Abstract
Antimicrobial resistance (AMR) is a public health issue attributed to the misuse of antibiotics in human and veterinary medicine. Since AMR surveillance requires a One Health approach, we sampled nine interconnected compartments at a hydrological open-air lab (HOAL) in Austria to obtain six bacterial species included in the WHO priority list of antibiotic-resistant bacteria (ARB). Whole genome sequencing-based typing included core genome multilocus sequence typing (cgMLST). Genetic and phenotypic characterization of AMR was performed for all isolates. Eighty-nine clinically-relevant bacteria were obtained from eight compartments including 49 E. coli, 27 E. faecalis, 7 K. pneumoniae and 6 E. faecium. Clusters of isolates from the same species obtained in different sample collection dates were detected. Of the isolates, 29.2% were resistant to at least one antimicrobial. E. coli and E. faecalis isolates from different compartments had acquired antimicrobial resistance genes (ARGs) associated with veterinary drugs such as aminoglycosides and tetracyclines, some of which were carried in conjugative and mobilizable plasmids. Three multidrug resistant (MDR) E. coli isolates were found in samples from field drainage and wastewater. Early detection of ARGs and ARB in natural and farm-related environments can identify hotspots of AMR and help prevent its emergence and dissemination along the food/feed chain.
Keywords: antimicrobial resistance, One Health, whole genome sequencing, antimicrobial resistance genes
1. Introduction
Antimicrobial resistance (AMR) in bacteria is a leading cause of death worldwide, with Escherichia coli, Staphylococcus aureus and Klebsiella pneumoniae being the top three resistant pathogens [1]. In the European region alone, the incidence of bacterial AMR in 2019 was equal to 67.7 per 100,000 people and according to new estimations, the global number of deaths associated with AMR may reach up to 10 million by 2050 [2,3]. For Austria in particular, the AURES resistance report 2020 showed that the resistance rates in human E. coli and K. pneumoniae isolates remained stable (high) in comparison to 2019, or decreased for fluoroquinolones, third generation cephalosporins and aminoglycosides. The rate of K. pneumoniae resistance to carbapenemases, for example, remained stable in 2020 compared to the previous year in contrast to the rest of Europe. For Enterococci, the resistance rate to aminopenicillin and aminoglycosides remained stable or decreased, respectively, while MRSA continued its decreasing trend over the last 5 years [4].
In 2017 the World Health Organization (WHO) published a list of antibiotic-resistant “priority pathogens“ including three categories ranked from 1 to 3 [5]. These categories referred to the greater or lesser need to develop new antibiotics to treat infections caused by the bacteria on this list, whose treatment options are increasingly limited by the emerging resistance [6]. Priority 1, named critical priority or known by the acronym ESKAPE, grouped Gram-negative carbapenem-resistant and/or extended-spectrum beta-lactamases (ESBL)-producing Enterobacteriaceae. Priority 2, also named high priority, included diverse Gram-negative fluoroquinolone-resistant bacteria, such as Salmonellae and Gram-positive vancomycin-resistant bacteria, such as Enterococcus faecium or methicillin-resistant S. aureus. Lastly, priority 3 bacteria also included both Gram-negative and positive bacteria of medium priority, such as penicillin-resistant Streptococcus pneumoniae. In order to combat AMR, surveillance studies on clinically-relevant bacteria included in the abovementioned WHO list are increasingly incorporating One Health approaches [7]. One Health is a multidisciplinary concept created in 2004 that aims to sustainably balance and optimize the health of people, animals and ecosystems [8]. Several drivers of geographical, ecological, anthropogenic and agricultural nature modulate the intra and interrelationships of the One Health components [9].Thus, this holistic approach is essential for reducing the emergence and spread of resistance and maintaining the effectiveness of antibiotics. Antimicrobial resistance genes (ARGs) are naturally present in the environment, but antibiotic usage selects for antibiotic resistant bacteria (ARB) and, hence, for ARGs [6]. However, less attention is paid to the environment, with most studies focusing exclusively on human and the animal (mostly livestock) components. Evidence shows that some ARGs such as the blaCTX encoding for extended spectrum betalactamases have their origin in bacterial species that are common in the environment and these can easily be transferred to human and animal bacteria [10]. Likewise, wildlife is to some extent ignored in the surveillance of clinically-relevant bacteria [11].
To our knowledge, no studies have been performed in Austria to monitor the occurrence of ARB in the three One Health components simultaneously using whole genome sequencing (WGS). To address this issue, we aimed in this study to assess the phenotypic and genetic resistance of six bacterial species included in the WHO priority list of antibiotic-resistant bacteria across environmental compartments representing the food/feed chain.
2. Results
2.1. Isolates by Species and Compartment
Out of 61 samples collected from nine compartments over a one-year-growing period, a total of 89 clinically-relevant isolates were obtained from 40 of those samples belonging to eight compartments and were selected for phenotypic and genotypic AMR analysis. In the other 19 samples, only non-clinically relevant isolates were obtained and were therefore excluded from the study. Forty-nine (55.1%) of the clinically-relevant isolates were E. coli, 27 (30.33%) were E. faecalis, 7 (7.86%) K. pneumoniae and 6 (6.74%) E. faecium. No MRSA nor Salmonella spp. isolates were found. By compartment, wildlife (n = 29) and wastewater (n = 28) accounted for the majority of the isolates (Table 1). One E. coli isolate was obtained from feed and no clinically-relevant species were detected in crops. Wastewater had the highest species diversity, comprising four of the abovementioned bacterial species, followed by groundwater, with three species. E. coli was found in all compartments except crops. E. faecalis was found in all compartments except crops, feed and field drainage water. E. faecium was isolated from ground- and wastewater and K. pneumoniae only from wastewater.
Table 1.
Number of isolates gathered by species and compartment in Petzenkirchen, Austria, 2020–2021.
| Species | Feed | Field Drainage | Groundwater | Pig Manure | River | Soil | Wastewater | Wildlife | Crops | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | 1 | 5 | 2 | 1 | 5 | 4 | 12 | 19 | 0 | 49 |
| E. faecalis | 0 | 0 | 1 | 5 | 2 | 3 | 6 | 10 | 0 | 27 |
| K. pneumoniae | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 7 |
| E. faecium | 0 | 0 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 6 |
| Total | 1 | 5 | 7 | 5 | 7 | 7 | 28 | 29 | 0 | 89 |
Most of the isolates had been retrieved from samples collected in July (n = 30), September or October (14 and 18 respective isolates), with E. coli being more than half (n = 16) of those (Figure S1).
2.2. Phylogenetic Relationships
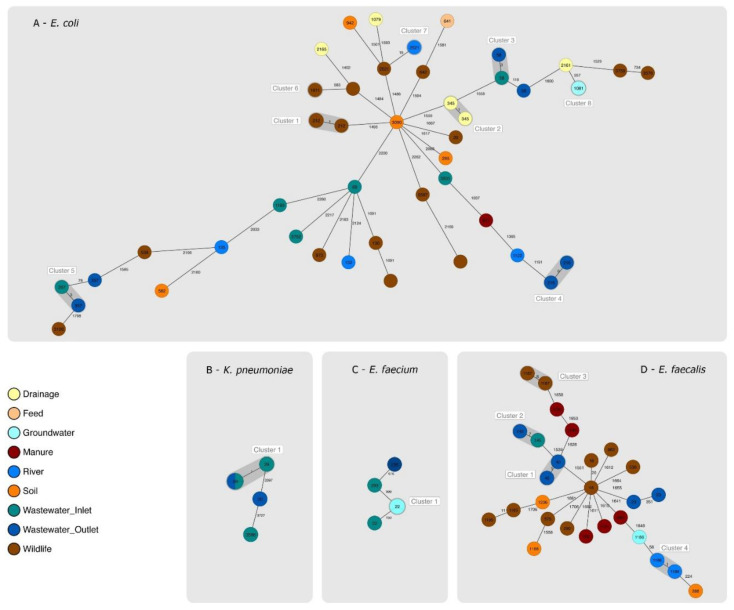
The 49 E. coli isolates resolved into 36 STs and 44 cgMLST (CT) types. Three isolates presented new STs, and all isolates belonged to a novel CT (Table S1). We detected four cgMLST clusters (clusters 1, 3, 4 and 5) and four pseudo-clusters (clusters 2, 6, 7 and 8) (Figure 1A).
Figure 1.
Genetic relatedness between isolates of the same species based on their core genomes. STs are indicated inside each circle, which represents one or more isolates. Empty circles correspond to isolates with new STs. Clusters are highlighted in grey. Colors identify the compartment from which each isolate was gained. The number of allelic differences between two or more isolates is shown on the connecting lines between them. (A) Minimum spanning tree (MST) displaying the collected E. coli isolates; (B) MST displaying the collected K. pneumoniae isolates; (C) MST displaying the collected E. faecium isolates; (D) MST displaying the collected E. faecalis isolates.
Cluster 1, grouped four E. coli ST212 wildlife fecal isolates collected in September from two different fecal samples. Cluster 3 grouped two ST58 isolates from two different wastewater subcompartments (inlet and outlet), which had been collected in July. Cluster 4 grouped two wastewater ST216 isolates from two different wastewater samples (subcompartment outlet) collected in July and October. Cluster 5 grouped two ST357 wastewater isolates collected in January 2021 from the inlet and the outlet. Among the singletons, we detected major STs such as ST69 and ST1193, both from wastewater.
The seven K. pneumoniae isolates were resolved into three STs (20, 29 and 3586). Five ST29 isolates obtained from five different samples and three sampling dates (April, July and October 2020) were grouped in a cluster. Three had been obtained from the inlet and two from the outlet (Figure 1B and Table S2).
