Abstract
A Taenia solium metacestode cDNA expression library in the lambda ZAPII vector was screened with pooled sera from patients with neurocysticercosis. Sixty primary clones were identified and shown to belong to two classes. The clones NC-3 and NC-9 did not reveal any significant homologies to sequences deposited in the databases and were further characterized. Both recombinant antigens were expressed as glutathione S-transferase fusion proteins and applied for serological diagnosis of human cysticercosis. An enzyme-linked immunosorbent assay was established and evaluated with 27 serum samples of La Réunion and Madagascar patients with cysticercosis. Diagnosis in these patients was established with radiological and serological procedures. For antigen NC-3 a sensitivity of 96.3% and a specificity of 91.5% for the serodiagnosis were achieved. In contrast, the sensitivity of antigen NC-9 was only 33.3%.
The larval stage of the pork tapeworm Taenia solium is the causative agent of cysticercosis. Human infections occur by ingestion of eggs excreted with the feces of individuals harboring the adult tapeworm T. solium in the intestinal tract. Infection may result from contaminated food or water or, alternatively, through the anus-to-mouth auto-infective route. After ingestion the eggs are induced to hatch (34), and the hatched larvae, called oncospheres, subsequently penetrate the intestinal mucosa. The larvae migrate throughout the body, invade skeletal muscle, subcutaneous tissue, or the central nervous system (CNS) and encyst to form cysticerci. Infection of the CNS is called neurocysticercosis (NCC), which is the most frequent parasitosis of the CNS (4, 34). In Mexico, Central and South America, and parts of sub-Saharan Africa, as well as in the non-Muslim islands of the Indian Ocean, including Madagascar and La Réunion, NCC is endemic (7, 9, 14, 17, 22). In 1987, a survey conducted with an unbiased sample of sera from inhabitants living on La Réunion indicated a high prevalence (8.2%) of serum samples reacting with cysticercal antigens (14). In addition, La Réunion is characterized by the virtual absence of any other major parasitosis (14, 29). Due to the high numbers of immigrants from endemic areas as well as increased international travel the occurrence of NCC is no longer restricted to developing countries (34). This is especially true for parts of the southwestern United States with large immigrant populations from Latin America (12, 28).
Clinical manifestations of cerebral cysticercosis include seizures, hydrocephalus, and mental disorders (1, 24, 32). However, no pathognomonic clinical manifestations exist, and the observation of patients remaining asymptomatic despite long-term infections precludes a reliable diagnosis based on clinical criteria alone (32, 34). Treatment of cysticercosis with praziquantel or albendazol should be individualized and governed by factors such as location of cysts, the degree of host inflammatory response, and the presence of seizures or hydrocephalus (24, 31). Neuroimaging studies such as computed tomography (CT) or MRT are the main methods for diagnosing NCC (3, 5). However, these methods may lead to false-positive results, since neuroimaging studies may show nonspecific findings (5); also, CT scanning fails in some cases with intraventricular cysts (26). In addition, such sophisticated diagnostic procedures are too cost-intensive to determine the prevalence of the disease in different populations or to evaluate new therapeutic approaches in the endemic areas (5, 29). This provides the impetus for developing cost-effective sensitive and specific immunodiagnostic tests. Currently, the most effective method for the detection of specific anticysticercal antibodies in serum is the enzyme-linked immunotransfer blot (EITB). According to the Centers for Disease Control and Prevention, the EITB has a specificity of 100%, a sensitivity of 98% for patients with multiple cerebral lesions, and a sensitivity of 60 to 85% for patients with a single cystic lesion (33). In addition, we have developed an EITB demonstrating a 100% sensitivity and specificity depending on the parasite involution stage (16). Recently, a commercially available enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against T. solium cysticerci in serum was evaluated (30). This test has a lower sensitivity and specificity than EITB (30). Both detection methods require whole cysticerci as their source of antigen. These usually have to be dissected from the muscles of heavily infested pigs. In the case of the EITB, crude antigen (16) or purified antigen (2, 33) is used. The ELISA uses cyst fluid from the cysticerci (30) or crude extract as antigen (13, 15). Since antigens from T. solium cysticerci are scarce and difficult to obtain, antigens from the related species Taenia crassiceps either in a crude or recombinant form were tested as a substitute for those of T. solium (6, 8). Here, we describe the use of recombinant antigens from the larval stage of the tapeworm T. solium for the highly sensitive and specific serological diagnosis of cysticercosis.
