Abstract
The use of filter paper is an inexpensive and convenient method for collecting, storing, and transporting blood samples for serological studies. In addition, samples occupy little space and can be readily transported without refrigeration. Rickettsial diseases often evolve according to an epidemic mode and are now considered reemerging diseases, especially in developing countries, under conditions where fieldwork could be difficult. The suitability of collecting whole-blood specimens on filter paper discs for rickettsial antibody assay was evaluated. Dried blood specimens from 64 individuals with antibodies to Coxiella burnetii, Bartonella quintana, or Rickettsia conorii were tested for rickettsial antibodies by microimmunofluorescence. Although occasional titers were 1 or 2 dilutions lower than those of tested serum samples, no statistically significant differences were observed. Among patients with negative serology, no false positives were found. This study demonstrated that the recovery of antibodies from finger-stick blood dried on filter paper after elution produces results comparable to those obtained by recovering antibodies from serum. Storing paper samples for 1 month at room temperature or at 4°C did not significantly affect the level of antibodies recovered. This report shows the utility of this sample collection method in developing countries where refrigeration is not possible and venipuncture is problematic.
There are considerable problems for field epidemiological studies and serological diagnoses of infectious diseases in developing countries. Difficulties of work in the field include a lack of medical facilities, which makes serum samples difficult to obtain. Venipuncture requires nurses or other qualified personnel, is expensive, requires disposable items such as test tubes, syringes, and needles, and is invasive. Moreover, blood samples need to be centrifuged and refrigerated, and test tubes can be broken. Accurate serological diagnosis requires an organized health care system with laboratory capabilities. Most often, there is no specialized laboratory operating in the developing area because tests are not only costly but also require sophisticated techniques and expertise. The use of blood, easily collected by pricking a finger or heel, spotted on filter paper could be a convenient and cheap alternative. For several years, blood spot specimens on blotting paper have frequently been used for the diagnosis and seroepidemiologic investigation of infectious diseases. This method has been applied to the diagnosis and seroepidemiologic survey of bacterial, viral, and parasitic diseases (2, 4, 5, 7–11, 15–23, 26, 27, 31, 33, 35–37, 39, 43). For rickettsial disease, one study was performed with scrub typhus (18) and another was performed to check the storage stabilities of different antibody species against Rickettsia prowazekii antigens in two samples of blood dried on filter paper discs (9).
The range of rickettsioses is wide and comprises roughly two types of infections: eruptive fevers (typhus, spotted fevers, and trench fever) whose transmission is due to an arthropod and Q fever, which is rarely transmitted by arthropods. Most rickettsioses usually occur as epidemics. Large outbreaks of Q fever due to Coxiella burnetii have been reported in the Basque country of Spain (1), Switzerland (14), Great Britain (41), and Berlin, Germany (32). Trench fever due to Bartonella quintana in homeless people has been described (6). Outbreaks of epidemic typhus due to R. prowazekii persist, especially in African and Russian populations suffering from poverty, famine, and lack of hygiene, which are particularly acute in refugee camps that favor the propagation of lice (29, 38, 42).
Laboratory support is essential for the assessment and management of rickettsial diseases, and reference laboratories can play a role by testing the dry samples sent from developing countries. For this purpose, we have applied a microimmunofluorescence technique adapted to dried samples collected on blotting paper after finger-sticks. However, significant delays can occur between collection and laboratory testing; this delay might expose the blood spots to hot, humid conditions and could possibly compromise the test results. The purpose of this study was to assess the accuracy of diagnosing rickettsial diseases by using whole blood collected on blotting paper and the potential of this technique for field use. For this effort, we chose to examine the accuracy of testing dried samples from patients infected by C. burnetii, B. quintana, or Rickettsia conorii and to compare the results with those obtained by microimmunofluorescence testing of regular serologic samples.
MATERIALS AND METHODS
Antigen preparation.