The six E. faecium isolates were resolved into three STs (22, 236 and 293). One cgMLST pseudo-cluster with three ST22 groundwater isolates was detected. Another ST22 isolate, not clustering with any other isolate, was obtained from an inlet wastewater sample (Figure 1C and Table S3).
For E. faecalis, the 27 isolates were resolved into 11 known STs and eight new STs. One cgMLST two-isolate cluster was detected (cluster 2), which had two ST145 wastewater isolates. One isolate had been obtained from a wastewater sample from the outlet and another one from the inlet. Clusters 1, 3 and 4 were pseudoclusters (Figure 1D and Table S4).
2.3. Phenotypic AMR
The screening for antimicrobial susceptibility by disk diffusion detected 29 (32.6%) resistant isolates to at least one of the antimicrobials tested. One E. coli isolate (id: 510423-20) was identified as an ESBL producer (amoxicillin-clavulanic acid diameter >5 mm with respect to that of the cefotaxime).
By E-test, the overall prevalence of AMR was 29.2% (26/89), including the resistance to those antimicrobials tested after confirming ARG presence by WGS. In total, 13.4% (n = 12) of the isolates were resistant to one antimicrobial, 10.1% (n = 9) to two antimicrobials, 2.24% (n = 2) to three antimicrobials, 2.24% (n = 2) to six antimicrobials and one isolate (1.12%) was resistant to 10 antimicrobials. There were 63 isolates (70.8%) susceptible to all antimicrobials tested. E-test patterns of phenotypic resistance by species are given in Table 2.
Table 2.
Antimicrobial susceptibility patterns of the 26 isolates confirmed as resistant by E-test.
| Species | AMR Pattern | Isolates, n (%) | |
|---|---|---|---|
| E. coli | AMP/AZT*/CEP/CIP/CTX/ERY*/GEN/MOX*/ STR*/TS* |
1 | 9/49 (18.3%) |
| AMP/CIP/MOX*/STR*/TET*/TS* | 2 | ||
| AMC/AMP | 1 | ||
| STR*/TET* | 2 | ||
| AMP | 1 | ||
| TET* | 2 | ||
| K. pneumoniae | AMP/FOS* | 5 | 7/7 (100%) |
| AMP | 2 | ||
| E. faecium | AMP | 1 | 1/6 (16.7%) |
| E. faecalis | KAN*/STR*/TET* | 2 | 9/24 (33.3%) |
| ERY*/TET* | 1 | ||
| CLY*/TET* | 1 | ||
| TET* | 5 | ||
* Antimicrobials used in the second round of E-test for isolates with one or more ARGs possibly linked to phenotypic resistance. AMP = ampicillin, AZT = azitromycin, CEP = cefepime, CIP = ciprofloxacin, CLY = clindamycin, CTX = cefotaxime, ERY = erythromycin, FOS = fosfomycin, GEN = gentamicin, MOX = moxifloxacin, STR= streptomycin, TET = tetracyclin.
Overall, only macrolide- and tetracycline-resistant E. coli and E. faecalis isolates were detected in soil, feed and manure samples from farmers 1 and 2.
In E. coli, all the cluster-related isolates were confirmed as susceptible to all tested antimicrobials. Among singletons and pseudo-clusters, resistance to tetracycline (n = 6, MIC: 32–128 µg/mL), ampicillin (n = 5, MIC: ≥256 µg/mL), streptomycin (n = 5, MIC: 32–192 µg/mL), ciprofloxacin (n = 3, MIC: 8–32µg/mL), moxifloxacin (n = 3, MIC: 8 µg/mL), trimethoprim-sulfamethoxazole (n = 3, MIC: ≥32 µg/mL), amoxicillin-clavulanate (n = 1, MIC: 24 µg/mL), azithromycin (n = 1, MIC: ≥256 µg/mL), cefotaxime (n = 1, MIC: ≥32 µg/mL), erythromycin (n = 1, MIC: ≥256 µg/mL) and gentamicin (n = 1, MIC: 32 µg/mL) were found. One isolate showed intermediate resistance to cefepime (n = 1, MIC: 4 µg/mL).
All K. pneumoniae (n = 7) presented low-level ampicillin resistance (MIC: 12–24 µg/mL) and the five ST29-cluster isolates were highly resistant (HLAR) to fosfomycin (MIC: ≥1024 µg/mL). All isolates were susceptible against amikacin, cefoxitin, colistin, ertapenem and kanamycin.
Nine E. faecalis isolates out of 27 were at least resistant to tetracycline (MIC: 8–96 µg/mL). Two of those were also resistant to streptomycin (MIC: 768 µg/mL) and kanamycin (MIC: ≥256 µg/mL), one to erythromycin (MIC: ≥256 µg/mL) and another one to clindamycin (MIC: 24µg/mL).
Among E. faecium we only detected one resistant isolate, which presented low-level resistance to ampicillin (MIC: 64 µg/mL).
2.4. ARGs, VGs and Plasmids
Species-specific differences were observed in ARG content between the isolates. Thus, E. coli isolates carried a mean of 46.3 ARGs, followed by K. pneumoniae, with 20.6 ARGs, E. faecium with 3.3 and E. faecalis, with 2.4 each. By compartment, isolates obtained from drainage samples carried, on average, 48.6 genes, followed by feed (47 genes), river (33 genes), wildlife (32.1 genes), soil (28 genes), wastewater (25.8 genes), groundwater (14.1 genes) and manure (11.8 genes).
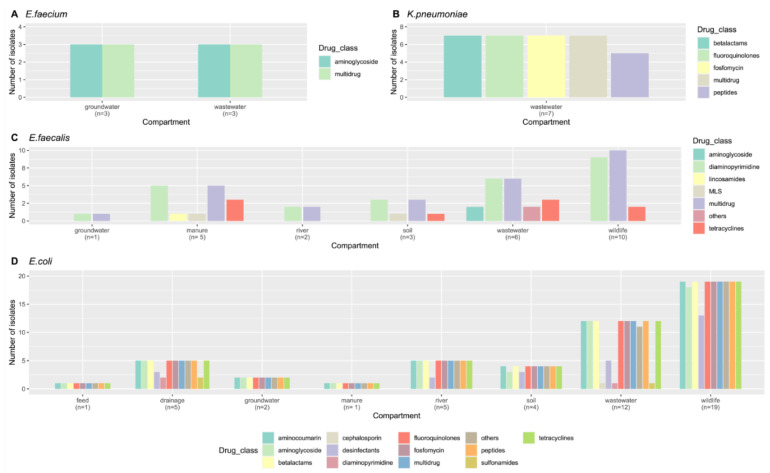
After categorizing the ARGs by drug class, the largest group in all four species was the multidrug group, accounting for 70% of the ARGs in E. faecium, 65.28% in K. pneumoniae, 54.7% in E.coli and 34.38% in E. faecalis. The second most frequently found drug class varied among species. Thus, aminoglycosides represented 30% of the ARGs found in E. faecium, diaminopyrimidines represented 32.9% of the ARGs in E. faecalis, followed by tetracyclines (18.8%), while betalactams grouped 18.1% of the ARGs in K. pneumoniae. All other drug classes were represented by less than 10% of ARGs in all four species.
Overall, manure and wastewater isolates were the most diverse, with ARGs in five or more different drug classes (Figure 2). The proportion of isolates presenting ARGs in each of the above-described categories also varied among species but was similar between compartments and collection dates.
Figure 2.
Number of isolates obtained in each species ((A)—E. faecium, (B)—K. pneumoniae, (C)—E. faecalis, (D)—E. coli) and compartment, harboring ARGs allocated to different drug classes.
All ARGs, VGs and plasmids detected in each isolate by species and compartment are shown in Tables S1–S4. The presence and absence of a given ARG in each isolate can also be visualized in Figure S2a–d.
In E. coli isolates, ARGs associated with intrinsic resistance such as the AcrA efflux system, chromosomal ampC or ampC-like genes or target replacement genes such as bacAor eptA, among others, were detected in all isolates. Acquired aminoglycoside ARGs were detected in six (12.2%) isolates, five of which were confirmed as streptomycin-resistant. Two of those isolates were ST345 obtained from drainage water (ids: 510655-20, 510656-20) and carried aadA2 and aph(6)-Id (also known as strB) genes, while one isolate of ST1193 (id: 510423-20) belonged to a wastewater-inlet sample and carried aac(3)-IId, aadA5, aph(3”)-Ib and aph(6)-Id. This isolate had been also confirmed as resistant to gentamicin. Two other isolates from soil (meadow; id: 510520-20) and manure (id: 510473-20), which were confirmed as moderately resistant to streptomycin, carried aph(3”)-Ib and aph(6)-Id and aph(3”)-Ib alone. Lastly, one streptomycin-susceptible isolate from wastewater (id: 510667-20) carried aph(3”)-Ib and aph(6)-Id. The six tetracycline-resistant isolates carried tet genes, including tetA or tetB plus tetR, and five of them (ids: 510655-20, 510656-20, 510520-20, 510473-20, 510667-20) were carrying aminoglycoside genes. The sixth tetracycline-resistant isolate was obtained from a feed sample (id: 510063-21). In addition, the abovementioned drainage isolates (ids: 510655-20, 510656-20) and wastewater isolate (id: 510423-20) carried either dfrA12 or dfrA17 genes and the three harbored the sulfonamide genes sul1 and sul2, being resistant to trimethoprim-sulfamethoxazol. In addition, these three isolates had point mutations in gyrA (D87N) and parC (S80I) associated with resistance to moxifloxacin and ciprofloxacin and carried the betalactamase gene blaTEM-1 responsible for ampicillin resistance. BlaTEM-1 was also found in a wastewater-inlet isolate (id: 510245-20), which was also phenotypically resistant to ampicillin. The wastewater isolate 510423-20 harbored a blaCTX-M-55 as well, which had a transposase IS26 followed by an ISECp1 287bp upstream from the start of the gene. Downstream from the betalactamase gene, a WubC-encoding gene was detected. This was the cefepime and cefotaxime resistant isolate, which also carried the macrolide resistant gene mphA linked to its high level resistance to azithromycin. Lastly, one fosfomycin susceptible soil isolate from a field fertilized with pig manure (id: 510299-20) carried the fosfomycin gene fosA2.
