Abstract
Background
Knowledge regarding the long-term impact of invasive mechanical ventilation on the inspiratory muscles and functional outcomes in COVID-19 survivors is limited.
Methods
In this single-centre prospective cohort study, we evaluated invasively ventilated patients with COVID-19 pneumonia 3 and 6 months post-intensive care unit (ICU) discharge. Outcomes included: maximal inspiratory pressure (MIP), ultrasound parameters for diaphragm function, 6-min walk distance (6MWD), dyspnoea and quality of life. We evaluated associations between MIP and duration of mechanical ventilation with follow-up outcomes.
Results
50 COVID-19 survivors discharged from ICU between 15 October 2020 and 1 April 2021 were enrolled. Overall, survivors showed a recovery trajectory over time. However, impaired MIP remained in 24 (48%) and 12 (24%) at 3 and 6 months, respectively. Diaphragm dysfunction was not observed. At 3 months, 23 (46%) had impaired functional capacity versus 10 (20%) at 6 months. Dyspnoea persisted in 44 (88%) patients at 3 months and 38 (76%) at 6 months. Quality of life was slightly decreased at 3 months with further improvements at 6 months. MIP was correlated to 6MWD, 6MWD % predicted, dyspnoea across follow-up, and quality of life at 3 months. The duration of invasive ventilation was correlated with 6MWD and 6MWD % predicted.
Conclusion
In invasively ventilated COVID-19 survivors, inspiratory muscle strength impairments persisted 6 months after ICU discharge, while maintaining normal diaphragm function. Decreased functional capacity, dyspnoea and slightly reduced health status were observed. Early screening of survivors is of utmost importance to identify those with impairments and at risk of delayed or incomplete recovery.
Short abstract
A proportion of #COVID19 survivors who undergo invasive mechanical ventilation show inspiratory muscle weakness 6 months after ICU discharge. Early screening and rehabilitation may be beneficial for optimal recovery after ICU discharge. https://bit.ly/3TepU29
Introduction
Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, vast numbers of patients have required admission to an intensive care unit (ICU) worldwide due to pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Patients with COVID-19 infection may rapidly develop acute respiratory distress syndrome (ARDS), which may necessitate life support with invasive mechanical ventilation. There is growing evidence on the short- to long-term outcomes following hospitalisation for acute COVID-19 infection that indicate new disability across all areas of functioning [2–5]. While most studies have investigated mixed severity cohorts of non-ICU and ICU patients with varying requirements for respiratory failure support, the specific functional outcomes of mechanically ventilated COVID-19 survivors are not yet fully understood.
Moreover, the impact of critical illness on inspiratory muscle function has received little attention in follow-up studies investigating physical function outcomes of COVID-19 ICU patients. Mechanically ventilated patients with COVID-19 often require prolonged invasive support, frequent neuromuscular blockade and corticosteroid therapy [6–8]. These are known risk factors for developing common neuromuscular complications for critically ill individuals, such as peripheral muscle weakness [9] and respiratory muscle weakness, also known as ventilator-induced diaphragm dysfunction [10]. Prior research indicates that inspiratory muscle weakness is present twice as often as peripheral muscle weakness in general populations of critically ill patients [11]. Weakness of the inspiratory muscles is associated with delays in weaning [12], long-term mortality [13] and worse physical function and quality of life at 5 years in ICU survivors [14]. Thus, a better understanding of the role of the inspiratory muscles in the overall recovery from critical disease in invasively ventilated COVID-19 patients is essential to identify functional impairments and to design effective rehabilitation interventions.
To address these research gaps, we conducted an observational longitudinal study to describe the functional outcomes and time course of recovery of a cohort of invasively ventilated COVID-19 survivors 3 and 6 months after ICU discharge. Post-ICU functional outcomes included inspiratory muscle strength and function, as our primary outcome, together with functional capacity, dyspnoea and health-related quality of life. Secondary aims were to examine possible associations between inspiratory muscle strength and the duration of invasive mechanical ventilation with post-ICU outcomes.
Methods
Study design and participants
This was an observational, prospective, single-centre study investigating the 3- and 6-month health outcomes of a cohort of adult (≥18 years old) critically ill COVID-19 ICU survivors from a tertiary public hospital in Madrid, Spain. Consecutive adults who were discharged from ICU between 15 October 2020 and 1 April 2021 following ≥48 h of invasive mechanical ventilation due to laboratory-confirmed SARS-CoV-2 pneumonia were assessed for eligibility.
Patients who were unable to perform follow-up tests due to medical contraindications or any health issues, had cognitive/communication deficit or known baseline independent mobility impairments were excluded.
Ethical approval was obtained from the institutional Clinical Research Ethics Committee (approval reference 419/20). Written informed consent was obtained on the first follow-up visit. The study was conducted and reported in line with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [15] and was registered in ClinicalTrials.gov (identifier NCT04853940).
Procedures
Eligible patients were contacted by telephone by the research coordinator and were invited to attend follow-up appointments 3 and 6 months post-ICU discharge at hospital facilities. Free hospital parking and fully funded transportation was available for all patients.