MATERIALS AND METHODS
Isolation and characterization of the recombinant antigen used in this study.
Antigens NC-3 and NC-9 were isolated from a cDNA expression library of the larval stage of T. solium by immunoscreening with pooled sera obtained from three patients suffering from neurocysticercosis. For construction of the cDNA library, 100 μg of total RNA was isolated from T. solium cysticerci. The cysticerci were dissected from the muscles of infested pigs from Madagascar. The cDNA was synthesized, starting from a total amount of 5 μg of mRNA, with the Stratagene cDNA synthesis kit by using Moloney murine leukemia virus reverse transcriptase and ligated unidirectionally into the lambda ZAPII vector (Stratagene, Heidelberg, Germany) as previously described (19, 20). After being packaged in vitro, Escherichia coli SURE cells (Stratagene) were infected. Expression of the recombinant antigens in E. coli SURE cells was performed as previously described (11, 19). Immunoscreening with the pooled sera was carried out with peroxidase-conjugated goat anti-human antibody. Horseradish peroxidase signal was detected by enhanced chemiluminescence with ECL Western blotting products (Amersham Life Science, Little Chalfont, England) according to the instructions of the manufacturer. Two individual cDNA clones, NC-3 and NC-9, were isolated and sequenced by using the T7 DNA sequencing kit (Pharmacia, Heidelberg, Germany).
Synthesis and purification of glutathione S-transferase (GST) fusion proteins.
For expression of fusion proteins the putative open reading frames (ORFs) of NC-3 and NC-9 were amplified by PCR and cloned in frame into the BamHI and EcoRI sites of the expression vector pGEX-3X (Pharmacia Biosystems). The sequence and the integrity of the junctions between vector and inserts were confirmed by sequence analysis. Purification of the glutathione fusion proteins was performed as previously described (11, 19).
ELISA for the diagnosis of cysticercosis.
Polystyrene microtiter plates (Dynex Laboratory, McLean, Va.) were coated overnight at 4°C with 100 μl of fusion protein per well (1 μg/ml in phosphate-buffered saline [PBS], pH 7.4). Wells were washed five times with 100 μl each of 5% (wt/vol) bovine serum albumin (BSA)–0.05% Tween 20 in PBS and subsequently saturated for 1 h at 37°C with washing buffer, followed by five additional washes. Then, 100 μl of patient sera, diluted 1:200 in washing buffer, was added, and the mixture was left for 2 h at 37°C. Plates were subsequently washed five times. The presence of antibodies in the sera directed against antigen NC-3 or NC-9 was detected by the addition of 100 μl of horseradish peroxidase-labeled goat anti-human immunoglobulin G (Biosys) diluted 1:2,000 in washing buffer and incubated for 2 h at 37°C, followed by six washes and then one final wash with 5% BSA in PBS. Next, 100 μl of substrate solution was added. The substrate solution consisted of 0.48 mg of o-phenylenediamine (Sigma, Deisenhofen, Germany) per ml and 0.036% H2O2 in 100 mM sodium citrate buffer (pH 5.5). After 30 min at 37°C the reaction was stopped with 100 μl of 2.5 N H2SO4. The extinction was measured at 492 nm in a Multiscan Plus reader (Labsystems, Helsinki, Finland). All serum samples were tested in triplicate. In order to assess the reproducibility the mean (m) and the standard deviation (ς) for positive and negative controls were calculated in 19 consecutive independent tests. The coefficient of variation was calculated as follows: ς × 100/m. As a control, all sera were tested in an additional ELISA which was identical to the one described above, except that the fusion proteins were replaced by GST as the antigen to eliminate false-positive reactions due to recognition of GST alone by the serum. The extinction values obtained for the control assays were subtracted from the values for the ELISA, which was performed with the recombinant antigens. The cutoff value was determined as the mean value for 38 negative sera tested in each ELISA plus two standard deviations.