Overall, three E. coli isolates were designated as MDR (id: 510423-20, 510655-20, 510656-20), since they carried acquired ARGs from five to seven drug classes and were confirmed as phenotypically resistant to all of them (id: 510423-20, 510655-20, 510656-20). The MDR isolate from wastewater (id: 510423-20) carried up to four plasmids with ARGs, namely an IncB/O/K/Z conjugative plasmid carrying aac(3)-IId, aadA5, blaCTX-M-55, mph(A), sul1 and dfrA17, a col(pHAD28) mobilizable plasmid carrying aph(3’’)-Ib, aph(6)-Id and sul2. For the two drainage isolates (ids: 510655-20, 510656-20) the presence of a mobilizable plasmid incQ1 harboring aadA2, aph(3”)-Ib, aph(6)-Id, blaTEM-1B, sul1, sul2, tet(A) and dfrA12 genes was confirmed. The manure isolate 510473-21 carried a conjugative plasmid with aph(3”)-Ib, aph(6)-Id and tet(A), while the soil isolate 510520-21 carried aph(3”)-Ib and aph(6)-Id in a conjugative plasmid.
In K. pneumoniae, where low-level resistance to ampicillin was found in all isolates, chromosomally-encoded blaSHV (blaSHV-187, blaSHV-11) and ampC-like (ampH) genes were detected. The fosfomycin genes fosA5 and fosA6 were found in two and five isolates. Only those carrying fosA6 were resistant. We did not find evidence of fosA-carrying plasmids.
In E. faecium, the only phenotypically-resistant isolate (510426-20 ampicillin resistant) harbored up to thirteen simultaneous known point mutations and four unknown mutations in the chromosomal gene pbp5. None of the isolates carried acquired ARGs. All (n = 6) carried the chromosomal msrC, associated with macrolide–streptogramin B resistance, aac(6′)-Ii, responsible for low-level resistance to aminoglycosides and efmA, which codes for an efflux pump associated with macrolide and fluoroquinolone resistance. No known mutations in the 23S ribosomal genes associated with linezolid resistance were found. Likewise, neither linezolid (cfr, optrA, poxtA) nor vancomycin ARGs (van clusters) were detected.
In E. faecalis, the most frequently detected ARGs were the lincosamide gene lsa, which was present in all isolates, the dihydrofolate reductase gene dfrE (21/27) and efrA encoding for an ABC multidrug efflux pump (22/27). In addition, acquired tetracycline genes were detected in all the nine tetracycline-resistant isolates (tetM: 9/27 and tet45: 3/27). Two of those isolates (ids: 510672-20, 510673-20) were the ones confirmed as resistant to streptomycin and kanamycin by E-test and carried the aminoglycoside genes aad(6) and aph(3”)-IIIa and the streptothricin resistance gene sat-4, which were encoded on a mobilizable plasmid. The tet genes of those isolates were also integrated in mobilizable plasmids. Additionally, the erythromycin resistance gene ermB was detected in two unrelated isolates from manure (id: 510691-20) and soil (id: 510295-20). In both isolates the ermB was located in the transposon Tn917. The soil isolate was the only one confirmed as phenotypically resistant to erythromycin. The manure isolate carried the ermB together with tetracycline genes on a conjugative plasmid, while the soil isolate carried those genes on a mobilizable plasmid. The lincosamide resistance gene lnuG was detected in an isolate obtained from pig manure (id: 510683-20) located in the transposon tn6260. The isolate presented a MIC for clindamycin of 24 µg/mL.
In E. coli, the most frequently detected phylogroup was B1 (n = 28, 57.7%), followed by B2 (n = 8, 16.3%), D (n = 6, 12.2%), A (n = 5, 10.2%) and E (n = 2, 4.1%). Isolates of different phylogroups were found in different compartments (Table S3). E. coli phylogroup B2 isolates had, on average, 54 VGs, E isolates 38 VGs, and D, B1 and A isolates had 19, 13 and 9 respective VGs. Of the isolates, 90% had fimbrial-related genes. Thirteen isolates from the phylogroups B1, B2 and D were considered as potentially pathogenic. Seven out of the eight B2 isolates identified carried the chuA, fyuA and vat genes and were classified as UPEC. One of them was the MDR isolate 510423-20. The remaining B2 isolate, obtained from a meadow sample (soil), together with two B1 wildlife isolates were classified as atypical EPEC (ids: 510307-20, 510398-20, 510620-20), since they carried eae gene but not bfpA. In addition, one of the B1 isolates from wildlife was identified as a new STEC (id: 510632-20), which carried the stx2c variant, the enterohaemolysin gene ehxA, the subtilase gene subAB, was eae-negative and belonged to serotype O142:H16. The ST was newly designated as ST12789. Lastly, two isolates from wastewater (id: 510245-20) and wildlife (id: 510402-20-WH) were identified as presumptive ExPEC, which carried the ExPEC-specific markers kpsMII and iutA. The isolate from wastewater belonged to the major E. coli group ST69 and was one of the ampicillin resistant isolates. In total, 36 isolates were designated as commensal and did not carry the required marker combinations to be considered as E. coli pathotypes.
In K. pneumoniae, VGs were detected in all the seven isolates, including enterobactin (n = 7, 100%), salmochelin (n = 7, 100%) and yersiniabactin genes (n = 1, 14.3%). The aerobactin gene iuc and the hypermucoid phenotype gene rmpA were not detected in any isolate. Additionally, isolates had capsule types typical of non-hpkv strains (capsular types other than K1 and K2).
In E. faecium, the only VG detected was the adhesin-encoding gene acm.
Regarding E. faecalis, the presence of VGs varied among the isolates. BopD, cpsA, cpsB, ebpA, ebpB, ebpC, efaA and srtC genes were detected in all the 27 strains. Other frequently found genes (>70%) were EF0818, fss1, gelE and sprE. Cyl genes were found in only two isolates from river water (ids: 510416, 510417) and two isolates from wastewater (ids: 510672, 510673). The latter also had the asa1 gene. The esp gene coding for a surface protein was not found in the isolates.
3. Discussion
In this study, we aimed to obtain clinically-relevant bacteria from connected environmental compartments. We detected conjugative and mobilizable plasmids and other mobile genetic elements that can spread via horizontal gene transfer. Although we only found resistances to macrolides and tetracyclines in isolates obtained from samples directly associated with farmers 1 and 2 (manure, soil and feed), we found resistances and ARGs in other connected compartments such as the field drainage, mainly associated with veterinary drugs. This highlights the importance of monitoring for AMR and ARB emergence on farms and farm-related environments.
Overall, our work has used a One Health approach, allowing us to detect four of the six targeted clinically-relevant bacterial species, some of which carried acquired ARGs, were phenotypically resistant to one or more antimicrobials and/or were present in several samples. Although there is recent evidence that pig manure increases the persistence of Salmonella sp. in the soil, from where it can penetrate into crops, we did not find Salmonella spp. in any of the compartments studied. Nevertheless, certain types of soil and plants seem to be more associated with persistence and more frequent detection of Salmonella [12]. Likewise, previous reports have confirmed that up to 11% of the MRSA acquired in the community come from crops fertilized with pig manure [13]. However, we did not detect MRSA in our HOAL samples. A reason for this could be that the prevalence of MRSA is lower in small-scale farm systems compared to industrial ones [14].
We obtained a higher number of isolates in July. This observation does not seem to be related to the application of pig manure to the field, which occurred in March, and could be due to the diverse representation of compartments among the samples collected in July.
In addition, only E. faecalis and E. coli were detected in the pig manure, but these were also the two most abundant species. Therefore, it is difficult to assess the role of pig manure on the bacterial population with so many potential factors affecting the diversity, and the same applies to the ARGs detected in the isolates. A recent longitudinal study also performed at the Austrian HOAL observed that the concentrations of a set of ARGs detected by qPCR decrease after manure application reaching the baseline levels in the sampled soils quickly [15]. Here, we did not observe differences between isolation dates and the diversity of ARGs in the isolates, but differences between isolates of different species and compartments, independent of the point in time. Thus, a study on other non-clinically relevant species would be of interest in order to assess the ARGs that are naturally present (not anthropogenic origin) in environmental bacteria, which can be hotspots of AMR with or without increased selective pressure from human-derived activities.
The low prevalence or absence of clinically-relevant bacteria in feed and crops detected here is in accordance with previous reports [16]. This and the geographical location of the HOAL, far away from big complex urban areas and the size of pig farms, might explain these results.