Data on demographics, comorbidities, ICU and hospital clinical characteristics were retrospectively retrieved from electronic patient records. Anthropometric data (height, weight and body mass index (BMI)) were collected during follow-up visits.
Outcomes
During follow-up visits, patients underwent physical examination to evaluate functional outcomes including inspiratory muscle strength and diaphragmatic function, as the main outcomes, and submaximal exercise capacity. Patient-reported outcomes for dyspnoea and health-related quality of life were also assessed. All evaluations were performed by a research team of five trained physiotherapists. Re-tests were not necessarily carried out by the same assessor.
Inspiratory muscle strength
Global inspiratory muscle strength was determined by measuring the maximal inspiratory pressure (MIP) using a hand-held commercial pressure transducer (RP Check; MD Diagnostics Ltd, Chatham, UK) via a single-patient use, flanged mouthpiece in accordance with guidelines [16]. Patients were instructed to perform a maximal inspiratory effort from residual volume in a seated position, without a nose clip. Encouragement was provided during the test. The highest value of three measurements within 20% variation was recorded. Measurements were reported as absolute values and as the percentage of predicted values by reference equations for the Spanish population [17]. Inspiratory muscle strength was considered impaired when MIP was lower than 70% predicted.
Diaphragm thickness and function
The diaphragm muscle was evaluated by real-time ultrasound imaging with a portable ultrasound system (Minisono; Alpinion Medical Systems, Anyang, South Korea) according to the method described by Boon et al. [18]. With the patient in a supine lying position, sonographic images of the diaphragm thickness at functional residual capacity, i.e. at rest and then at the end of a deep inspiratory effort, were obtained. The mean of three measurements was recorded and the diaphragm thickening ratio was subsequently calculated according to the formula: diaphragm thickness at the end of deep inspiration/diaphragm thickness at rest. Two researchers (the examiner and another member of the research team) reviewed the sonographic images and reached a consensus on the measurements if there were any disagreements.
Diaphragm thickness has been associated with diaphragmatic inspiratory strength in healthy subjects [19]. The diaphragm thickening ratio is an indirect measure of inspiratory effort and an index of diaphragm dysfunction [20]. Established lower limit of normality (LLN) values for diaphragm thickness (<0.15 cm) and diaphragm thickening ratio (<1.2) were used as thresholds for diaphragm thickness abnormality and diaphragm dysfunction, respectively [18].
Functional capacity
Submaximal functional exercise capacity was evaluated with the self-paced, field six-min walk test (6MWT) on a 30-m clear course [21]. Each participant performed the test once at each follow-up visit. The distance walked in 6 min (6MWD) was obtained. Absolute values and percentage of predicted according to published regression equations [22] were reported. Impaired functional capacity was established at 70% predicted.
Heart rate, oxyhaemoglobin saturation (SpO2) and the modified Borg rating of perceived exertion scale for shortness of breath and lower limb fatigue (range 0–10, higher scores indicate greater degree of perceived exertion) [23] were recorded pre-test and immediately post-test. Exercise-induced desaturation was defined as a fall in SpO2 of ≥4% at end-test [24]. Patients who were on continuous supplemental oxygen or who were walking-aid users were allowed to perform the test with these aids according to their own preference.
Patient-reported outcomes
The modified British Medical Research Council scale (mMRC) [25] was used to classify the impact of dyspnoea on daily activities into five possible grades from 0 to 4, with 4 being the worst degree. Patients were asked for symptoms of new or worsened dyspnoea over the last week in relation to their pre-COVID-19 status. Grade 2 was adapted to “usual” instead of “people my age” to allow reporting of breathlessness related to their pre-COVID-19 health status [26].
Health-related quality of life was determined with the respiratory specific St George's Respiratory Questionnaire (SGRQ) [27] (score range 0–100, lower values indicate better health status) based on three domains: symptoms, activity and impact. The validated Spanish version was administered in interview format at each assessment visit, taking care not to influence patient's responses. The recall period was adapted from “the last year” to “the last 4 weeks”. Scores were calculated with the application available at http://sgrq.github.io/.
Statistics
Descriptive data are presented as the median (25th−75th percentiles) for continuous variables and frequencies (percentages) for categorical data. Normality of distribution was determined by the Kolmogorov–Smirnov test. Comparisons between timepoints data were conducted using paired parametric (t-test) and non-parametric (Mann–Whitney U-test and chi-squared test) tests, as appropriate. The correlation analysis was performed using Spearman's rank correlation coefficients. Two-sided p-values <0.05 were considered statistically significant for all tests. Analysis was performed in SPSS Version 25.0 (IBM SPSS Statistics for Macintosh, Armonk, NY, USA).
