Abstract
Cancer is one of the leading causes of morbidity and mortality around the globe and is likely to become the major cause of global death in the coming years. As per World Health Organization (WHO) report, every year there are over 10 and 9 million new cases and deaths from this disease. Chemotherapy, radiotherapy, and surgery are the three basic approaches to treating cancer. These approaches are aiming at eradicating all cancer cells with minimum off-target effects on other cell types. Most drugs have serious adverse effects due to the lack of target selectivity. On the other hand, resistance to already available drugs has emerged as a major obstacle in cancer chemotherapy, allowing cancer to proliferate irrespective of the chemotherapeutic agent. Consequently, it leads to multidrug resistance (MDR), a growing concern in the scientific community. To overcome this problem, in recent years, nanotechnology-based drug therapies have been explored and have shown great promise in overcoming resistance, with most nano-based drugs being explored at the clinical level. Through this review, we try to explain various mechanisms involved in multidrug resistance in cancer and the role nanotechnology has played in overcoming or reversing this resistance.
Keywords: cancer, chemotherapy, multidrug resistance, nanotechnology, nanomedicine
1. Introduction
Cancer is a global burden, and as per the latest GLOBOCAN 2020, over 19.3 and 10 million new cases and deaths occurred in 2020, respectively; female breast cancer has surpassed lung cancer and is now the most commonly diagnosed cancer (11.7%), followed by lung cancer (11.4%), colorectal cancer (10%), and prostate cancer (7.3%) [1]. In mortality, lung cancer remains at the top [1]. As per World Health Organization (WHO) statistics 2019, in 112 out of 183 countries in the world, people die of cancer before attaining the age of 70 years [2]. Despite the world having advanced in science and technology, chemotherapy remains a promising option to treat cancer [3]. Conventional chemotherapy has greatly improved the decline in the mortality rate of several dreadful cancers, but its major problem is the killing of cancerous and noncancerous cells causing serious off-target effects such as hair loss, bone marrow depression, and other toxic effects [4]. Therefore, a significant percentage of cancer-related research over the past few decades has focused on creating medications that more precisely target tumor cells rather than normal cells [5]. Precision therapy has greatly advanced thanks to the development of targeted therapy, but there are still numerous unavoidable side effects, and drug resistance has long been an issue [6].
Over 90% of failures in chemotherapy are due to the development of resistance to the already available drugs; this resistance resembles infectious disease treatment resistance and is the most challenging aspect of treating and preventing cancers [7]. This has emerged as a major obstacle and allows cancer to proliferate in presence of a chemotherapeutic agent [8]. Significant resistance develops generally to repeated treatment with one kind of anticancer agent and then develops further towards similar or completely different drugs having a similar mechanism of action. This mechanism, known as multidrug resistance (MDR), can be intrinsic or acquired [9].
To overcome this problem, in recent years, nanotechnology-based drug dosage forms have been explored, which have shown great promise [10]. Most of these nanomedicines are heading toward clinical trials [11]. Nanotechnology has been used in medicine more and more over the past few decades, including applications for safer and more efficient tumor targeting, detection, and treatment [12,13,14,15,16,17,18,19,20]. Drug delivery methods based on nanoparticles (NPs) have demonstrated a number of benefits in the treatment of cancer, including good pharmacokinetics, accurate targeting of tumor cells, a decrease in adverse effects, and reduced drug resistance [21,22,23,24,25]. Nevertheless, nanomedicine-based formulations have some demerits, such as difficulty in physical handling due to smaller size, particle aggregation, limited drug loading, and burst release [19,20]. This review outlines the different mechanisms of cancer chemotherapy resistance and describes the different mechanisms such as the use of nanotechnology in overcoming multiple drug resistance.
2. Cancer Chemotherapy Resistance and Mechanism
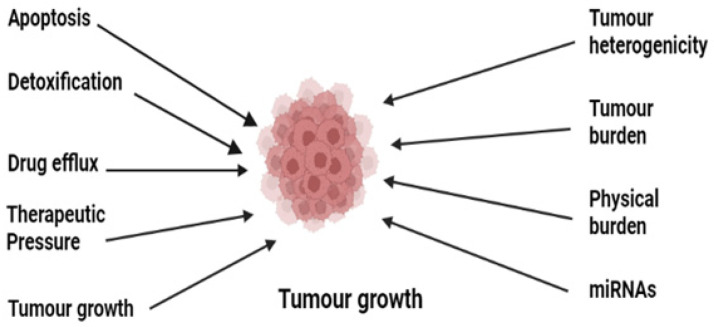
Cancer chemoresistance is a growing concern in medical oncology. Some cancers, including Hodgkin’s lymphomas, acute promyelocytic leukemia, and chronic myeloid leukemia, have been successfully understood and treated despite their complex pathophysiology [26]. The development of anticancer agents against these complex cancers has been achieved by understanding the deep mechanisms, and various drugs have been developed [7]. These mainly include the stimulation of immune response using interferon-alpha (IF-α) and inhibition of oncogenes or oncoproteins [27,28,29,30,31,32,33,34,35]. Many of them are still in practice; however, resistance has developed to the majority of them, which has ultimately affected patient survival [36]. There are various reported resistance mechanisms associated with cancer chemotherapy such as drug efflux, detoxification, stem cells, epithelial-to-mesenchymal transition, inactivation of the drugs before reaching the target, multidrug resistance, inhibiting cell death (apoptosis suppression), augmenting gene amplification and DNA repair of oncogenes, and alteration in the metabolism of drugs. Figure 1 shows the illustration of different possible mechanisms of chemotherapy resistance [37]. Drug resistance in cancer is believed to be due to intrinsic and acquired resistance; however, most cancers in clinical settings have become resistant owing to combinations of these factors [38]. In this review, we will describe a few important drug resistance mechanisms in cancer and will then focus our view on the use of nanotechnology in overcoming drug resistance in cancer.
Figure 1.
Illustration of the various possible underlying mechanisms in the development of drug resistance in cancer.
2.1. Role of Drug Efflux Pumps in Cancer Drug Resistance
The human genome encodes 48 members of drug efflux proteins called ATP-binding cassettes which are further classified into seven subgroups (ABCA, ABCB ABCC, ABCD, ABCE, ABCF, and ABCG) [39]. These proteins have a significant role in the development of drug resistance [40]. These proteins expel the drug out of the cell, thereby reducing the therapeutic concentration of the drug inside the cell [41]. Enough evidence suggests the overexpression of these proteins, especially multiple drug resistance protein 1 (MDR 1) known as P-glycoprotein, multiple drug resistance-associated protein (MDRA), and breast cancer resistant protein (BCRP), on cellular surfaces [42]. Normally these transporters help in pumping out toxins and foreign substances [43]. These transporters in general and P-pg in particular transport a range of substances including anticancer agents out of the cell, causing depletion of therapeutic concentration. Overexpression of P-pg in patients causes efflux of paclitaxel and doxorubicin and leads to resistance to these drugs [44]. This is evidenced by a study conducted on a genetically engineered mouse model (GEMM), where the tumor recurred due to upregulation of ABCB1a and b and was found to be cross-resistant to docetaxel also [39,45]. Another study shows the non-responsiveness of the tumor to olaparib, a PARP inhibitor, due to the overexpression of ABC1 a/b [46,47], thus confirming the overexpression of these efflux proteins in drug-resistant cancers.
2.2. Suppression of Apoptosis
Although apoptosis and autophagy are altogether different, they ultimately contribute to cell death [48]. Two different mechanisms in apoptosis contribute to cell death: (a) intrinsic, which involves the mitochondrial-mediated bcl2 proteins, Akt, and caspase-9, and (b) extrinsic, which involves death receptors on the cellular surface [49]. Ample evidence supports the initiation of human cancer from cancer stem cells (CSCs), and it is believed that these apoptotic pathways become dysregulated and lead to cancer chemotherapy resistance and tumor recurrence [50]. High levels of antiapoptotic proteins which are considered the hallmarks of cancer have been seen in drug-resistant cancers [51]. The antiapoptotic protein family which includes Bcl-2, Mcl-1, and Bcl-xL has been seen at raised levels compared to proapoptotic proteins Bax, Puma, Noxa, Bak, Bil, and Bid, causing an imbalance between the pro- and antiapoptotic proteins which ultimately leads to cancer drug resistance [52]. The formation of the mitochondrial apoptosis-induced channel (MAC), which is formed by binding of tBid with Bax and Bak through activated caspase-8, is hindered by the downregulation of proapoptotic and upregulation of antiapoptotic proteins, which leads to the formation of resistant cancers by inhibiting the release of cytochrome C, a key protein for electron transfer in mitochondria [53]. This overexpression of antiapoptotic proteins is responsible for drug resistance in multiple cancers [54]. Additionally, overexpression of Nf-kB, P53, and PI3/AKT cell death-related receptors is also involved in chemoresistance [55]. In addition, apoptosis evasion through aberrant autophagy is another factor in the development of multiple drug resistance [56].
2.3. Drug Inactivation
Before a drug reaches the gastrointestinal tract or systemic circulation, some drugs that are in prodrug form interact with certain proteins which partially degrade, modify, and form complexes with other endogenous substances, leading to the activation of a drug [57]. Certain cancers have developed resistance due to decreased activation of prodrugs to active drugs [58]; the most prominent example is the mutation and downregulation of phosphorylation events in the conversion of AraC into AraC-triphosphate which is used in the treatment of acute myelogenous leukemia [8,59]. Several drugs metabolizing enzymes such as uridine diphosphate-glucuronosyltransferase, the glutathione-S-transferase family, and cytochrome P450 are muted one way or another and ultimately lead to resistance to already available drugs [60]. The overactivity of cytochrome p450 has been reported to lead to its resistance in breast cancer [61]. Detoxification of drugs by overproduction of glutathione has led to the development of resistance to many platinum compounds and alkylating agents such as cisplatin and doxorubicin [62]. Thus, mutations in phase I and phase II reactions either reduce the activity of drugs by increasing their detoxification or lead to the development of drug resistance by inactivating certain drugs.