In general, isolates from manure and wastewater were more often phenotypically resistant to the antimicrobials tested than the isolates gained from other compartments, and had more ARGs per isolate. In addition, we observed that for these two compartments, ARGs detected could be distributed in a wider range of drug class categories than those from other compartments. This indicates that manure and wastewater isolates, and in particular, E. coli, presented more ARGs, both intrinsic and acquired, contributing to a higher degree of phenotypic AMR. E. coli isolates also showed high diversity in terms of STs, with three isolates having unknown STs. The detection of four clusters composed of isolates obtained from samples of different compartments and/or isolation dates among such a diverse isolate collection and low sample size confirms the spread of different clones between compartments, some of which could be pathogenic. ST357 (cluster 5), classified as possible UPEC here, has been found to be one of the most frequent UPEC clones causing UTI in humans [17]. Cluster 3, composed by ST58 wastewater isolates, has been reported in different hosts and sources, including wastewater, as one of the top 20 ExPEC clones able to cause urosepsis [18] and it is newly described as a possibly zoonotic ST [19]. However, we reported it here as commensal, since it did not fulfill the criteria for ExPEC designation. According to the literature, isolates that carry ≥2 or more of chuA, fyuA, vat, and yfcV genes are considered as UPEC, while isolates that carry ≥2 or more of apaH/papC, sfa-focDE, afa-draBC, iutA and kpsMII are considered as ExPEC [20]. ST212 (cluster 1-wildlife) is not only a major E. coli clone but has been associated with wildlife, in particular vultures, and has been reported to disseminate across the human–animal-environment interface [21]. In addition, it has been detected in patients with UTI, although here we described it as commensal [22]. In agreement with other authors, ST216 was described here as commensal E. coli, matching their phylogroup (B1) and source of isolation with those in the literature, where they were found in the environment and wildlife [23,24]. Among the singletons, the detection of an UPECST1193 O75:H11 MDR clone was remarkable. ST1193 is an emerging fluoroquinolone resistance strain genetically related to the MDR pandemic clone ST131 [25,26], not detected in our study. The sequence of our ST1193 isolate was described in our previous report [26] and differed by only 13 alleles from an UPEC isolated from a male patient with urinary tract infection in Australia in 2011 (Uberstrain accession: ESC_PA1198AA, strain MS10745) and by 17 alleles from a MDR strain isolated in the US causing neonatal meningitis (accession: CP030111). Although the Australian strain was also MDR and carried most of the acquired ARGs detected in our strain (sul1/sul2, blaTEM-1, aph(6)-Id, aac(3)-IId, qacE), it lacked blaCTX-M-55, therefore being cephalosporin susceptible. In addition, we detected a ST69 isolate from wastewater, which is also a major ExPEC clone that can cause sepsis and urinary tract infections [18]. In agreement with other authors, ST69 carried blaTEM-1 and was susceptible to extended-spectrum cephalosporins [27]. However, we did not find the mcr-9 gene, which has been associated with this clone in particular [28]. We also found an eae-negative STEC O142:H16 isolate (id: 510632-20). Recently, an STEC strain belonging to the same serotype but of an unknown source was sequenced by CDC (SRR14150468), differing from our strain by 65 alleles in the core genome. While our strain was isolated from a wildlife fecal sample from a brown hare (Lepus europaeus), there are other reports of this serotype obtained from raw milk, mozzarella cheese [29] and free ranging deer [30]. Like the deer isolate, our isolate lacked eae and had the stx2c variant, ehxA and other virulence genes such as subAB, indicating that it was potentially pathogenic for humans [31]. In addition, the three isolates that had eae genes but no shigatoxin can be considered in the absence of stx genes and bfpA as aEPEC [32], but could be equally pathogenic, since some aEPEC such as the O103, also detected here in a soil sample, have been linked to human disease but originated from animals [33].
As for AMR in our E.coli isolates, it can be seen in general that those strains with acquired ARGs mostly expressed a resistant phenotype associated with the ARG in question. An exception for this was the only AMC resistant isolate (id: 510298-20), which was also AMP resistant, and for which no blaTEM gene was found. AMC and AMP resistance are known to be associated with TEM-1 overproduction, which suggests that in the absence of this gene, hyperproduction of the chromosomal AmpC may occur, causing the AMC-AMP resistant phenotype [34]. It is also noteworthy that one of the farmers interviewed had administered tetracyclines, sulfonamides and penicillins, coinciding with the resistances seen in most of the resistant E. coli. Indeed, tetracycline ARGs but also aminoglycoside genes, a drug class not administered to the HOAL pigs in principle, were detected in isolates from at least three compartments simultaneously. According to the European medicines agency antimicrobial expert group (EMA-AMEG), the use of aminoglycosides in animals should be limited to scenarios when no other alternative antimicrobials can be used, since the development of resistances to this drug class represents a high risk to public health [35]. Different types of enzymes, such as those encoded in the aadA2 or aad5 genes, confer a lower level of resistance to streptomycin, while those encoded in the aph(3”)-Ib and aph(6)-Id confer a higher level of resistance. Accordingly, we detected combinations of the two aminoglycoside genes that corresponded with the highest resistance, while strains with only one type of gene were more susceptible to this antibiotic. Moreover, the gentamicin-resistant isolate (id: 510423-20) was the only one carrying aac(3”)-IId, a determinant for gentamicin resistance, while the absence of resistance markers such as aph(3’)-Ia, which is responsible for kanamycin resistance, explained the phenotypic susceptibility against this antimicrobial [36,37]. Isolates carrying tet genes were obtained from five compartments, including pig manure, and highlight that the use of tetracyclines in pigs select for these tetracycline genes [38]. Moreover, in wildlife isolates the only ARGs detected were tet genes. This lack of AMR in wildlife contrasted with a previous study carried out in Germany, where although low, AMR against cephalosporins, fluoroquinolones and colistin was detected [39].
K. pneumoniae was only detected in wastewater samples, although this species is adapted to other environments such as soil and vegetation [40]. Despite the low number of retrieved isolates, we were able to identify a cluster of five ST29 isolates from five different samples and three sampling dates, which indicates the persistence of this clone over time and in both the inlet and the outlet of the wastewater treatment plant. Unlike in previous studies, our ST29 isolates did not carry ESBL genes, which seems to be uncommon in this ST [41]. In any case, the discharge of K. pneumoniae clones (MDR and/or pathogenic) via the effluent of the wastewater treatment plant into a downstream body of water and further on to the crops via irrigation using river water cannot be excluded.
Regarding phenotypic resistance, the low-level ampicillin resistance observed in all the K. pneumoniae isolates is known to be of an intrinsic origin and encoded in the chromosome by blaSHV-1 and ampH genes [42,43], both detected in our isolates. The high level of fosfomycin resistance observed in five of our isolates was most likely associated with fosA6 genes encoded in the chromosome. Research shows that these genes can be located either in the chromosome or in plasmids via mobilization through IS10 in both K. pneumoniae and E. coli, and there is prior evidence of high level fosfomycin resistance due to fosA6 in E. coli [44,45]. However, IS10 was not found in any of these isolates in the surroundings of the fosA6 gene nor was a plasmid detected, pointing to a chromosomal location. On the contrary, the two fosA5 isolates were fosfomycin-susceptible, in agreement with other authors, and although IncR and/or one IncFIB_K plasmids were detected in these isolates, they did not harbor the fosA5, therefore indicating a possible chromosomal location [46].
Although all K. pneumoniae isolates carried enterobactin genes and the iroE gene, which is part of the locus that encodes for salmochelin, in order to designate isolates as hvKp, at least four of the following genes need to be present in a K. pneumoniae isolate: rmpA, rmpA2, iucA, iroB and peg-344 [47,48]. Moreover, other authors differentiated commensal K. pneumoniae from hvKp due to the presence in hvKp of salmochelin and aerobactin [49], as well as the presence of specific capsule serotypes for hvKp [50]. None of the isolates detected here matched the hvKp definition, in accordance with the VGs found.
All the ARGs found in E. faecium isolates are known to be intrinsic resistance determinants in this species [51,52], which is in agreement with the lack of phenotypic resistance found here, except for one ST236 isolate, whose ampicillin resistance was due to point mutations in pbp5 genes. In contrast, other authors obtained non-resistant isolates of this ST from environmental samples [53]. Conversely, other works have reported ST22 isolates obtained from human and animal sources as optrA carriers [54], while our ST22 isolates were not phenotypically resistant nor did they carry any ARG. Regarding the only VG found in E. faecium, the acm gene, this has recently been described in clinical strains of patients with endocarditis, indicating pathogenic potential.
In this study, we found a high diversity of STs for E. faecalis, some of which have been described as zoonotic. Indeed, ST16 and ST40 isolates identified here in wildlife and wastewater have been detected before in hospitalized and non-hospitalized patients, food production animals, wild birds and the environment, namely wastewater [51,55,56,57].
Regarding AMR, E. faecalis is known to be intrinsically resistant to lincosamides via efflux pumps, all cephalosporins, quinupristin-dalfopristin, low level aminoglycosides and trimethoprim [52,58]. Here, all E. faecalis isolates carried lsa, responsible for intrinsic resistance to lincosamides and streptogramin A [59]. However, the detection of lnu(G) in a pig manure isolate could also explain the clindamycin MIC of 24 µg/mL observed, which is ten times higher than previously reported [60]. Lnu(G) was first identified in swine located on the lincosamide nucleotidyl transferase transposon Tn6260 of E. faecalis. Unlike lincomycin, which is frequently used in veterinary medicine, clindamycin is often prescribed in human medicine. Therefore, the detection of lnu(G) in pig manure could lead to an increase in the resistance to lincosamides when disseminated and acquired via the food/feed chain. The ermB gene, detected in another manure and a soil isolate could be responsible for the erythromycin resistance detected in the manure isolate. Interestingly, the pig manure from which this isolate was gained belonged to the same farmer, as did the crop field from which the soil sample was taken (corn fertilized with manure). As reported elsewhere [61], pig manure can be the origin of ermB and lnu(G) in E. faecalis obtained from soil at nearby pig farms.
In agreement with recent reports, most of our E. faecalis isolates carried dfrE, which, unlike dfrF, is considered intrinsic [62,63]. Conversely, only the two E. faecalis isolates from wastewater showing high level streptomycin and kanamycin resistance had acquired aminoglycosides genes, namely, aph(3’)-IIIa [64]. As for tetracycline resistance, the tetM gene, a resistance marker that can be transferred by HGT [65] was detected here and it has been proved to increase in soils fertilized with manure. Indeed, in addition to three manure isolates carrying tetM in our study, the only tetracycline resistant E. faecalis obtained from soil belonged to a field fertilized with manure.