Results
Study cohort
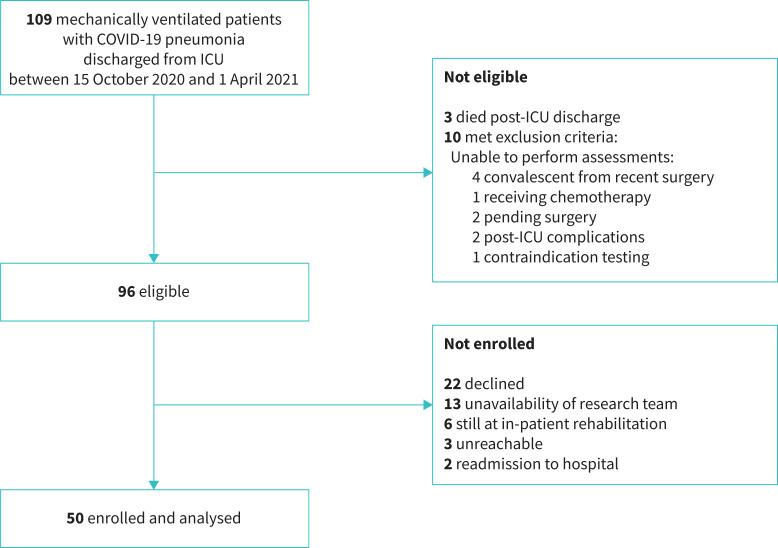
Between 15 October 2020 and 1 April 2021, a total of 109 mechanically ventilated patients with COVID-19 pneumonia were consecutively discharged from ICU. Three (2.7%) patients had died following hospital discharge at initial study contact. Survivors were screened for eligibility. A total of 50 patients were enrolled and completed the assessments at 3 and 6 months. The flow-chart of participants is depicted in figure 1.
FIGURE 1.
Study flow diagram. ICU: intensive care unit.
Median (25th–75th percentile) days from ICU discharge were 89 (86–93) and 174 (113–133) for the first and second follow-up visit, respectively; and 119 (113–183) and 205 (109−222) from symptom onset.
An overview of patients’ characteristics is summarised in table 1. Median (25th–75th percentile) age was 61 (52–68) years, 26 (52%) were men and median BMI was 28 (27−34) kg·m−2. With regards to medical history, seven (14%) had a diagnosis of COPD or asthma, and 12 (24%) were former or current smokers. Duration of invasive ventilation was 11 (8–22) days, with 13 (26%) receiving prolonged mechanical ventilation (≥21 days). All patients were treated with a course of intravenous dexamethasone during their admission, and almost all (46; 92%) received continuous neuromuscular blockade during mechanical ventilation. Upon hospital discharge, 13 (26%) patients were referred to an inpatient rehabilitation facility.
TABLE 1.
Baseline and clinical characteristics of invasively ventilated COVID-19 survivors (n=50)
| Baseline characteristics | |
| Age years | 61 (52–68) |
| Sex, male | 26 (52) |
| BMI kg·m−2 | 28 (27–34) |
| BMI group | |
| Normal (18.5–24.9 kg·m−2) | 5 (10) |
| Overweight (25–29.9 kg·m−2) | 26 (52) |
| Obese (>30 kg·m−2) | 19 (38) |
| Comorbidities | |
| Hypertension | 21 (42) |
| Dyslipidaemia | 14 (28) |
| Diabetes mellitus | 12 (24) |
| COPD | 4 (8) |
| Asthma | 3 (6) |
| Active malignancy | 3 (6) |
| SAHS | 3 (6) |
| Ischaemic heart disease | 1 (2) |
| Chronic renal disease | 1 (2) |
| Smoking history | |
| Never | 33 (66) |
| Former | 10 (20) |
| Current | 2 (4) |
| Unknown | 5 (10) |
| Charlson Comorbidity Index | 2 (1–4) |
| Clinical characteristics | |
| SOFA | 6.5 (4.5–7) |
| Invasive mechanical ventilation days | 11 (8–22) |
| Prolonged mechanical ventilation (≥21 days) | 13 (26) |
| Tracheostomy | 11 (22) |
| Re-intubation | 6 (12) |
| Pulmonary thromboembolism | 9 (18) |
| Continuous neuromuscular blockade | 46 (92) |
| Systemic corticosteroids during admission# | 50 (100) |
| Length of stay in ICU days | 15 (12–29) |
| Length of stay post-ICU days | 7.5 (6–13) |
| Length of stay in hospital days | 27.5 (23–45) |
| Supplemental O2 | |
| Hospital discharge | 27 (54) |
| 3 months follow-up visit | 3 (6) |
| 6 months follow-up visit | 2 (4) |
| Hospital discharge destination | |
| Home | 20 (40) |
| Home with follow-up physiotherapy | 15 (30) |
| Inpatient rehabilitation | 13 (26) |
| Home with outpatient physiotherapy | 2 (4) |
Data are presented as median (25th–75th percentile) for continuous variables and n (%) for categorical variables. BMI: body mass index; SAHS: sleep apnoea–hypopnoea syndrome; SOFA: sequential organ failure assessment; ICU: intensive care unit. #: course of intravenous dexamethasone.
Table 2 presents the results for the outcome assessments at 3 and 6 months.
TABLE 2.