2.4. Role of miRNAs in Cancer Drug Resistance
miRNAs are processed from RNA hairpin structures, which regulate genes in cancer, especially in resistant ones [63]. They are involved in apoptosis, cellular proliferation, stress tolerance, the cell cycle, and immune response [64]. Around 30% of human genes are regulated by miRNAs and have a role in tumor development [63]. Some act as protumor genes, some act as suppressor genes, and some act as both [65]. Studies conducted by various researchers provide evidence of miRNAs being involved in cancer drug resistance by either enhancing tumor cancer genes or having involvement in genes that are related to apoptosis, cellular proliferation, and the cell cycle [66]. Due to their tissue specificity, one kind of microRNA could be targeted by multiple microRNAs; hence the same miRNA can either promote or inhibit resistance to chemotherapy [67,68]. In breast cancer, upregulation of miRNA-21 downregulates phosphatase tensin homolog (PTEN) and thereby decreases the susceptibility of doxorubicin to cancer cells, while overexpression of PTEN inhibits miRNA-21 and reduces the resistance of breast cancer cells to doxorubicin [69]. Table 1 reports some of the miRNAs that regulate cancer chemotherapeutic drug resistance [70,71,72,73,74,75,76,77,78,79,80].
Table 1.
List of some miRNAs that regulate cancer chemoresistance.
| miRNA | Target | Cancer Type | Drug Target | Reference |
|---|---|---|---|---|
| miR-7 | MDR1 | SCLC | Anthracyclines | [70] |
| miR-21 | PTEN | Breast | Trastuzumab | [71] |
| miR-20a | MAPK1 | Colorectal | 5-Fluorouracil | [72] |
| miR-103/107 | P-gp | Gastric | Doxorubicin | [73] |
| miR-196a | MDR1/MRP1 | NSCLC | Cisplatin | [74] |
| miR-17-5p | PHIPP2 | MCL | Topotecan | [75] |
| microRNA-34a | SIRT1, Bcl-2 | Prostate | Paclitaxel | [76] |
| miR-96 | XIAP | Colorectal | 5-Fluorouracil | [77] |
| miR-499a | UBE2V2 | Cervical | 5-Fluorouracil | [78] |
| miR-RNA-449 | NOTCH1 | Ovarian | Doxorubicin | [79] |
| miR-320c | SMARCC1 | Pancreatic | Gemcitabine | [80] |
Abbreviations: MDR1: multidrug resistance mutation 1; PTEN: phosphatase tensin homolog; MAPK1: mitogen-activated protein kinase 1; P-gp: P-glycoprotein; MRP1: multidrug resistance-associated protein 1; PHIPP2: phage phi-PP2; SIRT1: sirtuin 1; XIAP: X-linked inhibitor of apoptosis protein; Bcl-2: B-cell lymphoma-2; UBE2V2: ubiquitin conjugated enzyme E2V2; NOTCH1: human gene; SMARCC1: protein; SCLC: small cell lung carcinoma; NSCLC: non-small-cell lung carcinoma; MCL: mantle cell lymphoma.
2.5. Tumor Microenvironment (TME)
The TME leads to the development of resistance by providing an environment rich in the stroma, immune cells, and vasculature which helps in the development of resistance by several mechanisms such as hampering drug absorption, restricting immune clearing of cancer cells, and stimulating factors for cancer cell proliferation [81]. Lactic acid produced by intermediate glycolytic intermediates results in a change in pH in cancer cells; this change in pH gradient results in neutralization and protonation of certain anticancer agents such as doxorubicin, thereby preventing them from entering the target site [82,83]. Hypoxia is a key factor in cancer drug resistance; its transcription factor hypoxia-inducible factor (HIF) is expressed in many cancers [84], which is supported by numerous studies involving HIF inhibitors as chemosensitizing agents in cancer. The TME also helps tumors to become resistant by providing an environment for metabolic reprogramming, DNA repair, and the immune microenvironment [85].
3. Nanotechnology and Cancer
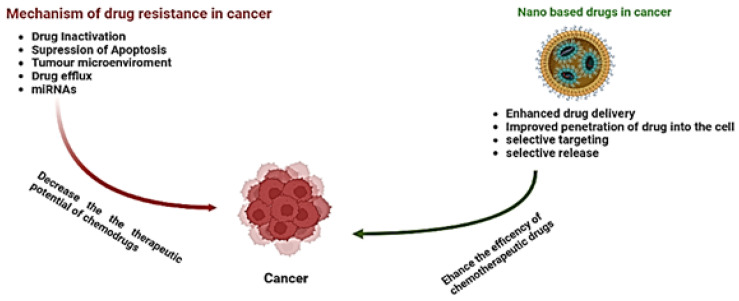
Nanotechnology is an interdisciplinary field that has recently emerged as one of the most promising fields in the treatment of cancer [86]. Owing to this, an urgent need arises for the development of novel and innovative technologies and methodologies that could assist in characterizing tumors, recognizing micrometastasis and residuary tumor cells, and ascertaining whether or not a certain tumor has been removed completely [87]. The focus on nanotechnology for in vitro diagnostics and drug delivery has surged in recent years. This technology comprises only a few sections, albeit analytic ones, which are being assembled to triumph over the war against cancer [88]. Studies focusing on nano-based drugs have achieved tremendous achievements in reversing drug resistance either by active or passive mechanisms [89]. These nano-based drugs have successfully improved the efficacy of drugs, reduced off-target effects, and overcome drug resistance [90]. The advantage of nano-based drugs over conventional therapies is the selectivity of the target [91]. Over time, a number of nanoparticles have been designed and studied, such as metal nanoparticles, polymer-based nanoparticles, and nanovesicles such as liposomes and dendrimers which have greatly overcome chemoresistance in cancer [10]. Figure 2 and Figure 3 show the possible mechanisms of resistance and how nanotechnology helped in achieving efficient chemotherapy and overcoming resistance.
Figure 2.
Illustration of the influence of nanotechnology on multidrug resistance in cancer.
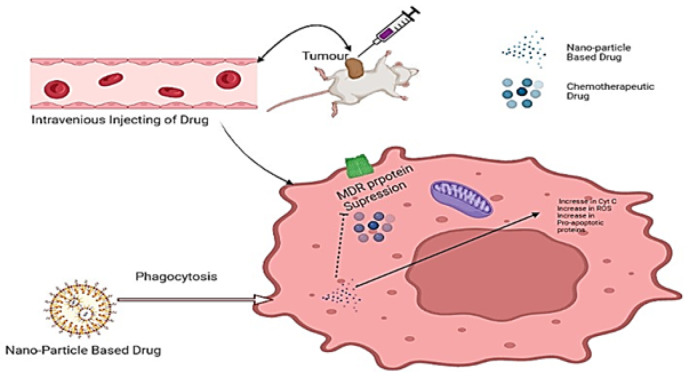
Figure 3.
Illustration of the use of a nanoparticle-based drug delivery system in overcoming multidrug-resistant cancer.
As already discussed, chemotherapy faces several unavoidable problems such as short half-life, cytotoxicity, lack of selective targeting, poor solubility, and multiple drug resistance. Nanomaterial-based chemotherapy, chemodynamic therapy (CDT), photodermal therapy (PDT), molecular therapy, sonodynamic therapy (SDT), photothermal therapy (PTT), and targeted therapy are currently being used in cancer treatment. In addition, an ample number of studies on certain therapies such as signal modification therapy, immunotherapy, nucleic-acid-based therapy, anti-angiogenesis therapy, therapies regulating apoptosis, and molecular therapy have been conducted in recent years [92]. Table 2 summarizes the different types of nanoparticles that have been explored in cancer research [93,94,95,96,97,98,99,100].
Table 2.
Type of nanoparticles in cancer research.
| Modification | Payload | Therapy Involved | Outcome | Reference |
|---|---|---|---|---|
| PLGA NP | PTX | Chemotherapy | There was improved efficiency in drug delivery compared with free PTX | [93] |
| PEG, transferrin-modified NP | Nucleic acids | Nucleic-acid-based therapy | Transfected leukemia cells with K562 showed high efficiency compared to nontargeted particles | [94] |
| Trastuzumab-modified NP | Docetaxel | Targeted therapy, chemotherapy | There was an overall increase in cytotoxicity in HER2-positive BT474 cells with no or minimal effect in but not in HER2-negative MCF7 cells | [95] |
| Trastuzumab-modified NP | PTX | Targeted therapy, chemotherapy | There was much better efficacy in treatment with low cytotoxicity to human breast epithelial cells | [96] |
| PLGA NP | Alantolactone Erlotinib | Targeted therapy | Significant induction of apoptosis was seen in cancer treated with NP-loaded drug | [97] |
| Exosome | Doxorubicin | Chemotherapy | Accumulation of the drug in heart of mice was reduced and an increase in cytotoxicity of doxorubicin was seen | [98] |
| Gold NP-encapsulated IONPs/Ag cores | ONPs/Ag | PTT | Gold NP complex acted | [99] |
| Trithiol-terminated poly-meth-acrylic acid-modified nanorods | Fe2P | SDT, PTT | It showed photodermal and therapeutic potential | [100] |
Abbreviations: PLGA: poly(lactic-co-glycolic) acid; NP: nanoparticle; PEG: polyethylene glycol; IONPs: iron oxide nanoparticles; Ag: silver; PTX: paclitaxel; ONPs: organic nanoparticles; Fe2P: iron phosphide; PTT: photothermal therapy; SDT: sonodynamic therapy.
3.1. Targeting Mechanism of Nanoparticles in Chemotherapy
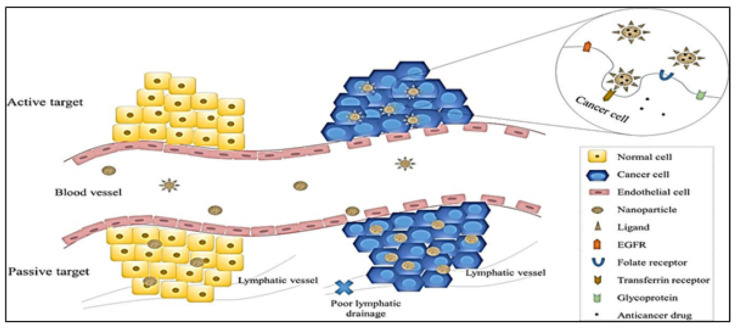
Several studies have been performed to determine the mechanism through which nanoparticles execute their action against tumor cells. Before knowing the mechanism of action, it is crucial to know the interaction between cancer cells and nanoparticle-based drugs. Nanocarriers execute their mechanism of action in two ways, namely active targeting and passive targeting. Figure 4 illustrates the two types of targeting mechanisms of nanoparticles in cancer.