ELISA for the serological diagnosis of cystic and alveolar echinococcosis.
Sera from patients with NCC were tested in an ELISA for the serological differentiation between cystic and alveolar echinococcosis by use of the recombinant antigens EM10 and EG55 from the larval stage of the cestodes Echinococcus multilocularis and Echinococcus granulosus as previously described (11).
Patient sera.
A collection of 27 serum samples from cysticercosis patients was subjected to serologic testing with the recombinant antigens. Of these 27 serum samples, 9 were from La Réunion cysticercosis patients and the remaining 18 were from Madagascar cysticercosis patients. All of the 27 samples reacted positive in an EITB and ELISA with crude (29) or purified cysticercal antigen (33). All of the La Réunion sera were from CT-confirmed NCC cases. Of the 18 serum samples from Madagascar, 6 were CT-confirmed NCC cases. Analysis of stool samples from the 27 patients with NCC did not reveal the presence of any intestinal parasites. Analysis of multiple urine samples from the 18 patients with NCC from Madagascar did not demonstrate any Schistosoma haematobium eggs. The following 35 serum samples from patients with other confirmed parasitic infections were tested in the NC-3 ELISA as controls: Plasmodium falciparum (n = 4), Hymenolepis nana (n = 3), Ascaris lumbricoides (n = 4), Trichuris trichuris (n = 4), Wuchereria bancrofti (n = 3), Taenia sp. (n = 3), Schistosoma mansoni (n = 4), S. haematobium (n = 4), Echinococcus multilocularis (n = 4), and simultaneous infections with A. lumbricoides and H. nana (n = 1) and with A. lumbricoides, T. trichuris, and Taenia sp. (n = 1). An additional 38 serum samples were from individuals from Madagascar and La Réunion island with no clinical sign of a parasitic infection and a normal CT scan. These samples were negative in the previously described ELISA and EITB analyses for the detection of anticysticercal antibodies (29).
RESULTS AND DISCUSSION
Immunoscreening of T. solium cysticercal cDNA library and characterization of immunogenic clones.
Pooled sera from three inhabitants of Madagascar affected by cerebral cysticercosis were used for immunoscreening of a T. solium metacestode cDNA library. The sera were chosen because they exhibited high reactivity against antigens from cysticerci and displayed a heterogeneous band pattern in Western blot analysis (data not shown) (29). Sixty immunogenic clones were isolated by screening 4 × 103 recombinant phages. Cross-hybridization analysis revealed that the 60 immunoreactive phages harbored inserts belonging to a class of two individual clones named NC-3 and NC-9 (GenBank accession no., AJ012669 and AJ012670). Clone NC-3 contained a putative ORF of 419 bp coding for a protein with a theoretical molecular mass of 8 kDa (data not shown). A FASTA search of the GenBank database (21) did not reveal any significant homologies to known sequences (data not shown). Clone NC-9 contained a putative ORF of 429 bp coding for a protein with a theoretical molecular mass of 13 kDa (data not shown). As with NC-3, NC-9 did not demonstrate any homology to sequences deposited in the databases. Both antigens were expressed as GST-fusion proteins and were purified by affinity chromatography by using their glutathione moieties.
ELISA with the recombinant antigens NC-3 and NC-9.