R. conorii (Moroccan strain, ATCC VR 141) was cocultivated with Vero cells and was purified as previously described (28). B. quintana (Oklahoma) was cocultivated with ECV 304 and was purified as previously described (25). C. burnetii (Nine Mile, ATCC VR 615) was cocultivated with L929 cells and was purified as previously described (13, 40). The products were resuspended in the smallest possible volumes of sterile water, and the protein contents of the purified organisms were determined by UV spectrophotometry and adjusted to 1 mg/ml. The antigens were subsequently stored at −20°C until immunofluorescence tests were performed.
Immunofluorescence tests.
Sera were tested with a microimmunofluorescence test (24). Rickettsial antigens were applied by pen point to 18-well microscope slides (Dynatech Laboratories, Billingshurt, United Kingdom). After application to slides, antigens were air dried for 30 min and fixed at room temperature for 10 min, in acetone for C. burnetii and R. conorii and in methyl alcohol for B. quintana. An appropriate positive- or negative-control serum was added to the antigen sets in the upper left corner of each slide. Twofold dilutions of sera were prepared in 3% nonfat dry milk in phosphate-buffered saline (PBS), placed onto the antigen slides, incubated in a moist chamber for 30 min at 37°C, and washed in two changes of PBS for 10 min each and in distilled water for 5 min. After the washings, the slides were treated with specific fluorescein isothiocyanate-conjugated goat anti-human γ chain and μ chain immunoglobulins (BioMérieux, Marcy l’Etoile, France) and rabbit anti-human α chain immunoglobulins (Behring, Marburg, Germany) for C. burnetii under the same conditions. After the conjugate was added, slides were incubated for 30 min at 37°C, washed in two changes of PBS for 10 min each and for 5 min in distilled water, and mounted in buffered glycerol. Endpoints for each antigen were the lowest concentrations in serum that definitely conferred fluorescence on bacteria. The diagnostic cutoff titers for R. conorii have been established as 1/128 for immunoglobulin G (IgG) and 1/64 for IgM (24). A cutoff titer of 1/100 has been demonstrated for B. quintana diagnosis (25). Cutoff values for C. burnetii were anti-phase II IgG titers of ≥200 and anti-phase IgM titers of ≥50 for the diagnosis of acute Q fever and anti-phase I IgG titers of ≥800 for the diagnosis of chronic Q fever (13).
Selection of patients.
Forty-one patients with antibody to C. burnetii, 14 patients with antibody to B. quintana, and 9 patients with antibody to R. conorii were tested. Blood was collected by venipuncture into tubes without anticoagulant for serum samples and into tubes containing EDTA-anticoagulant for samples on blotting paper.
Negative-control groups of sera, containing 30 samples each for C. burnetii, B. quintana, and R. conorii, were also tested as dried samples to assess false-positive results.
Blotting-paper test.
Blotting paper with a weight of 0.02 g/cm2 was obtained from Fischer Scientific (Elancourt, France). Spots of blood (75 μl) were dispensed on the paper. The blood spots were dried at ambient laboratory temperature for 4 h prior to storage. Discs were cut with a card punch to obtain 6-mm-diameter blood-impregnated discs. Blood spots were then eluted overnight in 250 μl of PBS and Tween 20 at 4°C. Eluted samples were used immediately or stored at −20°C until used. According to Bailey et al., a 6-mm-diameter disc saturated with blood yields an eluate equivalent to a 1/25 dilution of serum (3). This technique was standardized by concurrent tests on serum and blotting paper.
Storage of dried blood spots on filter paper.
Specimens were stored in two environments. The first environment was a refrigerator at 4°C, and the second was a non-air-conditioned room in the laboratory at a temperature ranging from 30 to 40°C. Blood-spotted papers were stored in paper envelopes. All specimens were tested at 4 weeks. Serological tests with serum samples and dried blood spots on filter paper were performed in parallel.
Statistical analyses.
The χ2 test and the Student’s t test (with all variances homogenous) were used. A P value of ≤0.05 was considered significant.
