Abstract
The aim was to evaluate the outcome of stenting in patients with isolated distal internal carotid artery (ICA) stenosis or post-surgical restenosis, as no data are currently available in the literature. Sixty-six patients (men, N = 53; median age: 66 [IQR, 61–73] years) with ≥50% distal ICA (re)stenosis were included in this single-center retrospective study. The narrowest part of the (re)stenosis was at least 20 mm from the bifurcation in all patients. Patients were divided into two etiological groups, atherosclerotic (AS, N = 40) and post-surgical restenotic (RES, N = 26). Postprocedural neurological events were observed in two patients (5%) in the AS group and in two patients (7.7%) in the RES group. The median follow-up time was 40 (IQR, 18–86) months. Three patients (7.5%) in the AS group had an in-stent restenosis (ISR) ≥ 50%, but none in the RES group. Three patients (7.5%) in the AS group and seven patients (26.9%) in the RES group died. None of the deaths in the RES group were directly related to stenting itself. The early neurological complication rate of stenting due to distal ICA (re)stenoses is acceptable. However, the mid-term mortality rate of stenting for distal ICA post-surgical restenoses is high, indicating the vulnerability of this subgroup.
Keywords: internal carotid artery, atherosclerosis, restenosis, stenting, outcome, stroke, in-stent restenosis, patency, mortality, survival
1. Introduction
The most common sites of atherosclerotic lesions of the carotid arteries are the bifurcation, the 10–15 mm proximal segment of the internal carotid artery (ICA), and the origin/proximal third of the left common carotid artery (CCA). Atherosclerosis rarely affects the distal part of the ICA [1]. Invasive therapy for atherosclerotic carotid stenoses includes open surgery, stenting, or a combination of both [2,3]. Distal ICA lesions can only be approached with great difficulty by open surgery, either from the retromandibular fossa or by other means (e.g., the mobilization of the parotid gland, double mandibular osteotomy, or mandibular subluxation with styloidectomy) [4,5,6,7]. For this reason, stenting rather than open surgery is the invasive option for these patients, even for those who are symptomatic [2].
After open carotid surgery, restenosis occurs in 0.3–9% of cases [8]. Like atherosclerotic stenoses, restenoses can localize to the distal ICA [9]. Stenting is also the main invasive therapy for distal ICA restenoses [2].
Since there are no literature data on the short- and mid-term efficacy of stenting in atherosclerotic or post-surgical restenotic distal ICA stenoses, our aim was to provide information on this topic.
2. Materials and Methods
2.1. Study Design
This single-center retrospective study analyzed patients (N = 66) who underwent stenting for atherosclerotic or post-surgical restenotic isolated distal ICA stenosis between January 2001 and January 2020.
2.2. Stenting Process
For each patient, the vascular team at our center decided on the need for stenting based on the European Society for Vascular Surgery guidelines in force at the time. Patients were considered symptomatic if they had any ischemic neurological event (amaurosis fugax, transient ischemic attack [TIA], minor or major stroke) in the ipsilateral carotid territory within 6 months before the intervention [10].
Stenting was performed in the standard manner [11] by three interventional radiologists with more than 10 years of experience, with the implantation of self-expanding stents and embolic protection. If the patient was not on antiplatelet therapy or was on monotherapy only, 100–300 mg acetylsalicylic acid and/or 75 mg clopidogrel daily was started at least 72 h before the procedure. In urgent cases, a loading dose of 250–500 mg acetylsalicylic acid and/or 300–600 mg clopidogrel was given. In the absence of cardiological or other indications, dual antiplatelet therapy was continued for 1 month after the intervention, followed by monotherapy for an indefinite period [12].
Stenting was technically successful if no extravasation, dissection, or >30% residual stenosis was seen on final digital subtraction angiography (DSA) images [11].
2.3. Follow-Up Visits
Follow-up visits were scheduled for the 6th week after the intervention, the 6th and 12th month, and then once a year. However, due to complaints, contralateral invasive carotid procedure, or other reasons, these dates could be changed. Follow-up visits consisted of interviewing the patient and ultrasound examination of the cervical arteries. Restenosis was defined as 50–69% if the peak systolic velocity (PSV) inside the stent or at either end of the stent was 225–350 cm/s and ≥ 70% if PSV was >350 cm/s [13]. If the distal part of the stent was not visible by ultrasound but indirect signs (ICA flow volume <159 mL/min, ICA PSV < 33 cm/s, and/or CCA PSV < 42 cm/s) suggested a ≥70% in-stent restenosis (ISR) [14], the patient was submitted to computed tomography angiography (CTA).
2.4. Analyzed Parameters
Cardiovascular risk factors and comorbidities (female sex, age ≥ 80 years, hypertension, hyperlipidemia, diabetes mellitus, and chronic kidney disease), previous invasive vascular therapies, lesion- and intervention-related parameters, neurological events (amaurosis fugax, TIA, minor or major stroke) before and after the stenting, ISR characteristics and primary patency and mortality rates were assessed. For a definition of cardiovascular risk factors and comorbidities, see another publication by our research group [15]. The parameters of the lesions (localization, grade and length of stenosis, presence, and severity of calcification) were determined on preprocedural CTA scans. By localization, the affected side and the distance of the narrowest part of the ICA stenosis from the bifurcation was meant. The percentage of stenosis was calculated using the formula in the North American Symptomatic Carotid Endarterectomy Trial [16]. The length of the lesion was defined as the distance between the proximal and distal points where the grade of stenosis decreased to 80% of its maximum [17]. The severity of calcification was classified according to Woodcock and four types, such as absent, mild (thin, discontinuous), moderate (thin, continuous or thick, discontinuous), and severe (thick, continuous), were distinguished [18]. Among the intervention-related parameters, the puncture site, the type of embolic protection device, the manufacturer, diameter and length of the balloons and stents, and the complications were collected. Regarding the definition of neurological events, reference is made to a guideline [19]. ISR characteristics included the ultrasonographic grade, localization (in-stent, peristent, or both), and pattern (focal or diffuse) of restenotic lesions. The ISR was considered focal if it was shorter than 10 mm. Primary patency was defined as freedom from ≥50% ISR or occlusion.