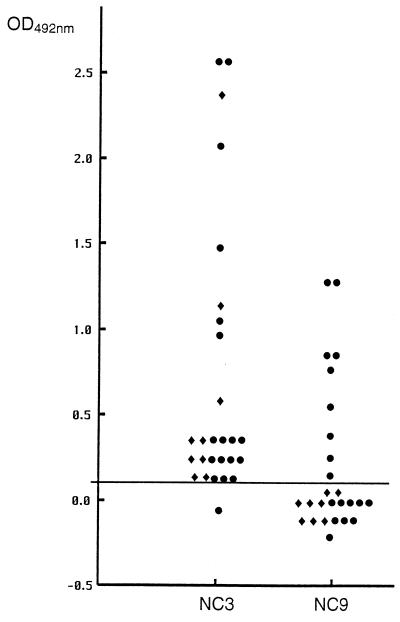
To determine the cutoff value for both ELISAs, we tested 38 serum samples from healthy inhabitants of Madagascar and La Réunion island who did not present signs of a parasitic infection. The mean plus two standard deviations was subsequently chosen as the cutoff. As shown in Fig. 1, 26 of 27 serum samples (96.3%) from cysticercosis patients living in La Réunion or Madagascar reacted with the recombinant antigen NC-3. All of the serum samples from the CT-confirmed cases of NCC provided by inhabitants of La Réunion island reacted with antigen NC-3 (Fig. 1). Previously, it has been reported that false-positive results in the serodiagnosis of NCC can result from cysticerci localized outside the CNS or, alternatively, from the presence of the adult tapeworm T. solium in the intestinal tract (23, 25). None of the patients from La Réunion either had a history of subcutaneous cysts or demonstrated cysts upon physical examination. Furthermore, the examination of stool samples did not reveal the presence of any intestinal parasites. Based on the CT data obtained from these patients and the fact that other major parasitic diseases are virtually absent in La Réunion (14, 29), therefore, the positive reaction in the ELISA with the recombinant antigen NC-3 was thought to be due to the localization of cysts in the CNS. Of the 18 serum samples, from patients with cysticercosis living in Madagascar, 17 (94%) reacted with antigen NC-3 (Fig. 1). Of these 17 positive samples, 6 were from CT-confirmed cases of NCC. The one nonreactive serum sample in the NC-3 ELISA was from a case not confirmed by CT (data not shown). Since all of the serum samples from Madagascar tested positive in the EITB conducted at the Institut Pasteur de Madagascar and were also found to be positive at the Centers for Disease Control and Prevention, the discrepancy between the ELISA with antigen NC-3 and the EITB remains unclear. Three patients from Madagascar presented with a history of subcutaneous nodules. Although these were never diagnosed as cysticercus through biopsy, we cannot rule out the possibility that the positive reaction with antigen NC-3 was caused by antibodies directed against these subcutaneously localized cysts.
FIG. 1.
Extinction values obtained in the NC-3 and NC-9 ELISAs with 27 serum samples from patients with cysticercosis. Symbols: ⧫, patients from La Réunion; ●, patients from Madagascar. The cutoff value, indicated by the horizontal line, was determined for both ELISAs by testing 38 negative sera. All sera were tested three times with identical results.
In contrast to NC-3, only 9 of the 27 serum samples (33.3%) reacted with the recombinant antigen NC-9 (Fig. 1). Thus, antigen NC-3 was used for further studies. The variation coefficient for representative positive control sera calculated in 19 independent tests was 3.2%, thus demonstrating the high reproducibility of this test. This coefficient could not be calculated for the negative control sera, as the mean optical density value was approaching 0.0.
Thirty-five serum samples from patients with other parasitic infections were used in the study for cross-reactivities. Of the 35 samples, 3 (8.5%) reacted with antigen NC-3. Among these three samples provided by inhabitants from Madagascar, one came from a patient with P. falciparum malaria, one came from a patient with documented S. mansoni infection, and one came from a patient with intestinal taeniasis. Due to the high prevalence of cysticercosis in Madagascar (10, 17), we cannot rule out the possibility of a clinical nonapparent cysticercosis (1). However, these three patients were not available for follow-up examinations. None of the four serum samples from patients with alveolar echinococcosis reacted with antigen NC-3. Cross-reactions decreased the specificity (91.5%), which was 100% in patients without parasitic diseases when antigen NC-3 was used in the ELISA.