RESULTS
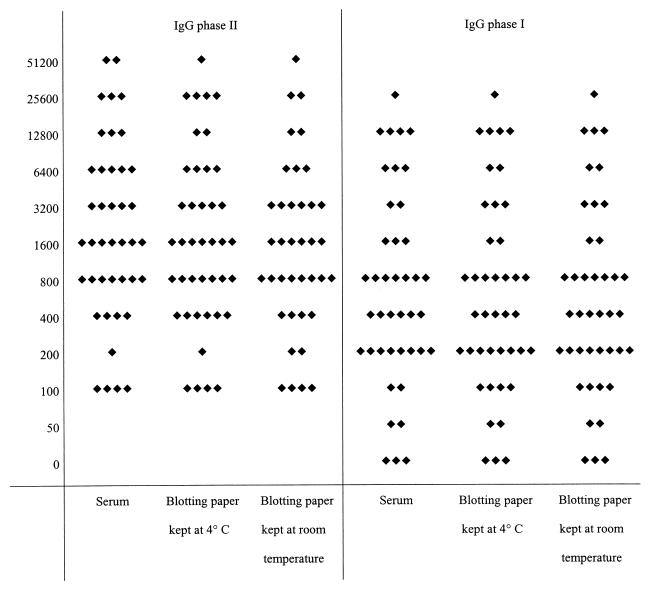
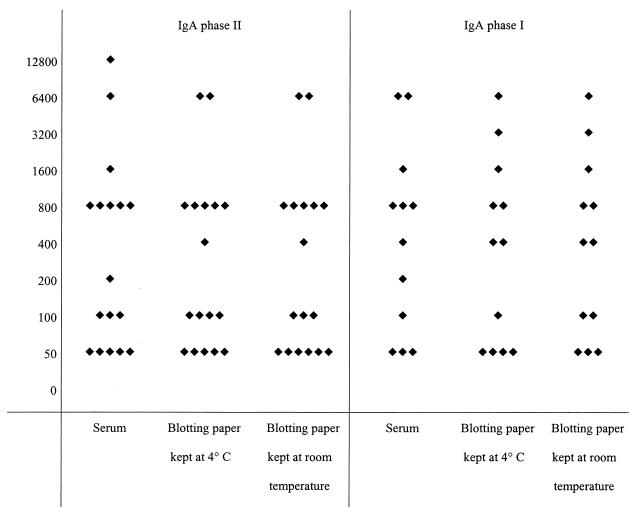
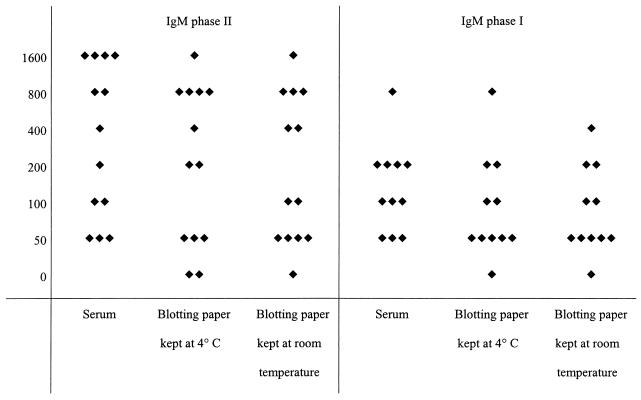
The results of serological tests performed with serum samples and dried blood spots on blotting paper kept at 4°C or at room temperature are presented in Fig. 1, 2, and 3 for C. burnetii, in Fig. 4 for B. quintana, and in Fig. 5 for R. conorii. For the 41 patients with a positive serology for C. burnetii (Fig. 1), the titers were most often equal (within 1 dilution in all but two cases) and the differences did not alter the final results; furthermore, the statistical analyses did not show significant differences between groups. For the patients with IgA phase II and I antibodies of C. burnetii (Fig. 2), all titers but one were within 1 dilution. The statistical analyses did not show significant differences. For the 13 patients testing positive for IgM (Fig. 3), the titers were within 1 dilution for all but one patient. For two of the three patients who had C. burnetii IgM at 1/50, serological tests of the blotted blood spots were negative. In one, both samples tested negative, and in the other, only the sample kept at 4°C tested negative.
FIG. 1.
Serological results for 41 patients with positive IgG phase II and I serology for Q fever. Each diamond represents the titer of IgG antibody to C. burnetii. The left and right panels show titers of IgG phase II and phase I antibodies, respectively, for serum samples and dried blood samples on blotting paper.