Regarding VGs in E. faecalis, isolates with more VGs (>23) were from wildlife feces or pig manure, while in E. coli, wastewater isolates carried more VGs (>45). Among the E. faecalis VGs typically associated with disease in humans, we only detected three of them, namely asa1, cylA and gelE, alone or in combination with each other [66]. In agreement with other authors, gelE was the most frequent VG among that group of genes, while cylA was not detected in animals, but in the environment (river and wastewater) [66]. Nevertheless, isolates with more VGs(>23) did not carry cylA nor asa1, and at the same time, these and other VGs have also been isolated from E. faecalis obtained from healthy humans. Thus, caution is advised when designating isolates as pathogenic [67].
In conclusion, we have demonstrated that some clones are detected at different time-points, but we have not seen the same clone in more than one compartment. We detected the same ARGs in distinguishable isolates from different compartments, most of them as phenotypically resistant to at least one antimicrobial. In addition, although the HOAL symbolizes the exchange of ARGs and ARBs between different compartments existing in nature, the mere presence of two pig farms in this HOAL is not representative of the totality of existing farming practices. Different results might be obtained when choosing industrial breeding farms, which would contribute more significantly to the environmental antibiotic resistance pool. Additionally, the presence of larger urban centers near the HOAL would have a greater impact on ARG exchange. Likewise, it is uncertain whether some ARGs have been exchanged between isolates of different compartments after applying manure to the field or if this is purely coincidental. Taken together, the detection of the same ARGs in different compartments warrants further attention. Moreover, some of the gaps in our knowledge can be better addressed by using culture-free methodologies such as shotgun metagenomics in addition to WGS on the bacterial cultures, in order to characterize the environmental resistome, as well as the microbial diversity in the different compartments.
4. Materials and Methods
4.1. Sampling
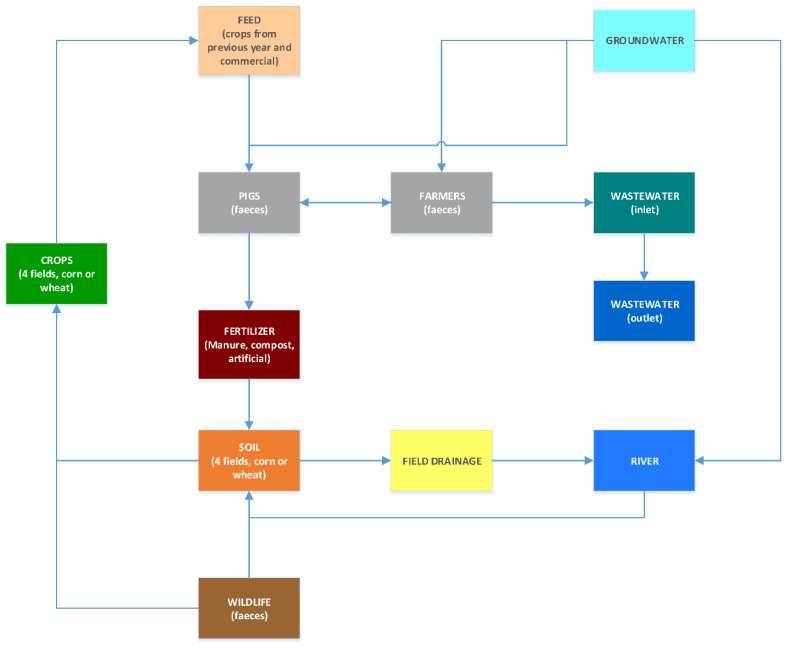
As part of the OHEJP FED-AMR project, we collected 61 samples from nine out of 11 different but interconnected environmental compartments in an annual longitudinal study (Figure 3) from March 2020 to January 2021 in the area of Petzenkirchen, Austria.
Figure 3.
Graphical representation of the relationship between the different HOAL compartments from which samples were collected. Grey compartments are those from which no sample could be obtained.
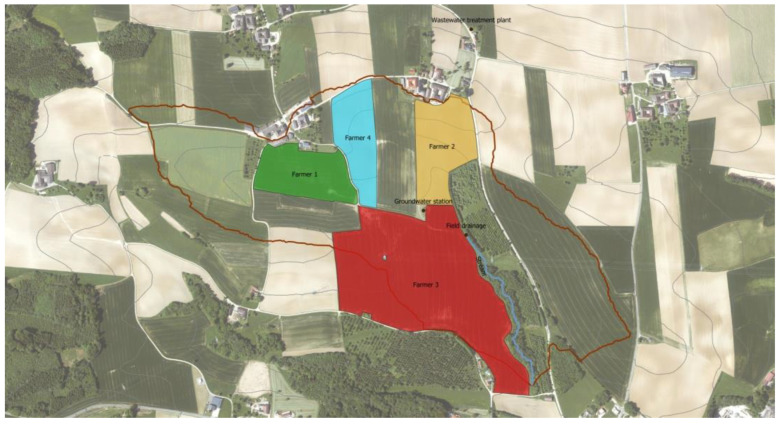
The sampling area was part of a hydrological open-air laboratory (HOAL) (Figure 4). The intended purpose is to understand water flow related processes by investigating their microorganisms [68].
Figure 4.
Aerial view of the HOAL in Petzenkirchen, Lower Austria. The limit of the HOAL is depicted by the red line. The sampled fields are colored by farmer. Green, yellow and red fields are sown with corn. The field in blue is sown with wheat. The blue line represents a stream (i.e., compartment “river”). Alongside there is a field drainage and groundwater station. The wastewater treatment plant is outside the catchment area, next to the house of farmer 2.
Three corn fields and one winter wheat field, each from a different farmer, were chosen for collecting soil and crop samples. Two of the corn fields were fertilized with pig manure plus artificial fertilizer (farmer 1 and 2), while the third one was nourished with compost plus artificial fertilizer (farmer 3). The winter wheat field (farmer 4) was enriched only with artificial fertilizer. In the catchment area, there were two pig farms from two different farmers (1 and 2). Data for the use of antibiotics on these two farms were retrieved from the official veterinary records (Table S5). Feed given to the pigs originated from a mixture of crops from the previous year (2019). These crops had received artificial fertilizer, manure or combination of both. Additionally, farmers purchased commercial feed with additives (mineral feed). At the time of the first sampling, pig manure was less than a year old and the slurry pits were emptied every autumn. That is, pig manure was a mixture of manure accumulating since fall of the previous year. Fertilization either with swine manure, compost or commercial artificial fertilizer took place in March–April 2020.
Next to farmer 3’s field was a field drainage and a small river from which samples were also collected. Wildlife feces were easily accessible from the ground of the field nourished with compost (farmer 3), the largest field. In addition, samples were taken from the wastewater treatment plant collecting the effluents of nine agricultural households right outside the catchment area; two of these belonged to farmers 1 and 2. Samples from ground water supplying these households and the pig farms for both human and animal consumption were also obtained. The sample collection procedure for each compartment and the transportation conditions are available at Zenodo (doi:10.5281/zenodo.5081756).
4.2. Isolate Selection and Sequencing
From the samples, 0.5 g were introduced in 15 mL sterile tubes with glass beads and 4.5 mL 0.1% buffer Na4P2O7.10H2O buffer at pH 7.0. Serial dilutions 1:10 were made and 100 µL of each dilution were plated on CCA agar for the detection of E. coli and K. pneumoniae (Chromocult® Coliform Agar, Chromocult, Merck, Feltham, UK); on CHROMagar™ MRSA II (Becton Dickinson and Company, Sparks, MD, USA) for MRSA detection; on Columbia CAN agar with 5% sheep blood (BioMérieux, Marcy l’Etoile, France) for Enterococcus spp. Plates were subsequently incubated for 24 h at 37 °C in aerobiosis. We selected up to four colonies with different morphologies from each sample and subcultivated them in Columbia COS agar with 5% sheep blood (BioMérieux, Marcy l’Etoile, France). Bacterial species were identified with matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS, Bruker, Ettlingen, Germany) with the Software IVD MBT Compass v4.2.90. When either E. coli, K. pneumoniae, E. faecium, E.faecalis, S. aureus or Salmonella spp. were detected, isolates were subsequently used for DNA extraction, WGS-based typing and characterization of genetic and phenotypic antimicrobial resistances.
4.3. Whole Genome Sequencing and Typing
We performed whole-genome sequencing (WGS) on the selected isolates as described previously [69]. High molecular weight (HMW) DNA was extracted using the MagAttract HMW DNA kit (Qiagen, Hilden, Germany). Library preparation was carried out with Nextera XT kit (Illumina, San Diego, CA, USA) and 2 × 250-bp sequencing was carried out on an Illumina MiSeq device. The quality of the FASTQ reads was assessed with FastQC v0.11.9 [70] and the adapters, the last 10 bp of each sequence and sequences below a quality score of 20 were removed or trimmed with Trimmomatic v0.36 [71]. Genome assemblies were generated using SPAdes v3.11.1 [72]. Ridom SeqSphere+ v8.2.0 (Ridom, Münster, Germany) [73,74] was used for WGS-based typing, including species identification, assessment of the genetic relatedness with multilocus sequence typing (MLST) and core genome MLST (cgMLST). For E. coli, we used the Warwick cgMLSTv1 scheme hosted at Enterobase, while for E. faecium and E. faecalis we used the schemes from PubMLST [75,76]. For K. pneumoniae we used the stable cgMLST scheme for K.pneumoniae sensu lato defined by Seqpshere+. Genome sequences from isolates with unknown alleles and STs were submitted to PubMLST (Enterococcus sp.) or EnteroBase (Enterobacteriaceae) for the assignment of new alleles and STs by database curators. For each species, MSTs were generated to visualize clusters and the number of allelic differences between the isolates. The default cluster thresholds provided in each of the cgMLST schemes were used. We defined as pseudo-clusters those clusters grouping two or more isolates obtained from the same sample.