2.5. Statistical Analysis
Statistical analysis was performed using SPSS Statistics for Windows (Version 25.0.; IBM Corp., Armonk, NY, USA) and GraphPad Prism 7.01 (GraphPad Software Inc., La Jolla, CA, USA) software. Continuous data were presented as median and interquartile range (IQR) and compared using Mann–Whitney U test. Categorical data were expressed as numbers (percentages) and compared using Fisher’s exact test. Kaplan–Meier analysis was performed to determine primary patency and mortality rates. Follow-up was maximized at 48 months. Survival curves were compared using a log-rank test. All statistical tests were two-tailed. The threshold for statistical significance was p ≤ 0.05.
3. Results
3.1. Patient Data
The median age of the 66 patients (women, N = 13; men, N = 53) was 66 years (IQR, 61–73 years). Patients were divided into two etiological groups, atherosclerotic (AS, N = 40 [60.6%]; median age: 67 years [IQR, 61–74 years]) and post-surgical restenotic (RES, N = 26 [39.4%]; median age: 64.5 years [IQR, 60.5–71 years]). There was no significant difference (p = 0.541) in median age between the AS and RES groups. The carotid surgery in all patients was an eversion endarterectomy. The median time between carotid surgery and stenting was 80 months (IQR, 22–148 months). Stenting was carried out in nine patients within 48 months after endarterectomy. Patient-related parameters are shown in Table 1. Of the 66 patients, 15 (22.7%) had some neurological symptoms before stenting. There was no significant difference (p > 0.999) in preprocedural neurological events between the two groups. The RES group had significantly more women (p = 0.003) and significantly more patients with hypertension (p = 0.010), contralateral carotid invasive treatment (p = 0.015), and lower extremity arterial reconstruction (p = 0.046).
Table 1.
Preprocedural neurological events, cardiovascular risk factors, comorbidities, and previous invasive vascular therapies.
| Patient-Related Parameters | AS Group (N = 40) |
RES Group (N = 26) |
p-Value |
|---|---|---|---|
| Preprocedural neurological events, N (%) | 9 (22.5) | 6 (23.1) | >0.999 |
| Amaurosis fugax, N (%) | 3 (7.5) | 0 (0) | 0.273 |
| TIA, N (%) | 3 (7.5) | 6 (23.1) | 0.139 |
| Minor stroke, N (%) | 3 (7.5) | 0 (0) | 0.273 |
| CV risk factors, comorbidities | |||
| Female sex, N (%) | 3 (7.5) | 10 (38.5) | 0.003 |
| Age ≥ 80 years, N (%) | 5 (12.5) | 2 (7.7) | 0.695 |
| Hypertension, N (%) | 28 (70) | 25 (96.2) | 0.010 |
| Hyperlipidemia, N (%) | 13 (32.5) | 8 (30.8) | >0.999 |
| Diabetes mellitus, N (%) | 8 (20) | 8 (30.8) | 0.384 |
| Chronic kidney disease, N (%) | 2 (5) | 4 (15.4) | 0.202 |
| Previous invasive vascular therapies | |||
| Coronary artery invasive treatment, N (%) | 11 (27.5) | 3 (11.5) | 0.217 |
| Contralateral carotid artery invasive treatment, N (%) | 8 (20) | 13 (50) | 0.015 |
| Subclavian artery invasive treatment, N (%) | 0 (0) | 1 (3.8) | 0.394 |
| Visceral artery invasive treatment, N (%) | 1 (2.5) | 2 (7.7) | 0.557 |
| Aortic invasive treatment, N (%) | 0 (0) | 1 (3.8) | 0.394 |
| Lower extremity arterial invasive treatment, N (%) | 7 (17.5) | 11 (42.3) | 0.046 |
AS, Atherosclerotic; CV, cardiovascular; N, number; RES, restenotic; TIA, transient ischemic attack.
3.2. Lesion Data
Lesion characteristics can be found in Table 2. The narrowest part of the ICA stenosis was at least 20 mm from the bifurcation in all patients. Among lesion-related parameters, only length was significantly different between the two groups; AS lesions were significantly longer (p = 0.002) than RES lesions.
Table 2.
Lesion characteristics.
| Lesion-Related Parameters | AS Group (N = 40) |
RES Group (N = 26) |
p-Value |
|---|---|---|---|
| Right side, N (%) | 15 (37.5) | 14 (53.8) | 0.214 |
| Distance from the bifurcation (mm), median (IQR) | 20.4 (20.1–21.4) | 21.5 (20.1–24) | 0.126 |
| Stenosis grade (%), median (IQR) | 90 (80–90) | 90 (85–95) | 0.099 |
| Stenosis length (mm), median (IQR) | 8.1 (6.1–12) | 5.1 (4.1–7.5) | 0.002 |
| Calcification, N (%) | 25 (62.5) | 11 (42.3) | 0.133 |
| Mild, N (%) | 14 (35) | 8 (30.8) | 0.794 |
| Moderate, N (%) | 8 (20) | 1 (3.8) | 0.077 |
| Heavy, N (%) | 3 (7.5) | 2 (7.7) | >0.999 |
AS, Atherosclerotic; IQR, interquartile range; N, number; RES, restenotic.