FIG. 2.
Serological results for 17 patients with positive IgA phase II and I serology for Q fever. Each diamond represents the titer of IgA antibody to C. burnetii. The left and right panels show titers of IgA phase II and phase I antibodies, respectively, for serum samples and dried blood samples on blotting paper.
FIG. 3.
Serological results for 13 patients with positive IgM phase II and I serology for Q fever. Each diamond represents the titer of IgM antibody to C. burnetii. The left and right panels show titers of IgM phase II and phase I antibodies, respectively, for serum samples and dried blood samples on blotting paper.
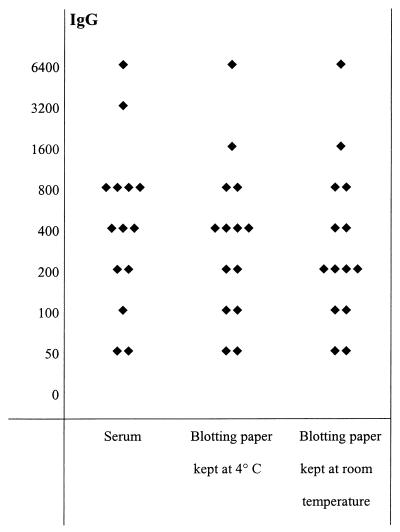
FIG. 4.
Serological results for 14 patients with positive IgG serology for B. quintana. Diamonds represent titers of IgG antibody for serum samples and dried blood samples on blotting paper.
FIG. 5.
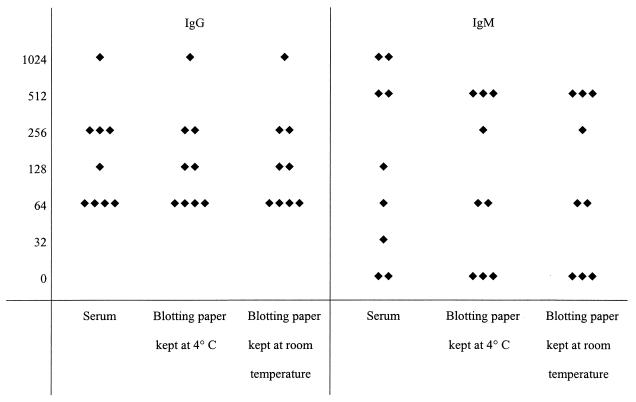
Serological results for nine patients with positive IgG and IgM serology for Mediterranean spotted fever. The left and right panels show titers of IgG and IgM antibodies, respectively, for serum samples and dried blood samples on blotting paper.
For B. quintana, all the tested serum samples were positive for IgG only. Eight of fourteen dried samples tested had the same titers as serum samples under all storage conditions, four had titers 1 dilution lower under all storage conditions, one sample had titers 1 dilution lower when stored at 4°C, and 2 samples had titers 1 dilution lower when stored at room temperature. However, none of the differences resulted in a changed serologic diagnosis.
Of the nine dried samples positive for IgG to R. conorii, seven had the same titers as serum samples under all storage conditions, one had titers 1 dilution lower when stored at 4°C, and one sample had a 1-dilution decrease under both storage conditions. Of the seven samples taken from patients positive for IgM antibodies to R. conorii, two dried samples had the same titers as serum samples, two had titers 1 dilution lower under all storage conditions, one had titers 1 dilution lower when kept at 4°C and 2 dilutions lower when stored at room temperature, and another had a 1-dilution decrease when stored at 4°C.
We also compared serum lacking antibodies to rickettsiae with blood dried on blotting paper to look for false positives. In all cases where serum samples were antibody negative, the corresponding blood samples dried on blotting paper tested negative after 4 weeks of storage at 4°C or at room temperature.