Outcomes at 3 and 6 months post-intensive care unit (ICU) discharge for mechanically ventilated COVID-19 survivors and significance of change over time
| Outcome | 3 months | 6 months | p-value |
| Subjects n | 50 | 50 | |
| Days from ICU discharge | 89 (86–93) | 174 (113–133) | |
| Days from symptom onset | 119 (113–183) | 205 (194–222) | |
| Inspiratory muscle strength | |||
| MIP cmH2O | 73 (51.5–102) | 86.5 (67.5–111) | ≤ 0. 001 |
| MIP % predicted | 70 (60–89) | 87.6 (70–99) | ≤ 0. 001 |
| MIP <70% predicted | 24 (48) | 12 (24) | ≤ 0. 001 |
| Diaphragm ultrasound imaging | |||
| Diaphragm thickness mm | 0.20 (0.16–0.28) | 0.24 (0.17–0.28) | 0.051 |
| Diaphragm thickness <LLN | 4 (8) | 1 (2) | 0.100 |
| Diaphragm thickening ratio | 1.54 (1.42–1.72) | 1.65 (1.52–1.79) | 0. 009 |
| Diaphragm thickening ratio <LLN | 0 (0) | 0 (0) | |
| Functional capacity | |||
| 6MWD | 442.5 (384–537) | 520 (405–588) | ≤ 0. 001 |
| 6MWD % predicted | 71 (66–82) | 82 (71.5–90) | ≤ 0. 001 |
| 6MWD <70% predicted | 23 (46) | 10 (20) | ≤ 0. 001 |
| SpO2 pre-test | 96 (96–98) | 96 (95–97) | 0. 043 |
| SpO2 immediately post-test | 95 (93–96) | 95 (93–97) | 0.223 |
| ΔSpO2 | 2.5 (1–4) | 1 (−0.25–4) | 0. 05 |
| Post-test desaturation (ΔSpO2 ≥4%) | 14 (28) | 14 (28) | |
| Breathlessness Borg score | 3 (2–4) | 3 (2–4) | 0.935 |
| Lower limb fatigue Borg score | 1 (0–3) | 2 (0–3) | 0.723 |
| Dyspnoea | |||
| mMRC score | 1 (1–2) | 1 (0.75–1) | ≤ 0. 001 |
| mMRC score grade | ≤ 0. 001 | ||
| 0 | 5 (10) | 12 (24) | |
| 1 | 24 (48) | 27 (54) | |
| 2 | 13 (26) | 8 (16) | |
| 3 | 3 (6) | 1 (2) | |
| 4 | 5 (10) | 2 (4) | |
| Health-related quality of life | |||
| SGRQ total score | 20.53 (9.74–30.70) | 13.05 (3.85–21.38) | ≤ 0. 001 |
| SGRQ symptoms domain | 0.0 (0.0–15.78) | 0.0 (0.0–9.21) | 0.049 |
| SGRQ activity domain | 37.75 (18.73–55.58) | 25.9 (6.07–45.91) | 0. 001 |
| SGRQ impact domain | 6.85 (2.42–22.83) | 4.4 (0.0–12.04) | 0. 001 |
Data are presented as median (25th–75th percentile) for continuous variables and n (%) for categorical variables. MIP: maximal inspiratory pressure; LLN: lower limit of normality; 6MWD: 6-min walk distance; SpO2: pulse oximeter oxyhaemoglobin saturation; mMRC: modified Medical Research Council; SGRQ: St George's Respiratory Questionnaire. Bold values denote statistical significance p<0.05.
Inspiratory muscle strength and diaphragm ultrasound imaging
Absolute MIP significantly increased between follow-ups. However, median relative MIP remained lower compared to healthy population norms with 70% (60–89%) and 87.6% (70–99%) predicted at 3 and 6 months, respectively. Overall, the number of patients with impaired muscle strength was 24 (48%) and 12 (24%) at each sequential timepoint.
Regarding sonographic assessments, diaphragm thickness at rest and diaphragm thickening ratio significantly increase between follow-ups. Values of diaphragm thickness below LLN, i.e. 5th percentile of a healthy population, were present in four (8%) and one (2%) patients at sequential follow-up visits. Overall, none of the observed individual measures for diaphragm thickening ratio identified diaphragmatic dysfunction at any timepoint.
Functional capacity
Overall, 6MWD significantly increased over time. Nevertheless, the median distance walked was reduced to 71% (66–82%) and 82% (71.5–90.0%) predicted at 3 and 6 months, respectively. A total of 23 (46%) and 10 (20%) COVID-19 ICU survivors had functional capacity lower than 70% predicted at each sequential assessment. Two patients performed the 6MWT with light portable O2 equipment at 3 months, and one of them at 6 months. One patient performed tests using a walking frame. Borg score at end-test was moderate for breathlessness, and mild for leg fatigue at 3 and 6 months. Exercise-induced desaturation was present in 14 (28%) patients at 3 and 6 months. 10 (25%) patients had an unchanged desaturation response between assessments. Four of them sustained pulmonary embolism during ICU admission and one had COPD.
Patient-reported outcomes
Median mMRC dyspnoea scores significantly improved over the 6 months follow-up. Nonetheless, 24 (48%) survivors reported a persistent degree of mild (grade 1) dyspnoea with daily activities at 3 months and 27 (54%) at 6 months. The number of patients reporting moderate-to-severe dyspnoea (mMRC ≥2) decreased from 21 (42%) to 11 (22%) over the 6-month period.