ARGs were extracted via the CARD database v4.0 with strict and perfect mode [77]. All ARGs detected were allocated to artificially-created drug classes. Genes encoding for multidrug efflux pumps and other markers whose product conferred resistance to more than one drug class were allocated to the category “multidrug”. In order to plot bar charts with isolate counts the package ggplot2 v3.3.6 from the R statistical software v.4.1.1 was used [78]. Neighbor-joining trees obtained via Seqsphere displaying the cgMLST of each species were imported into R and visualized with the package ggtree v3.4.2 [79]. Afterwards, the gheatmap function was used to annotate the trees for the presence or absence of ARGs in each isolate. Virulence genes (VGs) were extracted from the WGS data using the VFDB database v2020 [80]. Plasmids were detected with PlasmidFinder v2.1.1 [81] and the prediction of the possible location (chromosome/plasmid) of acquired ARGs was identified with the Ellipsis pipeline (https://github.com/NorwegianVeterinaryInstitute/Ellipsis). For K. pneumoniae isolates, the tool Kaptive v3 was used to identify K capsule types typically associated with hypervirulent (hvKp) clones [82]. For E. coli isolates, phylogroups were detected using EzClermont [83] and serogroups with SeroTypeFinder v2.0 [84].
4.4. Phenotypic Resistance
Isolates were first screened for resistances by disk diffusion to critically and highly important antimicrobials (see Table S6). For each isolate, a Mueller–Hinton plate prepared in-house was used to inoculate a 0.5 McFarland suspension of the isolate with a disposable swab. Up to six discs with different antimicrobials (Oxoid/ThermoFisher Scientific, Basingstoke, UK) were placed on the plates with a disc dispenser. After 24 h of incubation at 37 °C, the inhibition halos were measured and compared with the EUCAST breakpoint tables for disk diffusion. Possible clavulanate synergy with cefotaxime, ceftazidime or both was assessed in order to look for ESBL producers [85]. We also looked for cefoxitin resistance to identify AmpC producers. Resistant isolates were then tested by E-test (Biomérieux, France) to confirm and quantify (minimum inhibitory concentration or MIC) their phenotypic resistance (Table S7).
An extended antimicrobial susceptibility test (AST) with E-test was also performed for some isolates using additional antimicrobials when acquired ARGs were detected by WGS.
4.5. Sequence Data Availability
This whole-genome shotgun project has been deposited in DDBJ/ENA/GenBank under BioProject accession no. PRJNA873263. The version described in this paper is the first version. The raw sequence reads have been deposited in the sequence read archive (SRA). Accession numbers of the E. coli, K. pneumoniae, E. faecium and E. faecalis genomes can be consulted in Tables S1–S4.
Acknowledgments
We thank Shakeel Mowlaboccus from The University of Western Australia for assigning STs to our E. faecalis isolates via PubMLST.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms231911276/s1.
Author Contributions
Conceptualization, M.W. and W.R.; Data curation, A.C. (Adriana Cabal); Formal analysis, A.C. (Adriana Cabal); Investigation, A.C. (Adriana Cabal), S.S.M. and M.C.; Methodology, A.C. (Adriana Cabal), G.R., B.D.-P., A.S., N.P., A.C. (Ali Chakeri), H.B., K.F., J.S., K.R., P.H., S.S. and P.S.; Writing—original draft, A.C. (Adriana Cabal); Writing—review and editing, A.C. (Adriana Cabal), F.A. and W.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the manuscripts or the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was partially funded by the European Union’s Horizon 2020 Research and Innovation program, grant agreement No 773830: One Health European Joint Program.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murray C.J.L., Ikuta K.S., Sharara F., Swetschinski L., Robles Aguilar G., Gray A., Han C., Bisignano C., Rao P., Wool E., et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson T.P., Bu D.P., Carrique-Mas J., Fèvre E.M., Gilbert M., Grace D., Hay S.I., Jiwakanon J., Kakkar M., Kariuki S., et al. Antibiotic resistance is the quintessential One Health issue. Trans. R. Trop. Med. Hyg. 2016;110:377–380. doi: 10.1093/trstmh/trw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassini A., Högberg L.D., Plachouras D., Quattrocchi A., Hoxha A., Simonsen G.S., Colomb-Cotinat M., Kretzschmar M.E., Devleesschauwer B., Cecchini M., et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apfalter P., Berning L., Eigentler A., El-Belazi G., Fellinger F., Klemens F., Fuchs R., Hain C., Hartl R., Igler G., et al. Resistenzbericht Österreich AURES. Bundesministerium für Soziales, Gesundheit, Pflege und Konsumentenschutz (BMSGPK); Wien, Austria: 2020. [Google Scholar]
- 5.Mancuso G., Midiri A., Gerace E., Biondo C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens. 2021;10:1310. doi: 10.3390/pathogens10101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Oliveira D.M.P., Forde B.M., Kidd T.J., Harris P.N.A., Schembri M.A., Beatson S.A., Paterson D.L., Walker M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020;33:e00181-19. doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslam B., Khurshid M., Arshad M.I., Muzammil S., Rasool M., Yasmeen N., Shah T., Chaudhry T.H., Rasool M.H., Shahid A., et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell. Infect. Microbiol. 2021;11:1153. doi: 10.3389/fcimb.2021.771510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Destoumieux-Garzón D., Mavingui P., Boetsch G., Boissier J., Darriet F., Duboz P., Fritsch C., Giraudoux P., Le Roux F., Morand S., et al. The One Health Concept: 10 Years Old and a Long Road Ahead. Front. Vet. Sci. 2018;5:14. doi: 10.3389/fvets.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calistri P., Iannetti S., Danzetta M.L., Narcisi V., Cito F., Di Sabatino D., Bruno R., Sauro F., Atzeni M., Carvelli A., et al. The Components of ‘One World—One Health’ Approach. Transbound. Emerg. Dis. 2013;60:4–13. doi: 10.1111/tbed.12145. [DOI] [PubMed] [Google Scholar]
- 10.Huijbers P.M.C., Flach C.-F., Larsson D.G.J. A conceptual framework for the environmental surveillance of antibiotics and antibiotic resistance. Environ. Int. 2019;130:104880. doi: 10.1016/j.envint.2019.05.074. [DOI] [PubMed] [Google Scholar]
- 11.Dolejska M., Literak I. Wildlife Is Overlooked in the Epidemiology of Medically Important Antibiotic-Resistant Bacteria. Antimicrob. Agents Chemother. 2019;63:e01167-19. doi: 10.1128/AAC.01167-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jechalke S., Schierstaedt J., Becker M., Flemer B., Grosch R., Smalla K., Schikora A. Salmonella Establishment in Agricultural Soil and Colonization of Crop Plants Depend on Soil Type and Plant Species. Front. Microbiol. 2019;10:967. doi: 10.3389/fmicb.2019.00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey J.A., Curriero F.C., Cosgrove S.E., Nachman K.E., Schwartz B.S. High-density livestock operations, crop field application of manure, and risk of community-associated methicillin-resistant Staphylococcus aureus infection in Pennsylvania. JAMA Intern. Med. 2013;173:1980–1990. doi: 10.1001/jamainternmed.2013.10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalupahana R.S., Duim B., Verstappen K.M., Gamage C.D., Dissanayake N., Ranatunga L., Graveland H., Wagenaar J.A. MRSA in Pigs and the Environment as a Risk for Employees in Pig-Dense Areas of Sri Lanka. Front. Sustain. Food Syst. 2019;3:967. doi: 10.3389/fsufs.2019.00025. [DOI] [Google Scholar]
- 15.Radu E., Woegerbauer M., Rab G., Oismüller M., Strauss P., Hufnagl P., Gottsberger R.A., Krampe J., Weyermair K., Kreuzinger N. Resilience of agricultural soils to antibiotic resistance genes introduced by agricultural management practices. Sci. Total Environ. 2021;756:143699. doi: 10.1016/j.scitotenv.2020.143699. [DOI] [PubMed] [Google Scholar]
- 16.Hölzel C.S., Tetens J.L., Schwaiger K. Unraveling the Role of Vegetables in Spreading Antimicrobial-Resistant Bacteria: A Need for Quantitative Risk Assessment. Foodborne Pathog. Dis. 2018;15:671–688. doi: 10.1089/fpd.2018.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsukawa M., Igarashi M., Watanabe H., Qin L., Ohnishi M., Terajima J., Iyoda S., Morita-Ishihara T., Tateda K., Ishii Y., et al. Epidemiology and genotypic characterisation of dissemination patterns of uropathogenic Escherichia coli in a community. Epidemiol. Infect. 2019;147:e148. doi: 10.1017/S0950268819000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manges A.R., Geum H.M., Guo A., Edens T.J., Fibke C.D., Pitout J.D.D. Global Extraintestinal Pathogenic Escherichia coli (ExPEC) Lineages. Clin. Microbiol. Rev. 2019;32:e00135-18. doi: 10.1128/CMR.00135-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Royer G., Darty M.M., Clermont O., Condamine B., Laouenan C., Decousser J.