Figure 4.
Schematic diagram of active and passive targeting of nanocarriers in cancer cells. Image reproduced with permission from reference [101].
3.1.1. Passive Targeting
Shreds of evidence support that the proliferation of tumor cells causes neovascularization and very large pores in vessels, leading to a decrease in the permeability of tumor cells compared to normal cells [102]. Passive targeting is achieved by the enhanced permeability and retention (EPR) (one of the driving forces for passive targeting) effect caused by retention of NPs due to poor lymphatic drainage associated with cancer cells, thus allowing nanocarriers to release the drug at the target site [103]. In addition, this EPR effect is achieved by the small particle size of NPs, which have better permeability [104,105] compared to larger particles such as conventional drugs which are likely expelled from the cell by the immune system [106].
3.1.2. Active Targeting
Receptors or molecules such as siRNAs, proteins, vitamins, amino acids, monoclonal antibodies, and peptides that are expressed on the surfaces of cancer cells are utilized to achieve the active target mechanism of nanoparticles; in other words, this is achieved by the direct interaction between ligands and receptors. The ligand-mediated target of nanoparticles in cancer cells helps these particles in distinguishing between tumor cells and healthy cells [107,108]. This interaction leads to receptor-mediated endocytosis allowing NPs to release the drug at the target site [109,110]. The targeting ligands used for active targeting are usually monoclonal antibodies or antibody fragments or non-antibody ligands.
3.2. Polymeric Nanoparticles
Polymeric nanoparticles are defined as colloidal nanocarriers, having a submicron size of 10–1000 nm. Sustained release to the target site is achieved by these nanoparticles [111]. The dimensions of nanocarriers are very important for targeting cancer. The dimension of colloidal nanocarriers for reaching cancer cells should be less than 200 nm [112]. It is difficult for nanoparticles with larger dimensions to reach cancer cells. API is encapsulated on the surface of polymeric nanoparticles, forming a nanosphere and nanocapsule. Polyacrylamide, polystyrene, polymethyl methacrylate, and polyacrylate are some of the non-biodegradable nanomaterials that have been used to fabricate these nanoparticles [113,114]. As there were certain limitations with the use of these materials, such as toxicity and other pharmacokinetic problems, biodegradable polymers such as poly(lactic-co-glycolic acid), poly amino acid, and polylactic acid have been introduced to overcome these limitations. Table 3 lists some of the polymeric nanoformulations that have been recently investigated with a positive outcome [115,116,117,118,119,120].
Table 3.
Some of the polymeric nanoparticle formulations that have been recently explored.
| Type | Drug | Targeting Agent | Name of Polymer Used | Result | Reference |
|---|---|---|---|---|---|
| Polymeric nanoparticle | Cisplatin | Cytokeratin-specific monoclonal antibody | Poly(d,l-lactide-co-glycolide) and polyethene glycol | Prevent metastasis | [115] |
| Polymeric nanoparticle | Paclitaxel | Monoclonal antibodies (antiHERT2) | Poly(d,l-lactic acid) | Selective targeting | [116] |
| Polymeric nanoparticle | Paclitaxel | Folic acid | Polylactic acid and polyethylene glycol | Enhanced drug accumulation in tumor | [117] |
| Polymer micelle | Doxorubicin | Folic acid | PEG-co-poly(lactic-co-glycolic acid) | Increased cellular uptake and cytotoxicity | [118] |
| Polymer micelle | Doxorubicin | Folic acid | PEG-poly(aspartate hydrazine doxorubicin) | Increased endocytotic cellular uptake | [118] |
| Polymeric nanoparticle | Doxorubicin | Cyclo-(1,12)-penITDGEATDGC (cLABL) |
PGLA Poly d,l-lactic-co-glycolic acid |
It showed enhanced cellular uptake | [119] |
| Polymeric nanoparticle | Mitomycin | Folic acid | mPEG poly(ethylene glycol) methyl ether |
Targeted cellular uptake and enhanced tumor tissue distribution of the drug were achieved | [120] |
3.3. Extracellular Vesicles
Extracellular vesicles are used in long-distance communications because they contain protein, RNA, and DNA [121]. They have lipids similar to the cell, thus enabling them to escape the immune surveillance of the body and interact with the target site easily [122]. There are several reports on the use of these extracellular vesicles in combatting multiple drug resistance in several cancers [98,123]. Hadla et al. and colleagues reported that exosomes loaded with doxorubicin showed better cytotoxicity and reduced accumulation of doxorubicin in the heart when compared with free doxorubicin [98]. Another study conducted by Jeong et al. and colleagues studied the use of exosomes to deliver mRNA-497 in a lung cancer cell line (A549) and found that tumor growth, as well as expression of associated genes, was suppressed, indicating this exosome-mediated miRNA therapeutic can be used in targeted cancer therapy to reduce cancer drug resistance [124]. Table 4 lists some of the EVs developed for overcoming MDR in cancer [125,126,127,128,129,130,131].
Table 4.
Some of the extracellular vesicles used in chemotherapy.
| Nanocarrier | Drug/System | Cancer Type | Results | Reference |
|---|---|---|---|---|
| Acryl acid polyethylene glycol-modified exosome | Paclitaxel | Lung cancer | High loading capacity, better accumulation of cancer cells, and improved therapeutic outcome are the advantages | [125] |
| Exosome | Doxorubicin | Osteosarcoma | The anticancer effect was increased while cytotoxicity was reduced in myocardial cells when compared to free doxorubicin | [126] |
| Exosome | miR-497 | Lung cancer | Suppression of tumor growth as well as a decrease in expression of genes associated with tumors | [127] |
| Microvesicle | Therapeutic mRNA/protein | Schwannoma | Microvesicles loaded with miRNA led to the conversion of the prodrug into active form and resulted in cell death | [128] |
| Extracellular vesicle | miR-101 | Osteosarcoma | Inhibition and suppression of migration and cell invasion after administration of miR-101-loaded extracellular vesicles | [129] |
| Exosome–liposome hybrid NP | CRISPR/Cas9 system | Osteosarcoma | These hybrid nanoparticles can deliver the CRISPR/Cas9 system and have the potential to be used for cancer therapy | [130] |
| Exosome | Interferon-γ fusion protein | Prostate cancer | Induction of immune response against prostate cancer-derived exosomes and inhibition of tumor growth by exosomal vaccines | [131] |
3.4. Using Nanocarriers in the Delivery of Pooled siRNAs in Combatting MDR in Cancer
In addition to the strategies mentioned above, pooling siRNAs using nanocarriers has emerged as a novel tool for overcoming multidrug resistance, which is confirmed by a study conducted by Chen and coworkers in 2015 [132,133]. This technique inhibits the no flux and efflux-related protein pumps which are involved or overexpressed in multidrug resistance, thereby enhancing the efficacy of already existing drugs. In order to improve the treatment result against resistant tumors, He et al. (2014) carried out a study on the loading of siRNA with cisplatin into nanoscale metal–organic frameworks (NMOFs) [134]. Bcl-2, P-gp, and survivin siRNAs were employed by He et al. to silence genes. In this study, cisplatin (prodrug-based bisphosphonate bridging ligand) was linked with Zn2+ metal and coated with a cationic lipid layer, which enabled the adsorption of pooled siRNA. This increased drug release increased the cellular uptake of pooled siRNA and cisplatin [135]. Table 5 lists some pooled siRNAs combined with gene therapy to overcome MDR in cancer [136,137,138,139,140,141,142,143,144].
Table 5.
Summary of some of the nanocarriers that can be combined with gene therapy and chemotherapy for overcoming multidrug resistance in cancer.
| Target | Gene | Nanocarrier | Chemoagent | Drug-Resistant Cell Line | Reference |
|---|---|---|---|---|---|
| P-gp | siRNA | PDA-coated mesenchymal stem cell (MSC) | Doxorubicin | MCF-7/ADR | [136] |
| Chitosan nanoparticle | Doxorubicin | HepG2/ADR | [137] | ||
| Polymeric NP | Doxorubicin | MCF-7/ADR | [138] | ||
| mRNA | Molecular beacon-based micelle | Doxorubicin | OVCAR-8/ADR | [139] | |
| Survivin | siRNA | Hyaluronic acid NP | Cisplatin | A549/DDP | [140] |
| Bcl-2 | siRNA | Polymeric NP | Doxorubicin | HepG2/ADR MCF-7/ADR |
[141] |
| GAPDH | siRNA | Liposome | Paclitaxel | HeLa, MCF-7 | [142] |
| Autophagy | siRNA | Polymeric NP | Doxorubicin | A549/ADR | [143] |
| P-gp, Bcl-2, survivin | siRNA | Coordination polymerMOF | Cisplatin | SKOV-3 | [144] |
3.5. Using Nanoparticle-Based Combination Therapies in Overcoming Multidrug Resistance in Cancer
Since conventional therapies have developed resistance to various classes of drugs by various mechanisms as described above, combination therapies using nanosystems that are sensitive to changes in pH, certain enzymes, and ROS in cancer cells have gained importance in cancer therapeutics [145]. Li and colleagues have reported that these systems efficiently release the drug into the tumor cell [146]. The tumor microenvironment acts as a physical and biological barrier to the drug as it controls and regulates its accumulation in and outside the cell, thereby helping in inducing drug resistance [147]. Table 6 summarizes the list of nanoparticle-based combinations as a strategic tool in overcoming drug resistance in cancer [144,148,149,150,151,152].
Table 6.