DISCUSSION
Storage of blood on filter paper is a frequently used technique after blood collection in human serologic surveys. Serologic surveys are currently being used to study microbial ecology and to help to define the etiology and extent of epidemic and endemic diseases, especially in areas where modern laboratory facilities are not available. Rickettsial diseases are reemerging, and factors influencing this reemergence include the immunodepression associated with declines in social conditions brought about by factors such as poor hygiene, poverty, or war (30). Such conditions are experienced by those in a wide range of situations worldwide, ranging from the refugees of Central Africa to the inner-city homeless and poverty stricken in Western Europe and the United States (6). Testing blood blotted on paper would be a good first step to explore and confirm outbreaks of rickettsial diseases all over the world. Whole-blood collection on filter paper for rickettsial-antibody assays offers numerous advantages over serum sampling. Equipment requirements are minimal; inexpensive sterile lancets and filter cards replace the syringes, tubes, centrifuges, refrigerators, freezers, and electricity which are needed for serum collection and storage. The filter cards are light, cannot be broken or split, can be stored at room temperature for several weeks, require minimal storage space, and can be shipped by mail. The blood spot technique can be performed anywhere by minimally trained personnel and therefore is suited to screening programs in developing regions such as Africa. Little is known about the recovery of rickettsial antibodies from blood samples dried on filter paper (9, 18). The limitations of field techniques employed in serologic surveys must be documented to ensure the appropriate interpretation of collected information. Storage temperatures, durations of storage, and elution conditions must be determined to avoid declines in antibody activity.
Detection of rickettsial antibodies in whole-blood spots that were collected on filter paper and stored over a 4-week period at 4°C and at room temperature was not significantly affected by environmental conditions. In some cases, titers that were 1 or 2 dilutions lower were obtained, especially with IgM; however, the final diagnosis (positive or negative) was not changed except for two patients with IgM for C. burnetii at 1/50. Furthermore, statistical analysis did not confirm any significant differences. IgM immunofluorescence tests are sometimes confounded by the presence of rheumatoid factors which require the adsorption of sera before IgM determination (24). Unfortunately, we did not have enough sample volume to perform this procedure.
Overall, the results suggest that whole-blood collection on filter paper can be effectively substituted for serum sampling in rickettsial-antibody assays in field studies. It is important to note that the source of filter paper is critical, and only paper certified for whole-blood collection should be used. The stability of the samples on filter paper allows them to be collected and stored without refrigeration and tested centrally in laboratories with the appropriate equipment. As local facilities are often not adequately equipped, filter paper specimens could be sent to an appropriate laboratory within the time limits identified in this report. Although collecting, drying, and storing whole blood on filter paper simplifies sample collection, the careful and precise performance of laboratory procedures by trained persons in a well-equipped laboratory with an efficient quality assurance program in place is still required. This method of specimen collection provides an economical way to obtain and transport specimens for large-scale seroepidemiological and outbreak studies without sacrificing sensitivity or specificity. We have begun to apply this approach to the diagnosis of rickettsioses in the field. In 1996, we used the blood-on-blotting-paper technique to investigate the seroprevalence of C. burnetii antibodies among 843 persons during a Q fever epidemic in the south of France (unpublished data), and we examined 188 dried samples on blotting paper taken from various sources and clinical entities during the typhus epidemic in Burundi in 1997 (29, 42). The samples dried on blotting paper were shipped by standard airmail in an envelope and reached France in 7 to 15 days. These studies showed that, under field conditions, the sizes of the blood spots were variable and sometimes smaller than the required 6-mm discs. We propose two solutions to resolve this problem. First, a 3-mm saturated blood spot eluted overnight in 250 μl of PBS-Tween was found to be equivalent to a 1/50 dilution of serum. Second, in order to standardize results, we measured the optical densities of a number of dilutions of hemoglobin to yield a standard curve. The optical density of all elutes was measured, allowing an estimation of the dilution of each sample to be made. When the dilution factor of the eluate was ≥0.75, serological results were not altered; however, if the dilution factor was between 0.375 and 0.75, the serological titer obtained was doubled, and if the dilution factor was <0.375, the titer was quadrupled (unpublished data). In conclusion, whenever it is difficult to obtain and properly store sera for antibody detection, as is commonly the case in developing countries or during large epidemics, collection of whole-blood spots on filter paper is an excellent alternative method, and it is surprising that this technique has not gained wide acceptance in microbiology.
ACKNOWLEDGMENTS
We thank P. Kelly and S. Dumler for critical review of the manuscript and Herve Tissot-Dupont for technical assistance.
