Infection. Viruses. 2017;9:289. doi: 10.3390/v9100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Dyavar Shetty R., Velu V., Titanji K., Bosinger S.E., Freeman G.J., Silvestri G., Amara R.R. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J. Clin. Investig. 2012;122:1712–1716. doi: 10.1172/JCI60612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Palmer C.S., Henstridge D.C., Yu D., Singh A., Balderson B., Duette G., Cherry C.L., Anzinger J.J., Ostrowski M., Crowe S.M. Emerging Role and Characterization of Immunometabolism: Relevance to HIV Pathogenesis, Serious Non-AIDS Events, and a Cure. J. Immunol. 2016;196:4437–4444. doi: 10.4049/jimmunol.1600120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gay C.L., Bosch R.J., Ritz J., Hataye J.M., Aga E., Tressler R.L., Mason S.W., Hwang C.K., Grasela D.M., Ray N., et al. Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J. Infect. Dis. 2017;215:1725–1733. doi: 10.1093/infdis/jix191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tzeng H.T., Tsai H.F., Liao H.J., Lin Y.J., Chen L., Chen P.J., Hsu P.N. PD-1 blockage reverses immune dysfunction and hepatitis B viral persistence in a mouse animal model. PLoS ONE. 2012;7:e39179. doi: 10.1371/journal.pone.0039179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gardiner D., Lalezari J., Lawitz E., DiMicco M., Ghalib R., Reddy K.R., Chang K.M., Sulkowski M., Marro S.O., Anderson J., et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS ONE. 2013;8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kaufmann D.E., Walker B.D. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J. Immunol. 2009;182:5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cecchinato V., Tryniszewska E., Ma Z.M., Vaccari M., Boasso A., Tsai W.P., Petrovas C., Fuchs D., Heraud J.M., Venzon D., et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J. Immunol. 2008;180:5439–5447. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Nakamoto N., Kaplan D.E., Coleclough J., Li Y., Valiga M.E., Kaminski M., Shaked A., Olthoff K., Gostick E., Price D.A., et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–1937. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ma S.D., Xu X., Jones R., Delecluse H.J., Zumwalde N.A., Sharma A., Gumperz J.E., Kenney S.C. PD-1/CTLA-4 Blockade Inhibits Epstein-Barr Virus-Induced Lymphoma Growth in a Cord Blood Humanized-Mouse Model. PLoS Pathog. 2016;12:e1005642. doi: 10.1371/journal.ppat.1005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tinoco R., Carrette F., Barraza M.L., Otero D.C., Magaña J., Bosenberg M.W., Swain S.L., Bradley L.M. PSGL-1 Is an Immune Checkpoint Regulator that Promotes T Cell Exhaustion. Immunity. 2016;44:1190–1203. doi: 10.1016/j.immuni.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Cook K.D., Whitmire J.K. LAG-3 Confers a Competitive Disadvantage upon Antiviral CD8+ T Cell Responses. J. Immunol. 2016;197:119–127. doi: 10.4049/jimmunol.1401594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Blackburn S.D., Shin H., Haining W.N., Zou T., Workman C.J., Polley A., Betts M.R., Freeman G.J., Vignali D.A., Wherry E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ye B., Li X., Dong Y., Wang Y., Tian L., Lin S., Liu X., Kong H., Chen Y. Increasing LAG-3 expression suppresses T-cell function in chronic hepatitis B: A balance between immunity strength and liver injury extent. Medicine. 2017;96:e5275. doi: 10.1097/MD.0000000000005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sada-Ovalle I., Chávez-Galán L., Torre-Bouscoulet L., Nava-Gamiño L., Barrera L., Jayaraman P., Torres-Rojas M., Salazar-Lezama M.A., Behar S.M. The Tim3-galectin 9 pathway induces antibacterial activity in human macrophages infected with Mycobacterium tuberculosis. J. Immunol. 