Some of the latest nano-based drug combinations to overcome MDR in cancer.
| Drug Delivery System | Treatment Strategy | Loaded with | Cancer Type | Reference |
|---|---|---|---|---|
| Nanoparticulate targeting mitochondria | Downregulation of pump-related proteins that are involved in drug resistance | Mitochondrial complex, P-gp siRNA | Breast cancer | [144] |
| Nanoparticle–peptide drug biconjugate | Enhancement of efficient drug delivery and release | Doxorubicin peptides | H69AR | [148] |
| Folate-decorated polymersome | Combining chemotherapy with P-gp inhibitors | Paclitaxel, doxorubicin, and tariquidar | MDR breast cancer | [149] |
| Polymer–drug conjugate | Bypassing of pumps related to drug efflux | Doxorubicin | Breast cancer | [150] |
| Zinc oxide nanoparticle | Synergistic autophagy with increased reactive oxygen species generation | Doxorubicin and zinc oxide | MCF-7 | [151] |
| Liposome | Its controlled drug release promotes drug accumulation in cancers | Docetaxel (DTX) and dexamethasone (DEX) | KBv Human epidermoid carcinoma |
[152] |
3.6. Application of Nanotechnology in Antibody-Mediated Targeting in Cancer
The immune response educed by tumor cells lacks adequate strength to counter the cellar response to external stimuli, and thus monoclonal antibodies have gained importance and have been used to counter tumor cells. Nanoparticles have been conjugated with antibodies that are specific against different tumor antigens [153]. These conjugated antibodies execute anticancer activity through various mechanisms, namely the inhibition of growth and the induction of apoptosis which is usually suppressed in resistant cancers [154]. These NP-mediated antibody targetings induce both complement and antibody-mediated cytotoxicity [155]. Researchers encapsulated the hepatic cancer therapeutic gene (HSV1tk) by preparing hollow protein nanoparticles. These nanoparticles were modified to carry the surface antigen of the hepatitis B virus so that its cells recognize it and form particles. This was tested in an animal model, and the results were satisfactory in delivering the gene into the target site after intravenous administration [155]. Another study conducted by Wartlick and coworkers designed and developed a biodegradable nanoparticle whose surface was altered by attaching a biotin-binding protein called NeutrAvidin; trastuzumab (HER2 receptor-specific antibody) was then conjugated to its surface for specific targeting of HER2-overexpressing cells, and this effect was confirmed by confocal laser scanning microscopy [156,157].
3.7. Application of Natural Polyphenol Nanotechnology in Reducing Multidrug Resistance in Cancer
Many studies showed that natural polyphenols have a great role in overcoming MDR in cancer. The commonly used polyphenols for this purpose are curcumin, resveratrol, and epigallocatechin-3-gallate (EGCG) [158,159]. Nanotechnology-based formulations of natural polyphenols attracted much attention due to several reasons. Several investigations have been carried out on the role of polyphenols in designing nanoformulations for drug targeting [158]. Gold nanoparticles based on gelatin–doxorubicin and EGCG were prepared and characterized for targeting prostate cancer. These gold nanoparticles coated with gelatin–doxorubicin and EGCG were found to increase the cellular uptake of doxorubicin [160]. Some studies also revealed that EGCG is able to reduce gold ions; this enabled the enhancement of gold nanoparticles, which showed higher drug uptake by tumor cells [161,162,163]. EGCG-based nanoformulations have also been found suitable for targeting drugs to non-small-cell lung carcinoma cells [164]. Curcumin-based nanoparticles have been found suitable for targeting drugs to brain and breast cancer [165,166]. Resveratrol nanoparticles have been shown to provide protection against UV light in various targets [167]. Liposomal-based formulations of resveratrol have been found effective in targeting drugs to brain tumors in several studies [167,168,169]. Resveratrol-based nanoformulations have also been found effective in the treatment of other tumors, such as lung cancer, colorectal cancer, glioma carcinoma, hepatocarcinoma, and breast cancer [170,171,172,173,174].
4. Conclusions
Resistance to chemotherapy is as similar to infectious disease drug resistance and is a complex phenomenon that is educed by the suppression of apoptosis-related proteins, enhancement of DNA repair, inactivation of drugs, overexpression of efflux proteins, and miRNAs and leads to the failure of already available drugs. Thus, overcoming this resistance is a hot topic in cancer research currently. Nanotechnology has gained importance in recent years in many diseases and has been applied to cancer therapy as well to overcome this multidrug resistance either through passive or active targeting mechanisms. The use of nanocarriers to pool siRNAs, nano-based drug combination with chemotherapy, and antibody-mediated target action have been explored in many cancers. When compared to conventional drugs, these nano-based drugs have successfully improved biocompatibility, target selectivity, pharmacokinetics, and stability, while also simultaneously helping in reducing systemic toxicity and overcoming the burden of multidrug resistance. In addition, nanocarriers are platforms for combination therapy, thereby helping to combine targeting agents with cytotoxic agents to achieve the reversal of drug resistance. Based on evidence-based literature, nanotechnology has the potential to revolutionize cancer chemotherapy through its robust mechanisms and target selectivity. Nevertheless, most of the nanotechnology-based formulations have been tested in animal models instead of human models. Therefore, more studies are required on human beings in order to reach the commercialization of these nanotechnology-based formulations.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through large groups (RGP.2/251/43).
Author Contributions
Conceptualization, S.A.M., A.S., and F.S.; methodology, L.H., G.N.B., and P.A.; software, M.Y.A.; validation, G.N.B. and M.R.; formal analysis, M.Y.A.; investigation, S.A.M., G.N.B., and M.R.; resources, A.S.; data curation, L.H.; writing—original draft preparation, S.A.M.; writing—review and editing, A.S., F.S., and M.R.; visualization, P.A.; supervision, F.S.; project administration, F.S.; funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
Funding Statement
This work was funded by the Deanship of Scientific Research (DSR) at King Khalid University through large groups under grant number RGP.2/251/43. The APC was funded by DSR.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Laversanne M., Weiderpass E., Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 3.Falzone L., Salomone S., Libra M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018;9:1300. doi: 10.3389/fphar.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senapati S., Mahanta A.K., Kumar S., Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018;3:7. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zitvogel L., Apetoh L., Ghiringhelli F., Kroemer G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 6.Xie Y.-H., Chen Y.-X., Fang J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020;5:E22. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodman L.S., Wintrobe M.M. Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J. Am. Med. Assoc. 1946;132:126–132. doi: 10.1001/jama.1946.02870380008004. [DOI] [PubMed] [Google Scholar]
- 8.Housman G., Byler S., Heerboth S., Lapinska K., Longacre M., Snyder N., Sarkar S. Drug Resistance in Cancer: An Overview. Cancers. 2014;6:1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozben T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett. 2006;580:2903–2909. doi: 10.1016/j.febslet.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., del Pilar Rodriguez-Torres M., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua S., de Matos M.B.C., Metselaar J.M., Storm G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018;9:790. doi: 10.3389/fphar.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin C., Wang K., Oppong-Gyebi A., Hu J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci. 2020;17:2964–2973. doi: 10.7150/ijms.49801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalam M.A., Raish M., Ahmed A., Alkharfy K.M., Mohsin K., Alshamsan A., Al-Jenoobi F.I., Al-Mohizea A.M., Shakeel F. Oral bioavailability enhnacement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater. Sci. Eng. C. 2017;76:319–329. doi: 10.1016/j.msec.2017.03.088. [DOI] [PubMed] [Google Scholar]
- 14.Alshahrani S.M., Alshetaili A.S., Alalaiwe A., Alsulays B.B., Anwer M.K., Al-Shdefat R., Imam F., Shakeel F. Anticancer Efficacy of Self-Nanoemulsifying Drug Delivery System of Sunitinib Malate. AAPS PharmSciTech. 2018;19:123–133. doi: 10.1208/s12249-017-0826-x. [DOI] [PubMed] [Google Scholar]
- 15.Shazly G.A., AlShehri S., Ibrahim M.A., Tawfeek H.M., Razik J.A., Hassan Y.A., Shakeel F. Development of Domperidone Solid Lipid Nanoparticles: In Vitro and In Vivo Characterization. AAPS PharmSciTech. 2018;19:1712–1719. doi: 10.1208/s12249-018-0987-2. [DOI] [PubMed] [Google Scholar]
- 16.Hussain A., Shakeel F., Singh S.K., Alsarra I.A., Faruk A., Alanazi F.K., Christoper G.P. Solidified SNEDDS for the oral delivery of rifampicin: Evaluation, proof of concept, in vivo kinetics, and in silico GastroPlusTM simulation. Int. J. Pharm. 2019;566:203–217. doi: 10.1016/j.ijpharm.2019.05.061. [DOI] [PubMed] [Google Scholar]