REFERENCES
- 1.Aguirre Errasti C, Montjedo Baranda M, Villate Navarro J L, Sobradillo Pena V. An outbreak of Q fever in the Basque country. Can Med Assoc J. 1984;131:48–49. [PMC free article] [PubMed] [Google Scholar]
- 2.al-Tukhi M H, Ackers J P, al-Ahdal M N, Peters W. Enzyme-linked immunosorbent assay for the detection of anti-Giardia specific immunoglobulin G in filter paper blood samples. Trans R Soc Trop Med Hyg. 1993;87:36–38. doi: 10.1016/0035-9203(93)90412-j. [DOI] [PubMed] [Google Scholar]
- 3.Bailey N M, Cunningham M P, Kimber C B. The indirect fluorescent antibody technique applied to dried blood, for use as a screening test in the diagnosis of human trypanosomiasis in Africa. Trans R Soc Trop Med Hyg. 1967;61:696–700. doi: 10.1016/0035-9203(67)90135-6. [DOI] [PubMed] [Google Scholar]
- 4.Beebe J L, Briggs L C. Evaluation of enzyme-linked immunoassay systems for detection of human immunodeficiency virus type 1 antibody from filter paper disks impregnated with whole blood. J Clin Microbiol. 1990;28:808–810. doi: 10.1128/jcm.28.4.808-810.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brody J A, McAlister R, Haseley R, Lee P. Use of dried whole blood on filter paper disks in adenovirus complement fixation and measles hemagglutination inhibition tests. J Immunol. 1964;92:854–857. [PubMed] [Google Scholar]
- 6.Brouqui P, La Scola B, Roux V, Raoult D. Bartonella quintana chronic bacteremia in paucisymptomatic homeless. N Engl J Med. 1998;340:184–189. doi: 10.1056/NEJM199901213400303. [DOI] [PubMed] [Google Scholar]
- 7.Chanteau S, Plichart R, Boutin J P, Roux J, Cartel J L. Finger-prick blood collection and computer-assisted enzyme-linked immunosorbent assay for large-scale serological studies on leprosy. Trans R Soc Trop Med Hyg. 1989;83:414–416. doi: 10.1016/0035-9203(89)90523-3. [DOI] [PubMed] [Google Scholar]
- 8.Chishty S M. Filter paper blood samples used in serological studies of respiratory virus infections in children. J Clin Pathol. 1971;24:41–43. doi: 10.1136/jcp.24.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen A B, Natgi J N, Wisseman C L., Jr Storage stability of different antibody species against arbovirus and rickettsial antigens in blood dried on filter paper discs. Trans R Soc Trop Med Hyg. 1968;89:345–352. doi: 10.1093/oxfordjournals.aje.a120947. [DOI] [PubMed] [Google Scholar]
- 10.Condorelli F, Stivala A, Gallo R, Marino A, Battaglini C M, Messina A, Russo G, Castro A, Scalia G. Use of a microquantity enzyme immunoassay in a large-scale study of measles, mumps and rubella immunity in Italy. Eur J Clin Microbiol Infect Dis. 1998;17:49–52. doi: 10.1007/BF01584365. [DOI] [PubMed] [Google Scholar]
- 11.Das P C, de Vries A H, McShine R L, Smit Sibinga T. Dried sera for confirming blood-borne virus infections (HCV, HTLV-I, HIV and HbsAg) Transfus Med Rev. 1996;6:319–323. doi: 10.1111/j.1365-3148.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 12.Drancourt M, Mainardi J L, Brouqui P, Vandenesch F, Carta A, Lehnert F, Etienne J, Goldstein F, Acar J, Raoult D. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med. 1995;332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 13.Dupont H T, Thirion X, Raoult D. Q fever serology: cutoff determination for microimmunofluorescence. Clin Diagn Lab Immunol. 1994;1:189–196. doi: 10.1128/cdli.1.2.189-196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupuis G, Petite J, Peter O, Vouilloz M. An important outbreak of human Q fever in a Swiss Alpine Valley. Int J Epidemiol. 1987;16:282–287. doi: 10.1093/ije/16.2.282. [DOI] [PubMed] [Google Scholar]