A mild reduction was noted in the self-reported scores for overall health status, as shown by a decrease in the SGRQ score, with statistical improvement from 3 to 6 months. All three individual questionnaire domains also improved over time. At 3 and 6 months, the activity domain showed the greatest impairment, followed by impact and symptoms domains.
Associations
Table 3 summarises the associations between MIP and follow-up outcomes, and between the duration of invasive ventilation and inspiratory muscle parameters. MIP showed a significant correlation with 6MWD, 6MWD % predicted and a negative correlation with dyspnoea mMRC, across follow-up; and with SGRQ scores at 3 months, and diaphragm thickening ratio at 6 months. The duration of mechanical ventilation was inversely correlated with the 6MWD, 6MWD % predicted and dyspnoea scores at 3 months.
TABLE 3.
Spearman's correlation coefficients between maximal inspiratory pressure and follow-up outcomes, and between duration of mechanical ventilation and inspiratory muscle outcomes
| Variable | 3 months | 6 months |
| Maximal inspiratory pressure | ||
| Diaphragm thickness | 0.312* | 0.273 |
| Diaphragm thickening ratio | 0.180 | 0.308* |
| 6MWD | 0.657** | 0.622** |
| 6MWD % predicted | 0.380** | 0.284* |
| Dyspnoea mMRC score | −0.365** | −0.379** |
| Borg dyspnoea | −0.097 | −0.015 |
| SGRQ | −0.349* | −0.277 |
| Duration of mechanical ventilation | ||
| Maximal inspiratory pressure | −0.182 | −0.162 |
| Maximal inspiratory pressure, % predicted | −0.105 | −0.029 |
| Diaphragm thickness | −0.257 | −0.163 |
| Diaphragm thickening ratio | −0.106 | −0.137 |
| 6MWD | −0.389** | −0.365** |
| 6MWD % predicted | −0.427** | −0.389** |
| Dyspnoea mMRC score | 0.424** | 0.183 |
| SGRQ | 0.160 | 0.127 |
6MWD: 6-min walk distance; mMRC: modified Medical Research Council; SGRQ: St George's Respiratory Questionnaire. *: p<0.05; **: p<0.01.
Discussion
This single-centre prospective study provides insight into the post-ICU recovery of severe, invasively ventilated COVID-19 survivors. While patients experienced an overall recovery trajectory over the initial 6 months, impaired inspiratory muscle strength was present in 24 (48%) patients at 3 months and remained in 12 (24%) at 6 months. Despite this, ultrasound imaging did not identify diaphragm dysfunction. Functional capacity impairment was observed in 46% and 20% of survivors at subsequent follow-up timepoints. Mild to moderate dyspnoea and mild reductions in quality of life persisted at 6 months. Significant negative correlations were found between inspiratory muscle strength with functional capacity and dyspnoea across follow-up, and with health status at 3 months. The duration of mechanical ventilation was correlated with functional capacity at 3 and 6 months, and dyspnoea at 3 months, but not with inspiratory muscle outcomes. To our knowledge, the present study is the first reporting on longitudinal outcomes of inspiratory muscle strength and diaphragmatic function in a homogeneous cohort of mechanically ventilated COVID-19 survivors.
Current evidence about the impact of invasive ventilation on the inspiratory muscles in patients with COVID-19 pneumonia is confined to a few studies reporting outcomes for heterogeneous cohorts including small subsets of invasive ventilated individuals. In line with our results, impaired inspiratory muscle strength was found in 42% at 3 months in ICU survivors, including 14 intubated patients [28], and in 53% of 17 severe patients at 30 days [3]. Reduced values (68% predicted) were also reported in intubated patients at 4 months, with no significant difference with non-ventilated survivors [29]. It is noteworthy that high rates of peripheral muscle weakness have also been reported in mechanically ventilated COVID-19 individuals during the acute hospitalisation phase [30, 31]. Our study suggests that neuromuscular complications of mechanical ventilation may similarly impact the inspiratory muscles in a substantial proportion of COVID-19 survivors.
Despite this, diaphragmatic parameters for structure and function remained mostly within the normal limits in our patients. In contrast, in a small cohort of mechanically ventilated COVID-19 patients admitted to inpatient rehabilitation, Farr et al. [32] found that 18% had lower resting diaphragm thickness compared to non-COVID-19 ICU survivors, and that 66% had reduced diaphragm thickening ratio. Differences with our results may be explained by the longer duration of mechanical ventilation (mean±sd 48±35 versus 16±12 days) and earlier testing. It should be noted that the duration of invasive ventilation did not show a significant linear correlation to any of the inspiratory muscle physiology measurements in our cohort. Whether COVID-19 patients who require exceptionally long periods of mechanical ventilation may sustain greater diaphragm impairment, and its clinical significance, requires further investigation.