2012;189:5896–5902. doi: 10.4049/jimmunol.1200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Blackburn S.D., Wherry E.J. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 278.Brockman M.A., Kwon D.S., Tighe D.P., Pavlik D.F., Rosato P.C., Sela J., Porichis F., Le Gall S., Waring M.T., Moss K., et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114:346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Porichis F., Hart M.G., Zupkosky J., Barblu L., Kwon D.S., McMullen A., Brennan T., Ahmed R., Freeman G.J., Kavanagh D.G., et al. Differential impact of PD-1 and/or interleukin-10 blockade on HIV-1-specific CD4 T cell and antigen-presenting cell functions. J. Virol. 2014;88:2508–2518. doi: 10.1128/JVI.02034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Brooks D.G., Ha S.J., Elsaesser H., Sharpe A.H., Freeman G.J., Oldstone M.B. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc. Natl. Acad. Sci. USA. 2008;105:20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Grint D., Tedaldi E., Peters L., Mocroft A., Edlin B., Gallien S., Klinker H., Boesecke C., Kokordelis P., Rockstroh J.K. Hepatitis C virus (HCV) RNA profiles among chronic HIV/HCV-coinfected individuals in ESPRIT; spontaneous HCV RNA clearance observed in nine individuals. HIV Med. 2017;18:430–434. doi: 10.1111/hiv.12466. [DOI] [PubMed] [Google Scholar]
- 282.Zhen A., Rezek V., Youn C., Lam B., Chang N., Rick J., Carrillo M., Martin H., Kasparian S., Syed P., et al. Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J. Clin. Investig. 2017;127:260–268. doi: 10.1172/JCI89488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bengsch B., Johnson A.L., Kurachi M., Odorizzi P.M., Pauken K.E., Attanasio J., Stelekati E., McLane L.M., Paley M.A., Delgoffe G.M., et al. Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8+ T Cell Exhaustion. Immunity. 2016;45:358–373. doi: 10.1016/j.immuni.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Schurich A., Pallett L.J., Jajbhay D., Wijngaarden J., Otano I., Gill U.S., Hansi N., Kennedy P.T., Nastouli E., Gilson R., et al. Distinct Metabolic Requirements of Exhausted and Functional Virus-Specific CD8 T Cells in the Same Host. Cell Rep. 2016;16:1243–1252. doi: 10.1016/j.celrep.2016.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Nehme J., Borghesan M., Mackedenski S., Bird T.G., Demaria M. Cellular senescence as a potential mediator of COVID-19 severity in the elderly. Aging Cell. 2020;19:e13237. doi: 10.1111/acel.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Xu M., Pirtskhalava T., Farr J.N., Weigand B.M., Palmer A.K., Weivoda M.M., Inman C.L., Ogrodnik M.B., Hachfeld C.M., Fraser D.G., et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kakkola L., Denisova O.V., Tynell J., Viiliäinen J., Ysenbaert T., Matos R.C., Nagaraj A., Ohman T., Kuivanen S., Paavilainen H., et al. Anticancer compound ABT-263 accelerates apoptosis in virus-infected cells and imbalances cytokine production and lowers survival rates of infected mice. Cell Death Dis. 2013;4:e742. doi: 10.1038/cddis.2013.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Singh A.K., McGuirk J.P. CAR T cells: Continuation in a revolution of immunotherapy. Lancet Oncol. 2020;21:e168–e178. doi: 10.1016/S1470-2045(19)30823-X. [DOI] [PubMed] [Google Scholar]