- 15.Eaton R B, Petersen E, Seppanem H, Tuuminen T. Multicenter evaluation of a fluorometric enzyme immunocapture assay to detect toxoplasma-specific immunoglobulin M in dried blood filter paper specimens from newborns. J Clin Microbiol. 1996;34:3147–3150. doi: 10.1128/jcm.34.12.3147-3150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Harith A, Kolk A H, Leeuwenburg J, Muigai R, Huigen E, Jelsma T, Kager P A. Improvement of a direct agglutination test for field studies of visceral leishmaniasis. J Clin Microbiol. 1988;26:1321–1325. doi: 10.1128/jcm.26.7.1321-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evengard B, Hagi H, Linder E. A filter-paper technique for the detection of IgG and IgM class schistosome-specific antibodies in an endemic area. Ann Trop Med Parasitol. 1988;82:307–309. doi: 10.1080/00034983.1988.11812248. [DOI] [PubMed] [Google Scholar]
- 18.Gan E, Cadigan F C, Jr, Walker J S. Filter paper collection of blood for use in a screening and diagnostic test for scrub typhus using the IFAT. Am J Epidemiol. 1972;66:588–593. doi: 10.1016/0035-9203(72)90304-5. [DOI] [PubMed] [Google Scholar]
- 19.Guerrero I C, Chin W, Collins W E. A survey of malaria in Indochinese refugees arriving in the United States, 1980. Am J Trop Med Hyg. 1982;31:897–901. doi: 10.4269/ajtmh.1982.31.897. [DOI] [PubMed] [Google Scholar]
- 20.Hopkins D R. Fluorescent treponema antibody absorption (FTA-ABS) test using blood samples collected on filter paper. Am J Trop Med Hyg. 1977;26:188–189. doi: 10.4269/ajtmh.1977.26.188. [DOI] [PubMed] [Google Scholar]
- 21.Huang Z, Dwyer B, Kaldor J. Helicobacter pylori serology using specimens collected on filter paper. J Clin Pathol. 1991;44:167–169. doi: 10.1136/jcp.44.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jafri H S, Torrico F, Noh J C, Bryan R T, Balderrama F, Pilcher J B, Tsang V C. Application of the enzyme-linked immunoelectrotransfer blot to filter paper blood spots to estimate seroprevalence of cysticercosis in Bolivia. Am J Trop Med Hyg. 1998;58:313–315. doi: 10.4269/ajtmh.1998.58.313. [DOI] [PubMed] [Google Scholar]
- 23.Kenny J V, MacCabe R J. Sero-epidemiology of hydatid disease in the non-intervention area of north-east Turkana. Ann Trop Med Parasitol. 1993;87:451–457. doi: 10.1080/00034983.1993.11812795. [DOI] [PubMed] [Google Scholar]
- 24.La Scola B, Raoult D. Laboratory diagnosis of rickettsioses: current approaches to diagnoses of old and new rickettsial diseases. J Clin Microbiol. 1997;35:2715–2727. doi: 10.1128/jcm.35.11.2715-2727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurin M, Raoult D. Bartonella (Rochalimaea) quintana infections. Clin Microbiol Rev. 1996;9:273–292. doi: 10.1128/cmr.9.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miezan T, Doua F, Cattand P, de Raadt P. Evaluation of Tetstryp CATT applied to blood samples on filter paper and on diluted blood in a focus of trypanosomiasis due to Trypanosoma brucei gambiense in the Ivory Coast. Bull W H O. 1991;69:603–606. [PMC free article] [PubMed] [Google Scholar]
- 27.Mirchamsy H, Nazari F, Stellman C, Esterabady H. The use of dried whole blood absorbed on filter-paper for the evaluation of diphtheria and tetanus antitoxins in mass surveys. Bull W H O. 1968;38:665–671. [PMC free article] [PubMed] [Google Scholar]