Previously, we reported the usefulness of recombinant antigens for the serological differentiation between alveolar and cystic echinococcosis (11). In that study we showed that 17.6% of sera from patients with NCC cross-reacted with the recombinant echinococcal antigen EM10 (11). The 27 serum samples from the patients with cysticercosis used in the present investigation were evaluated for cross-reactions with the recombinant antigens EM10 and EG55 from the larval stage of the tapeworms E. multilocularis and E. granulosus. Only 1 of the 27 cysticercosis sera of the present study (3.7%) reacted with antigen EM10 from E. multilocularis causing alveolar echinococcosis. In accordance with the previous study, none of the tested sera reacted with antigen EG55 from E. granulosus causing cystic echinococcosis (data not shown). NCC and alveolar echinococcosis can usually be easily distinguished by clinical criteria. In the past, extensive serologic cross-reactions were reported with sera from patients with cystic echinococcosis and cysticercosis (27). Thus, the recombinant antigens NC-3 and EG55 may provide means for the serological differentiation between cysticercosis and cystic echinococcosis.
Human cysticercosis is a pleomorphic disease, and diagnosis remains a challenge. Simple and reliable serological methods are required in order to permit diagnosis and to gain information about the prevalence of this disease. It was shown previously that ELISAs with crude, unfractionated antigens are of limited value for serodiagnosis (23). Currently, EITB appears to be the most reliable method for detecting anticysticercal antibodies in serum (16, 29, 33). However, a common problem faced by manufacturers of immunodiagnostic kits is the difficulty in obtaining T. solium cysticerci that provide sufficient antigen of constant quality. The application of recombinant DNA methods may alleviate problems with quality control and supply of antigens derived directly from parasites. By using the recombinant antigen NC-3 from T. solium cysticerci in an ELISA for the serodiagnosis of cysticercosis, a high sensitivity almost comparable to the sensitivity of the EITB as the gold standard was achieved. Production of antibodies against cysticerci localized within the CNS may be restricted to within the blood-brain barrier (18, 24). Thus, we are currently evaluating whether we can further improve the accuracy of the NC-3 ELISA in the detection of NCC through cerebrospinal fluid analysis.
ACKNOWLEDGMENT
This work was supported by grant Fr689/9-2 from Deutsche Forschungsgemeinschaft (M.F.).
REFERENCES
- 1.Andriantsimahavandy A, Lesbordes J L, Rasoaharimalala B, Peghini M, Rabarijaona L, Roux J, Boisier P. Neurocysticercosis: a major aetiological factor of late-onset epilepsy in Madagascar. Trop Med Int Health. 1997;8:741–746. doi: 10.1046/j.1365-3156.1997.d01-379.x. [DOI] [PubMed] [Google Scholar]
- 2.Andriantsimahavandy A, Michelaut A, Andrianirina W, Rakotondrazaka J, Rabarijaona L, Boisier P, Chanteau S. 4èmes Actualités du Pharo et de l’Hopital Laveran, Marseille, France. 1997. Evaluation d’un antigène glycoprotéique purifié pour le diagnostic de la cysticercose: application à la séroépidémiologie. [Google Scholar]
- 3.Camargo C A, Maeshall W H. Radiological diagnosis of neurocysticercosis. Parasitol Today. 1987;3:30–31. doi: 10.1016/0169-4758(87)90097-4. [DOI] [PubMed] [Google Scholar]