3.3. Procedure Data
In the AS group, the access site was femoral in 28 cases (70%) and radial in 12 cases (30%), while in the RES group, the access site was femoral in 13 cases (50%), radial in 10 cases (38.5%), and brachial in three cases (11.5%). In the AS group, embolic protection was distal type (FilterWire EZ; Boston Scientific Corp., Marlborough, MA, USA) in 38 patients (95%) and proximal type (Mo.Ma; Medtronic Inc., Minneapolis, MN, USA) in two patients (5%). In the RES group, all patients had distal type embolic protection. Six cases (15%) in the AS group and one case (3.8%) in the RES group required predilation. Five different types of self-expanding stents were used. Twenty-eight (42.4%) of the stents were located only in the ICA and did not extend into the bifurcation and CCA. All stents were postdilated. Technical success was achieved in 100% of cases. The types, diameters, and lengths of balloons and stents are listed in Table 3.
Table 3.
Balloon and stent characteristics.
| Balloon- and Stent-Related Parameters | AS Group (N = 40) |
RES Group (N = 26) |
|---|---|---|
| Predilation balloons | ||
| Maverick (Boston Scientific Corp., Marlborough, MA, USA), N (%) | 4 (10) | 0 (0) |
| Emerge (Boston Scientific Corp.), N (%) | 1 (2.5) | 0 (0) |
| Pantera Pro (Biotronik SE & Co. KG, Berlin, Germany), N (%) | 1 (2.5) | 0 (0) |
| Sprinter Legend Rx (Medtronic Inc., Minneapolis, MN, USA), N (%) | 0 (0) | 1 (3.8) |
| Diameter (mm), range | 2.5–4 | 2.5 |
| Length (mm), range | 20–40 | 12 |
| Postdilation balloons | ||
| Sterling (Boston Scientific Corp.), N (%) | 25 (62.5) | 12 (46.2) |
| Maverick (Boston Scientific Corp.), N (%) | 8 (20) | 5 (19.2) |
| Viatrac 14 Plus (Abbott Vascular Inc., Santa Clara, CA, USA), N (%) | 5 (12.5) | 6 (23.1) |
| Ultra-Soft SV (Boston Scientific Corp.), N (%) | 2 (5) | 3 (11.5) |
| Diameter (mm), range | 4–6 | 4–5 |
| Length (mm), range | 20–40 | 20–40 |
| Stents | ||
| Wallstent (Boston Scientific Corp.), N (%) | 32 (80) | 25 (96.2) |
| Xact (Abbott Vascular Inc.), N (%) | 4 (10) | 0 (0) |
| Roadsaver (Terumo Corp., Tokyo, Japan), N (%) | 1 (2.5) | 1 (3.8) |
| Precise Pro Rx (Cordis Corp., Johnson & Johnson Co., Miami, FL, USA), N (%) | 2 (5) | 0 (0) |
| Exponent (Medtronic Inc.), N (%) | 1 (2.5) | 0 (0) |
| Diameter (mm), range | 5–9 | 5–9 |
| Length (mm), range | 25–50 | 30–50 |
AS, Atherosclerotic; N, number; RES, restenotic.
3.4. Early (≤30 Days) Postprocedural Period
There were five intervention-related complications: one inguinal haematoma (1.5%) not requiring evacuation and four neurological events (6.1%; AS group, one TIA and one major stroke; RES group, two TIAs). The parameters of patients with postprocedural neurological complications can be seen in Table 4. TIAs presented as contralateral upper and/or lower limb paresis or dysarthria and lasted no longer than 15 min. None of the TIA patients had an acute ischemic or hemorrhagic brain lesion on post-stenting CT or magnetic resonance images. The time between carotid surgery and stenting was 103 months in Patient 3 and 178 months in Patient 4. The major stroke patient became unconscious 2 h after an uneventful stenting procedure. The emergency CT scan showed extensive bleeding in the ipsilateral frontal and parietal lobes. The patient died on day 37 after stenting.
Table 4.
Parameters of patients with postprocedural neurological complications.
| Parameters | Patient 1 with Postprocedural TIA |
Patient 2 with Postprocedural Major Stroke | Patient 3 with Postprocedural TIA |
Patient 4 with Postprocedural TIA |
|---|---|---|---|---|
| Sex | Male | Male | Female | Female |
| Age | 59 years | 87 years | 67 years | 86 years |
| Etiological group | AS | AS | RES | RES |
| Preprocedural symptom | No | TIA | Minor stroke | TIA |
| Contralateral ICA stenosis/occlusion | Occlusion | No | Stenosis | Stenosis |
| Ipsilateral preprocedural stenosis grade | 90% | 95% | 90% | 95% |
| Ipsilateral preprocedural stenosis length | 6.2 mm | 16.8 mm | 3.3 mm | 4.5 mm |
| Calcification | Mild | Absent | Absent | Mild |
| Predilation | No | Yes | No | No |
| Stent type | Wallstent | Wallstent | Wallstent | Wallstent |
| Postprocedural ultrasound | Patent stent | Patent stent | Patent stent | Patent stent |
AS, Atherosclerotic; RES, restenotic; TIA, transient ischemic attack.