- 17.Kazi M., Alhajri A., AlShehri S.M., Elzayat E.M., Al Meanazel O.T., Shakeel F., Noman O., Altamimi M.A., Alanazi F.K. Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, In Vivo and Stability Evaluations. Pharmaceutics. 2020;12:749. doi: 10.3390/pharmaceutics12080749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abushal A.S., Aleanizy F.S., Alqahtani F.Y., Shakeel F., Iqbal M., Haq N., Alsarra I.A. Self-Nanoemulsifying Drug Delivery System (SNEDDS) of Apremilast: In Vitro Evaluation and Pharmacokinetics Studies. Molecules. 2022;27:3085. doi: 10.3390/molecules27103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soliman N.M., Shakeel F., Haq N., Alanazi F.K., Alshehri S., Bayomi M., Alenazi A.S.M., Alsarra I.A. Development and Optimization of Ciprofloxacin HCl-Loaded Chitosan Nanoparticles Using Box–Behnken Experimental Design. Molecules. 2022;27:4468. doi: 10.3390/molecules27144468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoaib A., Azmi L., Pal S., Alqahtani S.S., Rahamathulla M., Hani U., Alshehri S., Ghoneim M.M., Shakeel F. Integrating nanotechnology with naturally occurring phytochemicals in neuropathy induced by diabetes. J. Mol. Liq. 2022;350:118189. doi: 10.1016/j.molliq.2021.118189. [DOI] [Google Scholar]
- 21.Badran M.M., Mady M.M., Ghannam M.M., Shakeel F. Preparation and characterization of polymeric nanoparticles surfacemodified with chitosan for target treatment of colorectal cancer. Int. J. Biol. Macromol. 2017;95:643–649. doi: 10.1016/j.ijbiomac.2016.11.098. [DOI] [PubMed] [Google Scholar]
- 22.Dadwal A., Baldi A., Kumar Narang R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018;46:295–305. doi: 10.1080/21691401.2018.1457039. [DOI] [PubMed] [Google Scholar]
- 23.Javed S., Alshehri S., Shoaib A., Ahsan W., Sultan M., Alqahtani S., Kazi M., Shakeel F. Chronicles of Nanoerythrosomes: An Erythrocyte-Based Biomimetic Smart Drug Delivery System as a Therapeutic and Diagnostic Tool in Cancer Therapy. Pharmaceutics. 2021;13:368. doi: 10.3390/pharmaceutics13030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alshammari R.A., Aleanizy F.S., Aldarwesh A., Alqahtani F.Y., Mahdi W.A., Alquadeib B., Alqahtani Q.H., Haq N., Shakeel F., Abdelhady H.G., et al. Retinal Delivery of the Protein Kinase C-β Inhibitor Ruboxistaurin Using Non-Invasive Nanoparticles of Polyamidoamine Dendrimers. Pharmaceutics. 2022;14:1444. doi: 10.3390/pharmaceutics14071444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan S., Mansoor S., Rafi Z., Kumari B., Shoaib A., Saeed M., Alshehri S., Ghoneim M.M., Rahamathulla M., Hani U., et al. A review on nanotechnology: Properties, applications, and mechanistic insights of cellular uptake mechanisms. J. Mol. Liq. 2022;348:118008. doi: 10.1016/j.molliq.2021.118008. [DOI] [Google Scholar]
- 26.Siegel R., DeSantis C., Virgo K., Stein K., Mariotto A., Smith T., Cooper D., Gansler T., Lerro C., Fedewa S., et al. Cancer treatment and survivorship statistics, 2012. CA A Cancer J. Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 27.Raderer M., Scheithauer W. Treatment of advanced colorectal cancer with 5-fluorouracil and interferon-α: An overview of clinical trials. Eur. J. Cancer. 1995;31:1002–1008. doi: 10.1016/0959-8049(95)00078-X. [DOI] [PubMed] [Google Scholar]
- 28.Guilhot F., Chastang C., Michallet M., Guerci A., Harousseau J.-L., Maloisel F., Bouabdallah R., Guyotat D., Cheron N., Nicolini F., et al. Interferon Alfa-2b Combined with Cytarabine versus Interferon Alone in Chronic Myelogenous Leukemia. New Engl. J. Med. 1997;337:223–229. doi: 10.1056/NEJM199707243370402. [DOI] [PubMed] [Google Scholar]
- 29.Druker B.J., Talpaz M., Resta D.J., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., et al. Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. N. Engl. J. Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 30.Tallman M.S., Nabhan C., Feusner J.H., Rowe J.M. Acute promyelocytic leukemia: Evolving therapeutic strategies. Blood. 2002;99:759–767. doi: 10.1182/blood.V99.3.759. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien S.G., Guilhot F., Larson R.A., Gathmann I., Baccarani M., Cervantes F., Cornelissen J.J., Fischer T., Hochhaus A., Hughes T., et al. Imatinib Compared with Interferon and Low-Dose Cytarabine for Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia. N. Engl. J. Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 32.Sawyers C. Targeted cancer therapy. Nature. 2004;432:294–297. doi: 10.1038/nature03095. [DOI] [PubMed] [Google Scholar]
- 33.Ferrantini M., Capone I., Belardelli F. Interferon-α and cancer: Mechanisms of action and new perspectives of clinical use. Biochimie. 2007;89:884–893. doi: 10.1016/j.biochi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Chin L., Gray J.W. Translating insights from the cancer genome into clinical practice. Nature. 2008;452:553–563. doi: 10.1038/nature06914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sellers W.R. A Blueprint for Advancing Genetics-Based Cancer Therapy. Cell. 2011;147:26–31. doi: 10.1016/j.cell.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Wilson T., Johnston P., Longley D. Anti-Apoptotic Mechanisms of Drug Resistance in Cancer. Curr. Cancer Drug Targets. 2009;9:307–319. doi: 10.2174/156800909788166547. [DOI] [PubMed] [Google Scholar]
- 37.Longley D.B., Johnston P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 38.Qin S., Jiang J., Lu Y., Nice E.C., Huang C., Zhang J., He W. Emerging role of tumor cell plasticity in modifying therapeutic response. Signal Transduct. Target. Ther. 2020;5:228. doi: 10.1038/s41392-020-00313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robey R.W., Pluchino K.M., Hall M.D., Fojo A.T., Bates S.E., Gottesman M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer. 2018;18:452–464. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szakacs G., Paterson J.K., Ludwig J.A., Booth-Genthe C., Gottesman M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 41.Fernández L., Hancock R.E.W. Adaptive and Mutational Resistance: Role of Porins and Efflux Pumps in Drug Resistance. Clin. Microbiol. Rev. 2012;25:661–681. doi: 10.1128/CMR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottesman M.M., Fojo T., Bates S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 43.Choi C.-H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005;5:30. doi: 10.1186/1475-2867-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X., Zhang H., Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2:141–160. doi: 10.20517/cdr.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kersten K., Visser K.E., Miltenburg M.H., Jonkers J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol. Med. 2017;9:137–153. doi: 10.15252/emmm.201606857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi Y.E., Meghani K., Brault M.-E., Leclerc L., He Y., Day T.A., Elias K.M., Drapkin R., Weinstock D.M., Dao F., et al. Platinum and PARP Inhibitor Resistance Due to Overexpression of MicroRNA-622 in BRCA1-Mutant Ovarian Cancer. Cell Rep. 2016;14:429–439. doi: 10.1016/j.celrep.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weil M.K., Chen A.P. PARP Inhibitor Treatment in Ovarian and Breast Cancer. Curr. Probl. Cancer. 2011;35:7–50. doi: 10.1016/j.currproblcancer.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L., Zeng Y., Zhou S.-F. Role of Apoptosis in Cancer Resistance to Chemotherapy. Program. Cell Death. InTech. 2018 doi: 10.5772/intechopen.80056. [DOI] [Google Scholar]
- 49.Fulda S., Debatin K.-M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 50.Safa A.R. Resistance to Cell Death and Its Modulation in Cancer Stem Cells. Crit. Rev. Oncog. 2016;21:203–219. doi: 10.1615/CritRevOncog.2016016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Llambi F., Green D.R. Apoptosis and oncogenesis: Give and take in the BCL-2 family. Curr. Opin. Genet. Dev. 2011;21:12–20. doi: 10.1016/j.gde.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu B., Yuan B., Zhang L., Mu W., Wang C. ROS/p38/p53/Puma signaling pathway is involved in emodin-induced apoptosis of human colorectal cancer cells. Int. J. Clin. Exp. Med. 2015;8:15413–15422. [PMC free article] [PubMed] [Google Scholar]
- 53.Meng X., Carlson N.R., Dong J., Zhang Y. Oncogenic c-Myc-induced lymphomagenesis is inhibited non-redundantly by the p19Arf-Mdm2-p53 and RP-Mdm2-p53 pathways. Oncogene. 2015;34:5709–5717. doi: 10.1038/onc.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng H.-C. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8:59950–59964. doi: 10.18632/oncotarget.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang H.-J., Wang M., Wang L., Cheng B.-F., Lin X.-Y., Feng Z.-W. NF-κB regulates caspase-4 expression and sensitizes neuroblastoma cells to Fas-induced apoptosis. PLoS ONE. 2015;10:E0117953. doi: 10.1371/journal.pone.0117953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubinstein A.D., Kimchi A. Life in the balance—A mechanistic view of the crosstalk between autophagy and apoptosis. J. Cell Sci. 2012;125:5259–5268. doi: 10.1242/jcs.115865. [DOI] [PubMed] [Google Scholar]
- 57.Zubay G., Druker B.J., Talpaz M., Resta D.J., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 2001;344:1038–1042. doi: 10.1056/nejm200104053441402. [DOI] [PubMed] [Google Scholar]