- 4.Del Brutto O H. Neurocysticercosis. Curr Opin Neurol. 1997;10:268–272. doi: 10.1097/00019052-199706000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Del Brutto O H, Wadia N H, Dumas M, Cruz M, Tsang V C W, Schantz P M. Proposal of diagnostic criteria for human cysticercosis and neurocysticercosis. J Neurol Sci. 1996;42:1–6. doi: 10.1016/0022-510x(96)00130-x. [DOI] [PubMed] [Google Scholar]
- 6.Fischer C, Nosratian R, Habtemichael N, Tolle R, Riemenschneider V, Geyer E. Preparation and sequence analysis of Taenia crassiceps metacestode recombinant antigens with potential for immunodiagnosis of human cerebral cysticercosis. Trop Med Parasitol. 1994;45:324–328. [PubMed] [Google Scholar]
- 7.Flisser A. Neurocysticercosis in Mexico. Parasitol Today. 1988;4:131–137. doi: 10.1016/0169-4758(88)90187-1. [DOI] [PubMed] [Google Scholar]
- 8.Garcia E, Ordonez G, Sotelo J. Antigens from Taenia crassiceps cysticerci used in complement fixation, enzyme-linked immunosorbent assay, and Western blot (immunoblot) for diagnosis of neurocysticercosis. J Clin Microbiol. 1995;33:3324–3325. doi: 10.1128/jcm.33.12.3324-3325.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia H H, Gilman R H, Tovar M A, Flores E, Jo R, Tsang V C W, Diaz F, Torres P, Miranda E The Cysticercosis Working Group in Peru. Factors associated with Taenia solium cysticercosis: analysis of nine hundred forty-six Peruvian neurologic patients. Am J Trop Med Hyg. 1995;52:145–148. doi: 10.4269/ajtmh.1995.52.145. [DOI] [PubMed] [Google Scholar]
- 10.Grill J, Rakotomalala, Andriantsimahavandy A, Boisier P, Guyon P, Roux J, Esterre P. High prevalence of serological markers among epileptic Malagasy children. Ann Trop Pediatr. 1996;16:185–191. doi: 10.1080/02724936.1996.11747824. [DOI] [PubMed] [Google Scholar]
- 11.Helbig M, Frosch P, Kern P, Frosch M. Serological differentiation between cystic and alveolar echinococcosis by use of recombinant larval antigens. J Clin Microbiol. 1993;31:299–303. doi: 10.1128/jcm.31.12.3211-3215.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormick G F, Zee C S, Heiden J. Cysticercosis cerebri. Review of 127 cases. Arch Neurol. 1982;39:534–539. doi: 10.1001/archneur.1982.00510210004002. [DOI] [PubMed] [Google Scholar]
- 13.Michault A, Coubes P, Laporte J P, Bouillant-Linet E, Leroy D. Diagnostic de la cysticercose encéphalique à l’ile de la Réunion par méthode immunoenzymologique. Pathol Biol. 1986;36:266–270. [PubMed] [Google Scholar]
- 14.Michault A, Duval G, Bertil G, Folio G. Etude séroépidémiologique de la cysticercose à l’ile de la Réunion. Bull Soc Pathol Exot. 1990;83:82–97. [PubMed] [Google Scholar]
- 15.Michault A, Leroy D, Coubes P, Laporte J P, Bertil G, Mignard C. Diagnostic immunologique dans le liquide céphaloarachidien et le sérum de la cysticercose encéphalique évolutive. Pathol Biol. 1989;37:249–253. [PubMed] [Google Scholar]
- 16.Michault A, Riviere B, Fressy P, Laporte J P, Bertil G, Mignard C. Apport de l’enzyme-linked immuno-electrotransfert blot assay au diagnostic de la neurocysticercose humaine. Pathol Biol. 1990;38:119–125. [PubMed] [Google Scholar]
- 17.Michel P, Callies P, Raharison H, Guyon P, Holvoet L, Genin C. Epidémioloie de la cysticercose à Madagascar. Bull Soc Pathol Exot. 1993;86:62–67. [PubMed] [Google Scholar]
- 18.Miller B L, Staugaitis S M, Tourtellotte W W, Shapshak P, Goldberg M, Heiner D, Weil M. Intra-blood-brain-barrier IgG synthesis in cerebral cysticercosis. Arch Neurol. 1985;42:782–784. doi: 10.1001/archneur.1985.04210090046013. [DOI] [PubMed] [Google Scholar]
- 19.Mühlschlegel F, Sygulla L, Frosch P, Massetti P, Frosch M. Paramyosin of Echinococcus granulosus: cDNA sequence and characterization of a tegumental antigen. Parasitol Res. 1993;79:660–666. doi: 10.1007/BF00932508. [DOI] [PubMed] [Google Scholar]