3.5. Follow-Up Period
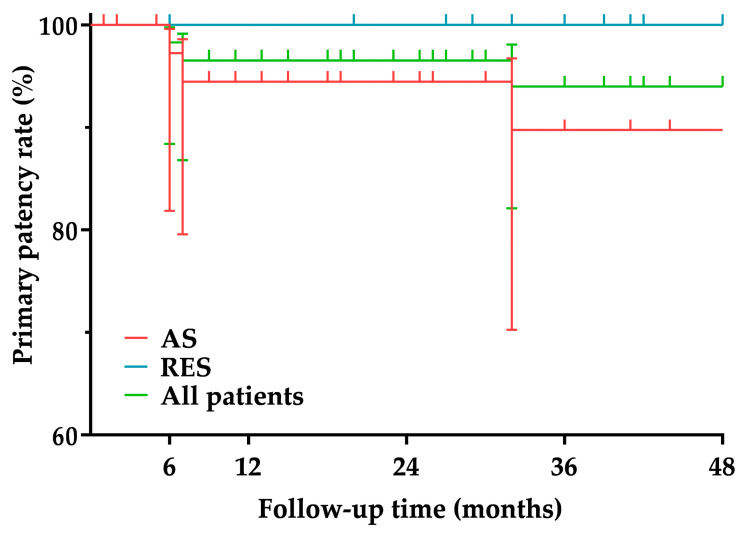
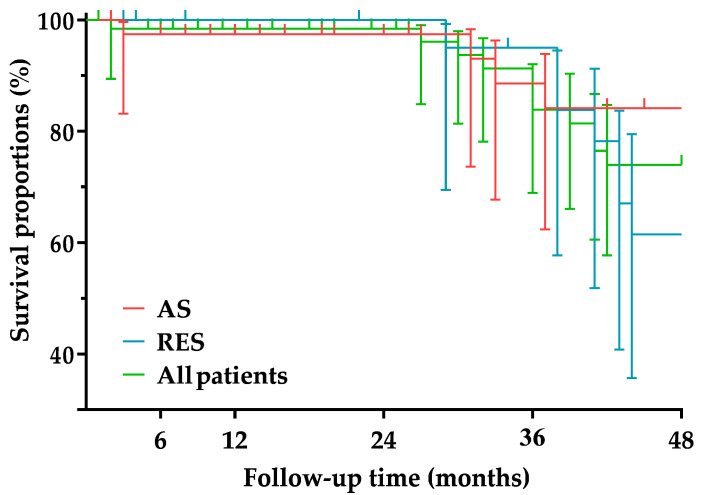
The median follow-up time was 34 months (IQR, 15–87 months) in the AS group and 41 months (IQR, 28–74 months) in the RES group. There was no significant difference (p = 0.708) in follow-up time between the two groups. In the AS group, two cases (5%) of 50–69% ISR and one case (2.5%) of ≥70% ISR were detected. All ISRs were located within the stent and were of the focal type. Patients with ISR were asymptomatic. The patient with ≥70% ISR underwent reintervention with a plain balloon (Trek; Abbott Vascular Inc., Santa Clara, CA, USA; size, 4 mm × 20 mm). No one in the RES group had ISR. The primary patency rate was 97.2% at 6 months, 94.4% at 12 and 24 months, and 89.7% at 36 and 48 months in the AS group and 100% over the entire follow-up period in the RES group. The primary patency rates of the two groups were not significantly different (p = 0.528) (Figure 1 and Table 5). During follow-up, three patients (7.5%) in the AS group and seven patients (26.9%) in the RES group died. The cause of death was myocardial infarction in three patients, heart failure in two patients, malignancy in two patients, major stroke in one patient, chronic obstructive pulmonary disease in one patient, and gastrointestinal bleeding in one patient. The survival proportion was 97.4% at 6, 12, and 24 months and 84.1% at 36 and 48 months in the AS group and 100% at 6, 12, and 24 months, 83.8% at 36 months, and 61.5% at 48 months in the RES group. The survival proportions of the two groups were not significantly different (p = 0.289) (Figure 2 and Table 6).
Figure 1.
Primary patency. AS, Atherosclerotic; RES, restenotic.
Table 5.
Primary patency.
| 6 Months | 12 Months | 24 Months | 36 Months | 48 Months | ||
|---|---|---|---|---|---|---|
| All patients | % | 98.3 | 96.5 | 96.5 | 94 | 94 |
| 95% CI | 88.4–99.7 | 86.8–99.1 | 86.8–99.1 | 82.1–98.1 | 82.1–98.1 | |
| Number at risk | 58 | 52 | 45 | 35 | 26 | |
| AS group | % | 97.2 | 94.4 | 94.4 | 89.7 | 89.7 |
| 95% CI | 81.9–99.6 | 79.5–98.5 | 79.5–98.5 | 70.2–96.7 | 70.2–96.7 | |
| Number at risk | 36 | 31 | 25 | 18 | 16 | |
| RES group | % | 100 | 100 | 100 | 100 | 100 |
| 95% CI | - | - | - | - | - | |
| Number at risk | 22 | 22 | 21 | 17 | 11 | |
AS, Atherosclerotic; CI, confidence interval; RES, restenotic.
Figure 2.
Survival proportions. AS, Atherosclerotic; RES, restenotic.
Table 6.