- 58.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 59.Galmarini C.M., Mackey J.R., Dumontet C. Nucleoside analogues: Mechanisms of drug resistance and reversal strategies. Leukemia. 2001;15:875–890. doi: 10.1038/sj.leu.2402114. [DOI] [PubMed] [Google Scholar]
- 60.Longo-Sorbello G.S., Bertino J.R. Current understanding of methotrexate pharmacology and efficacy in acute leukemias. Use of newer antifolates in clinical trials. Haematologica. 2001;86:121–127. [PubMed] [Google Scholar]
- 61.Inaba H., Greaves M., Mullighan C.G. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943–1955. doi: 10.1016/S0140-6736(12)62187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jansen B.A., Brouwer J., Reedijk J. Glutathione induces cellular resistance against cationic dinuclear platinum anticancer drugs. J. Inorg. Biochem. 2002;89:197–202. doi: 10.1016/S0162-0134(02)00381-1. [DOI] [PubMed] [Google Scholar]
- 63.Garzon R., Fabbri M., Cimmino A., Calin G.A., Croce C.M. MicroRNA expression and function in cancer. Trends Mol. Med. 2006;12:580–587. doi: 10.1016/j.molmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Schickel R., Boyerinas B., Park S.-M., Peter M.E. MicroRNAs: Key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27:5959–5974. doi: 10.1038/onc.2008.274. [DOI] [PubMed] [Google Scholar]
- 65.Jones M.F., Lal A. MicroRNAs, wild-type and mutant p53: More questions than answers. RNA Biol. 2012;9:781–791. doi: 10.4161/rna.20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gambari R., Brognara E., Spandidos D.A., Fabbri E. Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: Νew trends in the development of miRNA therapeutic strategies in oncology (Review) Int. J. Oncol. 2016;49:5–32. doi: 10.3892/ijo.2016.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Selbach M., Schwanhäusser B., Thierfelder N., Fang Z., Khanin R., Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 68.Wu S., Huang S., Ding J., Zhao Y., Liang L., Liu T., Zhan R., He X. Multiple microRNAs modulate p21Cip1/Waf1 expression by directly targeting its 3′ untranslated region. Oncogene. 2010;29:2302–2308. doi: 10.1038/onc.2010.34. [DOI] [PubMed] [Google Scholar]
- 69.Wang Z.-X., Lu B.-B., Wang H., Cheng Z.-X., Yin Y.-M. MicroRNA-21 Modulates Chemosensitivity of Breast Cancer Cells to Doxorubicin by Targeting PTEN. Arch. Med. Res. 2011;42:281–290. doi: 10.1016/j.arcmed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 70.Liu H., Wu X., Huang J., Peng J., Guo L. miR-7 modulates chemoresistance of small cell lung cancer by repressing MRP1/ABCC1. Int. J. Exp. Pathol. 2015;96:240–247. doi: 10.1111/iep.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Mattos-Arruda L., Bottai G., Nuciforo P.G., Di Tommaso L., Giovannetti E., Peg V., Losurdo A., Pérez-Garcia J., Masci G., Corsi F., et al. MicroRNA-21 links epithelial-to-mesenchymal transition and inflammatory signals to confer resistance to neoadjuvant trastuzumab and chemotherapy in HER2-positive breast cancer patients. Oncotarget. 2015;6:37269–37280. doi: 10.18632/oncotarget.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Si W., Shen J., Du C., Chen D., Gu X., Li C., Yao M., Pan J., Cheng J., Jiang D., et al. A miR-20a/MAPK1/c-Myc regulatory feedback loop regulates breast carcinogenesis and chemoresistance. Cell Death Differen. 2018;25:406–420. doi: 10.1038/cdd.2017.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y., Qu X., Li C., Fan Y., Che X., Wang X., Cai Y., Hu X., Liu Y. miR-103/107 modulates multidrug resistance in human gastric carcinoma by downregulating Cav-1. Tumor Biol. 2015;36:2277–2285. doi: 10.1007/s13277-014-2835-7. [DOI] [PubMed] [Google Scholar]
- 74.Li J.-H., Luo N., Zhong M.-Z., Xiao Z.-Q., Wang J.-X., Yao X.-Y., Peng Y., Cao J. Inhibition of microRNA-196a might reverse cisplatin resistance of A549/DDP non-small-cell lung cancer cell line. Tumor Biol. 2016;37:2387–2394. doi: 10.1007/s13277-015-4017-7. [DOI] [PubMed] [Google Scholar]
- 75.Rao E., Jiang C., Ji M., Huang X., Iqbal J., Lenz G., Wright G., Staudt L.M., Zhao Y., McKeithan T., et al. The miRNA-17∼92 cluster mediates chemoresistance and enhances tumor growth in mantle cell lymphoma via PI3K/AKT pathway activation. Leukemia. 2012;26:1064–1072. doi: 10.1038/leu.2011.305. [DOI] [PubMed] [Google Scholar]
- 76.Liu X., Luo X., Wu Y., Xia D., Chen W., Fang Z., Deng J., Hao Y., Yang X., Zhang T., et al. MicroRNA-34a attenuates paclitaxel resistance in prostate cancer cells via direct suppression of JAG1/notch1 axis. Cell. Physiol. Biochem. 2018;50:261–276. doi: 10.1159/000494004. [DOI] [PubMed] [Google Scholar]
- 77.Kim S.-A., Kim I., Yoon S.K., Lee E.K., Kuh H.-J. Indirect modulation of sensitivity to 5-fluorouracil by microRNA-96 in human colorectal cancer cells. Arch. Pharmacal Res. 2014;38:239–248. doi: 10.1007/s12272-014-0528-9. [DOI] [PubMed] [Google Scholar]
- 78.Chen Y., Song Y., Mi Y., Jin H., Cao J., Li H., Han L., Huang T., Zhang X., Ren S., et al. microRNA-499a promotes the progression and chemoresistance of cervical cancer cells by targeting SOX6. Apoptosis. 2020;25:205–216. doi: 10.1007/s10495-019-01588-y. [DOI] [PubMed] [Google Scholar]
- 79.Tormo E., Ballester S., Adam-Artigues A., Burgués O., Alonso E., Bermejo B., Menéndez S., Zazo S., Madoz-Gúrpide J., Rovira A., et al. The miRNA-449 family mediates doxorubicin resistance in triple-negative breast cancer by regulating cell cycle factors. Sci. Rep. 2019;9:E5316. doi: 10.1038/s41598-019-41472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iwagami Y., Eguchi H., Nagano H., Akita H., Hama N., Wada H., Kawamoto K., Kobayashi S., Tomokuni A., Tomimaru Y., et al. miR-320c regulates gemcitabine-resistance in pancreatic cancer via SMARCC1. Br. J. Cancer. 2013;109:502–511. doi: 10.1038/bjc.2013.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Z.-W., Dalton W.S. Tumor microenvironment and drug resistance in hematologic malignancies. Blood Rev. 2006;20:333–342. doi: 10.1016/j.blre.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 82.Liberti M.V., Locasale J.W. The warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi S.Y.C., Collins C.C., Gout P.W., Wang Y. Cancer-generated lactic acid: A regulatory, immunosuppressive metabolite? J. Pathol. 2013;230:350–355. doi: 10.1002/path.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jun J.C., Rathore A., Younas H., Gilkes D., Polotsky V.Y. Hypoxia-Inducible Factors and Cancer. Curr. Sleep Med. Rep. 2017;3:1–10. doi: 10.1007/s40675-017-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sormendi S., Wielockx B. Hypoxia Pathway Proteins As Central Mediators of Metabolism in the Tumor Cells and Their Microenvironment. Front. Immunol. 2018;9:40. doi: 10.3389/fimmu.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sengupta S., Eavarone D., Capila I., Zhao G., Watson N., Kiziltepe T., Sasisekharan R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 87.Misra R., Acharya S., Sahoo S.K. Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discov. Today. 2010;15:842–850. doi: 10.1016/j.drudis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 88.Heath J.R., Davis M.E. Nanotechnology and cancer. Annu. Rev. Med. 2008;59:251–265. doi: 10.1146/annurev.med.59.061506.185523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jahangirian H., Ghasemian lemraski E., Webster T.J., Rafiee-Moghaddam R., Abdollahi Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017;12:2957–2978. doi: 10.2147/IJN.S127683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yetisgin A.A., Cetinel S., Zuvin M., Kosar A., Kutlu O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules. 2020;25:2193. doi: 10.3390/molecules25092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao C.-Y., Cheng R., Yang Z., Tian Z.-M. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules. 2018;23:826. doi: 10.3390/molecules23040826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Le Broc-Ryckewaert D., Carpentier R., Lipka E., Daher S., Vaccher C., Betbeder D., Furman C. Development of innovative paclitaxel-loaded small PLGA nanoparticles: Study of their antiproliferative activity and their molecular interactions on prostatic cancer cells. Int. J. Pharm. 2013;454:712–719. doi: 10.1016/j.ijpharm.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 94.Bellocq N.C., Pun S.H., Jensen G.S., Davis M.E. Transferrin-Containing, Cyclodextrin Polymer-Based Particles for Tumor-Targeted Gene Delivery. Bioconjugate Chem. 2003;14:1122–1132. doi: 10.1021/bc034125f. [DOI] [PubMed] [Google Scholar]
- 95.Zhang X., Liu J., Li X., Li F., Lee R.J., Sun F., Li Y., Liu Z., Teng L. Trastuzumab-Coated Nanoparticles Loaded With Docetaxel for Breast Cancer Therapy. Dose Response. 2019;17 doi: 10.1177/1559325819872583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abedin M.R., Powers K., Aiardo R., Barua D., Barua S. Antibody–drug nanoparticle induces synergistic treatment efficacies in HER2 positive breast cancer cells. Sci. Rep. 2021;11:7347. doi: 10.1038/s41598-021-86762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bao S., Zheng H., Ye J., Huang H., Zhou B., Yao Q., Lin G., Zhang H., Kou L., Chen R. Dual Targeting EGFR and STAT3 With Erlotinib and Alantolactone Co-Loaded PLGA Nanoparticles for Pancreatic Cancer Treatment. Front. Pharmacol. 2021;12:E625084. doi: 10.3389/fphar.2021.625084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hadla M., Palazzolo S., Corona G., Caligiuri I., Canzonieri V., Toffoli G., Rizzolio F. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine. 2016;11:2431–2441. doi: 10.2217/nnm-2016-0154. [DOI] [PubMed] [Google Scholar]