- 20.Mühlschlegel F, Frosch P, Castro A, Apfel H, Müller A, Frosch M. Molecular cloning and characterization of an Echinococcus multilocularis and Echinococcus granulosus stress protein homologous to the mammalian 78-kDa glucose-regulated protein. Mol Biochem Parasitol. 1995;74:245–250. doi: 10.1016/0166-6851(95)02501-4. [DOI] [PubMed] [Google Scholar]
- 21.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preux P M, Melaku Z, Druet-Cabanac M, Avode G, Grunitzky E K, Bouteille M, Dumas M. Cysticercosis and neurocysticercosis in Africa: current status. Neurol Infect Epidemiol. 1996;1:63–68. [Google Scholar]
- 23.Ramos-Kuri M, Montoya R M, Padilla A, Govezensky T, Diaz M L, Sciutto E, Sotelo J, Larralde C. Immunodiagnosis of neurocysticercosis. Disappointing performance of serology (enzyme-linked immunosorbent assay) in an unbiased sample of neurological patients. Arch Neurol. 1992;49:633–636. doi: 10.1001/archneur.1992.00530300069012. [DOI] [PubMed] [Google Scholar]
- 24.Rolfs A, Mühlschlegel F, Jansen-Rosseck R, Martins A R, Bedaque E A, Tamburus W M, Pedretti L, Schulte G, Feldmeier H, Kremsner P. Clinical and immunologic follow-up study of patients with neurocysticercosis after treatment with praziquantel. Neurology. 1995;45:532–538. doi: 10.1212/wnl.45.3.532. [DOI] [PubMed] [Google Scholar]
- 25.Rosas N, Sotelo J, Nieto D. ELISA in the diagnosis of neurocysticercosis. Arch Neurol. 1989;43:353–356. doi: 10.1001/archneur.1986.00520040039016. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld E A, Byrd S E, Shulman S T. Neurocysticercosis among children in Chicago. Clin Infect Dis. 1996;23:262–268. doi: 10.1093/clinids/23.2.262. [DOI] [PubMed] [Google Scholar]
- 27.Schantz P M, Shanks D, Wilson M. Serologic cross-reactions with sera from patients with echinococcosis and cysticercosis. Am J Trop Med Hyg. 1980;29:609–612. doi: 10.4269/ajtmh.1980.29.609. [DOI] [PubMed] [Google Scholar]
- 28.Shandera W X, White A C, Jr, Chen J C, Diaz P, Armstrong R. Neurocysticercosis in Houston, Texas. A report of 112 cases. Medicine. 1994;73:37–52. doi: 10.1097/00005792-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Simac C, Michel P, Andriantsimahavandy A, Esterre P, Michault A. Use of enzyme-linked immunosorbent assay and enzyme-linked immunoelectrotransfer blot for the diagnosis and monitoring of neurocysticercosis. Parasitol Res. 1995;81:132–136. doi: 10.1007/BF00931618. [DOI] [PubMed] [Google Scholar]
- 30.Sloan L, Schneider S, Rosenblatt J. Evaluation of enzyme-linked immunoassay for serological diagnosis of cysticercosis. J Clin Microbiol. 1995;33:3124–3128. doi: 10.1128/jcm.33.12.3124-3128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sotelo J, Del Brutto O H, Penagos P, Escobedo F, Torres B, Rodriguez-Carbajal J, Rubio-Donadieu F. Comparison of therapeutic regimen of anticysticercal drugs for parenchymal brain cysticercosis. J Neurol. 1990;237:69–72. doi: 10.1007/BF00314663. [DOI] [PubMed] [Google Scholar]
- 32.Sotelo J, Guerrero V, Rubio F. Neurocysticercosis: a new classification based on active and inactive forms. Arch Intern Med. 1985;145:442–445. [PubMed] [Google Scholar]
- 33.Tsang V C W, Brand J A, Boyer A E. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) J Infect Dis. 1989;159:50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 34.White A C., Jr Neurocysticercosis: a major cause of neurological disease worldwide. Clin Infect Dis. 1997;24:101–115. doi: 10.1093/clinids/24.2.101. [DOI] [PubMed] [Google Scholar]