In accordance with published reports including subsets of ICU patients, we found similar reductions in functional capacity that improved gradually over time [33–35]. In mixed cohorts of hospitalised COVID-19 patients, ICU survivors showed greater functional capacity deficits than non-ICU patients at earlier post-acute phases [36, 37], but differences evened out after 1 year in most patients [38]. In our cohort, impaired physical performance remained in 46% and 20% of subjects throughout timepoints. Functional capacity was strongly associated with inspiratory muscle strength over time. In fact, inspiratory muscle weakness after critical illness was an independent predictor of long-term lower functional capacity in general ICU populations [14]. This finding highlights that inspiratory muscle weakness must not be overlooked when evaluating the impact of critical illness in COVID-19 patients.
Similarly, our patients presented with ongoing mild/moderate dyspnoea and slight reductions in self-reported quality of life, with the highest impact on the physical status domain, in line with previous studies [36, 39, 40]. Importantly, we found that lower MIP was moderately correlated with higher mMRC dyspnoea and worse self-reported health status. Moreover, moderate exertional dyspnoea as measured by the Borg scale, and exercise-induced desaturations remained throughout the 6 months follow-up. Vitacca et al. [41] found that mMRC dyspnoea scores but not Borg dyspnoea were correlated with exercise-induced desaturation in post-COVID-19 lung disease, including 35% of intubated survivors. We also found contradictory results as mMRC dyspnoea was correlated with MIP but not with Borg scores. The underlying mechanisms of persistent dyspnoea in COVID-19 are yet to be fully elucidated. Residual lung fibrosis and pulmonary vascular pathology have been identified as possible causes [42]. Additional research should look at the potential contribution of inspiratory muscle weakness in survivors with invasive support requirements. Meanwhile, inspiratory muscle strength training has established effects in improving perceived exertion and quality of life in critically ill patients [43], as well as submaximal exercise capacity in chronic respiratory disease populations [44]. Our findings suggest that invasively ventilated COVID-19 survivors with poorer functional outcomes may benefit from multimodal rehabilitation programmes that include inspiratory muscle strength training.
A question that remains is whether inspiratory muscle weakness plays a role in the abnormal lung function parameters described in severe COVID-19 survivors, or whether the latter can be exclusively attributed to lung parenchymal changes. Although reduced diffusing capacity is the most common lung function abnormality reported in COVID-19 patients [45], mild restrictive physiology is also seen in the first few months post infection [3, 28, 46, 47] lasting up to 12 months [36]. In a multicenter study, severe/critical COVID-19 patients, including mechanically ventilated survivors, paradoxically showed higher MIP but lower total lung capacity compared to younger patients with mild/moderate disease [37]. Anastasio et al. [29] reported similar results for mechanically ventilated compared to non-ventilated COVID-19 survivors. It is unclear why critically ill COVID-19 patients experienced a lesser impact on inspiratory muscle strength than less severe patients. Cohort heterogeneity, the number of missing observations and other methodological issues may explain these unexpected results. Since we did not perform pulmonary function testing or radiological assessments, we were not able to evaluate whether restrictive lung volumes or fibrotic lesions were present in our cohort. However, a proportion of our patients experienced notable impairments in inspiratory muscle strength, with MIP absolute values as low as 25–45 cm H2O in some cases. These findings could indicate that unrecovered inspiratory muscle strength may contribute to restrictive physiology in invasively ventilated COVID-19 survivors, which might be multifactorial otherwise. Consequently, our results underline the importance of screening COVID-19 survivors to identify those who would benefit from further rehabilitation intervention with the aim of restoring optimal inspiratory muscle function.
Limitations
Generalisability is inherently limited in single-centre observational designs. Besides, this study has other limitations. First, only 50 out of 109 COVID-19 ICU survivors were enrolled. Hence, results may not be representative of the whole cohort. Additionally, some eligible patients were still at a rehabilitation centre or required further admission to hospital. Therefore, our findings may not capture the full spectrum of outcomes, particularly of those patients with greater post-ICU disability. Second, pre-morbid baseline assessments were not available. As a consequence, we cannot ascertain whether the observed impairments can be attributed to post-COVID-19 critical illness or were pre-existing. Lastly, the multiplicity of assessors may have potentially limited the reproducibility of measurements.
Conclusions
Despite showing an overall recovery over the 6 months after ICU discharge, a substantial proportion of mechanically ventilated COVID-19 survivors experienced persisting impairments in inspiratory muscle strength, while maintaining normal diaphragmatic function. In addition, reduced functional capacity, ongoing mild-to-moderate dyspnoea and slight decrease in quality of life were frequent over the follow-up period. Early screening of residual impairments in survivors is of utmost importance to identify those who may benefit form rehabilitation interventions, with the aim to achieve optimal and timely recovery after COVID-19 critical illness.
Acknowledgements
The authors thank the patients for their participation in this study.
Provenance: Submitted article, peer reviewed.
This article has an editorial commentary: https://doi.org/10.1183/23120541.00521-2022
Data availability: The dataset used during the current study is available from the corresponding author on reasonable request.
Author contributions: M.N. Núñez-Seisdedos was responsible for conceptualisation, methodology, formal analysis, writing (original draft, and revision and editing), supervision and project administration. M.N. Núñez-Seisdedos, D. Valcárcel-Linares, M.T. Gómez-González, I. Lázaro-Navas and L. López-González contributed to data collection. M.N. Núñez-Seisdedos, L. López-González and I. Lázaro-Navas contributed to funding acquisition. I. Rodríguez-Costa and D. Pecos-Martín reviewed the original draft. All authors approved the final version of the manuscript.