Survival proportions.
| 6 Months | 12 Months | 24 Months | 36 Months | 48 Months | ||
|---|---|---|---|---|---|---|
| All patients | % | 98.4 | 98.4 | 98.4 | 83.9 | 73.9 |
| 95% CI | 89.4–99.7 | 89.4–99.7 | 89.4–99.7 | 68.9–92.1 | 55.7–84.7 | |
| Number at risk | 58 | 54 | 46 | 37 | 28 | |
| AS group | % | 97.4 | 97.4 | 97.4 | 84.1 | 84.1 |
| 95% CI | 83.1–99.6 | 83.1–99.6 | 83.1–99.6 | 62.3–93.8 | 62.3–93.8 | |
| Number at risk | 37 | 33 | 26 | 20 | 18 | |
| RES group | % | 100 | 100 | 100 | 83.8 | 61.5 |
| 95% CI | - | - | - | 57.7–94.5 | 35.7–79.5 | |
| Number at risk | 22 | 22 | 21 | 17 | 11 | |
AS, Atherosclerotic; CI, confidence interval; RES, restenotic.
4. Discussion
Most studies have separately analyzed the short- and mid-/long-term outcomes of carotid artery stenting (CAS) for atherosclerosis and post-surgical restenosis [19,20], but we found eight studies that did so comparatively [21,22,23,24,25,26,27,28]. The two main indicators of the short-term success of CAS are the rate of new or recurrent neurological events and mortality. The rate of stroke within 30 days after stenting ranges from 0% to 9.8%, while the rate of all-cause mortality within 30 days after stenting ranges from 0% to 1.3% for atherosclerotic ICA stenoses [19]. The same rates for post-surgical ICA restenosis stenting range from 0% to 18% and 0% to 2%, respectively [20]. In three of the eight comparative studies, peri- and postprocedural neurological complications were more frequent in patients undergoing stenting for atherosclerotic ICA stenoses [22,23,24]. The other five studies showed no significant difference in neurological events within 30 days after stenting between the two etiological groups [21,25,26,27,28]. The etiology of ICA lesions had no effect on CAS 30-day mortality in any of the comparative studies [21,22,23,24,25,26,27,28]. However, to the best of our knowledge, no study has specifically investigated the outcome of CAS in distal ICA lesions. In our AS group, the rate of neurological complications within 30 days after stenting was 5%, resulting from one TIA and one hemorrhagic stroke; the patient with hemorrhagic stroke died on day 37 after the intervention. The underlying cause of the hemorrhagic stroke was presumably hyperperfusion syndrome. After CAS, hyperperfusion syndrome occurs in 0–21.2% of cases and consequential hemorrhagic stroke in 0–100% of cases [29]. In our RES group, compared to our AS group, a non-significantly higher proportion of patients, 7.7%, developed neurological symptoms within 30 days after stenting, but no deaths were recorded in the early postprocedural period. Thus, the short-term success rates for stenting distal ICA (re)stenoses are not worse than the rates reported for stenting ICA (re)stenoses in general (without defining the lesion location).
The mid-/long-term outcome of CAS is best characterized by ISR and mortality rates. In some publications, both the ISR ≥ 50% rate and the ISR ≥ 70% rate are given [30,31,32], while in others, only the ISR ≥ 70% rate is mentioned [33,34,35,36,37,38]. Based on literature data, the prevalence of ISR ≥ 50% after stenting for atherosclerotic ICA lesions is between 0% and 37% [30,31,32], while the prevalence of ISR ≥ 70% is between 0% and 9.8% [33,34,35,36,37,38]. For post-surgical ICA restenosis stenting, these incidences range from 0% to 15% [39,40] and 0% to 9.5% [41,42], respectively. Of the eight comparative studies, only two examined ISR (one considered ISR ≥ 50% [21], the other considered ISR ≥ 70% as the endpoint [26]), and none revealed a significant difference in the prevalence of ISR between the two etiological groups [21,26]. In our patient population, the incidence of ISR was non-significantly higher in the AS group (ISR ≥ 50%, 7.5% and ISR ≥ 70%, 2.5%) than in the RES group (ISR ≥ 50%, 0% and ISR ≥ 70%, 0%). Thus, the ISR rates for stenting distal ICA (re)stenoses (such as the short-term results) are not worse than the rates reported for stenting ICA (re)stenoses in general (without defining the lesion location).
Only a few publications were found that included mid-/long-term mortality rates for CAS. For CAS performed for atherosclerotic ICA stenoses, the mid-/long-term mortality rate ranges from 12.1% to 35% [18,20,30,32,42,43], while for CAS performed for post-surgical ICA restenoses, the mid-/long-term mortality rate ranges from 9.6% to 11.8% [20,41,44]. Of the eight comparative studies, only one study aimed to determine the mid-term (4-year) mortality rate [21]. In this study, there was no significant difference in the 4-year mortality rate between CAS for atherosclerosis (12.1%) and CAS for post-surgical restenosis (11.8%) [21]. The mid-term mortality rate of 7.5% in our AS group is low, while the mid-term mortality rate of 26.9% in our RES group is quite high in light of the literature. It is important to note, however, that none of the deaths in our RES group were directly related to CAS itself; the deaths were the result of other serious comorbidities in the patients.
Our study has two main limitations: its retrospective nature and the relatively small number of patients.
5. Conclusions
The early complication and ISR rates of distal ICA stenting are acceptable and are not influenced by the etiology of the lesion. However, the mid-term mortality rate of the RES group is high. The lower survival is probably not due to the stenting procedure but rather to the more complex comorbidity profile of the RES population.