-W., Vallenet D., Lefort A., de Lastours V., Denamur E., et al. Phylogroup stability contrasts with high within sequence type complex dynamics of Escherichia coli bloodstream infection isolates over a 12-year period. Genome Med. 2021;13:77. doi: 10.1186/s13073-021-00892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tetzschner A.M.M., Johnson J.R., Johnston B.D., Lund O., Scheutz F., Dekker J.P. In Silico Genotyping of Escherichia coli Isolates for Extraintestinal Virulence Genes by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2020;58:e01269-20. doi: 10.1128/JCM.01269-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Carvalho M.P.N., Fernandes M.R., Sellera F.P., Lopes R., Monte D.F., Hippólito A.G., Milanelo L., Raso T.F., Lincopan N. International clones of extended-spectrum β-lactamase (CTX-M)-producing Escherichia coli in peri-urban wild animals, Brazil. Transbound. Emerg. Dis. 2020;67:1804–1815. doi: 10.1111/tbed.13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decano A.G., Pettigrew K., Sabiiti W., Sloan D.J., Neema S., Bazira J., Kiiru J., Onyango H., Asiimwe B., Holden M.T.G. Pan-Resistome Characterization of Uropathogenic Escherichia coli and Klebsiella pneumoniae Strains Circulating in Uganda and Kenya, Isolated from 2017–2018. Antibiotics. 2021;10:1547. doi: 10.3390/antibiotics10121547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarabai H., Wyrsch E.R., Bitar I., Dolejska M., Djordjevic S.P. Epidemic HI2 Plasmids Mobilising the Carbapenemase Gene blaIMP-4 in Australian Clinical Samples Identified in Multiple Sublineages of Escherichia coli ST216 Colonising Silver Gulls. Microorganisms. 2021;9:567. doi: 10.3390/microorganisms9030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loong S.K., Mahfodz N.H., Che Mat Seri N.A., Mohamad Wali H.A., Abd Gani S.A., Wong P.F., AbuBakar S. Genetic characterization of commensal Escherichia coli isolated from laboratory rodents. Springerplus. 2016;5:1035. doi: 10.1186/s40064-016-2745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valenza G., Werner M., Eisenberger D., Nickel S., Lehner-Reindl V., Höller C., Bogdan C. First report of the new emerging global clone ST1193 among clinical isolates of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli from Germany. J. Glob. Antimicrob. Resist. 2019;17:305–308. doi: 10.1016/j.jgar.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Cabal A., Peischl N., Rab G., Stöger A., Springer B., Sucher J., Allerberger F., Ruppitsch W. Draft Genome Sequence of a Multidrug-Resistant Escherichia coli Sequence Type 1193 Pandemic Clone Isolated from Wastewater in Austria. Microbiol. Resour. Announc. 2021;10:e0076221. doi: 10.1128/MRA.00762-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doumith M., Day M., Ciesielczuk H., Hope R., Underwood A., Reynolds R., Wain J., Livermore D.M., Woodford N. Rapid identification of major Escherichia coli sequence types causing urinary tract and bloodstream infections. J. Clin. Microbiol. 2015;53:160–166. doi: 10.1128/JCM.02562-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieffer N., Royer G., Decousser J.-W., Bourrel A.-S., Palmieri M., Rosa J.-M.O.D.L., Jacquier H., Denamur E., Nordmann P., Poirel L. mcr-9, an Inducible Gene Encoding an Acquired Phosphoethanolamine Transferase in Escherichia coli, and Its Origin. Antimicrob. Agents Chemother. 2019;63:e00965-19. doi: 10.1128/AAC.00965-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nobili G., Franconieri I., Basanisi M.G., La Bella G., Tozzoli R., Caprioli A., La Salandra G. Short communication: Isolation of Shiga toxin-producing Escherichia coli in raw milk and mozzarella cheese in southern Italy. J. Dairy Sci. 2016;99:7877–7880. doi: 10.3168/jds.2016-11613. [DOI] [PubMed] [Google Scholar]
- 30.Eggert M., StüBer E., Heurich M., Fredriksson-Ahomaa M., Burgos Y., Beutin L., Märtlbauer E. Detection and characterization of Shiga toxin-producing Escherichia coli in faeces and lymphatic tissue of free-ranging deer. Epidemiol. Infect. 2013;141:251–259. doi: 10.1017/S0950268812000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newton H.J., Sloan J., Bulach D.M., Seemann T., Allison C.C., Tauschek M., Robins-Browne R.M., Paton J.C., Whittam T.S., Paton A.W., et al. Shiga toxin-producing Escherichia coli strains negative for locus of enterocyte effacement. Emerg. Infect. Dis. 2009;15:372–380. doi: 10.3201/eid1503.080631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Söderlund R., Hurel J., Jinnerot T., Sekse C., Aspán A., Eriksson E., Bongcam-Rudloff E. Genomic comparison of Escherichia coli serotype O103:H2 isolates with and without verotoxin genes: Implications for risk assessment of strains commonly found in ruminant reservoirs. Infect. Ecol. Epidemiol. 2016;6:30246. doi: 10.3402/iee.v6.30246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandes R.T., Elias W.P., Vieira M.A.M., Gomes T.A.T. An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol. Lett. 2009;297:137–149. doi: 10.1111/j.1574-6968.2009.01664.x. [DOI] [PubMed] [Google Scholar]
- 34.Doi Y., Paterson D.L. Detection of plasmid-mediated class C β-lactamases. Int. J. Infect. Dis. 2007;11:191–197. doi: 10.1016/j.ijid.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 35.European Medicines Agency . Use of Aminoglycosides in Animals in the European Union: Development of Resistance and Impact on Human and Animal Health. European Medicines Agency (EMA); Amsterdam, The Netherlands: 2018. [Google Scholar]
- 36.Awad A., Arafat N., Elhadidy M. Genetic elements associated with antimicrobial resistance among avian pathogenic Escherichia coli. Ann. Clin. Microbiol. Antimicrob. 2016;15:59. doi: 10.1186/s12941-016-0174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sunde M., Norström M. The genetic background for streptomycin resistance in Escherichia coli influences the distribution of MICs. J. Antimicrob. Chemother. 2005;56:87–90. doi: 10.1093/jac/dki150. [DOI] [PubMed] [Google Scholar]
- 38.Yoshizawa N., Usui M., Fukuda A., Asai T., Higuchi H., Okamoto E., Seki K., Takada H., Tamura Y. Manure Compost Is a Potential Source of Tetracycline-Resistant Escherichia coli and Tetracycline Resistance Genes in Japanese Farms. Antibiotics. 2020;9:76. doi: 10.3390/antibiotics9020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plaza-Rodríguez C., Alt K., Grobbel M., Hammerl J.A., Irrgang A., Szabo I., Stingl K., Schuh E., Wiehle L., Pfefferkorn B., et al. Wildlife as Sentinels of Antimicrobial Resistance in Germany? Front. Vet. Sci. 2020;7:627821. doi: 10.3389/fvets.2020.627821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wareth G., Neubauer H. The Animal-foods-environment interface of Klebsiella pneumoniae in Germany: An observational study on pathogenicity, resistance development and the current situation. Vet. Res. 2021;52:16. doi: 10.1186/s13567-020-00875-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L., Feng Y., Wei L., Xiao Y., Zong Z. KPC-2-Producing Carbapenem-Resistant Klebsiella pneumoniae of the Uncommon ST29 Type Carrying OXA-926, a Novel Narrow-Spectrum OXA β-Lactamase. Front. Microbiol. 2021;12:436. doi: 10.3389/fmicb.2021.701513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyres K.L., Holt K.E. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr. Opin. Microbiol. 2018;45:131–139. doi: 10.1016/j.mib.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Heinz E., Ejaz H., Bartholdson Scott J., Wang N., Gujaran S., Pickard D., Wilksch J., Cao H., Haq I.-U., Dougan G., et al. Resistance mechanisms and population structure of highly drug resistant Klebsiella in Pakistan during the introduction of the carbapenemase NDM-1. Sci. Rep. 2019;9:2392. doi: 10.1038/s41598-019-38943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang T.-Y., Lu P.-L., Tseng S.-P. Update on fosfomycin-modified genes in Enterobacteriaceae. J. Microbiol. Immunol. Infect. 2019;52:9–21. doi: 10.1016/j.jmii.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Ma Y., Xu X., Guo Q., Wang P., Wang W., Wang M. Characterization of fosA5, a new plasmid-mediated fosfomycin resistance gene in Escherichia coli. Lett. Appl. Microbiol. 2015;60:259–264. doi: 10.1111/lam.12366. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y.-P., Chen Y.-H., Hung I.-C., Chu P.-H., Chang Y.-H., Lin Y.-T., Yang H.-C., Wang J.-T. Transporter Genes and fosA Associated With Fosfomycin Resistance in Carbapenem-Resistant Klebsiella pneumoniae. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.816806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cubero M., Grau I., Tubau F., Pallarés R., Dominguez M.A., Liñares J., Ardanuy C. Hypervirulent Klebsiella pneumoniae clones causing bacteraemia in adults in a teaching hospital in Barcelona, Spain (2007–2013) Clin. Microbiol. Infect. 2016;22:154–160. doi: 10.1016/j.cmi.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 48.Patro L.P.P., Sudhakar K.U., Rathinavelan T. K-PAM: A unified platform to distinguish Klebsiella species K- and O-antigen types, model antigen structures and identify hypervirulent strains. Sci. Rep. 2020;10:16732. doi: 10.1038/s41598-020-73360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu J., Wang T., Chen L., Du H. Virulence Factors in Hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2021;12:642484. doi: 10.3389/fmicb.2021.642484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lam M.M.C., Wick R.R., Watts S.C., Cerdeira L.T., Wyres K.L., Holt K.E. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat. Commun. 2021;12:4188. doi: 10.1038/s41467-021-24448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aun E., Kisand V., Laht M., Telling K., Kalmus P., Väli Ü., Brauer A., Remm M., Tenson T. Molecular Characterization of Enterococcus Isolates From Different Sources in Estonia Reveals Potential Transmission of Resistance Genes Among Different Reservoirs. Front. Microbiol. 