Conflict of interest: M.N. Núñez-Seisdedos has nothing to disclose.
Conflict of interest: D. Valcárcel-Linares has nothing to disclose.
Conflict of interest: M.T. Gómez-González has nothing to disclose.
Conflict of interest: I. Lázaro-Navas has nothing to disclose.
Conflict of interest: L. López-González has nothing to disclose.
Conflict of interest: D. Pecos-Martín has nothing to disclose.
Conflict of interest: I. Rodríguez-Costa has nothing to disclose.
Support statement: This study was supported by the Colegio Profesional de Fisioterapeutas de la Comunidad de Madrid Ayudas a la Investigación en Fisioterapia y COVID and Biomedical Research Foundation of the Ramón y Cajal University Hospital, Spain, grant FiBioHRC 2020/0351. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Jo hns Hopkins Coronavirus Resource Center. Weekly Hospitalization Trends. https://coronavirus.jhu.edu/data/hospitalization-7-day-trend Date last accessed: 19 June 2022.
- 2.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. . More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021; 11: 16144. doi: 10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Y, Tan C, Wu J, et al. . Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res 2020; 21: 163. doi: 10.1186/s12931-020-01429-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodgson CL, Higgins AM, Bailey MJ, et al. . The impact of COVID-19 critical illness on new disability, functional outcomes and return to work at 6 months: a prospective cohort study. Crit Care 2021; 25: 382. doi 10.1186/s13054-021-03794-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Huang L, Wang Y, et al. . 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lönnqvist PA, Bell M, Karlsson T, et al. . Does prolonged propofol sedation of mechanically ventilated COVID-19 patients contribute to critical illness myopathy? Br J Anaesth 2020; 125: e334–e336. doi: 10.1016/j.bja.2020.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courcelle R, Gaudry S, Serck N, et al. . Neuromuscular blocking agents (NMBA) for COVID-19 acute respiratory distress syndrome: a multicenter observational study. Crit Care 2020; 24: 446. doi: 10.1186/s13054-020-03164-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health. COVID-19 Treatment Guidelines: Corticosteroids. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/ Date last updated: 31 May 2022. Date last accessed: 19 June 2022. [PubMed]
- 9.Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 2011; 10: 931–941. doi: 10.1016/S1474-4422(11)70178-8 [DOI] [PubMed] [Google Scholar]
- 10.Supinski GS, Morris PE, Dhar S, et al. . Diaphragm dysfunction in critical illness. Chest 2018; 153: 1040–1051. doi: 10.1016/j.chest.2017.08.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dres M, Dubé BP, Mayaux J, et al. . Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med 2017; 195: 57–66. doi: 10.1164/rccm.201602-0367OC [DOI] [PubMed] [Google Scholar]
- 12.Jung B, Moury PH, Mahul M, et al. . Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med 2016; 42: 853–861. doi: 10.1007/s00134-015-4125-2 [DOI] [PubMed] [Google Scholar]
- 13.Medrinal C, Prieur G, Frenoy É, et al. . Respiratory weakness after mechanical ventilation is associated with one-year mortality – a prospective study. Crit Care 2016; 20: 231. doi: 10.1186/s13054-016-1418-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Aerde N, Meersseman P, Debaveye Y, et al. . Five-year outcome of respiratory muscle weakness at intensive care unit discharge: secondary analysis of a prospective cohort study. Thorax 2021; 76: 561–567. doi: 10.1136/thoraxjnl-2020-216720 [DOI] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, et al. . Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806–808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Thoracic Society/European Respiratory Society . ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002; 166: 518–624. doi: 10.1164/rccm.166.4.518 [DOI] [PubMed] [Google Scholar]
- 17.Morales P, Sanchis J, Cordero PJ, et al. . Presiones respiratorias estáticas máximas en adultos. Valores de referencia de una población caucasiana mediterránea. [Maximum static respiratory pressures in adults. The reference values for a Mediterranean Caucasian population.] Arch Bronconeumol 1997; 33: 213–219. doi: 10.1016/s0300-2896(15)30609-8 [DOI] [PubMed] [Google Scholar]
- 18.Boon AJ, Harper CJ, Ghahfarokhi LS, et al. . Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle Nerve 2013; 47: 884–889. doi: 10.1002/mus.23702 [DOI] [PubMed] [Google Scholar]
- 19.Goligher EC, Dres M, Fan E, et al. . Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med 2018; 197: 204–213. doi: 10.1164/rccm.201703-0536OC [DOI] [PubMed] [Google Scholar]
- 20.Boussuges A, Rives S, Finance J, et al. . Assessment of diaphragmatic function by ultrasonography: current approach and perspectives. World J Clin Cases 2020; 8: 2408–2424. doi: 10.12998/wjcc.v8.i12.2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 22.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998; 158: 1384–1387. doi: 10.1164/ajrccm.158.5.9710086 [DOI] [PubMed] [Google Scholar]
- 23.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–381. [PubMed] [Google Scholar]
- 24.Poulain M, Durand F, Palomba B, et al. 6-Minute walk testing is more sensitive than maximal incremental cycle testing for detecting oxygen desaturation in patients with COPD. Chest 2003; 123: 1401–1407. 10.1378/chest.123.5.1401. [DOI] [PubMed] [Google Scholar]