- 99.Lin A.Y., Young J.K., Nixon A.V., Drezek R.A. Encapsulated Fe3O4/Ag complexed cores in hollow gold nanoshells for enhanced theranostic magnetic resonance imaging and photothermal therapy. Small. 2014;10:3246–3251. doi: 10.1002/smll.201303593. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y., Zhen W., Wang Y., Liu J., Jin L., Zhang T., Zhang S., Zhao Y., Song S., Li C., et al. One-Dimensional Fe2 P Acts as a Fenton Agent in Response to NIR II Light and Ultrasound for Deep Tumor Synergetic Theranostics. Angew. Chem. Int. Ed. 2019;58:2407–2412. doi: 10.1002/anie.201813702. [DOI] [PubMed] [Google Scholar]
- 101.Yao Y., Zhou Y., Liu L., Xu Y., Chen Q., Wang Y., Wu S., Deng Y., Zhang J., Shao A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020;7:E193. doi: 10.3389/fmolb.2020.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 103.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001;41:189–207. doi: 10.1016/S0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 104.Caritá A.C., Eloy J., Chorilli M., Lee R.J., Leonardi G. Recent Advances and Perspectives in Liposomes for Cutaneous Drug Delivery. Curr. Med. Chem. 2018;25:606–635. doi: 10.2174/0929867324666171009120154. [DOI] [PubMed] [Google Scholar]
- 105.Torchilin V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 106.Sykes E.A., Chen J., Zheng G., Chan W.C. Investigating the Impact of Nanoparticle Size on Active and Passive Tumor Targeting Efficiency. ACS Nano. 2014;8:5696–5706. doi: 10.1021/nn500299p. [DOI] [PubMed] [Google Scholar]
- 107.Shi J., Xiao Z., Kamaly N., Farokhzad O.C. Self-Assembled Targeted Nanoparticles: Evolution of Technologies and Bench to Bedside Translation. Accounts Chem. Res. 2011;44:1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- 108.Kamaly N., Xiao Z., Valencia P.M., Radovic-Moreno A.F., Farokhzad O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Farokhzad O.C., Langer R. Impact of Nanotechnology on Drug Delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 110.Danhier F., Feron O., Préat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 111.Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C. 2016;60:569–578. doi: 10.1016/j.msec.2015.11.067. [DOI] [PubMed] [Google Scholar]
- 112.Suner S.S., Sahiner M., Mohapatra S., Ayyala R.S., Bhethanabotla V.R., Sahiner N. Degradable poly(catechin) nanoparticles as a versatile therapeutic agent. Int. J. Polym. Mater. Polym. Biomater. 2022;71:1104–1115. doi: 10.1080/00914037.2021.1941957. [DOI] [Google Scholar]
- 113.Shastri V.P. Non-Degradable Biocompatible Polymers in Medicine: Past, Present and Future. Curr. Pharm. Biotechnol. 2003;4:331–337. doi: 10.2174/1389201033489694. [DOI] [PubMed] [Google Scholar]
- 114.Vijayan V., Reddy K.R., Sakthivel S., Swetha C. Optimization and charaterization of repaglinide biodegradable polymeric nanoparticle loaded transdermal patchs: In vitro and in vivo studies. Colloids Surfaces B Biointerfaces. 2013;111:150–155. doi: 10.1016/j.colsurfb.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 115.Kos J., Obermajer N., Doljak B., Kocbek P., Kristl J. Inactivation of harmful tumour-associated proteolysis by nanoparticulate system. Int. J. Pharm. 2009;381:106–112. doi: 10.1016/j.ijpharm.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 116.Cirstoiu-Hapca A., Buchegger F., Bossy L., Kosinski M., Gurny R., Delie F. Nanomedicines for active targeting: Physico-chemical characterization of paclitaxel-loaded anti-HER2 immunonanoparticles and in vitro functional studies on target cells. Eur. J. Pharm. Sci. 2009;38:230–237. doi: 10.1016/j.ejps.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 117.Patil Y.B., Toti U.S., Khdair A., Ma L., Panyam J. Single-step surface functionalization of polymeric nanoparticles for targeted drug delivery. Biomaterials. 2009;30:859–866. doi: 10.1016/j.biomaterials.2008.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brewer E., Coleman J., Lowman A. Emerging Technologies of Polymeric Nanoparticles in Cancer Drug Delivery. J. Nanomater. 2010;2011:E408675. doi: 10.1155/2011/408675. [DOI] [Google Scholar]
- 119.Chittasupho C., Xie S.-X., Baoum A., Yakovleva T., Siahaan T.J., Berkland C.J. ICAM-1 targeting of doxorubicin-loaded PLGA nanoparticles to lung epithelial cells. Eur. J. Pharm. Sci. 2009;37:141–150. doi: 10.1016/j.ejps.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hou Z., Zhan C., Jiang Q., Hu Q., Li L., Chang D., Yang X., Wang Y., Li Y., Ye S., et al. Both FA- and mPEG-conjugated chitosan nanoparticles for targeted cellular uptake and enhanced tumor tissue distribution. Nanoscale Res. Lett. 2011;6:E563. doi: 10.1186/1556-276X-6-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Batrakova E.V., Kim M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 123.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 124.Jeong K., Yu Y.J., You J.Y., Rhee W.J., Kim J.A. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab Chip. 2020;20:548–557. doi: 10.1039/C9LC00958B. [DOI] [PubMed] [Google Scholar]
- 125.Kim M.S., Haney M.J., Zhao Y., Yuan D., Deygen I., Klyachko N.L., Kabanov A.V., Batrakova E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomed. Nanotechnol. Biol. Med. 2018;14:195–204. doi: 10.1016/j.nano.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 126.Wei H., Chen J., Wang S., Fu F., Zhu X., Wu C., Liu Z., Zhong G., Lin J. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019;14:8603–8610. doi: 10.2147/IJN.S218988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wei W., Ao Q., Wang X., Cao Y., Liu Y., Zheng S.G., Tian X. Mesenchymal Stem Cell–Derived Exosomes: A Promising Biological Tool in Nanomedicine. Front. Pharmacol. 2021;11:E590470. doi: 10.3389/fphar.2020.590470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mizrak A., Bolukbasi M.F., Ozdener G.B., Brenner G.J., Madlener S., Erkan E.P., Ströbel T., Breakefield X.O., Saydam O. Genetically Engineered Microvesicles Carrying Suicide mRNA/Protein Inhibit Schwannoma Tumor Growth. Mol. Ther. 2013;21:101–108. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang K., Dong C., Chen M., Yang T., Wang X., Gao Y., Wang L., Wen Y., Chen G., Wang X., et al. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics. 2020;10:411–425. doi: 10.7150/thno.33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin Y., Wu J., Gu W., Huang Y., Tong Z., Huang L., Tan J. Exosome-Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs. Adv. Sci. 2018;5:1700611. doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi X., Sun J., Li H., Lin H., Xie W., Li J., Tan W. Antitumor efficacy of interferon-γ-modified exosomal vaccine in prostate cancer. Prostate. 2020;80:811–823. doi: 10.1002/pros.23996. [DOI] [PubMed] [Google Scholar]
- 132.Chen W., Liu X., Xiao Y., Tang R. Overcoming Multiple Drug Resistance by Spatial-Temporal Synchronization of Epirubicin and Pooled siRNAs. Small. 2015;11:1775–1781. doi: 10.1002/smll.201402377. [DOI] [PubMed] [Google Scholar]
- 133.Chen Q., Xu M., Zheng W., Xu T., Deng H., Liu J. Se/Ru-Decorated Porous Metal–Organic Framework Nanoparticles for The Delivery of Pooled siRNAs to Reversing Multidrug Resistance in Taxol-Resistant Breast Cancer Cells. ACS Appl. Mater. Interfaces. 2017;9:6712–6724. doi: 10.1021/acsami.6b12792. [DOI] [PubMed] [Google Scholar]
- 134.He C., Lu K., Liu D., Lin W. Nanoscale Metal–Organic Frameworks for the Co-Delivery of Cisplatin and Pooled siRNAs to Enhance Therapeutic Efficacy in Drug-Resistant Ovarian Cancer Cells. J. Am. Chem. Soc. 2014;136:5181–5184. doi: 10.1021/ja4098862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He C., Liu D., Lin W. Self-assembled nanoscale coordination polymers carrying siRNAs and cisplatin for effective treatment of resistant ovarian cancer. Biomaterials. 2015;36:124–133. doi: 10.1016/j.biomaterials.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cheng W., Nie J., Gao N., Liu G., Tao W., Xiao X., Jiang L., Liu Z., Zeng X., Mei L. A multifunctional nanoplatform against multidrug resistant cancer: Merging the best of targeted chemo/gene/photothermal therapy. Adv. Func. Mater. 2017;27:E1704135. doi: 10.1002/adfm.201704135. [DOI] [Google Scholar]
- 137.Zhu Q.-L., Zhou Y., Guan M., Zhou X.-F., Yang S.-D., Liu Y., Chen W.-L., Zhang C.-G., Yuan Z.-Q., Liu C., et al. Low-density lipoprotein-coupled N-succinyl chitosan nanoparticles co-delivering siRNA and doxorubicin for hepatocyte-targeted therapy. Biomaterials. 2014;35:5965–5976. doi: 10.1016/j.biomaterials.2014.03.088. [DOI] [PubMed] [Google Scholar]
- 138.Wang T., Luo Y., Lv H., Wang J., Zhang Y., Pei R. Aptamer-Based Erythrocyte-Derived Mimic Vesicles Loaded with siRNA and Doxorubicin for the Targeted Treatment of Multidrug-Resistant Tumors. ACS Appl. Mater. Interfaces. 2019;11:45455–45466. doi: 10.1021/acsami.9b16637. [DOI] [PubMed] [Google Scholar]
- 139.Yang Z., Li J., Feng G., Gao S., Wang Y., Zhang S., Liu Y., Ye L., Li Y., Zhang X. MicroRNA-145 Modulates N6-Methyladenosine Levels by Targeting the 3′-Untranslated mRNA Region of the N6-Methyladenosine Binding YTH Domain Family 2 Protein. J. Biol. Chem. 2017;292:3614–3623. doi: 10.1074/jbc.M116.749689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ganesh S., Iyer A.K., Gattacceca F., Morrissey D.V., Amiji M.M. In vivo biodistribution of siRNA and cisplatin administered using CD44-targeted hyaluronic acid nanoparticles. J. Control. Release. 2013;172:699–706. doi: 10.1016/j.jconrel.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun W., Chen X., Xie C., Wang Y., Lin L., Zhu K., Shuai X. Co-delivery of doxorubicin and anti-BCL-2 siRNA by pH-responsive polymeric vector to overcome drug resistance in in vitro and in vivo HepG2 hepatoma model. Biomacromol. 2018;19:2248–2256. doi: 10.1021/acs.biomac.8b00272. [DOI] [PubMed] [Google Scholar]