2021;12:601490. doi: 10.3389/fmicb.2021.601490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hollenbeck B.L., Rice L.B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence. 2012;3:421–433. doi: 10.4161/viru.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rathnayake I., Hargreaves M., Huygens F. SNP diversity of Enterococcus faecalis and Enterococcus faecium in a South East Queensland waterway, Australia, and associated antibiotic resistance gene profiles. BMC Microbiol. 2011;11:201. doi: 10.1186/1471-2180-11-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Timmermans M., Bogaerts B., Vanneste K., De Keersmaecker S.C.J., Roosens N.H.C., Kowalewicz C., Simon G., Argudín M.A., Deplano A., Hallin M., et al. Large diversity of linezolid-resistant isolates discovered in food-producing animals through linezolid selective monitoring in Belgium in 2019. J. Antimicrob. Chemother. 2021;77:49–57. doi: 10.1093/jac/dkab376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee T., Jordan D., Sahibzada S., Abraham R., Pang S., Coombs G.W., O’Dea M., Abraham S. Antimicrobial Resistance in Porcine Enterococci in Australia and the Ramifications for Human Health. Appl. Environ. Microbiol. 2021;87:e03037-20. doi: 10.1128/AEM.03037-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pöntinen A.K., Top J., Arredondo-Alonso S., Tonkin-Hill G., Freitas A.R., Novais C., Gladstone R.A., Pesonen M., Meneses R., Pesonen H., et al. Apparent nosocomial adaptation of Enterococcus faecalis predates the modern hospital era. Nat. Commun. 2021;12:1523. doi: 10.1038/s41467-021-21749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.León-Sampedro R., Del Campo R., Rodriguez-Baños M., Lanza V.F., Pozuelo M.J., Francés-Cuesta C., Tedim A.P., Freitas A.R., Novais C., Peixe L., et al. Phylogenomics of Enterococcus faecalis from wild birds: New insights into host-associated differences in core and accessory genomes of the species. Environ. Microbiol. 2019;21:3046–3062. doi: 10.1111/1462-2920.14702. [DOI] [PubMed] [Google Scholar]
- 58.Chilambi G.S., Nordstrom H.R., Evans D.R., Kowalski R.P., Dhaliwal D.K., Jhanji V., Shanks R.M.Q., Van Tyne D. Genomic and phenotypic diversity of Enterococcus faecalis isolated from endophthalmitis. PLoS ONE. 2021;16:e0250084. doi: 10.1371/journal.pone.0250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holman D.B., Klima C.L., Gzyl K.E., Zaheer R., Service C., Jones T.H., McAllister T.A. Antimicrobial Resistance in Enterococcus Spp. Isolated from a Beef Processing Plant and Retail Ground Beef. Microbiol. Spectr. 2021;9:e0198021. doi: 10.1128/Spectrum.01980-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu X.Q., Wang X.M., Li H., Shang Y.H., Pan Y.S., Wu C.M., Wang Y., Du X.D., Shen J.Z. Novel lnu(G) gene conferring resistance to lincomycin by nucleotidylation, located on Tn6260 from Enterococcus faecalis E531. J. Antimicrob. Chemother. 2017;72:993–997. doi: 10.1093/jac/dkw549. [DOI] [PubMed] [Google Scholar]
- 61.Li L., Sun J., Liu B., Zhao D., Ma J., Deng H., Li X., Hu F., Liao X., Liu Y. Quantification of lincomycin resistance genes associated with lincomycin residues in waters and soils adjacent to representative swine farms in China. Front. Microbiol. 2013;4:364. doi: 10.3389/fmicb.2013.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coque T.M., Singh K.V., Weinstock G.M., Murray B.E. Characterization of dihydrofolate reductase genes from trimethoprim-susceptible and trimethoprim-resistant strains of Enterococcus faecalis. Antimicrob. Agents Chemother. 1999;43:141–147. doi: 10.1128/AAC.43.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaheer R., Cook S.R., Barbieri R., Goji N., Cameron A., Petkau A., Polo R.O., Tymensen L., Stamm C., Song J., et al. Surveillance of Enterococcus spp. reveals distinct species and antimicrobial resistance diversity across a One-Health continuum. Sci. Rep. 2020;10:3937. doi: 10.1038/s41598-020-61002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diab M., Salem D., El-Shenawy A., El-Far A., Abdelghany A., Awad A.R., El Defrawy I., Shemis M. Detection of high level aminoglycoside resistance genes among clinical isolates of Enterococcus species. Egypt. J. Med. Hum. Genet. 2019;20:28. doi: 10.1186/s43042-019-0032-3. [DOI] [Google Scholar]
- 65.Akhtar M., Hirt H., Zurek L. Horizontal transfer of the tetracycline resistance gene tetM mediated by pCF10 among Enterococcus faecalis in the house fly (Musca domestica L.) alimentary canal. Microb. Ecol. 2009;58:509–518. doi: 10.1007/s00248-009-9533-9. [DOI] [PubMed] [Google Scholar]
- 66.Ferguson D.M., Talavera G.N., Hernández L.A.R., Weisberg S.B., Ambrose R.F., Jay J.A. Virulence Genes among Enterococcus faecalis and Enterococcus faecium Isolated from Coastal Beaches and Human and Nonhuman Sources in Southern California and Puerto Rico. J. Pathog. 2016;2016:3437214. doi: 10.1155/2016/3437214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bakshi U., Sarkar M., Paul S., Dutta C. Assessment of virulence potential of uncharacterized Enterococcus faecalis strains using pan genomic approach—Identification of pathogen–specific and habitat-specific genes. Sci. Rep. 2016;6:38648. doi: 10.1038/srep38648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blöschl G., Blaschke A.P., Broer M., Bucher C., Carr G., Chen X., Eder A., Exner-Kittridge M., Farnleitner A., Flores-Orozco A., et al. The Hydrological Open Air Laboratory (HOAL) in Petzenkirchen: A hypothesis-driven observatory. Hydrol. Earth Syst. Sci. 2016;20:227–255. doi: 10.5194/hess-20-227-2016. [DOI] [Google Scholar]
- 69.Pietzka A., Murer A., Lennkh A., Hauser K., Vötsch K., Springer B., Allerberger F., Ruppitsch W. Draft Genome Sequences of Two Listeria monocytogenes Strains Isolated from Invasive Snails (Arion vulgaris) in Austria in 2019. Microbiol. Resour. Announc. 2021;10:e0037521. doi: 10.1128/MRA.00375-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. [(accessed on 19 September 2022)]. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 71.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jünemann S., Sedlazeck F.J., Prior K., Albersmeier A., John U., Kalinowski J., Mellmann A., Goesmann A., Von Haeseler A., Stoye J., et al. Updating benchtop sequencing performance comparison. Nat. Biotechnol. 2013;31:294–296. doi: 10.1038/nbt.2522. [DOI] [PubMed] [Google Scholar]
- 74.Ondov B.D., Treangen T.J., Melsted P., Mallonee A.B., Bergman N.H., Koren S., Phillippy A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016;17:132. doi: 10.1186/s13059-016-0997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neumann B., Prior K., Bender J.K., Harmsen D., Klare I., Fuchs S., Bethe A., Zühlke D., Göhler A., Schwarz S., et al. A Core Genome Multilocus Sequence Typing Scheme for Enterococcus faecalis. J. Clin. Microbiol. 2019;57:e01686-18. doi: 10.1128/JCM.01686-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Been M., Pinholt M., Top J., Bletz S., Mellmann A., van Schaik W., Brouwer E., Rogers M., Kraat Y., Bonten M., et al. Core Genome Multilocus Sequence Typing Scheme for High- Resolution Typing of Enterococcus faecium. J. Clin. Microbiol. 2015;53:3788–3797. doi: 10.1128/JCM.01946-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alcock B.P., Raphenya A.R., Lau T.T.Y., Tsang K.K., Bouchard M., Edalatmand A., Huynh W., Nguyen A.-L.V., Cheng A.A., Liu S., et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2019;48:D517–D525. doi: 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer; New York, NY, USA: 2016. [Google Scholar]
- 79.Yu G., Smith D.K., Zhu H., Guan Y., Lam T.T.-Y. ggtree: An r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017;8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- 80.Liu B., Zheng D., Jin Q., Chen L., Yang J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2018;47:D687–D692. doi: 10.1093/nar/gky1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carattoli A., Hasman H. PlasmidFinder and In Silico pMLST: Identification and Typing of Plasmid Replicons in Whole-Genome Sequencing (WGS) Methods Mol. Biol. 2020;2075:285–294. doi: 10.1007/978-1-4939-9877-7_20. [DOI] [PubMed] [Google Scholar]
- 82.Wick R.R., Heinz E., Holt K.E., Wyres K.L. Kaptive Web: User-Friendly Capsule and Lipopolysaccharide Serotype Prediction for Klebsiella Genomes. J. Clin. Microbiol. 2018;56:e00197-18. doi: 10.1128/JCM.00197-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waters N.R., Abram F., Brennan F., Holmes A., Pritchard L. Easy phylotyping of Escherichia coli via the EzClermont web app and command-line tool. Access Microbiol. 2020;2:acmi000143. doi: 10.1099/acmi.0.000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joensen K.G., Tetzschner A.M., Iguchi A., Aarestrup F.M., Scheutz F. Rapid and Easy In Silico Serotyping of Escherichia coli Isolates by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2015;53:2410–2426. doi: 10.1128/JCM.00008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.EFSA (European Food Safety Authority) Aerts M., Battisti A., Hendriksen R., Kempf I., Teale C., Tenhagen B.-A., Veldman K., Wasyl D., Guerra B., et al. Technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food-producing animals and food. EFSA J. 2019;17:e05709. doi: 10.2903/j.efsa.2019.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the manuscripts or the Supplementary Materials.