- 28.Raoult D, Dasch G A. Line blot and Western blot immunoassays for diagnosis of Mediterranean spotted fever. J Clin Microbiol. 1989;27:2073–2079. doi: 10.1128/jcm.27.9.2073-2079.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raoult D, Ndihokubwayo J B, Tissot-Dupont H, Roux V, Faugere B, Abegbinni R, Birtles R J. Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet. 1998;352:353–358. doi: 10.1016/s0140-6736(97)12433-3. [DOI] [PubMed] [Google Scholar]
- 30.Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10:694–719. doi: 10.1128/cmr.10.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed D, Broody J A. Use of blood collected on filter paper disks in neutralization tests for poliovirus antibody. Public Health Rep. 1965;80:1100–1102. [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider T, Jahn H U, Steinhoff D, Guschoreck H M, Liesenfeld O, Mater-Bohm H, Wesirow A L, Lode H, Ludwig W D, Dissmann T. A Q fever epidemic in Berlin. The epidemiological and clinical aspects. Dtsch Med Wochenschr. 1993;118:689–695. doi: 10.1055/s-2008-1059379. [DOI] [PubMed] [Google Scholar]
- 33.Sharma M, Ghosh S, Singal A K, Anand B S, Talwar G P. Use of micro samples of finger prick blood dried on filter paper for a quick and simple dipstick dot-EIA for diagnosis of amebic liver abscess. J Clin Lab Anal. 1994;8:96–98. doi: 10.1002/jcla.1860080207. [DOI] [PubMed] [Google Scholar]
- 34.Spach D H, Kanter A S, Dougherty M J, Larson A M, Coyle M B, Brenner D J, Swaminathan B, Matar G M, Welch D F, Root R K, Stamm W E. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med. 1995;332:424–428. doi: 10.1056/NEJM199502163320703. [DOI] [PubMed] [Google Scholar]
- 35.Sundharagiati B, Harinasuta C. Determination of leptospiral antibodies in dry blood on filter paper. Trans R Soc Trop Med Hyg. 1965;59:607–608. [Google Scholar]
- 36.Tada I, Korenaga M, Shiwaku K, Ogunba E O, Ufomadu G O, Nwoke B E. Specific serodiagnosis with adult Onchocerca volvulus antigen in an enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1987;36:383–386. doi: 10.4269/ajtmh.1987.36.383. [DOI] [PubMed] [Google Scholar]
- 37.Takkouche B, Iglesias J, Alonso-Fernandez J R, Fernandez-Gonzalez C, Gestal-Otero J J. Detection of Brucella antibodies in eluted dried blood: a validation study. Immunol Lett. 1995;45:107–108. doi: 10.1016/0165-2478(94)00247-o. [DOI] [PubMed] [Google Scholar]
- 38.Tarasevich I, Rydkina E, Raoult D. Outbreak of epidemic typhus in Russia. Lancet. 1998;352:1151. doi: 10.1016/S0140-6736(05)79799-3. [DOI] [PubMed] [Google Scholar]
- 39.Terhell A J, Haarbrink M, Abadi K, Bronneberg D C, Tieleman M C, Asri M, Yazdanbakhsh M. A filter paper technique for the detection of anti-filarial IgG4 in lymphatic filariasis. Trans R Soc Trop Med Hyg. 1996;90:196–198. doi: 10.1016/s0035-9203(96)90140-6. [DOI] [PubMed] [Google Scholar]
- 40.Williams J C, Peacock M G, McPaul T F. Immunological and biological characterization of Coxiella burnetii, phases I and II, separated from host components. Infect Immun. 1981;32:840–851. doi: 10.1128/iai.32.2.840-851.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winner S J, Eglin R P, Moore V I, Mayon-White R T. An outbreak of Q fever affecting postal workers in Oxfordshire. J Infect. 1987;14:255–261. doi: 10.1016/s0163-4453(87)93560-2. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. A large outbreak of epidemic louse-borne typhus in Burundi. Weekly Epidemiol Rec. 1997;21:152–153. [PubMed] [Google Scholar]
- 43.Zoulek G, Bürger P, Deinhardt F. Markers of hepatitis viruses A and B: direct comparison between whole serum and blood spotted on filter paper. Bull W H O. 1985;63:935–939. [PMC free article] [PubMed] [Google Scholar]