- 142.Guan J., Sun J., Sun F., Lou B., Zhang D., Mashayekhi V., Sadeghi N., Storm G., Mastrobattista E., He Z. Hypoxia-induced tumor cell resistance is overcome by synergistic GAPDH-siRNA and chemotherapy co-delivered by long-circulating and cationic-interior liposomes. Nanoscale. 2017;9:9190–9201. doi: 10.1039/C7NR02663C. [DOI] [PubMed] [Google Scholar]
- 143.Yang B., Hao A., Chen L. Mirror siRNAs loading for dual delivery of doxorubicin and autophagy regulation siRNA for multidrug reversing chemotherapy. Biomed. Pharmacother. 2020;130:110490. doi: 10.1016/j.biopha.2020.110490. [DOI] [PubMed] [Google Scholar]
- 144.Chen W., Shi K., Chu B., Wei X., Qian Z. Mitochondrial Surface Engineering for Multidrug Resistance Reversal. Nano Lett. 2019;19:2905–2913. doi: 10.1021/acs.nanolett.8b05188. [DOI] [PubMed] [Google Scholar]
- 145.Hu C.-M.J., Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem. Pharmacol. 2012;83:1104–1111. doi: 10.1016/j.bcp.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 146.Li R., Xie Y. Nanodrug delivery systems for targeting the endogenous tumor microenvironment and simultaneously overcoming multidrug resistance properties. J. Control. Release. 2017;251:49–67. doi: 10.1016/j.jconrel.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 147.Klemm F., Joyce J.A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sangtani A., Petryayeva E., Susumu K., Oh E., Huston A.L., Aragonés G.L., Medintz I.L., Algar W.R., Delehanty J.B. Nanoparticle–Peptide–Drug Bioconjugates for Unassisted Defeat of Multidrug Resistance in a Model Cancer Cell Line. Bioconjugate Chem. 2019;30:525–530. doi: 10.1021/acs.bioconjchem.8b00755. [DOI] [PubMed] [Google Scholar]
- 149.Qin Y., Zhang Z., Huang C., Fan F., Liu L., Lu L., Wang H., Liu Z., Yang J., Wang C., et al. Folate-Targeted Redox-Responsive Polymersomes Loaded with Chemotherapeutic Drugs and Tariquidar to Overcome Drug Resistance. J. Biomed. Nanotechnol. 2018;14:1705–1718. doi: 10.1166/jbn.2018.2623. [DOI] [PubMed] [Google Scholar]
- 150.Zhou M., Li L., Li L., Lin X., Wang F., Li Q., Huang Y. Overcoming chemotherapy resistance via simultaneous drug-efflux circumvention and mitochondrial targeting. Acta Pharm. Sin. B. 2018;9:615–625. doi: 10.1016/j.apsb.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hu Y., Zhang H.-R., Dong L., Xu M.-R., Zhang L., Ding W.-P., Zhang J.-Q., Lin J., Zhang Y.-J., Qiu B.-S., et al. Enhancing tumor chemotherapy and overcoming drug resistance through autophagy-mediated intracellular dissolution of zinc oxide nanoparticles. Nanoscale. 2019;11:11789–11807. doi: 10.1039/C8NR08442D. [DOI] [PubMed] [Google Scholar]
- 152.Zhang L., Su H., Liu Y., Pang N., Li J., Qi X.-R. Enhancing solid tumor therapy with sequential delivery of dexamethasone and docetaxel engineered in a single carrier to overcome stromal resistance to drug delivery. J. Control. Release. 2018;294:1–16. doi: 10.1016/j.jconrel.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 153.Sutradhar K.B., Amin M.L. Nanoemulsions: Increasing possibilities in drug delivery. Eur. J. Nanomedicine. 2013;5:97–110. doi: 10.1515/ejnm-2013-0001. [DOI] [Google Scholar]
- 154.Walko C.M., West H. Antibody Drug Conjugates for Cancer Treatment. JAMA Oncol. 2019;5:E1648. doi: 10.1001/jamaoncol.2019.3552. [DOI] [PubMed] [Google Scholar]
- 155.Praetorius N., Mandal T. Engineered nanoparticles in cancer therapy. Rec. Pat. Drug Deliv. Formul. 2007;1:37–51. doi: 10.2174/187221107779814104. [DOI] [PubMed] [Google Scholar]
- 156.Wartlick H., Michaelis K., Balthasar S., Strebhardt K., Kreuter J., Langer K. Highly Specific HER2-mediated Cellular Uptake of Antibody-modified Nanoparticles in Tumour Cells. J. Drug Target. 2004;12:461–471. doi: 10.1080/10611860400010697. [DOI] [PubMed] [Google Scholar]
- 157.Majidinia M., Mirza-Aghazadeh-Attari M., Rahimi M., Mihanfar A., Karimian A., Safa A., Yousefi B. Overcoming multidrug resistance in cancer: Recent progress in nanotechnology and new horizons. IUBMB Life. 2020;72:855–871. doi: 10.1002/iub.2215. [DOI] [PubMed] [Google Scholar]
- 158.Dana P.M., Sadoughi F., Asemi Z., Yousefi B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell. Mol. Biol. Lett. 2022;27:1–26. doi: 10.1186/s11658-021-00301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sharifi-Rad J., Quispe C., Mukazhanova Z., Knut E., Turgumbayeva A., Kipchakbayeva A., Seitimova G., Mahomoodally M.F., Lobine D., Koay A., et al. Resveratrol-Based Nanoformulations as an Emerging Therapeutic Strategy for Cancer. Front. Mol. Biosci. 2021;8:E649395. doi: 10.3389/fmolb.2021.649395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tsai L.-C., Hsieh H.-Y., Lu K.-Y., Wang S.-Y., Mi F.-L. EGCG/gelatin-doxorubicin gold nanoparticles enhance therapeutic efficacy of doxorubicin for prostate cancer treatment. Nanomedicine. 2016;11:9–30. doi: 10.2217/nnm.15.183. [DOI] [PubMed] [Google Scholar]
- 161.Viator J.A., Gupta S., Goldschmidt B.S., Bhattacharyya K., Kannan R., Shukla R., Dale P.S., Boote E., Katti K. Gold Nanoparticle Mediated Detection of Prostate Cancer Cells Using Photoacoustic Flowmetry with Optical Reflectance. J. Biomed. Nanotechnol. 2010;6:187–191. doi: 10.1166/jbn.2010.1105. [DOI] [PubMed] [Google Scholar]
- 162.Shukla R., Chanda N., Zambre A., Upendran A., Katti K., Kulkarni R.R., Nune S.K., Casteel S.W., Smith C.J., Vimal J., et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proc. Natl. Acad. Sci. USA. 2012;109:12426–12431. doi: 10.1073/pnas.1121174109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chavva S.R., Deshmukh S.K., Kanchanapally R., Tyagi N., Coym J.W., Singh A.P., Singh S. Epigallocatechin Gallate-Gold Nanoparticles Exhibit Superior Antitumor Activity Compared to Conventional Gold Nanoparticles: Potential Synergistic Interactions. Nanomaterials. 2019;9:396. doi: 10.3390/nano9030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jiang P., Wu X., Wang X., Huang W., Feng Q. NEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin sensitivity in lung cancer cells. Oncotarget. 2016;7:43337–43351. doi: 10.18632/oncotarget.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kuo Y.-C., Wang L.-J., Rajesh R. Targeting human brain cancer stem cells by curcumin-loaded nanoparticles grafted with anti-aldehyde dehydrogenase and sialic acid: Colocalization of ALDH and CD44. Mater. Sci. Eng. C. 2019;102:362–372. doi: 10.1016/j.msec.2019.04.065. [DOI] [PubMed] [Google Scholar]
- 166.Zhou Q., Ye M., Lu Y., Zhang H., Chen Q., Huang S., Su S. Curcumin Improves the Tumoricidal Effect of Mitomycin C by Suppressing ABCG2 Expression in Stem Cell-Like Breast Cancer Cells. PLoS ONE. 2015;10:e0136694. doi: 10.1371/journal.pone.0136694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Summerlin N., Soo E., Thakur S., Qu Z., Jambhrunkar S., Popat A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015;479:282–290. doi: 10.1016/j.ijpharm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 168.Jhaveri A., Deshpande P., Pattni B., Torchilin V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release. 2018;277:89–101. doi: 10.1016/j.jconrel.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lian B., Wu M., Feng Z., Deng Y., Zhong C., Zhao X. Folate-conjugated human serum albumin-encapsulated resveratrol nanoparticles: Preparation, characterization, bioavailability and targeting of liver tumors. Art. Cells Nanomed. Biotechnol. 2019;47:154–165. doi: 10.1080/21691401.2018.1548468. [DOI] [PubMed] [Google Scholar]
- 170.Wang X.-X., Li Y.-B., Yao H.-J., Ju R.-J., Zhang Y., Li R.-J., Yu Y., Zhang L., Lu W.-L. The use of mitochondrial targeting resveratrol liposomes modified with a dequalinium polyethylene glycol-distearoylphosphatidyl ethanolamine conjugate to induce apoptosis in resistant lung cancer cells. Biomaterials. 2011;32:5673–5687. doi: 10.1016/j.biomaterials.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 171.Mohanty R.K., Thennarasu S., Mandal A.B. Resveratrol stabilized gold nanoparticles enable surface loading of doxorubicin and anticancer activity. Colloids Surfaces B Biointerfaces. 2014;114:138–143. doi: 10.1016/j.colsurfb.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 172.Huang X., Dai Y., Cai J., Zhong N., Xiao H., McClements D.J., Hu K. Resveratrol encapsulation in core-shell biopolymer nanoparticles: Impact on antioxidant and anticancer activities. Food Hydrocoll. 2016;64:157–165. doi: 10.1016/j.foodhyd.2016.10.029. [DOI] [Google Scholar]
- 173.Kamal R., Chadha V.D., Dhawan D.K. Physiological uptake and retention of radiolabeled resveratrol loaded gold nanoparticles (99mTc-Res-AuNP) in colon cancer tissue. Nanomedicine. 2018;14:1059–1071. doi: 10.1016/j.nano.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 174.Pund S., Thakur R., More U., Joshi A. Lipid based nanoemulsifying resveratrol for improved physicochemical characteristics, in vitro cytotoxicity and in vivo antiangiogenic efficacy. Colloids Surfaces B Biointerfaces. 2014;120:110–117. doi: 10.1016/j.colsurfb.2014.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not report any data.