Author Contributions
Conceptualization, D.T.N. and E.D.; methodology, D.T.N.; software, D.T.N.; validation, Á.B., B.B.N. and Á.S.; formal analysis, D.T.N.; investigation, D.T.N. and M.P.; resources, E.D.; data curation, B.B.N. and Á.S.; writing—original draft preparation, D.T.N.; writing—review and editing, E.D.; visualization, Á.B.; supervision, E.D.; project administration, M.P.; funding acquisition, Á.B. and B.B.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Semmelweis University (approval number 222/2017).
Informed Consent Statement
Due to the retrospective nature of the study, no informed consent for analysis of data was obtained from patients.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to reasons pertaining to patient privacy.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the EFOP-3.6.3-VEKOP-16-2017-00009 project (Á.B. and B.B.N.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bonati L.H., Brown M.M. Carotid artery disease. In: Grotta J.C., Albers G.W., Broderick J.P., Kasner S.E., Lo E.H., Mendelow A.D., Sacco R.L., Wong L.K.S., editors. Stroke: Pathophysiology, Diagnosis, and Management. Elsevier—Health Sciences Division; Amsterdam, The Netherlands: 2016. p. 326. [Google Scholar]
- 2.Naylor A.R., Ricco J.B., de Borst G.J., Debus S., de Haro J., Halliday A., Hamilton G., Kakisis J., Kakkos S., Lepidi S., et al. Editor’s Choice—Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS) Eur. J. Vasc. Endovasc. Surg. 2018;55:3–81. doi: 10.1016/j.ejvs.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Kwolek C.J., Jaff M.R., Leal J.I., Hopkins L.N., Shah R.M., Hanover T.M., Macdonald S., Cambria R.P. Results of the ROADSTER multicenter trial of transcarotid stenting with dynamic flow reversal. J. Vasc. Surg. 2015;62:1227–1234. doi: 10.1016/j.jvs.2015.04.460. [DOI] [PubMed] [Google Scholar]
- 4.Buckley M., Carlson E., Heidel E., McNally M., Hodge A., Fahmy M., Arnold J., Grandas O., Freeman M. Double Mandibular Osteotomy for Access to High-Carotid Pathology. Ann. Vasc. Surg. 2021;70:202–212. doi: 10.1016/j.avsg.2020.08.093. [DOI] [PubMed] [Google Scholar]
- 5.Mock C.N., Lilly M.P., McRae R.G., Carney W.I., Jr. Selection of the approach to the distal internal carotid artery from the second cervical vertebra to the base of the skull. J. Vasc. Surg. 1991;13:846–853. doi: 10.1016/0741-5214(91)90050-5. [DOI] [PubMed] [Google Scholar]
- 6.Izci Y., Moftakhar R., Pyle M., Başkaya M.K. Retromandibular fossa approach to the high cervical internal carotid artery: An anatomic study. Neurosurgery. 2008;62:ONS363–ONS370. doi: 10.1227/01.neu.0000326020.07187.6b. discussion ONS369–ONS370. [DOI] [PubMed] [Google Scholar]
- 7.Naylor A.R., Moir A. An aid to accessing the distal internal carotid artery. J. Vasc. Surg. 2009;49:1345–1347. doi: 10.1016/j.jvs.2008.12.076. [DOI] [PubMed] [Google Scholar]
- 8.Szabo A., Brazda E., Dosa E., Apor A., Szabolcs Z., Entz L. Long-term restenosis rate of eversion endarterectomy on the internal carotid artery. Eur. J. Vasc. Endovasc. Surg. 2004;27:537–539. doi: 10.1016/j.ejvs.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Gaudry M., Bartoli J.M., Bal L., Giorgi R., De Masi M., Magnan P.E., Piquet P. Anatomical and Technical Factors Influence the Rate of In-Stent Restenosis following Carotid Artery Stenting for the Treatment of Post-Carotid Endarterectomy Stenosis. PLoS ONE. 2016;11:e0161716. doi: 10.1371/journal.pone.0161716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timaran C.H., McKinsey J.F., Schneider P.A., Littooy F. Reporting standards for carotid interventions from the Society for Vascular Surgery. J. Vasc. Surg. 2011;53:1679–1695. doi: 10.1016/j.jvs.2010.11.122. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen D.T., Vokó B., Nyárádi B.B., Munkácsi T., Bérczi Á., Vokó Z., Dósa E. Restenosis rates in patients with ipsilateral carotid endarterectomy and contralateral carotid artery stenting. PLoS ONE. 2022;17:e0262735. doi: 10.1371/journal.pone.0262735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aboyans V., Ricco J.B., Bartelink M.E.L., Björck M., Brodmann M., Cohnert T., Collet J.P., Czerny M., De Carlo M., Debus S., et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: The European Stroke Organization (ESO), the Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) Eur. Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 13.Stanziale S.F., Wholey M.H., Boules T.N., Selzer F., Makaroun M.S. Determining in-stent stenosis of carotid arteries by duplex ultrasound criteria. J. Endovasc. Ther. 2005;12:346–353. doi: 10.1583/04-1527.1. [DOI] [PubMed] [Google Scholar]
- 14.Lee J.D., Ryu S.J., Chang Y.J., Hsu K.C., Chen Y.C., Huang Y.C., Lee M., Hsiao M.C., Lee T.H. Carotid ultrasound criteria for detecting intracranial carotid stenosis. Eur. Neurol. 2007;57:156–160. doi: 10.1159/000098467. [DOI] [PubMed] [Google Scholar]