- 25.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93: 580–586. doi: 10.1378/chest.93.3.580 [DOI] [PubMed] [Google Scholar]
- 26.Tong A, Baumgart A, Evangelidis N, et al. . Core outcome measures for trials in people with coronavirus disease 2019: respiratory failure, multiorgan failure, shortness of breath, and recovery. Crit Care Med 2021; 49: 503–516. doi: 10.1097/CCM.0000000000004817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med 1991; 85: Suppl B, 25–31. doi: 10.1016/s0954-6111(06)80166-6 [DOI] [PubMed] [Google Scholar]
- 28.Truffaut L, Demey L, Bruyneel AV, et al. . Post-discharge critical COVID-19 lung function related to severity of radiologic lung involvement at admission. Respir Res 2021; 22: 29. doi: 10.1186/s12931-021-01625-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anastasio F, Barbuto S, Scarnecchia E, et al. . Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J 2021; 58: 2004015. Doi: 10.1183/13993003.04015-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Aerde N, Van den Berghe G, Wilmer A, et al. . Intensive care unit acquired muscle weakness in COVID-19 patients. Intensive Care Med 2020; 46: 2083–2085. doi: 10.1007/s00134-020-06244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Núñez-Seisdedos MN, Lázaro-Navas I, López-González L, et al. . Intensive care unit-acquired weakness and hospital functional mobility outcomes following invasive mechanical ventilation in patients with COVID-19: a single-centre prospective cohort study. J Intensive Care Med 2022; 37: 1005–1014. doi: 10.1177/08850666221100498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farr E, Wolfe AR, Deshmukh S, et al. . Diaphragm dysfunction in severe COVID-19 as determined by neuromuscular ultrasound. Ann Clin Transl Neurol 2021; 8: 1745–1749. doi: 10.1002/acn3.5141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien K, Townsend L, Dowds J, et al. . 1-year quality of life and health-outcomes in patients hospitalised with COVID-19: a longitudinal cohort study. Respir Res 2022; 23: 115. doi: 10.1186/s12931-022-02032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latronico N, Peli E, Calza S, et al. . Physical, cognitive and mental health outcomes in 1-year survivors of COVID-19-associated ARDS. Thorax 2022; 77: 300–303. doi: 10.1136/thoraxjnl-2021-218064 [DOI] [PubMed] [Google Scholar]
- 35.van Gassel RJJ, Bels J, Remij L, et al. . Functional outcomes and their association with physical performance in mechanically ventilated coronavirus disease 2019 survivors at 3 months following hospital discharge: a cohort study. Crit Care Med 2021; 49: 1726–1738. doi: 10.1097/CCM.0000000000005089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorent N, Vande Weygaerde Y, Claeys E, et al. . Prospective longitudinal evaluation of hospitalised COVID-19 survivors 3 and 12 months after discharge. ERJ Open Res 2022; 8: 00004-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guler SA, Ebner L, Aubry-Beigelman C, et al. . Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J 2021; 57: 2003690.doi: 10.1183/13993003.03690-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang L, Yao Q, Gu X, et al. . 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021; 398: 747–758. doi: 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taboada M, Moreno E, Cariñena A, et al. . Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br J Anaesth 2021; 126: e110–e113. doi: 10.1016/j.bja.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Writing Committee for the COMEBAC Study Group . Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021; 325: 1525–1534. doi: 10.1001/jama.2021.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vitacca M, Paneroni M, Brunetti G, et al. . Characteristics of COVID-19 pneumonia survivors with resting normoxemia and exercise-induced desaturation. Respir Care 2021; 66: 1657–1664. doi: 10.4187/respcare.09029 [DOI] [PubMed] [Google Scholar]
- 42.Dhawan RT, Gopalan D, Howard L, et al. . Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19. Lancet Respir Med 2021; 9: 107–116. doi: 10.1016/S2213-2600(20)30407-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bissett BM, Leditschke IA, Neeman T, et al. . Inspiratory muscle training to enhance recovery from mechanical ventilation: a randomised trial. Thorax 2016; 71: 812–819. doi: 10.1136/thoraxjnl-2016-208279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gosselink R, De Vos J, van den Heuvel SP, et al. . Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J 2011; 37: 416–425. doi: 10.1183/09031936.00031810 [DOI] [PubMed] [Google Scholar]
- 45.Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, et al. . Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology 2021; 27: 328–337. doi: 10.1016/j.pulmoe.2020.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker AJ, Humbir A, Tiwary P, et al. . Recovery after critical illness in COVID-19 ICU survivors. Br J Anaesth 2021; 126: e217–e219. doi: 10.1016/j.bja.2021.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramani C, Davis EM, Kim JS, et al. . Post-ICU COVID19 outcomes: a case series. Chest 2021; 159: 215–218. doi: 10.1016/j.chest.2020.08.2056 [DOI] [PMC free article] [PubMed] [Google Scholar]