- 289.Ellebrecht C.T., Bhoj V.G., Nace A., Choi E.J., Mao X., Cho M.J., Di Zenzo G., Lanzavecchia A., Seykora J.T., Cotsarelis G., et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kansal R., Richardson N., Neeli I., Khawaja S., Chamberlain D., Ghani M., Ghani Q.U., Balazs L., Beranova-Giorgianni S., Giorgianni F., et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci. Transl. Med. 2019;11:eaav1648. doi: 10.1126/scitranslmed.aav1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Amor C., Feucht J., Leibold J., Ho Y.J., Zhu C., Alonso-Curbelo D., Mansilla-Soto J., Boyer J.A., Li X., Giavridis T., et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583:127–132. doi: 10.1038/s41586-020-2403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Schumann K., Lin S., Boyer E., Simeonov D.R., Subramaniam M., Gate R.E., Haliburton G.E., Ye C.J., Bluestone J.A., Doudna J.A., et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc. Natl. Acad. Sci. USA. 2015;112:10437–10442. doi: 10.1073/pnas.1512503112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Pereira B.I., Devine O.P., Vukmanovic-Stejic M., Chambers E.S., Subramanian P., Patel N., Virasami A., Sebire N.J., Kinsler V., Valdovinos A., et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8. Nat. Commun. 2019;10:2387. doi: 10.1038/s41467-019-10335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sagiv A., Burton D.G., Moshayev Z., Vadai E., Wensveen F., Ben-Dor S., Golani O., Polic B., Krizhanovsky V. NKG2D ligands mediate immunosurveillance of senescent cells. Aging. 2016;8:328–344. doi: 10.18632/aging.100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Childs B.G., Gluscevic M., Baker D.J., Laberge R.M., Marquess D., Dananberg J., van Deursen J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017;16:718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zhang Q., Lenardo M.J., Baltimore D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Horwitz B.H., Scott M.L., Cherry S.R., Bronson R.T., Baltimore D. Failure of lymphopoiesis after adoptive transfer of NF-kappaB-deficient fetal liver cells. Immunity. 1997;6:765–772. doi: 10.1016/S1074-7613(00)80451-3. [DOI] [PubMed] [Google Scholar]
- 298.Park J.M., Greten F.R., Wong A., Westrick R.J., Arthur J.S., Otsu K., Hoffmann A., Montminy M., Karin M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis--CREB and NF-kappaB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 299.Nagashima K., Sasseville V.G., Wen D., Bielecki A., Yang H., Simpson C., Grant E., Hepperle M., Harriman G., Jaffee B., et al. Rapid TNFR1-dependent lymphocyte depletion in vivo with a selective chemical inhibitor of IKKbeta. Blood. 2006;107:4266–4273. doi: 10.1182/blood-2005-09-3852. [DOI] [PubMed] [Google Scholar]
- 300.Schmidt-Supprian M., Tian J., Ji H., Terhorst C., Bhan A.K., Grant E.P., Pasparakis M., Casola S., Coyle A.J., Rajewsky K. I kappa B kinase 2 deficiency in T cells leads to defects in priming, B cell help, germinal center reactions, and homeostatic expansion. J. Immunol. 2004;173:1612–1619. doi: 10.4049/jimmunol.173.3.1612. [DOI] [PubMed] [Google Scholar]
- 301.Lavon I., Goldberg I., Amit S., Landsman L., Jung S., Tsuberi B.Z., Barshack I., Kopolovic J., Galun E., Bujard H., et al. High susceptibility to bacterial infection, but no liver dysfunction, in mice compromised for hepatocyte NF-kappaB activation. Nat. Med. 2000;6:573–577. doi: 10.1038/75057. [DOI] [PubMed] [Google Scholar]
- 302.Justice J.N., Nambiar A.M., Tchkonia T., LeBrasseur N.K., Pascual R., Hashmi S.K., Prata L., Masternak M.M., Kritchevsky S.B., Musi N., et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Hickson L.J., Langhi Prata L.G.P., Bobart S.A., Evans T.K., Giorgadze N., Hashmi S.K., Herrmann S.M., Jensen M.D., Jia Q., Jordan K.L., et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Friedman A.A., Letai A., Fisher D.E., Flaherty K.T. Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer. 2015;15:747–756. doi: 10.1038/nrc4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Soukas A.A., Hao H., Wu L. Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol. Metab. 2019;30:745–755. doi: 10.1016/j.tem.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.