- 15.Dósa E., Hirschberg K., Apor A., Járányi Z., Entz L., Acsády G., Hüttl K. Echolucent or predominantly echolucent femoral plaques predict early restenosis after eversion carotid endarterectomy. J. Vasc. Surg. 2010;51:345–350. doi: 10.1016/j.jvs.2009.08.080. [DOI] [PubMed] [Google Scholar]
- 16.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Barnett H.J.M., Taylor D.W., Haynes R.B., Sackett D.L., Peerless S.J., Ferguson G.G., Fox A.J., Rankin R.N., Hachinski V.C., et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 17.Bonati L.H., Ederle J., Dobson J., Engelter S., Featherstone R.L., Gaines P.A., Beard J.D., Venables G.S., Markus H.S., Clifton A., et al. Length of carotid stenosis predicts peri-procedural stroke or death and restenosis in patients randomized to endovascular treatment or endarterectomy. Int. J. Stroke. 2014;9:297–305. doi: 10.1111/ijs.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodcock R.J., Jr., Goldstein J.H., Kallmes D.F., Cloft H.J., Phillips C.D. Angiographic correlation of CT calcification in the carotid siphon. AJNR Am. J. Neuroradiol. 1999;20:495–499. [PMC free article] [PubMed] [Google Scholar]
- 19.Müller M.D., Lyrer P., Brown M.M., Bonati L.H. Carotid artery stenting versus endarterectomy for treatment of carotid artery stenosis. Cochrane Database Syst. Rev. 2020;2:CD000515. doi: 10.1002/14651858.CD000515.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Texakalidis P., Giannopoulos S., Jonnalagadda A.K., Kokkinidis D.G., Machinis T., Reavey-Cantwell J., Armstrong E.J., Jabbour P. Carotid Artery Endarterectomy versus Carotid Artery Stenting for Restenosis After Carotid Artery Endarterectomy: A Systematic Review and Meta-Analysis. World Neurosurg. 2018;115:421–429.e1. doi: 10.1016/j.wneu.2018.02.196. [DOI] [PubMed] [Google Scholar]
- 21.AbuRahma A.F., Abu-Halimah S., Bensenhaver J., Nanjundappa A., Stone P.A., Dean L.S., Keiffer T., Emmett M., Tarakji M., AbuRahma Z. Primary carotid artery stenting versus carotid artery stenting for postcarotid endarterectomy stenosis. J. Vasc. Surg. 2009;50:1031–1039. doi: 10.1016/j.jvs.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 22.White R.A., Sicard G.A., Zwolak R.M., Sidawy A.N., Schermerhorn M.L., Shackelton R.J., Siami F.S., SVS Outcomes Committee Society of vascular surgery vascular registry comparison of carotid artery stenting outcomes for atherosclerotic vs nonatherosclerotic carotid artery disease. J. Vasc. Surg. 2010;51:1116–1123. doi: 10.1016/j.jvs.2009.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuadra S., Hobson R.W., Lal B.K., Goldstein J., Chakhtoura E., Jamil Z. Outcome of carotid artery stenting for primary versus restenotic lesions. Ann. Vasc. Surg. 2009;23:330–334. doi: 10.1016/j.avsg.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Kahlberg A., Ardita V., Spertino A., Mascia D., Bertoglio L., Baccellieri D., Lembo R., Melissano G., Chiesa R. Propensity-Matched Comparison for Carotid Artery Stenting in Primary Stenosis versus after Carotid Endarterectomy Restenosis. Ann. Vasc. Surg. 2021;70:332–340. doi: 10.1016/j.avsg.2020.06.063. [DOI] [PubMed] [Google Scholar]
- 25.Hynes B.G., Kennedy K.F., Ruggiero N.J., 2nd, Kiernan T.J., Margey R.J., Rosenfield K., Garasic J.M. Carotid artery stenting for recurrent carotid artery restenosis after previous ipsilateral carotid artery endarterectomy or stenting: A report from the National Cardiovascular Data Registry. JACC Cardiovasc. Interv. 2014;7:180–186. doi: 10.1016/j.jcin.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Mousa A.Y., AbuRahma A.F., Bozzay J., Broce M., Kali M., Yacoub M., Stone P., Bates M.C. Long-term Comparative Outcomes of Carotid Artery Stenting Following Previous Carotid Endarterectomy vs De Novo Lesions. J. Endovasc. Ther. 2015;22:449–456. doi: 10.1177/1526602815581597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arhuidese I.J., Rizwan M., Nejim B., Malas M. Outcomes of Primary and Secondary Carotid Artery Stenting. Stroke. 2017;48:3086–3092. doi: 10.1161/STROKEAHA.117.016963. [DOI] [PubMed] [Google Scholar]
- 28.Fokkema M., de Borst G.J., Nolan B.W., Lo R.C., Cambria R.A., Powell R.J., Moll F.L., Schermerhorn M.L., Vascular Study Group of New England Carotid stenting versus endarterectomy in patients undergoing reintervention after prior carotid endarterectomy. J. Vasc. Surg. 2014;59:e1–e2. doi: 10.1016/j.jvs.2013.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huibers A.E., Westerink J., de Vries E.E., Hoskam A., den Ruijter H.M., Moll F.L., de Borst G.J. Editor’s Choice—Cerebral Hyperperfusion Syndrome After Carotid Artery Stenting: A Systematic Review and Meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2018;56:322–333. doi: 10.1016/j.ejvs.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann A., Taschner C., Engelter S.T., Lyrer P.A., Rem J., Radue E.W., Kirsch E.C. Carotid artery stenting versus carotid endarterectomy. A prospective, randomised trial with long term follow up (BACASS) Schweiz. Arch. Neurol. Psychiatr. 2006;157:191–192. [Google Scholar]
- 31.Steinbauer M.G., Pfister K., Greindl M., Schlachetzki F., Borisch I., Schuirer G., Feuerbach S., Kasprzak P.M. Alert for increased long-term follow-up after carotid artery stenting: Results of a prospective, randomized, single-center trial of carotid artery stenting vs carotid endarterectomy. J. Vasc. Surg. 2008;48:93–98. doi: 10.1016/j.jvs.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 32.Bonati L.H., Gregson J., Dobson J., McCabe D.J.H., Nederkoorn P.J., van der Worp H.B., de Borst G.J., Richards T., Cleveland T., Müller M.D., et al. Restenosis and risk of stroke after stenting or endarterectomy for symptomatic carotid stenosis in the International Carotid Stenting Study (ICSS): Secondary analysis of a randomised trial. Lancet Neurol. 2018;17:587–596. doi: 10.1016/S1474-4422(18)30195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mas J.L., Arquizan C., Calvet D., Viguier A., Albucher J.F., Piquet P., Garnier P., Viader F., Giroud M., Hosseini H., et al. Long-term follow-up study of endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis trial. Stroke. 2014;45:2750–2756. doi: 10.1161/STROKEAHA.114.005671. [DOI] [PubMed] [Google Scholar]
- 34.Liu C.W., Liu B., Ye W., Wu W.W., Li Y.J., Zheng Y.H., Wu J.D., Zeng R., Guan H. [Carotid endarterectomy versus carotid stenting: A prospective randomized trial] Zhonghua Wai Ke Za Zhi. 2009;47:267–270. [PubMed] [Google Scholar]
- 35.Eckstein H.H., Ringleb P., Allenberg J.R., Berger J., Fraedrich G., Hacke W., Hennerici M., Stingele R., Fiehler J., Zeumer H., et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: A multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902. doi: 10.1016/S1474-4422(08)70196-0. [DOI] [PubMed] [Google Scholar]
- 36.Brott T.G., Howard G., Roubin G.S., Meschia J.F., Mackey A., Brooks W., Moore W.S., Hill M.D., Mantese V.A., Clark W.M., et al. Long-Term Results of Stenting versus Endarterectomy for Carotid-Artery Stenosis. N. Engl. J. Med. 2016;374:1021–1031. doi: 10.1056/NEJMoa1505215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang P., Liang C., Du J., Li J. Effects of carotid endarterectomy and carotid artery stenting on high-risk carotid stenosis patients. Pak. J. Med. Sci. 2013;29:1315–1318. doi: 10.12669/pjms.296.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mannheim D., Karmeli R. A prospective randomized trial comparing endarterectomy to stenting in severe asymptomatic carotid stenosis. J. Cardiovasc. Surg. 2017;58:814–817. doi: 10.23736/S0021-9509.16.09513-6. [DOI] [PubMed] [Google Scholar]
- 39.Antonello M., Deriu G.P., Frigatti P., Amistà P., Lepidi S., Stramanà R., Battocchio P., Dall’Antonia A., Grego F. Does the type of carotid artery closure influence the management of recurrent carotid artery stenosis? Results of a 6-year prospective comparative study. Surgery. 2008;143:51–57. doi: 10.1016/j.surg.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 40.AbuRahma A.F., Abu-Halimah S., Hass S.M., Nanjundappa A., Stone P.A., Mousa A., Lough E., Dean L.S. Carotid artery stenting outcomes are equivalent to carotid endarterectomy outcomes for patients with post-carotid endarterectomy stenosis. J. Vasc. Surg. 2010;52:1180–1187. doi: 10.1016/j.jvs.2010.06.074. [DOI] [PubMed] [Google Scholar]
- 41.Dorigo W., Pulli R., Fargion A., Pratesi G., Angiletta D., Aletto I., Alessi Innocenti A., Pratesi C. Comparison of open and endovascular treatments of post-carotid endarterectomy restenosis. Eur. J. Vasc. Endovasc. Surg. 2013;45:437–442. doi: 10.1016/j.ejvs.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 42.Marques de Marino P., Martinez Lopez I., Hernandez Mateo M.M., Cernuda Artero I., Cabrero Fernandez M., Reina Gutierrez M.T., Serrano Hernando F.J. Open Versus Endovascular Treatment for Patients with Post-Carotid Endarterectomy Restenosis: Early and Long-term Results. Ann. Vasc. Surg. 2016;36:159–165. doi: 10.1016/j.avsg.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 43.Rinaldo L., Bhargav A.G., Arnold Fiebelkorn C.E., Lanzino G. Long-Term Mortality After Carotid Stenting. World Neurosurg. 2020;141:e589–e599. doi: 10.1016/j.wneu.2020.05.264. [DOI] [PubMed] [Google Scholar]
- 44.Wen L., Wang S., Liu L., Chen L., Geng J., Kuang L., Qian G., Su J., Chen K., Zhou Z. The Long-Term Efficacy and Safety of Carotid Artery Stenting among the Elderly: A Single-Center Study in China. Behav. Neurol. 2018;2018:4707104. doi: 10.1155/2018/4707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to reasons pertaining to patient privacy.